Abstract
Oxytocin neurons have a physiological role in food intake and energy balance. Several studies have shown that central histaminergic and adrenergic systems synapse on oxytocin neurons but there is no information for their interaction on food intake regulation in birds. The purpose of this study was to examine the effects of intracerebroventricular (ICV) injection of α-fluoromethylhistidine (α-FMH, histidine decarboxylase inhibitor), chlorpheniramine (histamine H1 receptors antagonist), famotidine (histamine H2 receptors antagonist), thioperamide (histamine H3 receptors antagonist), prazosin (α1 receptor antagonist), yohimbine (α2 receptor antagonist), metoprolol (β1 adrenergic receptor antagonist), ICI 118,551 (β2 adrenergic receptor antagonist) and SR59230R (β3 adrenergic receptor antagonist) on oxytocin-induced hypophagia in 3-h food-deprived (FD3) neonatal broiler chicken. In Experiment 1, 3 h-fasted chicks were given an ICV injection of saline, α-FMH (250 nmol), oxytocin (10 μg) and co-injection of α-FMH + oxytocin. Experiments 2–9 were similar to experiment 1 except birds were injected with chlorpheniramine (300 nmol), famotidine (82 nmol), thioperamide (300 nmol), prazosin (10 nmol), yohimbine (13 nmol), metoprolol (24 nmol), ICI 118,551(5 nmol) and SR59230R (20 nmol) instead of α-FMH, respectively. After injection cumulative food intake was measured until 120 min post injection. According to the results, ICV injection of oxytocin significantly decreased food intake in broiler chickens (P < 0.001). ICV injection of α-FMH significantly attenuated hypophagic effect of oxytocin (P < 0.001). Also, co-injection of chlorpheniramine plus oxytocin significantly decreased the effect of oxytocin on food intake (P < 0.001). Co-administration of thioperamide and oxytocin significantly amplified hypophagic effect of oxytocin in chickens (P < 0.001). In addition, ICI 118,551 attenuated hypophagic effect of oxytocin (P < 0.001); while famotidine, prazosin, yohimbine, metoprolol and SR59230R had no effect on oxytocin- induced food intake in FD3 broiler chickens. These results suggest that the effect of oxytocin on food intake is probably mediated by histaminergic (via H1 and H3 receptors) and noradrenergic (via β2 receptors) systems in broiler chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds, like mammals, have complex mechanisms that regulate food intake. Given a choice between more than one diet, turkeys, broilers, layers, and other avian species display the ability to self-select a diet adequate for growth or production (Denbow 1999). Food intake regulation is highly conserved across animals, and thus neural and endocrine networks controlling this behavior are similarly conserved. The hypothalamus has emerged as the major site of food intake (Hussain and Bloom 2013). The hypothalamus receives signals from peripheral tissues as well as other parts of the brain, and it integrates these inputs (Richards 2003). As in mammals, lesioning the medial hypothalamus of avian species increases food intake, whereas lesioning the lateral hypothalamic area (LHA) decreases food intake (Kuenzel et al. 1999). While these sites were traditionally considered the satiety and feeding centers, respectively, it is currently believed that they are considered as parts of larger neural circuits involved in food intake regulation.
In the central nervous system (CNS), oxytocin (OT) is synthesized mainly in the supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus as well as in hypothalamic magnocellular accessory neurons located between the PVN and SON (Adan et al. 2011). The OT receptor is a typical class I G protein-coupled receptor that is primarily coupled via Gq proteins to phospholipase C (Gimpl and Fahrenholz 2001). In the brain, OT receptors are expressed in several areas including ventromedial hypothalamus, bed nucleus of the stria terminalis, ventral pallidum, paraventricular nucleus, and dorsal part of the supraoptic nucleus (Adan et al. 2011). Previous studies showed that ICV injection of OT decreases food intake in mammals (Arletti et al. 1990) and birds dose-dependently (Jonaidi et al. 2003).
Histamine is derived from the decarboxylation of the amino acid histidine and is released as a neurotransmitter in brain. Histaminergic neurons are found in the tuberomammillary nuclei and regulate various activities of the brain, such as the arousal state, brain energy metabolism, locomotor activity, neuroendocrine, autonomic and vestibular functions, feeding, drinking, sexual behavior, and analgesia (Wada et al. 1991; Brown et al. 2001). The histamine receptors are a class of G protein–coupled receptors (Hill et al. 1997). H1 and H2 receptors are mainly expressed postsynaptically and H1 receptors are coupled positively to phospholipase C and high densities are found especially in the limbic system, while H2 receptors are coupled positively to adenylyl cyclase and are highly expressed in hippocampus, amygdala and basal ganglia (Brown et al. 2001). Also, H3 receptors are particularly presynaptically located and are negatively coupled to adenylyl-cyclase and high densities are expressed in the basal ganglia (Brown et al. 2001). Previous studies have indicated that histamine decreases food intake through H1 but not H2 receptors in broiler chickens (Taati et al. 2009). Also, histamine H3 receptor antagonist through stimulation of synthesis and release of neuronal histamine can decrease food intake in broiler chickens (Taati et al. 2009).
Norepinephrine (NE) is an organic chemical in the catecholamine family and functions as a neurotransmitter inside the brain. The effects of norepinephrine are manifested in alertness, arousal, and readiness for action (Sara and Bouret 2012). Norepinephrine has two main classes of adrenergic receptors, α and β, with several subtypes. Alpha receptors are divided into subtypes α1 (a Gq coupled receptor) and α2 (a Gi coupled receptor) (Qin et al. 2008). Beta receptors are divided into subtypes: β1, β2, and β3 all three are linked to Gs proteins (Chen-Izu et al. 2000).
Based on previous studies noradrenergic system, similar to histaminergic system has an undeniable role in control of food intake in mammals and birds (Wellman 2000; Bungo et al. 2002). Pharmacologic manipulations that raise NE can enhance or suppress food intake, depending on the site and type of NE manipulation (Wellman 2000). Previous studies showed that NE exerts orexigneic effects through α2 adrenergic receptor in layer type chicks (Bungo et al. 2002) and anorexigenic effects via β2-adrenergic receptors in broilers (Zendehdel and Hassanpour 2014; Baghbanzadeh et al. 2010; Wellman 2000).
Hypothalamic histamine may also play a role in the physiological regulation of OT release (Knigge and Warberg 1991). On the other hand, activation of histaminergic and noradrenergic neurons in the brain stimulates release of OT (Knigge et al. 1999a, b). ICV administration of histamine and noradrenaline in rats increases both systemic and intranuclear release of OT. It has previously been reported that infused ICV histamine in rats stimulated OT secretion almost 6-fold and norepinephrine stimulated OT secretion about 5-fold (Knigge et al. 1999a, b). Based on the literature there is not any report about central effects of histaminergic and noradrenergic systems on OT induced food intake in broiler chickens. So, in this study we tried to investigate the possible interaction of histaminergic and noradrenergic systems with OT on feeding behavior in meat-type chickens.
Material and method
Animals
Broiler (Ross 308) chickens were obtained from a local hatchery (Mahan Morgh. Co., Tehran, Iran). These chickens first were housed for 2 days as flocks, then randomly distributed into individual cages, temperature controlled (30 ± 1) °C with 23-h light, 1-h dark cycle, and with 50 ± 2% humidity until 5 days of age (Olanrewaju et al. 2006). The birds had free access to food and tap water. Chickens were supplied with a mash diet (21% crude protein and 2.870 kcal/kg of metabolizable energy) and fresh water. The birds were food deprived (FD3) 3-h prior to intracerebroventricular (ICV) injection but had free access to water.
Experimental drugs
Experimental drugs including Oxytocin, α-FMH (histidine decarboxylase inhibitor), Chlorpheniramine (histamine H1 receptors antagonist), Famotidine (histamine H2 receptors antagonist), Thioperamide (histamine H3 receptors antagonist), Prazosin (α1 receptors antagonist), Yohimbine (α2 receptors antagonist), Metoprolol (β1 adrenergic receptors antagonist), ICI 118,551 (β2 adrenergic receptor antagonist), SR 59230R (β3 adrenergic receptor antagonist) and Evans blue were purchased from Sigma Co. (Sigma, USA) and Tocris Bioscience Co. (Tocris, UK). All these compounds were dissolved in a 0.1% Evans Blue solution, which was prepared in 0.85% saline or first dissolved in absolute Dimethyl sulfoxide (DMSO) then diluted with 0.85% saline containing Evans blue at a ratio of 1/250. DMSO with this ratio does not have a cytotoxic effect. Saline containing Evans Blue was used as a control.
ICV injection protocol
Intracerebroventricular (ICV) injections were administered at 5 days of age. A total of 9 experiments were designed to investigate the effects of histaminergic and adrenergic systems on oxytocin- induced food intake in neonatal birds. Before the start of the investigation, all chickens were weighed and distributed into treatment groups according to their body weight. So, mean weight across the group was made as uniform as possible. Each experiment included 4 treatment groups with 11 replicates per group (n = 44 chickens per experiment). Each chicken received ICV injected once in each experiment. ICV injections were done without any anesthesia by using a microsyringe (Hamilton, Switzerland) (Davis et al. 1979; Furuse et al. 1997). In this method, we put the head of the chickens in an acrylic device in which the bill holder was 45° and the calvarium was parallel to the surface of the table (Van Tienhoven and Juhasz 1962). An orifice was made in a plate that was placed over the skull of chicken instantly over the right lateral ventricle. Afterward a microsyringe was inserted into the right ventricle through the orifice in the plate and the tip of the needle penetrated only 4 mm below the skin of the skull. This technique does not have any physiological stress in chicks (Saito et al. 2005). The control group received control solution as 10 μL of saline containing Evan’s blue (Furuse et al. 1999). After injection the chick was immediately returned to its cage and fresh food and water were supplied. Cumulative food intake (gr) was measured at 30, 60 and 120 min after the injection. 3 h before the injection, birds were food deprived (FD3). Each bird was used once in each experiment. At the end of the experiments, to recognize the accuracy of injection, chicken were killed by decapitation. Accuracy of placement of the injection in the ventricle was verified by the presence of Evans blue followed by slicing the frozen brain tissue. Only data from individuals in which dye was present in their lateral ventricle were used for analysis. All experimental procedures were done from 8:00 A.M. until 3:30 P.M. Also, the time course of food consumption was selected based on previous studies (Jonaidi and Noori 2012; Zendehdel and Hassanpour 2014; Zendehdel et al. 2016).
Feeding experiment
Experiments 1–9 were designed to examine the effects of ICV injection of histaminergic and adrenergic receptors antagonist on oxytocin- induced feeding behavior in FD3 neonatal meat-type chicks. In experiment 1, each treatment group received either control solution, α-fluoromethylhistidine (α-FMH, histidine decarboxylase inhibitor; 250 nmol), OT (10 μg) or a co-injection of α-FMH (250 nmol) and OT (10 μg). Experiments 2–4 were similar to experiment 1, except birds were injected with chlorpheniramine (histamine H1 receptors antagonist; 300 nmol), famotidine (histamine H2 receptors antagonist; 82 nmol) and thioperamide (histamine H3 receptors antagonist; 300 nmol), instead of α-FMH, respectively. In experiment 5, each treatment group received either control solution, prazosin (α1 receptor antagonist; 10 nmol), OT (10 μg) or a co-injection of prazosin (10 nmol) and OT (10 μg). Experiments 6–9 were similar to experiment 5, except yohimbine (α2 receptor antagonist; 13 nmol), metoprolol (β1 adrenergic receptor antagonist; 24 nmol), ICI 118,551 (β2 adrenergic receptor antagonist; 5 nmol) and SR59230R (β3 adrenergic receptor antagonist; 20 nmol) were used instead of prazosin, respectively. Experiments 1–4 were designed to examine the interaction of central histaminergic system and OT on food intake in neonatal meat-type chicks. In addition, experiments 5–9 examined the interaction of central adrenergic system and OT on food intake in chicks. In the co-injections, both substances were administered in a unique injection. To examine the possible effects of central histaminergic and adrenergic systems on feeding behavior induced by OT the effective and sub-effective doses of the pharmacological agents were administered in the co-injections. Thus, the sub-effective doses of antagonists and effective dose of OT were administered in experiments 1–9. Cumulative food intake (g) was measured at 30, 60 and 120 min after the injection. Food consumption was calculated as a percentage of body weight (%BW) to minimize effect of body weight on the amount of food intake. Drug doses were calculated based on previous studies as well as our pilot studies (Jonaidi et al. 2003; Taati et al. 2009, 2010; Tachibana et al. 2003; Bungo et al. 2010; Zendehdel et al. 2015, 2016).
Statistical analysis
Cumulative food intake is expressed as mean ± SEM (standard error of mean) and p < 0.05 is the defined level for statistical significance. Data were processed by commercially available software Graph Pad Prism 6.0. The mean food intakes of different groups were compared by repeated measure two-way ANOVA followed by Bonferroni’s multiple comparisons test, to specify the treatment and time effects on feeding behaviour.
Result
Central effects of histaminergic and adrenergic systems on oxytocin- induced food intake in FD3 neonatal meat-type chicks are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9.
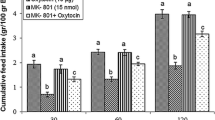
Effect of ICV injection of α-FMH (250 nmol), oxytocin (10 μg) and their combination on cumulative food intake (% BW) in neonatal meat- type chickens. α-FMH: alpha fluoromethyl histidine (inhibitor of histidine decarboxylase). Data are expressed as mean ± SEM. a = p < 0.0001 oxytocin vs control; b = p < 0.001 α-FMH + oxytocin vs oxytocin
Effect of ICV injection of chlorpheniramine (300 nmol), oxytocin (10 μg) and their combination on cumulative food intake (% BW) in neonatal meat- type chickens. Chlorpheniramine: histamine H1 receptors antagonist. Data are expressed as mean ± SEM. a = p < 0.0001 oxytocin vs control; b = p < 0.05 chlorpheniramine + oxytocin vs oxytocin
Effect of ICV injection of thioperamide (300 nmol), oxytocin (10 μg) and their combination on cumulative food intake (% BW) in neonatal meat- type chickens. Thioperamide: histamine H3 receptors antagonist. Data are expressed as mean ± SEM. a = p < 0.0001 oxytocin vs control; b = p < 0.001 thioperamide + oxytocin vs oxytocin
Effect of ICV injection of ICI 118,551 (5 nmol), oxytocin (10 μg) and their combination on cumulative food intake (% BW) in neonatal meat- type chickens. ICI 118,551: β2 adrenergic receptors antagonist. Data are expressed as mean ± SEM. a = p < 0.0001 oxytocin vs control; b = p < 0.05 ICI 118,551 + oxytocin vs oxytocin
In experiment 1, ICV injection of sub effective dose of α-FMH (irreversible-inhibitor of histidine decarboxylase, 250 nmol) had no significant effect on food intake compared to control group in FD3 neonatal broiler. The ICV injection of an effective dose of OT (10 μg) significantly decreased food consumption at 30, 60 and 120 min post-injection. Co-injection of α-FMH (250 nmol) and OT (10 μg) significantly attenuated hypophagic effect of OT at 30, 60 and 120 min post-injection [treatment effect: F (3, 80) = 363.2, p < 0.0001; time effect: F (2, 80) = 1235, p < 0.0001; treatment and time interaction: F (6, 80) = 5.198; p < 0.0001; Fig. 1].
The results of experiment 2 showed that ICV injection of sub effective dose of chlorpheniramine (H1 receptor antagonist, 300 nmol) had no significant effect on cumulative food intake in FD3 chicks. Likewise, ICV injection of 10 μg OT significantly decreased food intake at 30, 60 and 120 min post-injection. Co-administration of chlorpheniramine and OT significantly attenuated hypophagic effect of OT in FD3 neonatal broiler [treatment effect: F (3, 80) = 312.8, p < 0.0001; time effect: F (2, 80) = 1048, p < 0.0001; treatment and time interaction: F (6, 80) = 7.290; p < 0.0001; Fig. 2].
In experiment 3, it was determined that ICV injection of famotidine (H2 receptor antagonist, 82 nmol) had no significant effect on food intake in broilers. ICV injection of OT (10 μg) had a hypophagic effect at 30, 60 and 120 min post-injection. However, there was no significant effect on OT- induced feeding behavior by combine injection of famotidine plus OT [treatment effect: F (3, 80) = 64.57, p < 0.0001; time effect: F (2, 80) = 891.7, p < 0.0001; treatment and time interaction: F (6, 80) = 8.598; p < 0.0001; Fig. 3].
According to the results of experiment 4, ICV injection of thioperamide (H3 receptor antagonist, 300 nmol) had no significant effect on food consumption in comparison to control group. OT (10 μg) significantly decreased cumulative food intake in chickens. In addition, the anorexigenic effect of OT (10 μg) was significantly amplified in the co-injection of thioperamide (300 nmol) and OT (10 μg) in neonatal chicks [treatment effect: F (3, 80) = 108.9, p < 0.0001; time effect: F (2, 80) = 727.8, p < 0.0001; treatment and time interaction: F (6, 80) = 34.14; p < 0.0001; Fig. 4].
In experiment 5, it was determined that ICV injection of prazosin (α1 adrenergic receptor antagonist, 10 nmol) had no significant effect in cumulative food intake in chickens. Also, 10 μg OT significantly decreased cumulative food intake in neonatal chicks. Co-injection of prazosin (10 nmol) and OT (10 nmol) had no effect on OT-induced hypophagia at 30, 60 and 120 min post-injection [treatment effect: F (3, 80) = 51.83, p < 0.0001; time effect: F (2, 80) = 917.8, p < 0.0001; treatment and time interaction: F (6, 80) = 5.697; p < 0.0001; Fig. 5].
In experiment 6, 13 nmol yohimbine (α2 adrenergic receptor antagonist) had no significant effect on cumulative food intake in comparison with control group. In addition, co-administration of yohimbine plus OT had no significant effect on hypophagic effect of OT in chickens injection [treatment effect: F (3, 80) = 38.59, p < 0.0001; time effect: F (2, 80) = 899.1, p < 0.0001; treatment and time interaction: F (6, 80) = 9.157; p < 0.0001; Fig. 6].
In experiment 7, administration of metoprolol (β1 adrenergic receptor antagonist, 24 nmol) had no significant effect on food consumption in comparison to control group. ICV injection of 10 μg OT induced hypophagia and this effect of OT was not altered by co-injection of metoprolol (24 nmol) and OT (10 μg) at 30, 60 and 120 min post-injection [treatment effect: F (3, 80) = 50.03, p < 0.0001; time effect: F (2, 80) = 522.6, p < 0.0001; treatment and time interaction: F (6, 80) = 4.695; p < 0.0004; Fig. 7].
In experiment 8, there was no significant effect on cumulative food intake by administration of 5 nmol ICI 118,551 (β2 adrenergic receptor antagonist), but OT (10 μg) significantly decreased food intake in chickens. Furthermore, the hypophagic effect of OT was significantly attenuated by co-injection of ICI 118,551 plus OT at 30, 60 and 120 min post-injection [treatment effect: F (3, 80) = 53.81, p < 0.0001; time effect: F (2, 80) = 746.2, p < 0.0001; treatment and time interaction: F (6, 80) = 11.31; p < 0.0001; Fig. 8].
According to the results of experiment 9, ICV injection of sub effective dose of SR 59230R (β3 adrenergic receptor antagonist, 20 nmol) had no significant effect on food intake compared to control group in FD3 neonatal broiler. The ICV injection of an effective dose of OT (10 μg) significantly decreased food consumption at 30, 60 and 120 min post-injection. Co-injection of SR 59230R (20 nmol) and OT (10 μg) had no effect on Oxytocin- induced hypophagia at 30, 60 and 120 min post-injection [treatment effect: F (3, 80) = 41.36, p < 0.0001; time effect: F (2, 80) = 490.1, p < 0.0001; treatment and time interaction: F (6, 80) = 3.469; p < 0.0042; Fig. 9].
Discussion
We found that central administration of the α-FMH, chlorpheniramine and ICI 118,551 attenuated the hypophagic effect of OT but thioperamide amplified the hypophagic effect of OT in broiler chickens. The results of this study support previous observations in association with OT, HA and NE (Jonaidi et al. 2003; Taati et al. 2009; Zendehdel and Hassanpour 2014; Zendehdel et al. 2015, 2016). But to our knowledge it is the first study on the central effects of histaminergic and noradrenergic systems on oxytocin-induced food intake in neonatal meat-type chicks.
Based on the literature this study is the first report on the relationship between OT and histaminergic and adrenergic systems on food intake in FD3 neonatal broiler chickens. According to the data OT had a hypophagic effect (Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9) which is in agreement with previous reports in rats (Arletti et al. 1990) and meat chickens (Jonaidi et al. 2003). The purpose of this study was to define the central effects of histaminergic and adrenergic systems on OT- induced feeding behavior in broiler chicken. The main place of OT gene expression is the magnocellular neurons of the hypothalamic PVN and SON (Hazell et al. 2012). OT directly inhibits food and water intake in rat (Arletti et al. 1990), with this effect being specifically mediated by brain OT receptors. Previously, Jonaidi et al. (2003) reported, ICV injection of OT caused a dose-dependent decrease in feed intake and feeding time in birds. Thus, brain oxytocinergic system plays an important role in the regulation of ingestive behavior in both mammals and birds.
In this study, we found that alpha fluoromethyl histidine (α-FMH; irreversible inhibitor of histidine decarboxylase) significantly attenuated hypophagic effect of OT in neonatal meat-type chicks. α-FMH is a specific and potent inhibitor of histidine decarboxylase, which converts histidine to histamine (Watanabe et al. 1990). So histamine depletion is caused by α-FMH. Intracerebroventricular administration of α-FMH stimulates feeding in rat (Ookuma et al. 1993). Kjaer et al. (1995) showed that depletion of neuronal histamine with α-FMH or blockade of histamine receptors prevents OT release in CNS (Kjaer et al. 1995).
As seen in this study, ICV injection of H1 receptor antagonist (chlorpheniramine) significantly attenuated oxytocin- induced hypophagia in neonatal meat- type chicks, while H2 receptor antagonist (famotidine) had no effect. In this regard, it was previously observed that H1 receptor agonist decreased but H1 receptor antagonist (chlorpheniramine) increased food intake in broiler chickens, however H2 receptors had no effect on food intake in broilers (Taati et al. 2009; Zendehdel et al. 2015). H1 receptor activation causes excitation in most brain sites (brainstem, thalamus, hypothalamus, cortex, amygdala, striatum) through Gq protein and a direct block of a leak potassium conductance or phospholipase C, inositol trisphosphate (IP3) and diacylglycerol (DAG) mediation (Hill et al. 1997). Autoradiographic surveys have revealed that in the SON and PVN, H1 and H3 receptors are found in high-moderate density, while H2 receptors are found in lower density (Bouthenet et al. 1988; Ruat et al. 1991). Also, ventricular administration of histamine enhances expression of both OT mRNA and c-fos in magnocellular neurosecretory cells (Yang and Hatton 1994). ICV infused histamine or H1 receptor agonist stimulated OT secretion almost 6-fold in conscious male rats (Knigge et al. 1999a, b). Our result showed that hypophagic effect of OT may be mediated by histaminergic system in broiler chickens and blockade of central H1 receptors attenuated hypophagic effect of OT while H2 receptors had no effect.
Furthermore, in this study, we found that hypophagic effect of OT is amplified by H3 receptor antagonist (thioperamide) in neonatal chicks. Thioperamide negatively controls the release of histamine, and increases the activity of histaminergic neurons by blocking H3 receptors, leading to greater release of histamine. In this regard, Taati et al. (2009) reported that anorexigenic effect of thioperamide is significantly attenuated by chlorpheniramine in birds. Also, it was demonstrated that thioperamide through stimulation of synthesis and release of neuronal histamine and then stimulation of H1 receptor decreases food intake in birds (Taati et al. 2009). Several studies have shown that HA-containing neurons synapse on OT neurons (Yang and Hatton 1994; Luckman and Larsen 1997). Functionally, pharmacological blockade of central H1 receptors and depletion of neuronal HA with α-fluoromethylhistidine prevents systemic OT (Schagen et al. 1996). Taken together, these results demonstrate that there is an interaction between histamine and OT in CNS. Furthermore, administration of H1 antagonist with HA prevented the increase in PVN OT release evoked by HA alone. These data demonstrate that activation of H1 receptors is necessary for HA-induced release of PVN OT (Bealer and Crowley 1999). Our findings showed that oxytocin- induced hypophagia is partially mediated via H1 and H3 receptors in neonatal meat- type chicks.
As seen in this study, β2 adrenergic receptor antagonist (ICI 118,551) significantly attenuated hypophagic effect of OT in FD3 chicks. Noradrenergic system has important effects in feeding behavior. In this regard, Bungo et al. (2010) reported that ICV injection of Noradrenaline significantly stimulated feeding behavior in layer chicks at 30 min post injection (Bungo et al. 2010). Also, feed intake was increased by Noradrenaline in the pigeon (Ravazio et al. 1990). In the chicken, β1 and β3 receptor antagonists had no effect on food intake (Zendehdel and Hassanpour 2014). It is reported, ICV injection of propranolol (a nonselective β receptor antagonist) in the third ventricle increases food intake by a tonic inhibitory influence of the β1 or β2 adrenergic receptors in rat (Tsujii and Bray 1998).
The present investigation does not elucidate the site(s) of action in the brain where the interaction between the histaminergic and oxytocinergic system and between the noradrenergic and oxytocinergic system occurs. However, other investigations may to some extent explain these place(s). The histaminergic neurons in the tuberomamillary nucleus (TMN) project to other brain areas as well as to other hypothalamic sites including the SON and PVN (Brown et al. 2001). Dense histaminergic nerve fibers are in contact with oxytocineric neurons in PVN and SON (Weiss et al. 1989) and Histamine is a main neurotransmitter in the brain and plays an important role in feeding behavior (Hill et al. 1997). Also, noradrenergic neurons originate from the brain stem to the SON and PVN (Plotsky et al. 1989). Noradrenergic and adrenergic fibers make contact with oxytocinergic neurons in PVN and SON in hypothalamus (Michaloudi et al. 1997). There is considerable functional evidence that norepinephrine is a primary excitatory neurotransmitter in regulation of OT secretion and the magnocellular nuclei receive dense noradrenergic innervation from the medulla (Michaloudi et al. 1997). Both the PVN and SON contain OT magnocellular populations, whereas the PVN hosts as well as parvocellular OT neurons. Magnocellular neurons release OT primarily to the general circulation via the neurohypophysis. Additionally, parvocellular OT neurons send their axons not only to the neurohypophysis, but also to a variety of central sites (Gimpl and Fahrenholz 2001). Furthermore, based on IHC (immunohistochemistry), ISHH (in situ hybridization histochemistry) and Arrays (DNA microarrays) detection, co-localization of OT and HA (H1 and H3) receptors occurs in the OT neurons in the PVN and SON (Georgina et al. 2012; Hatton 2002; Lemonick et al. 2001; Markovic and Challiss 2009). In addition, arrays detection has shown that co-location of OT and β2-adrenergic receptors takes place in the OT neurons in the SON (Georgina et al. 2012; Hatton 2002). So, it is more likely that the interaction of histaminergic and noradrenergic systems with oxytocin on feeding behavior in broilers occurs in SON and PVN. As mentioned previously, oxytocinergic system has an undeniable role in food intake and many of the brain areas targeted by the OT system are involved in the regulation of food intake: for example, the components of the reward system (the NAc and VTA) as well as the energy balance-related CNS regions (for example, NTS and the dorsal motor nucleus of the vagus) (Blevins and Baskin 2010). Therefore, CNS-derived OT controls feeding as well as other physiological and behavioral outcomes via its receptors distributed throughout the CNS as well as in peripheral organs (Swanson and Sawchenko 1983; Morris and Ludwig 2004).
In this study it seems that effect of ICV injection of OT on cumulative food intake is probably mediated through H1, H3 and β2 receptors in 5-day-old broiler chickens. Previous studies have shown that there is a significant interaction between histaminergic and adrenergic systems in the central regulation of OT secretion (Knigge et al. 1999b). In this regard, histamine increases OT release in the PVN by stimulating noradrenaline release (Knigge et al. 1999b). Also, ICV infusion of H1 receptors antagonist significantly inhibited the OT response to noradrenaline. So there is an interaction between histaminergic and noradrenergic systems in the central control of OT release in PVN (Knigge et al. 1999b). Thus, it seems that histaminergic and noradrenergic neurotransmitter systems are important in control of intranuclear OT release in PVN.
This data suggest that there is probably neurotransmitter interaction between histaminergic and oxytocinergic systems in the central regulation of food intake through H1 and H3 receptors and also between adrenergic and oxytocinergic systems through β2 adrenergic receptors in broiler chickens. The findings of current study can be used as basic information and further researches are required to clarify any direct interaction of cellular and molecular signaling pathways in the interconnection between histaminergic and noradrenergic systems with OT on feeding behavior in avian.
References
Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP (2011) Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology 136(9):4022–4028
Arletti R, Benelli A, Bertolini A (1990) Oxytocin inhibits food and fluid intake in rats. Physiol Behav 486:825–830
Baghbanzadeh A, Hajinezhad MR, Shohreh B, Maleklou R (2010) Intralateral hypothalamic area injection of isoproterenol and propranolol affects food and water intake in broilers. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 1963:221–226
Bealer SL, Crowley WR (1999) Stimulation of central and systemic oxytocin release by histamine in the paraventricular hypothalamic nucleus: evidence for an interaction with norepinephrine. Endocrinology 1403:1158–1164
Blevins JE, Baskin DG (2010) Hypothalamic-brainstem circuits controlling eating. Forum Nutr 63:133–140
Bouthenet M, Ruat M, Sales N, Garbarg M, Schwartz JC (1988) A detailed mapping of histamine H1-receptors in Guinea-pig central nervous system established by autoradiography with [125I] iodobolpyramine. Neuroscience 26:553–600
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672
Bungo T, Higaki T, Ueda H, Furuse M (2002) Intracerebroventricular administration of octopamine stimulates food intake of chicks through alpha(2)-adrenoceptor. Physiol Behav 76(4-5):575–578
Bungo T, Yanagita K, Shiraishi J (2010) Feed intake after infusion of noradrenaline, dopamine or its precursor into the lateral ventricles in neonatal chicks. J Anim Vet Adv 94:760–763
Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H (2000) G (I)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca (2+) channels. Biophys J 795:2547–2556
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1999) Food intake regulation in birds. J Exp Zool 283:333–338
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, Shimojo M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 405:698–700
Georgina GJ, Charles CH, George RP, James AR, Stafford LL, David M, Carroll AM, Stephen JL (2012) G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei – serpentine gateways to neuroendocrine homeostasis. Front Neuroendocrinol 33(1):45–66
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 812:629–683
Hatton GI (2002) Glial–neuronal interactions in the mammalian brain. Adv Physiol Educ 26:225–237
Hazell GGJ, Hindmarch C, Pope GR, Roper JA, Lightman SL, Murphy D, Carroll AM, Lolait SJ (2012) G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei- serpentine gateways to neuroendocrine homeostasis. Front Neuroendocrinol 331:45–66
Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL (1997) International union of pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev 49(3):253–278
Hussain SS, Bloom SR (2013) The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes 37:625–633
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198(11):827–832
Jonaidi H, Oloumi MM, Denbow DM (2003) Behavioral effects of intracerebroventricular injection of oxytocin in birds. Physiol Behav 794-5:725–729
Kjaer A, Knigge U, Warberg J (1995) Involvement of oxytocin in histamine- and stress-induced ACTH and prolactin secretion. Neuroendocrinology 61(6):704–713
Knigge U, Warberg J (1991) The role of histamine in the neuroendocrine regulation of pituitary hormone secretion. Acta Endocrinol 1246:609–619
Knigge U, Soe-Jensen P, Jørgensen H, Kjær A, Møller M, Warberg J (1999a) Stress-induced release of anterior pituitary hormones: effect of H3 receptormediated inhibition of histaminergic activity or posterior hypothalamic lesion. Neuroendocrinology 69:44–53
Knigge U, Willems E, Kjær A, Jørgensen H, Warberg J (1999b) Histaminergic and catecholaminergic interactions in the central regulation of vasopressin and oxytocin secretion. Endocrinology 140(8):3713–3719
Kuenzel WJ, Beck MM, Teruyama R (1999) Neural sites and pathways regulating food intake in birds: a comparative analysis to mammalian systems. J Exp Zool 283:348–364
Lemonick MD, Cray D, Park A, Thomas CB, Thompson D (2001) Brave new pharmacy, time magazine, US 157
Luckman SM, Larsen PJ (1997) Evidence for the involvement of histaminergic neurones in the regulation of the rat oxytocinergic system during pregnancy and parturition. J Physiol Lond 501:649–655
Markovic D, Challiss RA (2009) Alternative splicing of G protein-coupled receptors: physiology and pathophysiology. Cell Mol Life Sci 66:3337–3352
Michaloudi HC, Majdoubi ME, Poulain DA, Papadopoulos GC, Theodosis DT (1997) The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. J Neuroendocrinol 9:17–23
Morris JF, Ludwig M (2004) Magnocellular dendrites: prototypic receiver/transmitters. J Neuroendocrinol 16(4):403–408
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broilers production. Int J Poult Sci 5(4):301–308
Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H (1993) Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res 628:235–242
Plotsky PM, Cunningham-Et J, Widmaier EP (1989) Catecholaminergic modulation of corticotropin-releasing factor, and adrenocorticotropin secretion. Endocr Rev 10:437–458
Qin K, Sethi PR, Lambert NA (2008) Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins. FASEB J 22(8):2920–2927
Ravazio MR, Canllo M, Paschoalini MA, Marino-Neto J (1990) Behavioral effects of adrenaline and noradrenaline injected into the lateral ventricle of the pigeon. Braz J Med Biol Res 23:1133–1137
Richards MP (2003) Genetic regulation of feed intake and energy balance in poultry. Poult Sci 82:907–916
Ruat M, Traiffort E, Arrang JM, Leurs R, Schwartz JC (1991) Cloning and tissue expression of a rat histamine H2-receptor gene. Biochem Biophys Res Commun 179(3):1470–1478
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Sara SJ, Bouret S (2012) Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 761:130–141
Schagen FHE, Knigge U, Kjaer A, Larsen PJ, Warberg JG (1996) Involvement of histamine in suckling-induced release of oxytocin, prolactin, and adrenocorticotropin in lactating rats. Neuroendocrinology 63:550–558
Swanson LW, Sawchenko PE (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
Taati M, Babapour V, Kheradmand A, Tarrahi MJ (2009) The role of central endogenous histamine and H1, H2 and H3 receptors on food intake in broiler chickens. Iran J Vet Res 123:192–198
Taati M, Nayebzadeh H, Khosravinia H, Cheraghi J (2010) The role of the histaminergic system on the inhibitory effect of ghrelin on feed intake in broiler chickens. Iran J Vet Res 111:38–45
Tachibana T, Takagi T, Saito ES, Tomonaga S, Zhang R, Koga Y, Kido Y, Michael Denbow D, Furuse M (2003) Beta 3-adrenergic receptor is involved in feeding regulation in chicks. Comp Biochem Physiol A Mol Integr Physiol 135(3):403–409
Tsujii S, Bray GA (1998) A beta-3 adrenergic agonist (BRL-37,344) decreases food intake. Physiol Behav 63:723–728
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Wada H, Inagaki N, Yamatodani A, Watanabe T (1991) Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci 149:415–418
Watanabe T, Yamatodani A, Maeyama K, Wada H (1990) Pharmacology of alpha-fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends Pharmacol Sci 119:363–367
Weiss M, Yang QZ, Hatton GI (1989) Magnocellular tuberomammillary nucleus input to the supraoptic nucleus in the rat: anatomical and in vitro electrophysiological investigations. Neuroscience 31:299–311
Wellman PJ (2000) Norepinephrine and the control of food intake. Nutrition 1610:837–842
Yang QZ, Hatton GI (1994) Histamine mediates fast synaptic inhibition of rat supraoptic oxytocin neurons via chloride conductance activation. Neuroscience 61:955–964
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 645:383–391
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on Nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 21:179–186
Zendehdel M, Parvizi Z, Hassanpour S, Baghbanzadeh A, Hamidi F (2016) Interaction between Nociceptin/orphanin FQ and adrenergic system on food intake in neonatal chicken. J Physiol Sci. doi:10.1007/s10989-016-9548-2
Acknowledgements
The authors thank the Research Council of the Faculty of Veterinary Medicine, Tehran University for financial support. This research is conducted as a part of the PhD thesis of the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Vahid Mirnaghizadeh, Morteza Zendehdel and Vahab Babapour declare that they have no conflict of interest.
Informed consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and animal rights
All experiments were executed according to the Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Mirnaghizadeh, S.V., Zendehdel, M. & Babapour, V. Involvement of histaminergic and noradrenergic receptors in the oxytocin-induced food intake in neonatal meat-type chicks. Vet Res Commun 41, 57–66 (2017). https://doi.org/10.1007/s11259-016-9672-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-016-9672-7