Abstract
The common steroid hormones are estrone (E1), 17β-estradiol (E2), estriol (E3), 17α-ethinylestradiol (EE2), and testosterone (T). These steroids are reported to contaminate the environment through wastewater treatment plants. Steroid estrogens are widespread in the aquatic environment and therefore pose a potential risk, as exposure to these compounds has adverse impacts on vertebrates. Excessive exposure to steroid estrogens causes endocrine disruption in aquatic vertebrates, which affects the normal sexual life of these animals. Steroid pollutants also cause several health problems in humans and other animals. Microbial degradation is an efficient method for removing hormone pollutants from the environment by remediation. Over the last two decades, microbial metabolism of steroids has gained considerable attention due to its higher efficiency to reduce pollutants from the environment. The present review is focused on the major causes of steroid pollution, concentrations of these pollutants in surface water, groundwater, drinking water, and wastewater, their effect on humans and aquatic animals, as well as recent efforts by various research groups that seek better ways to degrade steroids by aerobic and anaerobic microbial systems. Detailed overview of aerobic and anaerobic microbial biotransformation of steroid estrogens and testosterone present in the environment along with the active enzyme systems involved in these biotransformation reactions is described in the review article, which helps readers to understand the biotransformation mechanism of steroids in depth. Other measures such as co-metabolic degradation, consortia degradation, algal, and fungal steroid biotransformation are also discussed in detail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Steroid hormones directly interact with the normal functioning of endocrine systems, thus affecting reproduction and development in aquatic wildlife (Kumar et al. 2012). Some common steroid hormones (natural and synthetic) found in the environment include (E1), 17β-estradiol (E2), estriol (E3), 17α-ethinylestradiol (EE2), mestranol (MeEE 2), and testosterone (T) (Table 1) (Ting and Praveena 2017). Both natural and synthetic estrogens have a tetracyclic network consisting of a phenolic, two cyclohexane, and one cyclopentane ring. The difference in the configurations of the D ring at the C16 and 17 positions of estrogens, give rise to different compounds (Hamid and Eskicioglu 2012). Testosterone also has cyclopentanoperhydrophenanthrene nucleus as in estrogenic substances. Steroid hormones enter the environment through human and animal excretions (urine and feces) as well as from waste generated by pharmaceutical industries (Zheng et al. 2008). Waste containing estrogens are carried with wastewater into wastewater treatment plants (WWTPs) and then released into the aquatic environment, including drinking water (Sang et al. 2012), as WWTPs without biological treatment facilities are unable to efficiently remove estrogens (Ting and Praveena 2017). Increasing industrialization and modernization means that more estrogen would end up contaminating the aquatic environments (Jiang et al. 2013), thereby interfering with sexual development and reproduction in aquatic animals (Yu et al. 2013). Many studies have reported that the feminization of male fish is a result of estrogen contamination (Liu et al. 2016). Estrogen pollution affects intracellular estrogen receptors (ERs), which regulate the transcription of responsive genes, resulting in the initiation of rapid, non-genomic reactions. Malfunctional ERs disrupt the normal functioning of the estrogen system and causes defective homeostasis in fishes (Pinto et al. 2014). In aquatic systems, estrogens also act as endocrine-disrupting compounds (EDCs) because they interfere with the normal functioning of the endocrine system of aquatic animals by mimicking, and antagonizing the effect of endogenous hormones as well as disrupting the anabolism and catabolism of endogenous hormones (Silva et al. 2012; Liu et al. 2016). Estrogen contaminants also affect the immune, cardiovascular, and neurological systems in humans (McKinlay et al. 2008; Chighizola and Meroni 2012; Woclawek-Potocka et al. 2013). For instance, in humans, exposure to endogenous or exogenous estrogenic compounds leads to lower sperm count, declining male reproductive health, and feminization of men (Sumpter and Jobling 2013). Excessive exposure to estrogen contamination also leads to susceptibility to several types of cancer such as prostate cancer in men and breast cancer in women (Trevino et al. 2015; Adeel et al. 2017). Some studies have also revealed that intake of a single estrogen or multiple estrogens in combination with progesterone lowers the intraocular pressure (IOP) inside the eye, which could damage the optical nerve and therefore lead to glaucoma (Adeel et al. 2017). Apart from estrogens, environmental androgen, i.e., testosterone, is a potential micropollutant, which interferes with the endocrine systems of organisms, even in trace amounts (Davis et al. 2000; Seki et al. 2004; Fu et al. 2019). Moreover, androgen can interfere with the reproductive development of aquatic animals and adversely affect the structure and function of microbial communities (Barbosa et al. 2008; Kang et al. 2008). The major source of environmental androgen contamination comes from human and animal excreta, similar to estrogen contamination (Lange et al. 2002; Lorenzen et al. 2004; Arnon et al. 2008). Increasing levels of androgen contamination in the aquatic environment have also been observed in recent years (Zheng et al. 2008; Sang et al. 2012; Fu et al. 2019) and have been detected in surface water, groundwater, rivers, and sediments (Zheng et al. 2008; Sang et al. 2012). Androgens promote a high proportion of males in some aquatic animals (Orn et al. 2006) and cause masculinization or virilization in females and reduce their reproductive capabilities (Orlando et al. 2004; Kang et al. 2008), which has consequential effects on aquatic ecosystem.
Several physical, biological, and chemical methods can be used to control estrogen and testosterone pollution. The most common methods used include photocatalytic degradation, advanced oxidation processes (AOPs), adsorption, and biological degradation or biotransformation (Zhang et al. 2015a). Among these methods, biological degradation is the most common, successful, and economical method. Some groups of bacteria and white-rot fungi can efficiently degrade or transform these pollutants in contaminated environments. These microbial systems use the estrogen and testosterone as carbon and energy sources, thereby degrading or transforming them into other less harmful or neutral compounds (Zhang et al. 2015b; Li et al. 2017). Studies have shown that the use of microbial consortium rather than a single pure culture is a more effective way of estrogen biotransformation (Johnson et al. 2014). Similarly, some microbial strains (bacterial and algal) in the environment can biotransform testosterone into other less-harmful by-products (Yang et al. 2010; Fu et al. 2019).
Presence of steroid hormones
Natural and synthetic estrogens are generated from human and animal excretions, pharmaceutical, dairy, poultry, and meat industries (Adeel et al. 2017). Since the rise in global industrialization, the release of steroid estrogens in wastewater increases tremendously, which further raises the steroid estrogen level in various water bodies (Jiang et al. 2013). This enhanced steroid estrogen pollution became a major threat to aquaculture along with humans too. These estrogens can be detected in feces, liquid manure, solid waste collected from cattle, lagoon effluent, and in manure applied directly to agricultural land (Biswas et al. 2013). For these reasons, four of the most commonly found natural and synthetic steroid estrogens distributed in various parts of the earth include E1, E2, E3, and EE2. These estrogens are also referred to as micropollutants as they are present in minute quantities but can affect aquatic animals due to their continuous exposure (Kim and Zoh 2016). The molar effective concentrations (EC50) and relative potency factor (RPF) with reference compound 17β-estradiol for E1, E2, E3, and EE2 are 1.2e−12, 1.8e−12, 7.3e−12, and 1.0e−12 and 1.4, 1.0, 0.23, and 1.7, respectively (Bermudez et al. 2012; Conley et al. 2017). Various studies have confirmed the presence of these steroid estrogens globally in freshwater, groundwater, and wastewater. Conley et al. (2017) reported the occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in stream water in the USA. Estrogens are released into the environment from animal excretions (Zheng et al. 2008), with pigs and poultry industry waste releasing about 58% E2 in feces, while 96% EE2 and 69% E3 are excreted through urine (Adeel et al. 2017). The distribution of E1, E2, E3, and EE2 and testosterone in the surface water of 12 different countries found in four subcontinents is shown in Table 2. Total estrogen concentration in surface water, suspended particulate matter, and sediments also vary with season. In the Yangtze estuary of China, the total estrogen concentration varies throughout the year, with the pattern of estrogen concentration in four successive seasons of the year being 3.92 to 14.54 ng/L in July, 3.22 to 16.36 ng/L in October, 10.42 to 20.61 ng/L in January, and 5.03 to 10.77 ng/L in May. Moreover, levels of synthetic estrogens were higher than natural estrogens throughout the year (Nie et al. 2015). The presence of steroid estrogens in groundwater in various countries has also been analyzed, with the results revealing that in China the concentrations of E3 and EE2 in groundwater varies between 0.03 and 0.09 ng/L (Li et al. 2013), whereas E1 and E2 were not detected (Chen et al. 2011). In European countries (France and Spain), the concentration of E1, E2, and EE2 in groundwater varied between 1.3 and 3.5 ng/L in France (Vulliet et al. 2008), with no estrogens detected in Spain’s groundwater (Vulliet et al. 2008). In North America (USA and Mexico), no traces of estrogen were detected in Mexico groundwater (Felix-Canedo et al. 2013), but E1 (n.d.–79 ng/L), E2 (n.d.–147 ng/L), E3 (n.d.–1745 ng/L), and EE2 (n.d.–230 ng/L) were detected in the groundwater of USA (Karnjanapiboonwong et al. 2011). The presence of steroid estrogens in drinking water has also been evaluated, with the drinking water of mainland China reported to contain traces of E1 (n.d.–9.9 ng/L), E2 (n.d.–0.1 ng/L), and EE2 (n.d.–0.3 ng/L) (Fan et al. 2013; Zhang et al. 2011; Zhang et al. 2013). However, European (France, Italy, Spain) and American countries (Brazil and the USA) had no traces of estrogen in drinking water (Devier et al. 2013; Maggioni et al. 2013; Esteban et al. 2014b; Wang et al. 2011; Jardim et al. 2012; Falconer et al. 2006). In these studies, testosterone was only detected in China’s (n.d.–480 ng/L) and Brazil’s (n.d.–329 ng/L) groundwater and drinking water (Yang et al. 2014a; Montagner et al. 2019). Whereas in Japan, Malaysia, and Australia’s groundwater and drinking water, its concentrations were below detection limits (Praveena et al. 2016). In Europe (France, Italy, Netherlands, Spain) and America (Mexico, the USA, Argentina), no testosterone traces were found in groundwater and drinking water (Table 2).
Fate of steroid hormones
Steroid estrogens E1, E2, E3, and EE2 pollutants in wastewater are usually treated (biological or chemical) before releasing into the aquatic environment. Estrogens can be degraded by microbial communities present in soil and water under aerobic and anaerobic conditions. The half-life of estrogens varies with environmental conditions and concentration in a particular environment. The half-life of estrogens also depends on their degradation rate and oxygen availability (Adeel et al. 2017). Under aerobic conditions in soil, the half-lives of E1, E2, and E3 were found to be 2.8–4.9, 0.8–0.11, and 0.7–1.7 days, respectively (Biswas et al. 2013). Whereas in river water under aerobic conditions, the half-life of E1 and E2 were found to be 2–3 and 2–4 days, respectively (Jurgens et al. 2002; Ying et al. 2002; Adeel et al. 2017).
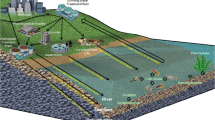
Liu et al. (2009b) determined the average concentration of steroid estrogens and natural androgens in municipal sewage wastewater treatment plants. The total excretion rate of estrogens E1, E2, and E3 was only 66–82% of total waste excretion. The concentration of these estrogens and natural androgen in urinary excretion varies with age and gender. For example, the estrogen excretion rates are different in premenopausal, pregnant, and postmenopausal women. The urinary excretion rate of E1, E2, and E3 for premenopausal, pregnant, and postmenopausal women were 10.73, 4.71, and 8.12, 1194, 347, and 24,078, and 5, 2.78, and 2.78 μg/day, respectively, whereas for men it was 3.9, 1.5, and 1.5 μg/day, respectively (Liu et al. 2009a). The urinary excretory rate of natural androgen (i.e., testosterone) for a man was 56.65 and 6.78 μg/day for women (Liu et al. 2009a). Thus, the total concentration of steroid estrogens in sewage wastewater also depends on the gender ratio of that particular area. Estrogen removal during wastewater treatment is a well-practiced process and has been described by several research groups (Racz and Goel 2010; Xu et al. 2012; Luo et al. 2014; Liu et al. 2015). The diagrammatic illustration of estrogen’s fate in a wastewater treatment plant is shown in Fig. 1. After mixing with activated sludge in the WWTPs, estrogens are either adsorbed onto solids in the wastewater-activated sludge or undergo biodegradation by various microbial systems (Racz and Goel 2010). Biodegradation is the major method used in estrogen removal from wastewater, which includes deconjugation, use of estrogen as carbon source by heterotrophic microorganisms, co-metabolism with nitrifying biomass, followed by another co-metabolism (Ke et al. 2007; Liu et al. 2016; Yu et al. 2016; Fernandez et al. 2017). There are variations in estrogen degradation or removal rate which mainly depend on different factors including operating conditions, geological location of the treatment plant, estrogen concentration in effluent, and the type of biological system used (Yu et al. 2013). It has been reported that WWTPs equipped with biological treatment systems are much more effective in estrogen degradation as compared with those without biological treatments. More estrogen degradation has been observed in treatment plants that have long solid retention time (SRT) and long hydraulic retention time (HRT). Long SRT and HRT provide sufficient time for the growth of slow-growing estrogen degraders and longer contact time for estrogen-adsorbent materials (Maeng et al. 2013). Complete microbial degradation or biotransformation of steroids (estrogens and androgens) is challenging because of their complex chemical structure, low solubility in water, low number of functional groups, and the presence of four alicyclic rings, as well as two quaternary carbon atoms (Olivera and Luengo 2019). It has been shown that estrogens E1, E2, and EE2 removal ranges from 19 to 94, 76 to 92, and 83 to 87%, respectively in WWTPs (Baronti et al. 2000). The descending order of biodegradation rate of steroid estrogens in WTTPs are E3 > E2 > E1 > EE2 (Garcia et al. 2019). In activated sludge, up to 98% E1 can be removed (Racz and Goel 2010; Zhou et al. 2012; Garcia et al. 2019), 99.9% E2 capable of being removed (Racz and Goel 2010; Heffron et al. 2016; Garcia et al. 2019), almost 100% E3 can be removed (Racz and Goel 2010; Garcia et al. 2019), while EE2 removal varies between 34% and almost 100% (Muller et al. 2008; Racz and Goel 2010; Garcia et al. 2019). Studies have also shown that estrogens concentration in effluent fluctuates daily, with no uniform or clear temporal trend despite consistency in wastewater flow (Williams et al. 2003; Racz and Goel 2010; Heffron et al. 2016). Even after activated sludge treatment, there is still a certain environmentally significant concentration of estrogens in the wastewater. For example, the biotransformation of E2 leads to the formation of E1. Generally, the microorganisms that biotransform E2 are unable to biotransform E1 further, which means that E1 removal from polluted water is relatively poor compared with E2 (Samir et al. 2006). It is partly, for this reason, that E1 starts to accumulate in water bodies and its concentration increase in the aquatic environment. Furthermore, it has been observed that E1 concentration in the surface water is much higher than other natural estrogens (Sami and Fatima 2019). In WWTPs, the fate of androgen can be tracked by metabolite analysis with isotope-labeled substrates. Under aerobic degradation of testosterone, most of the bacteria adopt 9,10-seco pathway. It has been shown that Comamonas spp. and Pseudomonas spp. are dominant in sewage that has testosterone traces. The meta-cleavage dioxygenase gene (tesB) of various proteobacteria is used to track this essential catabolic gene in sewage (Chen et al. 2016). In anaerobic degradation pathway, bacteria degrade androgens through the 2,3-seco pathway by the bifunctional 1-testosterone hydratase/dehydrogenase (Yang et al. 2016). Furthermore, to ensure efficient estrogen removal from wastewater, additional chemical treatment technologies such as activated carbon treatment, chlorination, ozonation, and ultraviolet irradiation, etc. are also carried out (Bila et al. 2007; Racz and Goel 2010; Hartmann et al. 2014; Li et al. 2016).
Fate of steroid estrogens in wastewater treatment plants (modified from Racz and Goel 2010)
Studies using LC-MS/MS and GC-MS have revealed the presence of steroid estrogens E1, E2, and E3 and testosterone in WWTP-activated sludge along with their concentrations (ng/L) in influent, effluent as well as their removal percentage in five different countries, i.e., Japan, Italy, Australia, the USA, and Canada (see Table 3) (Liu et al. 2009b). Steroid estrogens and natural androgens are released into the environment as urinary (90–95%) and fecal (5–10%) waste from humans and animals. These estrogens are released into the environment in either conjugated or unconjugated forms, with most of the estrogen released with fecal matter being unconjugated, whereas estrogens released with urinary waste are mostly conjugated (Ternes et al. 1999; Ascenzo et al. 2003; Liu et al. 2009a; Racz and Goel 2010). There are nine types of estrogen conjugates found in WWTP environment (Liu et al. 2009b). Recently, Yu et al. (2019) published a review on estrogen conjugates concentration (ng/L) present in various WWTP influents and effluents. The common estrogen conjugates along with their concentration (ng/L) in influent and effluent and their removal percentage in WWTPs are shown in Table 4. Data analysis showed that in WWTPs, the average removal rate of estriol, estradiol, and estrone conjugates are 90, 66, and 46%, respectively. Furthermore, it has been shown that the detection of any micropollutant in a medium depends on the limit of detection (LOD) or limit of quantification (LOQ) of that particular instrument. Thus, estrogen pollutants might not be detected in effluents (due to the detection range of the instrument), which does not mean that 100% of the steroid pollutants have been removed from the effluent after treatment (Naldi et al. 2016).
Mechanism of steroid hormone biotransformation
Microorganisms can degrade or biotransform steroid hormones by three major mechanisms: (i) Growth linked (metabolic): where estrogens are used as the sole carbon and energy source by the microorganisms. (ii) Non-growth linked (co-metabolic): where microorganisms grow by using other carbon and energy sources found in the media and produce enzymes which catalyze estrogen biotransformation to form various products (Yu et al. 2013; Zhang et al. 2015b). Co-metabolism also refers to the simultaneous degradation of non-growth substrates by microorganisms using a nutrient substrate (Fernandez-Fontaina et al. 2014). Studies have shown that co-metabolism can initiate reactions, convert persistent compounds into potentially more biodegradable intermediates, and participate in central metabolic pathways for further biotransformation (Groning et al. 2007; Tran et al. 2013). Municipal wastewater contains numerous micropollutants such as antibiotics and estrogens but due to their small quantity, they cannot support microbial growth but can induce microbial genes related to enzymes and cofactors which are involved in biodegradation (Fischer and Majewsky 2014). (iii) Convert steroid to metabolites but does not degrade the metabolites. Dehydrogenation of steroids is considered as a detoxication process (Hamid and Eskicioglu 2012). Most of the estrogen degradation is growth linked, where microorganisms use estrogen as a sole carbon source for their growth. Abiotic nitration or oxidation is also observed in some microbial systems. This mechanism is adopted by microorganisms in the presence of higher ammonia concentration (Yu et al. 2013). The rate of microbial degradation of estrone changes under the influence of background nitrogen and carbon. It has been seen that the use of ammonia as a nitrogen source enhances the estrone biotransformation rate significantly, probably because the presence of ammonia promotes tyrosine synthesis by promoting the GS-GOGAT pathway. The presence of acetic acid (AA) and humic acid (HA) also boost estrone biotransformation because the presence of these acids (AA and HA) triggers the up-regulation of tyrosine synthesis enzymes (Du et al. 2017). Besides, a higher concentration of ammonia also enhances EE2 biotransformation because ammonium oxidation provides more reducing power. Enhanced EE2 biotransformation has been observed in ammonia enriched sludge (Jantanaprasartporn et al. 2017).
Biotransformation of steroid estrogens
The complete degradation or transformation of steroids has been studied extensively due to their potential effects on the aquatic environment. The metabolic degradation of steroid estrogens is related to the degradation mechanism adopted by microorganisms. In this process, microorganisms use steroids as a sole carbon and energy source. This degradation mechanism is adopted only with the presence of high steroid concentration. Estrogens found in the environment can be degraded by both aerobic and anaerobic metabolic degradation pathways. The details of these (aerobic and anaerobic) microbial degradation pathways are given in subsequent sections.
Aerobic microbial biotransformation
The aerobic microbial biotransformation of steroid estrogen is common in nature. It has been observed that aerobic degradation is faster in summers than in winters (Vieno et al. 2005). Aerobic biotransformation is carried out by bacterial, fungal, and algal species.
Aerobic steroid biotransformation by bacteria
Steroid estrogen biotransformation by bacteria species is one of the most common and prominent ways of microbial degradation. Over the last decade, a large number of steroids transforming bacteria species have been isolated from marine, river, compost, sludge, sandy aquifer (Ke et al. 2007), and soil samples. E2-biotransforming strains Rhodococcus sp. JX-2 and Rhodococcus sp. DS201were mainly isolated from activated sludge samples (Liu et al. 2016; Yu et al. 2016). Both of these bacterial species effectively degrade 17β-estradiol (E2) from the environment. Another bacteria strain Novosphingobium sp. E2S with E2 transformation capability was also isolated from activated sludge samples. This strain transforms 66% of E2 after 7 days of incubation (Li et al. 2017). Due to the marine oligotrophic environment and high levels of salinity, strains that transform estrogen are difficult to isolate. So far, Buttiauxella sp. (Zhang et al. 2011), Vibrio sp. (Sang et al. 2012), Rhodococcus (Ye et al. 2017), Virgibacillus, and Bacillus (Fernandez et al. 2017) are the main estrogen-transforming strains isolated from the marine environment. Fernandez et al. (2017) isolated five 17β-estradiol anaerobic transforming bacterial strains (F1–F5) from deep-sea sediments.
Aerobic steroid biotransformation by fungi
The non-bacterial microorganisms that are capable of transforming steroid estrogens are fungi. Several fungal species are reported to transform steroids. Mascoti et al. (2016) explored the biotransformation of dehydro-epi-androsterone in Aspergillus parasiticus. This fungal strain was able to effectively biotransform bicycle [3.2.0] hept-2-en-6-one, the standard Baeyer-Villiger monooxygenase (BVMO) substrate to produce testololactone and the homo-lactone 3β-hydroxy-17a-oxa-D-homoandrost-5-en-17-one. Hunter et al. (2006) reported that Aspergillus tamari was a unique fungus that transforms progesterone into testololactone in high yield (about 70%) through a four-step enzymatic pathway, which is flexible for a range of steroidal substrates. A mycelium named Curvularia lunata, displayed a good capability of hydroxylation of steroids. This fungal strain transforms 16Δ5–3βhydroxy- and Δ4-3-ketosteroids of androstane and pregnane classes into 20 monohydroxy and dihydroxy-metabolites (Andrushina et al. 2011). Also, this fungus can dehydrogenate a wide range of different classes of steroids, because 17β-hydroxysteroid dehydrogenase from this strain (17β-HSDcl) could oxidize and reduce both estrogens and androgens, including estrone, 4-estrene-3,17-dione, 4-androstene-3,17-dione, and 5a-androstane-3,17-dione (Lanisnik Rizner et al. 2001). The fungus Fusarium moniliforme can be used for transforming 3-hydroxy-steroids into their 7α-hydroxylated derivatives (Cotillon and Morfin 1990). An inducible microsomal 7β-hydroxylase was characterized by this strain and was shown to be able to hydroxylate dehydroepiandrosterone (DHEA), pregnenolone, epiandrosterone, and estradiol at the 7α-position. Furthermore, many other fungal strains that are capable of transforming steroids including Fusavium oxysporum var. cubense, which causes the Panama disease of bananas (Musa sp.) and Exophiala jeanselmei var. lecanii-cod, a contaminant of the ginger plant (Zingiber ofjcinale). These fungal strains are responsible for 7α and 15α hydroxylation of steroids and side-chain degradation as well as 1,2- and 1,4-reduction of steroidal enones, respectively (Wilson et al. 1999; Reese 2007).
Aerobic steroid biotransformation by algae
Apart from bacterial and fungal strains, some algae can also efficiently transform steroid estrogens. The freshwater bacteria-free microalgae Raphidocelis subcapitata exhibits a strong ability to remove E2 and diethylstilbestrol (DES) by biotransformation (Liu et al. 2018b). Furthermore, the presence of DES enhances the removal of E2, which might be that DES stimulates enzymes such as glutathione S-transferase (GST), cytochrome P450, and peroxidase, which actively participate in E2 biotransformation (Shi et al. 2010; Peng et al. 2014; Gao and Chi 2015; Liu et al. 2018b). Wang et al. (2017) also reported an enhanced removal of EE2 by green microalgae in the presence of E2. Another freshwater alga, Chlorella vulgaris, can transform steroid estrogens present in sewage water. Steroid biotransformation rate depends on the concentration of algal cells (C. vulgaris) as well as the substrate concentration (Lai et al. 2002). Similarly, Shi et al. (2010) achieved efficient steroid estrogen transformation from pond water with the help of a complex inoculum of six different algal species along with duckweed. The six algal species, Anabaena cylindrica, Chlorococcus, Spirulina platensis, Chlorella, Scenedesmus quadricauda, and Anaebena var., were surprisingly able to biotransform E1, E2, and EE2, when present in nanogram concentrations (Shi et al. 2010). Besides, another algal species, Scenedesmus dimorphus, was able to efficiently biotransform steroid estrogens, with biotransformation efficiencies of about 85% for 17α-estradiol and estrone and 95% for 17β-estradiol and estriol over 8 days (Zhang et al. 2014). Thus, based on present findings, the most general transformation processes of hazardous pollutants by microalgae, including hydroxylation, glycosylation, and methylation (Liu et al. 2018b). In general, the cytochrome P450 monooxygenase (CYP450) was found to be involved in the detoxification of steroid estrogens in microalgae (Torres et al. 2008).
Anaerobic steroid microbial biotransformation
The biological transformation of steroids under anaerobic environment is considered recalcitrant (Czajka and Londry 2006). Since steroid biotransformation rate is much slower in an anoxic environment, these steroids become recalcitrant and start accumulating in sediments (Racz and Goel 2010). Also, the absence of active substances such as dissolved organic matter and Fe(III) in the anaerobic environment adversely affect the biotransformation rate of steroids, for which reasons anaerobic microbial degradation of steroids is challenging (Gu et al. 2018). Very little is known about the anaerobic biotransformation mechanism of steroids. In the last two decades, only a few anaerobic steroids transforming microbial systems have been discovered (Dermer and Fuchs 2012). Anaerobic catabolism of steroids involves oxygen-independent steps that are well studied in the anaerobic breakdown of cholesterol. It has been discovered that anaerobic steroid transformation is biphasic, i.e., rapid and slow transformation phases, both of which can be described by first-order degradation kinetics (Zhang et al. 2015b). During anaerobic transformation, E2 is degraded much faster than E1 and EE2, while EE2 is almost non-biodegradable (Zhang et al. 2015a). Some research groups have successfully demonstrated the anaerobic transformation of testosterone (Chiang et al. 2010). It has been shown that anoxic transformation of steroids takes place during enterohepatic circulation in various mammals by intestinal bacteria. In this anoxic environment, the cleavage of alkyl aryl ether linkages, dehydroxylation, and oxidation or reduction at C-17 was found, but the breakdown of the steroid nucleus by these bacteria to obtain energy was not clearly described (Groh et al. 1993). In the denitrifying steps of wastewater treatment and anoxic river sediments, mineralization of estradiol has been observed but the exact mechanism of mineralization used by bacteria is still unclear (Fahrbach 2006). Unfortunately, there is currently not much information on the mineralization of estrogens under anoxic conditions. However, the anaerobic transformation of E2 into E1 has been observed in acetone enriched activated sludge samples (Lee and Liu 2002). Besides, facultative anaerobes isolated from the deep sea (Fernandez et al. 2017) and sandy aquifer (Ke et al. 2007) could also transform E2 into E1 in an anaerobic medium. The list of some anaerobic microbial steroid degraders is given in Table 5.
Anaerobic steroid transforming microbial consortia
Microbial consortia are a mixture of different microbial strains which are capable to degrade or transform a particular substrate present in the environment (Clark et al. 2009). In the consortium, more than one microbial strains are growing symbiotically and simultaneously participates in the transforming mechanism. The enrichment culture technique is considered as a powerful tool to obtain microbial consortia with desired degradation capabilities (Feng et al. 2011; Okeke and Lu 2011). Microbial consortia developed by this method are much closer to the consortia that are functioning in nature (Feng et al. 2011). In steroid-degrading consortia experiment, microbial consortia took from activated sludge. The microbial consortium were firstly enriched in mineral salt media spiked with steroids. After successful enrichment of these strains, species present in consortia were identified and characterized by using different molecular biology tools (Agarwal et al. 2015; Edet et al. 2017). Yu et al. (2005) first time used the quantitative fingerprinting method and the real-time-RFLP to determine three different microbial systems present in activated sludge samples which were responsible for the transformation of 17α-estradiol, 17β-estradiol, and estrone. Secondly, Zanga et al. (2008) determined various phylogenic groups present in activated sludge samples by using micro autoradiography-fluorescence in situ hybridization (MR-FISH) technique. After that Real-time PCR assays were developed to determine estrogen degrading bacteria from consortia, this RT-PCR assay technique was further used to determine the average cell number of strain JEM-1 present in activated sludge (Hashimoto et al. 2010).
Co-metabolic steroid transformation by nitrifying microorganisms
Most microbial systems use metabolic degradation pathways to degrade a particular substrate when its concentration is high in the environment. At lower substrate concentrations, the co-metabolic pathway is used by microorganisms to degrade/transform steroids. In co-metabolic pathways, steroids are transformed with the help of some enzymes released by the microbial system. In these cases, they are not used as a sole carbon and energy source. Thus, a primary growth substrate is required for sustainable microbial growth. Nitrifying bacteria can efficiently metabolize steroids by this pathway. The transformation of EE2 in nitrification tanks using ammonium monooxygenase (AMO) enzyme is an example of a co-metabolic transformation of steroids (Andersen et al. 2003). It has been proposed that the AMO gene product is responsible for steroid transformation under anoxic conditions, but it is still not clear that whether nitrifying bacteria or heterotrophic bacteria is responsible for steroid transformation (Kayee 2014). Various molecular biology techniques have been used to identify and isolate the genes involved in co-metabolic steroid transformation. Through this, several research groups have been able to successfully isolate, clone and express steroid transforming genes in suitable hosts to enhance the co-metabolic steroid transformation (Shao et al. 2016; Wang et al. 2017).
Enzyme systems involved in estrogen and testosterone transformation
Microbial transformation of estrogen and testosterone is a complex physiological process that requires a series of catalytic reactions (Knol et al. 2008; Ye et al. 2017). The major enzymatic reactions involved in steroid transformation are hydroxylation (Van der Geize et al. 2008), isomerization (Talalay and Wang 1955), oxylation (Samavat and Kurzer 2015), acylation and hydrolysis. Major enzymes which catalyze these reactions are dehydrogenase (Genti-Raimondi et al. 1991; Ye et al. 2017), cytochrome P450 (Venkataraman et al. 2015), ring-cleavage dioxygenase, hydroxylase, monooxygenase, isomerase, hydratase, and demethylase (Kristan and Rizner 2012), etc. Also, it has been observed that various proteins such as electron transfer proteins, receptor proteins, signaling proteins as well as regulatory proteins are involved in the active transformation of steroid hormones (Penning 2003; Miller and Auchus 2011; Xu et al. 2017; Acconcia and Marino 2018). The most important enzymes that regulate steroid hormone biotransformation are hydroxylase and dioxygenase.
Short-chain dehydrogenase
The short-chain dehydrogenase/reductase (SDR) is a large enzyme family that is primarily involved in the metabolism of various hormones such as steroids, retinoids, etc. Some essential enzymes found in human beings of this family are epimerase, hydratase and NAD (P)H-dependent oxidoreductase (Simard et al. 1995). Most of the proteins of this family are made up of 250–300 amino acid residues and mostly consist of at least 2 domains, which first binds to coenzyme and the second binds to the substrate. The common microbial enzymes involved in steroid degradation from this family are HSD i.e., 17β-hydroxysteroid dehydrogenase and 3α-hydroxysteroid dehydrogenase. HSD has been characterized as the main or the sole enzyme that initiates the catabolism of cholesterol or other sterols such as estradiol in Mycobacteria, Nocardia, and Rhodococcus sp. (Wipperman et al. 2014; Kreit 2017; Ye et al. 2017).
17β-Hydroxysteroid dehydrogenase
3β, 17β-hydroxysteroid dehydrogenase (3β, 17β-HSD, EC 1.1.1.51) is directly related to steroid metabolism. The first step in steroid transformation, i.e., conversion of estradiol to estrone (Fernand et al. 1997) was catalyzed by this enzyme (Fig. 2). Studies have revealed that 17β-HSD plays a major role in the conversion of the active form of steroid hormones into non-active forms. In filamentous fungus, 17β-HSDcl from Cochliobolus lunatus can catalyze the same reactions similar to some human enzymes (Rizner et al. 1996).
Dehydrogenation of common estrogen with 17β-HSD enzyme (modified from Ye et al. 2019)
Cassetta et al. (2005) demonstrated that the Tyr167 amino acid residue was an active center of fungal 17β-HSDcl. Besides, 17β-HSD plays an active role in Comamonas testosterone and causes the complete oxidative degradation of steroid skeleton (Yu et al. 2015). Furthermore, Ye et al. (2017) also described the presence of 17β-HSD homologs in Rhodococcus sp. P14, which effectively converted toxic estradiol into less toxic estrone. In Pseudomonas putida SJTE-1, two genes (crgA and oxyR) adjacent to 17β-HSD encoded the potential CrgA and OxyR regulators, which are under the regulation of the 17β-HSD gene. CrgA could enhance the transcription of 17β-HSD, while oxyR represses 17β-HSD expression (Fernand et al. 1997).
3α-Hydroxysteroid dehydrogenase
3α-hydroxysteroid dehydrogenase (3α-HSD, E.C. 1.1.1.50) acts on various steroid hormones by catalyzing the redox reaction of the hydroxylatone group at position 3 of C19–27 on steroids. The strain C. testosteroni (Pseudomonas testis) expresses this dehydrogenase (Oppermann et al. 1993; Abalain et al. 1995), which can reduce the ketone group present at position 3 of testosterone. Also, this strain expresses the isoenzyme 3α-hydroxysteroid dehydrogenase/carbonyl reductase (3α-HSD/CR) (M.W. 49.4 kDa), which further reduces the toxicity of testosterone (Maser et al. 2000). X-ray crystallographic studies revealed that each asymmetric unit of the enzyme is a homodimer in nature.
Hydroxylase
Bacterial hydroxylase is one of the important components of the steroid catabolic pathway and plays an important role in steroid transformation. These enzymes help in steroid breakdown by opening the B-ring. Hydroxylation of ethinylestradiol by strain Selenastrum capricornutum, Scenedesmus quadricauda, and Ankistrodesmus braunii is shown in Fig. 3 (Greca et al. 2008). The most common hydroxylases involved in steroid transformation are Rieske-type non-heme iron oxygenase and Cytochrome P450.
Hydroxylation of Ethinylestradiol by strain Selenastrum capricornutum, Scenedesmus quadricauda and Ankistrodesmus braunii (modified from Greca et al. 2008)
Rieske-type non-heme iron oxygenase
These types of enzymes (3-ketosteroid 9α-hydroxylase (KSH)) are involved in the 9,10-seco pathway of testosterone biotransformation. This is the only known core aerobic pathway of steroid transformation by bacteria (Horinouchi et al. 2012). The opening of the steroid structure is initiated by the alpha-hydroxylation of C9 position. Microbial species such as Nocardia (Strijewskim 1982), Rhodococcus sp. (Knol et al. 2008), and Mycobacterium neoauram (Yao et al. 2014) express KSH enzymes. These KSH enzymes are mainly of two types, i.e., oxygenase (KshA) and flavin-dependent ferredoxin reductase (KshB), both of which are required for the KSH activity (Van der Geize et al. 2008; Petrusma et al. 2009). Studies have revealed that KSH works along-side 3-anthrone-Δ1-dehydrogenase (KstD) to form 9α-hydroxyandrost-1,4-diene-3,17-dione (9α-OH-ADD), where KSH is responsible for cleaving the CC bond at positions 9 and 10 or AD alone which produces 9α-hydroxyandrost-4-ene-3,17-dione (9α-OH-AD) (Yao et al. 2014).
Cytochrome P450
Cytochrome P450 (CYP) is a class of heme and thiol-rich proteins which is widely distributed among living organisms. On the bases of their involvement in electron transport during a catalytic reaction, cytochrome P450 can be categorized into four different categories: the first type is a FAD-containing reductase and iron-sulfur protein. This is the most abundant type of CYP and so far most of the bacterial enzymes found to belong to this class. The second type of enzymes requires P450 reductase containing FAD and FMN, which are electron transport systems found in microsomes. The third type of enzyme does not require any electron donor, whereas, in the fourth type of enzyme, electrons are acquired directly from NAD(P)H (Urlacher and Girhard 2012).
In prokaryotic organisms, cytochrome P450 is a soluble protein found in the cytosol whereas in eukaryotic organisms it is membrane-bound. Cytochrome P450 is a typical heme protein that catalyzes the hydroxylation of aromatic and aliphatic substrates (Bernhardt and Urlacher 2014). Monooxygenase cytochrome P450 was discovered for the first time to be involved in aerobic hydroxylation of steroid hormones in 1963 (Cooper et al. 1963), while the first bacterial cytochrome P450 was discovered in Bacillus megaterium in the mid-1970s (Berg et al. 1975). A limited number of bacteria cytochrome P450 involved in estrogen conversion have been discovered. The commonly found CYP450 are Bacillus megaterium (Berg et al. 1975; Kille et al. 2011; Schmitz et al. 2014; Jozwik et al. 2016), Nocardia farcinica IFM 10152 (Bracco et al. 2013), and Streptomyces griseus (Makino et al. 2014).
Structural studies of the cytochrome P450 families revealed that the primary structure has fewer similarities but the spatial structure of all P450 enzymes belonging to various families are highly conserved and have higher similarities with each other (Tsuchiya et al. 2005; Nelson 2005). Usually, a typical cytochrome P450 structure contains 13 different α-helices (A–L) and four β-sheet domains from the N-terminus to the C-terminus, which form an inverted triangle structure. Detailed studies of cytochrome P450 showed that its complex protease system requires electron transport chains in addition to the terminal oxygenases. The conserved three-dimensional structure of cytochrome P450 seems to have some connection with its catalytic activity for various organic pollutants (Szaleniec et al. 2018).
Ring cleavage dioxygenase
Biological transformation of aromatic compounds such as steroids is mostly initiated by the enzyme ring-hydroxylating dioxygenase, which converts them into diol intermediates compounds (Martin and Mohn 1999; Shindo et al. 2007). Further, ring cleavage dioxygenase enzyme could cleave the A ring/B ring of these diol intermediate compounds (Horinouchi et al. 2012; Chen et al. 2018). Various studies concluded that different microbial species followed different steroid metabolizing pathways, but these all metabolizing pathways produce some common intermediates such as protocatechuic acid, catechol (Guevara et al. 2019), gentisic acid, hydroquinone, propionyl-CoA (Liu et al. 2018a), acetyl-CoA (Xu et al. 2017), tyrosine (Li et al. 2012a; b), etc. The cyclic cleavage dioxygenase acts on these common intermediate metabolites and opens their benzene rings. Chen et al. (2017) described for the first time, the role of enzymes which actively participated in the ring cleavage of estrogens. The initial reaction of the estrogen catabolic pathway by strain KC8 was demonstrated. It was demonstrated that E1 transformation is initiated with the oxygenolytic degradation of the aromatic A ring through the 4-hydroxylation and the subsequent meta-cleavage reactions. It is well known that E2 is being transformed into E1 with the help of 17β-estradiol dehydrogenase enzyme, which is the product of OecA. Furthermore, this E1 was transformed into 4-hydroxyestrone in the presence of estrone 4-hydroxylase enzyme which is the product of OceB (Gene cluster I). In strain KC8, OecC was found up-regulated which indicates that OecC plays an important role in estrogen catabolism. This OecC belongs to the type I extradiol dioxygenase family, which uses ferrous ion to catalyze meta-cleavage of catechols and their analogs, while under abiotic conditions it leads to the production of pyridinestrone acid. The details of the enzymatic estrogen ring cleavage are shown in Fig. 4 (Chen et al. 2017). Also, aromatization of A-ring of testosterone can be carried out by a meta-cleavage enzyme (Horinouchi et al. 2012). Especially, tesB from Comamonas testosteroni TA441 is necessary for testosterone degradation (Horinouchi et al. 2001).
Over all compilation of estrogen degradation by various microbial enzymes. (References: ①, Badawi et al. (2001); ②, Kurisu et al. (2010); ③, Tsuchiya et al. (2005); ④, Ye et al. (2017); ⑤, Ye et al. (2019); ⑥, Chen et al. (2017); ⑦, Kisselev et al. (2005); ⑧, Chen et al. (2018); ⑨, Lee et al. (2003); ⑩, Li et al. (2012a), b); ⑪, Breton et al. 1996; ⑫, Moeller and Adamski (2006); ⑬, Lee and Liu (2002); ⑭, Ye et al. (unpublished work))
Summary of microbial enzymes involved in estrogen biotransformation
Estrogen biotransformation is a broad topic, with studies on estrogen-metabolizing enzymes mainly carried out in humans. Studies on bacterial estrogen-transforming enzymes were initially focused on dehydrogenation reactions (Ye et al. 2017; Ye et al. 2019). Chen et al. (2017) proposed a complete microbial degradation metabolic pathway of estradiol in Sphingomonas sp. strain KC8, by detecting intermediate components, genomic analysis, transcriptome analysis and other techniques to study the enzyme involved in various reactions. In strain KC8, 4,5-seco pathway, an aerobic estrogen catabolic pathway having three estrogen catabolic gene clusters, i.e., oecA; KC8_09390, oecB; KC8_16650, and oecC; KC8_05325 was described. These three gene clusters were responsible for the expression of enzymes 3β, 17β-hydroxysteroid dehydrogenase, estrone 4-hydroxylase, and 4-hydroxyestrone 4, 5-dioxygenase, respectively, which were directly involved in the meta-cleavage of estrogen A ring (Chen et al. 2017). Besides, Lee et al. (2007) also tried to detect the metabolic pathway of estradiol degradation in Stenotrophomonas maltophilia ZL1 by using genomic and proteomic analysis techniques and revealed the up-regulation of aromatic-amino-acid transaminase. Extensive studies on 17β-estradiol transforming enzymes (both microbial and human) has been carried out in previous couple of years which resulted the characterization or identification of several new intermediate compounds during these biotransformation reactions (Greca et al. 2008; Urlacher and Girhard 2012; Bracco et al. 2013; Bernhardt and Urlacher 2014; Ye et al. 2017; Ye et al. 2019). A combined diagrammatic representation of all intermediates formed during 17β-estradiol transformation by both human and microbial enzymes is compiled in Fig. 4.
Summary of microbial enzymes involved in testosterone biotransformation
Microbial biotransformation of testosterone has been one of the hot research topics for the last two decades, and a large number of research articles have been published on this topic. The most common microbial species that are capable of transforming testosterone are Steroidobacter denitrificans DSMZ18526 (Fahrbach et al. 2008; Chiang et al. 2010; Wang et al. 2014), Rhodococcus erythropolis SQ1 (Knol et al. 2008; Van der Geize et al. 2008), Nocardia farcinica IFM 10152 (Ishikawa et al. 2004; Bracco et al. 2013), and Comamonas testosteroni TA441 (Horinouchi et al. 2003; Horinouchi et al. 2012). The Comamonas testosteroni TA441 is a classical organism that has several enzymes that aerobically transform testosterone (Fig. 5). The production of androstenedione (AD) by 17β-hydroxysteroid dehydrogenase, followed by Δ1-dehydrogenase (Δ1-dehydrogenase, TesH), which is further converted to androst-1,4-diene-3,17-dione (ADD). The ADD is responsible for the introduction of hydroxyl group at C-9 through ORF17, which causes 9α-hydroxylation (the process causes the generation of a very unstable intermediate), at the same time the B-ring cleavages take place accompanied by aromatization of the A-ring. This process generates 3-hydroxy-9,10-diol-1,3,5(10)-triene-9,17-dione (3-hydroxy-9,10-seco-androsta-1,3,5(10)-triene-9,17-dione (3-HSA)) as a product. Furthermore, TesA1A2 enzyme complex hydroxylate C-4 cleaves the core ring and produces 4-dihydroxy-9,10-nonanediol-1,3,5(10)-triene-9,17-dione (3,4-dihydroxy-9,10-seco-androst-1,3,5(10)-triene-9,17-dione (3,4-DHSA)). Next, TesB Bio-oxygenase splits the A ring to 4,5-9,10-diseco-3-hydroxy-5,9,17-trioxoandorosta-1(10),2-dien-4-oic acid (4,9-DSHA). Finally, after multiple steps, the testosterone is mineralized into carbon dioxide and water. It shows that the TesB gene cluster includes 18 different types of androgen transforming genes (Horinouchi et al. 2003). This cluster is widely found in androgen transforming bacterial species such as Burkholderia, Comamonas, Cupriavidus, Glaciecol, Hydrocarboniphaga, Marinobacterium, Novosphingobium, Pseudoalteromonas, Pseudomonas, Shewanella, and Sphingomonas (Chen et al. 2016; Olivera et al. 2018). This method of testosterone metabolism by the aerobic bacterial system is known as the 9-10-seco pathway.
Over all degradation of androgen along with their intermediates by various microbial enzymes. (References: ①, Mindnich et al. (2004); ②, Ye et al. (2019); ③, Horinouchi et al. (2012); ④, Yoshimoto and Guengerich (2014); ⑤, Chen et al. (2012); ⑥, Yang et al. (2016); ⑦, Vanden et al. 1993); ⑧, Badawi et al. (2001); ⑨, Schmitz et al. (2014); ⑩, Van der Geize et al. (2008); ⑪, Venkataraman et al. (2015); ⑫, Bracco et al. (2013); ⑬, Wang et al. (2013); ⑭, Ye et al. (Unpublished work))
In addition to the aerobic 9-10-seco pathway, an anaerobic catabolic pathway was also observed in Steroidobacter denitrificans DSMZ18526. This pathway is different from the aerobic pathway. In this transformation route, under controlled conditions, testosterone was first converted into 1-dehydrotestosterone by the action of 3-ketosteroidΔ1-dehydrogenase. This first step is the divergent step between the aerobic and anaerobic pathways of testosterone transformation. Under anoxic conditions, the catabolic process starts from ring A. This bacterial species encodes the gene of the molybdenum protein of the xanthine oxidase family, AtacABC (anaerobic testosterone catabolism), which introduces a hydroxyl group at C-1 and oxidizes the C-1 hydroxyl group to form 17-hydroxy-androstane-1,3-dione. Phylogenetic analysis of this gene indicates that the enzyme belongs to the family of xanthine oxidases containing molybdenum, FAD, and iron clusters (Yang et al. 2016). Besides, studies revealed that anaerobic biotransformation of testosterone was also carried out by 2,3-seco pathway (Yang et al. 2016). For instance, CYP154C5 from N. farcinica could catalyze T into 16α-OH-T (Bracco et al. 2013) and DHEA could be catalyzed by CYP106A2 to form 7β-OH-DHEA (Schmitz et al. 2014). Interestingly, androgens could be converted to estrogens by the steroid aromatase, CYP19A1 (Yoshimoto and Guengerich 2014). Androgen metabolism by various microbial enzymes and their intermediate compounds (identified and unidentified) are shown in Fig. 5 (Talalay and Wang 1955; Nobel et al. 2001; Morris et al. 2003; Penning 2003; Horinouchi et al. 2003; Ishikawa et al. 2004; Fahrbach et al. 2008; Van der Geize et al. 2008; Chiang et al. 2010; Horinouchi et al. 2012; Bracco et al. 2013; Wang et al. 2014; Chen et al. 2016; Yang et al. 2016; Ye et al. 2017; Ye et al. 2019).
Genetic adaptation mediated steroid biotransformation
Microbial systems are designed by nature to be adaptable, as bacterial cells can adapt to different habitats, including contaminated and extreme environments and still be capable of performing various physiological activities (Dash et al. 2013). It has been observed that when some microbial strains are subjected to the high-stress environments (PAH and steroid pollution), they adapt to that environment and start growing by using the stress elements as energy and carbon source (Haritash and Kaushik 2009; Wang et al. 2018). This is termed adaptation, as pre-exposure to a high dose of contaminants increases the potential of the microbial community to metabolize or oxidize pollutants (Wang et al. 2018). Several studies have reported that adaptation increases the rate of organic pollutant transformation (Bergstrand et al. 2016). The genetic adaptation process that might lead to the induction and repression of enzymes, genetic changes, and selective enrichment has been defined as three mechanisms for adaptation of microbial communities to chemical contaminants (Leahy and Colwell 1990). The primary mechanism for detecting genetic adaptation capability of the microbial community is the identification and amplification of genes that are directly or indirectly involved in the metabolism of the organic contaminant by selective enrichment and gene transfer. The monitoring and identification of adaptation to steroid contamination are made possible by the generation of DNA probes specific for the genes encoding steroid catabolic pathways. Furthermore, the identification of genetic adaptation can be carried out by comparative genomic analysis (Bergstrand et al. 2016).
Current state of steroid biotransformation
The above discussion shows that steroid pollution is harmful to aquatic animals and aquatic ecosystem as these pollutants have potential effects on sexual development and, the reproduction process in these animals. As society develops, there is a corresponding increase in steroid pollution. Presently, several microbiological research groups all over the world are working on the mechanisms of steroid biotransformation.
In the last couple of years, a significant number of research papers describing the microbial transformation of estradiol, estriol, and 17α-ethinylestradiol have been published. Li et al. (2012a), b) reported the transformation of E2 into E1 by strain Stenotrophomonas maltophilia ZL1; proteomic data analysis revealed that strain S. maltophilia ZL1 can convert E1 to amino acid tyrosine through ring cleavage on a saturated ring of the E1 molecule and further use this tyrosine in protein biosynthesis. They also observed that the presence of the environmental tyrosine could affect the biotransformation pathway of E2/E1 by causing the feedback inhibition process. Besides, Yoneda et al. (2016) compared the 33rd and 40th passage generations of Rhodococcus opacus PD630 by using comparative genomics and transcriptomics methods and then identified an up-regulation in the expression of degradation genes, which helped to clarify the action of phenol transporter genes. The evolutionary relationship between these two strains was analyzed, with the comparative genomics data revealing that the use of toxic compounds as sole carbon source accelerated the accumulation of single nucleotide polymorphisms (SNPs), which further caused targeted mutations. Using various techniques to decipher information on estrogen-degrading genes and degradation products helps to understand the biodegradation of organic pollutants. Further, the strain Pseudomonas putida Pf5 was used for estradiol degradation and the expression of functional proteins was analyzed using iTRAQ (Du et al. 2017). The results revealed that various factors such as stress responses, energy metabolism, transport, chemotaxis, cell motility and changes in protein production by carbon metabolism, especially over-expression of carbon-metabolizing proteins lead to the activation of nucleotide metabolic pathways along with carbohydrate pathways, which are mainly associated with energy metabolism (Du et al. 2017). For further investigation, environmental estrogen biotransformation kinetics was analyzed by quantitative proteomics analysis of Hydrogenophaga atypica ZD1 under various nitrogen and carbon backgrounds. It was observed that branched-chain amino acid aminotransferase (IlvE) might play an important role in E1 biodegradation (Xu et al. 2017). Similarly, transcriptomics and lipidomics analysis of R. rubber strain, an efficient polyethylene degrader, showed that this strain can change its membrane composition to control the rate of polyethylene transformation (Gravouil et al. 2017). Chen et al. (2017) identified and validated three estrogen catabolic gene clusters in Sphingomonas sp. KC8 strain and also successfully demonstrated its natural estrogen-transforming activity by steroid 4,5-seco pathway. To investigate sterol biotransformation in wild-type Mycobacterium neoaurum ATCC 25795, Liu et al. (2018a) used integrated transcriptomics and proteomics to identify the key genes and key metabolic activities of mutant strains producing steroid intermediates. Our group also reported a novel 17β-hydroxysteroid dehydrogenase (17β-HSD) enzyme from Rhodococcus sp. P14, which is capable of transforming E2 into E1. This enzyme (17β-HSD) is for the first time, reported in bacterial species which can oxidize E2 into E1 (Ye et al. 2017). As revealed in most of the reports, microbial systems can transform estradiol, estriol, and 17α-ethinylestradiol into estrone. Estrone is less harmful than other steroids, due to its low concentration in nature. Most of the steroids are being transformed into E1, which ultimately raises E1 concentration in the environment. The higher concentration of E1 also has endocrine-disrupting effects on aquatic animals. Unfortunately, very few studies have reported on or explained E1 biodegradation. Most of the E1 degradation reports are associated with some physical degradation techniques. Recently, our group reported a new microbial strain that is capable of biotransforming E1, along with this transformation process, two new non-accumulating intermediate metabolites, i.e., 3-hydroxyandrosta-5,7,9(11)-trien-17-one and androsta-1,4,6-triene-3,17-dione (ATD) were also characterized (Pratush et al. 2019). We hope to intensify our research to identify and characterize more E1-transforming microbial systems that could be used to remediate E1 concentration in aquatic systems.
Conclusion and future perspective
In this review, we collected various information on the generation of steroids, their entry into the environment, and their effects on aquatic animals as well as on humans. In recent years, the scientific community is very much aware of steroid pollution. This review highlights the widespread contamination of the aquatic environment by different types of steroid estrogens. In the past few years, several steroid-transforming microorganisms have been isolated from soil, water, sediments, and marine water. These microorganisms are believed to transform steroids by metabolic and co-metabolic pathways, while the mechanisms involved are not clear, hence there is a need for further research on this. It has been also observed that the WWTPs are unable to degrade estrogens without biological treatment systems, for which reason these steroids find their way into water bodies. On the other hand, the WWTPs equipped with a biological treatment system also has some limitations, as the microbial systems have very low transforming capability; therefore, these systems need long SRT and HRT, which increase the load on these treatment setups. To overcome these problems, microbial systems with high and fast degrading/transforming capabilities, which are also environmentally friendly, would have to be engineered or generated. Research should also be focused on degradation/ transformation kinetics to improve overall steroid metabolism. Also, modern techniques such as multi-omics analysis, isotope labeling would have to be used to detect new products. Presently, a large number of oxic (aerobic) microbial steroid transforming systems have been isolated from different sources. The oxic steroid transformation found to be much more efficient than anoxic transformation. However, only very few anoxic microbial species are identified that could effectively transform steroid estrogens. Thus, for effective estrogen transformation in anoxic environments, new anoxic microbial species must be isolated. Furthermore, studies on anoxic metabolism of steroids by bacteria should address the following issues: (i) whether the cleavage of the core ring system of steroid compounds begins from the A-ring, (ii) whether the elimination of C17-alkyl side chain precedes cleavage of the core ring system in the anoxic cholesterol catabolic pathway, (iii) if a common pathway for steroid metabolism by denitrifying bacteria is potentially involved in both oxic and anoxic conditions, and (iv) the purification and characterization of novel steroid-transforming enzymes (Ismail and Chiang 2011). The researcher would also have to focus their work on finding facultative anaerobic microbial strains, which can transform steroids in both the presence and absence of oxygen. For both microbial transformations (aerobic and anaerobic), many challenges still exist in steroid biotransformation. The major challenge is the low biotransformation productivity of microbial strains, which is not economically viable to use for industrial applications. The other problems that need to be solved include low steroid solubility, insufficient substrate availability, and in some cases, the toxicity of the substrate/product to microbial cells. Apart from scientific efforts, strict regulations must be put in place to check steroid pollution.
References
Abalain JH, Di Stefano S, Abalain-Colloc ML, Floch HH (1995) Cloning, sequencing and expression of Pseudomonas testosteroni gene encoding 3α-hydroxysteroid dehydrogenase. J Steroid Biochem Mol Biol 55:233–238. https://doi.org/10.1016/0960-0760(95)00170-5
Acconcia F, Marino M (2018) Steroid hormones: synthesis, secretion, and transport. In: Belfiore A, LeRoith D (eds) Principles of endocrinology and hormone action, Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-44675-2_4
Adeel M, Song X, Francis D, Yang Y (2017) Environmental impact of estrogens on human, animal and plant life: a critical review. Environ Int 99:107–119. https://doi.org/10.1016/j.envint.2016.12.010
Agarwal PK, Agrawal S, Shrivastava R (2015) Modern molecular approaches for analyzing microbial diversity from mushroom compost ecosystem. Biotechnology 5:853–866. https://doi.org/10.1007/s13205-015-0289-2
Andersen H, Siegrist H, Halling-Sorensen B, Ternes TA (2003) Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37(18):4021–4026. https://doi.org/10.1021/es026192a
Andrushina VA, Druzhinina AV, Yaderets VV, Stitsenko TS, Voishvillo NE (2011) Hydroxylation of steroids by Curvularia lunata mycelium in the presence of methyl-β-cyclodextrine. Appl Biochem Micro 47(1):42–48. https://doi.org/10.1134/S0003683811010029
Arnon S, Dahan O, Elhanany S, Cohen K, Pankratov I, Gross A, Ronen Z, Baram S, Shore LS (2008) Transport of testosterone and estrogen from dairy-farm waste lagoons to groundwater. Environ Sci Technol 42:5521–5526. https://doi.org/10.1021/es800784m
Ascenzo GD, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, Samperi R (2003) Fate of natural estrogen congugates in municipal sewage transport and treatment facilities. Sci Total Environ 302:199–209. https://doi.org/10.1016/S0048-9697(02)00342-X
Badawi AF, Cavalieri EL, Rogan EG (2001) Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism 50(9):1001–1003. https://doi.org/10.1053/meta.2001.25592
Barbosa IR, Nogueira AJA, Soares AMVM (2008) Acute and chronic effects of testosterone and 4-hydroxyandrostenedione to the crustacean Daphnia magna. Ecotox Environ Saf 71:757–764. https://doi.org/10.1016/j.ecoenv.2008.02.020
Baronti C, Curini R, D’Ascenzo G, Corcia AD, Gentili A, Samperi R (2000) Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol 40:2181–2189
Ben WW, Zhu B, Yuan XJ, Zhang Y, Yang M, Qiang ZM (2017) Transformation and fate of natural estrogens and their conjugates in wastewater treatment plants: influence of operational parameters and removal pathways. Water Res 124:244–250. https://doi.org/10.1016/j.watres.2017.07.065
Ben WW, Zhu B, Yuan XJ, Zhang Y, Yang M, Qiang ZM (2018) Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: comparison of wastewater treatment processes. Water Res 130:38–46. https://doi.org/10.1016/j.watres.2017.11.057
Berg A, Carlstrom K, Gustafsson JA, Ingelman-Sundberg M (1975) Demonstration of a cytochrome P-450-dependent steroid 15β-hydroxylase in Bacillus megaterium. Biochem Biophys Res Commun 66:1414–1423. https://doi.org/10.1016/0006-291X(75)90517-3
Bergstrand LH, Cardenas E, Holert J, Van Hamme JD, Mohn WW (2016) Delineation of steroid-degrading microorganisms through comparative genomic analysis. mBio:7(2). https://doi.org/10.1128/mBio.00166-16
Bermudez DS, Gray LE, Wilson VS (2012) Modeling defined mixtures of environmental oestrogens found in domestic animal and sewage treatment effluents using an in vitro oestrogen-mediated transcriptional activation assay (T47D-KBluc). Int J Androl 35(3):397–406. https://doi.org/10.1111/j.1365-2605
Bernhardt R, Urlacher VB (2014) Cytochromes P450 as promising catalysts for biotechnological application: chances and limitations. Appl Microbiol Biotechnol 98:6185–6203. https://doi.org/10.1007/s00253-014-5767-7
Bila D, Montalvao AF, de A. Azevedo D, Dezotti M (2007) Estrogenic activity removal of 17β-estradiol by ozonation and identification of by-products. Chemosphere 69(5):736–746. https://doi.org/10.1016/j.chemosphere.2007.05.016
Biswas S, Shapiro C, Kranz W, Mader T, Shelton D, Snow D, Bartelt-Hunt S, Tarkalson D, Van Donk S, Zhang T (2013) Current knowledge on the environmental fate, potential impact, and management of growth-promoting steroids used in the US beef cattle industry. J Soil Water Con 68:325–336. https://doi.org/10.2489/jswc.68.4.325
Bracco P, Janssen DB, Schallmey A (2013) Selective steroid oxyfunctionalisation by CYP154C5, a bacterial cytochrome P450. Microb Cell Factories 12(1):95. https://doi.org/10.1186/1475-2859-12-95
Breton RD, Mazza HC, Fontecilla-Camps JC (1996) The structure of a complex of human 17beta-hydroxysteroid dehydrogenase with estradiol and NADP+ identifies two principal targets for the design of inhibitors. Structure 4(8):905–915. https://doi.org/10.1016/s0969-2126(96)00098-6
Cargouet M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levi Y (2004) Assessment of river contamination by estrogenic compounds in Paris area (France). Sci Total Environ 324:55–66. https://doi.org/10.1016/j.scitotenv.2003.10.035
Cassetta A, Budefeld T, Rizner TL, Kristan K, Stojan J, Lamba D (2005) Crystallization, X-ray diffraction analysis and phasing of 17beta-hydroxysteroid dehydrogenase from the fungus Cochliobolus lunatus. Acta Crystallogr Sect F Struct Biol Cryst Commun 61:1032–1034. https://doi.org/10.1107/S1744309105034949
Chen F, Ying GG, Kong LX, Wang L, Zhao JL, Zhou LJ, Zhang LJ (2011) Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ Poll 159:1490–1498. https://doi.org/10.1016/j.envpol.2011.03.016
Chen MM, Wang FQ, Lin LC, Yao K, Wei DZ (2012) Characterization and application of fusidane antibiotic biosynethsis enzyme 3-ketosteroid-1-dehydrogenase in steroid transformation. Appl Microbiol Biotechnol 96(1):133–142. https://doi.org/10.1007/s00253-011-3855-5
Chen YL, Wang CH, Yang FC, Ismail W, Wang PH, Shih CJ, Wu YC, Chiang YR (2016) Identification of Comamonas testosteroni as an androgen degrader in sewage. Sci Rep UK 6:35386. https://doi.org/10.1038/srep35386
Chen YL, Yu CP, Lee TH, Goh KS, Chu KH, Wang PH, Ismail W, Shih CJ, Chiang YR (2017) Biochemical mechanisms and catabolic enzymes involved in bacterial estrogen degradation pathways. Cell Chem Biol 24:1–13. https://doi.org/10.1016/j.chembiol.2017.05.012
Chen YL, Fu HY, Lee TH, Shih CJ, Huang L, Wang YS, Ismail W, Chiang YR (2018) Estrogen degraders and estrogen degradation pathway identified in an activated sludge. Appl Environ Microbiol 84(10). https://doi.org/10.1128/AEM.00001-18
Chiang YR, Fang JY, Ismail W, Wang PH (2010) Initial steps in anoxic testosterone degradation by Steroidobacter denitrificans. Microbiol 156:2253–2259. https://doi.org/10.1099/mic.0.037788-0
Chighizola C, Meroni PL (2012) The role of environmental estrogens and autoimmunity. Autoimmun Rev 11(6–7):A493–A501. https://doi.org/10.1016/j.autrev.2011.11.027
Clara M, Kreuzinger N, Strenn B, Gans O, Kroiss H (2005) The solids retention time—a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res 39:97–106. https://doi.org/10.1016/j.watres.2004.08.036
Clark DP, Dunlap PV, Madigan MT, Martinko JM (2009) Brock biology of microorganisms. Pearson, San Francisco, p 485
Conley JM, Evans N, Cardon MC, Rosenblum L, Iwanowicz LR, Hartig PC, Schenck KM, Bradley PM, Wilson VS (2017) Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ Sci Technol 51:4781–4791. https://doi.org/10.1021/acs.est.6b06515
Cooper DY, Estabrook RW, Rosenthal O (1963) The stoichiometry of C21 hydroxylation of steroids by adrenocortical microsomes. J Biol Chem 238:1320–1323
Cotillon AC, Morfin R (1990) Transformation of 3-hydroxy-steroids by Fusarium moniliforme 7α-hydroxylase. J Steroid Biochem & Mol Biol 68:229–237. https://doi.org/10.1016/S0960-0760(99)00035-7
Czajka CP, Londry KL (2006) Anaerobic biotransformation of estrogens. Sci Total Environ 367(2):932–941. https://doi.org/10.1016/j.scitotenv.2006.01.021
Dash HR, Mangwani N, Chakraborty J, Kumari S, Das S (2013) Marine bacteria: potential candidates for enhanced bioremediation. Appl Microbiol Biotechnol 97:561–571. https://doi.org/10.1007/s00253-012-4584-0
Davis KB, Morrison J, Galvez JI (2000) Reproductive characteristics of adult channel catfish treated with trenbolone acetate during the phenocritical period of sex differentiation. Aquaculture 189:351–360. https://doi.org/10.1016/S0044-8486(00)00378-1
Dermer J, Fuchs G (2012) Molybdoenzyme that catalyzes the anaerobic hydroxylation of a tertiary carbon atom in the side chain of cholesterol. J Biol Chem 287(44):36905–36916. https://doi.org/10.1074/jbc.M112.407304
Devier MH, Le Menach K, Viglino L, Di Gioia L, Lachassagne P, Budzinski H (2013) Ultra-trace analysis of hormones, pharmaceutical substances, alkylphenols and phthalates in two French natural mineral waters. Sci Total Environ 443:621–632. https://doi.org/10.1016/j.scitotenv.2012.10.015
Du Z, Chen Y, Li X (2017) Quantitative proteomic analyses of the microbial degradation of estrone under various background nitrogen and carbon conditions. Water Res 123:361–368. https://doi.org/10.1016/j.watres.2017.06.070
Edet UO, Antai SP, Brooks AA, Asitok AD, Enya O, Japhet FH (2017) An overview of cultural, molecular and metagenomic techniques in description of microbial diversity. J Adv Microbiol 7(2):1–19. https://doi.org/10.9734/JAMB/2017/37951
Esteban S, Gorga M, Petrovic M, Gonzalez-Alonso S, Barcelo D, Valcarcel Y (2014a) Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci Total Environ 466-467:939–951. https://doi.org/10.1016/j.scitotenv.2013.07.101
Esteban S, Gorga M, Gonzalez-Alonso S, Petrovic M, Barcelo D, Valcarcel Y (2014b) Monitoring endocrine disrupting compounds and estrogenic activity in tap water from Central Spain. Environ Sci Pollut Res 21:9297–9310. https://doi.org/10.1007/s11356-014-2847-2
Fahrbach M (2006) Anaerobic degradation of steroid hormones by novel denitrifying bacteria. Thesis 1–115
Fahrbach M, Kuever J, Remesch M, Huber BE, Kampfer P, Dott W, Hollender J (2008) Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int J Syst Evol Microbiol 58:2215–2223. https://doi.org/10.1099/ijs.0.65342-0
Falconer IR, Chapman HF, Moore MR, Ranmuthugala G (2006) Endocrine-disrupting compounds: a review of their challenge to sustainable and safe water supply and water reuse. Environ Toxicol 21:181–191. https://doi.org/10.1002/tox.20172
Fan Z, Hu J, An W, Yang M (2013) Detection and occurrence of chlorinated byproducts of bisphenol a, bonylphenol, and estrogens in drinking water of China: comparison to the parent compounds. Environ Sci Technol 47:10841–10850. https://doi.org/10.1021/es401504a
Felix-Canedo TE, Duran-Alvarez JC, Jimenez-Cisneros B (2013) The occurrence and dstribution of a group of organic micropollutants in Mexico City’s water sources. Sci Total Environ 454:109–118. https://doi.org/10.1016/j.scitotenv.2013.02.088
Feng Y, Yu Y, Fig X, Qu Y, Li D, He W, Kim B (2011) Degradation of raw corn stover powder (RCSP) by an enriched microbial consortium and its community structure. Bioresour Technol 102(2):742–747. https://doi.org/10.1016/j.biortech.2010.08.074
Fernand L, The VL, Lin SX, Labrie C, Simard J, Breton R, Brlanger A (1997) The key role of 17β -hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148–158. https://doi.org/10.1016/s0039-128x(96)00174-2
Fernandez MP, Ikonomou MG, Buchanan I (2007) An assessment of estrogenic organic contaminants in Canadian wastewaters. Sci Total Environ 373:250–269. https://doi.org/10.1016/j.scitotenv.2006.11.018
Fernandez L, Louvado A, Esteves VI, Gomes NCM, Almeida A, Cunha A (2017) Biodegradation of 17β-estradiol by bacteria isolated from deep sea sediments in aerobic and anaerobic media. J Hazard Mater 323:359–366. https://doi.org/10.1016/j.jhazmat.2016.05.029
Fernandez-Fontaina E, Carballa M, Omil F, Lemma JM (2014) Modelling cometabolic biotransformation of organic micropollutants in nitrifying reactors. Water Res 15(65):371–383. https://doi.org/10.1016/j.watres.2014.07.048
Fischer K, Majewsky M (2014) Cometabolic degradation of organic wastewater micropollutants by activated sludge and sludge-inherent microorganisms. Appl Microbiol Biotechnol 98(15):6583–6597. https://doi.org/10.1007/s00253-014-5826-0
Fu M, Deng B, Lu H, Yao W, Su S, Wang D (2019) The bioaccumulation and biodegradation of testosterone by Chlorella vulgaris. Int J Environ Res Public Health 16(7):1253. https://doi.org/10.3390/ijerph16071253
Fujii K, Satomi M, Morita N, Motomura T, Tanaka T, Kikuchi S (2003) Novosphingobium tardaugens sp. nov., an oestradiol-degrading bacterium isolated from activated sludge of a sewage treatment plant in Tokyo. Int J Syst Evol Micr 53:47–52. https://doi.org/10.1099/ijs.0.02301-0
Furuichi T, Kannan K, Giesy JP, Masunaga S (2004) Contribution of known endocrine disrupting substances to the estrogenic activity in Tama River water samples from Japan using instrumental analysis and in vitro reporter gene assay. Water Res 38:4491–4501. https://doi.org/10.1016/j.watres.2004.08.007
Gao J, Chi J (2015) Biodegradation of phthalate acid esters by different marine microalgal species. Mar Pollut Bull 99:70–75. https://doi.org/10.1016/j.marpolbul.2015.07.061
Garcia TC, Curtis TP, Mrozik WR, Davenport RJ (2019) Enhanced estrogen removal in activated sludge processes through the optimization of the hydraulic flow pattern. Water Res 164:114905. https://doi.org/10.1016/j.watres.2019.114905
Genti-Raimondi S, Tolmasky ME, Patrito LC, Flury A, Actis LA (1991) Molecular cloning and expression of the β-hydroxysteroid dehydrogenase gene from Pseudomonas testosteroni. Gene 105:43–49. https://doi.org/10.1016/0378-1119(91)90512-A
Gravouil K, Ferru-Clement R, Colas S, Helye R, Kadri L, Bourdeau L, Moumen B, Mercier A, Ferreira T (2017) Transcriptomics and lipidomics of the environmental strain Rhodococcus ruber point out consumption pathways and potential metabolic bottlenecks for polyethylene degradation. Environ Sci Technol 51(9):5172–5181. https://doi.org/10.1021/acs.est.7b00846
Greca MD, Pinto G, Pistillo P, Pollio A, Previtera L, Temusii F (2008) Biotransformation of ethinylestradiol by microalgae. Chemosphere. 70:2047–2053. https://doi.org/10.1016/j.chemosphere.2007.09.011
Groh H, Schad K, Horhold-Schube C (1993) Steroid metabolism with intestinal microorganisms. J Basic Microbiol 33(1):59–72. https://doi.org/10.1002/jobm.3620330115
Groning J, Held C, Garten C, Clasussnitzer U, Kaschabek SR, Schlomann M (2007) Transformation of diclofenac by the indigenous microflora of river sediments and identification of a major intermediate. Chemosphere 69(4):509–516. https://doi.org/10.1016/j.chemosphere.2007.03.037
Gu L, Huang B, Lai C, Xu Z, He H, Pan X (2018) The microbial transformation of 17β-estradiol in an anaerobic aqueous environment is mediated by changes in the biological properties of natural dissolved organic matter. Sci Total Environ 631–632:641–648. https://doi.org/10.1016/j.scitotenv.2018.03.056
Guevara G, Lopez MC, Alonso S, Perera J, Navarro-Llorens JM (2019) New insights into the genome of Rhodococcus ruber strain Chol-4. BMC Genomics 20(1):332. https://doi.org/10.1186/s12864-019-5677-2
Hamid H, Eskicioglu C (2012) The fate of estrogenic hormones in wastewater and sludge treatment: a review of properties and analytical detection techniques in sludge matrix. Water Res 46:5813–5833. https://doi.org/10.1016/j.watres.2012.08.002
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15. https://doi.org/10.1016/j.jhazmat.2009.03.137
Hartmann J, Beyer R, Harm S (2014) Effective removal of estrogens from drinking water and wastewater by adsorption technology. Environ Process 1:87–94. https://doi.org/10.1007/s40710-014-0005-y
Hashimoto T, Onda K, Morita T, Luxmy BS, Tada K, Miya A, Murakami T (2010) Contribution of the estrogen-degrading bacterium Novosphingobium sp. strain JEM-1 to estrogen removal in wastewater treatment. J Environ Eng 136(9):890–896. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000218
Heffron KT, Gaines KF, Novak JM, Canam T, Collard DA (2016) 17β-Estradiol influent and effluent concentrations in wastewater: demographic influences and the risk to environmental health. Environ Monit Assess 188:288. https://doi.org/10.1007/s10661-016-5292-5
Horinouchi M, Yamamoto T, Taguchi K, Arai H, Kudo T (2001) Meta-cleavage enzyme gene tesB is necessary for testosterone degradation in Comamonas testosteroni TA441. Microbiol 147(Pt 12):3367–3375. https://doi.org/10.1099/00221287-147-12-3367
Horinouchi M, Hayashi T, Yamamoto T, Kudo T (2003) A new bacterial steroid degradation gene cluster in Comamonas testosteroni TA441 which consists of aromatic-compound degradation genes for seco-steroids and 3-ketosteroid dehydrogenase genes. Appl Environ Microbiol 69:4421–4430. https://doi.org/10.1128/AEM.69.8.4421-4430.2003
Horinouchi M, Hayashi T, Kudo T (2012) Steroid degradation in Comamonas testosteroni. J Steroid Biochem Mol Biol 129:4–14. https://doi.org/10.1016/j.jsbmb.2010.10.008
Hunter AC, Elsom J, Ross L, Barrett R (2006) Ring-B functionalized androst-4-en-3-ones and ring-C substituted pregn-4-en-3-ones undergo a differential transformation in Aspergillus tamarii KITA: ring-A transformation with all C-6 substituted steroids and ring-D transformation with C-11 substituents. Biochim Biophys Acta 1761:360–366. https://doi.org/10.1016/j.bbalip.2006.02.011
Ishikawa J, Yamashita A, Mikami Y, Hoshino Y, Kurita H, Hotta K, Shiba T, Hattori M (2004) The complete genomic sequence of Nocardia farcinica IFM 10152. Proc Natl Acad Sci U S A 101:14925–14930. https://doi.org/10.1073/pnas.0406410101
Ismail W, Chiang YR (2011) Oxic and anoxic metabolism of steroids by bacteria. J Bioremed Biodegrad S1:001. https://doi.org/10.4172/2155-6199.S1-001
Jantanaprasartporn A, Maneerat S, Rongsayamanont C (2017) Importance of culture history on 17α-ethinylestradiol cometabolism by nitrifying sludge. Environ Eng Res 23(1):28–35. https://doi.org/10.4491/eer.2017.044
Jardim WF, Montagner CC, Pescara IC, Umbuzeiro GA, Bergamasco AMDD, Eldridge ML, Sodre FF (2012) An integrated approach to evaluate emerging contaminants in drinking water. Sep Purif Technol 84:3–8. https://doi.org/10.1016/j.seppur.2011.06.020
Jiang JQ, Zhou Z, Sharma VK (2013) Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in wastewater—a review of global views. Microchem J 110:292–300. https://doi.org/10.1016/j.microc.2013.04.014
Johnson DR, Helbling DE, Lee TK, Park J, Fenner K, Kohler Hans-Peter E, Ackermann M (2014) Association of biodiversity with the rates of micropollutant biotransformations among full-scale wastewater treatment plant communities. Appl Environ Microb 81(2):1–43. https://doi.org/10.1128/AEM.03286-14
Jozwik IK, Kiss FM, Gricman L, Abdulmughni A, Brill E, Zapp J, Pleiss J, Bernhardt R, Thunnissen AW (2016) Structural basis of steroid binding and oxidation by the cytochrome P450 CYP109E1 from Bacillus megaterium. FEBSJ 283(22):4128–4148. https://doi.org/10.1111/febs.13911
Jurgens MD, Holthaus KI, Johnson AC, Smith JJ, Hetheridge M, Williams RJ (2002) The potential for estradiol and ethinylestradiol degradation in English rivers. Environ Toxicol Chem 21:480–488
Kang IJ, Yokota H, Oshima Y, Tsuruda Y, Shimasaki Y, Honjo T (2008) The effects of methyltestosterone on the sexual development and reproduction of adult medaka (Oryzias latipes). Aquat Toxicol 87:37–46. https://doi.org/10.1016/j.aquatox.2008.01.010
Karnjanapiboonwong A, Suski JG, Shah AA, Cai Q, Morse AN, Anderson TA (2011) Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water Air Soil Poll 216:257–273. https://doi.org/10.1007/s11270-010-0532-8
Kayee P (2014) Degradation of EE2 by different consortium of enriched nitrifying activated sludge. Int J Environ Chem Ecol Geol Geophy Eng 8(1):37–42. https://doi.org/10.5281/zenodo.1336574
Ke J, Zhuang W, Ginm KY-H, Reinhard M, Hoon LT, Tay J-H (2007) Characterization of estrogen-degrading bacteria isolated from an artificial sandy aquifer with ultrafiltered secondary effluent as the medium. Appl Microbiol Biotechnol 75(5):1163–1171. https://doi.org/10.1007/s00253-007-0923-y
Kille S, Zilly FE, Aceved JP, Reetz MT (2011) Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nat Chem 3(9):738–743. https://doi.org/10.1038/nchem.1113
Kim MK, Zoh KD (2016) Occurrence and removals of micropollutants in water environment. Environ Eng Res 21(4):319–332. https://doi.org/10.4491/eer.2016.115
Kisselev P, Schunck WH, Roots I, Schwarz D (2005) Association of CYP1A1 polymorphisms with differential metabolic activation of 17β-estradiol and Estrone. Cancer Res 65(7):2972. https://doi.org/10.1158/0008-5472.CAN-04-3543
Knol J, Bodewits K, Hessels GI, Dijkhuizen L, Van der Geize R (2008) 3-keto-5alpha-steroid Delta(1)-dehydrogenase from Rhodococcus erythropolis SQ1 and its orthologue in Mycobacterium tuberculosis H37Rv are highly specific enzymes that function in cholesterol catabolism. Biochem J 410:339–346. https://doi.org/10.1042/BJ20071130
Kobayashi Y, Okuda T, Yamashita N, Tanaka H, Tanaka S, Fuji S (2006) The behavior of free/conjugated estrogens during advanced wastewater treatment. Environ Sanit Eng Res 20:55–58 (in Japanese with English Abstract)
Kolodziej EP, Gray JL, Sedlak DL (2009) Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ Toxicol Chem 22:2622–2629. https://doi.org/10.1897/03-42
Kolok AS, Snow DD, Kohno S, Sellin MK, Guillette LJ Jr (2007) Occurrence and biological effect of exogenous steroids in the Elkhorn river, Nebraska, USA. Sci Total Environ 388:104–115. https://doi.org/10.1016/j.scitotenv.2007.08.001
Komori K, Tanaka H, Okayasu Y, Yasojima M, Sato C (2004) Analysis and occurrence of estrogen in wastewater in Japan. Water Sci Technol 50:93–100. https://doi.org/10.2166/wst.2004.0314
Kreit J (2017) Microbial catabolism of sterols: focus on the enzymes that transform the sterol 3β-hydroxy-5-en into 3-keto-4-en. FEMS Microbiol Lett 364(3):fnx007. https://doi.org/10.1093/femsle/fnx007
Kristan K, Rizner TL (2012) Steroid-transforming enzymes in fungi. J Steroid Biochem Mol Biol 129:79–91. https://doi.org/10.1016/j.jsbmb.2011.08.012
Kumar V, Nakada N, Yasojima M, Yamashita N, Johnson AC, Tanaka H (2009) Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography-tandem mass spectrometry. Chemosphere 77(10):1440–1446. https://doi.org/10.1016/j.chemosphere.2009.08.052
Kumar AK, Reddy MV, Chandrasekhar K, Srikanth S, Mohan SV (2012) Endocrine disruptive estrogens role in electron transfer: bio-electrochemical remediation with microbial mediated electrogenesis. Bioresour Technol 104:547–556. https://doi.org/10.1016/j.biortech.2011.10.037
Kurisu F, Ogura M, Saitoh S, Yamazoe A, Yagi O (2010) Degradation of natural estrogen and identification of the metabolites produced by soil isolates of Rhodococcus sp. and Sphingomonas sp. J Biosci Bioeng 109(6):576–582. https://doi.org/10.1016/j.jbiosc.2009.11.006
Lai KM, Scrimshaw MD, Lester JN (2002) Biotransformation and bioconcentration of steroid estrogens by Chlorella vulgaris. Appl Environ Microb 68(2):859–864. https://doi.org/10.1128/AEM.68.2.859-864.2002
Lange IG, Daxenberger A, Schiffer B, Witters H, Ibarreta D, Meyer HHD (2002) Sex hormones originating from different livestock production systems: fate and potential disrupting activity in the environment. Anal Chim Acta 473:27–37. https://doi.org/10.1016/S0003-2670(02)00748-1
Lanisnik Rizner T, Stojan J, Adamski J (2001) Searching for the physiological function of 17β-hydroxysteroid dehydrogenase from the fungus Cochliobolus lunatus: studies of substrate specificity and expression analysis. Mol Cell Endocrinol 171(1):193–198. https://doi.org/10.1016/S0303-7207(00)00424-X
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. FEMS Microb Rev 54(3):305–315 0146-0749/90/030305-11$02.00/0
Lee HB, Liu D (2002) Degradation of 17β-estradiol and its metabolites by sewage bacteria. Water Air Soil Pollut 134:353–368. https://doi.org/10.1023/A:1014117329403
Lee AJ, Conney AH, Zhu BT (2003) Human cytochrome P450 3A7 has a distinct high catalytic activity for the 16α-hydroxylation of estrone but not 17β-estradiol. Cancer Res 63(19):6532
Lee YC, Wang LM, Xue YH, Ge NC, Yang XM, Chen GH (2007) Natural estrogens in the surface water of Shenzhen and the sewage discharge of Hong Kong. Hum Ecolog Risk Assess An Int J 12:301–312. https://doi.org/10.1080/10807030500533394
Li G, Zu L, Wong PK, Hui X, Lu Y, Xiong JT, An T (2012a) Biodegradation and detoxification of bisphenol A with one newly-isolated strain Bacillus sp. GZB: kinetics, mechanism and estrogenic transition. Bioresour Technol 114:224–230. https://doi.org/10.1016/j.biortech.2012.03.067
Li Z, Nandakumar R, Madayiputhiya N, Li X (2012b) Proteomic analysis of 17β-estradiol degradation by Stenotrophomonas maltophilia. Environ Sci Technol 46(11):5947–5955. https://doi.org/10.1021/es300273k
Li J, Fu J, Zhang H, Li Z, Ma Y, Wu M, Liu X (2013) Spatial and seasonal variations of occurrences and concentrations of endocrine disrupting chemicals in unconfined and confined aquifers recharged by reclaimed water: a field study along the Chaobai River, Beijing. Sci Total Environ 450-451:162–168. https://doi.org/10.1016/j.scitotenv.2013.01.089
Li M, Xu B, Liungai Z, Hu HY, Chen C, Qiao J, Lu Y (2016) The removal of estrogenic activity with UV/chlorine technology and identification of novel estrogenic disinfection by-products. J Hazard Mater 15(307):119–126. https://doi.org/10.1016/j.jhazmat.2016.01.003
Li S, Juan L, Sun M, Ling W, Zhu X (2017) Isolation, characterization, and degradation performance of the 17β-estradiol-degrading bacterium Novosphingobium sp. E2S. Inter J Envi Res Public Health 14(115):1–13. https://doi.org/10.3390/ijerph14020115
Liu ZH, Ito M, Kanjo Y, Yamamoto A (2009a) Profile and removal of endocrine disrupting chemicals (EDCs) in two municipal wastewater treatment plants by using an ER(AR) competitive ligand binding assay and chemical analyses. J Environ Sci. https://doi.org/10.1016/S1001-0742(08)62356-6
Liu Z-H, Kanjo Y, Mizutani S (2009b) Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: a review. Sci Total Environ 407:4975–4985. https://doi.org/10.1016/j.scitotenv.2009.06.001
Liu ZH, Lu GN, Yin H, Dang Z, Rittmann B (2015) Removal of natural estrogens and their conjugates in municipal wastewater treatment plants: a critical review. Environ Sci Technol 49(9):5288–5300. https://doi.org/10.1021/acs.est.5b00399
Liu J, Liu J, Xu D, Ling W, Li S, Chen M (2016) Isolation, immobilization, and degradation performance of the 17β-estradiol-degrading bacterium Rhodococcus sp. JX-2. Water Air Soil Pollut 227(422):1–13. https://doi.org/10.1007/s11270-016-3122-6
Liu M, Xiong LB, Tao X, Liu QH, Wang FQ, Wei DZ (2018a) Integrated transcriptome and proteome studies reveal the underlying mechanisms for sterol catabolism and steroid production in Mycobacterium neoaurum. J Agric Food Chem 66(34):9147–9157. https://doi.org/10.1021/acs.jafc.8b02714
Liu W, Chen Q, He N, Sun K, Sun D, Wu X, Duan S (2018b) Removal and biodegradation of 17β-estradiol and diethylstilbestrol by the freshwater microalgae Raphidocelis subcapitata. Inter J Envi Res Public Health 15(3):E452. https://doi.org/10.3390/ijerph15030452
Lorenzen A, Hendel JG, Conn KL, Bittman S, Kwabiah AB, Lazarovitz G, Masse D, McAllister TA, Topp E (2004) Survey of hormone activities in municipal biosolids and animal manures. Environ Toxicol 19:216–225. https://doi.org/10.1002/tox.20014
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641. https://doi.org/10.1016/j.scitotenv.2013.12.065
Maeng SK, Choi BG, Lee KT, Song KG (2013) Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res 47:3151–3162. https://doi.org/10.1016/j.watres.2013.03.014
Maggioni S, Balaguer P, Chiozzotto C, Benfenati E (2013) Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ Sci Pollut Res 20:1649–1660. https://doi.org/10.1007/s11356-012-1075-x
Makino T, Katsuyama Y, Otomatsu T, Misawa N, Ohnishi Y (2014) Regio- and stereospecific hydroxylation of various steroids at the 16alpha position of the D ring by the Streptomyces griseus cytochrome P450 CYP154C3. Appl Environ Microbiol 80(4):1371–3179. https://doi.org/10.1128/AEM.03504-13
Martin VJ, Mohn WW (1999) A novel aromatic-ring-hydroxylating dioxygenase from the diterpenoid-degrading bacterium Pseudomonas abietaniphila BKME-9. J Bacteriol 181(9):2675–2682
Mascoti ML, Palazzolo MA, Bisogno FR, Kurina-Sanz M (2016) Biotransformation of dehydro-epi-androsterone by Aspergillus parasiticus: metabolic evidences of BVMO activity. Steroids 109:44–49. https://doi.org/10.1016/j.steroids.2016.03.018
Maser E, Mobus E, Xiong G (2000) Functional expression, purification, and characterization of 3alpha-hydroxysteroid dehydrogenase/carbonyl reductase from Comamonas testosterone. Biochem Biophys Res Comm 272:622–628. https://doi.org/10.1006/bbrc.2000.2813
McKinlay R, Plant JA, Bell JN, Voulvoulis N (2008) Calculating human exposure to endocrine disrupting pesticides via agricultural and non-agricultural exposure routes. Sci Total Environ 398(1–3):1–12. https://doi.org/10.1016/j.scitotenv.2008.02.056
Miller WL, Auchus RJ (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32(1):81–151. https://doi.org/10.1210/er.2010-0013
Mindnich R, Moller G, Adamski J (2004) The role of 17 beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 218(1–2):7–20. https://doi.org/10.1016/j.mce.2003.12.006
Moeller G, Adamski J (2006) Multi functionality of human 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 248(1):47–55. https://doi.org/10.1016/j.mce.2005.11.031
Montagner CC, Sodre FF, Acayada RD, Vidal C, Campestrini I, Locatelli MA, Pescara IC, Albuquerque AF, Umbuzeiro GA, Jardim WF (2019) Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from São Paulo state, Brazil. J Braz Chem Soc 30(3). https://doi.org/10.21577/0103-5053.20180232
Morris DJ, Brem AS, Ge R, Jellinck PH, Sakai RR, Hardy MP (2003) The functional roles of 11 beta-HSD1: vascular tissue, testis and brain. Mol Cell Endocrinol 203(1–2):1–12. https://doi.org/10.1016/S0303-7207(03)00094-7
Muller M, Rabenoelina F, Balaguer P, Patureau D, Lemenach K, Budzinski H, Barcelo D, Lopez de Alda M, Kuster M, Delgenes JP, Hernandez-Raquet G (2008) Chemical and biological analysis of endocrine-disrupting hormones and estrogenic activity in an advanced sewage treatment plant. Environ Toxicoln Chem 27(8):1649–1658. https://doi.org/10.1897/07-519
Naldi AC, Fayad PB, Prevost M, Sauve S (2016) Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry. Chem Cent J 10(30):1–17. https://doi.org/10.1186/s13065-016-0174-z
Nelson DR (2005) Cytochrome P450: structure, mechanism, and biochemistry, 3rd edn. Edited by Paul R. Ortiz de Montellano (University of California, San Francisco). Kluwer Academic/Plenum Publishers: New York. ISBN 0-306-48324-6. J American Chem Soc 127:12147–12148
Nie M, Yan C, Dong W, Liu M, Zhou J, Yang Y (2015) Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary. Chemosphere 127:09–116. https://doi.org/10.1016/j.chemosphere.2015.01.021
Nobel S, Abrahmsen L, Oppermann U (2001) Metabolic conversion as a pre-receptor control mechanism for lipophilic hormones. Eur J Biochem 268:4113–4125. https://doi.org/10.1046/j.1432-1327.2001.02359.x
Okeke BC, Lu J (2011) Characterization of a defined cellulolytic and xylanolytic bacterial consortium for bioprocessing of cellulose and hemicelluloses. Appl Biochem Biotechnol 163:869–881. https://doi.org/10.1007/s12010-010-9091-0
Olivera ER, Luengo JM (2019) Steroids as environmental compounds recalcitrant to degradation: genetic mechanisms of bacterial biodegradation pathways. Genes 10:512. https://doi.org/10.3390/genes10070512
Olivera ER, de la Torre M, Barrientos A, Luengo MJ (2018) Steroid catabolism in bacteria: genetic and functional analyses of stdH and stdJ in Pseudomonas putida DOC21. Can J Biotech 2(1):88–99. https://doi.org/10.24870/cjb.2018-000119
Oppermann UCT, Netter KJ, Maser E (1993) Carbonyl reduction by 3α-HSD from Comamonas Testosteroni-new properties and its relationship to the SCAD family. In: Weiner H, Crabb DW, Flynn TG (eds) Enzymology and molecular biology of carbonyl metabolism 4. Springer US, Boston, MA, pp 379–390
Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, Gray LE, Soto AM, Guillette LJ (2004) Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ Health Persp 112:353–358. https://doi.org/10.1289/ehp.6591
Orn S, Svenson A, Viktor T, Holbech H, Norrgren L (2006) Male-biased sex ratios and vitellogenin induction in zebrafish exposed to effluent water from a Swedish pulp mill. Arch Environ Contam Toxicol 51:445–451. https://doi.org/10.1007/s00244-005-0199-0
Patrolecco L, Capri S, Ademollo N (2014) Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ Sci Pol Res. https://doi.org/10.1007/s11356-014-3765-z
Peng FQ, Ying GG, Yang B, Liu S, Lai HJ, Liu YS, Chen ZF, Zhou GJ (2014) Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): transformation kinetics and products identification. Chemosphere 95:581–588. https://doi.org/10.1016/j.chemosphere.2013.10.013
Penning TM (2003) Hydroxysteroid dehydrogenases and pre-receptor regulation of steroid hormone action. Hum Reprod Update 9:193–205. https://doi.org/10.1093/humupd/dmg022
Petrusma M, Dijkhuizen L, Van der Geize R (2009) Rhodococcus rhodochrous DSM 43269 3-ketosteroid 9alpha-hydroxylase, a two-component iron-sulfur-containing monooxygenase with subtle steroid substrate specificity. App Environ Microbiol 75:5300–5307. https://doi.org/10.1128/AEM.00066-09
Pinto PIS, Estevao MD, Power DM (2014) Effects of estrogens and estrog enic disrupting compounds on fish mineralized tissues. Marine Drugs 12:4474–4494. https://doi.org/10.3390/md12084474
Pratush A, Yang Q, Peng T, Huang TW, Hu Z (2019) Identification of non-accumulating intermediate compounds during estrone (E1) metabolism by a newly isolated microbial strain BH2-1 from mangrove sediments of the South China Sea. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06894-1
Praveena SM, Lui TS, Hamin NA, Razak SQNA, Aris AZ (2016) Occurrence of selected estrogenic compounds and estrogenic activity in surface water and sediment of Langat River (Malaysia). Environ Monit Assess 188:442. https://doi.org/10.1007/s10661-016-5438-5
Racz L, Goel RK (2010) Fate and removal of estrogens in municipal wastewater. J Enviorn Monit 12(1):58–70. https://doi.org/10.1039/b917298j
Reddy S, Iden CR, Brownawell B (2005) Analysis of steroid conjuages in sewage influent and effluent by liquid chromatography-tandem mass spectrometry. Anal Chem 77:7032–7038. https://doi.org/10.1021/ac050699x
Reese PB (2007) Biotransformation of terpenes and steroids by fungi. Nat Prod:71–76. https://doi.org/10.1142/9789812707444_0006
Rizner TL, Zakelj-Mavric M, Plemenitas A, Zorko M (1996) Purification and characterization of 17β-hydroxysteroid dehydrogenase from the filamentous fungus Cochliobolus lunatus. J Steroid Biochem Mol Biol 59(2):205–214. https://doi.org/10.1016/S0960-0760(96)00098-2
Robert FC, Suri RPS, Fu HX (2007) Free synthetic and natural estrogen hormones in influent and effluent of three municipal wastewater treatment plants. Water Environ Res 79:969–974. https://doi.org/10.2175/106143007X175843
Samavat H, Kurzer MS (2015) Estrogen metabolism and breast cancer. Cancer Lett 356:231–243. https://doi.org/10.1016/j.canlet.2014.04.018
Sami N, Fatima T (2019) Studies on estrone biodegradation potential of cyanobacterial species. Biocatal Agric Biotechnol 17:576–582. https://doi.org/10.1016/j.bcab.2019.01.022
Samir KL, Xie B, Thompson ML, Sung S, Ong SK, Leeuwen JHV (2006) Fate, transport, and biodegradation of natural estrogens in the enviornment and engineered systems. Enviorn Sci Technol 40(21):6537–6546. https://doi.org/10.1021/es0607739
Sang Y, Xiong G, Maser E (2012) Identification of a new steroid degrading bacterial strain H5 from the Baltic Sea and isolation of two estradiol inducible genes. J Steroid Biochem Mol Biol 129:22–30. https://doi.org/10.1016/j.jsbmb.2011.01.018
Schmitz DJ, Zapp J, Bernhardt R (2014) Steroid conversion with CYP106A2 production of pharmaceutically interesting DHEA metabolites. Microb Cell Factories 13:81. https://doi.org/10.1186/1475-2859-13-81
Seki M, Yokota H, Matsubara H, Maeda M, Tadokoro H, Kobayashi K (2004) Fish full life-cycle testing for androgen methyltestosterone on medaka (Oryzias latipes). Environ Toxicol Chem 23:774–781. https://doi.org/10.1897/03-26
Servos MR, Bennie DT, Burnison BK, Jurkovie A, McInnis R, Neheli T, Schnell A, Seto P, Smyth SA, Ternes TA (2005) Distribution of estrogens,17β-estradiol and estrone, in Canadian municipal wastewater treatment plants. Sci Total Environ 336:155–170. https://doi.org/10.1016/j.scitotenv.2004.05.025
Shao M, Sha Z, Zhang X, Zao Z, Xu M, Yang T, Xu Z, Yang S (2016) Efficient androst-1,4-diene-3,17-dione production by co-expressing 3-ketosteroid-D 1-dehydrogenase and catalase in Bacillus subtilis. J Appl Microbiol 122:119–128. https://doi.org/10.1111/jam.13336
Shi W, Wang L, Rousseau DP, Lens PN (2010) Removal of estrone, 17α-ethinylestradiol, and 17β-estradiol in algae and duckweed-based wastewater treatment systems. Environ. Sci Pollut Res Int 17:824–833. https://doi.org/10.1007/s11356-010-0301-7
Shindo K, Osawa A, Kasai Y, Lba N, Saotome A, Misawa N (2007) Hydroxylations of substituted naphthalenes by Escherichia coli expressing aromatic dihydroxylating dioxygenase genes from polycyclic aromatic hydrocarbon-utilizing marine bacteria. J Mol Catal B Enzym 48:77–83. https://doi.org/10.1016/j.molcatb.2007.06.007
Silva CP, Otero M, Esteves V (2012) Processes for the elimination of estrogenic steroid hormones from water: a review. Environ Pollut 165:38–58. https://doi.org/10.1016/j.envpol.2012.02.002
Simard AJ, Sanchez R, Durocher F, Rheaume E, Turgeon C, Labrie Y, Luu-The V, Mebarki F, Morel Y, de Launoit Y, Labrie F (1995) Structure-function relationships and molecular genetics of the 3β-hydroxysteroid dehydrogenase gene family. J Steroid Biochem Mol Biol 55:489–505. https://doi.org/10.1016/0960-0760(95)00198-0
Sodre FF, Pescara IC, Montagner CC, Jardim WF (2010) Assessing selected estrogens and xenoestrogens in Brazilian surface waters by liquid chromatography–tandem mass spectrometry. Microchem J 96:92–98. https://doi.org/10.1016/j.microc.2010.02.012
Strijewskim A (1982) The steroid-9 alpha-hydroxylation system from Nocardia species. Eur J Biochem 128:125–135. https://doi.org/10.1111/j.1432-1033.1982.tb06942.x
Sueka M, Tanabe K, Ohiwa T, Komori K, Suzuki Y, Tanaka H (2005) Determination of conjugated-estrogens in wastewater by LC-MS/MS. Environ Eng Res 42:265–275 (in Japanese with English Abstract)
Sumpter JP, Jobling S (2013) The occurrence, causes, and consequences of estrogens in the aquatic environment. Environ Toxicol Chem 32:249–251. https://doi.org/10.1002/etc.2084
Szaleniec M, Wojtkiewicz AM, Bernhardt R, Borowski T, Donova M (2018) Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms. App Microbiol Biotechnol 102(19):8153–8171. https://doi.org/10.1007/s00253-018-9239-3
Talalay P, Wang VS (1955) Enzymic isomerization of delta5-3-ketosteroids. Biochim Biophys Acta 18(2):300–301
Ternes TA, Kreckel P, Mueller J (1999) Behaviour and occurrence of estrogens in municipal sewage treatment plants--II. Aerobic batch experiments with activated sludge. Sci Total Environ 225:91–99. https://doi.org/10.1016/s0048-9697(98)00335-0
Ting YF, Praveena SM (2017) Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: a mini review. Environ Monit Assess 189(178):1–19. https://doi.org/10.1007/s10661-017-5890-x
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotox Environ Safe 71(1):1–15. https://doi.org/10.1016/j.ecoenv.2008.05.009
Tran NH, Urase T, Ngo HH, Hu J, Ong SL (2013) Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour Technol 146:721–731. https://doi.org/10.1016/j.biortech.2013.07.083
Trevino LS, Wang Q, Walker CL (2015) Hypothesis: activation of rapid signaling by environmental estrogens and epigenetic reprogramming in breast cancer. Reprod Toxicol 54:136–140. https://doi.org/10.1016/j.reprotox.2014.12.014
Tsuchiya Y, Nakajima M, Yokoi T (2005) Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227(2):115–124. https://doi.org/10.1016/j.canlet.2004.10.007
Urlacher VB, Girhard M (2012) Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol 30:26–36. https://doi.org/10.1016/j.tibtech.2011.06.012
Valdes ME, Marino DJ, Wunderlin DA, Somoza GM, Ronco AE, Carriquiribode P (2015) Screening concentration of E1, E2 and EE2 in sewage effluentsand surface waters of the “Pampas” region and the “Río de la Plata” Estuary (Argentina). Bull Environ Contam Toxicol 94:29–33. https://doi.org/10.1007/s00128-014-1417-0
Van der Geize R, Hessels GI, Nienhuis-Kuiper M, Dijkhuizen L (2008) Characterization of a second Rhodococcus erythropolis SQ1 3-ketosteroid 9alpha-hydroxylase activity comprising a terminal oxygenase homologue, KshA2, active with oxygenase-reductase component KshB. Appl Environ Microbiol 74:7197–7203. https://doi.org/10.1128/AEM.00888-08
Vanden B, Marichal HP, Jeune LL, Coene MC, Gorrens J, Cools W (1993) Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother 37(10):2101–2105. https://doi.org/10.1128/aac.37.10.2101
Venkataraman H, Te Poele EM, Rosloniec KZ, Vermeulen N, Commandeur JN, Van der Geize R, Dijkhuizen L (2015) Biosynthesis of a steroid metabolite by an engineered Rhodococcus erythropolis strain expressing a mutant cytochrome P450 BM3 enzyme. Appl Microbiol Biotechnol 99:4713–4721. https://doi.org/10.1007/s00253-014-6281-7
Vethaak AD, Lahr J, Schrap SM, Belfroid AC, Rijs GBJ, Gerritsen A, Boer J, Bulder AS, Grinwis GCM, Kuiper RV, Legler J, Murk TAJ, Peijnenburg W, Verhaar HJM, Voogt P (2005) An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere 59:511–524. https://doi.org/10.1016/j.chemosphere.2004.12.053
Vieno NM, Tuhkanen T, Kronberg L (2005) Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ Sci Technol 39(21):8220–8226. https://doi.org/10.1021/es051124k
Volodymr I, Lim JJW, Stabnikova O, Gin KYH (2010) Biodegradation of estrogens by facultative anaerobic iron-reducing bacteria. Process Biochem 45:284–287. https://doi.org/10.1016/j.procbio.2009.09.017
Vulliet E, Wiest L, Baudot R, Grenier-Loustalot MF (2008) Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J Chromat A 1210:84–91. https://doi.org/10.1016/j.chroma.2008.09.034
Wang C, Shi H, Adams CD, Gamagedara S, Stayton I, Timmons T, Ma Y (2011) Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water Res 45(4):1818–1828. https://doi.org/10.1016/j.watres.2010.11.043
Wang PH, Leu YL, Ismail W, Tang SL, Tsai CY, Chen HJ, Kao AT, Chiang YR (2013) Anaerobic and aerobic cleavage of the steroid core ring structure by Steroidobacter denitrificans. J Lipid Res 54(5):1493–1504. https://doi.org/10.1194/jlr.M034223
Wang PH, Yu CP, Lee TH, Lin CW, Ismail W, Wey SP, Kuo AT, Chiang YR (2014) Anoxic androgen degradation by the denitrifying bacterium Sterolibacterium denitrificans via the 2,3-seco pathway. Appl Environ Microbiol 80(11):3442–3452. https://doi.org/10.1128/AEM.03880-13
Wang W, Ge F, Ma C, Li J, Ren Y, Li W, Fu J (2017) Heterologous expression and characterization of a 3-ketosteroid- D 1-dehydrogenase from Gordonia neofelifaecis and its utilization in the bioconversion of androst-4,9(11)-dien-3,17-dione. 3 Biotech 7(19):3–9. https://doi.org/10.1007/s13205-017-0601-4
Wang H, Zhang S, Pratush A, Ye X, Xie J, Wei H, Sun C, Hu Z (2018) Acclimation of Culturable bacterial communities under the stresses of different organic compounds. Front Microbiol 9:225. https://doi.org/10.3389/fmicb.2018.00225
Williams RJ, Johnson AC, Smith JJL, Kanda R (2003) Steroid estrogens profiles along river stretches arising from sewage treatment works discharges. Environ Sci Technol 37(9):1744–1750. https://doi.org/10.1021/es0202107
Wilson MR, Gallimore WA, Reese PB (1999) Steroid transformations with Fusarium oxysporum var. cubense and Colletotrichum musae. Steroids 64(12):834–843. https://doi.org/10.1016/S0039-128X(99)00067-7
Wipperman MF, Sampson NS, Thomas ST (2014) Pathogen roid rage: cholesterol utilization by Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol 49:269–293. https://doi.org/10.3109/10409238.2014.895700
Woclawek-Potocka I, Mannelli CD, Boruszewska I, Kowalczyk-Zieba T, Wasniewski T, Skarzynski DJ (2013) Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol:1–15. https://doi.org/10.1155/2013/650984
Xu N, Xu YF, Xu S, Li J, Tao HC (2012) Removal of estrogens in municipal wastewater treatment plants: a Chinese perspective. Environ Pollut 165:215–224. https://doi.org/10.1016/j.envpol.2011.12.025
Xu W, Yan W, Huang W, Miao L, Zhong L (2014) Endocrine-disrupting chemicals in the Pearl River Delta and coastal environment: sources, transfer, and implications. Environ Geochem Health 36:1095–1104. https://doi.org/10.1007/s10653-014-9618-3
Xu J, Zhang L, Hou J, Wang X, Liu H, Zheng D, Liang R (2017) iTRAQ-based quantitative proteomic analysis of the global response to 17β-estradiol in estrogen-degradation strain Pseudomonas putida SJTE-1. Sci Rep 7(41682):1–14. https://doi.org/10.1038/srep41682
Yang YY, Borch T, Young RB, Goodridge LD, Davis JG (2010) Degradation kinetics of testosterone by manure-borne Bacteria: influence of temperature, pH, glucose amendments, and dissolved oxygen. J Environ Qual 39(4):1153–1160. https://doi.org/10.2134/jeq2009.0112
Yang G, Fan M, Zhang G (2014a) Emerging contaminants in surface waters in China—a short review. Environ Res Lett 9(7):4018. https://doi.org/10.1088/1748-9326/9/7/074018
Yang J, Li H, Ran Y, Chan K (2014b) Distribution and bioconcentration of endocrine disrupting chemicals in surface water and fish bile of the Pearl River Delta, South China. Chemosphere 107:439–446. https://doi.org/10.1016/j.chemosphere.2014.01.048
Yang FC, Chen YL, Tang SL, Yu CP, Wang PH, Ismail W, Wang CH, Ding JY, Yang CY, Yang CY, Chiang YR (2016) Integrated multi-omics analyses reveal the biochemical mechanisms and phylogenetic relevance of anaerobic androgen biodegradation in the environment. ISME J 10(8):1967–1983. https://doi.org/10.1038/ismej.2015.255
Yao K, Xu LQ, Wang FQ, Wei DZ (2014) Characterization and engineering of 3-ketosteroid- big up tri, open1-dehydrogenase and 3-ketosteroid-9alpha-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9alpha-hydroxy-4-androstene-3,17-dione through the catabolism of sterols. Metab Eng 24:181–191. https://doi.org/10.1016/j.ymben.2014.05.005
Ye X, Wang H, Kan J, Li J, Huang T, Xiong G, Hu Z (2017) A novel 17beta-hydroxysteroid dehydrogenase in Rhodococcus sp. P14 for transforming 17beta-estradiol to estrone. Chem Biol Interact 276:105–112. https://doi.org/10.1016/j.cbi.2017.06.010
Ye X, Peng T, Feng J, Yang Q, Pratush A, Xiong G, Huang TW, Hu Z (2019) A novel dehydrogenase 17β-HSDx from Rhodococcus sp. P14 with potential application in bioremediation of steroids contaminated environment. J Hazar Mat 362:170–177. https://doi.org/10.1016/j.jhazmat.2018.09.023
Ying GG, Kookana RS, Ru YJ (2002) Occurrence and fate of hormone steroids in the environment. Environ Int 28:545–551. https://doi.org/10.1016/S0160-4120(02)00075-2
Ying GG, Kookana RS, Kumar A, Mortimer M (2009) Occurrence and implications of estrogens and xenoestrogens in sewage effluents and receiving waters from south East Queensland. Sci Total Environ 407:5147–5155. https://doi.org/10.1016/j.scitotenv.2009.06.002
Yoneda A, Henson WR, Goldner NK, Park KJ, Forsberg KJ, Kim SJ, Pesesky MW, Foston M, Dantas G, Moon TS (2016) Comparative transcriptomics elucidates adaptive phenol tolerance and utilization in lipid-accumulating Rhodococcus opacus PD630. Nucleic Acids Res 44(5):2240–2254. https://doi.org/10.1093/nar/gkw055
Yoshimoto FK, Guengerich FP (2014) Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. J Am Chem Soc 136(42):15016–15025. https://doi.org/10.1021/ja508185d
Yu CP, Ahuja R, Sayler G, Chu KH (2005) Quantitative molecular assay for fingerprinting microbial communities of wastewater and estrogen degrading consortia. Appl Environ Microbiol 71(3):1433–1444. https://doi.org/10.1128/AEM.71.3.1433-1444.2005
Yu CP, Deeb RA, Chu KH (2013) Microbial degradation of steroidal estrogens. Chemosphere 91:1225–1235. https://doi.org/10.1016/j.chemosphere.2013.01.112
Yu Y, Liu C, Wang B, Li Y, Zhnag H (2015) Characterization of 3,17β-hydroxysteroid dehydrogenase in Comamonas testosteroni. Chem Biol Interact 234:221–228. https://doi.org/10.1016/j.cbi.2015.01.005
Yu Q, Wang P, Liu D, Gao R, Shao H, Zhao H, Ma Z, Wang D, Huo H (2016) Degradation characteristics and metabolic pathway of 17β-estradiol (E2) by Rhodococcus sp. DS201. Biotechnol Bioprocess Eng 21:804–813. https://doi.org/10.1007/s12257-016-0283-5
Yu W, Du B, Yang L, Zhang Z, Yang C, Yuan S, Zhang M (2019) Occurrence, sorption, and transformation of free and conjugated natural steroid estrogens in the environment. Environ Sci Pollut Res 26:9443–9468. https://doi.org/10.1007/s11356-019-04402-z
Zanga K, Kurisu F, Kasuga I, Furumai H, Yagie O (2008) Analysis of the phylogenetic diversity of estrone-degrading bacteria in activated sewage sludge using microautoradiography–fluorescence in situ hybridization. Sys Appl Microbiol 31:206–214. https://doi.org/10.1016/j.syapm.2008.03.005
Zhang T, Xiong G, Maser E (2011) Characterization of the steroid degrading bacterium S19-1 from the Baltic Sea at Kiel, Germany. Chemi Biol Interact 191:83–88. https://doi.org/10.1016/j.cbi.2010.12.021
Zhang HC, Xu T, Hu XL, Pang WH, Yin DQ (2013) The distributions, removals and estrogenic effects of selected endocrine disrupting chemicals in two drinking water factories in China. J Water Health 11:41–50. https://doi.org/10.2166/wh.2012.121
Zhang Y, Habteselassie MY, Resurreccion EP, Mantripragada V, Peng S, Bauer S, Colosi LM (2014) Evaluating removal of steroid estrogens by a model alga as a possible sustainability benefit of hypothetical integrated algae cultivation and wastewater treatment systems. ACS Sustain Chem Eng 2:2544–2553. https://doi.org/10.1021/sc5004538
Zhang C, Li Y, Wang C, Niu L, Cai W (2015a) Occurrence of endocrine disrupting compounds in the aqueous environment and their bacterial degradation: a review. Crit Rev Enviorn Sci Technol 46(1):1–59. https://doi.org/10.1080/10643389.2015.1061881
Zhang Z, Gao P, Su H, Zhan P, Ren N, Feng Y (2015b) Anaerobic biodegradation characteristics of estrone, estradiol, and 17α-ethinylestradiol in activated sludge batch tests. Desalin Water Treat 53(4):985–993. https://doi.org/10.1080/19443994.2013.848415
Zheng W, Yates SR, Bradford SA (2008) Analysis of steroid hormones in a typical dairy waste disposal system. Environ Sci Technol 42(2):530–535. https://doi.org/10.1021/es071896b
Zhou Y, Zha J, Wang Z (2012) Occurrence and fate of steroid estrogens in the largest wastewater treatment plant in Beijing, China. Environ Monit Assess 184:6799–6813. https://doi.org/10.1007/s10661-011-2459-y
Zhu B, Ben WW, Yuan XJ, Zhang Y, Yang M, Qiang ZM (2015) Simultaneous detection of endocrine disrupting chemicals including conjugates in municipal wastewater and sludge with enhanced sample pretreatment and UPLC-MS/MS. Environ Sci-Proc Imp 17(8):1377–1385. https://doi.org/10.1039/c5em00139k
Funding
This work was supported by grants from the National Natural Science Foundation of China (31870104, 41706143, 31770130, and 31670117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pratush, A., Ye, X., Yang, Q. et al. Biotransformation strategies for steroid estrogen and androgen pollution. Appl Microbiol Biotechnol 104, 2385–2409 (2020). https://doi.org/10.1007/s00253-020-10374-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10374-9