Abstract
The present study was designed to examine the effects of intracerebroventricular injection of para-chlorophenylalanine (PCPA) (cerebral serotonin depletive), fluoxetine (selective serotonin reuptake inhibitor), 8-OH-DPAT (5-HT1A autoreceptor agonist) and SB 242084 (5-HT2c receptor antagonist) on nociceptin/orphanin FQ (N/OFQ) induced feeding response in chickens. A guide cannula was surgically implanted into the lateral ventricle of chickens. Before the experiments, 3-h fasting periods had been given to all experimental birds. In experiment 1, chickens were injected with PCPA (1.5 μg) followed by an N/OFQ injection (16 nmol) intracerebroventricularly. In experiment 2, birds received fluoxetine (10 μg) prior to the injection of N/OFQ. In experiment 3, chickens were administered with N/OFQ after the 8-OH-DPAT administration (15.25 nmol). In experiment 4, birds were injected with SB 242084 (1.5 μg) followed by an N/OFQ injection. Cumulative food intake was measured at 3 h post injection. The results of this study show that N/OFQ increases food intake in broiler cockerels (P < 0.05) and that this effect is amplified by pretreatment with PCPA and SB 242084 in an additive manner (P < 0.05). The effect of N/OFQ is not changed by pretreatment with 8-OH-DPAT (P > 0.05). Furthermore, the stimulatory effect of N/OFQ on food intake was significantly attenuated by pretreatment with fluoxetine. These results suggest that N/OFQ induced hyperphagia is mediated by serotonergic mechanisms, and possibly imply an interaction between N/OFQ and the serotonergic system (via 5-HT2C receptors) on food intake in chickens.
Similar content being viewed by others
Introduction
The brain integrates diverse signals from the mouth, gastrointestinal system and other organs to regulate food intake. Elaborate physiological mechanisms control feeding behaviors, including those dependent on interplay between neuropeptidergic pathways [1]. Importantly, a single neuropeptide does not act alone in the process of food intake regulation; instead, a wide distributed neural network that hosts a variety of peptides appears to control the feeding status of the humans and animals. Via the organization of this system, neuropeptides interact with each other; for example, by affecting the release of each other or by reaching the same target cells [1].
Nociceptin or orphanin FQ (N/OFQ) is widely distributed in the central nervous system (CNS). It is found in many regions of the hypothalamus, brainstem and forebrain as well as in the ventral horn and dorsal horn of the spinal cord [2]. The term “N/OFQ” refers to heptadecapeptide orphanin FQ (OFQ), where orphanin refers to the peptide affinity for the newly cloned orphan opioid receptor; F (phenylalanine) and Q (glutamine) denote the first and the last amino acids of the peptide, respectively, previously described by Reinscheid et al. [3]. Nociceptin acts at the nociceptin receptor (NOP1), formerly known as ORL-1. The receptor is also widely distributed in the brain, including the cortex, anterior olfactory nucleus, lateral septum, hypothalamus, hippocampus, amygdala, central gray, pontine nuclei, interpeduncular nucleus, substantia nigra, raphe complex, locus coeruleus and spinal cord [2]. It is derived from the prepronociceptin protein, including two further peptides, nocistatin and NocII [4]. Nociceptin/orphanin FQ, a neuropeptide closely related to the opioid family, induces a mild orexigenic response in a way similar to classical opioids to some extent. N/OFQ is capable of initiating and maintaining food intake. However, it does not appear to mediate rewarding properties of consumption [1]. This peptide affects food intake by acting through the CNS. Data suggest that the orexigenic properties of N/OFQ originate from its interactions with other feeding related neuropeptidergic systems [2]. It has been reported that intracerebroventricular (ICV) administration of N/OFQ increases food intake in food deprived rats [1].
Neurons of the raphe nuclei are the principal source of serotonin (5-HT) release in the brain. The raphe nuclei are neurons grouped into nearly nine pairs and distributed along the entire length of the brainstem, centered near the reticular formation [5]. Neural axons in raphe nuclei form a neurotransmitter system, reaching almost every part of the CNS. Axons of neurons in the lower raphe nuclei are terminated in the cerebellum and spinal cord, while axons of the higher nuclei spread out in the entire brain, including the arcuate nucleus [5]. The 5-HT-ergic system action is terminated primarily via uptake of 5-HT from the synapse. This is accomplished through the specific monoamine transporter for 5-HT (SERT) on the presynaptic neurons. Various agents can inhibit 5-HT reuptake, including MDMA (ecstasy), amphetamine, cocaine, dextromethorphan (an antitussive), tricyclic antidepressants (TCAs) and selective 5-HT reuptake inhibitors (SSRIs) [6].
It is well recognized that fluoxetine tends to decrease serotonin reuptake in animals and humans. In contrast, SERT is of considerable interest as a target molecule for a variety of antidepressants such as serotonin-selective reuptake inhibitors (SSRIs) including citalopram, fluoxetine and paroxetine. SERT is a domain protein that cotransports one 5-HT molecule inwardly in protonated form together with one Na+ and one Cl− ion. An internal K+ ion is bound and counter transported after the dissociation of 5-HT, Na+ and Cl−, as SERT returns to its initial state [7]. The pharmacological action of fluoxetine (a selective serotonin reuptake inhibitor) is observed by blocking 5-HT transporter mediated reuptake; therefore, causing enhancement of extracellular 5-HT levels [8].
5-HT has been used in the control of eating behavior and body weight gain. The 5-HT-ergic system is known to decrease food intake in mammalian species, and experimental situations that increase 5-HT system activity decrease feeding behaviors [9–11]. In chickens, 5-HT-ergic systems are involved in the regulation of various physiological functions such as an inhibitory effect on feeding behavior [12–15]. ICV injection of 5-HT induces a potent decrease in food intake in chickens and turkeys [16, 17]. Studies with agonists have demonstrated that 5-HT acts on different receptor subtypes [10, 11, 15].
The effects of central injection of 5-HT and its interaction with other neuropeptides in avian species, have been sparsely studied. Based on previous studies, 5-HT stimulates corticotropin releasing factor (CRF) secretion, and the hyperphagic effect of N/OFQ is mediated by the activation of central glucocorticoid receptors [18, 19]. In the current study, the interaction of N/OFQ and the central 5-HT-ergic system on feeding behavior in chickens has been assessed.
Materials and methods
Animals
Ninety-six broiler cockerels (each six birds in a replicate) (Eshragh Co., Iran) were reared in heated batteries with continuous lighting until 3 weeks of age. Before the experiments, birds were weighed and randomly assigned into experimental groups (average live body weight (LBW) 950 ± 30 g in each group). Birds were provided with water and a mash diet (21 % protein and 2,869 kcal/kg of metabolizable energy) ad libitum. The birds were transferred to individual cages at approximately 2 weeks of age. The temperature and relative humidity of the animal room were maintained at 22 ± 1 °C and 50 %, respectively.
Drugs
N/OFQ, para-chlorophenylalanine (PCPA) (cerebral 5-HT depletive), fluoxetine (selective 5-HT reuptake inhibitor), 8-OH-DPAT (5-HT1A autoreceptor agonist) and SB 242084 (5-HT2c receptor antagonist) were purchased from Sigma, USA. All solutions were prepared in pyrogen-free 0.9 % NaCl solution (saline), which was also used as the control.
Surgical preparation
Three-week-old birds were anesthetized with xylazin (1 mg/kg body weight, IM) and ketamin (30 mg/kg body weight, IM) [20] to insert a 23-gauge thin-wall stainless steel guide cannula into the right lateral ventricle stereotaxically [21]. The stereotaxic specifications were AP = 6.7 mm, L = 0.7 mm and H = 3.5–4 mm with the oriented head [22]. Three stainless steel screws were placed in calvarias to protect the guide cannula, then acrylic dental cement (Pars acryl, Iran) was applied to the screws and guide cannula. While the chickens were not being used in experiments, an orthodontic #014 wire (American Orthodontics, USA) trimmed to the exact length of the guide cannula, was inserted into it. Lincospectin (Razak, Iran) was applied to the incision to prevent possible infections. The birds were allowed a minimum of 5 days of recovery prior to injections [23].
Experimental procedures
For the possible involvement of central serotonergic system on N/OFQ induced eating response, the effects of centrally administered PCPA, fluoxetine, 8-OH-DPAT and SB 242084 were assessed in chickens. Injections were carried out with a 29-gauge, thin-wall stainless steel injection cannula which extends 1 mm beyond the guide cannula. This injection cannula was connected through a 60-cm-long PE-20 tube to a 10-μl Hamilton syringe. Solutions were injected over a period of 60 s. An additional time period of 60 s was applied to allow the solution to diffuse from the tip of the cannula into the ventricle. All experiments were performed between 10:00 a.m. and 2:00 p.m. Birds were mock injected daily during the 5-day recovery period to get acquainted with the injection procedure. Three hours of fasting had been given for all experimental birds before the injections. After the injection, birds were returned to their cages immediately. Then, fresh water and food were supplied and the food intake was recorded at 15, 30, 60, 120 and 180 min after the injection.
In experiment 1, each bird received two injections. The first injection consisted of either 0 or 1.5 μg of PCPA in 5 μl of saline and the second injection consisted of either 0 or 16 nmol of N/OFQ in 5 μl of saline as described in Table 1 (n = 6 for each group). In the control group, the first and the second injections consisted of 5 μl of saline. The interval time for these injections was 15 min.
Experiments 2, 3 and 4 were conducted similar to the first one except that the chickens received 0 or 10 μg of fluoxetine in experiment 2; 0 or 15.25 nmol of 8-OH-DPAT in experiment 3 and 0 or 1.5 μg of SB 242084 in experiment 4 instead of PCPA. Each bird was only used in one experiment. Animals were killed painlessly following the study according to the guidelines mentioned. Placement of the guide cannula into the ventricle was verified by the presence of cerebrospinal fluid and ICV injection of methylene blue was followed by slicing the frozen brain tissue at the end of each experiment.
In the current study, the selection of PCPA, fluoxetine, 8-OH-DPAT and SB 242084 doses were chosen based on the preliminary and previous studies [24–28]. These doses alone had no effects on food intake in FD3 chickens. Selection of N/OFQ dose was based on a study by Tajalli et al. [29]. According to our previous findings, injection of 1.5 μg PCPA diminished brain serotonin levels without causing any suppression in food intake in chickens [30].
Statistical analysis
Cumulative food intake was analyzed by two-way analysis of variance (ANOVA), and is presented as mean ± SEM. For treatments showing a main effect by ANOVA, means were compared using post hoc Bonferroni and Dunnett tests. P < 0.05 was considered as significant differences between the treatments.
Results
In all experiments, injected N/OFQ (16 nmol) into the lateral ventricle of chickens caused an increase in food consumption 180 min post injection when compared with control groups (F3, 25 = 18.43) (P < 0.05) (Figs. 1, 2, 3, and 4).
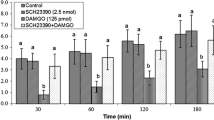
Effect of intracerebroventricular injection of PCPA (1.5 μg) followed by N/OFQ (16 nmol) on food intake in chickens: Saline 0.9 % NaCl, PCPA parachlorophenylalanine, N/OFQ nociceptin/orphanin FQ. Data are presented as mean ± SEM. There are significant differences between groups with different codes in a column in each time (superscript letters a, b, c; p < 0.05)
Effect of intracerebroventricular injection of fluoxetine (10 μg) followed by N/OFQ (16 nmol) on food intake in chickens: Saline 0.9 % NaCl, N/OFQ nociceptin/orphanin FQ. Data are presented as mean ± SEM. There are significant differences between groups with different codes in a column in each time (superscript letters a, b, c and d; p < 0.05)
Effect of intracerebroventricular injection of 8-OH-DPAT (15.25 nmol) followed by N/OFQ (16 nmol) on food intake in chickens: Saline 0.9 % NaCl, N/OFQ nociceptin/orphanin FQ. Data are presented as mean ± SEM. There are significant differences between groups with different codes in a column in each time (superscript letters a and b; p < 0.05)
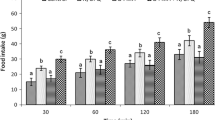
Effect of intracerebroventricular injection of SB242084 (1.5 μg) followed by N/OFQ (16 nmol) on food intake in chickens: Saline 0.9 % NaCl, N/OFQ nociceptin/orphanin FQ. Data are presented as mean ± SEM. There are significant differences between groups with different codes in a column in each time (superscript letters a, b, c; p < 0.05)
In experiment 1, the stimulatory effect of N/OFQ on food intake was amplified by 1.5 μg PCPA pretreatment (F3, 25 = 16.28) (P < 0.05), but 1.5 μg PCPA alone failed to change the food intake in FD3 chickens (F3, 25 = 1.63) (P > 0.05) (Fig. 1).
In experiment 2, the effect of N/OFQ was significantly attenuated by the administration of 10 μg of fluoxetine 60–180 min post injection in FD3 chickens (F3, 25 = 23.58) (P < 0.05) (Fig. 2), while 10 μg of fluoxetine alone had no effects on food intake (F3, 25 = 1. 08) (P > 0.05).
In experiment 3, 8-OH-DPAT (15.25 nmol) had no effects on food intake and failed to change the N/OFQ induced food intake in FD3 chickens (F3, 25 = 2.39) (P > 0.05) (Fig. 3).
In experiment 4, the hyperphagic effect of N/OFQ was significantly increased by SB 242084 pretreatment in FD3 chickens (F3, 25 = 32. 47) (P < 0.05) (Fig. 4). Furthermore, 1.5 μg of SB 242084 alone had no effects on food intake in chickens (F3, 25 = 1.09) (P > 0.05).
Discussion
The present study was designed to investigate the possible involvement of 5-HT-ergic paths in N/OFQ induced hyperphagia in chickens. A hyperphagic effect of N/OFQ was observed after its administration into the third ventricle and ARC but not in the central amygdale (CA), VMH, hypothalamic paraventricular nucleus (PVN) or fourth ventricle [31]. Furthermore, microinjection of N/OFQ into the shell of the nucleus accumbens (NA) and ventromedial hypothalamus (VMH) caused a significant increase in food intake [32]. Central injection of N/OFQ induces feeding in satiated rats which can be blocked by the peripheral administration of opioid antagonist naloxone [33]. In all experiments in this study, injecting N/OFQ into the lateral ventricle of chickens caused an increase in food consumption. It has been stated that the hyperphagic effect of N/OFQ is mediated by the activation of central glucocorticoid receptors, and central injection of glucocorticoid receptor antagonist RU486, 30 min prior to injection of N/OFQ significantly reduces N/OFQ induced food intake [18]. The effect of ICV injection of CRF on various behaviors in chickens was assessed at 15-min intervals over a 30-min time period. Food intake of the chickens was significantly decreased, and pecking rhythm was significantly delayed by the CRF during the first 15 min post injection [34]. Multiple studies have reported the effects of 5-HT on food intake in avian species. 5-HT exerts an inhibitory effect on feeding behavior in animals, including birds [26]. Cell bodies of 5-HT-secreting neurons are identified primarily in the midline raphe region and reticular system of the medulla, pons and upper brain stem [35, 36]. Axons of the neurons in the lower raphe nuclei are terminated in the cerebellum and spinal cord while axons of the higher nuclei are spread out in the entire brain including the arcuate nucleus [5].
Studies with agonists have demonstrated that 5-HT acts on various receptor subtypes [9, 37–41] and the hypophagic effects of ICV injection of 5-HT can be mediated by 5-HT2a and 5-HT2c receptors. It is worth noting that ICV injection of 5-HT1a agonists (8-OH-DPAT) in 24FD pigeons had no effects on amount of food intake but elevated the duration of food consumption [15]. Additionally, ICV injection of 5-HT was able to induce a decrease in food consumption in 24-h food-deprived or satiated leghorn chickens, turkeys and pigeons [13, 16, 26]. In a similar study, it has been observed that central injection of 5-HT led to a modest decrease in food consumption in satiated broiler chickens. However, no effects were observed in 24-h food-deprived chickens [12, 21]. In the current study, we showed that the stimulatory effect of N/OFQ on food intake was significantly strengthened by PCPA (Fig. 1), and that this effect was attenuated with pretreatment of fluoxetine (selective 5-HT reuptake inhibitor SSRI) (Fig. 2). The increased food consumption induced by the ICV injection of N/OFQ was not significantly fortified by pretreatment with 8-OH-DPAT (5-HT1A receptor agonists) (Fig. 3), but this effect of N/OFQ significantly increased with pretreatment with SB 242084 (selective antagonist for the 5-HT2C receptor) (Fig. 4).
Based on the current study, there is possibly an interaction between N/OFQ and 5-HT neurons and feeding behavior in chickens. In birds, central injection of a glucocorticoid receptor antagonist apparently affects food intake. The effects of serotonergic agonists and antagonists on CRF secretion have been investigated by explanted rat hypothalamus. 5-HT stimulates CRF secretion in explanted rat hypothalami and this effect appears to be mediated mainly through a 5-HT2 receptor mechanism [19].
In chickens, both CRF and 5-HT stimulate the hypothalamo-pituitary-adrenal (HPA) axis and the stimulatory role of 5-HT on CRF secretion is mediated mainly by a 5-HT2 receptor [42]. It has been reported that pharmacological blockade of 5-HT reuptake by fluoxetine increases CRF in portal plasma [43]. Furthermore, based on the previous studies, the hyperphagic effect of N/OFQ is mediated by the activation of central glucocorticoid receptors [18]. These results indicate that central N/OFQ and central serotonergic systems affect food intake and presumably these effects occur on the same pathway by interacting with endogenous CRF and its receptors.
References
Olszewski PK, Levine AS (2004) Minireview: characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology 145(6):2627–2632
Mollereau C, Simons MJ, Soularue P, Liners F, Vassart G, Meunier JC, Parmentier M (1996) Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc Natl Acad Sci USA 93(16):8666–8670
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270(5237):792–794
Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S (1998) Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature 392(6673):286–289
Frazer A, Hensler JG (1999) Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter. In: Siegel GJ, Agranoff BW, Albers WR, Fisher SK, Uhler MD (eds) Basic neurochemistry: molecular, cellular, and medical aspects, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 264–268
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301(5631):386–389
Larsen MB, Elfving B, Wiborg O (2004) The chicken serotonin transporter discriminates between serotonin-selective reuptake inhibitors. A species-scanning mutagenesis study. J Biol Chem 279(40):42147–42156
Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG (2006) Paradoxical striatal cellular signaling responses to psycho stimulants in hyperactive mice. J Biol Chem 281(43):32072–32080
Simansky KJ (1996) Serotonergic control of the organization of feeding and satiety. Behav Brain Res 73(1–2):37–42
De Vry J, Schreiber R (2000) Effects of selected serotonin 5-HT(1) and 5-HT(2) receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev 24(3):341–353
Da Silva RA, de Oliveira ST, Hackl LP, Spilere CI, Faria MS, Marino-Neto J, Paschoalini MA (2004) Ingestive behaviors and metabolic fuels after central injections of 5-HT1A and 5-HT1D/1B receptor agonists in the pigeon. Brain Res 1026(2):275–283
Denbow DM, Van Krey HP, Cherry JA (1982) Feeding and drinking response of young chicks to injections of serotonin into the lateral ventricle of the brain. Poult Sci 61(1):150–155
Denbow DM, Van Krey HP, Lacy MP, Dietrick TJ (1983) Feeding, drinking and body temperature of Leghorn chicks: effects of ICV injections of biogenic amines. Physiol Behav 31(1):85–90
Blundell JE (1984) Serotonin and appetite. Neuropharmacology 23(12B):1537–1551
Saadoun A, Cabrera MC (2002) Effect of the 5-HT(1A) receptor agonist 8-OH-DPAT on food and water intake in chickens. Physiol Behav 75(3):271–275
Denbow DM (1984) Body temperature and food intake of turkeys following ICV injections of serotonin. Nutr Behav 1:301–304
Denbow DM (1989) Peripheral and central control of food intake. Poult Sci 68(7):938–947
Nicholson JR, Akil H, Watson SJ (2002) Orphanin FQ-induced hyperphagia is mediated by corticosterone and central glucocorticoid receptors. Neuroscience 115(2):637–643
Calogero AE, Bernardini R, Margioris AN, Bagdy G, Gallucci WT, Munson PJ, Tamarkin L, Tomai TP, Brady L, Gold PW et al (1989) Effects of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides 10(1):189–200
Thurmon JC, Tranquilli WJ, Benson GJ, Lumb W, Jones E (1996) Lumb & Jones Veterinary Anesthesia, 3rd edn. John Wiley & Sons, Toronto pp 686–735
Denbow DM, Cherry JA, Siegel PB, Van Krey HP (1981) Eating, drinking and temperature response of chicks to brain catecholamine injections. Physiol Behav 27(2):265–269
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Abbasnejad M, Jonaidi H, Denbow DM, Pour Rahimi AM (2005) Feeding and locomotion responses to centrally injected nociceptin/orphanin FQ in chicks. Physiol Behav 85(4):383–386
Medeiros MA, Costa-e-Sousa RH, Olivares EL, Cortes WS, Reis LC (2005) A reassessment of the role of serotonergic system in the control of feeding behavior. Anais da Academia Brasileira de Ciencias 77(1):103–111
von Meyenburg C, Langhans W, Hrupka BJ (2003) Evidence that the anorexia induced by lipopolysaccharide is mediated by the 5-HT2C receptor. Pharmacol Biochem Behav 74(2):505–512
Steffens SM, Casas DC, Milanez BC, Freitas CG, Paschoalini MA, Marino-Neto J (1997) Hypophagic and dipsogenic effects of central 5-HT injections in pigeons. Brain Res Bull 44(6):681–688
Lazartigues E, Brefel-Courbon C, Bagheri H, Costes S, Gharib C, Tran MA, Senard JM, Montastruc JL (2000) Fluoxetine-induced pressor response in freely moving rats: a role for vasopressin and sympathetic tone. Fundam Clin Pharmacol 14(5):443–451
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DL-AP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67(2):217–223
Tajalli S, Jonaidi H, Abbasnejad M, Denbow DM (2006) Interaction between nociceptin/orphanin FQ (N/OFQ) and GABA in response to feeding. Physiol Behav 89(3):410–413
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37(1):37–41
Polidori C, de Caro G, Massi M (2000) The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides 21(7):1051–1062
Stratford TR, Holahan MR, Kelley AE (1997) Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. NeuroReport 8(2):423–426
Pomonis JD, Billington CJ, Levine AS (1996) Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. NeuroReport 8(1):369–371
Ohgushi A, Bungo T, Shimojo M, Masuda Y, Denbow DM, Furuse M (2001) Relationships between feeding and locomotion behaviors after central administration of CRF in chicks. Physiol Behav 72(1–2):287–289
Hagen CJ, Newmyer BA, Webster RI, Gilbert ER, Siegel PB, Tachibana T, Cline MA (2013) Stimulation of food intake after central galanin is associated with arcuate nucleus activation and does not differ between genetically selected low and high body weight lines of chickens. Neuropeptides. http://dx.doi.org/10.1016/j.npep.2012.11.003
Brown RE (1994) An introduction to neuroendocrinology. Cambridge University Press, UK, p 59
Aghajanian GK, Sprouse JS, Rasmussen K (1987) Physiology of the mesencephalic serotonin system. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven Press, New York, pp 141–149
Mundey MK, Fletcher A, Marsden CA (1994) Effect of the putative 5-HT1A antagonists WAY100135 and SDZ 216–525 on 5-HT neuronal firing in the guinea-pig dorsal raphe nucleus. Neuropharmacology 33(1):61–66
Bovetto S, Richard D (1995) Functional assessment of the 5-HT 1A-, 1B-, 2A/2C-, and 3-receptor subtypes on food intake and metabolic rate in rats. Am J Physiol 268(1 Pt 2):R14–R20
Brewerton TD (1995) Toward a unified theory of serotonin dysregulation in eating and related disorders. Psychoneuroendocrinology 20(6):561–590
Dourish CT, Clark ML, Fletcher A, Iversen SD (1989) Evidence that blockade of post-synaptic 5-HT1 receptors elicits feeding in satiated rats. Psychopharmacology 97(1):54–58
Zhang R, Tachibana T, Takagi T, Koutoku T, Denbow DM, Furuse M (2004) Serotonin modifies corticotropin-releasing-factor-induced behaviors of chicks. Behav Brain Res 151(1–2):47–52
Gibbs DM, Vale W (1983) Effect of the serotonin reuptake inhibitor fluoxetine on corticotropin-releasing factor and vasopressin secretion into hypophysial portal blood. Brain Res 280(1):176–179
Acknowledgments
This research was supported by a grant from the Research Council of the Faculty of Veterinary Medicine, University of Tehran. Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and to the Iranian codes of practice for the use of laboratory animals.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zendehdel, M., Mokhtarpouriani, K., Babapour, V. et al. The effect of serotonergic system on nociceptin/orphanin FQ induced food intake in chicken. J Physiol Sci 63, 271–277 (2013). https://doi.org/10.1007/s12576-013-0263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-013-0263-x