Abstract
Central regulatory mechanisms for neurotransmitters of food intake vary among animals. Endocannabinoids have crucial role on central food intake regulation in mammals but its role has not been studied in layer-type chicken. Thus, in this study 6 experiments designed to evaluate effects of intracerebroventricular (ICV) administration of 2-AG (2-Arachidonoylglycerol, selective CB1 receptors agonist), SR141716A (selective CB1 receptors antagonist), JWH015 (selective CB2 receptors agonist), AM630 (selective CB2 receptors antagonist) on feeding behavior in 3 h food deprived neonatal layer-type chickens. In experiment 1, birds ICV injected with control solution and 2-AG (0.25, 0.5 and 1 μg). In experiment 2: control solution, SR141716A (6.25, 12.5 and 25 μg) were ICV injected to birds. In experiment 3 animals received: control solution, SR141716A (6.25 μg), 2-AG (1 μg) and co-injection of SR141716A+2-AG. In experiment 4, chickens received control solution and JWH015 (6.25, 12.5 and 25 μg). In experiment 5, control solution and AM630 (1.25, 2.5 and 5 μg) were injected. In experiment 6, the birds received control solution, AM630 (1.25 μg), JWH015 (25 μg) and co-administration of AM630+JWH015. Then, cumulative food intake was recorded until 120 min after injection. According to the results, 2-AG dose dependently increased cumulative food intake while SR141716A reduced appetite compared to control group (P < 0.05). Injection of 2-AG (1 μg) amplified food intake and its effect minimized by SR141716A (6.25 μg) (P < 0.05). Also, ICV injection of JWH015 (25 μg) dose dependently increased food intake and co-injection of JWH015+AM630 decreased JWH015-induced food intake (P < 0.05). These results suggest CB1 and CB2 receptors have an important role on ingestive behavior in FD3 neonatal layer-type chicken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several factors with complicated neurological mechanisms are responsible for food intake regulation in animals (Levine 2006). Feeding behavior is modulated by neurochemical mediators known as neurotransmitters in several parts of the brain such as striatum, hypothalamus, amygdala, nucleus tractus solitaries (NTS) and arcuate nucleus (ARC) (Parker et al. 2014).
Cannabinoids are originally known as psychoactive ingredients of marijuana (∆9-tetrahydrocannabinol, THC) (Novoseletsky et al. 2011). It has been used as medication and recreational purposes for thousands of years (Di Marzo et al. 2001). The endocannabinoids (ECBs) are derivatives of arachidonic acid, resembling other lipid transmitters such as prostaglandins or leukotrienes. ECBs are conjugated with ethanolamine to form fatty acid amides or with glycerol to generate monoacylglycerols, N-arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG) (D’Addario et al. 2014). In the central nervous system (CNS) and peripheral tissues, 2-AG and AEA, the ECBs ligands were identified (López 2010). To date, two cannabinoid (CB) receptors have been cloned: CB1 and CB2 both belong to G-protein coupled receptors (GPCRs) (Sharkey et al. 2014). CB1 receptors are expressed abundantly in the CNS. CB2 receptors are plentiful in peripheral nervous system (PNS), immune cells and tissues (Pertwee 2005) but also identified in the brain (Onaivi et al. 2012). It is reported EBCs regulate several physiological functions such as pain relief, thermoregulation and hypotension, motor control, learning and memory formation (Sanudo-Pena et al. 2000; Di Marzo et al. 2001).
So far, several researches done to determine effect of neurotransmitters on feeding behavior in mammals, but aspects of food intake regulation in avian still unclear (Zendehdel and Hassanpour 2014). Given the estimated 300 million years of evolutionary distance between mammals and avians, it is not surprising that significant differences have been found in the activities of a number of components involved in the regulation of energy homeostasis (Novoseletsky et al. 2011). For example, ICV injection of μ-opioid receptors agonist decreased food intake in chicks (Bungo et al. 2005; Alimohammadi et al. 2015) but increased in rat (Le Merrer et al. 2009; Kaneko et al. 2012). For instance, ghrelin, is an orexigenic peptide in rat (Wren et al. 2000; Kaiya et al. 2011) while known as an anorexigenic neurotransmitter in birds (Saito et al. 2005; Zendehdel and Hassanpour 2014; Zendehdel et al. 2013). Furthermore, interestingly comparative physiological studies suggested there are differences on appetite regulation pathways between the meat-type (broiler) and layer-type (hens) chickens (Denbow 1994; Shiraishi et al. 2011).
In the ARC nucleus of hypothalamus, neuropeptide Y (NPY)/agouti related protein (AgRP) and pro-opiomelanocortin (POMC) and cocaine/amphetamine regulated transcript (CART) are the main sites which regulate appetite. NPY and AgRP neurons have orexigenic effect of in mammalian. Interestingly, AgRP increased cumulative food intake by layer but not by broiler chicken (Saneyasu et al. 2011).
Following the detection of ECBs and CB receptors in the prefrontal cortex (PFC), amygdala, septohippocampal system, nucleus accumbens (NAcc), hypothalamus and ARC associated with ingestive behavior (Di Marzo et al. 2001) researchers proved the involvement of CBs in central food intake regulation (Wiley et al. 2012). ICV administration of CB agonists stimulated food intake in rodents while SR141716A (selective CB1 receptors antagonist) or AM251 (CB1 cannabinoid receptors inverse agonist) decreased food consumption in rat (Chen et al. 2006). Little is known about the role of ECBs on feeding behavior in domestic fowl. Scarce reports exist on effects of CB1 receptors in chicken. It is reported CB1 receptors involved in memory recall in chicks (Adam et al. 2008) and might have no role on food intake in neonatal broilers (Emadi et al. 2011). In a sole study, Novoseletsky et al. (2011) reported intravenous injection of AM251 (inverse CB1 receptors agonist) leads to transient attenuation on food intake in meat-type chicken.
Most of the present knowledge regarding on behavioral effects of CBs on food intake has been derived from studies in mammalian species. On the basis of comparative physiology it is important to determine the role of ECBs in other species. No report exists on involvement of the CBergic system on food intake regulation and energy balance in neonatal layer-type chicken. Therefore, the possible contribution of CB1 and CB2 receptors on reward and consumption behavior will employ in the layer industry to enhance hen’s productivity by manipulating appetite and lessen malnutrition concerns. So, the objective of this study was to assess role of the CBergic system on feeding behavior in 3 h food deprived (FD3) neonatal layer-type chicken.
Materials and methods
Animals
In this study to determine role of central the CBergic system on food intake, 288 day-old layer-type (Hy-Line) chickens were used (Morghak Co. Iran). Animal handling and experimental procedures were performed according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care. Birds at first were kept for 2 days as flocks. Then, randomly distributed into individual cages at a temperature of 30 ± 1 °C with 50 ± 2 % humidity until 5 days of age (Olanrewaju et al. 2006). A mesh diet contains 21 % crude protein and 2850 kcal/kg of metabolizeable energy (Chineh Co. Iran) were provided for animals. During the study birds had ad libitum access to food and fresh water. A 3-h prior the intracerebroventricular (ICV) injections, animals were food deprived (FD3) but given free access to water. ICV injections were done on day 5 of age.
Experimental drugs
2-AG (2-Arachidonoylglycerol, a selective CB1 receptors agonist), SR141716A (a selective CB1 receptors antagonist), JWH015 (a selective CB2 receptors agonist), AM630 (a selective CB2 receptors antagonist) and Evans blue were purchased from Sigma Co. (Sigma, USA). Drugs except 2-AG at first dissolved in absolute dimethyl sulfoxide (DMSO) then diluted with 0.85 % saline containing Evans blue at a ratio of 1/250. 2-AG dissolved in 0.1 % Evans blue solution which was prepared in either 0.85 % saline.
ICV injection procedures
Before the initiation of the study, chickens were weighed and allocated into treatment groups based on their body weight. So, the mean body weight between treatment groups was as uniform as possible. In this study, 6 experiments (each includes 4 treatment groups within 11 replicates in each group; n = 44 birds per experiment) designed to assume the role of central the CBergic system on food intake in layer-type chicken. ICV injections were done using a Microsyringe (Hamilton, Switzerland) without anesthesia in accordance to Davis et al. (1979) and Furuse et al. (1997). Briefly in this technique, head of the bird was held with an acrylic device which the bill holder was 45° and calvarium parallel to the surface of the table (Van Tienhoven and Juhasz 1962). A hole was drilled in a plate where the skull over the right lateral ventricle directly overlaid through this plate. For injection, the Microsyringe inserts into the right ventricle using this hole where the tip of the needle penetrated 4 mm beneath the skin of the skull. It is well documented there is no ICV injection induced physiological stress using this method in neonatal chicks (Saito et al. 2005). Each chick received an ICV injection (with vehicle or drug solution) in a volume of 10 μl. Right away after injection, FD3 birds returned to their individual cages and supplied fresh water and food (pre-weighed). Cumulative food intake (gr) was measured at 30, 60 and 120 min post the injection. Food consumption was calculated as a percentage of body weight to minimize impact of body weight on the amount of food intake. Each bird just used once in each experimental group. At the end of the experiments, to recognize the accuracy of injection, chicks were sacrificed by decapitation and accuracy of placement of the injection in the lateral ventricle was verified by the presence of Evans blue followed by slicing the frozen brain tissue. In each group, 11 birds received injection, but just data of those individuals were used for analysis where dye was present in their lateral ventricle (8–11 chickens per group). All experimental procedures were done from 8:00 A.M. until 3:30 P.M.
Feeding experiments
Experiment 1 designed to investigate effect of CB1 receptors agonist on food intake in FD3 chickens where control group intracerebroventriculary injected with saline whereas other groups received an ICV injection of 2-AG at doses of 0.25, 0.5 and 1 μg, respectively. In experiment 2, effect of the CB1 receptors antagonist was studied on appetite. FD3 chickens received ICV injections as follows: control solution, SR141716A at doses of 6.25, 12.5 and 25 μg, respectively. In experiment 3, fasted chickens were ICV injected with control solution, SR141716A (6.25 μg), 2-AG (1 μg) and co-injection of SR141716A+2-AG. In experiment 4, effect of CB2 receptors agonist on food intake was investigated where injection groups received vehicle and JWH015 at levels of 6.25, 12.5 and 25 μg, respectively. In experiment 5, the birds received vehicle, AM630, a selective CB2 receptors antagonist at doses of 1.25, 2.5 and 5 μg, respectively. In experiment 6, birds received ICV injection of control solution, AM630 (1.25 μg), JWH015 (25 μg) and co-injection of AM630+JWH015. These doses of drugs were determined according to the previous and our pilot studies (Chen et al. 2006; Irwin et al. 2008; Onaivi et al. 2008; Emadi et al. 2011; Novoseletsky et al. 2011).
Statistical analysis
Cumulative food intake as percent of body weight was analyzed by repeated measure two-way analysis of variance (ANOVA) using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). Data is presented as mean±SEM. For treatment showing a main effect by ANOVA, means compared by Tukey-Kramer test. P < 0.05 was considered as significant differences between treatments.
Results
Effects of central the CBergic system on cumulative food intake in FD3 neonatal layer-type chicks is shown in Figs. 1, 2, 3, 4, 5 and 6.
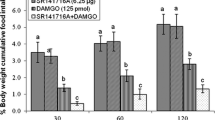
Effect of ICV injection of SR141716A (6.25 μg), 2-AG (1 μg) and their combination on cumulative food intake (% BW) in neonatal layer type chicken. 2-AG: a selective CB1 receptors agonist; SR141716A: a selective CB1 receptors antagonist. There are significant differences between groups with different superscripts in a column (a, b and c; P < 0.05)
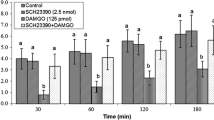
Effect of ICV injection of AM630 (1.25 μg), JWH015 (25 μg) and their combination on cumulative food intake (% BW) in neonatal layer type chicken. AM630: a selective CB2 receptors antagonist; JWH015: a selective CB2 receptors agonist. There are significant differences between groups with different superscripts in a column (a, b and c; P < 0.05)
In experiment 1, ICV injection of 2-AG (a selective CB1 receptors agonist, 0.25 μg) had no significant effect on cumulative food intake (% BW) in comparison with control group [F (l,87) = 3.12, P > 0.05]; but 0.5 and 1 μg of 2-AG in a dose-dependent manner increased cumulative food intake [F (l,87) = 98.42, P < 0.01] (Fig. 1).
In experiment 2, ICV injection of SR141716A (a selective CB1 receptors antagonist) at levels of 12.5 and 25 μg but not 6.25 μg significantly increased food consumption compared to control group in chicken [F (l,87) = 124.07, P < 0.01] (Fig. 2).
In experiment 3, ICV injection of 2-AG (1 μg) significantly amplified food intake [F (l,87) = 179.65, P < 0.01]. Also, CB1 receptors-induced hyperphagia diminished by co-injection of 2-AG and SR141716A (6.25 μg) [F (l,87) = 109.31, P < 0.01] (Fig. 3). Thus, it seems that CB1 receptors have an important role on food intake control in layer-type chick.
According to the results of experiment 4, ICV injection of JWH015 (a selective CB2 receptors agonist, 6.25 μg) had no significant effect on cumulative food intake (% BW) in comparison with control group [F (l,87) = 1.73, P >0.05]; but levels of 12.5 and 25 μg doses dependently increased food intake [F (l,87) = 117.01, P < 0.01] (Fig. 4).
As seen in experiment 5, ICV injection of AM630 (at doses of 2.5 and 5 μg a selective CB2 receptors antagonist) significantly induced hypophagia in FD3 neonatal layer-type chicks compared to control group at 30, 60 and 120 min after injection [F (l,87) = 79.58, P < 0.01] (Fig. 5). However, 1.25 μg of AM630 had no effect on food intake [F (l,87) = 5.06, P > 0.05] (Fig. 5).
In experiment 6, ICV injection of JWH015 (25 μg) significantly increased food intake compared to control group [F (l,87) = 136.14, P < 0.01]. However, Co-injection of AM630 (1.25 μg)+JWH015 significantly attenuated JWH015-induced hyperphagia in FD3 layer-type chicken [F (l,87) = 94.18, P < 0.01] (Fig. 6).
Discussion
To our knowledge this paper is the first report on the specific role of CB1 and CB2 receptors on feeding behavior in neonatal layer-type chicken. Also, this study provides the first demonstration of hyperphagia induced by ICV injection of a selective CB1 receptor agonist in layer-type chicks. More than 40 years passed from the isolation of specific CB receptors and several researches have done on the role of the CBergic system in the CNS but several physiologic roles of the CBergic system remains unclear. Evidences imply ECBs regulate several behavior and emotional states, such as food intake, pain, anxiety and go forth (López 2010).
Earlier studies had shown that pharmacological stimulation of CB receptors by systemic administration of plant-derived or ECBs stimulate eating and produce anabolic effects (D’Addario et al. 2014).
As observed in this study food intake increased via both CB1 and CB2 receptors layer-type chicks which was similar to mammals (Di Marzo et al. 2001; Chen et al. 2006; Wiley et al. 2012) but dissimilar to broilers which only CB2 receptors interact on feeding (Emadi et al. 2011; Novoseletsky et al. 2011). ICV injection of SR141716SA or AM251 reduced food intake in normal mice, but not in CB1 knockout mice where contributing to the role of the ECBs system on appetite by activating CB1 receptors (Di Marzo et al. 2001). Also, AM251 has been shown to decrease food intake in food-restricted rats (Irwin et al. 2008). In the present study, we have evaluated the potential effect of SR141716A (an analogue of AM251) on food intake in FD3 birds.
There are different between ECBs and other neurotransmitters. Almost all neurotransmitters are water-soluble and stored in high concentrations in vesicles. In depolarization, neurotransmitter release from the presynaptic terminal into the synaptic cleft and binds to its specific receptors on postsynaptic neuron (Nicoll and Alger 2004). In mammals and birds, CB1 receptors are expressed in presynaptic terminals of both inhibitory and excitatory nerves (Sharkey et al. 2014). ECBs are synthesized in postsynaptic neurons and binds to CB receptors on the presynaptic membrane. These receptors belong to G protein, inhibits Ca2+ influx which decreases the release of other neurotransmitters (Williams and Kirkham 2002). Additionally, CB1 receptors may interact at the signal transduction mechanism with cyclic adenosine monophosphate (cAMP) where SR141716A increase cAMP synthesis via an effect on Gi proteins (Verty et al. 2004). In the brain, the ECB system tonically reinforces food consumption by interacting with the mesolimbic pathways engaged in reward mechanisms (D’Addario et al. 2014). Interconnections reported between ECBs with orexigenic and anorexigenic neurotransmitters (Chen et al. 2006; Tedesco et al. 2008; Alimohammadi et al. 2015; Zendehdel et al. 2015). Ventromedial, dorsomedial and lateral hypothalamus, together with paraventricular and ARC nuclei are the hypothalamic areas involved in food intake control. In such a framework, these regions are interconnected by the neuronal pathways with other neurotransmitters. Recent studies have found EBCs reinforces release of orexigenic neurotransmitters on these areas (D’Addario et al. 2014). Numerous hypotheses have been postulated to explain the mechanisms underlying the direct action for how ECBs regulates food intake, despite accuracy of these theories remain unclear. One of the suggested pathways is that ECBs via CB1 receptors modulate signaling in POMC neurons, anorexigenic neurotransmitter in the ARC (Hentges et al. 2005). However, in this study, POMC level was not measured after ICV injection of CBs receptors agonists or antagonists. Presumably, CB1 receptors impress their hyperphagic role by blocking POMC neurons in the ARC nucleus (D’Addario et al. 2014). In our opinion, additional studies will uncover possible interconnection(s) in layer-type chicken. Also, CB1 receptors are expressed in presynaptic terminals of excitatory nerves (Sharkey et al. 2014). ICV injection of the AEA or CB1 receptors increases NPY levels (Verty et al. 2004; Gamber et al. 2005; Lim et al. 2010). C-Fos expression increases by ICV injection of CB1 receptors in the lateral hypothalamus, suggesting the existence of a connection on hypothalamus feeding centers (Soria-Gomez et al. 2007). Therefore, the CBergic system not only modulates neurotransmitter release, but it may also influence the expression and/or release of other feeding related neurotransmitters (Emadi et al. 2011). Of particular new findings of the current study is that CB1 receptors play important role on food intake regulation in layers compared to cockerels. Layer has indirectly been selected for slow growth and low body weight while broilers have been genetically selected for rapid muscle and body weight gain. Genetic selection altered chicken’s brain neurological pathways associated with food intake (Richards 2003). Broilers have higher feed consumption, basal metabolic rate and energy expenditure, possibly because of a genetically altered mechanism of food intake control (Denbow 1994). For example, brain AMP-activated protein kinase (AMPK) systems response to food intake regulation altered because of genetic selection among domestic fowl (Pingwen et al. 2011). This inconsistency may be due to the differences in localization, affinity or expression of CB1 receptors in layer- and meat-type chicks (Emadi et al. 2011; Novoseletsky et al. 2011). In this study, we were not able to find information about alteration on CB receptors in meat- and layer-type chickens. The mechanisms by which CB1 receptors influence the food intake in layer chicks have still to be explored in much greater detail.
There are no reports on effect of CB2 agonist, JWH015, on food intake in neonatal layer-type chicken. Despite researches showed CB2 receptor is expressed mainly in the immune system, but they are cloned from brain derived immune cells (Van Sickle et al. 2005). It is now believed CB2 receptors carry out important functions in the brain. It this study hyperphagia observed after ICV injection of CB2 receptors agonist in FD3 neonatal layer-type birds. Previously, Emadi et al. (2011) reported food intake increases following ICV injection of CB65 (a CB2 receptors agonist) in FD3 neonatal broiler chicken. Following 12-h food deprivation, peripheral injection of AM630 (selective CB2 receptors antagonist) stimulated appetite in rat (Onaivi et al. 2008). There is only one research describing the presence of a CB2-like protein in the CNS of chicken embryos, which disappears in adult chicken (Fowler et al. 2001). Perhaps observed effect of CB2 receptors might relate to indirect effects of these receptors on the digestive system (Emadi et al. 2011). Stimulation of CB2 receptors reduces inflammatory response by suppressing brain microglial activation (Ehrhart et al. 2005). In the current study, we focused on the role of central the CBergic system on feeding behavior in chicken. Evidence indicated ECBs promote food intake through peripheral sites (Galiegue et al. 1995). Although the mechanisms whereby the cannabinoids influence food intake remains unclear, results suggest that the CB system will be an important target in future studies in obesity. The authors recommend further research needed to clarify any direct cellular and molecular signaling pathway on appetite regulation in layer-type chicken.
References
Adam AS, Wenger T, Csillag A (2008) The cannabinoid CB1 receptor antagonist rimonabant dose-dependently inhibits memory recall in the passive avoidance task in domestic chicks (Gallus domesticus). Brain Res Bull 76:272–274
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun. doi:10.1007/s11259-015-9631-8
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2005) Effects of various lμ-, δ- and κ-opioid ligands on food intake in the meat type chick. Physiol Behav 85:519–523
Chen RZ, Frassetto A, Fong TM (2006) Effects of the CB1 cannabinoid receptor inverse agonist AM251 on food intake and body weight in mice lacking μ-opioid receptors. Brain Res 1108:176–178
D’Addario C, Micioni Di Bonaventura MV, Puccia M, Romano A, Gaetani S, Ciccocioppo R, Cifani C, Maccarrone M (2014) Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev 47:203–224
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Di Marzo VGS, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G (2001) Leptin regulated endocannabinoids are involved in maintaining food intake. Nature 410:822–825
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation 2:29
Emadi L, Jonaidi H, Hosseini Amir Abad E (2011) The role of central CB2 cannabinoid receptors on food intake in neonatal chicks. J Comp Physiol A 197:1143–1147
Fowler CJ, Nilsson O, Andersson M, Disney G, Jacobsson SO, Tiger G (2001) Pharmacological properties of cannabinoid receptors in the avian brain: similarity of rat and chicken cannabinoid1 receptor recognition sites and expression of cannabinoid2 receptor-like immunoreactivity in the embryonic chick brain. Pharmacol Toxicol 88:213–222
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232:54–61
Gamber KM, Macarthur H, Westfall TC (2005) Cannabinoids augment therelease of neuropeptide Y in the rat hypothalamus. Neuropharmacology 49:646–652
Hentges ST, Low MJ, Williams JT (2005) Differential regulation of synaptic inputsby constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25:9746–9751
Irwin N, Hunter K, Frizzell N, Flatt PR (2008) Antidiabetic effects of sub-chronic administration of the cannabinoid receptor (CB1) antagonist, AM251, in obese diabetic (ob/ob) mice. Eur J Pharmacol 581:226–233
Kaiya H, Miyazato M, Kangawa K (2011) Recent advances in the phylogenetic study of ghrelin. Peptides 32:2155–2174
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-Involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Levine AS (2006) The animal model in food intake regulation: examples from the opioid literature. Physiol Behav 89:92–96
Lim CT, Kola B, Korbonits M (2010) AMPK as a mediator of hormonal signaling. J Mol Endocrinol 44:87–97
López HH (2010) Cannabinoid–hormone interactions in the regulation of motivational processes. Horm Behav 58:100–110
Nicoll RA, Alger BE (2004) The brain’s own marijuana. Sci Am 291:68–75
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, Benno R (2008) Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci 1139:426–433
Onaivi ES, Ishiguro H, Gu S, Liu QR (2012) CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol 26:92–103
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res 260:131–138
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol 168:1–51
Pingwen X, Siegel PB, Denbow DM (2011) Genetic selection for body weight in chickens has altered responses of the brain’s AMPK system to food intake regulation effect of ghrelin, but not obestatin. Behav Brain Res 221:216–226
Richards MP (2003) Genetic regulation of feed intake and energy balance in poultry. Poult Sci 82:907–916
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Saneyasu T, Honda K, Kamisoyama H, Ikura A, Nakayama Y, Hasegawa S (2011) Neuropeptide Y effect on food intake in broiler and layer chicks. Comp Biochem Physiol A Physiol 159:422–426
Sanudo-Pena MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM (2000) Activational role of cannabinoids on movement. Eur J Pharmacol 391:269–274
Sharkey KA, Darmani NA, Parker LA (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146
Shiraishi J, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami S, McMurtry JP, Bungo T (2011) Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiol Behav 103:233–239
Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O (2007) Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol 151:1109–1116
Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B, Pagotto U, Nisoli E (2008) Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 57:2028–2036
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Verty ANA, McFarlane JR, McGregor IS, Mallet PE (2004) Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology 145(7):3224–3231
Wiley JL, Marusich JA, Zhang Y, Fulp A, Maitra R, Thomas BF, Mahadevan A (2012) Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice. Eur J Pharmacol 695:62–70
Williams CM, Kirkham TC (2002) Reversal of ∆9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav 71:333–340
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S et al (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37:37–41
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther. doi:10.1007/s10989-014-9445-5
Conflict of interest
Abbas Alizadeh, Morteza Zendehdel, Vahab Babapour, Saeed Charkhkar and Shahin Hassanpour declare that they have no conflict of interest.
Informed consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and animal rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the institutional animal ethics committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alizadeh, A., Zendehdel, M., Babapour, V. et al. Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun 39, 151–157 (2015). https://doi.org/10.1007/s11259-015-9636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-015-9636-3