Abstract
Appetite is controlled by a complex system of central and peripheral signals interacting to modulate the ingestion response. Several brain mediators with complex networks adjust food intake in birds. Based on the available literature, these mediators have interactions with a number of other neurotransmitters (NTS) involved in feed intake. It means that, NTS regulate feeding behavior through mediating other peptide and NTs activity. In birds, insulin known as a hypophagic hormone that is interplaying with neuropeptide Y (NPY), pro-opiomelanocortin (POMC), and corticotropin-releasing factor (CRF) in brain. Another hormone ghrelin, inhibits food intake in birds and other mediators, such as glutamate, endocannabinoid system (ECS), serotonin (5-hydroxytryptamine, 5-HT), and norepinephrine (NE), which play a key role in ghrelin-induced hypophagia. Another involved peptide on feeding behavior in chickens called nociceptin/orphanin FQ (N/OFQ) is modulated by histamine, glutamate, dopamine (DA), gamma-aminobutyric acid (GABA), agouti-related protein (AgRP), and cocaine and amphetamine-regulated transcript (CART). Some of the NTS such as opioid have both orexigenic and anorexigenic effects in birds while has interaction with NE, glutamate, histamine, DA, and cannabinoids (CBs). Thus, interaction among mediators is a prominent process needs to be considered in order to understanding the mechanisms underlying feed intake regulation in birds. This review aims to investigate the role of major regulators and their mediatory interactions with one another in poultry feeding behavior. According to mentioned interactions, it seems that dopamine, serotonin, and glutamate have the most interactions with other NT systems. Therefore, they play an axial role in the central regulation of food intake in CNS.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Appetite modulation is a complicated physiologic phenomenon that is made of various central and peripheral signal integration at the CNS. Different CNS compartments such as hypothalamus, nucleus accumbens (NAcc), amygdala, ventral tegmental area (VTA), and nucleus of the tractus solitaries have effect on appetite modulation (Barnes et al. 2006). In this view, the hypothalamic subregions the arcuate nucleus (ARC), paraventricular nucleus (PVN), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and lateral hypothalamic area (LHA) have underpinning role in regulate food consumption (Fig. 1) (Wynne et al. 2005; Yousefvand and Hamidi 2021). Cerebral mechanisms by neural mediators control food intake in the hypothalamus (Zendehdel et al. 2014). It has been demonstrated that the mediators in the CNS, have different stimulatory, inhibitory, and modulatory roles in order to regulate various physiologic behaviors, such as perception, pleasure, excitement, memory, and learning.
Schematic figure containing the special nuclei which regulate appetite. AM amygdala, ARC arcuate nucleus, CCX cerebral cortex, DMN dorsomedial nucleus, FX fornix, CC corpus callosum, HI hippocampus, LHA lateral hypothalamic area, ME median eminence, PFA perifornical area, OC optic chiasm, PVN paraventricular nucleus, 3 V third ventricle, TH thalamus, VMN ventromedial nucleus, SE septum (Yu and Kim 2012)
It is worth mentioning that appetite is adjusted by a vast scattered network of neurons via different NTS (Alizadeh et al. 2015; Hassanpour et al. 2015). NTS are kinds of mediators secreted by neuronal terminals, influencing pre-/postsynaptically. Some of them, such as DA, 5-HT, and ghrelin, decrease food intake in birds, while N/OFQ, CBs, and GABA increase it (Denbow et al. 2000; Bungo et al. 2009; Zendehdel et al. 2014a, 2017c, 2020, 2013c, 2019, 2017a, 2008). Among the NTS involved with the food intake regulation, epinephrine, NE, DA, 5-HT, and histamine belong to the subgroup of biogenic amines; however, GABA and glutamate are amino acids, NPY and opioids are recognized as neuropeptides. Given the importance of food consumption in several physiological processes, such as growth, immunity, and production, realizing the NTS effect on ingestion behavior has been one of the interesting fields of study over the last decades. On this line, the interaction between NTS is a noticeable research field in which different investigations have revealed its significant effect on the modulation of food intake. For instance, the previous studies have shown that the hypophagic effect induced by the melanocortin receptors (MCRs) is modulated by the serotonin and glutamate in the chicken’s brain (Khodadadi et al. 2017; Zendehdel et al. 2016; Shiraishi et al. 2008). In this aspect of view, the crosstalk between above-mentioned NTS and the other mediators such as leptin and insulin is a remarkable subject which is under evaluation. Considering the importance of interactions regulating feed intake, this review intends to study the role of mediators and the interactions between them in the central regulation of food intake in birds.
Study Methodology
In this review, various valid papers from electronic sources have been used in order to investigate the crosstalk between brain mediators regulating food intake behavior in birds. Authentic articles were indexed in the Web of Science, Scopus, PubMed, SID, Google scholar, and ISI databases by using of the key words: Central regulation of food intake, Brain neurotransmitters, Bird food intake, and Feeding behavior studied.
Biogenic Amines
Dopamine (DA)
DA is the primary catecholamine neurotransmitter in the central nervous system (CNS), controlling several physiological operations, such as emotion, locomotor activity, cognition, and food intake. DA is an important anorexigenic neurotransmitter regulating reward function through its projections from VTA into NAcc and ARC (Volkow et al. 2011). Nowadays, five different subtypes of DA receptors are recognized (D1–5), appertain to G protein-coupled receptor subtypes (GPCRs). D1, like receptor subtypes D1 and D5, connects to the stimulatory G protein (Gs) by adenylyl cyclase pathway, whereas D2, like subfamily D2, D3, and D4, performs via inhibiting adenylyl cyclase and activating K channels (Ikemoto 2007). D1 and D2 receptors are ampler than the other DA receptors in the brain areas (Cadet et al. 2010). It has been revealed that DA decreases food intake via the D1 receptor (Bungo et al. 2010; Zendehdel et al. 2014a). Also, it has been reported that D2 has a mediatory role in appetite regulation (Khodadadi et al. 2017). The mediatory function of D1 and D2 receptors in food desire, induced by other central systems, has been confirmed (Mahzouni et al. 2016). Previous studies have shown DA interaction with other brain mediators. In this regard, Taherian et al. (2016) have reported that DA-induced hypophagia is mediated via NMDA and mGlu1 receptors in chicken. Also, Zendehdel et al. (2014a) have shown that DA performs its effect through the 5-HT receptor, 5-HT2c. Based on recent studies, yohimbine (α2 receptor antagonist) and ICI 118,551 (β2 adrenergic receptor antagonist) amplify and inhibit DA-induced hypophagia, respectively (Zanganeh et al. 2020). The anorexigenic effect of DA agonists is enhanced by the precursor of Nitric oxide (NO), showing a connection between them (Zendehdel et al. 2017a). Besides, there is a neurological interplay between µ and D1 receptors in appetite regulation (Zendehdel et al. 2016). Pretreatment with the cannabinoid receptor agonist increases feed consumption mediated through the DA receptor antagonist (Khodadadi et al. 2017). Moreover, the interplay between GABA and DAergic systems in feeding behavior has been demonstrated. Accordingly, research on birds has shown that the GABAA receptor agonist-hyperphagic effect amplifies by D1 receptor antagonization (Hashemzadeh et al. 2018). In addition, GhandForoushan et al. (2017) have indicated that H1 and H2 receptors antagonist respectively attenuates and amplifies the DA hypophagic effect on food intake. All above-mentioned results accentuate the interconnection between DA and the glutamate, 5-HT, NE, NO, opioid, CBs, GABA and histamine systems in regulation of food intake behavior in birds.
Serotonin (5-Hydroxytryptamine, 5-HT)
5-HT acts as a mediator in many processes in both the peripheral and central nervous systems and has various effects on food desire, general metabolism, and sleep (Caliendo et al. 2005). Based on our knowledge, 5-HT receptors can be categorized into seven subtypes (5-HT1-5-HT7), considering amino acid sequence, pharmacological property, signal transduction, and molecular cloning (Hoyer et al. 2002). Almost all 5-HT receptors, except for the 5-HT3 subtype as a ligand-gated ion channel, form a subset of G protein-coupled receptors (GPCRs) (Bikker et al. 1998). In CNS, 5-HT originates mainly from the midbrain raphe' nuclei (Ciranna 2006; Takahashi et al. 2010). Further, 5-HT is known to regulate mood, although 5-HT affects the central regulation of feeding behavior in mammals and avian species (Bechtholt et al. 2007; Fang et al. 2013). Reduction effect and mediatory contribution of 5-HT in food intake have been reported through different researches; 5-HT reduces food desire by connecting to POMC neurons (Zendehdel et al. 2012a). Also, an interplay has been observed between DA and 5-HT; hypophagic effect of D1 was attenuated by 5-HT2C antagonist (Zendehdel et al. 2014a). Besides, anorexigenic effect of this ligand is amplified by GABAA receptor antagonist and α2 receptor antagonist whereas attenuated by NMDA, AMPA/Kainate receptor antagonist, and β2 receptor antagonist (Zendehdel et al. 2017c; Mortezaei et al. 2013). Additionally, some receptors of 5-HT (5-HT2a and 5-HT2c) can decrease the anorexigenic effect of harmaline on food desire (Zendehdel et al. 2013b). The receptors of 5-HT (5-HT2c) and glutamate (NMDA) diminished the hypophagic effect of lipopolysaccharides (LPS) regulated via CRF (Zendehdel et al. 2012b; Jonaidi et al. 2019).
Norepinephrine (NE)
Norepinephrine (NE), as a catecholamine, plays a significant role in response to the stressful stimuli in chicken. Based on the studies, catecholamines have a regulatory role by adjusting appetite in birds' production. Also, the regulatory activity of NE in the brain-gut-microbiome axis has been emphasized in chicken (Dennis et al. 2016). In overall, it has been well documented that this ligand intracranioventricularly changes food desire in birds similar to mammals (Denbow and Sheppard 1993). Although the ICV injection of this catecholamine increases food intake in chicken (Denbow 1983), the same treatment in turkey shows controversial results; interestingly, the same study in leghorn does not show any effect on appetite in this strain (Denbow et al. 1983). NE has also a mediatory role in food intake in relation with the other brain mediators. For example, the effect of ghrelin and leptin is adjusted by this ligand receptor. In this respect, the significant attenuating effect of β2 antagonist on hypophagia induced by ghrelin and leptin has been detected in broiler chickens (Zendehdel and Hassanpour 2014a, b; Zendehdel et al. 2020). Moreover, as mentioned earlier, NE interacts with opioid, 5-HT, oxytocin (OT), and DA (Nayebzadeh et al. 2020; Zendehdel et al. 2017c; Mirnaghizadeh et al. 2017; Zanganeh et al. 2020).
Histamine
Histamine is a biogenic amine with a central function in the Nervous System. Its receptors include H1, H2, H3, and H4 (Masaki et al. 2005). The central histaminergic system is implicated in adjusting several physiological aspects, e.g., food consumption, thermos-regulation, and locomotor activity (Swiergiel et al. 1999). According to studies, histamine diminished food desire in birds and also it has regulatory effect in feeding (Zendehdel et al. 2008); the anorexigenic effect of LPS is lessened by the H1 receptor of this amine (Zendehdel et al. 2015a). Further, the effect of nesfatin-1, an endogenous anorectic peptide, and GABA is attenuated by H1 and H3 receptors antagonist (Heidarzadeh et al. 2018; Morteza et al. 2013).
Amino Acids
Glutamate
One of the major stimulative NTs in the CNS, playing an important role in reward processes and hypothalamic centers, is glutamate (McFadden et al. 2014). According to pharmacological properties of the glutamate receptors, they can be divided into two categories, including the ionotropic and metabotropic receptors (mGluRs). Main glutamate receptors called N-methyl-D-aspartate (NMDA), Kainate, AMPA, and the metabotropic receptors (mGluRs) with different subtypes (Charles et al. 2014). Glutamate has hypophagic effect, as well as many interactions with other mediators, showing its significant role in food intake regulation in birds. Based on studies, feed intake in pigeon is affected by the injection of NMDA and AMPA-kainite receptor antagonists (Da Silva et al. 2003). It has been well documented that the receptor antagonist of NMDA increases food intake (Taati et al. 2011). In chickens, DA performs its effect via some receptors of glutamate (Taherian et al. 2016). Also, the glutamate hypophagic effect is mediated through melanocortin system, CRF, GABA, and histamine. As reported, receptor antagonist of MCRS and CRFS adjust the effect of glutamate on feed desire in a manner that the administration of the receptors MC3,4 antagonists, similar to that of in CRF1,2 receptors, attenuated the hypophagic effect of glutamate. These results suggested that the hypophagic effect of glutamate mediates via CRF1,2 and MC3,4 receptors in chickens (Ahmadi et al. 2019). Moreover, it has been shown that the effect of glutamate on feeding behavior is enhanced through the GABAA receptor antagonist (Zendehdel et al. 2009). Recently, an interplay between glutamate and receptors of histamine H1,3 has been observed. Accordingly, in contrary to H3 antagonist, H1 antagonist can reduce the hypophagic influence of glutamate in layer chicken (Mobarhan et al. 2020). In addition, previous studies have demonstrated that the anorexigenic properties of ghrelin and leptin is modulated through the glutamic system (Taati et al. 2011; Adeli et al. 2020).
Gamma-Aminobutyric Acid (GABA)
GABA is an important neurotransmitter with many physiological roles such as respiration and appetite control, anti-convulsion, memory and sleep regulation beside the pain modulation (Chen et al. 2015). GABAA, GABAB, and GABAC are three receptors that the GABAergic system acts through (Stratford and Wirtshafter 2013). GABAA and GABAC belong to a macromolecular complex coupled to a Cl-ionophore, whereas GABAB, a metabotropic receptor, is a member of G-protein-coupled receptors (GPCRs). Feeding behavior by proposed receptors is the subject of many studies. Based on previous studies, feeding is enhanced by GABAA and GABAB agonists (Zendehdel et al. 2017c; Bungo et al. 2003). However, GABAB agonist does not affect broiler food desire (Zendehdel and Hassanpour 2014a, b). In addition, GABA stimulates food intake in the turkey (Denbow 1991). It has been shown that GABAA has orexigenic effect on appetite in birds and this effect is adjusted by Nitrol-arginine methyl ester (L-NAME) and N/OFQ (Mokhtarpouriani et al. 2016a, b; Tajalli et al. 2006); orexigenic effect of GABAA decreased by CB1 receptor antagonist and amplified by D1 receptor antagonist (Hashemzadeh et al. 2018; Zendehdel et al. 2017c).
Glycine
Glycine is an inhibitory neuronal mediator in the CNS where several functions are accomplished by this ligand such as synaptic transmission (Colin et al. 1998; Scain et al. 2010), important role in motor control (Rees et al. 2003), pain perception (Harvey et al. 2004), and food intake (Rahimi et al. 2021; Reidelberger et al. 2011; Sorrels and Bostock 1992). Research on chicken has revealed that glycine decreases food intake and this effect is amplified by DA. Also, recently, it has been reported that this interaction is exerted through D1 receptors (Rahimi et al. 2021).
Peptides
In the bellow chapters, the effects of well-known peptides and their interactions with different NTs systems on regulation of feeding behavior will be described. According to the fact that these peptides may have anorexigenic and/or orexigenic effects in avian and other species, the following classification is considered in which the peptides with hypophagic, hyperphagic, and both hypo- and hyperphagic properties have been categorized in anorexigenic, orexigenic, and orexigenic/anorexigenic subtypes respectively.
Anorexigenic Peptides
Corticotropin-Releasing Factor (CRF)
CRF is a 41 amino acid neurotransmitter which acts on the anterior pituitary to stimulate the secretion of corticotropin and regulates the synthetic/secretory activity of the adrenal cortex (Vale et al. 1981). This peptide in the central nervous system and in the periphery, has various actions such as regulating anxiety, mood, feeding, inflammation, gastric emptying, and blood pressure (Dautzenberg and Hauger 2002). Regarding its effect on feeding, feed intake in mammals and chickens is affected and decreased by ICV injection of CRF (Contarino and Gold 2002; Furuse et al. 1997; Denbow et al. 1999; Zhang et al. 2001). From the interactional perspective, it has been documented that CRF has interplay with LPS, ghrelin, RFamide-related peptide-3 (RFRP-3), and glutamate and adjusts their effects on feeding in chicken. As a result of these interactions, anorexigenic effect of LPS, ghrelin, RFRP-3, and glutamate is attenuated by central injection of CRF receptors antagonist. Moreover, as mentioned earlier, there is an interplay between 5-HT (5-HT2c) and CRF (Kooshki et al. 2019; Saito et al. 2005; Moosadoost et al. 2020; Ahmadi et al. 2019).
Melanocortins
The melanocortins include adrenocorticotropic hormone (ACTH), and α-, β-, and γ-melanocyte-stimulating hormones (α-, β-, γ-MSHs) which are derived from the cleavage of the precursor POMC (Wang et. al. 2019). ACTH is an important component of the hypothalamic–pituitary–adrenal axis and is often produced in response to biological stress. It has shown that this peptide changes food intake and can induce anorexia in animals (Stevenson et al. 1970; Van Putten et al. 1953; Schulz et al. 2010; Deviche and Delius 1981). α-MSH performs a similar role to ACTH in feeding behavior and energy homeostasis (Kawakami et al. 2000; Zendehdel et al. 2012a); in avian, α-MSH has five subtypes of MCR expressed throughout the body which are a family of G protein-coupled receptors (Dores et al. 2013). In this relevant, MCR subtypes 3 and 4 (MC3,4) are presented more abundant in the brain (Shojaei et. al. 2020) and perform a hypophagic effect in chicken (Zendehdel et al. 2012a). In terms of the studying the role of brain mediators interaction in poultry food intake behavior, several lines of studies have revealed the interplay between melanocortin receptors and various mediators such as 5-HT, glutamate, insulin, leptin, RFRP-3, and Neuropeptide FF (NPFF). In this view, there is a neurological interaction between serotoninergic and melanocortin systems, affecting the feeding behavior. It is further reported that the 5-HT anorexigenic effect is mediated by receptors of melanocortin (Zendehdel et al. 2012a). Moreover, the hypophagic effect of glutamate is decreased through MC3,4 receptors antagonist (Ahmadi et al. 2019; Shiraishi et al. 2008). Besides, it has been depicted that, central injection of insulin in chicks significantly increased expression of POMC mRNA which leads to diminish food intake (Shiraishi et al. 2008). Also, previous research on broiler chickens has demonstrated that the expression of hypothalamic gene of MCR subtypes 4 and 5 was significantly reduced by the infusion of leptin (Dridi et al. 2005). This, in turn, postulated that the crosstalk between melanocortin system and leptin can regulate food intake in birds through inducing hypophagia. In addition, it has been proved that the anorexic neuropeptide RFRP-3, which belongs to arginine-phenylalanine-amide (RFamide) peptide family, decreases food intake in chicken, and its effect is mediated via MC4 and CRF2 receptors. Also, authors have suggested a modulatory role for receptors of the Neuropeptide FF (NPFF), a member of RFamide family, in food intake induced by RFRP-3 in chickens brain (Moosadoost et al. 2019, 2020).
Oxytocin (OT)
OT is synthesized in supraoptic and PVN nuclei, as well as hypothalamic magnocellular accessory neurons (Adan et al. 1995). Receptors of OT are located in the PVN and VMH nucleus, the stria terminals, and the dorsal part of the supraoptic nucleus (Adan et al. 1995). The OT injection declines food desire in mammals and birds (Jonaidi et al. 2003; Arletti et al. 1990). Further, OT performs its effect through receptors of opioid (μ and κ opioidergic receptors) and glutamate (NMDA and AMPA receptors) (Jalali et al. 2019; Raji-Dahmardeh et al. 2019). Finally, its effect on food consumption is adjusted via receptors of histamine (H1 and H3 receptors) and NE (β2 receptors) in birds (Mirnaghizadeh et al. 2017).
Leptin
Leptin, known as an obesity gene product, is a small (16 kDa) peptide hormone secreted from adipose tissue. Its central injection reduces food intake and enhances energy expenditure. In CNS, leptin has a significant role in food desire (Valassi et al. 2008). Chicken's leptin cDNA (CLEP) (Genebank AF 012727, AF082500) is cloned and sequenced in birds. Sequencing the leptin gene in chicken has confirmed 145 amino acids instead of 146 in mammals. It is noted that leptin is in chicken even before its sequencing in this species (Ashwell et al. 1999). Farkašová et al. (2016) confirm the presence of the LEP gene in birds after long-term debates on the absence (Lovell et al., 2015) or the presence of CLEP sequence in the chicken genome. Similar to mammals, the leptin reduction effect on feed intake in avian is reported (Denbow et al. 2000). As mentioned above, previous research on feeding regulation demonstrated the crosstalk between leptin and the melanocortin system. In this view, under stimulation of leptin, hypothalamic gene expression of MC4/MC5 has significantly become downregulated (Dridi et al. 2005). Remarkably, the recombinant chicken leptin has also decreased the gene expression of the orexigenic neuroprptides NPY, orexin and orexin receptor in broiler chicken hypothalamus (Dridi et al. 2005). This latter demonstrates the leptin interaction with NPY and orexin system. Interestingly, leptin and ghrelin in a wild bird decrease hoarding behavior (Henderson et al. 2018). In addition, it has been well documented that the anorexigenic effect of leptin is attenuated by β2-selective adrenoceptor antagonist displaying the interaction between leptin and NE system (Zendehdel et al. 2020). Furthermore, in terms of the interplay between leptin and other brain mediator systems, recently the receptors of glutamate (NMDA and AMPA) have been shown to modulate the effect of leptin (Adeli et al. 2020).
Insulin
Insulin is a hormone secreted from Beta cells of the pancreas that controls blood sugar. In the brain, it affects the central regulation of food desire and energy consumption (Plum et al. 2005). A number of studies have shown the effect of insulin on appetite adjustment. In general, insulin mainly has anorexigenic properties through having interaction with different peptides with orexigenic or anorexigenic effects. In this view, it is stated that mRNA levels of POMC, CART, and CRF are upregulated by insulin. To have more explanation, it has been reported that α-MSH known as an anorexigenic peptide, forms from the post-translational cleavage of POMC at the hypothalamus, CART and CRF have hypophagic effect on ingestion behavior in birds (Honda et al. 2007). Interestingly, it has been demonstrated that the antagonization of melanocortin receptors can prohibit the hopophagia induced by insulin in chicks (Shiraishi et al. 2008). Therefore, as in mammals, the central melanocortin system mediates insulin-induced hypophagia in birds (Fig. 2). In another aspect, the central injection of this hormone, namely insulin, in chicks decreases NPY mRNA (Shiraishi et al. 2008). Also, its ICV injection affecting food intake is mediated via the interaction with the receptors of NPY (Yousefvand et al. 2018, 2019). NPY1 receptor antagonist potentiates decreasing effect of insulin on food consumption; while, this decreasing effect of insulin is prevented by antagonizing NPY2 receptor (Yousefvand et al. 2020).
The POMC and NPY neurons situated in ARC nucleus as well as receptors of insulin found on these neurons. Also, CRF, receptors of POMC (MC3,4) and NPY1 are located on the PVN. The ICV administration of insulin stimulated neurons of CART and POMC along with suppressing NPY neurons. Consequently, increased level of α-MSH resulted from POMC neurons stimulated the melanocortin receptors and resulted hypophagia. In another aspect, insulin-induced decreased level of NPY in synaptic space caused reduction activity of NPY1 receptor. Besides, there is an interaction between insulin and CRF neuron in PVN. Consequently, anorexigenic output is exported by PVN, and caused a hypophagic effect on food consumption in bird under effect of insulin and mentioned interactions (Honda et al. 2007; Shiraishi et al. 2008; Yousefvand et al. 2018 and 2019)
Orexigenic Peptides
Agouti-Related Peptide (AgRP)
AgRP is a peptide which is made up of 112 amino acids and synthesized in the ARC, projects to other key hypothalamic nuclei and sites involved in feeding (Bagnol et al. 1999; Haskell-Luevano et al. 1999; Lu et al. 1994). AgRP potentiates food intake behavior and this orexigenic effect of AgRP has documented in birds and mice (Boswell and Dunn 2017; Takahashi and Cone 2005). Functionally, AgRP binds to the melanocortin receptors MC3,4 and therefore is considered as a member of central melanocortin system (Boswell and Dunn 2017). Research on mammals has revealed that AgRP exerts its effect through agonizing Gi protein- coupled MC4 which results in inversing the effect of Gs protein- coupled MC4 agonists (i.e., α-MSH); subsequently, decreasing the levels of cAMP and the synthesis of CRF and Thyroid releasing hormone (TRH) in hypothalamus (Baldini and Phelan 2019; Sarkar et al. 2002). This inverse agonistic effect of AgRP leads to increase in food intake behavior (Baldini and Phelan 2019). The biased agonism of AgRP is also reported toward MC3 (Yang and Tao 2017). Beside of the decreasing effect of CRF on feeding, which was mentioned before, the similar effect is also produced by subcutaneous and ICV administration of TRH in rodents (Choi et al. 2002; Vijayan and McCann 1977). It has been proposed that this effect is independent of hypothalamus-pituitary-thyroid, HPT, axis and is exerted via central mechanisms (Yoo et al. 2021). In this respect, TRH increases the histamine turnover in tuberomammillary, PVN, VMH nuclei, and activates GABAergic neurons in lateral hypothalamic area resulting melanin-concentrating hormone, an orexigenic peptide, expressing neurons suppression (Zhang et al. 2012; Gotoh et al. 2007). Interestingly, in another aspects of view, it has been shown that AgRP neurons in ARC can be excited by glutamatergic projections originated from TRH expressing neurons in PVN introducing TRH orexigenic effects (Krashes et al. 2014). Reciprocally, NPY/AgRP neurons along with POMC neurons in ARC develop synaptic inputs to PVN TRH neurons (Yoo et al. 2021). Since the most of TRH neurons in PVN express MC4, and in consideration of the agonistic effect of α-MSH on Gs protein- coupled MC4 along with agonistic effect of AgRP on Gi protein- coupled MC4, the regulatory role of central melanocortin system consists of AgRP and α-MSH through MC4 on TRH neurons is plausible. These mechanisms remain to be determined in the future studies on birds.
Moreover, there is interaction between AgRP and other mediators which have key role in food intake such as opioids and N/OFQ. For instance, the concentration of AgRP mRNA in the diencephalon increased after central injection of N/OFQ (Hagan et al. 2001; Bungo et al. 2009).
Neuropeptide Y (NPY)
NPY is one of the amplest peptides in the nervous system affecting food intake. Structurally, it consists of 36 amino acids with a single different residue between avian and mammalian amino acid sequences. NPY gene regulates food consumption and reproductive activity (Fraley and Kuenzel 1993). NPY is the most potent orexigenic peptide functioning through NPY1 and NPY2 receptors. The ARC is well presented as the major center for controlling appetite at the hypothalamus in mammalian. Further, it is shown to be almost permeable to NPY and able to receive peripheral inputs from lateral ventricle fluid. It has been indicated that the first-order orexigenic neurons located in the Arc are responsible for NPY secretion. NPY has been reported to have the orexigenic effect in broiler and Leghorn (Denbow et al. 1988; Cline and Furuse 2013). It increases food intake in chicken, while the injection of anti-chicken NPY antibody reduces it in early hatched chickens (Chen et al. 2016). Research on rodents has unraveled that the stimulation of the NPY receptor subtype 1 potentiates feeding activity; in contrary, agonizing the NPY2 receptor presents hypophagic behavior. For sake of clarity, NPY2 receptor exerts its action as an autoreceptor resulting the inhibition of NPY biosynthesis and release (Ortiz et al. 2007). Likewise, the extracted data of further studies on chickens are in agreement with mentioned results (Yousefvand et al. 2019). The mediatory role of NPY and related receptors in adjusting food desire in birds has been documented. NPY has interaction with insulin so that the hypophagic effect of insulin are regulated by the receptors of NPY, NPY1 and NPY2 (Yousefvand et al. 2018, 2019). In another aspect, insulin can reduce the mRNA expression of NPY highlighting the interplay between NPY system and insulin (Shiraishi et al. 2008). Moreover, research work on chickens has shown that somatostatin has orexigenic properties. Interestingly, on this line, somatostatin has interaction with the receptor NPY1 (Yousefvand et al. 2018).
Nociceptin/Orphanin FQ (N/OFQ)
N/OFQ is an endogenous ligand for the opioid-like GPCR1 or nociceptin receptor (NOP) (Alt et al. 2012). As reported in earlier researches, food desire is increased under effect of N/OFQ (Zendehdel et al. 2013a; Abbasnejad et al. 2005). This ligand involves GABA-induced hyperphagia through receptors of GABAA in birds (Tajalli et al. 2006). 5-HT amplifies the influence of this neuropeptide in chicken by receptors of 5HT2C (Zendehdel et al. 2013a). Also, based on findings, the orexigenic effect of N/OFQ is mediated through CART, AgRP, glutamate, NE, DA, and histamine in chicken. It is stated that this ligand increases the concentration of AgRP mRNA while declines CART mRNA. α-MSH blocks this neuropeptide effect, indicating that the AgRP and the CART neurons may mediate such a hyperphagic effect, as in mammals (Bungo et al. 2009). The receptor of NE (β2) and glutamate (NMDA and AMPA) can increase the hyperphagic effect of N/OFQ (Zendehdel et al. 2017b; Abolghasempour et al. 2019); the N/OFQ effect is enhanced by receptors of DA (D1 and D2) and histamine (H1), but it is attenuated by H3 (Zendehdel et al. 2019, 2015b).
Somatostatin
Another peptide which consists of 14 amino acids and was isolated from the ovine hypothalamus in 1973 for the first time, is somatostatin (Stengel et al. 2015). It acts as an inhibitory neurotransmitter, distributed in the brain especially in the ARC and the PVN nuclei (Stengel et al. 2015), plays an important role in stimulating food intake in animal (Schneeberger et al. 2014; Stengel et al. 2010b, 2010a; Karasawa et al. 2014). It has shown that this peptide has interplay with other madiators. For instance, somatostatin, in chickens through opioidergic-μ and adrenergic α-2-receptors, stimulates food intake (Tachibana et al. 2009). Also, the somatostatin- induced hyperphagia is significantly declined by NPY1 antagonist in chicken (Yousefvand et al. 2019).
Anorexigenic/Orexigenic Peptides
Ghrelin
As an endogenous ligand for growth hormone (GH), ghrelin was isolated from rat and human's stomach about 15 years ago. It, as one of the most important appetite-regulating peptides, has shown a stimulatory effect on food intake and GH release in the brain. Gene expression of ghrelin and its receptor GHS-R1a in the hypothalamus, liver, and abdominal fat of chicken's body has been measured recently. Although ghrelin is a stimulant factor for the secretion of human and avian GH in the brain, it prevents food intake in avian (Kaiya et al. 2011). In addition, it is confirmed that ghrelin has a reduction effect in avian (Taati et al. 2011; Zendehdel et al. 2013c). According to previous research, ghrelin-induced hypophagia is mediated via glutamate, CBs, 5-HT, NE, DA, CRF, and GABA. When it comes to the mediatory role of glutamate, NMDA receptor antagonist enhances the anorexigenic effect of ghrelin (Taati et al. 2011). Concerning the ghrelin interaction with CBs, the receptor antagonist of CBs can modulate its anorexigenic effect (Taherian et al. 2019). In mediation with 5-HT receptors, it has been demonstrated that the 5-HT2C receptor antagonist attenuates the effect of ghrelin (Zendehdel et al. 2013c). Besides, ghrelin performs its effect by interacting with the receptor of NE, namely β2 (Zendehdel and Hassanpour 2014a, b). Based on recent studies, cannabinoid receptor antagonists enhance the influence of ghrelin on appetite, and its impact is mediated by the DA receptor (Farrokhi et al. 2020). Ghrelin may diminish food desire in chicks by declining GABA synthesis due to its reduction effect on glutamate decarboxylase 2 (GAD2) gene expression (Jonaidi et al. 2012). Also, the effect of ghrelin is modulated via CRF, which has anorexic effect (Saito et al. 2005). In this regard, it has been concluded that the ICV administration of ghrelin in chickens accentuates the release of CRF; subsequently, HPA axis and corticosterone release (Saito et al. 2005). This illustrated the interplay between the anorexigenic peptides ghrelin and CRF in birds.
Opioids
Opioids form a well-known subgroup of inhibitory NTs. Its receptors consist of µ, δ, and k, being homologous to GPCRs (Fichna et al. 2007; Erbs et al. 2015). There is a plethora of endogenous opioid peptides in CNS, playing a key role in controlling respiration, pain mechanism, and the immune system (Le Merrer et al. 2009; Bodnar and Klein 2006). According to studies, the endogenous opioidergic system contributes to food intake regulation in birds. For example, the ICV injections of DAMGO (µ-opioid receptors agonist) reduce appetite, whereas DPDPE (δ-opioid receptors agonist) and U-50488H (κ-opioid receptor agonist) boost feeding behavior in chicks (Bungo et al. 2004, 2005). The µ receptor of opioid declines food consumption in chicken; however, other receptors enhance food desire (Zendehdel et al. 2016). There are some interactions between opioids and other brain mediators such as 5-HT (Shojaei et al. 2015), histamine (Jaefari et al. 2018), NE (Nayebzadeh et al. 2020), CBs (Zendehdel et al. 2015c), glutamate (Torkzaban et al. 2018), nitric oxide (NO) (Alimohammadi et al. 2015), and OT (Raji-Dahmardeh); given this, the reduction effect of DAMGO (µ-opioid receptor agonist) is amplified by CB1 and CB2 receptors antagonist, H3 receptor antagonist, and L-arginine (the precursor of NO) whereas that is diminished by NMDA and mGlu1 receptors antagonist, OT antagonist, β2 receptor antagonist, H1 receptors antagonist, and 5-HT2c receptor antagonist. Also, DPDPE (δ-opioid receptors agonist) induced hyperphagia is decreased by α1 receptor antagonist, while that is increased by AMPA glutamate receptors antagonist. In addition, orexigenic effect of U-50488H (κ-opioid receptor agonist) is attenuated by α2 receptor antagonist and has no interaction with the other drugs mentioned above (Raji-Dahmardeh et al. 2019).
RFamide Peptides
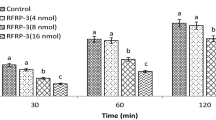
RFamide-related peptide-1 (RFRP-1) and RFamide-related peptide-3 (RFRP-3) belong to the arginine-phenylalanine- amide (RF amide) family and 5 peptide groups of this family such as Neuropeptide FF, (PQRFa) PrRP, LPXRFamides (RFRPs), Kisspeptin and QFRP (26RFa) have been recognized yet, playing a crucial role in food intake, puberty, and reproduction activity (Tsutsui et al. 2010). These peptides have hyperphagic and hypophagic effects in animals; it has been reported that an increasing trend in food intake occurs in mice after administration of 26RFamide (Chartrel et al. 2003) whereas NPFF and PRrP decrease feeding (Murase et al. 1996; Lawrence et al. 2000). Also, RFRP-3 increases food intake in mammals (Johnson et al. 2007; Murakami et al. 2008; Dockray 2004), but has hypophagic effect in chicken (Moosadoost et al. 2020). Some interactions between this family members and other mediators have been shown. For example, NPFF has crosstalk with DAMGO (μ-opioid receptor agonist), which regulates anorexigenic effect of this ligand in rats (Murase et al.1996; Nicklous and Simansky 2003). Also, in chicken, RFRP-3 induced hypophagia is mediated via corticotropin (CRF2) and melanocortin receptors (MC4) (Moosadoost et al. 2020).
Cocaine and Amphetamine-Regulated Transcript (CART)
CART is a neuropeptide distributed in the central nervous system including hypothalamus nuclei, PVN and ARC (Douglass and Daoud 1996; Gautvik et al. 1996; Couceyro et al. 1997). Previous studies documented that CART not only plays a significant role in feeding, but also it has different effects on it; ICV administration of CART reduces appetite (Kristensen et al. 1998; Larsen et al. 2000) while its administration directly into the PVN increases food intake (Smith et al. 2008; Yousefvand and Hamidi 2020). Also, in chickens, ICV injection of CART peptide is reported to inhibit food intake (Tachibana et al. 2003; Honda et al. 2007; Cai et al. 2015). Studies have also reported that this peptide has a mediatory role in food intake (Kristensen et al. 1998; Lambert et al. 1998; Vrang et al. 1999); in chicken, CART interacts with NPY in the central nervous system which leads to regulate feeding through the attenuation of NPY hyperphagic effects (Tachibana et al. 2003). Also, increasing CART/TRH through injection of CCK and leptin decreases food intake (Akieda-Asai et al. 2014).
Endocannabinoid System (ECS)
Marijuana (9-tetrahydrocannabinol, THC) or psychoactive ingredients of the Cannabis sativa plant is called CBs (Novoseletsky et al. 2011). It has been confirmed that glucose homeostasis, eating behaviors, lipogenesis, and energy balance all are controlled by the ECS in humans, rodents, and poultry (KEYSHAMS et al. 2016; Alizadeh et al. 2015). Two cannabinoid receptor proteins, i.e., CB1 and CB2, belong to the G-protein-coupled family of receptors. Generally, in mammals and birds, CB1 receptors are situated in presynaptic terminals of the inhibitory and excitatory nerves in the CNS (Sharkey et al. 2014a, b; Novoseletsky et al. 2011). In contrast, CB2 receptors are mainly related to the immune cell function located in the brain (Sharkey et al. 2014a, b). Risen food intake via the CB2 receptor has been reported in layer-type chickens (Alizadeh et al. 2015). In addition, both mentioned receptors have shown increased food intake in mammals and layer-type chickens (Alizadeh et al. 2015; Pertwee 2005). In broilers, CB1 has shown a different trend since its injection has no effect, while CB2 remains increasing as in previous cases (Emadi et al. 2011). The CBs interact with glutamate and DA in chicken; CB1 agonist- induced hyperphagia is increased by NMDA antagonist whereas is decreased by D2 antagonist. Also, orexigenic effect of CB2 is amplified through the antagonization of D2 and AMPA/kainate receptors (Keyshams et al. 2016; Khodadadi et al. 2017).
Nitric Oxide (NO)
NO which has important physiological functions in the CNS, is produced from L-arginine by NO synthase (NOS). It has been reported that the central inhibition and stimulation of NO synthesis change feeding behavior in both meat- and layer-type chicken in different manners (Zendehdel et al 2015a, 2015b, 2015c; Khan et al. 2007; Choi et al. 1994; Clonidine Choi et al. 1995). In comparison with layers, the broiler chicks have genetically higher food consumption and energy expenditure in which the decremental alteration in feeding behavior has been observed through ICV injection of L-NNA but not L-NAME, the inhibitors of NO synthesis (Khan et al. 2007; Choi, et al. 1994). This effect was attenuated by the precursor of NO, namely L-arginine (Choi et al. 1994). Interestingly, ICV administration of L-NAME in layer chicks, has induced hyperphagia in dose dependent fashion. On this line, the hypophagic properties of L-arginine have been detected in layers (Alimohammadi et al. 2015). These controversial results extracted from different avian species can be ascribed to the effect of genetic diversity on the responsiveness of feeding regulatory pathways (Denbow, 1994). In this way, the precise mechanisms need to be elucidated in the future studies.
The interaction of NO with several brain mediators and neuronal pathways have been discussed in different literatures. Choi et al., have been shown that increased appetite induced by α2-receptor agonist, has been weakened by inhibition of NO synthesis in neonatal broiler chicks (Choi et al. 1995). In addition, an interplay between NO and cannabinoidergic systems has been accompanied by an increasing effect of L-NAME on CB1 agonist- induced hyperphagia in layer- type chickens (Hassanpour et al. 2015). Also, during several studies on layers, hypophagia- induced by OT, amphetamine (an indirect agonist of DA), and DAMGO (μ-opioid receptor agonist) significantly decreased by the central inhibition of NO synthesis which highlighted the crosstalk between central nitrergic system with OT, DA, and opioidergic systems (Zendehdel et al. 2021, 2017a; Alimohammadi et al. 2015).
Conclusion
Central interaction between mediators is a process which has underpinning role in regulation of appetite and feeding behavior. In above- mentioned chapters the interaction between different mediators in brain modulating food intake behavior in birds has been described. In this respect, the interplay between the most important mediators in different classes including biogenic amines, aminoacids, peptides, ECS, nitrergic systems and different relevant subclasses were under debate. In terms of peptides, according to the fact that the well-known peptides and the relevant subtypes have anorexigenic and/or orexigenic effects in avian and other species, the mentioned classification is considered in this review. In conclusion, the interactions between NTs involved in food intake regulation in birds have been documented; among them the biogenic amines dopamine and serotonin, and also the amino acid glutamate have more notable mediatory role due to the further interactions they have with other NT systems.
In consideration of developing global food demands, poultry industry and chicks breeding is one of the most important processing to fulfill of food requirements. Increasing food intake behavior in chickens can improve the poultry industry; understanding the neuronal pathways and related crosstalk affecting chicks appetite leads to have a better outline of feeding behavior in birds which is effective factor to progress poultry industry.
During studying the interplay of neuronal pathways in the control of food consumption, several variations have been demonstrated not only among different animal species but also within different strains of birds (Table 1). It seems that these noticeable differences detected among different bird strains are dependent on genetical diversity resulted in variations in central neuronal pathways and their dominancy in appetite and feeding regulation. The exact molecular mechanisms underlying the relevant variations remains to be elucidated in the future studies.
In the field of surveying the interactions between neuronal pathways intervening poultry food intake, most research works have employed the ICV administration of different drugs in order to inducing the alterations in feeding behavior. In consideration of the rout of drug administration in these studies, the identification of the regions in CNS and the neuron locations, which have role in feeding regulation, is not possible. To fix this issue, the use of intranuclear drug injection is being recommended to be applied as a research method in the future studies in this field. Moreover, the most of conducted models have induced the alterations in neuronal activity via pharmalogical agonizing and antagonizing procedures (Table 1). Although, the useful and applicable data has been extracted out of pharmacological methods, it is suggested that the new neurological techniques such as chemogenetics and optogenetics be widely employed to control the firing of specific neuronal pathway more precisely. Undoubtedly, these precise methods are capable of simulating the research models closer to the physiological status in more controlled condition.
References
Abbasnejad M, Jonaidi H, Denbow D, Rahimi AP (2005) Feeding and locomotion responses to centrally injected nociceptin/orphanin FQ in chicks. Physiol Behav 85:383–386
Abolghasempour S, Zendehdel M, Panahi N, Jahandideh A, Gilanpour H (2019) Intracerebroventricular injection of the glutamatergic receptors antagonist affects N/OFQ-induced hyperphagia in neonatal broilers: role of NMDA and AMPA receptors. Int J Pept Res Ther 25:835–843
Adan R, Van Leeuwen F, Sonnemans M, Brouns M, Hoffman G, Verbalis J, Burbach J (1995) Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology 136:4022–4028
Adeli A, Zendehdel M, Babapour V, Panahi N (2020) Interaction between leptin and glutamatergic system on food intake regulation in neonatal chicken: role of NMDA and AMPA receptors. Int J Neurosci 130:713–721
Ahmadi F, Zendehdel M, Babapour V, Panahi N (2019) CRF1/CRF2 and MC3/MC4 receptors affect glutamate-induced food intake in neonatal meat-type chicken. Braz J Poult Sci 21(01)
Akieda-Asai S, Poleni P-E, Date Y (2014) Coinjection of CCK and leptin reduces food intake via increased CART/TRH and reduced AMPK phosphorylation in the hypothalamus. Am J Physiol 306:E1284–E1291
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39:105–113
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun 39:151–157
Alt C, Lam JS, Harrison MT, Kershaw KM, Samuelsson S, Toll L, D’andrea A (2012) Nociceptin/orphanin FQ inhibition with SB612111 ameliorates dextran sodium sulfate-induced colitis. Eur J Pharmacol 683:285–293
Arletti R, Benelli A, Bertolini A (1990) Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48:825–830
Ashwell CM, Czerwinski SM, Brocht DM, Mcmurtry JP (1999) Hormonal regulation of leptin expression in broiler chickens. Am J Physiol 276:R226–R232
Bagnol D, Lu X-Y, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ (1999) Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci 19:R26
Baldini G, Phelan KD (2019) The melanocortin pathway and control of appetite-progress and therapeutic implications. J Endocrinol 241:R1–R33
Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA (2006) Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides 27:3292–3298
Bechtholt AJ, Hill TE, Lucki I (2007) Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology 190:531–540
Berger AJ, Isaacson JS (1999) Modulation of motoneuron N-methyl-D-aspartate receptors by the inhibitory neurotransmitter glycine. J Physiol 93:23–27
Bikker JA, Trumpp-Kallmeyer S, Humblet C (1998) G-protein coupled receptors: models, mutagenesis, and drug design. J Med Chem 41:2911–2927
Bodnar RJ, Klein GE (2006) Endogenous opiates and behavior: (2005). Peptides 27:3391–3478
Boswell T, Dunn IC (2017) Regulation of agouti-related protein and pro-opiomelanocortin gene expression in the avian arcuate nucleus. Front Endocrinol 8:75
Bungo T, Izumi T, Kawamura K, Takagi T, Ueda H, Furuse M (2003) Intracerebroventricular injection of muscimol, baclofen or nipecotic acid stimulates food intake in layer-type, but not meat-type, chicks. Brain Res 993:235–238
Bungo T, Kawamura K, Izumi T, Dodo K-I, Ueda H (2004) Feeding responses to μ-, δ-and κ-opioid receptor agonists in the meat-type chick. Pharmacol Biochem Behav 78:707–710
Bungo T, Dodo K-I, Kawamura K, Izumi T, Ueda H (2005) Effects of various μ-and δ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85:519–523
Bungo T, Shiraishi J-I, Yanagita K, Ohta Y, Fujita M (2009) Effect of nociceptin/orphanin FQ on feeding behavior and hypothalamic neuropeptide expression in layer-type chicks. Gen Comp Endocrinol 163:47–51
Bungo T, Yanagita K, Shiraishi J-I (2010) Feed intake after infusion of Noradrenalin, Dopamine or its. J Anim Vet Adv 9:760–763
Cai G, Mo C, Huang L, Li J, Wang Y (2015) Characterization of the two CART genes (CART1 and CART2) in chickens (Gallus gallus). PLoS ONE 10:0127107
Caliendo G, Santagada V, Perissutti E, Fiorino F (2005) Derivatives as 5HT1A receptor ligands-past and present. Curr Med Chem 12:1721–1753
Charles J, Duva M, Ramirez G, Lara R, Yang C, Stanley B (2014) Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacology 79:59–65
Chartrel N, Dujardin C, Anouar Y, Leprince J, Decker A, Clerens S, Do-Rego J-C, Vandesande F, Llorens-Cortes C, Costentin J (2003) Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci 100:15247–15252
Chen Z, Xie J, Hu M, Tang J, Shao Z, Li M (2015) Protective effects of γ-aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J Anim Plant Sci 25:78–87
Chen G, Yang F, Wu T, Jiang J, Zhou W (2016) The stimulatory effect of cerebral intraventricular injection of cNPY on precocial feeding behavior in neonatal chicks (Gallus domesticus). PLoS ONE 11:e0153342
Choi Y-H, Furuse M, Okumura J-I, Denbow DM (1994) Nitric oxide controls feeding behavior in the chicken. Brain Res 654:163–166
Choi Y-H, Furuse M, Okumura J-I, Denbow DM (1995) The interaction of clonidine and nitric oxide on feeding behavior in the chicken. Brain Res 699:161–164
Choi Y-H, Hartzell D, Azain MJ, Baile CA (2002) TRH decreases food intake and increases water intake and body temperature in rats. Physiol Behav 77:1–4
Ciranna Á (2006) Serotonin as a modulator of glutamate-and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 4:101–114
Colin I, Rostaing P, Augustin A, Triller A (1998) Localization of components of glycinergic synapses during rat spinal cord development. J Comp Neurol 398:359–372
Contarino A, Gold L (2002) Targeted mutations of the corticotropin-releasing factor system: effects on physiology and behavior. Neuropeptides 36:103–116
Couceyro PR, Koylu EO, Kuhar MJ (1997) Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat 12:229–241
Da Silva AA, Marino-Neto J, Paschoalini MA (2003) Feeding induced by microinjections of NMDA and AMPA–kainate receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res 966:76–83
Dautzenberg FM, Hauger RL (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23:71–77
Denbow, DM (1983) Food intake and temperature response to injections of catecholamines into the lateral ventricle of the turkey brain. Poult Sci 62(6):1088–1092
Denbow DM (1991) Induction of food intake by a GABAergic mechanism in the turkey. Physiol Behav 49:485–488
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S-1354S
Denbow D, Sheppard B (1993) Food and water intake responses of the domestic fowl to norepinephrine infusion at circumscribed neural sites. Brain Res Bull 31:121–128
Denbow D, Van Krey H, Lacy M, Dietrick T (1983) Feeding, drinking and body temperature of Leghorn chicks: effects of ICV injections of biogenic amines. Physiol Behav 31:85–90
Denbow D, Duke G, Chaplin S (1988) Food intake, gastric secretion, and motility as affected by avian pancreatic polypeptide administered centrally in chickens. Peptides 9:449–454
Denbow DM, Snapir N, Furuse M (1999) Inhibition of food intake by CRF in chickens. Physiol Behav 66:645–649
Denbow DM, Meade S, Robertson A, Mcmurtry JP, Richards M, Ashwell C (2000) Leptin-induced decrease in food intake in chickens. Physiol Behav 69:359–362
Dennis R (2016) Adrenergic and noradrenergic regulation of poultry behavior and production. Domest Anim Endocrinol 56:S94–S100
Deviche P, Delius JD (1981) Short-term modulation of domestic pigeon (Columbia livia L.) behaviour induced by intraventricular administration of ACTH. Z Tierpsychol 55:335–342
Dockray GJ (2004) The expanding family of-RFamide peptides and their effects on feeding behaviour. Exp Physiol 89:229–235
Dores RM (2013) Observations on the evolution of the melanocortin receptor gene family: distinctive features of the melanocortin-2 receptor. Front Neurosci 7:28
Douglass J, Daoud S (1996) Characterization of the human cDNA and genomic DNA encoding CART: a cocaine-and amphetamine-regulated transcript. Gene 169:241–245
Dridi S, Swennen Q, Decuypere E, Buyse J (2005) Mode of leptin action in chicken hypothalamus. Brain Res 1047:214–223
Emadi L, Jonaidi H, Abad EHA (2011) The role of central CB2 cannabinoid receptors on food intake in neonatal chicks. J Comp Physiol A 197:1143–1147
Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, Koch M, Kessler P, Hentsch D, Birling M-C (2015) A mu–delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 220:677–702
Fang X-L, Shu G, Yu J-J, Wang L-N, Yang J, Zeng Q-J, Cheng X, Zhang Z-Q, Wang S-B, Gao P (2013) The anorexigenic effect of serotonin is mediated by the generation of NADPH oxidase-dependent ROS. PLoS ONE 8:53142
Farkašová H, Hron T, Pačes J, Pajer P, Elleder D (2016) Identification of a GC-rich leptin gene in chicken. Agri Gene 1:88–92
Farrokhi R, Babapour V, Zendehdel M, Asghari A, Gilanpour H (2020) The role of dopaminergic and cannabinoidergic receptors on ghrelin-induced hypophagia in neonatal chicken. Arch Razi Ins [Internet]. Available from: http://archrazi.areeo.ac.ir/article_122277.html
Fichna J, Janecka A, Costentin J, Do Rego J-C (2007) The endomorphin system and its evolving neurophysiological role. Pharmacol Rev 59:88–123
Fowler CJ, Nilsson O, Andersson M, Disney G, Jacobsson SO, Tiger G (2001) Pharmacological properties of cannabinoid receptors in the avian brain: similarity of rat and chicken cannabinoid1 receptor recognition sites and expression of cannabinoid2 receptor-like immunoreactivity in the embryonic chick brain. Pharmacol Toxicol 88:213–222
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–213
Furuse M, Yamane H, Tomonaga S, Tsuneyoshi Y, Denbow DM (2007) Neuropeptidergic regulation of food intake in the neonatal chick: a review. J Poult Sci 44:349–356
Gautvik KM, De Lecea L, Gautvik VT, Danielson PE, Tranque P, Dopazo A, Bloom FE, Sutcliffe JG (1996) Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci 93:8733–8738
Ghandforoushan M, Zendehdel M, Babpour V (2017) Interactions between Histamine H1 and H3 and Dopamine D1 Receptors on feeding behavior in chicken. Iran J Vet Med 11:63–73
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683
Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H (2007) Hypothalamic neuronal histamine mediates the thyrotropin-releasing hormone-induced suppression of food intake 1. J Neurochem 103:1102–1110
Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ (2001) Opioid receptor involvement in the effect of AgRP-(83–132) on food intake and food selection. Am J Physiol 280:R814–R821
Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM (2004) GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304:884–887
Hashemzadeh M, Zendehdel M, Babapour V, Panahi N (2018) Interaction between central GABAA receptor and dopaminergic system on food intake in neonatal chicks: role of D1 and GABAA receptors. Int J Neurosci 128:361–368
Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD (1999) Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140:1408–1415
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56:443–451
Heidarzadeh H, Zendehdel M, Babapour V, Gilanpour H (2018) The effect of Nesfatin-1 on food intake in neonatal chicks: role of CRF 1/CRF2 and H1/H3 receptors. Vet Res Commun 42:39–47
Henderson LJ, Cockcroft RC, Kaiya H, Boswell T, Smulders TV (2018) Peripherally injected ghrelin and leptin reduce food hoarding and mass gain in the coal tit (Periparus ater). Proc R Soc B 285:20180417
Honda K, Kamisoyama H, Saneyasu T, Sugahara K, Hasegawa S (2007) Central administration of insulin suppresses food intake in chicks. Neurosci Lett 423:153–157
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 56:27–78
Jaefari-Anari M, Zendehdel M, Gilanpour H, Asghari A, Babapour V (2018) Central opioidergic system interplay with histamine on food intake in neonatal chicks: role of µ-opioid and H1/H3 receptors. Braz J Poult Sci 20:595–604
Jalali M, Zendehdel M, Babapour V, Gilanpour H (2019) Interaction between central oxytocinergic and glutamatergic systems on food intake in neonatal chicks: role of NMDA and AMPA receptors. Int J Pept Res Ther 25:195–203
Johnson MA, Tsutsui K, Fraley GS (2007) Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51:171–180
Jonaidi H, Oloumi M, Denbow D (2003) Behavioral effects of intracerebroventricular injection of oxytocin in birds. Physiol Behav 79:725–729
Jonaidi H, Abbassi L, Yaghoobi M, Kaiya H, Denbow D, Kamali Y, Shojaei B (2012) The role of GABAergic system on the inhibitory effect of ghrelin on food intake in neonatal chicks. Neurosci Lett 520:82–86
Jonaidi H, Abbasnejad M, Soltaninejad M, Sharifimehr A, Kooshki R, Yousefi M, Aghapour M (2019) Interaction effects of peripheral and central administration of LPS with inhibition of CRF receptors on food intake in neonatal chicks. Iran J Vet Sci Technol 11(1):19–25
Kaiya H, Miyazato M, Kangawa K (2011) Recent advances in the phylogenetic study of ghrelin. Peptides 32:2155–2174
Kalkman H, Engel G, Hoyer D (1984) Three distinct subtypes of serotonergic receptors mediate the triphasic blood pressure response to serotonin in rats. J Hypertens 2:S143–S145
Karasawa H, Yakabi S, Wang L, Tache Y (2014) Orexin-1 receptor mediates the increased food and water intake induced by intracerebroventricular injection of the stable somatostatin pan-agonist, ODT8-SST in rats. Neurosci Lett 576:88–92
Katayama S, Tomonaga S, Sato M, Yamane H, Tsuneyoshi Y, Denbow DM, Furuse M (2010) Norepinephrine does not alter NPY and POMC mRNA expression in neonatal chicks. Comp Biochem Physiol A 156:143–146
Kawakami S-I, Bungo T, Ando R, Ohgushi A, Shimojo M, Masuda Y, Furuse M (2000) Central administration of α-melanocyte stimulating hormone inhibits fasting-and neuropeptide Y-induced feeding in neonatal chicks. Eur J Pharmacol 398:361–364
Keyshams N, Zendehdel M, Babapour V, Baghbanzadeh A (2016) Cannabinoid–glutamate interactions in the regulation of food intake in neonatal layer-type chicks: role of glutamate NMDA and AMPA receptors. Vet Res Commun 40:63–71
Khan MSI, Tachibana T, Hasebe Y, Masuda N, Ueda H (2007) Peripheral or central administration of nitric oxide synthase inhibitor affects feeding behavior in chicks. Comp Biochem Physiol A 148:458–462
Khodadadi M, Zendehdel M, Baghbanzadeh A, Babapour V (2017) Consequence of dopamine D2 receptor blockade on the hyperphagic effect induced by cannabinoid CB1 and CB2 receptors in layers. Br Poult Sci 58:585–593
Kooshki R, Abbasnejad M, Jonaidi H, Soltaninejad M, Sharifimehr A, Yosoufi M, Aghapour M (2019) Interactive effects of peripheral and central administration of LPS with inhibition of CRF receptors on food intake in neonatal chicks. Iran J Vet Sci Technol 11:19–25
Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N (2014) An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507:238–242
Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393:72–76
Fraley G, Kuenzel W (1993) Precocious puberty in chicks (gallus domesticus) induced by central injections of neuropeptide Y. Life Sciences 52(20):1649–1656
Lambert PD, Couceyro PR, Mcgirr KM, Dall Vechia SE, Smith Y, Kuhar MJ (1998) CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29:293–298
Larsen PJ, Vrang N, Petersen PC, Kristensen P (2000) Chronic intracerebroventricular administration of recombinant CART (42–89) peptide inhibits food intake and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res 8:590–596
Lawrence CB, Celsi F, Brennand J, Luckman SM (2000) Alternative role for prolactin-releasing peptide in the regulation of food intake. Nat Neurosci 3:645–646
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89(4):1379–1412
Lovell PV, Wirthlin M, Carbone L, Warren WC, Mello CV (2015) Response to Hron et al. Genome Biol 16:1–2
Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371:799–802
Cadet JL, Jayanthi S, Mccoy MT, Beauvais G, Sheng Cai N (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord 9:526–538
Mahzouni M, Zendehdel M, Babapour V, Charkhkar S (2016) Methylamine induced hypophagia is mediated via dopamine D1 and D2 receptors in neonatal meat chicks. Vet Res Commun 40:21–27
Masaki T, Chiba S, Tatsukawa H, Noguchi H, Kakuma T, Endo M, Seike M, Watanabe T, Yoshimatsu H (2005) The role of histamine H1 receptor and H2 receptor in LPS-induced liver injury. FASEB J 19:1245–1252
Mcfadden KL, Cornier M-A, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol 5:553
Mirnaghizadeh SV, Zendehdel M, Babapour V (2017) Involvement of histaminergic and noradrenergic receptors in the oxytocin-induced food intake in neonatal meat-type chicks. Vet Res Commun 41:57–66
Mobarhan Fard M, Vazir B, Zendehdel M, Asghari A (2020) Interaction of central glutamatergic and histaminergic systems on food intake regulation in layer chicken. Arch Razi Inst 76:2021
Mokhtarpouriani K, Zendehdel M, Jonaidi H, Babapour V, Shayan P (2016a) The interaction of central nitrergic and GABAergic systems on food intake in neonatal layer-type chicks. Amino Acids 48:1275–1283
Mokhtarpouriani K, Zendehdel M, Jonaidi H, Babapour V, Shayan P (2016b) The interaction of central nitrergic and GABAergic systems on food intake in neonatal layer-type chicks. Amino Acids 48:1275–1283
Moosadoost Y, Zendehdel M, Khodadadi M (2019) Modulatory role of neuropeptide FF receptor on hypophagy through melanocortin system in neonatal meat-type chickens. Iran J Physiol Pharmacol 3:223–214
Moosadoost Y, Zendehdel M, Khodadadi M (2020) The effect of RFamide-related peptide-3 (RFRP-3 or NPVF) on food intake in neonatal chickens: the role of MC3/MC4 and CRF1/CRF2 receptors. Int J Pept Res Therap 27:253–262
Mortezaei SS, Zendehdel M, Babapour V, Hasani K (2013) The role of glutamatergic and GABAergic systems on serotonin-induced feeding behavior in chicken. Vet Res Commun 37:303–310
Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K (2008) Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199:105
Murase T, Arima H, Kondo K, Oiso Y (1996) Neuropeptide FF reduces food intake in rats. Peptides 17:353–354
Nayebzadeh N, Vazir B, Zendehdel M, Asghari A (2020) Central opioidergic and adrenergic systems mediates food intake via α 1, α 2 and β 2 receptors in neonatal layer-type chicken. Int J Pept Res Ther 26:1–10
Nicklous DM, Simansky KJ (2003) Neuropeptide FF exerts pro-and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am J Physiol 285:R1046–R1054
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Ortiz AA, Milardo LF, Decarr LB, Buckholz TM, Mays MR, Claus TH, Livingston JN, Mahle CD, Lumb KJ (2007) A novel long-acting selective neuropeptide Y2 receptor polyethylene glycol-conjugated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents. J Pharmacol Exp Ther 323:692–700
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol (168):1–51
Plum L, Schubert M, Bruning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65
Rahimi J, Zendehdel M, Khodadadi M (2021) Mediatory role of the dopaminergic system through D1 receptor on glycine-induced hypophagia in neonatal broiler-type chickens. Amino Acids 53:461–470
Raji-Dahmardeh F, Vazir B, Zendehdel M, Asghari A, Panahi N (2019) Interaction between oxytocin and opioidergic system on food intake regulation in neonatal layer type chicken. Int J Pept Res Therap 53:461
Rees MI, Harvey K, Ward H, White JH, Evans L, Duguid IC, Hsu CC-H, Coleman SL, Miller J, Baer K (2003) Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J Biol Chem 278:24688–24696
Reidelberger R, Haver A, Chelikani P, Keire DA, Reeve Jr JR (2011) Effects of glycine-extended and serine13-phosphorylated forms of peptide YY on food intake in rats. Peptides 32:770–775
Richards MP, Poch SM (2003) Molecular cloning and expression of the turkey leptin receptor gene. Comp Biochem Physiol B 136:833–847
Saito E-S, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Sarkar S, Legrádi G, Lechan RM (2002) Intracerebroventricular administration of α-melanocyte stimulating hormone increases phosphorylation of CREB in TRH-and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res 945:50–59
Saxena PR (1995) Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol Ther 66:339–368
Scain A-L, Le Corronc H, Allain A-E, Muller E, Rigo J-M, Meyrand P, Branchereau P, Legendre P (2010) Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci 30:390–403
Schneeberger M, Gomis R, Claret M (2014) Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J Endocrinol 220:T25–T46
Schulz C, Paulus K, Lobmann R, Dallman M, Lehnert H (2010) Endogenous ACTH, not only α-melanocyte-stimulating hormone, reduces food intake mediated by hypothalamic mechanisms. Am J Physiol 298:E237–E244
Sharkey KA, Darmani NA, Parker LA (2014a) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146
Sharkey KA, Darmani NA, Parker LA (2014b) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146
Shiraishi J-I, Yanagita K, Fujita M, Bungo T (2008) Central insulin suppresses feeding behavior via melanocortins in chicks. Domest Anim Endocrinol 34:223–228
Shojaei M, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Opioid-induced hypophagia is mediated by 5-HT2c receptors in neonatal layer-type chicken. Czech J Anim Sci 60:400–410
Shojaei M, Yousefi A, Zendehdel M, Khodadadi M (2020) Food intake regulation in birds: the role of neurotransmitters and hormones. Iran J Vet Med 14:99–115
Smith KL, Gardiner JV, Ward HL, Kong WM, Murphy KG, Martin NM, Ghatei MA, Bloom SR (2008) Overexpression of CART in the PVN increases food intake and weight gain in rats. Obesity 16:2239–2244
Song X, Jiao H, Zhao J, Wang X, Lin H (2019) Ghrelin serves as a signal of energy utilization and is involved in maintaining energy homeostasis in broilers. Gen Comp Endocrinol 272:76–82
Sorrels TL, Bostock E (1992) Induction of feeding by 7-chlorokynurenic acid, a strychnine-insensitive glycine binding site antagonist. Brain Res 572:265–268
Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Tache Y (2010a) Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology 151:4224–4235
Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Tache Y (2010b) Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav 101:614–622
Stengel A, Karasawa H, Tache Y (2015) The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behavior. Horm Behav 73:15–22
Stevenson JA, Franklin C, Geddes J (1970) Effects of ACTH and corticosteroids in the regulation of food and water intake. Prog Brain Res 32:141–152
Stratford TR, Wirtshafter D (2013) Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res 1490:128–133
Swiergiel AH, Burunda T, Patterson B, Dunn AJ (1999) Endotoxin-and interleukin-1-induced hypophagia are not affected by adrenergic, dopaminergic, histaminergic, or muscarinic antagonists. Pharmacol Biochem Behav 63:629–637
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DL-AP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Tachibana T, Cline MA, Sugahara K, Ueda H, Hiramatsu K (2009) Central administration of somatostatin stimulates feeding behavior in chicks. Gen Comp Endocrinol 161:354–359
Tachibana T, Takagi T, Tomonaga S, Ohgushi A, Ando R, Denbow DM, Furuse M (2003) Central administration of cocaine-and amphetamine-regulated transcript inhibits food intake in chicks. Neurosci Lett 337:131–134
Taherian M, Baghbanzadeh A, Zendehdel M (2016) Dopamine-induced hypophagia is mediated via NMDA and mGlu1 receptors in chicken. Iranian Journal of Veterinary Medicine 10:191–199
Taherian M, Zendehdel M, Hassanpour S (2019) Role of Central Cannabinoidergic System on Ghrelin-Induced Hypophagia in Layer-Type Neonatal Chicken. Iranian Journal of Veterinary Medicine 13:151–161
Tajalli S, Jonaidi H, Abbasnejad M, Denbow D (2006) Interaction between nociceptin/orphanin FQ (N/OFQ) and GABA in response to feeding. Physiol Behav 89:410–413
Takahashi KA, Cone RD (2005) Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146:1043–1047
Takahashi A, Shimamoto A, Boyson CO, Debold JF, Miczek KA (2010) GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci 30:11771–11780
Torkzaban M, Zendehdel M, Babapour V, Panahi N, Hassanpour S (2018) Interaction between central opioidergic and glutamatergic systems on food intake in neonatal chicks: role of NMDA, AMPA and mGLU1 receptors. Int J Pept Res Ther 24:157–169
Tsutsui K, Bentley G, Kriegsfeld L, Osugi T, Seong JY, Vaudry H (2010) Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol 22:716–727
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213(4514):1394–1397
Van Putten L, Van Bekkum D, Querido A (1953) Influence of cortisone and ACTH on appetite. Eur J Endocrinol 12:159–166
Vijayan E, Mccann SM (1977) Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH). Endocrinology 100:1727–1730
Volkow ND, Wang G-J, Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15:37–46
Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P (1999) Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res 818:499–509
Wang W, Guo D-Y, Lin Y-J, Tao Y-X (2019) Melanocortin regulation of inflammation. Front Endocrinol 10:683
Wynne K, Stanley S, Mcgowan B, Bloom S (2005) Appetite control. J Endocrinol 184:291–318
Yang L-K, Tao Y-X (2017) Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim Biophys Acta 1863(10 Pt A):2486–2495
Yoefvand S, Hami F (2020) Role of paraventricular nucleus in regulation of feeding behaviour and the design of intranuclear neuronal pathway communications. Int J Pept Res Ther 26:1231–1242
Yoefvand S, Hamidi F (2021) The role of ventromedial hypothalamus receptors in the central regulation of food intake. Int J Pept Res Ther 27:689–702
Yoo E-S, Yu J, Sohn J-W (2021) Neuroendocrine control of appetite and metabolism. Exp Mol Med 53(4):505–516
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018) Hypophagic effects of insulin are mediated via NPY1/NPY2 receptors in broiler cockerels. Can J Physiol Pharmacol 96:1301–1307
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2019) Interaction of neuropeptide Y receptors (NPY1, NPY2 and NPY5) with somatostatin on somatostatin-induced feeding behaviour in neonatal chicken. Br Poult Sci 60:71–78
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2020) Survey the effect of insulin on modulating feed intake via NPY receptors in 5-day-old chickens. Int J Pept Res Ther 26:467–476
Yu JH, Kim M-S (2012) Molecular mechanisms of appetite regulation. Diab Metab J 36:391
Zanganeh F, Panahi N, Zendehdel M, Asghari A (2020) Interconnection between adrenergic and dopaminergic systems on feeding behavior in neonatal chicks. Arch Razi Inst 76(2):345–358
Zendehdel M, Hassanpour S (2014) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 1:e251
Zendehdel M, Hassanpour S (2014b) Ghrelin-induced hypophagia is mediated by the β 2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Babapour V, Jonaidi H (2008) Effects of central histamine receptors blockade on GABA (A) agonist-induced food intake in broiler cockerels. Pak J Biol Sci 11:416–421
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behavior in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Hamidi F, Babapour V, Mokhtarpouriani K, Fard RMN (2012a) The effect of melanocortin (Mc3 and Mc4) antagonists on serotonin-induced food and water intake of broiler cockerels. J Vet Sci 13:229
Zendehdel M, Taati M, Jonaidi H, Amini E (2012b) The role of central 5-HT 2C and NMDA receptors on LPS-induced feeding behavior in chickens. J Physiol Sci 62:413–419
Zendehdel M, Mokhtarpouriani K, Babapour V, Baghbanzadeh A, Pourrahimi M, Hassanpour S (2013a) The effect of serotonergic system on nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 63:271–277
Zendehdel M, Mokhtarpouriani K, Babapour V, Pourrahimi M, Hamidi F (2013b) The role of 5-HT2A and 5-HT2C receptors on harmalineinduced eating behavior in 24-h food-deprived broiler cockerels. Iran J Vet Res 14:94–99
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013c) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37:37–41
Zendehdel M, Hasani K, Babapour V, Mortezaei SS, Khoshbakht Y, Hassanpour S (2014) Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Vet Res Commun 38:11–19
Zendehdel M, Baghbanzadeh A, Aghelkohan P, Hassanpour S (2015a) Lipopolysaccharide and histaminergic systems interact to mediate food intake in broilers. Br Poult Sci. https://doi.org/10.1080/00071668.2015
Zendehdel M, Hamidi F, Hassanpour S (2015b) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21:179–186
Zendehdel M, Hassanpour S, Babapour V, Charkhkar S, Mahdavi M (2015c) Interaction between endocannabinoid and opioidergic systems regulates food intake in neonatal chicken. Int J Pept Res Ther 21:289–297
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2016) Interaction between opioidergic and dopaminergic systems on food intake in neonatal layer type chicken. Int J Pept Res Ther 22:83–92
Zendehdel M, Moosadoost Y, Masoumi R, Rostami B, Shahir MH, Hassanpour S (2017a) Endogenous nitric oxide and dopamine regulates feeding behavior in neonatal layer-type chicken. Ann Anim Sci 17(4):1029–1042
Zendehdel M, Sardari F, Hassanpour S, Rahnema M, Adeli A, Ghashghayi E (2017b) Serotonin-induced hypophagia is mediated via α2 and β2 adrenergic receptors in neonatal layer-type chickens. Br Poult Sci 58:298–304
Zendehdel M, Tirgari F, Shohre B, Deldar H, Hassanpour S (2017c) Involvement of GABA and cannabinoid receptors in central food intake regulation in neonatal layer chicks: role of CB1 and GABAA receptors. Braz J Poultry Sci 19:221–230
Zendehdel M, Ebrahimi-Yeganeh A, Hassanpour S, Koohi M (2019) Interaction of the dopaminergic and Nociceptin/Orphanin FQ on central feed intake regulation in chicken. Br Poult Sci 60:317–322
Zendehdel M, Khodadadi M, Vosoughi A, Mokhtarpouriani K, Baghbanzadeh A (2020) β2 adrenergic receptors and leptin interplay to decrease food intake in chicken. Br Poult Sci 61:156–163
Zendehdel M, Khodadadi M, Zandiyeh H, Mokhtarpouriani K, Rahmani B, Baghbanzadeh A (2021) A newly discovered interference of the central nitrergic system on oxytocin-induced hypophagia in layer-type chickens. Iran J Vet Sci Technol [Internet]. Available from: https://ijvst.um.ac.ir/article_39773.html
Zhang X, Van Den Pol AN (2012) Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implications for TRH-mediated anorexic and arousal actions. J Neurosci 32:3032–3043
Zhang R, Nakanishi T, Ohgushi A, Ando R, Yoshimatsu T, Denbow DM, Furuse M (2001) Suppression of food intake induced by corticotropin-releasing factor family in neonatal chicks. Eur J Pharmacol 427:37–41
Author information
Authors and Affiliations
Contributions
All authors have agreed to be named as authors on this manuscript. Any work (data, text, or theories) of others besides the authors has been properly acknowledged. The work is original and not previously published. All data are true and accurate to the knowledge of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest with the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahmani, B., Ghashghayi, E., Zendehdel, M. et al. The Crosstalk Between Brain Mediators Regulating Food Intake Behavior in Birds: A Review. Int J Pept Res Ther 27, 2349–2370 (2021). https://doi.org/10.1007/s10989-021-10257-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10257-1