Abstract
Recently, methylamine has been found as an endogenous amine, which is controling food intake in mammals. However, there is no evidence about the effect of methylamine on feeding behavior in poultry. So, the present study was designed to evaluate the effect of intracerebroventricular (ICV) injection of methylamine and involvement of central methylamine/dopaminergic systems on feeding behavior in neonatal meat type chicks. In experiment 1, chicks were ICV injected with different doses of methylamine (0.48, 0.96, 1.44, 1.92 and 2.40 μmol). In experiment 2, chicks received a dose of either the control solution, 2.40 μmol methylamine, 125 nmol L-DOPA (dopamine precursor) or a combination of methylamine plus L-DOPA. Experiments 3–7 were similar to experiment 2 except that 150 nmol 6-OHDA (dopamine synthase inhibitor), 5 nmol SCH23390 (D1 receptor antagonist), 5 nmol AMI-193 (D2 receptor antagonist), 6.4 nmol NGB2904 (D3 receptor antagonist) and 6 nmol L-741, 742 (D4 receptor antagonist) were used instead of 125 nmol L-DOPA, respectively. Cumulative food intake was determined until 2 h post-injection. According to the results, methylamine significantly decreased food intake in a dose dependent manner (p < 0.05). The inhibitory effect of methylamine on food intake was significantly attenuated by 6-OHDA, SCH23390 and AMI-193 (P < 0.05), but NGB2904 and L-741, 742 had no effect on food intake induced by methylamine. In addition, hypophagic effect of methylamine significantly amplified by L-DOPA (P < 0.05). These results suggest that methylamine decrease food intake and there is an interaction between methylamine and dopaminergic system via D1 and D2 receptors in chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feeding behavior is controlled by complex neurochemical mechanisms in several parts of brain such as striatum, hypothalamus, amygdala and so on (Parker et al. 2014; Boswell 2005). Several studies propose that many features of feeding regulation in chicks are similar to that in mammals but there are some differences in the neurochemical processes for feeding between them (Zendehdel and Hassanpour 2014b; Hassanpour et al. 2015). A powerful and complex physiological system exists to balance energy intake and expenditure, composed of both afferent signals and efferent effectors (Wynne et al. 2005). The homeostasis of this process is regulated by the hypothalamus through neuronal circuits using specific neurotransmitters and amines. Fluctuation in the levels of mediators in the periventricular hypothalamic (PH) nucleus causes hyperphagic or hypophagic behaviour (Stanley et al. 2005; Raimondi et al. 2007).
Methylamine (MET), is an endogenous short aliphatic amine, derives from the deamination of adrenaline, sarcosine, creatinine and lecithin, or from foods and drinks (Zeisel and Da Costa 1986; Buffoni 1995; Pirisino et al. 2004). In fasted rats, low methylamine doses (from 5 to 15 μg) stimulated food consumption and this effect decreased progressively at methylamine doses from 30 to 45 μg. In this respect, high doses of methylamine, from 60 to 80 μg, caused an anorexigenic activity (Raimondi et al. 2007). These findings in rats were different from those observed in mice where only a clear-cut, dose–related hypophagia is observed (Pirisino et al. 2001; Pirisino et al. 2004).
Like many other biologically active substances, dopamine exerts its effects by binding to and activating receptors located on the surface of cells (Grace 1991). There are at least five subtypes of dopamine receptors, D1, D2, D3, D4, and D5. The D1 and D5 receptors are members of the D1-like family of dopamine receptors, whereas the D2, D3 and D4 receptors are members of the D2-like family (Contreras et al. 2002). Consequently, it is incorrect to describe dopamine itself as either excitatory or inhibitory substance. Its effect on a target neuron depends on the type of the receptors present on the membrane of the neuron and also the internal responses of the neuron to cyclic AMP. D1 receptors are the most common dopamine receptors in the central nervous system, D2 receptors are the next, and D3, D4, and D5 receptors are at significantly lower levels (Grace 1991). Our previous study showed that intracerebroventricular (ICV) injection of dopamine decreases food intake in cockerels (Zendehdel et al. 2014a).
Previously, has been reported that methylamine elicites a hypophagic effect that is related to increasing extracellular levels of dopamine in rat (Raimondi et al. 2007). In mice, methylamine-induced hypophagia was found to depend on the brain expression levels of the Shaker-like Kv1.6 subtype potassium channels (Pirisino et al. 2004), suggesting that methylamine could evoke the release of some hypophagic mediator(s) by interacting at these channels. In addition, Pirisino et al. (2004) showed that both the dopamine release and the hypophagia were lowered by reducing the Kv1.6 potassium channel expression in mice. These results show the relationship between methylamine, hypophagia, dopamine efflux and density of brain Kv1.6 subtypes (Raimondi et al. 2007).
No report exists on the interconnection of methylamine and dopaminergic system on central control of food intake in avian. On the basis of comparative physiology it is important to determine the role of amines and neurotransmitters in other species (Zendehdel and Hassanpour 2014b). So, the purpose of the present study was to investigate the role of ICV injection of methylamine and dopamine receptors antagonist on food intake in 3 h food deprived (FD3) neonatal meat chicken.
Materials and methods
Animals
Three hundred and ninety six, one-day-old broiler cockerels were purchased from a local hatchery (Mahan Company, Tehran, Iran). Chickens were kept in stabilized electrically heated batteries at a temperature of 32 °C ± 1, 40–50 % relative humidity and 23:1 lighting/dark period (Olanrewaju et al. 2006). After 3 days, chickens transferred randomly into individual cages. During the experiment, animals were feed by a commercial starter diet containing 21 % crude protein and 2850 kcal/kg metabolizeable energy (Animal Science Research Institute Co. Tehran, Iran) and fresh water ad libitum. 5-day-old chickens were food deprived for 3 h (FD3) whereas there was no limitation to drink water. Experimental procedures were performed on animals according to the Guide for the Care and Use of Laboratory animals by the National Institutes of Health (USA) and the current laws of the Iranian government for animal care rules.
Drugs
Experimental drugs included methylamine, L-DOPA (dopamine precursor), 6-OHDA (dopamine synthase inhibitor), SCH 23390 (D1 receptor antagonist), AMI-193 (D2 receptor antagonist), NGB2904 (D3 receptor antagonist), L-741,742 (D4 receptor antagonist) and Evans blue were purchased from Sigma Co. (Sigma, USA). In order to prepare these solutions and control, 1 % Evans blue used. Evans blue was dissolved in absolute Dimethyl sulfoxide (DMSO) and then diluted with 0.85 % normal saline.
ICV injection protocol
Body weight was recorded from each chicken and then they fasted for 3 h. To find interaction of methylamine with dopaminergic system, seven experiments were designed. Each experiment included 4 treatment groups (n = 11 in each group). Intracerebroventricular injection was done by using a microsyringe (Hamilton, Switzerland) without anesthesia (Davis et al. 1979; Furuse et al. 1997). According to this procedure, head of the birds were held with an acrylic device in which the bill holder was 45° and the calvarium was parallel to the surface of table as explained by Van Tienhoven and Juhaz (1962). An orifice was made in a plate that which located over the skull immediately over the right lateral ventricle. A microsyringe was inserted into the ventricle through the orifice in the plate and the tip of the needle perforated only 4 mm below the skin of the skull (Jonaidi and Noori 2012). This technique does not induce any physiological stress in neonatal chicks (Saito et al. 2005). Each injection was performed once and in a volume of 10 μl (Furuse et al. 1999).
Experimental procedure
For finding the effective dose of methylamine, experiment 1 was designed to examine the effect of ICV injection of methylamine at doses of 0.48, 0.96, 1.44, 1.92 and 2.40 μmol on the food intake of FD3 chickens.
In the second experiment, chickens received 125 nmol L-DOPA, 2.40 μmol methylamine and combination of methylamine + L-DOPA. Experiment 3 was designed to examine the effect of ICV injection of 150 nmol 6-OHDA (dopamine synthase inhibitor), 2.40 μmol methylamine and methylamine +6-OHDA on food intake in chickens. Birds in Experiment 4, received ICV injection of 5 nmol SCH23390 (D1 receptor antagonist), 2.40 μmol methylamine and SCH23390 + methylamine. FD3 chicks in experiment 5, were injected through ICV with 5 nmol AMI-193 (D2 receptor antagonist), 2.40 μmol methylamine and AMI-193 + methylamine. In Experiment 6, chickens injected with 6.4 nmol NGB2904 (D3 receptor antagonist), 2.40 μmol methylamine and combination of NGB2904 + methylamin. Experiment 7 was carried on with 6 nmol L-741,742 (D4 receptor antagonist), 2.40 μmol methylamine and combination of L-741,742 + methylamine. Then chickens were transferred to their individual cages with water and pre-weighed food. Food consumption was measured at 30, 60 and 120 min post injection. Food consumption is expressed as a percentage of body weight that body weight impact on the amount of food intake to a minimum. At the end of the experiments, to recognize accuracy of injection, the chicks were sacrificed by decapitation. Only data from individual chicks were used for analysis that was confirmed by the existence of Evans Blue color in the lateral ventricle. In each experiment, control groups were injected like treatment groups with 10 μl saline containing Evans blue (n = 11) (Furuse et al. 1999). Each group included at least 11 chicks. Each bird was injected once only. All doses of drugs were calculated based on previous and pilot studies (Pirisino et al. 2001; Raimondi et al. 2007; Zendehdel et al. 2014a).
Statistical analysis
Cumulative food intake was analyzed by repeated measures two-way analysis of variance (ANOVA) and is presented as the mean ± SEM. In order to evaluate the effect of the treatments according to the ANOVA, mean values were compared with post hoc Tukey-Kramer test. P-values <0.05 were considered to indicate significant differences between the treatments.
Results
The food intake response to ICV injection of methylamine and evaluation of its possible interaction with L-DOPA, 6-OHDA, SCH 23390, AMI-193, NGB2904 and L-741, 742 in neonatal meat chickens are presented in Figs. 1, 2, 3, 4, 5, 6 and 7.
Effects of intracerebroventricular injection of control solution, L-DOPA (dopamine precursor), methylamine (MET) and a combination of L-DOPA plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, 6-OHDA (dopamine synthase inhibitor), methylamine (MET) and a combination of 6-OHDA plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
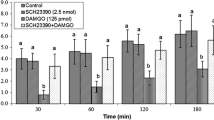
Effects of intracerebroventricular injection of control solution, SCH23390 (D1 receptor antagonist), methylamine (MET) and a combination of SCH23390 plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, AMI-193 (D2 receptor antagonist), methylamine (MET) and a combination of AMI-193 plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a, b and c) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, NGB2904 (D3 receptor antagonist), methylamine (MET) and a combination of NGB2904 plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
Effects of intracerebroventricular injection of control solution, L-741,742 (D4 receptor antagonist), methylamine (MET) and a combination of L-741,742 plus MET on cumulative food intake (% BW) in cockerels. Data are expressed as mean ± SEM. Different letters (a and b) indicate significant differences between treatments (P < 0.001)
In Experiment 1, ICV injection of 0.40 μmol methylamine had no significant effect on cumulative food intake (% BW) in comparison with control group [F(l,43) = 0.75, P > 0.05]; but 0.96, 1.44, 1.92 and 2.40 μmol of methylamine significantly decreased cumulative food intake in a dose-dependent manner [F(l,43) = 154.01, P < 0.001] (Fig. 1). Hence, to understand interaction between methylamine and dopaminergic system in food intake, 2.40 μmol methylamine was selected for the subsequent experiments.
In Experiment 2, ICV injection of methylamine (2.40 μmol) significantly decreased food intake compared to control group [F(l,36) = 140.06, P < 0.001]. The hypophagic effect of methylamine was significantly amplified by administration of 125 nmol L-DOPA [F(l,36) = 159.47, P < 0.001], while 125 nmol L-DOPA alone had no effects on food intake in FD3 chickens [F(l,36) = 3.86, P > 0.05] (Fig. 2).
In experiment 3, no significant alteration was observed after ICV injection of 6-OHDA (dopamine synthase inhibitor, 150 nmol) on cumulative food intake in chickens [F(l,36) = 5.46, P > 0.05]. Conversely, ICV injection of methylamine (2.40 μmol) significantly decreased food intake in FD3 chickens (P < 0.001). Also, according to data, combination of methylamine +6-OHDA weakened methylamine induced hypophagia [F(l,36) = 169.05, P < 0.001] (Fig. 3).
In experiment 4, intracerebroventicular administration of SCH 23390 (D1 receptor antagonist, 5 nmol) had no significant effect on food intake in contrast with control group at 30, 60 and 120 min post-injection [F(l,26) = 3.19, P > 0.05]; but significant alternation was found on food intake by injection of methylamine (2.40 μmol) [F(l,26) = 179.15, P < 0.001]. Additionally, injection of SCH 23390 + methylamine showed that dopamine D1 receptor antagonist could decrease hypophagia caused by methylamine [F(l,26) = 121.73, P < 0.001] (Fig. 4). It seems, dopamine D1 receptors interact with methylamine in food intake of chickens.
In Experiment 5, the application of 2.40 μmol methylamine significantly decreased food intake [F(l,34) = 154.16, P < 0.001]; but the AMI-193 (D2 receptor antagonist, 5 nmol) had no significant effect on food intake [F(l,34) = 2.64, P > 0.05]. Considerable hypophagic effect of methylamine was significantly attenuated by co-injection of AMI-193 and methylamine [F(l,34) = 114.08, P < 0.001] (Fig. 5). Perhaps, hypophagic effect of methylamine on food intake is mediated via dopamine D2 receptors in chickens.
In Experiment 6, ICV administration of methylamine (2.40 μmol) significantly decreased food intake compared to the control group [F(l,28) = 139.24, P < 0.001]. The mixture of NGB2904 (D3 receptor antagonist, 6.4 nmol) and methylamine failed to change the hypophagia induced by methylamine in FD3 chickens [F(l,28) = 1.83, P > 0.05] (Fig. 6). It seems, dopamine D3 receptors could not change hypophagic effect of methylamine in cockerels.
In Experiment 7, the hypophagic effect of methylamine (2.40 μmol) on food intake was not affected by concurrent injection of L-741, 742 (D4 receptor antagonist, 6 nmol) and methylamine in FD3 chickens [F(l,28) = 4.36, P > 0.05] (Fig. 7). Perhaps, dopamine D4 receptors have no role on hypophagic effect of methylamine in chicken.
Discussion
The present study was designed to investigate the role of ICV injection of methylamine on food intake in chickens and possible involvement of dopaminergic system on methylamine induced feeding behavior in neonatal meat-type chicks. To our knowledge this paper is the first report on the specific role of methylamine on feeding behavior in neonatal meat-type chicken. Methylamine has been shown to behave as an endogenous modulator and regulates numerous physiological functions such as the feeding behavior (Raimondi et al. 2007). Earlier studies had shown that central or peripheral administration of methylamine (as an endogenous amine) decreased food intake in normal or obese and type 2 diabetic starved mice (Pirisino et al. 2004; Cioni et al. 2006).
According to the results from Experiment 1, ICV injection of methylamine (from 0.96 to 2.40 μmol) significantly decreases cumulative food intake in a dose-dependent manner in FD3 chickens (Fig. 1). These findings propose that methylamine might act as one of anorexigenic mediators in the brain of chicken. Controversial results have been reported about the effect of methylamine on food intake in mammals. In accordance with our study, Pirisino et al. (2001) reported that ICV injection of methylamine dose-dependently reduced food intake in mice, suggesting that methylamine might be as an anorexigenic mediator in mice. This result was inconsistent with the data obtained from rats which ICV injection of methylamine showed biphasic dose-related effects on food consumption (Raimondi et al. 2007). In fasting rats, low methylamine doses (from 5 to 15 μg) increased food consumption whereas 60 and 80 μg had a hypophagic effect (Raimondi et al. 2007). This result, in the rat was different from those observed in mice and chicken where only a dose–dependent hypophagia is observed (Pirisino et al. 2001; Pirisino et al. 2004; Raimondi et al. 2007).
There is evidence central mechanisms for food intake regulation are different between mammalian and birds (Zendehdel and Hassanpour 2014b). For instance, effects of neurotransmitters such as ghrelin, leptin and adiponectin on feeding behavior regulation are somewhat dissimilar between mammals and avian (Zendehdel et al. 2015). Given the estimated 300 million years of evolutionary distance between mammals and avian, it is not surprising that significant dissimilarities have been found in the mechanisms of central food intake regulation (Novoseletsky et al. 2011). However, we think further research needed to investigate direct cellular and molecular signaling pathways of methylamine on dopaminergic system to determine possible difference among avian and mammalian.
In our study, the decrease in food intake caused by the injection of methylamine was amplified by ICV injection of L-DOPA (Fig. 2), whereas the combined administration of 6-OHDA and methylamine attenuated hypophagic effect of methylamine (Fig. 3). These results suggest that a possible relationship seems to be exist between methylamine and dopaminergic system involved in feeding behavior in chickens.
Dopamine is the predominant catecholamine neurotransmitter in the mammals and avian brain which controls variety of functions including locomotoractivity, cognition, emotion, positive reinforcement and food intake depending on the receptor subtype activated (Ikemoto 2007; Cooper and Plum 1987; Cooper et al. 2006; Zendehdel et al. 2014a). Dopaminergic neurons of the Substantia Nigra (SN), Pars Compacta, Ventral Tegmental Area and hypothalamus give origin to three main pathways, Nigrostriatal, Mesolimbocortical and Tuberoinfundibular in CNS. D1 receptor is more expressed and abundant than the other dopamine receptors in the human CNS (Cadet et al. 2010). The D2 receptor is identified in the striatum and olfactory tubercle in rat (Meador-Woodruff et al. 1992). Hence, distribution of D3 receptor is limbic areas such as ventromedial shell of the Nucleus Accumbens in rat (Bouthenet et al. 1991). Additionally, low level of D4 receptor mRNA has identified in the basal ganglia. It seems, this receptor highly expressed in the frontal cortex, amygdala, hippocampus, hypothalamus and mesencephalon (Van Tol et al. 1991).
In the present study, we used dopaminergic receptors antagonist in order to assay which one of dopaminergic receptors interact with methylamine in food intake of FD3 chicks. This study provides the first demonstration of hypophagia induced by central administration of methylamine is mediated by dopamine D1 and D2 receptors in neonatal meat chicks (Figs. 4 and 5); but D3 and D4 had no effect (Figs. 6 and 7). In trying to assess the interaction of methylamine and dopamine, previously (Raimondi et al. 2007), in agreement with our results, described that ICV injection of methylamine at hypophagic doses (60–80 μg), increased dopamine release from the periventricular hypothalamic (PH) nucleus in 12 h fasted rats. Moreover, pretreatment with a-methyl-p-tyrosine diminished methylamine induced hypophagia in rat. The periventricular hypothalamic nucleus nucleus has been shown to be the area involved in hypophagic effects of dopaminergic compounds (Parada et al. 1988; Leibowitz et al. 1989). Likewise, there is evidence that methylamine-induced hypophagia is modulated via brain expression levels of the Shaker-like Kv1.6 subtype potassium channels in mice. In this regard, methylamine stimulates release of some hypophagic mediator(s) by interacting at these channels (Pirisino et al. 2004). Decreased activity of Shaker-like Kv1.6 subtype potassium channels following to methylamine activation have been suggested as possible mechanisms that mediate methylamine-induced hypophagic effect. Consequently, it is probable that an increase in intracellular Ca+2 concentrations and thereby the release of dopamine modifies the methylamine hypophagic effect. In addition, NH3 releases mediators by increasing glutamate levels which elevates Ca2+ (Kitano et al. 2004; Monfort et al. 2004). This mechanism might be the consequence of the known ability of NH3 to reduce potassium conductance, a property that it shares with methylamine (Moroni et al. 1998; Hrnjez et al. 1999). In our study, L-DOPA potentiated and 6-OHDA inhibited hypophagic effect of methylamine.
According to our data, methylamine at the doses of 0.96–2.40 μmol (ICV), elicited a hypophagic effect that is maybe related to dopamine D1 and D2 receptors. Because the hypophagic effect of methylamine was significantly attenuated by SCH 23390 (D1 receptor antagonist, 5 nmol) and AMI-193 (D2 receptor antagonist, 5 nmol) in neonatal meat chicks. Raimondi et al. (2007) reveled that methylamine, at the doses of 60–80 mg (ICV), elicited a hypophagic effect that is related to increasing extracellular levels of dopamine, but not of 5-HT. As already observed in mice (Pirisino et al. 2004) and rat (Raimondi et al. 2007), dopamine release and the hypophagia were lowered by reducing the Kv1.6 potassium channel expression. These reports underline the close relationship between methylamine, hypophagia, dopamine efflux and density of brain Kv1.6 subtypes. Increased intracellular Ca2+, consequent on Kv1.6 closure, might be the mechanism responsible for dopamine release that provokes the hypophagic effect of methylamine. The extent of intraneuronal Ca2+ increase might account for the sequential stimulation of the dopaminergic responses evoked by increasing methylamine doses.
Most research on feeding behavior regulatory mechanisms have done in rat models whereas considering few investigations done in birds. Actually, there was no similar research to compare our results on mediatory role of methylamine on dopamine release as poultry model. Presumably central appetite regulation mechanisms are somewhat different among animals. These controversial data might be the consequence of injection methods and species difference. Additionally, it seems genetic selection for meat or egg production has altered chicken brain neurological pathways associated with appetite regulation. Also, it seems more researches needs on other physiological systems to clarify physiology of food intake regulation in poultry.
Conclusion
In summary, based on our findings, it seems methylamine-induced hypophagia is maybe modulated by dopaminergic system through the D1 and D2 receptors (not D3 or D4-dopamine receptors) in chicks. However, further investigation is required to elucidate the underlying cellular and molecular signaling pathways in the interconnection between methylamine and dopaminergic system on feeding behavior in neonatal meat chicks.
References
Boswell T (2005) Regulation of energy balance in birds by the neuroendocrine hypothalamus. J Poult Sci 42(3):161–181
Bouthenet ML, Soult MP, Martres P, Sokoloff B, Giros S, Schwartz JC (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Buffoni F (1995) Semicarbazide-sensitive amine oxidases: some biochemical properties and general considerations. Prog. Brain Res 106:323–331
Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS & Neurological Disorders—Drug Targets 9:526–538
Cioni L, De Siena G, Ghelardini C, Sernissi O, Alfarano C, Pirisino R, et al. (2006) Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur J Pharmacol 529:179–187
Contreras F, Fouillioux C, Bolívar A, Simonovis N, Hernández-Hernández R, Armas-Hernandez MJ, Velasco M (2002) Dopamine, hypertension and obesity. J Hum Hypertens 16(Suppl 1):13–17
Cooper AJ, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Cooper SJ, Al Naser HA, Clifton PG (2006) The anorectic effect of the selective dopamine D1-receptor agonist A-77636 determined by meal pattern analysis in free-feeding rats. Eur J Pharmacol 532:253–257
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the eitiology of schizophrenia. Neuroscience 41(1):1–24
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Hrnjez BJ, Song JC, Prasad M, Mayol JM, Matthews JB (1999) Ammonia blockade of intestinal epithelial K conductance. Am J Physiol 277:521–532
Ikemoto S (2007) Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Reviews 56(1):27–78
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198:827–832
Kitano T, Matsumura S, Seki T, Hikida T, Sakimura K, Nagano T, et al. (2004) Characterization of N-methyl – aspartate receptor subunits involved in acute ammonia toxicity. Neurochem Int 44:83–90
Leibowitz SF, Weiss GF, Walsh UA, Viswanath D (1989) Medial hypothalamic serotonin: role in circadian patterns of feeding and macronutrient selection. Brain Res 503:132–140
Meador-Woodruff MDK, Grandy SP, Damask O, Civelli S, Watson JR (1992) Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett 145:209–212
Monfort P, Munoz MD, Kosenko E, Llansola M, Sanchez-Perez A, Cauli O, et al. (2004) Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus: Alterations in hyperammonemia. Neurochem Int 45:895–901
Moroni A, Bardella L, Thiel G (1998) The impermeant ion methylammonium blocks Kþ and NH4þ currents through KAT1 channel differently: evidence for ion interaction in channel permeation. J Membr Biol 163:25–35
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J poultry Sci 5(4):301–308
Parada M, Hernandez L, Schwartz D, Hoebel BG (1988) Hypothalamic infusion of amphetamine increases serotonin, dopamine and norepinephrine. Physiol Behav 44:607–610
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res 260:131–138
Pirisino R, Ghelardini C, Banchelli G, Galeotti N, Raimondi L (2001) Methylamine and benzylamine induced hypophagia in mice: modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br J Pharmacol 134:880–886
Pirisino R, Ghelardini C, Pacini A, Galeotti N, Raimondi L (2004) Methylamine, but not ammonia, is hypophagic in mouse by interaction with brain Kv1.6 channel subtype. Br J Pharmacol 142:381–389
Raimondi L, Alfarano C, Pacini A, Livi S, Ghelardini C, DeSiena G, Pirisino R (2007) Methylamine- dependent release of nitric oxide and dopamine in the CNS modulates food intake in fasting rats. Brit J Pharmacol 150:1003–1110
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Stanley S, Wynne K, McGowan B, Bloom S (2005) Hormonal regulation of food intake. Physiol Rev 85:1131–1158
Van Tienhoven A, Juhaz LP (1962) The chicken telencephalon, diencephalons and mesencephalon in stereotaxic coordinates. J Comp Neural 118:185–197
Van Tol HHM, Bunzow JR, Guan H, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350:610–614
Wynne K, Stanley S, McGowan B, Bloom S (2005) Appetite control. J Endocrinol 184:291–318
Zeisel SH, da Costa K (1986) Increase in human exposure to methylamine precursors of N-nitrosamines after eating fish. Cancer Res 46:6136–6138
Zendehdel M, Hassanpour S (2014b) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 1:1–7
Zendehdel M, Hasani K, Babapour V, Seyedali Mortezaei S, Khoshbakht Y, Hassanpour S (2014a) Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Vet Res Commun 38:11–19
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2015) Interaction Between Opioidergic and Dopaminergic Systems on Food Intake in Neonatal Layer Type Chicken. Int J Pept Res Ther. doi:10.1007/s10989-015-9486-4
Acknowledgments
This research was performed in the Dr. Rastegar central Lab, Faculty of Veterinary Medicine, University of Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and Animal Rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Mahzouni, M., Zendehdel, M., Babapour, V. et al. Methylamine induced hypophagia is mediated via dopamine D1 and D2 receptors in neonatal meat chicks. Vet Res Commun 40, 21–27 (2016). https://doi.org/10.1007/s11259-015-9649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-015-9649-y