Abstract
The presence of nutrients and endocrine-disrupting chemical (EDC) hormones in the aquatic environment can affect wildlife and humans. Sewage treatment using biological processes followed by advanced oxidation processes can be a promising system for EDC removal, however, these have been little investigated in detail in the literature. This study evaluated the removal of nutrients, 17β-estradiol (E2), and 17α-ethynylestradiol (EE2) from sewage using an anaerobic/aerobic/anoxic sequential batch reactor (SBR). Furthermore, the treated sewage generated was subjected to post-treatment using a UV/H2O2 system. Hormones were added to the synthetic sewage only once, and it was submitted to treatment by SBR. The residues of E2 and EE2 were measured in the treated sewage generated by SBR for 13 consecutive cycles. The total removal rate of phosphorus and nitrogen by SBR was in average greater than 75% showing the simultaneous occurrence of nitrification, denitrification, and phosphorus biological removal. Seventeen percentage of the added E2 was detected in treated sewage, but 85% of EE2 was discharged into the same treated after 13 cycles of SBR. Although the literature has indicated the removal of EE2 by sorption and/or biodegradation in biological sewage treatment systems with nitrification, including full-scale ones, this study suggested that this removal has been apparent. The remaining residues of E2 and EE2 were completely degraded by the UV/H2O2 process. The removal of organic matter and nutrients by SBR facilitated the oxidative action of the UV/H2O2 system with lower consumption of peroxide. The integration of these two processes is very promising for EDC removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various endocrine-disrupting chemicals (EDCs) are commonly detected in aquatic environment matrices worldwide (Racz and Goel 2010; Zhou et al. 2019; Fan et al. 2021; Horak et al. 2021). Even at very low concentrations (ng L−1 to µg L−1), these compounds can interfere with the organic functions of wildlife and humans (Pereira et al. 2011). EDCs can act like hormones binding to specific receptors, potentiating or blocking their effects or even interfering with metabolization of these hormones (Sonnenschein and Soto, 1998). Exposure to these compounds reduces vitellogenin production and can cause feminization, intersex induction, and survival rate reduction in various fish species (Hamid and Eskicioglu 2012; Luo et al. 2014; Li et al. 2015). The hormones 17β-estradiol (E2) and 17α-ethynylestradiol (EE2) are the EDCs most found in the aquatic environment (Zhou et al. 2019; You and Song 2021). Some studies around the world have found the concentrations of E2 and EE2 in the range < 1 to 4000 ng L−1 in sanitary sewage (Cunha et al. 2017). Furthermore, it has been demonstrated that these compounds may be responsible for various reproductive effects in fish, crustaceans, amphibians, and gastropods (Birnbaum 2013; Giusti et al. 2014; Garmshausen et al. 2015; Luna et al. 2015). These hormones E2 is a natural hormone produced by women and is released during their lifetime, while EE2 is used in contraceptive pills and for hormone replacement therapy in older women (Cogliano et al. 2005).
According to the literature, sanitary sewage systems are one of the possible sources of E2 and EE2 hormones in the aquatic environment (Gabet-Giraud et al. 2010; Bilal et al. 2021; Deich et al. 2021). Sewage treatment systems (STSs) can represent a barrier against the direct release of these hormones into the environment; therefore, techniques to remove these substances from sanitary sewage systems need to be developed (Hashimoto and Murakami 2009; Verlicchi et al. 2012; Tang et al. 2021). Different biological processes are routinely employed in STSs, and some studies have discussed their efficiency for removing E2 and EE2 (Servos et al. 2005; Kim et al. 2007; Gabet-Giraud et al. 2010; Pessoa et al. 2014; Kent and Tay 2019; Castellanos et al. 2021; Komolafe et al. 2021) mainly with coupled processes involving biological nutrient removal (Roh et al. 2009; Khunjar et al. 2011; Hamid and Eskicioglu 2012; Chen et al 2018).
Biological nutrient removal by STSs requires the promotion of different metabolite phases during treatment. Changes between the anaerobic and aerobic stages can favor the development of phosphorus-accumulating organisms (PAO), which can remove phosphorus (Ahn 2006). PAO usually use organic matter during the anaerobic phase, producing polyhydroxybutyrate and releasing phosphates, whereas in the aerobic stage, these organisms reabsorb phosphates to produce energy (Bunce et al. 2018). The accumulated phosphate is discharged with sludge, and a low sludge retention time (SRT) can thus promote a high total phosphorus (TP) removal rate (Ratanatamskul and Katasomboon 2017). The biological removal of total nitrogen (TN) requires aerobic and anoxic phases. During the aerobic phase, ammonium is transformed into nitrite by the action of the enzyme ammonia monooxygenase (AMO), which is synthesized by ammonium-oxidizing bacteria (AOB), and nitrite is oxidized to nitrate by nitrite-oxidizing bacteria (NOB). Finally, denitrifying bacteria reduce nitrate to N2 gas under anoxic conditions (Ahn 2006; Wrage et al. 2001). Among biological treatment processes, sequencing batch reactors (SBRs) allow anaerobic–aerobic–anoxic phases to simultaneously exist in the same reactor, favoring organic matter and nutrient removal (Mace and Mata-Alvarez 2002; Zhang et al. 2005).
Sludge retention time (SRT) is an important control parameter in SBR processes, as it controls the development of microorganisms responsible for the biodegradation of nutrients, E2, and EE2 (Clara et al. 2005; Suárez et al. 2010; Verlicchi et al. 2012). Nitrification rates, specifically ammonium oxidation, have been identified as important factors for hormone removal (Andersen et al. 2003; Roh et al. 2009; Khunjar et al. 2011; Hamid and Eskicioglu 2012). Li et al. (2020), for example, detected E2 removal by employing AOB, with the co-metabolization of ammonia and E2 by AMO. Heterotrophic bacteria also play an important role in E2 degradation (Li et al. 2020). In contrast, Kassotaki et al. (2019) observed that an SBR cultivated with nitrifying activated sludge (mixed culture of AOB and NOB) was not able to remove EE2 (14% removal). Torresi et al. (2019) suggested that some EDCs can be removed during biological phosphorus removal and this removal occurs first through sorption and later by biodegradation (Kassotaki et al. 2019; Kent and Tay 2019). Therefore, the simultaneous removal of E2 and EE2 may be possible when using biological STSs for nutrient removal (Hertzier et al. 2010).
Although some biological processes can degrade hormones, the removal efficiency is usually unsatisfactory. Advanced oxidation processes (AOPs) such as O3, O3/UV, UV/TiO2, and UV/H2O2 processes can be interesting technological alternatives for the treatment of EDC-containing effluents (Esplugas et al. 2007; Yuan et al. 2009). AOPs can generate hydroxyl radicals (HO·) that react with refractory organic compounds (Chaves et al. 2020), such as carbon–carbon double bonds, and attack the phenolic ring responsible for estrogen activity (Zaviska et al. 2009). However, AOP technologies can represent up to 90% of the cost of wastewater treatments, so determining the process that should be employed is an important decision (Cédat et al. 2016). Moreover, heterogeneous processes, such as UV/TiO2, can efficiently remove EDCs; however, both the catalyst and particles present in sewage may compete for UV light (Zhang et al. 2010). As a result, UV/H2O2 is one of the most promising treatment technologies for estrogen removal and has already been evaluated for the removal of E2 and EE2 (Zhang et al. 2010; Frontistis et al. 2015; Ma et al. 2015; Cédat et al. 2016; Chaves et al. 2020). De la Cruz et al. (2013) and Besnault et al. (2014) reported that the UV/H2O2 process displays the best cost–benefit relationship among homogeneous processes. In addition to cost, one of the problems associated with the direct use of these processes is the presence of compounds that can affect the efficiency of hormone removal from sewage (Cédat et al. 2016; Chaves et al. 2020). The organic matter, turbidity, bicarbonate, and chloride present in sewage can react with hydroxyl radicals, producing chemical species with lower oxidation potentials (Sirtori et al. 2010). Moreover, inorganic compounds can precipitate when exposed to UV radiation, thereby decreasing the effective treatment energy (Glaze et al. 1995). Therefore, AOPs technologies for the post-treatment of treated sewage from biological STSs require further enhancements.
An alternative for the removal of E2 and EE2 is the combination of biological STSs and AOPs. Biological processes exhibit a favorable relationship between cost and efficiency in ecological terms (Henze et al. 2000), whereas AOPs can mineralize residual recalcitrant compounds as a tertiary treatment (Liu et al. 2013). Furthermore, the use of combined biological STP’s, and AOP’s at full scale requires more laboratory-scale studies to understand the safety of this practice and estimate costs. Combined SBR-AOPs are mostly based on the Fenton and photo-Fenton processes. For example, SBR-Fenton processes have been employed to treat industrial pharmaceutical effluents (Ganzenko et al. 2018), in sewage treatment for the bamboo industry (Wu et al. 2013) and for treating the insecticide thiamethoxam in effluents (Gomez-Herrero et al. 2019). In turn, SBR-photo-Fenton processes have been applied in the removal of herbicides (Farré et al. 2008) and treatment of industrial textile sewage (Blanco et al. 2014). However, no studies are available on EDC removal using SBR-UV/H2O2 systems.
The present study assessed the removal and/or degradation of E2 and EE2 by employing an SBR. Moreover, the impact of hormones on nutrient removal (TN and TP) was evaluated. Lastly, the efficiency of the SBR followed by UV/H2O2 was evaluated to remove the hormones completely.
Materials and methods
Reagents
E2 and EE2 (98% purity) were purchased from Sigma-Aldrich (São Paulo, Brazil). Purified water was obtained using a Milli-Q system (Millipore Corporation). Hydrogen peroxide (H2O2; 30% v/v) was supplied by Merck (Rio de Janeiro, Brazil).
SBR apparatus
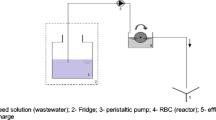
A laboratory-scale cylindrical reactor with an effective volume of 20 L was used as the SBR. Peristaltic pumps, a mixer, and air compressors controlled by an electronic system were used during the process, and a multiparametric probe model HI 9828 (Hanna Instruments Inc., Woonsocket, Rhode Island, 02895, USA) was used to measure the dissolved oxygen (DO), redox potential (ORP), and pH, as shown in Fig. 1A. The process was operated in batch mode with 8 h cycles, comprising filling (00:10 h), anaerobic phase (01:20 h), aerobic phase (02:30 h), anoxic phase (02:30 h), sedimentation (01:20 h), and emptying (00:10 h). A total of 10 L of the effluent were treated during each cycle. Synthetic sewage was prepared using casein peptone (320 mg L−1), meat extract (220 mg L−1), urea (60 mg L−1), potassium monoacid phosphate (56 mg L−1), sodium chloride (14 mg L−1), calcium chloride dehydrate (8 mg L−1), and magnesium sulfate heptahydrate (4 mg L−1), according to Holler and Trösch (2001) (Table 1). The biological sludge used in the SBR was collected from an activated sludge sewage treatment plant in Rio de Janeiro, Brazil (-22.8782486S;-43.2502422W). The SBR was acclimatized for three months. A total of 110 mL of the mixed liquor (sewage and sludge) were discharged at the end of the aerobic phase of each cycle. The sludge retention time (SRT) and hydraulic retention time (HRT) were fixed at 60 d and 8 h, respectively. The operating conditions applied were similar to those recommended by von Sperling (2007).
After the acclimation period, the process was monitored for four weeks, and raw and treated effluent samples were collected. Physicochemical characterization of the influent and treated effluent was conducted according to the standard methods for examination of water and wastewater (APHA 2017). The chemical oxygen demand (COD) (method 5220-D), dissolved organic carbon (DOC), turbidity (method 2130-B), nitrogen series (total nitrogen (TN), nitrite, nitrate and ammonium), and total phosphorus (TP) (method 4500-P) were analyzed during the process. Mixed liquor samples were placed in the SBR to determine the biomass content (mixed liquor volatile suspended solids (MLVSS) (methods 2540–D and –E). The determination of DOC and TN was carried out using a TOC and TN analyzer model TOC-L/TNM-L (Shimadzu Corporation, Kyoto, 604-8511, Japan). Nitrite and nitrate were determined by an ion chromatograph model 790 Personal (Methrohm AG, Ionenstrasse 9100 Herisau, Switzerland) and ammonium by an ammonia ion-selective electrode method coupled to pH-meter model Star 5 (ThermoFisher Scientific, Massachusetts, USA).
After monitoring, the synthetic influent was spiked only once with E2 and EE2 at 5 µg L−1. This concentration was chosen based on the environmental concentrations determined for raw sewage (Montagner and Jardim 2011; Cunha et al. 2017). E2 and EE2 solutions were prepared without solvents to avoid disturbing the SBR. Figure 2 shows a schematic of the sample withdrawal times for each SBR cycle. The treated effluent was collected for 13 cycles to determine the E2 and EE2 concentrations. These concentrations were converted into masses released in each cycle according to Eq. (1). The cumulative mass released in the effluent treated by the SBR for 13 cycles was calculated using Eq. (2).
where M is the mass of E2 or EE2 released during each cycle (1–13), C is the concentration of E2 or EE2 measured in the treated sewage for each cycle (1–13), and V is the volume of treated sewage discharged in each cycle (1–13).
where %AccMass is the cumulative mass percentage of E2 or EE2 released in the treated effluent by the SBR, ∑M (1 up to 13) is the total mass of E2 or EE2 released during cycles after the addition of E2 and EE2 (1 to 13), and M0 is the initial mass of E2 or EE2 added to the influent of the SBR (Cycle 0).
UV/H 2 O 2 reactor apparatus
The system used for the UV/H2O2 process consisted of a cylindrical closed glass reactor with a total volume capacity of 500 mL and illuminated area of 179 cm2. A lamp was placed on the centerline inside a quartz bulb, and magnetic stirrers were placed at the bottom of the reactor. The water recirculation system maintained a constant temperature of 25 °C according to the scheme presented in Fig. 1B. Additionally, 6 W lamps (21 cm length and 2.6 cm diameter) emitting radiation in the UVC spectra (Germicidal lamp—Osram PURITEC HNS G5, λ = 253.7 nm and 14.79 mW cm−2) were used. Radiant fluxes at 254 nm were measured using a radiometer (Cole-Parmer Instrument Co; model 9811-50). The UV lamps were heated for at least 30 min before the beginning of each experiment.
After biological treatment with the SBR, the treated effluent samples were placed in the UV/H2O2 reactor. The degradation process was evaluated at H2O2 doses of 3 and 6 mg L−1. The photodegradation time ranged from 30 to 90 min and the pH was maintained at 7.0. At the end of the H2O2 process, catalase was added to the samples to stop the reaction by decomposing hydrogen peroxide to water and oxygen, and the samples were stored at 4 °C in the dark until solid-phase extraction (SPE). The experimental conditions for E2 and EE2 degradation in the UV/H2O2 processes used in this study have been previously assessed by Chaves et al. (2020).
Analytical methods
E2 and EE2 were concentrated by solid-phase extraction (SPE) followed by chromatographic analysis. The analytical method was carried out according to Paula et al. (2023) with some modifications. Briefly, 500 ml of treated or raw effluent was filtered through glass microfiber (0.7 µm) and nylon membranes (0.45 µm). The residues retained in the membranes were extracted using methanol (3 × 10 mL) in an ultrasound bath. The extracts were dried, resuspended in purified water, and combined with filtered samples. This procedure allowed the determination of E2 and EE2 dissolved in water and adhered to particulate matter. The filtered samples and extracts were subjected to SPE using HLB Oasis (Water) cartridges (1 mL.min−1 flowrate) preconditioned with methanol (5 mL), acetonitrile (7 mL), and purified water (7 mL). After percolation, the cartridges were washed with a mixture of acetonitrile and purified water (30:70, v/v) and dried under vacuum for 20 min. The E2 and EE2 residues were eluted through a cartridge containing acetonitrile (20 mL), and the final purified extracts were dried under a gentle nitrogen flow and resuspended in a solution of acetonitrile and purified water (50:50 v/v).
E2 and EE2 concentrations were quantified using an HPLC-FLD model 1200 series (Agilent Technologies, Santa Clara, CA95051, USA), equipped with a C18 column model Zorbax Eclipse plus (5 μm, 4.6 × 250 mm) at an emission wavelength of 310 nm and excitation wavelength of 230 nm. The isocratic mobile phase consisted of ultrapure water (pH 3.0, adjusted with hydrochloric acid) and acetonitrile (50:50, v/v) at rate a 1.2 mL·min−1 and an injection volume of 100 μL. The limits of quantification (LOQ) were 24.89 and 30.28 ng L−1 for E2 and EE2, respectively, and the limits of detection (LOD) were 8.15 and 9.99 ng L−1, respectively.
Results and discussion
Nutrient removal performance by SBR
The average efficiency of organic matter removal was 92%, that for TP was 82%, and that for TN was 76% (Fig. 3A). Results showing the characteristics of treated sewage from SBR as well as the operating conditions and its efficiency are in Table 2 and S1 (Supplementary Materials). These results are similar to those obtained in previous studies (Jia et al. 2012; Yuan et al. 2016; Liu et al. 2020; Gomes et al. 2021). Jia et al. (2012) reported average removal rates of 91, 91, and 85% for COD, TP, and TN, respectively, in an SBR with simultaneous nitrification and denitrification. Yuan et al. (2016) obtained COD, PO43−-P, and TN removal efficiencies of 85, 96, and 83%, respectively, in an SBR with an SRT of 7 d. Liu et al. (2020) detected average removal efficiencies of 97, 94, and 96% for COD, TP, and TN, respectively, in an anaerobic–oxic–anoxic (AOA) SBR, with a cycle time of 6 h and SRT of 12 d. Although a higher SRT (60 d) was employed in this study, the strategy of conducting sludge (mixed liquor) discharge after the aerobic phase may have improved TP removal.
As exhibited in Fig. 3B, the average N-NHx, N-NO2−, and N-NO3− concentrations in treated effluent were 6.5, 0.16, and 12.0 mg L−1, respectively. The presence of a high concentration of N-NO3− indicates that only a part of the TN was removed by denitrification in the anoxic phase, and that nitrification was necessary. Generally, heterotrophic microorganisms are responsible for denitrification, and it is common to use an external carbon source to improve nitrogen removal (Ahn 2006) as observed by Liu et al. (2020). Although no external carbon source was added to the SBR, the average TN performance in this study was above 75%. Therefore, SBR removed organic matter and TN without the addition of external carbon in anoxic phase; furthermore, TP was removed due to the discharge of mixed liquor applied after the aerobic phase, even theory high SRT was adopted.
Residues of E2 and EE2 in treated sewage by SBR
The initial E2 and EE2 concentrations in the raw effluent were 3.18 and 3.88 µg L−1, respectively. After the first cycle (cycle 1), 0.35 and 0.94 µg L−1 of E2 and EE2 were detected in the treated effluent, respectively. These values corresponded to 11% and 24% of the initial concentrations of E2 and EE2, respectively, applied in the SBR (cycle 0) (Fig. 4A). This result suggests that SBR was capable of removing (or degrading) E2 and EE2 in the first cycle. However, throughout the process cycles, hormones were still discharged from the treated sewage. E2 residues were measured until cycle 5 (above the LOD), whereas EE2 was detected in the treated effluent until cycle 9 (above the LOQ) (Fig. 4A). This demonstrates that these hormones were periodically released with the treated effluent because E2 and EE2 were not continuously introduced into the system. Figure 4B shows the cumulative mass percentages of E2 and EE2 discharged into the treated effluent over 13 cycles. A total of 17% of E2 initially applied in the SBR were discharged with treated sewage until cycle 5, while 83% of the accumulated mass of EE2 was released until cycle 9, with. Therefore, E2 may have been biodegraded, whereas EE2 was initially removed, but later released.
Hashimoto and Murakami (2009) reported higher E2 removal rates (~ 99%) using batch-scale activated sludge under aerobic conditions. When the hydraulic retention time (HTR) was increased from 8 to 24 h, EE2 was not detected (below the limits of detection) in the treated effluent and settled sludge, and the authors suggested the biodegradation of EE2 as the main mechanism of its removal. Clara et al. (2004) observed that the removal efficiencies of E2 and EE2 were over 60% when the applied SRT was higher than 10 d. These authors suggested that SRT values between 5 and 10 d were critical for the removal of these compounds. Amin et al. (2018) observed an increase in the removal rates of different hormones (E1, E2, E3, and EE2) in an MBBR system with increasing HRT and SRT, reporting 98.6–99.9% E2 and 71–95% EE2 removal with an SRT from 11 to 46 d. Conversely, Servos et al. (2005) did not detect statistical correlations between HRT or SRT and E2 removal rates in different STPs in Canada, but the results suggested that the ones operating with nitrification had higher hormone removal rates. Kent and Tay (2019) reported EE2 adsorption as the main removal mechanism in aerobic granular sludge during sludge growth. According to the authors, after the sludge stabilized, all active spaces were occupied by adsorbed EE2, and that degradation became the main route of removal. Finally, Layton et al. (2000) studied the biodegradation capacity of sludge from different STSs in the USA to radiolabel estrogens under aerobic conditions, indicating that 75% of the applied 14C-E2 was mineralized after 24 h. Of the applied 14C-EE2, 40% was mineralized (14C–CO2) and only 20% was observed to be in the aqueous phase. In the present study, E2 and EE2 were added only once to the SBR, and HTR and SRT of 8 h and 60 d, respectively, were applied. Different behaviors were observed for the hormones as E2 was removed or biodegraded, whereas EE2 was initially adsorbed onto the settled sludge but released later (Fig. 4B). The Table 3 shows the difference between some studies found in the literature with the results of this work about E2 and EE2 removal. Therefore, the removal of these hormones by SBR was apparent, i.e., taking only the first cycle a high percentage of removal will be observed, however, a gradual disposal of their residues with treated sewage in subsequent cycles. From the researched literature, this is first report of this phenomenon.
Nitrification, denitrification, and nutrient removal are associated with hormone removal rates (Hamid and Eskicioglu 2012). Andersen et al. (2003) suggested that systems that promote denitrification and nitrification can biodegrade E2 and EE2, whereas studies carried out in Denmark reported that sludge sorption is not relevant to hormone removal (Andersen et al. 2005). Mechanisms that explain this phenomenon likely involve the co-metabolism of these compounds during ammonium oxidation by AMO (De Gusseme et al. 2009; Fernandez-Fontaina et al. 2016; Li et al. 2020). In contrast, Khunjar et al. (2011) suggested that AOB and heterotrophic bacteria may act cooperatively to degrade EE2, observing that AOB can degrade EE2 and form metabolites such as 4-hydroxy-EE2 and sulfo-EE2, and that these intermediates can be mineralized by heterotrophic bacteria. These authors also indicated that heterotrophic bacteria alone were capable of mineralizing EE2. Sheng et al. (2021) demonstrated that the co-metabolism of EE2 by Nitrosomonas europaea occurred only when high ammonium concentrations (> 38 mg L−1) were applied, above those found in typical sewage. These authors suggested that the use of alternative nitrogen sources could improve EE2 removal via co-metabolism. Larcher and Yargeau (2013) indicated that EE2 could be completely degraded by Rhodococcus species after 48 h in pure cultures, and that other heterotrophic bacteria, such as Bacillus subtilis, Pseudomonas aeruginosa, and Pseudomonas putida, were also able to degrade EE2 (averaging 25%), with metabolite formation. These bacteria are typically found in STSs, which may explain the difference in removal efficiencies observed in other studies, indicating that these microorganisms, as well as nitrifying bacteria, have the ability to degrade EE2 (Larcher and Yargeau 2013). Kassotaki et al. (2019) did not detect any association between nitrification rates and EE2 removal or biotransformation with increased ammonium concentrations in nitrification cultures. E2 was completely removed in a conventional activated sludge system (100%) by heterotrophic bacteria, probably through partial degradation, forming metabolites such as E1. However, EE2 degradation was negligible and its removal was prioritized through sorption, suggesting a possible elimination route. Finally, Kent and Tay (2019) investigated the initial EE2 adsorption to aerobic granular sludge through isotherm data and concluded that EE2 may be degraded after sorption to sludge.
In the present study, TP removal was observed in the SBR, with 83% of E2 removed or degraded, while EE2 remained in the system after the release of treated effluent. The low removal rate of EE2 could be related to the high removal of TP (> 80%). Chen et al. (2018) reported lower hormone removal efficiencies in processes that removed higher levels of TP. During anaerobiosis, heterotrophic bacteria are inhibited owing to the absence of oxygen, and PAOs can be developed using available organic matter (Bunce et al. 2018). In the aerobic step, the competition between heterotrophic and autotrophic nitrifying bacteria for oxygen is lower because of the lower amount of organic matter present in the medium; therefore, PAOs can use the previously accumulated polyhydroxybutyrate (stored organic matter) (Blackall et al. 2002; Bunce et al. 2018). Consequently, the success of TP removal involves the inhibition of heterotrophic bacteria, although this may impair EE2 SBR degradation. The EE2 metabolism requires the presence of heterotrophic bacteria whose development was inhibited by the initial anaerobic phase. This suggests that the co-metabolization of EE2 by AOB occurs only with the help of these bacteria. Therefore, the TP biological removal may impair the hormones removal in the sewage treatment plant.
Post-removal of E2 and EE2 by UV/H 2 O 2 system
The EDCs were not completely removed by the SBR. Thus, the UV/H2O2 process was combined for treating samples after biological treatment. E2 and EE2 degradation after biological treatment was performed using 3 mg L−1 H2O2 for 30, 60, and 90 min. Figure 5 indicates that the removal of both hormones was above 97% in the 30 min UV/H2O2 process. Frontistis et al. (2015) evaluated the degradation of EE2 (100 µg L−1) in STS in Greece and achieved 100% removal following UV/H2O2 application (10 mg L−1) for 15 min. Cédat et al. (2016) obtained an average removal rate higher than 90% for E1, E2, and EE2 in an effluent using UV/ H2O2 (40 mg L−1), whereas Ijpelaar et al. (2010) achieved the same results using 10 mg L−1 H2O2. Chaves et al. (2020) tested different concentrations of UV/H2O2 (3 and 30 mg L−1) for EE2 and E2 removal, achieving values below the limit of detection when employing 3 mg L−1 H2O2 for 90 min. In the present study, with a hydrogen peroxide concentration of 3 mg L−1, complete EDC removal was achieved in 30 min. The preceding biological SBR process facilitated hormone degradation as various components from sewage that can inhibit the UV/H2O2 process were removed, decreasing the concentrations of investigated hormones (Yuan et al. 2013; Venkiteshwaran et al. 2021).
Thus, the application of SBR could reduce the content of organic matter and nutrients, as well as other contaminants (suspended solids and turbidity), allowing the complete removal of hormone residues by the UV/H2O2 system. As higher treatment costs are observed when employing the UV/H2O2 process, owing to the high consumption of the oxidant (Cédat et al. 2016), this coupling of biological reactors and oxidative processes may be a viable alternative, and the decreased reaction times and lower input consumption may be economical for hormone removal in STSs (Cédat et al. 2016). Furthermore, the use of low oxidant concentrations reduces the loss of process efficiency (Ince 1999; Zhang et al. 2010), which occur because of cross reactions between hydroxyl radicals, creating a less reactive hydroperoxyl radical (HO2·) (Sharma et al. 2015).
The use of AOPs as post-treatment to remove EDCs may increase treatment efficiency, although no assessments employing biological systems coupled with UV/H2O2 have been reported in the literature. However, Estrada-Arriaga et al. (2016) observed reduced COD, TN, and TP concentrations and the removal of 11 EDCs using the Fenton process (Fe:H2O2 30:25 mg L−1) as a biological post-treatment system. De la Cruz et al. (2012, 2013) employed the neural photo-Fenton process to treat an effluent obtained from an activated sludge process, achieving the removal of 32 EDCs after 90 min of reaction. Nguyen et al. (2013) coupled a membrane bioreactor (MBR) with UV radiation to treat effluents containing 22 compounds. The MBR was able to remove hydrophobic substances and hydrophilic compounds, whereas the UV process removed 85% of the persistent compounds. Therefore, the use of UV/H2O2 processes for the post-treatment of effluent generated by the SBR was shown to be viable for the complete removal of E2 and EE2. The Table 4 presents the comparative results of different studies with UV and UV/H2O2 about E2 and EE2 removal.
Therefore, this study came to show that the mechanism of EE2 removal by biological processes capable of removing nitrogen and phosphorus is not yet well known. And even probable biodegradation of E2 by this process, there is a need to measure its main metabolites such as E1 and E3 that still have the ability to disturb the wildlife and humans. The main highlight was the possibility to degrade E2 and EE2 by UV/H2O2 process, which proved to be less costly when applied as a post-treatment of the biological process. The application of this combined SBR-UV/H2O2 system still depends on tests on a pilot and/or real scale using sanitary sewage.
Conclusion
This study assessed the removal of nutrients and hormones by SBR followed by post-treatment with UV/H2O2. TN was removed by the SBR anaerobic–aerobic–anoxic without the addition of external carbon during the anoxic phase. The strategy of releasing the mixed liquor after the aerobic phase helped in greater TP removal even adopting higher SRT. E2 was biodegraded into metabolites by SBR and metabolites such as E1 and E3 were probably generated, whereas EE2 was not biodegraded by SBR, but removed by sorption. However, its residues were disposed of with treated sewage throughout the SBR cycle. The results also verify that this removal was apparent. E2 and EE2 residues released with treated effluent from the SBR could be completely degraded by the UV/H2O2 process, employing a low amount of reagents and a short process time. The extensive use of the SBR-UV/H2O2 system still requires studies on larger scales to assess, for example, its performance on this scale and its operating cost.
Availability of data and materials
Not applicable.
References
Ahn Y-H (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721. https://doi.org/10.1016/j.procbio.2006.03.033
Amin MM, Bina B, Ebrahim K, Yavari Z, Mohammadi F (2018) Biodegradation of natural and synthetic estrogens in moving bed bioreactor. Chin J Chem Eng 26:393–399. https://doi.org/10.1016/j.cjche.2017.06.006
Andersen H, Siegrist H, Halling-Sørensen B, Ternes TA (2003) Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37:4021–4026. https://doi.org/10.1021/es026192a
Andersen HR, Hansen M, Kjølholt J, Stuer-Lauridsen F, Ternes T, Halling-Sørensen B (2005) Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment. Chemosphere 61:139–146. https://doi.org/10.1016/j.chemosphere.2005.02.088
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington
Besnault S, Ruel SM, Baig S, Esperanza M, Budzinski H, Miege C, Boucher C, Menach K, Le Coquery M (2014) Technical, economic and environmental evaluation of advanced tertiary treatment for micropollutants removal (oxidation and adsorption). In: Spec. conference on eco technologies for sewage treatment plants, Jun. 23–25. University of Verona, Verona, Italy. https://hal.archives-ouvertes.fr/hal-01068003
Bilal M, Barceló D, Iqbal HMN (2021) Occurrence, environmental fate, ecological issues, and redefining of endocrine disruptive estrogens in water resources. Sci Total Environ 800:149635. https://doi.org/10.1016/j.scitotenv.2021.149635
Birnbaum LS (2013) State of the science of endocrine disruptors. Environ Health Perspect 121:A107. https://doi.org/10.1289/ehp.1306695
Blackall LL, Crocetti GR, Saunders AM, Bond PL (2002) A review and update of the microbiology of enhanced biological phosphorus removal in wastewater treatment plants. Antonie Leeuwenhoek 81:681–691. https://doi.org/10.1023/A:1020538429009
Blanco J, Torrades F, Morón M, Brouta-Agnésa M, Garcia-Montaño J (2014) Photo-Fenton and sequencing batch reactor coupled to photo-Fenton processes for textile wastewater reclamation: feasibility of reuse in dyeing processes. Chem Eng J 240:469–475. https://doi.org/10.1016/j.cej.2013.10.101
Bunce JT, Ndam E, Ofiteru ID, Moore A, Graham DW (2018) A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front Environ Sci 6:1–15. https://doi.org/10.3389/fenvs.2018.00008
Castellanos RM, Bassin JP, Bila DM, Dezotti M (2021) Biodegradation of natural and synthetic endocrine-disrupting chemicals by aerobic granular sludge reactor: evaluating estrogenic activity and estrogens fate. Environ Pollut 274:116551. https://doi.org/10.1016/j.envpol.2021.116551
Cédat B, de Brauer C, Métivier H, Dumont N, Tutundjan R (2016) Are UV photolysis and UV/H2O2 process efficient to treat estrogens in waters? Chemical and biological assessment at pilot scale. Water Res 100:357–366. https://doi.org/10.1016/j.watres.2016.05.040
Chaves FP, Gomes G, Della-Flora A, Dallegrave A, Sirtori C, Saggioro EM, Bila DM (2020) Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: effect of pH, estrogenic activity, transformation products and toxicity. Sci Total Environ 746:141041. https://doi.org/10.1016/j.scitotenv.2020.141041
Chen Q, Li Z, Hua X (2018) Fate of estrogens in a pilot-scale step-feed anoxic/oxic wastewater treatment system controlling by nitrogen and phosphorus removal. Environ Sci Pollut Res Int 25:12981–12991. https://doi.org/10.1007/s11356-018-1584-3
Clara M, Strenn B, Saracevic E, Kreuzinger N (2004) Adsorption of bisphenol-A, 17 beta-estradiole and 17 alpha-ethinylestradile to sewage sludge. Chemosphere 56:843–851. https://doi.org/10.1016/j.chemosphere.2004.04.048
Clara M, Kreuzinger N, Strenn B, Gans O, Kroiss H (2005) The solids retention time—a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res 39:97–106. https://doi.org/10.1016/j.watres.2004.08.036
Cogliano V, Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi FE, WHO International Agency for Research on Cancer (2005) Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment. Lancet Oncol 6:552–553. https://doi.org/10.1016/S1470-2045(05)70273-4
Cunha DL, Paula LM, Silva SMC, Bila DM, Fonseca EM, Oliveira JLM (2017) Ocorrência e remoção de estrogênios por processos de tratamento biológico de esgotos. Rev Ambiente Água 12:249–262. https://doi.org/10.4136/ambi-agua.1992
De Gusseme BD, Pycke B, Hennebel T, Marcoen A, Vlaeminck SE, Noppe H, Boon N, Verstraete W (2009) Biological removal 17 alpha-ethinylestradiol by nitrifier enrichment culture in a membrane bioreactor. Water Res 43:2493–2503. https://doi.org/10.1016/j.watres.2009.02.028
De la Cruz N, Giménez J, Esplugas S, Grandjean D, de Alencastro LF, Pulgarín C (2012) Degradation of 32 emergent contaminants by UV and neutral photo-Fenton in domestic wastewater effluent previously treated by activated sludge. Water Res 46:1947–1957. https://doi.org/10.1016/j.watres.2012.01.014
De la Cruz N, Esquius L, Grandjean D, Magnet A, Tungler A, de Alencastro LF, Pulgarín C (2013) Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res 47:5836–5845. https://doi.org/10.1016/j.watres.2013.07.005
Deich C, Kanwischer M, Zhang R, Waniek JJ (2021) Natural and synthetic estrogenic compounds in the Pearl River Estuary and northern shelf of the South China Sea. Oceanologia. https://doi.org/10.1016/j.oceano.2021.08.001
Esplugas S, Bila DM, Krause LGT, Dezotti M (2007) Ozonation and advanced oxidation Technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater 149:631–642. https://doi.org/10.1016/j.jhazmat.2007.07.073
Estrada-Arriaga EB, Cortés-Muñoz JE, González-Herrera A, Calderón-Mólgora CG, de Lourdes Rivera-Huerta M, Ramírez-Camperos E, Montellano-Palacios L, Gelover-Santiago SL, Pérez-Castrejón S, Cardoso-Vigueros L, Martín-Domínguez A, García-Sánchez L (2016) Assessment of full-scale biological nutrient removal systems upgraded with physico-chemical processes for the removal of emerging pollutants present in wastewaters from Mexico. Sci Total Environ 571:1172–1182. https://doi.org/10.1016/j.scitotenv.2016.07.118
Fan D, Yin W, Gu W, Liu M, Liu J, Wang Z, Shi L (2021) Occurrence, spatial distribution and risk assessment of high concern endocrine-disrupting chemicals in Jiangsu Province, China. Chemosphere 285:131396. https://doi.org/10.1016/j.chemosphere.2021.131396
Farré MJ, Maldonado MI, Gernjak W, Oller I, Malato S, Domènech X, Peral J (2008) Coupled solar photo-Fenton and biological treatment for the degradation of diuron and linuron herbicides at pilot scale. Chemosphere 72:622–629. https://doi.org/10.1016/j.chemosphere.2008.02.043
Fernandez-Fontaina E, Gomes IB, Aga DS, Omil F, Lema JM, Carballa M (2016) Biotransformation of pharmaceuticals under nitrification, nitratation and heterotrophic conditions. Sci Total Environ 541:1439–1447. https://doi.org/10.1016/j.scitotenv.2015.10.010
Frontistis Z, Kouramanos M, Moraitis S, Chatzisymeon E, Hapeshi E, Fatta-Kassinos D, Xekoukoulotakis NP, Mantzavinos D (2015) UV and simulated solar photodegradation of 17α-ethynylestradiol in secondary-treated wastewater by hydrogen peroxide or iron addition. Cat Today 252:84–92. https://doi.org/10.1016/j.cattod.2014.10.012
Gabet-Giraud V, Miège C, Choubert JM, Ruel SM, Coquery M (2010) Occurrence and removal of estrogens and beta blockers by various processes in wastewater treatment plants. Sci Total Environ 408:4257–4269. https://doi.org/10.1016/j.scitotenv.2010.05.023
Ganzenko O, Trellu C, Papirio S, Oturan N, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2018) Bioelectro-Fenton: evaluation of a combined biological—advanced oxidation treatment for pharmaceutical wastewater. Environ Sci Pollut Res Int 25:20283–20292. https://doi.org/10.1007/s11356-017-8450-6
Garmshausen J, Kloas W, Hoffmann F (2015) 17α-Ethinylestradiol can disrupt hemoglobin catabolism in amphibians. Comp Biochem Physio c Toxicol Pharmacol 171:34–40. https://doi.org/10.1016/j.cbpc.2015.03.004
Giusti A, Lagadic L, Barsi A, Thomé J-P, Joaquim-Justo C, Ducrot V (2014) Investigating apical adverse effects of four endocrine active substances in the freshwater gastropod Lymnaea stagnalis. Sci Total Environ 493:147–155. https://doi.org/10.1016/j.scitotenv.2014.05.130
Glaze WH, Lay Y, Kang JW (1995) Advanced oxidation processes. A kinetic model for the oxidation of 1,2-dibromo-3-chloropropane in water by the combination of hydrogen peroxide and UV radiation. Ind Eng Chem Res 34:314–2323. https://doi.org/10.1021/ie00046a013
Gomes LCA, Pereira BC, Ribeiro RP, Oliveira JLM (2021) Nitrite build-up effect on nitrous oxide emissions in a laboratory-scale anaerobic/aerobic/anoxic/aerobic sequencing batch reactor. Rev Ambiente Água 16:1–11. https://doi.org/10.4136/ambi-agua.2634
Gomez-Herrero E, Lebik-ElHadi H, Ait-Amar H, Tobajas M, Rodriguez JJ, Mohedano AF (2019) Thiamethoxam removal by Fenton and biological oxidation. J Chem Technol Biotechnol 95:913–921. https://doi.org/10.1002/jctb.5953
Hamid H, Eskicioglu C (2012) Fate of estrogenic hormones in wastewater and sludge treatment: a review of properties and analytical detection techniques in sludge matrix. Water Res 46:5813–5833. https://doi.org/10.1016/j.watres.2012.08.002
Hashimoto T, Murakami T (2009) Removal and degradation characteristics of natural and synthetic estrogens by activated sludge in batch experiments. Water Res 43:573–582. https://doi.org/10.1016/j.watres.2008.10.051
Henze M, Harremoës P, La Cour Jansen J, Arvin E (2000) Wastewater treatment: biological and chemical processes, 3rd edn. Springer, Berlin
Hertzier P, Dufresne L, Randall C, Barnard J, Stensel D, Brown J (2010) Nutrient Control Design Manual. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-10/100
Holler S, Trösch W (2001) Treatment of urban wastewater in a membrane bioreactor at high organic loading rates. J Biotechnol 92:95–101. https://doi.org/10.1016/S0168-1656(01)00351-0
Horak I, Horn S, Pieters R (2021) Agrochemicals in freshwater systems and their potential as endocrine disrupting chemicals: a South African context. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.115718
Ijpelaar GF, Harmsen DJH, Beerendonk EF, van Leerdam RC, Metz DH, Knol AH, Fulmer A, Krijnen S (2010) Comparison of low pressure and medium pressure UV lamps for UV/H2O2 treatment of natural waters containing micro pollutants. Ozone Sci Eng 32:329–337. https://doi.org/10.1080/01919512.2010.508017
Ince NH (1999) “Critical” effect of hydrogen peroxide in photochemical dye degradation. Water Res 33:1080–1084. https://doi.org/10.1016/S0043-1354(98)00295-4
Jia W, Zhang J, Xie H, Yan Y, Wang J, Zhao Y, Xu X (2012) Effect of PHB and oxygen uptake rate on nitrous oxide emission during simultaneous nitrification denitrification process. Bioresour Technol 113:232–238. https://doi.org/10.1016/j.biortech.2011.10.095
Kassotaki E, Pijuan M, Rodriguez-Roda I, Buttiglieri G (2019) Comparative assessment of endocrine disrupting compounds removal in heterotrophic and enriched nitrifying biomass. Chemosphere 217:659–668. https://doi.org/10.1016/j.chemosphere.2018.11.012
Kent J, Tay JH (2019) Treatment of 17α-ethinylestradiol, 4-nonylphenol, and carbamazepine in wastewater using an aerobic granular sludge sequencing batch reactor. Sci Total Environ 652:1270–1278. https://doi.org/10.1016/j.scitotenv.2018.10.301
Khunjar WO, Mackintosh SA, Skotnicka-Pitak J, Baik S, Aga DS, Love NG (2011) Elucidating the relative roles of ammonia oxidizing and heterotrophic bacteria during the biotransformation of 17α-ethinylestradiol and trimetroprim. Environ Sci Technol 45:3605–3612. https://doi.org/10.1021/es1037035
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA (2007) Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res 41:1013–1021. https://doi.org/10.1016/j.watres.2006.06.034
Komolafe O, Mrozik W, Dolfing J, Acharya K, Vassalle L, Mota CR, Davenport R (2021) Occurrence and removal of micropollutants in full-scale aerobic, anaerobic and facultative wastewater treatment plants in Brazil. J Environ Manage 287:112286. https://doi.org/10.1016/j.jenvman.2021.112286
Larcher S, Yargeau V (2013) Biodegradation of 17α-ethinylestradiol by heterotrophic bacteria. Environ Pollut 173:17–22. https://doi.org/10.1016/j.envpol.2012.10.028
Layton AC, Gregory BW, Seward JR, Schultz TW, Sayler GS (2000) Mineralization of steroidal hormones by biosolids in wastewater treatment systems in Tennessee USA. Environ Sci Technol 34:3925–3931. https://doi.org/10.1021/es9914487
Li YY, Xu W, Chen XR, Lou QQ, Wei WJ, Qin ZF (2015) Low concentrations of 17β-trenbolone induce female-to-male reversal and mortality in the frog Pelophylax nigromaculatus. Aquat Toxicol 158:230–237. https://doi.org/10.1016/j.aquatox.2014.11.017
Li C, Lan L, Tadda MA, Zhu S, Ye Z, Liu D (2020) Interaction between 17β-estradiol degradation and nitrification in mariculture wastewater by Nitrosomonas europaea and MBBR. Sci Total Environ 705:135846. https://doi.org/10.1016/j.scitotenv.2019.135846
Liu S, Daigger GT, Liu B, Zhao W, Liu J (2020) Enhanced performance of simultaneous carbon, nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor (AOA-SBR) system by alternating the cycle times. Bioresour Technol 301:122750. https://doi.org/10.1016/j.biortech.2020.122750
Luna TO, Stephanie CP, Salice CJ (2015) Chronic effects of 17α-ethinylestradiol, fluoxetine, and the mixture on individual and population-level end points in Daphnia magna. Arch Environ Contam Toxicol 68:603–611. https://doi.org/10.1007/s00244-014-0119-2
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641. https://doi.org/10.1016/j.scitotenv.2013.12.065
Ma X, Zhang C, Deng J, Song Y, Li Q, Guo Y, Li C (2015) Simultaneous degradation of estrone, 17β-estradiol and 17α-ethinyl estradiol in an aqueous UV/H2O2 system. Int J Environ Res Public Health 12:12016–12029. https://doi.org/10.3390/ijerph121012016
Mace S, Mata-Alvarez J (2002) Utilization of SBR technology for wastewater treatment: an overview. Ind Eng Chem Res 41:5539–5553. https://doi.org/10.1021/ie0201821
Montagner CC, Jardim WF (2011) Spatial and seasonal variations of pharmaceuticals and endocrine disruptors in the Atibaia River, São Paulo State (Brazil). J Braz Chem Soc 22:1452–1462. https://doi.org/10.1590/S0103-50532011000800008
Nguyen LN, Hai FI, Kang J, Price WE, Nghiem LD (2013) Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int Biodeterior Biodegrad 85:474–482. https://doi.org/10.1016/j.ibiod.2013.03.014
Paula LM, Gonçalves ES, Gomes LCA, Moura Júnior JCR, Oliveira JLM (2023) Removal of 17α-ethinylestradiol and total phosphorus in a sequencing batch reactor under two different sludge retention-time conditions. Ambiente Agua, Rev. https://doi.org/10.4136/ambi-agua.2898
Pereira RO, Postigo C, de Alda ML, Daniel LA, Barceló D (2011) Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere 82:789–799. https://doi.org/10.1016/j.chemosphere.2010.10.082
Pessoa GP, De Souza NC, Vidal CB, Alves JAC, Firmino PIM, Nascimento RF, dos Santos AB (2014) Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci Total Environ 490:288–295. https://doi.org/10.1016/j.scitotenv.2014.05.008
Racz L, Goel RK (2010) Fate and removal of estrogens in municipal wastewater. J Environ Monit 12:58–70. https://doi.org/10.1039/B917298J
Ratanatamskul C, Katasomboon J (2017) Effect of sludge recirculation pattern on biological nutrient removal by a prototype IT/BF-MBR (inclined tube/biofilm-membrane bioreactor) and microbial population characteristics. Int Biodeterior Biodegrad 124:26–35. https://doi.org/10.1016/j.ibiod.2017.07.010
Roh H, Subramanya N, Zhao F, Yu CP, Sandt J, Chu KH (2009) Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 77:1084–1089. https://doi.org/10.1016/j.chemosphere.2009.08.049
Servos MR, Bennie DT, Burnison BK, Jurkovic A, McInnis R, Neheli T, Schnell A, Seto P, Smyth SA, Ternes TA (2005) Distribution of estrogens, 17β-estradiol and estrone, in Canadian municipal wastewater treatment plants. Sci Total Environ 336:155–170. https://doi.org/10.1016/j.scitotenv.2004.05.025
Sharma J, Mishra IM, Kumar V (2015) Degradation and mineralization of bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O8(2-) oxidation systems. J Environ Manage 156:66–275. https://doi.org/10.1016/j.jenvman.2015.03.048
Sheng Q, Yi M, Men Y, Lu H (2021) Cometabolism of 17α-ethynylestradiol by nitrifying bacteria depends on reducing power availability and leads to elevated nitric oxide formation. Environ Int 153:106528. https://doi.org/10.1016/j.envint.2021.106528
Sirtori C, Agüera A, Gernjak W, Malato S (2010) Effect of water-matrix composition on trimethoprim solar photodegradation kinetics and pathways. Water Res 44:2735–2744. https://doi.org/10.1016/j.watres.2010.02.006
Suárez S, Lema JM, Omil F (2010) Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res 44:3214–3224. https://doi.org/10.1016/j.watres.2010.02.040
Tang Z, Liu ZH, Wang H, Dang Z, Liu Y (2021) Occurrence and removal of 17α-ethynylestradiol (EE2) in municipal wastewater treatment plants: current status and challenges. Chemosphere 271:129551. https://doi.org/10.1016/j.chemosphere.2021.129551
Torresi E, Tang K, Deng J, Sund C, Smets BF, Christensson M, Andersen HR (2019) Removal of micropollutants during biological phosphorus removal: impact of redox conditions in MBBR. Sci Total Environ 663:496–506. https://doi.org/10.1016/j.scitotenv.2019.01.283
Venkiteshwaran K, Kennedy E, Graeber C, Mallick SP, McNamara PJ, Mayer BK (2021) Conversion of soluble recalcitrant phosphorus to recoverable orthophosphate form using UV/H2O2. Chemosphere 278:130391. https://doi.org/10.1016/j.chemosphere.2021.130391
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment – a review. Sci Total Environ 429:123–155. https://doi.org/10.1016/j.scitotenv.2012.04.028
von Sperling M (2007) Biological wastewater treatment series: activated sludge and aerobic biofilm reactors, 1st edn. IWA Publishing, Minas Gerais
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732
Wu D-L, Wang W, Guo Q-W, Shen Y-H (2013) Combined Fenton–SBR process for bamboo industry wastewater treatment. Chem Eng J 214:278–284. https://doi.org/10.11648/j.cej.2012.10.049
You HH, Song G (2021) Review of endocrine disruptors on male and female reproductive systems. Comput Biochem Physiol C Toxicol Pharmacol 244:109002. https://doi.org/10.1016/j.cbpc.2021.109002
Yuan F, Hu C, Hu X, Qu J, Yang M (2009) Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2. Water Res 43:1766–1774. https://doi.org/10.1016/j.watres.2009.01.008
Yuan H, Zhou X, Zhang Y-L (2013) Degradation of acid pharmaceuticals in the UV/H2O2 process: effects of humic acid and inorganic salts. Clean: Soil, Air, Water 41:43–50. https://doi.org/10.1002/clen.201200107
Yuan Y, Liu J, Ma B, Liu Y, Wang B, Peng Y (2016) Improving municipal wastewater nitrogen and phosphorous removal by feeding sludge fermentation products to sequencing batch reactor (SBR). Bioresour Technol 222:326–334. https://doi.org/10.1016/j.biortech.2016.09.103
Zaviska F, Drogui P, Mercier G, Blais J-F (2009) Procédés d’oxydation avancée dans le traitement des eaux et des effluents industriels : application à la dégradation des polluants réfractaires. Rev Sci Eau 22:535–564
Zhang H, Choi HJ, Huang CP (2005) Optimization of Fenton process for the treatment of landfill leachate. J Hazard Mater 125:166–174. https://doi.org/10.1016/j.jhazmat.2005.05.025
Zhang Z, Feng Y, Liu Y, Sun Q, Gao P, Ren N (2010) Kinetic degradation model and estrogenicity changes of EE2 (17α-ethinylestradiol) in aqueous solution by UV and UV/H2O2 technology. J Hazard Mater 181:1127–1133. https://doi.org/10.1016/j.jhazmat.2010.05.132
Zhou X, Yang Z, Luo Z, Li H, Chen G (2019) Endocrine disrupting chemicals in wild freshwater fishes: species, tissues, sizes and human health risks. Environ Pollut 244:462–468. https://doi.org/10.1016/j.envpol.2018.10.026
Acknowledgements
This study was financed in part by the National Health Foundation (Fundação Nacional da Saúde—FUNASA) with project code number 25380.100370/2018-87, Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES) with project code number 001/2021, and Research Support Foundation of the State of Rio de Janeiro (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—Faperj) with project code number E-26/210.882/2021. EMS thanks the “Young Scientist of Our State” scholarship granted by the Research Support Foundation of the State of Rio de Janeiro (FAPERJ—Project E-21/201.271/2021) and grant from CNPq (304571/2021-0).
Funding
This study was financed in part by the National Health Foundation (Fundação Nacional da Saúde—FUNASA) with project code number 25380.100370/2018–87, Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES) with project code number 001/2021, and Research Support Foundation of the State of Rio de Janeiro (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—Faperj) with project code number E-26/210.882/2021. EMS thanks the “Young Scientist of Our State” scholarship granted by the Research Support Foundation of the State of Rio de Janeiro (FAPERJ—Project E-21/201.271/2021) and grant from CNPq (304571/2021-0).
Author information
Authors and Affiliations
Contributions
Jaime Lopes da Mota Oliveira was involved in the supervision, conceptualization, writing—original draft preparation, and funding acquisition; Larissa Coelho Auto Gomes contributed to the resources, methodology, and writing—review and editing; Tayane Crispim contributed to the methodology, formal analysis and investigation; Enrico Mendes Saggioro assisted in the supervision, writing—review and editing, conceptualization, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval and consent to participate
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Mota Oliveira, J.L., de Souza, T.C., Gomes, L.C.A. et al. Removal of 17β-estradiol (E2) and 17α-ethynylestradiol (EE2) by a sequencing batch reactor following UV/H2O2 process. Int. J. Environ. Sci. Technol. 21, 7733–7748 (2024). https://doi.org/10.1007/s13762-024-05489-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-024-05489-9