Abstract
The endocannabinoids (ECBs) have diverse physiological functions including the regulation of food intake and metabolism. In mammals, ECBs regulate feeding primarily through the CB1 receptors within the brain whereas the CB2 receptors are primarily involved in the regulation of immune function by direct action on peripheral immune cells and central glia. The central effect of ECBs on feeding behavior has not been studied in non-mammalian species. Therefore, the present study investigated the effect of CB65, a selective CB2 receptors agonist, on food intake in the neonatal chicks. In addition, the effect of astressin, a CRF receptor antagonist, on CB65-induced food intake was also investigated. Intracerebroventricular injection of the CB65 (1.25 μg) increased the food intake at 30- and 60-min post-injection significantly as compared to the control group. Pretreatment with a selective CB2 receptor antagonist, AM630, but not astressin, significantly attenuated the CB65-induced food intake. These results suggested that CB2 receptor agonists act on the brain to induce food intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabinoid receptors, endocannabinoids and proteins catalyzing endocannabinoid biosynthesis and inactivation, constitute the endocannabinoid system (ECS) (Di Marzo et al. 2004). The history of this system refers to the mid-1960s, when the major psychoactive component of Cannabis sativa (marijuana), D9-tetrahydrocannabinol (D9-THC), was identified (Gaoni and Mecbonlam 1964). The next major achievement was finding out that D9-THC works by binding to a set of specific plasma membrane proteins, the ‘cannabinoid receptors’. To date, two cannabinoid receptors have been cloned: the cannabinoid receptor 1 (CB1 receptor), possibly the most abundant G-protein-coupled receptor in the central nervous system and the cannabinoid receptor 2 (CB2 receptor), expressed abundantly in several immune cells and tissues (Klein 2005; Pertwee 2005). Although brain CB1 receptors are coupled to the inhibition of neurotransmitter release, CB2 receptors seem to participate in the regulation of cytokine release and function (Klein 2005; Pertwee 2005). It is now believed that, in addition to CB1 receptors that also carry out important functions in peripheral tissues, CB2 receptors may be present in some brain regions as well (Pertwee 2005; Van Sickle et al. 2005). Regarding studies on avian species, few authors have reported cannabinoid receptors in birds (Soderstrom and Johnson 2000). It has been reported that CB1 receptor mRNA is expressed in various regions of chick brain (Stincic and Hyson 2008). However, there is only one report describing the presence of a CB2-like protein in the central nervous system (CNS) of chick embryos (Fowler et al. 2001) while, in adult chicken, no such evidence is available. The discovery of cannabinoid receptors suggested the existence of endogenous ligands capable of activating them, the ‘endocannabinoids’; the two best studied of which are anandamide (N-arachidonoylethanolamine; AEA) (Devane et al. 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al. 1995; Sugiura et al. 1995).
Following the detection of cannabinoid receptors in the hypothalamus, an important area in food intake regulation, several researchers provided evidence that the endocannabinoid system is involved in appetite regulation. To determine if CB1 receptors are involved in food intake, Ravinet Trillou et al. (2004) showed that the stimulation of CB1 receptors is a key component in the development of diet-induced obesity. Although many experiments indicated that endocannabinoids regulate food intake through the central nervous system, evidence suggests that they may also promote food intake via peripheral sites. Gomez et al. (2002) found that peripherally injected anandamide promoted hyperphagia in partially satiated, capsaicin differentiated rats.
Most of the present knowledge regarding the behavioral effect of endocannabinoids has been derived from studies in mammalian species. However, there are few CB1 related behavioral reports such as memory recall in chicks (Adam et al. 2008). Furthermore, Novoseletsky et al. (2011) recently reported that a single intravenous injection or ingestion of hydrocolloid carriers entrapping inverse agonist of CB1, AM251, leads to transient attenuation of food intake in chicks which is similar to its effect in mammals. In the present study, we investigated the central effect of a CB2 receptor selective agonist on food intake in the neonatal chickens for the first time. We also studied the ability of corticotropin-releasing factor (CRF) neurons, as an important key mediator of food intake regulation in birds, to mediate changes in food intake induced by CB2 receptors stimulation.
Materials and methods
Animals
A day-old-male Ross broiler chicks were purchased from a local hatchery (Mahan Company, Kerman, Iran). The chicks were housed in a temperature- and humidity-controlled battery (30°C, 45–50%, respectively) with continuous light. They had free access to water and a commercial starter diet (21% protein and 2,900 kcal metabolizable energy). On the day prior to injection, birds were placed in individual cages and 3-day-old chicks were used for intracerebroventricular (ICV) injection.
ICV injection
CB65, a selective CB2 receptors agonist, AM630, a selective CB2 receptors antagonist, and astressin, a non-selective CRF antagonist, were purchased from Tocris Bioscience Company (Missouri, Bristol, UK). CB65 and AM630 were dissolved in 5% dimethylsolfoxide (DMSO) containing 0.1% Evans Blue. Astressin was dissolved in 0.85% saline, containing 0.1% Evans Blue. Drugs were injected intracerebroventricularly in a volume of 10 μl, using a microsyringe as described by Davis et al. (1979) and Furuse et al. (1997) without anesthesia. Briefly, head of the chick was held with an acrylic device in which the bill holder was 45° and the calvarium was parallel to the surface of table as described by Van Tienhoven and Juhasz (1962). A hole was made in a plate overlying the skull immediately over the right lateral ventricle. Then a microsyringe was inserted into the right lateral ventricle through the hole and the test solution was injected. The top of the needle was penetrated only 4 mm below the skin of skull. This procedure was considered not to induce any physiological stress (Saito et al. 2005).
Feeding experiments
Before each experiment, birds were weighed and distributed into experimental groups based on their body weight so that the average weight between treatment groups was as uniform as possible. The birds were deprived of food for 3 h before ICV injections, but given free access to water. At the end of the experiment, chicks were killed with an ether overdose induction. The presence of Evans Blue dye in the lateral ventricle confirmed a successful injection. Birds with no trace of the dye in their lateral ventricle were not used for data analysis.
Experiment 1 was performed to examine whether ICV injection of the CB65 at a dose range of 0.312–5 μg affects food intake in chicks. Food intake was measured for 3 h after injection.
Experiment 2 was conducted to investigate the effects of the AM630 on the CB65-induced food intake. Each chick was injected once only with DMSO, CB65, AM630 and CB65 co-injected with AM630. The dose of CB65 applied in this experiment was chosen according to the experiment 1 which increased food intake significantly. The dose of AM630 (5 μg) used here was chosen according to the preliminary trials.
The involvement of CRF in the feeding effects of CB65 was determined by ICV co-injection of astressin (22 μg) and CB65 in experiment 3. This experiment was conducted similarly to the second experiment except that astressin was used in place of AM630. The dose of astressin was selected based on those of Saito et al. (2005).
Statistical analysis
The data were expressed as mean ± SEM. Feed intake was analyzed at each time period by analysis of variance (ANOVA) using the general linear modeling procedure. In experiments 2 and 3, the model included CB65 by CB2 antagonist or CRF antagonist interaction. Comparisons among treatment groups were made using Tukey’s multiple range tests.
A P value less than 0.05 was considered to be significant.
Results
Experiment 1 was conducted to study the effect of CB2 receptor stimulation on food intake (Fig. 1, panel a and b). Food intake was significantly increased in chicks given ICV with a dose of 1.25 μg CB65 at 30 and 60 min post-injection. In panel b, a tendency to increase in food intake was observed, but there was no significant difference between the treatments and control groups. These results suggested that CB65 as a selective CB2 receptor agonist affects food intake in a very limited dose range.
Cumulative food intake following ICV injection of CB65 with doses a greater than 1 μg and b lesser than 1 μg in neonatal chicks. Values are expressed as means ± SEM. Significant difference between treatments at each time point represented: a 30 min, F(3,33) = 2.912; 60 min, F(3,33) = 2.955; 120 min, F(3,32) = 1.57; 180 min, F(3,33) = 0.966; b 30 min, F(2,23) = 0.724; 60 min, F(2,22) = 3.904; 120 min, F(2,22) = 0.694; 180 min, F(2,24) = 1.26. The numbers of chicks used for the time-course experiment are as follows: Control (n = 9); CB65 0.325 μg (n = 9); CB65 0.612 μg (n = 7); CB65 1.25 μg (n = 11); CB65 2.5 μg (n = 10); CB65 5 μg (n = 7) P < 0.05. *Significant with Control #Significant with CB65 (1.25 μg)
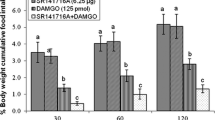
Experiment 2 was carried out to confirm the stimulatory effect of CB65 on food intake by blocking of CB2 receptors. The statistical analysis for feeding data in experiment 2 revealed that there is an interaction between the CB65 and AM630. Significant increase in food intake, induced by CB65 was reobserved in experiment 2 and 3 although these increasing effects lasted longer (Figs. 2, 3). The results also indicated that the effect of CB65 on food intake is attenuated by pretreatment with a selective CB2 antagonist AM630 (Fig. 2). AM630 alone did not alter food intake significantly as compared to the control group.
Cumulative food intake following ICV co-injection of CB65 (1.25 μg) and AM630 (5 μg) in neonatal chicks. Values are expressed as means ± SEM. Significant difference between treatments at each time point represented: 30 min, F(3,29) = 2.956; 60 min, F(3,32) = 3.155; 120 min, F(3,27) = 3.696; 180 min, F(3,30) = 5.036. The numbers of chicks used for the time-course experiment are as follows: control (n = 11); CB65 1.25 μg (n = 7); AM630 5 μg (n = 6); CB65 1.25 μg plus AM630 5 μg (n = 9) P < 0.05. *Significant with CB65 (1.25 μg)
Cumulative food intake following ICV co-injection of CB65 (1.25 μg) and astressin (22 μg) in neonatal chicks. Values are expressed as means ± SEM. Significant difference between treatments at each time point represented: 30 min, F(3,25) = 2.224; 60 min, F(3,26) = 3.97; 120 min, F(3,25) = 4.709; 180 min, F(3,26) = 4.683. The numbers of chicks used for the time-course experiment are as follows: control (n = 9); CB65 1.25 μg (n = 11); astressin 22 μg (n = 5); CB65 1.25 μg plus astressin 22 μg (n = 5) P < 0.05. *Significant with control
Experiment 3 was done to investigate whether the stimulatory effect of CB65 is mediated by CRF neurons. As shown in Fig. 3, administration of astressin, increased food intake in comparison with the control group but food intake was not significantly changed in chicks given CB65 plus astressin compared to chicks injected with the CB65 alone. Furthermore, there was no interaction between CB65 and astressin on food intake obtained by statistical analysis.
Discussion
This study provides the first demonstration of hyperphagia induced by central administration of a selective CB2 receptor agonist in chicks (Fig. 1). Also the attenuation of CB65 induced hyperphagia by a selective CB2 receptor antagonist, confirmed that CB2 receptors are involved in this effect (Fig. 2). In the current study, CB65 was effective at a limited dose in chicks. In mammals, CB2 receptors are present mainly on immune cells in peripheral tissues to modulate cytokine release (Svizenska et al. 2008). However, regarding their presence in the central nervous system, multiple recent investigations have revealed that CB2 receptors are expressed in brain microglia during neuroinflammation (Atwood and Mackie 2010). Though, there are controversial reports concerning the complete absence or widespread expression of CB2 receptors in neurons (Atwood and Mackie 2010). There are also controversial investigations regarding the central effect of ECS on feeding behavior of small rodents. Based on the study of Gomez et al. (2002), central CB1 receptors do not play a role in food intake of partially satiated rats. It has been shown that ICV injection of AM630 increases chow intake in overnight deprived rats (Werner and Koch 2003). Onaivi et al. (2008) have also shown that following 12-h food deprivation, a peripheral injection of CB2 agonist, (PEA) and a CB2 antagonist, (AM630) in mice decreases and increases food intake respectively, which is not consistent with the present study. It is assumed that the CB2 receptors may likely act on the immune system, which indirectly alters appetite through changes in the activity of the digestive system (Onaivi et al. 2008). In the current study, it seems that the central stimulatory effect of CB65 on food intake is a result of direct action on the brain tissues including either neural or glial cells. It was reported that the CB2 receptors agonist reduce inflammatory response in neurodegenerative diseases resulting from the activation of brain microglial cells. Stimulation of CB2 receptors suppresses microglial activation (Ehrhart et al. 2005). It is hard to ignore that the central injection of drugs, applied in the present study, is of particular importance for selecting between peripheral (potentially non-neural) and central (presumably neural) mechanisms of action. However, in chicks there is no evidence for expression or function of CB2 receptors in the microglia and neurons. The increases in food intake observed in the current study following ICV injection of 1.25 μg CB65, are difficult to interpret; because no existing literature, reports feeding behavior effects following central administration of CB2 receptor agonists or antagonists. The present data point to the need for further basic study of CB receptors in chicks.
CRF is a principal regulator of the hypothalamic–pituitary–adrenal axis and is suspected to play an important role in a variety of endocrine systems (Benoit et al. 2000). CRF and its family peptides are thought to be of great importance in the control of food intake in chickens. It is believed that central administration of CRF family peptides reduce food intake in chicks (Furuse et al. 1997; Denbow et al. 1999). Therefore, it can be expected that administration of CRF receptor antagonist, astressin, increases food intake as it is shown in Fig. 3. Evidence suggests that, in chickens, some regulatory neurotransmitters, including ghrelin, glucagons-like peptide-1 and bombesin, affect food intake through CRF neurons (Zhang et al. 2001; Meade and Denbow 2003; Saito et al. 2005). However, our finding showed no interaction between endocannabinoid CB2 receptor and CRF (Fig. 3). As a result, it may be concluded that the CB65 increases food intake through systems other than the CRF pathway.
In conclusion, our findings provided functional evidences for the presence of CB2 cannabinoid receptors in chick brain. Moreover, it was realized that these receptors are involved in increasing food intake and the CRF system does not play a role in this effect. The detail sites within the chick brain involved in the hyperphagic effect of the CB2 receptors and the mechanisms underlying this effect remains to be clarified. Nonetheless, our findings in neonatal chicks are inconsistent with the results seen in small rodents.
Abbreviations
- ECS:
-
Endocannabinoid system
- D9-THC:
-
D9-tetrahydrocannabinol
- CB1 receptor:
-
Cannabinoid receptor 1
- CB2 receptor:
-
Cannabinoid receptor 2
- CNS:
-
Central nervous system
- AEA:
-
N-Arachidonoylethanolamine
- 2-AG:
-
2-Arachidonoylglycerol
- CRF:
-
Corticotropin-releasing factor
- ICV:
-
Intracerebroventricular
- DMSO:
-
Dimethylsolfoxide
- ANOVA:
-
Analysis of variance
References
Adam AS, Wenger T, Csillag A (2008) The cannabinoid CB1 receptor antagonist rimonabant dose-dependently inhibits memory recall in the passive avoidance task in domestic chicks (Gallus domesticus). Brain Res Bull 76:272–274
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479
Benoit SC, Thiele TE, Heinrichs SC, Rushing PA, Blake KA, Steeley RJ (2000) Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides 21:345–351
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM, Snapir N, Furuse M (1999) Inhibition of food intake by CRF in chickens. Physiol Behav 66:645–649
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Marzo V, Bifulco M, De Petrocellis L (2004) The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3:771–784
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2:29
Fowler CJ, Nilsson O, Andersson M, Disney G, Jacobsson SO, Tiger G (2001) Pharmacological properties of cannabinoid receptors in the avian brain: similarity of rat and chicken cannabinoid1 receptor recognition sites and expression of cannabinoid2 receptor-like immunoreactivity in the embryonic chick brain. Pharmacol Toxicol 88:213–222
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Gaoni Y, Mecbonlam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647
Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F (2002) A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22:9612–9617
Klein TW (2005) Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5:400–411
Meade S, Denbow DM (2003) The interaction of bombesin and corticotropin-releasing hormone on ingestive behavior in the domestic fowl. Physiol Behav 78:611–614
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R (2008) Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci 1139:426–433
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168:1–51
Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Soderstrom K, Johnson F (2000) CB1 cannabinoid receptor expression in brain regions associated with zebra finch song control. Brain Res 857:151–157
Stincic TL, Hyson RL (2008) Localization of CB1 cannabinoid receptor mRNA in the brain of the chick (Gallus domesticus). Brain Res 1245:61–73
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Svizenska I, Dubovy P, Sulcova A (2008) Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav 90:501–511
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Werner NA, Koch JE (2003) Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in Lewis rats. Brain Res 967:290–292
Zhang R, Nakanishi T, Ohgushi A, Ando R, Yoshimatsu T, Denbow DM, Furuse M (2001) Interaction of corticotropin-releasing factor and glucagon-like peptide-1 on behaviors in chicks. Eur J Pharmacol 430:73–78
Acknowledgments
This study was a DVM thesis supported by “Shahid Bahonar University of Kerman”, Kerman, Iran. All experimental protocols were approved by the Research Ethic Committee of the Neuroscience Research Center of Kerman, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emadi, L., Jonaidi, H. & Hosseini Amir Abad, E. The role of central CB2 cannabinoid receptors on food intake in neonatal chicks. J Comp Physiol A 197, 1143–1147 (2011). https://doi.org/10.1007/s00359-011-0676-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0676-z