Abstract
The present study was designed to examine the role of opioidergic and glutamatergic systems on feeding behavior in neonatal meat-type chicken. In experiment 1, FD3 neonatal broilers ICV injected with (A) saline, (B) DAMGO (µ-opioid receptor agonist, 125 pmol), (C) MK-801 (NMDA glutamate receptors antagonist, 15 nmol) and (D) combination of DAMGO plus MK-801. Experiments 2–5 were similar to experiment 1, except FD3 chicks ICV injected with CNQX (AMPA glutamate receptors antagonist, 390 nmol), AIDA (mGLU1 receptors antagonist, 2 nmol), LY341495 (mGLU2 receptors antagonist, 150 nmol) and UBP1112 (mGLU3 receptors antagonist, 2 nmol) instead of MK-801, respectively. In experiments 6–10, FD3 chicks ICV injected as the same as procedure to the experiments 1–5, except to inject with DPDPE (δ-opioid receptor agonist, 40 nmol) instead of the DAMGO. The experiments 11–15 were similar to the experiments 1–5, except neonatal broilers ICV injected with U-50488H (κ-opioid receptor agonist, 30 nmol) instead of DAMGO. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of DAMGO, significantly decreased food intake (P < 0.05) while DPDPE and U-50488H increased feeding behavior compared to the control group (P < 0.05). Co-injection of the DAMGO + MK-801 and DAMGO + AIDA, significantly decreased DAMGO-induced hypophagia in neonatal chicks (P < 0.05). Also, co-injection of the DPDPE + CNQX significantly amplified DPDPE induced feeding behavior (P < 0.05). These results suggested interconnection between central opioidergic and glutamatergic systems on feeding behavior mediates via µ- and δ-opioid receptor with NMDA, AMPA and mGLU1 receptors in FD3 neonatal broilers. These findings may shed light on the circuitry underlying interconnection between central opioidergic and glutamatergic systems on feeding behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Feeding behavior is modulated by complex neurochemical pathways in numerous parts of the brain, including the striatum, hypothalamus and amygdala. To date, numerous neurotransmitters in the brain have been discovered where regulate food intake (Ladepeche et al. 2013). Opioids are inhibitory neurotransmitters which three subtypes of receptors identified, mu (µ), delta (δ) and kappa (κ), belonging to the G protein-coupled receptors (GPCRs) (Filizola and Devi 2013). Opioids take part in reward, pain modulation, respiratory, neuroendocrine and food intake regulation in the central nervous system (CNS) (Feng et al. 2012; Kaneko et al. 2012). For instance, intracerebroventricular (ICV) injection of [D-Ala2, NMe-Phe4, Gly5-ol]-enkephalin (DAMGO) and β-casomorphin (µ-opioid receptor agonists) inhibited food intake whereas [D-Pen2, 5]-enkephalin (DPDPE) (δ-opioid receptor agonist) and U-50488H (κ-opioid receptor agonist) increased food intake in neonatal layer and broiler chicks (Bungo et al. 2004; Alimohammadi et al. 2015; Zendehdel et al. 2015).
It is well documented that appetite regulates by the interaction of various neurotransmitters and complex network. Another feeding-regulatory factor known involved on food intake is glutamate (Taati et al. 2011). Glutamate is the main excitatory neurotransmitter in reward control in the hypothalamic centers (McFadden et al. 2014). Glutamate receptors classified into two groups, based on their pharmacology and mechanism. The ionotropic receptors include N-methyl-d-aspartate (NMDA), Kainate and AMPA and the metabotropic receptors (mGluRs) subtypes (Charles et al. 2014). Activation of lateral hypothalamic AMPA receptors increased feeding behavior in rats (Hettes et al. 2010). Injection of NMDA and AMPA-kainite receptor antagonists into ventral striatal and ventral pallidal areas induced food intake in the pigeon (Da Silva et al. 2003). Also, the ICV injection of NMDA receptor antagonist (DL-AP5) increased food consumption in FD3 broiler cockerels (Taati et al. 2011).
Based on the literature, interconnection exists between opiate and glutamate receptors (Farahmandfar et al. 2011). µ-opioid receptor activity can be affected by the presynaptic modulation of glutamate receptors (Lee and Ho 2013). Both NMDA and AMPA receptors are involved in the development phase of opioid sensitization (Sepehrizadeh et al. 2008a, b). Also, sensitization to opioids can alter the extracellular glutamate levels in the ventral tegmental area (VTA) and prefrontal cortex (Hao et al. 2007). Chronic exposure to opioids alters the glutamatergic synaptic transmission via NMDA receptor (Xu et al. 2003). Morphine administration changes the extracellular neurotransmitter concentration in the nucleus accumbens (NAcc), VTA and locus coeruleus (Farahmandfar et al. 2011). Glutamate NMDAR plays a pivotal role in the desensitization of opioid receptor by morphine in the CNS (Garzón et al. 2012). Opioids acts on µ-opioid receptor regulate glutamate activated NMDAR currents in the thalamus, locus coeruleus brainstem, medulla and hippocampal Cornu Ammonis (CA1) area (Guo et al. 2005; Garzón et al. 2012). Furthermore, opioid receptors are responsible for development of morphine addiction and NMDA glutamate receptor attenuate morphine withdrawal signs (Kamali et al. 2016). However, limit observation exists on co-localized AMPA glutamate receptors and µ-opioid receptor in the amygdala (Scavone et al. 2011).
However, researches were done on interconnection of the opioidergic and glutamatergic systems, but scarce information exists on food intake regulation. Additionally, despite various researches done to investigate central pathways responsible in appetite regulation in mammals, but aspects of appetite regulation in poultry remains quite limited (Denbow 1994). It is well documented central pathways for appetite regulation is differing between mammalian and birds (Zendehdel and Hassanpour 2014). So, it is logical to assume the regulatory mechanisms governing these processes in birds (Furuse 2002). So, this study was to find the possible interconnection of the opioidergic and glutamatergic systems on food intake in neonatal broilers.
Materials and Methods
Animals
In this study, 1-day-old meat-type male chickens (Ross 308) were purchased from local hatchery (Morghak Co. Iran). Birds were kept as flocks for 2 days then randomly transferred into individual cages at a temperature of 30 ± 1 °C with 50 ± 2 percent humidity (Olanrewaju et al. 2006). A commercial diet provided during the study containing 21% crude protein and 2850 kcal/kg of metabolizable energy (Chineh Co. Iran). All birds received ad libitum food and fresh water during the study. Just 3 h prior the ICV injections, chicken were food deprived (FD3) but had free access to water. The injections were applied to all birds at 5 days of age. Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care.
Experimental Drugs
DAMGO (µ-opioid receptor agonist), DPDPE (δ-opioid receptor agonist), U-50488H (κ-opioid receptor agonist), MK-801 (NMDA glutamate receptors antagonist), CNQX (AMPA glutamate receptors antagonist), AIDA (mGLU1 receptors antagonist), LY341495 (mGLU2 receptors antagonist), UBP1112 (mGLU3 receptors antagonist) and Evans blue were purchased from Sigma Co. (Sigma, USA) and Tocris Co. (UK). Drugs at first dissolved in absolute dimethyl sulfoxide (DMSO) then diluted with 0.85% saline containing Evans blue at a ratio of 1/250. DMSO with this ratio does not have cytotoxic effect (Blevins et al. 2002; Qi et al. 2008).
ICV Injection Procedures
In this study, 15 experiments designed to investigate interconnection of opioidergic and glutamatergic systems on cumulative food intake in neonatal meat-type birds (each experiment includes four groups within 11 replicates in each group). Prior to each experiment, the chicks were weighed and based on their body weight divided into experimental groups so the average weight between treatment groups was as uniform as possible. ICV injection applied using a microsyringe (Hamilton, Switzerland) without anesthesia according to the technique previously described by Davis et al. (1979) and Furuse et al. (1997) which head of the birds was held with an acrylic device while the bill holder was 45° and calvarium parallel to the surface of table (Van Tienhoven and Juhasz 1962). In a plate a hole was drilled which the skull over the right lateral ventricle immediately overlaid through this plate. A microsyringe was inserted into the right ventricle via the hole and tip of the needle penetrated 4 mm beneath the skin of the skull. It is revealed that, there is no injection-induced physiological stress using this method in neonatal chicks (Saito et al. 2005). Each chick received an ICV injection (with vehicle or drug solution) in a volume of 10 μL (Furuse et al. 1999). The control group received control solution (saline containing Evan’s blue 10 μL) (Furuse et al. 1999). Right away after injection, FD3 birds returned to their individual cages and supplied fresh water and food (pre-weighed). Cumulative food intake (gr) was measured at 30, 60 and 120 min post the injection. Food consumption was calculated as a percentage of body weight (BW) to minimize impact of BW on the amount of food intake. Each bird just used once in each experimental group. At the end of the experiments, accuracy of placement of the injection in the ventricle was verified by presence of Evans blue followed by slicing the frozen brain tissue. All experimental procedures were done from 8:00 a.m. until 3:30 p.m.
Feeding Experiments
In this study, 15 experiments were designed, each with four treatment groups (n = 44 in each experiment). In experiment 1, four groups of FD3 chicks received a dose of either the ICV injection of (A) control solution, (B) DAMGO (µ-opioid receptor agonist, 125 pmol), (C) MK-801 (NMDA glutamate receptors antagonist, 15 nmol) and (D) combination of DAMGO plus MK-801. Experiments 2–5 were similar to experiment 1, except FD3 chicks ICV injected with CNQX (AMPA glutamate receptors antagonist, 390 nmol), AIDA (mGLU1 receptors antagonist, 2 nmol), LY341495 (mGLUR2 receptors antagonist, 150 nmol) and UBP1112 (mGLU3 receptors antagonist, 2 nmol) instead of MK-801, respectively. In experiment 6, fowls ICV injected with (A) control solution, (B) DPDPE (δ-opioid receptor agonist, 40 nmol), (C) MK-801 (NMDA glutamate receptors antagonist, 15 nmol) and (D) combination of DPDPE plus MK-801. In experiments 7–10, FD3 chicks ICV injected as the same as procedure to the experiments 6, except injection with CNQX (390 nmol), AIDA (2 nmol), LY341495 (150 nmol) and UBP1112 (2 nmol) was done instead of MK-801, respectively. In experiment 11, FD3 birds ICV injected with (A) control solution, (B) U-50488H (κ-opioid receptor agonist, 30 nmol), (C) MK-801 (NMDA glutamate receptors antagonist, 15 nmol) and (D) combination of U-50488H plus MK-801. In experiments 12–15, were similar to experiment 11, except FD3 chicks ICV injected with CNQX (390 nmol), AIDA (2 nmol), LY341495 (150 nmol) and UBP1112 (2 nmol) instead of MK-801, respectively. Treatments procedure in experiments is presented in Table 1. Each bird was injected once only. These doses of drugs were calculated based on previous (Steinman et al. 1987; Zeni et al. 2000; Bungo et al. 2004, 2005; Baghbanzadeh and Babapour 2007; Shojaei et al. 2015; Zendehdel et al. 2009, 2012, 2015; Alimohammadi et al. 2015) and our pilot experiments (un-published data). Right away after injection, chickens were returned to their individual cages and provided ad libitum food (pre-weighed) and water. Cumulative food intake recorded at 30, 60 and 120 min after injection.
Statistical Analysis
Data is presented as mean ± SEM (standard error of the mean). Cumulative food intake (as percent of body weight) was analyzed by repeated measure two-way analysis of variance (ANOVA) using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). For treatment showing a main effect by ANOVA, means compared by Tukey–Kramer test. P < 0.05 was considered as significant differences between treatments.
Results
Effects and interactions of central opioidergic and glutamatergic systems on cumulative food intake in FD3 neonatal meat-type chicks are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15. In this study to examine the possible interaction between these two systems, effective and sub-effective doses of pharmacological agents were administered to confront nullifying effects of the agents.
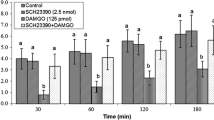
In experiment 1, ICV injection of an effective dose of DAMGO (µ opioid receptors agonist, 125 pmol) significantly decreased food intake until 120 min post injection compared to control group (P < 0.05). So, the 125 pmol DAMGO was selected to induce a decrease in food intake of chickens without affecting other non-ingestive behavioral parameters such as sedation (e.g., activity of chicks). ICV injection of sub effective dose of MK-801 (15 nmol) had no significant effect on food intake (P > 0.05). Also, co-administration of MK-801 and DAMGO significantly inhibited hypophagic effect of DAMGO in neonatal broilers (P < 0.05) [treatment effect: F(3, 80) = 401.5, P < 0.0001; time effect: F(2, 80) = 1035, P < 0.0001; treatment and time interaction: F(6, 80) = 6.347; P < 0.0001; Fig. 1].
Effects of intracerebroventricular injection of control solution, DAMGO (µ-opioid receptor agonist), MK-801 (NMDA glutamate receptors antagonist) and a combination of DAMGO plus MK-801 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 2, hypophagia observed after ICV injection of DAMGO (125 pmol) in FD3 neonatal chicken compared to control group (P < 0.05). There was no significant effect on food intake after ICV injection of 390 nmol CNQX (P > 0.05). Also, co-injection of DAMGO plus CNQX had no significant effect on cumulative food intake in neonatal chicks (P > 0.05) [treatment effect: F(3, 80) = 386.3, P < 0.0001; time effect: F(2, 80) = 873, P < 0.0001; treatment and time interaction: F(6, 80) = 4.173; P < 0.0001; Fig. 2].
Effects of intracerebroventricular injection of control solution, DAMGO (µ-opioid receptor agonist), CNQX (AMPA glutamate receptors antagonist) and a combination of DAMGO plus CNQX on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 3, significant decrease in feeding behavior observed after ICV injection of DAMGO (125 pmol) in FD3 neonatal birds until 120 min post injection compared to control group (P < 0.05). ICV injection of sub-effective dose of AIDA (2 nmol) had no significant effect on food intake (P > 0.05) while co-injection of the DAMGO + AIDA, significantly decreased DAMGO-induced hypophagia in neonatal chicks (P < 0.05) [treatment effect: F(3, 80) = 69.07, P < 0.0001; time effect: F(2, 80) = 834.1, P < 0.0001; treatment and time interaction: F(6, 80) = 6.19; P < 0.0001; Fig. 3].
Effects of intracerebroventricular injection of control solution, DAMGO (µ-opioid receptor agonist), AIDA (mGLUR1 glutamate receptors antagonist) and a combination of DAMGO plus AIDA on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments at each time (P < 0.05)
In experiment 4, ICV injection of effective dose of DAMGO (125 pmol) significantly decreased food intake in comparison to control group (P < 0.05). ICV injection of sub-effective dose of LY341495 (mGLU2 receptors antagonist, 150 nmol) had no significant effect on food intake (P > 0.05). Co-injection of the DAMGO plus LY341495 had no effect on DAMGO-induced hypophagia in neonatal broilers (P > 0.05) [treatment effect: F(3, 80) = 117.46, P < 0.0001; time effect: F(2, 80) = 509.7, P < 0.0001; treatment and time interaction: F(6, 80) = 7.26; P < 0.0001; Fig. 4].
Effects of intracerebroventricular injection of control solution, DAMGO (µ-opioid receptor agonist), LY341495 (mGLUR2 glutamate receptors antagonist) and a combination of DAMGO plus LY341495 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 5, hypophagia observed after ICV injection of DAMGO (125 pmol) in FD3 neonatal birds (P < 0.05). ICV injection of the sub-effective dose of the UBP1112 (mGLU3 receptors antagonist, 2 nmol) had no significant effect on food intake (P > 0.05). Co-administration of the DAMGO + UBP1112 had no significant effect on µ-opioid receptors agonist-induced hypophagia in neonatal chicks (P > 0.05) [treatment effect: F(3, 80) = 63.52, P < 0.0001; time effect: F(2, 80) = 927.13, P < 0.0001; treatment and time interaction: F(6, 80) = 5.74; P < 0.0001; Fig. 5].
Effects of intracerebroventricular injection of control solution, DAMGO (µ-opioid receptor agonist), UBP1112 (mGLUR3 glutamate receptors antagonist) and a combination of DAMGO plus UBP1112 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 6, ICV injection of the DPDPE (δ-opioid receptor agonist, 40 pmol) significantly increased food intake in neonatal chicks compared to control group (P < 0.05). ICV injection of the sub-effective dose of the NMDA glutamate receptors antagonist (MK-801, 15 nmol) had no effect on feeding behavior in neonatal chicks compared to control group (P > 0.05). Co-injection of DPDPE + MK-801 had no significant effect on cumulative food intake (P > 0.05) [treatment effect: F(3, 80) = 103.82, P < 0.0001; time effect: F(2, 80) = 641.49, p < 0.0001; treatment and time interaction: F(6, 80) = 4.91; P < 0.0001; Fig. 6].
Effects of intracerebroventricular injection of control solution, DPDPE (δ-opioid receptor agonist), MK-801 (NMDA glutamate receptors antagonist) and a combination of DPDPE plus MK-801 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
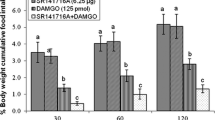
In experiment 7, hyperphagia observed after ICV injection of the DPDPE (40 pmol) in neonatal broilers compared to control (P < 0.05). Administration of the CNQX (AMPA glutamate receptors antagonist, 390 nmol) had no effect on the food consumption in FD3 neonatal birds (P > 0.05) while co-injection of the DPDPE + CNQX significantly amplified DPDPE induced feeding behavior (P < 0.05) [treatment effect: F(3, 80) = 57.04, P < 0.0001; time effect: F(2, 80) = 841.94, P < 0.0001; treatment and time interaction: F(6, 80) = 11.37; P < 0.0001; Fig. 7].
Effects of intracerebroventricular injection of control solution, DPDPE (δ-opioid receptor agonist), CNQX (AMPA glutamate receptors antagonist) and a combination of DPDPE plus CNQX on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments at each time (P < 0.05)
In experiment 8, ICV injection of the DPDPE (40 pmol) significantly increased food intake in neonatal broilers compared to control (P < 0.05). ICV administration of the AIDA (mGLU1 receptors antagonist, 2 nmol) had no significant effect on feeding behavior in comparison to the control group (P > 0.05). ICV injection of the AIDA with DPDPE could not significantly alter DPDPE-induced food intake in neonatal broilers (P > 0.05) [treatment effect: F(3, 80) = 149.82, P < 0.0001; time effect: F(2, 80) = 1032.08, P < 0.0001; treatment and time interaction: F(6, 80) = 5.91; P < 0.0001; Fig. 8].
Effects of intracerebroventricular injection of control solution, DPDPE (δ-opioid receptor agonist), AIDA (mGLUR1 glutamate receptors antagonist) and a combination of DPDPE plus AIDA on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 9, ICV injection of the DPDPE (40 pmol) significantly increased food intake in neonatal broilers compared to control (P < 0.05). ICV administration of the mGLU2 receptors antagonist (LY341495, 150 nmol) had no significant effect on feeding behavior (P > 0.05). Also, co-administration of LY341495 plus DPDPE had no effect on food intake induced by DPDPE (P > 0.05) [treatment effect: F(3, 80) = 85.17, P < 0.0001; time effect: F(2, 80) = 731.56, P < 0.0001; treatment and time interaction: F(6, 80) = 9.31; P < 0.0001; Fig. 9].
Effects of intracerebroventricular injection of control solution, DPDPE (δ-opioid receptor agonist), LY341495 (mGLUR2 glutamate receptors antagonist) and a combination of DPDPE plus LY341495 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 10, hyperphagia observed after ICV injection of the δ-opioid receptor agonist (DPDPE, 40 pmol) in FD3 neonatal broilers compared to control (P < 0.05). ICV administration of the mGLUR3 receptors antagonist (UBP1112, 2 nmol) had no significant effect on food intake in neonatal broilers compared to control (P > 0.05). In addition, co-injection of the UBP1112 + DPDPE was not able to fluctuate DPDPE-induced food intake (P > 0.05) [treatment effect: F(3, 80) = 70.66, P < 0.0001; time effect: F(2, 80) = 1018.35, P < 0.0001; treatment and time interaction: F(6, 80) = 5.95; P < 0.0001; Fig. 10].
Effects of intracerebroventricular injection of control solution, DPDPE (δ-opioid receptor agonist), UBP1112 (mGLUR3 glutamate receptors antagonist) and a combination of DPDPE plus UBP1112 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 11, ICV administration of the U-50488H (κ-opioid receptor agonist, 30 nmol) significantly increased food intake in FD3 neonatal broilers compared to control (P < 0.05). ICV injection of sub-effective dose of MK-801 (15 nmol) had no significant effect on food intake (P > 0.05). Also, co-administration of MK-801 and U-50488H had no effect on U-50488H-induced hyperphagia in neonatal broilers (P > 0.05) [treatment effect: F(3, 80) = 97.40, P < 0.0001; time effect: F(2, 80) = 642.18, P < 0.0001; treatment and time interaction: F(6, 80) = 10.36; P < 0.0001; Fig. 11].
Effects of intracerebroventricular injection of control solution, U-50488H (κ-opioid receptor agonist), MK-801 (NMDA glutamate receptors antagonist) and a combination of U-50488H plus MK-801 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 12, ICV injection of the U-50488H (30 nmol) significantly increased food intake in FD3 broilers compared to control (P < 0.05). There was no significant effect on food intake after ICV injection of 390 nmol CNQX (P > 0.05). Also, co-administration of the U-50488H plus CNQX had no significant effect on cumulative food intake in neonatal chicks (P > 0.05) (treatment effect: F(3, 80) = 73.59, P < 0.0001; time effect: F(2, 80) = 921.47, P < 0.0001; treatment and time interaction: F(6, 80) = 4.15; P < 0.0001; Fig. 12).
Effects of intracerebroventricular injection of control solution, U-50488H (κ-opioid receptor agonist), CNQX (AMPA glutamate receptors antagonist) and a combination of U-50488H plus CNQX on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 13, injection of the U-50488H (30 nmol) significantly increased food intake in FD3 broilers compared to control (P < 0.05). Administration of the AIDA (mGLU1 receptors antagonist, 2 nmol) had no significant effect on food intake (P > 0.05). Co-injection of the U-50488H plus AIDA had no significant effect on food intake induced by U-50488H in neonatal chicks (P > 0.05) [treatment effect: F(3, 80) = 132.06, P < 0.0001; time effect: F(2, 80) = 583.19, P < 0.0001; treatment and time interaction: F(6, 80) = 14.36; P < 0.0001; Fig. 13].
Effects of intracerebroventricular injection of control solution, U-50488H (κ-opioid receptor agonist), AIDA (mGLUR1 glutamate receptors antagonist) and a combination of U-50488H plus AIDA on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 14, hyperphagia observed after ICV injection of the U-50488H (30 nmol) in FD3 broilers compared to control (P < 0.05). Sub-effective dose of the LY341495 (mGLU2 receptors antagonist, 150 nmol) had no effect on cumulative food consumption in FD3 neonatal birds compared to the control group (P > 0.05). Co-injection of the U-50488H + LY341495 had no significant effect on U-50488H- induced hyperphagia in neonatal chicks (P > 0.05) [treatment effect: F(3, 80) = 70.24, P < 0.0001; time effect: F(2, 80) = 809.96, P < 0.0001; treatment and time interaction: F(6, 80) = 5.01; P < 0.0001; Fig. 14].
Effects of intracerebroventricular injection of control solution, U-50488H (κ-opioid receptor agonist), LY341495 (mGLUR2 glutamate receptors antagonist) and a combination of U-50488H plus LY341495 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
In experiment 15, injection of the U-50488H (30 nmol) significantly increased food intake in FD3 broilers compared to control group (P < 0.05). The ICV injection of the sub-effective level of the UBP1112 (mGLU3 receptors antagonist, 2 nmol) had no effect on feeding behavior in FD3 neonatal broiler (P > 0.05). Co-injection of the U-50488H + UBP1112 had no significant effect on U-50488H- induced food intake in neonatal chicks (P > 0.05) [treatment effect: F(3, 80) = 95.42, P < 0.0001; time effect: F(2, 80) = 954.13, P < 0.0001; treatment and time interaction: F(6, 80) = 8.27; P < 0.0001; Fig. 15].
Effects of intracerebroventricular injection of control solution, U-50488H (κ-opioid receptor agonist), UBP1112 (mGLUR3 glutamate receptors antagonist) and a combination of U-50488H plus UBP1112 on cumulative food intake (g/100 g BW) in neonatal chicks. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments at each time (P < 0.05)
Discussion
The present study was designed to investigate the possible interconnection of glutamatergic and opioidergic systems on food intake in neonatal broiler chicks. Obtained results imply ICV injection of DAMGO, significantly decreased food intake while DPDPE and U-50488H increased feeding behavior in FD3 neonatal broilers. As observed, injection of the MK-801 + DAMGO significantly inhibited hypophagic effect of DAMGO. Interconnection exists between µ-opioid receptors agonist and NMDA glutamate receptors in FD3 neonatal broilers. It is reported the NMDA receptors involved in the µ-opioid receptors sensitization in CA1 of the rat hippocampus (Farahmandfar et al. 2011). Also, behavioral sensitization to opioids can alter the glutamate level in the VTA and prefrontal cortex (Farahmandfar et al. 2011). Despite, the direct mechanism for how these two systems interacts is not fully elicited, but it is reported opiates impress their effects by activating µ-opioid receptors which are belongs to the GPCRs. These GPCRs regulate diverse effectors such as inwardly rectifying K+ channels, voltage-activated Ca2+ channels, NMDA, adenylyl cyclase and phospholipases C (Garzón et al. 2012). Interestingly, similar amino acid sequences have been identified in NMDA and µ-opioid receptors supporting a direct physical association between these systems (Garzón et al. 2012).
According to the results, perhaps interconnection exist between central opioidergic and glutamatergic systems on central food consumption by µ- and δ-opioid receptors with NMDA, AMPA and mGLU1 glutamate receptors in broilers. Co-injection of the DAMGO plus AIDA significantly decreased DAMGO-induced hypophagia in neonatal chicks. These results suggested interconnection between central µ-opioid and mGLUR1 glutamate receptors. It is reported, alteration in the glutamatergic system underlying the physical and psychological dependence on morphine. Based on the literature, ICV injection of the metabotropic glutamate receptors antagonist amplified feeding behavior in broiler cockerels (Baghbanzadeh and Babapour 2007). Neurons use glutamate as a co-transmitter which acts via AMPA/kainite mediated excitatory post synaptic potentials (EPSPs) (Liu and Salter 2010). Glutamate–opioid interactions are known one of the important neural pathways in the brain which play crucial role in stress, pain and addiction (Guo et al. 2005). It is known certain glutamatergic projection could be impacted by opioids (Guo et al. 2009). The mGLUR1 widely distributed in the NAcc providing the morphological evidence for their role in reward behaviors and drug addiction (Mitrano et al. 2008).
In this study, co-injection of the DPDPE plus CNQX, amplified δ-opioid receptor induced feeding behavior via AMPA glutamate receptors. It is reported, opioid receptor stimulation within the ventral medial prefrontal cortex (vmPFC) induces feeding via recruitment of glutamate signaling in rats (Mena et al. 2013). For instance, co-injection of DAMGO followed by blocking of the AMPA receptors within the NAcc shell enhanced food intake in rats (Mena et al. 2013). ICV injection of AMPA receptors agonist (but not NMDA) suppresses feeding behavior while simultaneously engendering locomotor hyperactivity and rearing behavior in rat (Mena et al. 2013). Also, the ICV injection of glutamate and HQCA (ionotropic glutamate antagonist) reduced food intake while MSPG (metabotropic glutamate receptors antagonist) increased feed intake and the latency to start feeding in broiler cockerels (Baghbanzadeh and Babapour 2007). Da Silva et al. (2003) reported microinjections of NMDA and AMPA-kainite receptors antagonists into ventral striatal and ventral pallidal areas of the pigeon induced feeding behavior. So, it seems there is difference in central food intake regulation between avian and mammals. Given the estimated 300 million years of evolutionary distance between avian and mammalian, it is not amazing differences involved in the central food intake regulation and energy expenditure (Novoseletsky et al. 2011). For example, ICV injection of μ-opioid receptors agonist decreased food intake in chicks (Bungo et al. 2005; Alimohammadi et al. 2015) but increased in the rat (Le Merrer et al. 2009; Kaneko et al. 2012).
In rat brain, in the lateral cerebroventricle regions, κ-opioid receptor systems are regulated by the AMPA glutamate receptor (Minowa et al. 2003). It is reported NMDA and AMPA receptors are involved in the opioid sensitization (Sepehrizadeh et al. 2008a, b). AMPA and NMDA receptors are localized to the postsynaptic membrane of glutamatergic synapses, where they are organized into large macromolecular signaling complexes (Garzón et al. 2012). However, the direct pathway responsible for interconnection of δ-opioid and AMPA receptors are unknown, mechanism(s) introduced for observed results. It is suggested opiates transiently activates the PI3K/Akt/nNOS pathway leading to recruitment of protein kinase C (PKC) and Raf-1 to the histidine triad nucleotide-binding protein 1 (HINT1) at the C terminus of opioid receptors in a redox and zinc dependent manner (Garzón et al. 2012). This mechanism(s) connect opioid receptors activation with that of the Raf-1/MAPK pathway and probably with the regulation of NMDAR function via AMPA receptors (Garzón et al. 2012).
Microinjection of AMPA receptors antagonist into the VTA attenuated morphine withdrawal symptoms, suggesting the AMPA receptors are involved in the morphine withdrawal process (Guo et al. 2009). Interestingly, opioid receptor and the AMPA subunit were frequently associated with common intracellular organelles, as well as adjacent areas of the surface membrane (Beckerman and Glass 2011). AMPA receptors are formed ligand-gated ion channels composed of various subunits termed as GluR1−4 (Guo et al. 2009). It is described alterations in AMPA-GluR1 in response to both acute and chronic morphine exposure in the VTA, a midbrain dopaminergic region integral to reward and reinforcement (Glass et al. 2008). Chronic opioid exposure induced an increased presence of AMPA-GluR1 immuno-reactivity at the plasma membrane and post-synaptic densities of the forebrain-projecting (motivation and drug-seeking) and of limbic structure projecting (locomotor and reward) VTA neurons (Glass et al. 2008). Opioid was shown to inhibit glutamate release through the reduction of Ca2+ influx into the terminal (Scavone 2011). As observed in this study, food intake amplified via co-injection of the δ-opioid receptors agonist and AMPA glutamate receptors antagonist. It seems, amplified hyperphagia might relate to the hyperphagic effect of the δ-opioid receptors agonist in one hand, and on the other hand the blockade of the AMPA glutamate receptors. However, there was no previous report on the interconnection of the opioidergic system and glutamate inotropic receptors on feeding behavior even in mammals. So, no report was found to compare the results with it. These findings may shed light on the circuitry underlying interconnection between central opioidergic and glutamatergic systems on feeding behavior. Obtained results indicate for functional interconnection of opioidergic and glutamatergic systems in appetite regulatory centers of the hypothalamus.
Dopamine has played key role in glutamate and opioids activity through the activation of D1 and mGLU1 receptors (Schotanus and Chergui 2008). The cellular mechanisms of the alteration of extracellular glutamate concentration in the CNS remain to be investigated. Glutamate releases from vesicles in presynaptic terminals by a Ca2+ dependent mechanism and it is controlled through a wide range of presynaptic receptors including opioid receptors (Guo et al. 2005). It is possible morphine may directly activate the opioid receptors in the appetite regulatory centers of the brain to regulate the level of glutamate (Guo et al. 2005). It is assumed there are interconnections between central glutamatergic, opioidergic and dopaminergic systems in the VTA. Dopamine modulates glutamatergic signals in the NAcc originating from the amygdala and hippocampus (Tzschentke and Schmidt 2003). ICV injection of Naloxone (opioid antagonist) decreased dopamine and increase glutamate in morphine-dependent rats (Guo et al. 2005). The dopamine releasing effect of glutamate in the NAcc may be predominantly mediated by AMPA (rather than NMDA) receptors in the amygdala and hippocampus (Tzschentke and Schmidt 2003). Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons in addicted mice (Branch et al. 2013). Recently, Taheriyan et al. (2016) reported dopamine-induced hypophagia is mediated via NMDA and mGlu1 receptors in FD3 neonatal meat-type chicken. On the other hand correlation reported between dopaminergic (DAergic) and opioidergic systems on feeding control where ICV injection of DAMGO significantly decreased food intake but co-injection of DAMGO plus D1 like receptors antagonist diminished DAMGO-induced hypophagia in neonatal layer (Zendehdel et al. 2016). Perhaps, several neurotransmitter systems modulate effects of opioidergic and glutamatergic neurons. So, further researches seem to be needed to determine possible pathways.
In conclusion, the results of the current results suggested ICV injection of the DAMGO plus MK-801 and DAMGO plus AIDA decreased DAMGO-induced hypophagia in neonatal chicks. Moreover, ICV administration of the DPDPE plus CNQX amplified DPDPE induced feeding behavior. These results suggested interconnection between central opioidergic and glutamatergic systems on feeding behavior mediates via µ- and δ-opioid receptors with NMDA, AMPA and mGLU1 receptors in FD3 neonatal broilers. There was no previous study on the role of central opioidergic and glutamatergic systems on food intake in avian. Most research on central food intake regulation has done with rat models, whereas considering few investigations done in birds. So, we were not able to compare our results with it. These observations can be used as base information on central food intake regulation in birds. The authors recommend further investigation need to clarify direct cellular and molecular signaling pathways of the opioidergic and glutamatergic systems with other receptors in physiology of food intake regulation in poultry.
References
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39:105–113
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res 62(4):125–129
Beckerman MA, Glass MJ (2011) Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala. Exp Neurol 227(1):149–158
Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71:277–282
Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ (2013) Food Restriction Increases Glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci 33(34):13861–13872
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2004) Feeding responses to µ-, δ- and κ-opioid receptor agonists in the meat-type chick. Pharmacol Biochem Behav 78: 707–710
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2005) Effects of various µ-, δ- and κ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85:519–523
Charles JR, Duva MA, Ramirez GJ, Lara RL, Yang CR, Stanley BG (2014) Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacology 79:59–65
Da Silva AA, Marino-Neto J, MA P (2003) Feeding induced by microinjections of NMDA and AMPA–kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res 966:76–83
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Farahmandfar M, Karimian SM, Zarrindast MR, Kadivar M, Afrouzi H, Naghdi N (2011) Morphine sensitization increases the extracellular level of glutamate in CA1 of rat hippocampus via µ-opioid receptor. Neurosci Lett 494: 130–134
Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y (2012) Current research on opioid receptor function. Curr Drug Target 13(2):230–246
Filizola M, Devi LA (2013) Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci 34(1):6–12
Furuse M (2002) Central regulation of food intake in the neonatal chick. Anim Sci J 73:83–94
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P (2012) Direct association of mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5:199–226
Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM (2008) Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol 210:750–761
Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G (2005) Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett 381: 12–15
Guo Y, Wang HL, Xiang XH, Zhao Y (2009) The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci Biobehav Rev 33:864–873
Hao Y, Yang JY, Wu CF, Wu MF (2007) Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial prefrontal cortex in mice. Pharmacol Biochem Behav 86:660–666
Hettes SR, GonzagaWJ, Heyming TW, Nguyen JK, Perez S, Stanley BG (2010) Stimulation of lateral hypothalamic AMPA receptors may induce feeding in rats. Brain Res 1346:112–120
Kamali M, Sahraei H, Khosravi M, Hassanpour S, Yaribeygi H (2016) Asymmetric involvement of central and the peripheral NMDA glutamate receptors in the expression of withdrawal syndrome in morphine-dependent mice. Physiol Pharmacol 19: 274–284
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Ladepeche L, Yang L, Bouchet D, Groc L (2013) Regulation of dopamine D1 receptor dynamics within the postsynaptic density of hippocampal glutamate synapses. PLoS ONE 8(9):e74512
Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Lee CWS, Ho IK (2013) Pharmacological profiles of oligomerized μ-opioid receptors. Cells 2, 689–714. doi:10.3390/cells2040689
Liu XJ, Salter MW (2010) Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci 32:278–289
McFadden KL, Cornier MA, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Frontiers In Psychol 5(553):1–7
Mena JD, Selleck RA, Baldo BA (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33(47):18540–18552
Minowa S, Ishihara S, Tsuchiya S, Horie S, Watanabe K, Murayama T (2003) Involvement of glutamate and c-amino-butyric acid receptor systems on gastric acid secretion induced by activation of κ-opioid receptors in the central nervous system in rats. Br J Pharmacol 138:1049–1058
Mitrano DA, Arnold C, Smith Y (2008) Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocainetreated rats. Neurosci 154: 653–666
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236:52–60
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Scavone JL, Asan E, Van Bockstaele EJ (2011) Unraveling glutamate-opioid receptor interactions using highresolution electron microscopy: implications for addictionrelated processes. Exp Neurol 229(2):207–213
Schotanus SM, Chergui K (2008) Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacol 54: 837–844
Sepehrizadeh Z, Bahrololoumi Shapourabadi M, Ahmadi S, Bozchlou Hashemi S, Zarrindast MR, Sahebgharani M (2008a) Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol 35:1321–1330
Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou Hashemi S, Zarrindast MR (2008b) Morphine-induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacol 81:333–343
Shojaei M, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Opioid-induced hypophagia is mediated by 5-HT2c receptors in neonatal layer-type chicken. Czech J Anim Sci 60(9):400–410
Steinman JL, Fujikawa DG, Wasterlain CG, Cherkin A, Morley JE (1987) The effects of adrenergic, opioid and pancreatic polypeptidergic compounds on feeding and other behaviors in neonatal leghorn chicks. Peptides 8: 585–592
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DLAP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Taheriyan MR, Baghbanzadeh A, Zendehdel M (2016) Dopamine- induced hypophagia is mediated via NMDA and mGlu1 receptors in chicken. Iranian J. Vet Med 10(3):191–199
Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatr 8:373–382
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G (2003) Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci 23:4775–4784
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2016) Interaction between opioidergic and dopaminergic systems on food intake in neonatal layer type chicken. Int J Pept Res Ther 22:83–92
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Hassanpour S, Babapour V, Charkhkar Mahdavi M (2015) Interaction between endocannabinoid and opioidergic systems regulates food intake in neonatal chicken. Int J Pept Res Ther 21:289–297
Zendehdel M, Taati M, Jonaidi H, Amini E (2012) The role of central 5-HT (2C) and NMDA receptors on LPS-induced feeding behavior in chickens. J Physiol Sci 62:413–419
Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA (2000) Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav 65(1):67–74
Acknowledgements
The authors thank the central laboratory (Dr. Rastegar Lab.) of the Faculty of Veterinary Medicine, University of Tehran for cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Participants
All experiments were executed according to the Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal ethics committee.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Torkzaban, M., Zendehdel, M., Babapour, V. et al. Interaction Between Central Opioidergic and Glutamatergic Systems on Food Intake in Neonatal Chicks: Role of NMDA, AMPA and mGLU1 Receptors. Int J Pept Res Ther 24, 157–169 (2018). https://doi.org/10.1007/s10989-017-9601-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9601-9