Abstract
Steroid estrogens, such as estrone (E1), 17β-estradiol (E2), estriol (E3), and 17α-ethinylestradiol (EE2), are natural and synthetic hormones released into the environment through incomplete sewage discharge. This review focuses on the sources of steroid estrogens in wastewater treatment plants (WWTPs). The mechanisms and fate of steroid estrogens throughout the entire wastewater treatment system are also discussed, and relevant information on regulatory aspects is given. Municipal, pharmaceutical industry, and hospitals are the main sources of steroid estrogens that enter WWTPs. A typical WWTP comprises primary, secondary, and tertiary treatment units. Sorption and biodegradation are the main mechanisms for removal of steroid estrogens from WWTPs. The fate of steroid estrogens in WWTPs depends on the types of wastewater treatment systems. Steroid estrogens in the primary treatment unit are removed by sorption onto primary sludge, followed by sorption onto micro-flocs and biodegradation by microbes in the secondary treatment unit. Tertiary treatment employs nitrification, chlorination, or UV disinfection to improve the quality of the secondary effluent. Activated sludge treatment systems for steroid estrogens exhibit a removal efficiency of up to 100%, which is higher than that of the trickling filter treatment system (up to 75%). Moreover, the removal efficiency of advance treatment systems exceeds 90%. Regulatory aspects related to steroid estrogens are established, especially in the European Union. Japan is the only Asian country that implements a screening program and is actively involved in endocrine disruptor testing and assessment. This review improves our understanding of steroid estrogens in WWTPs, proposes main areas to be improved, and provides current knowledge on steroid estrogens in WWTPs for sustainable development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various environmental chemicals have been identified as endocrine disruptors and have become serious problems to the environment. Natural and synthetic steroid hormones are recognized as the strongest endocrine disruptor compounds even at very low levels (Ying et al. 2002; Cui et al. 2006; Swart and Pool 2007). Natural steroid hormones, such as progestrogens, glucocorticoids, mineralocorticoids, androgens, and estrogens, are secreted by the ovary, testis, placenta, and adrenal cortex in both humans and animals (Ying et al. 2002; Swart and Pool 2007; Liu et al. 2012, Manickum and John 2015). Synthetic steroids, namely, ethinylestradiol (EE2) and mestranol (MeEE2), are commonly used in oral contraceptives and hormone replacement therapy (Ying et al. 2002; Cui et al. 2006; Ye et al. 2012). Steroid estrogens are chemically stable and are excreted as conjugates or in free form; as such, conjugated steroid estrogens can be readily biotransformed to their free forms (Swart and Pool 2007; Manickum and John 2014). The main pathway for conjugate excretion is either through glucuronides or sulfates in urine (Koh et al. 2007). All humans and animals excrete steroid estrogens in urine and feces, which are eventually secreted in the environment (Ying et al. 2002; Manickum and John 2014). The presence of these compounds in the environment has attracted global attention because of their interference with the normal functioning of the endocrine system in humans and animals (Cui et al. 2006; Swart and Pool 2007; Manickum and John 2014).
To date, few studies have investigated the occurrence and fate of steroid estrogens in environmental samples, such as groundwater, surface water, wastewater, and manure. Khanal et al. (2006) and Yu et al. (2013) focused on surface water and wastewater samples. Ying et al. (2002) and Combalbert and Hernandez-Raquet (2010) evaluated wastewater and manure samples. Jurado et al. (2012) studied groundwater samples, especially in Spain. Liu et al. (2011) and Pal et al. (2010) determined the occurrence and fate of synthetic estrogens derived from pharmaceuticals by using environmental samples. Luo et al. (2014) reviewed the occurrence and fate of steroid estrogens in environmental samples as well as other micropollutant categories, such as pharmaceuticals, personal care products, surfactants, industrial chemicals, and pesticides. However, literature reviews on steroid estrogens in wastewater treatment plants (WWTPs) remain limited. Thus far, Johnson and Sumpter (2001), Mes et al. (2005), Cajthaml et al. (2009), Hamid and Eskicioglu (2012), and Xu et al. (2012) reviewed the occurrence and fate of steroid estrogens in WWTPs. Johnson and Sumpter (2001) described the occurrence and fate of steroid estrogens in WWTPs with emphasis on other endocrine-disrupting compounds, such as alkylphenol. Mes et al. (2005) highlighted the elimination of natural steroid estrogens in WWTPs, and Cajthaml et al. (2009) focused on microbial transformation of synthetic estrogen (17α-ethinylestradiol) in WWTPs. Xu et al. (2012) reviewed the removal of steroid estrogens in WWTPs based on China’s perspective. Moreover, Hamid and Eskicioglu (2012) emphasized steroid estrogen removal in the sludge matrix of WWTPs. Thus, a review concerning the occurrence, mechanisms, and fate of both natural and synthetic steroid estrogens throughout wastewater treatment systems is crucial to determine the removal and incoming load of these compounds to the environment.
WWTPs have gained increased research attention because of their essential role in steroid estrogen removal; however, conventional wastewater treatment systems are not specifically optimized for steroid estrogen removal. Currently, a detailed understanding on the fate of steroid estrogens with various types of WWTPs remains unclear. Hence, this review aims to provide an overview of sources of steroid estrogens in WWTPs. Moreover, the mechanisms and fate for steroid estrogen removal involving WWTPs are discussed. Furthermore, relevant information on the regulatory aspects of steroid estrogens is highlighted. This review of steroid estrogens in WWTPs will provide a clear direction in identifying and prioritizing specific areas and transforming opportunities into actions for sustainable development and healthy population.
Sources of steroid estrogens in WWTPs
Figure 1 illustrates the fate of steroid estrogens in different parts of the environment. Steroid estrogens are potentially released into the environment through WWTP discharge and non-point source runoff. The non-point source runoff component of steroid estrogens is attributed to livestock feed lots and aquaculture. Livestock feed lots are potential sources of estrogenic compounds from the excretion of steroid estrogens in urine and manure (Soto et al. 2004; Sarmah et al. 2006). Moreover, spawning fish in the aquaculture sector may contribute to increased steroid estrogen concentration in the receiving river (Kolodziej et al. 2004).
WWTPs have limited capability to completely remove steroid estrogens, and the partially eliminated steroid estrogens during the wastewater systems are eventually released to the environment (Ma et al. 2007; Li et al. 2011; Silva et al. 2012). Thus, treated wastewater has been implicated as the most likely source of estrogenic compounds into the receiving environment such as river water (Sim et al. 2011). The actual sources are upstream discharges of natural and synthetic steroid estrogens that are flushed down home toilets, pharmaceutical industries, and hospitals (Ying et al. 2002; Cui et al. 2006; Leusch et al. 2006; Zhou et al. 2012). The presence of steroid estrogens in WWTPs mainly originated from municipal sources by human excretion as inactive glucuronides and sulfate conjugates or free forms (Sim et al. 2011; Xu et al. 2012; Zhou et al. 2012). Based on the study by Johnson et al. (2000), males were estimated to excrete 3.9 μg of E1, 1.6 μg of E2, and 1.5 μg of E3 in their urine per day. The daily excretions of steroid estrogens by pregnant women were 600 μg of E1, 259 μg of E2, and 6000 μg of E3, two orders of magnitude more hormones than menstruating females with 8 μg of E1, 3.5 μg of E2, and 4.8 μg of E3 in their urine per day. Moreover, menopausal females’ daily steroid estrogen excretions in urine were estimated to be 4, 2.3, and 1 μg for E1, E2, and E3, respectively. In addition, the daily excretion for women who used synthetic steroid estrogens (EE2) used in contraceptive pills was 35 μg per day. According to Mes et al. (2005) and Zhou et al. (2012), 80% of EE2 ingested is excreted as un-metabolized conjugates, of which 22–50% originated from urine and 30% originated from feces. Of the 80% EE2 excreted, Mes et al. (2005) emphasized that only 1 to 2% of EE2 can be de-ethynylated and transformed to E1, E2, and E3. On the other hand, steroid estrogen sources from the pharmaceutical industry are by-products or intermediate products of oral contraceptive pills discarded to WWTPs, and these products are difficult to degrade microbially due to the complexity and non-biodegradability of wastewater (Cui et al. 2006). For hospitals, the sources of steroid estrogens mainly originate from the diagnostic laboratory involving analyzed urine samples from patients and pregnant women which were discarded to WWTPs (Avbersek et al. 2011).
In river sediments, steroid estrogens are more likely to undergo biological uptake by plant, degrade and transform into more or less mobile forms which may move upward into the water column or downward toward groundwater (Campbell et al. 2006). Moreover, steroid estrogens in river water will undergo uptake by fish or be used as a source of raw water supply to be treated in drinking water treatment plants for the production of potable supplies to the community. Humans will eventually be exposed to steroid estrogens through drinking water consumption. Studies have reported the contamination of drinking water by steroid estrogens (Kuster et al. 2008; Pereira et al. 2011; Li et al. 2011).
Mechanisms of steroid estrogens
In general, the mechanisms of steroid estrogens in WWTPs involve volatilization, sorption, and biodegradation. The extent of volatilization of steroid estrogens can be predicted by a ratio of fractions of these estrogenic compounds dissolved in water and those in air, which known as the Henry’s law constant (H) (Khanal et al. 2006; Hamid and Eskicioglu 2012). Both natural and synthetic steroid estrogens have low vapor pressure, ranging from 2.3 × 10−10 to 6.7 × 10−15 mmHg (Lai et al. 2000), denoting the low volatility of these estrogenic compounds. Volatility is the tendency to evaporate, which is directly related to vapor pressure (Combalbert and Hernandez-Raquet 2010). Thus, they are likely to have very small Henry’s constants, making these estrogenic compounds less susceptible to volatilization under normal pressure and temperature, and their loss from the aqueous phase through volatilization likely to be negligible (Khanal et al. 2006; Combalbert and Hernandez-Raquet 2010; Liu et al. 2012).
According to Liu et al. (2012), Silva et al. (2012), Mohagheghian et al. (2014), and Pessoa et al. (2014), sorption and biodegradation processes are the main removal mechanisms for steroid estrogens in WWTPs. Briefly, the sorption elimination of steroid estrogens in wastewater treatment system is based on the absorption from the aqueous phase onto the associated solid phases such as sludge (Khanal et al. 2006). The relative low log octanol–water partition (K ow) value, which varies between 2.8 and 4.2, shows that steroid estrogens are hydrophobic organic compounds, indicating that steroid estrogens have low dissolution in water and favor sorption on sludge (Lai et al. 2000; Ying et al. 2002). However, Clara et al. (2004) highlighted that the sorption involved construction of the distribution coefficient (K d) and the Freundlich sorption isotherms. Hamid and Eskicioglu (2012) and Manickum and John (2014) defined distribution coefficient (K d) as a ratio of a dissolved adsorbate in a two-phase system involving sludge and wastewater at equilibrium. Based on the output of the Freundlich sorption isotherm model developed by Andersen et al. (2005), the sorption of steroid estrogens onto sludge can be described by linear adsorption, with the calculated log K d value varying from 2.60 to 2.84, and sorption is denoted as a relevant removal process. Silva et al. (2012) also reviewed and discovered that log K d value between 2 and 4 is relevant to removal mechanisms for sorption. The increasing sorption affinity order generally follows E2 < E1 < EE2 < E3 (Ren et al. 2007). The sorption process is spontaneous, rapid, and exothermic, and equilibrium is complete in less than 1 h or within 10 min (Racz and Goel 2009). Studies have shown that the removal rate of steroid estrogens by sorption is associated with the operating conditions of WWTPs. Li et al. (2011) investigated that anaerobic condition has favored the sorption of natural estrogens onto sludge as compared to anoxic and aerobic conditions, but was prone to accumulate recalcitrant estrogen like EE2 in sludge. Zeng et al. (2008) observed an increase in sorption with decreasing temperature (20% increase of K d with a reduction of 10 °C), suggesting reactions of an exothermic nature. Steroid estrogens can also desorb from sludge. Clara et al. (2005) found that 30 to 50% of initially adsorbed steroid estrogens were desorbed between pH 9 and 10. This may be due to the low binding energies of steroid estrogens which make physical sorption dominant over chemical sorption; thus, steroid estrogen sorption onto biomass is reversible to some extent, even at a rate slower than the initial sorption.
The presence of steroid estrogens in aqueous, solid, and mixed liquor revealed that the removal was attained by sorption onto solids followed by biodegradation. Biodegradation is the primary removal mechanism for steroid estrogens in WWTPs (Andersen et al. 2003; Braga et al. 2005; Servos et al. 2005). Biodegradation is described by first-order reaction kinetics (Ren et al. 2007; Zeng et al. 2008; Li et al. 2011), as well as pseudo-first-order kinetics (Khanal et al. 2006; McAdam et al. 2010). The bacteria present in wastewater have the capability to completely biodegrade steroid estrogens into harmless products (Khanal et al. 2006). The degradation of steroid estrogens is microbially mediated by deconjugation of sulfates and glucuronides by fecal coliform bacteria (Escherichia coli enzyme glucuronidase) and oxidation of E2 and EE2 to E1 as the main transformations (Lee and Liu 2002; Manickum and John 2014). Generally, natural steroid estrogen E2 is quite readily biodegradable, but synthetic EE2 is not easily biologically removed. Hamid and Eskicioglu (2012) highlighted that E2 can quantitatively oxidize to E1, except for EE2 which appeared to be more persistent to biodegradation. Layton et al. (2000) also observed mineralization of 20% for EE2 and 75% for E2 in 24 h. This finding may be attributed to the ethinyl group of EE2, which sterically hinders enzyme expression, substrate–receptor binding, and metabolism (Racz and Goel 2009). The ethinyl group at the same C atom which possesses the hydroxyl group is vulnerable to microbial attack, thereby complicating the cleavage of this ring and making EE2 to be recalcitrant (Silva et al. 2012; Manickum and John 2014). Figure 2 illustrates the degradation pathway of E2 and E1. 17β-estradiol (E2) is biodegraded from cyclopentane ring D at C17 into E1 during enzymatic degradation, and then E1 is further oxidized into a labile metabolite with a lactone structure (X1), and finally to carbon dioxide through the tricarboxylic acid cycle. Studies showed that the biodegradation rates of steroid estrogens are varied and associated with operating conditions of WWTPs (Esperanza et al. 2007; Li et al. 2011; Atkinson et al. 2012). Li et al. (2011) observed biodegradation of E2 in all three redox conditions (aerobic, anoxic, and anaerobic), but the accumulation of EE2 under anaerobic condition with potential to biodegrade at smaller extent in anoxic condition. Hamid and Eskicioglu (2012) also revealed that E1 and E2, as well as EE2, are biodegradable under all three redox conditions, with aerobic condition being the most favorable. In brief, biodegradation of natural steroid estrogens is more efficient under aerobic condition (Lee and Liu 2002; Esperanza et al. 2007; Manickum and John 2014), same goes to synthetic EE2 (Andersen et al. 2003). Moreover, elevated temperature favored the biodegradation of steroid estrogens by increased microbial activity (Zeng et al. 2008; Li et al. 2011; Atkinson et al. 2012).
Metabolic pathway of E2 and E1 by microorganisms in wastewater (Lee and Liu 2002)
Fate of steroid estrogens in WWTPs
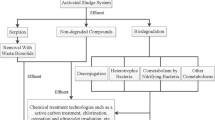
Figure 3 shows the typical layout of wastewater treatment plant involving primary, secondary, and tertiary treatment units. In every wastewater treatment unit, the secondary treatment plays the most important role in removing steroid estrogens (Racz and Goel 2009; Hamid and Eskicioglu 2012). As the main factor that affects the biodegradation and sorption mechanisms in wastewater treatment systems, the types of secondary treatment systems determine the fate of steroid estrogens in WWTPs (Koh et al. 2008; Combalbert and Hernandez-Raquet 2010; and Yu et al. 2013).
A primary treatment unit that involves removal of organic solids by gravity is mandatory prior to secondary treatment (Surujlal-Naicker and Bux 2013). Steroid estrogens at this stage are removed from the water phase by partitioning into fat, oil, and grease or sorption onto primary sludge, and subsequent removal of solid-bound steroid estrogens by flotation or sedimentation (Khanal et al. 2006). Since the log K ow value of steroid estrogens is greater than 2.81, they have stronger sorption capabilities and are more likely to have high partition coefficient between the organic solid and liquid phases (Zhang et al. 2011). Greater sorption was observed in suspended growth treatment systems because larger flocs are more porous and allow more intra-particle mass transfer (Racz and Goel 2009). However, the degree of steroid estrogen removal depends on the physicochemical properties of the compounds such as hydrophobicity and suspended solid content of wastewater, their settling characteristics, and their retention time in the settling tank (Koh et al. 2008). A sludge sorption model of hydrophobic steroid estrogens described by Khanal et al. (2006) indicated mass of primary sludge, partition coefficient of steroid estrogens, and hydraulic retention time (HRT) as the three principal factors for steroid estrogen removal in primary treatment. Zhang et al. (2011) and Huang et al. (2014) observed very little steroid estrogen removal rate in primary treatment, whereas Servos et al. (2005) and Liu et al. (2012) indicated no removal at all. Moreover, there are studies that determined an increasing E1 and descending trend of E2 and E3 after primary treatment (Nelson et al. 2007; Liu et al. 2012). This abnormal increase of E1 after primary treatment can be explained by the deconjugation of steroid conjugates such as glucuronides and sulfates in the aqueous phase by fecal bacteria (E. coli) during primary treatment or by oxidation of E2 to E1 under aerobic condition (Atkinson et al. 2012; Hamid and Eskicioglu 2012; Liu et al. 2012).
Secondary treatment, which involves organic matter removal, consists of suspended growth system, such as activated sludge, and attached growth systems, namely, trickling filter (TF) treatment system and rotating biological contactor (RBC) treatment system. Activated sludge treatment systems involved microorganisms to break down organic material with aeration and agitation (Aziz et al. 2014). For TF treatment system, biofilm grows when the wastewater trickles through a circular bed of plastic media or coarse stones, while RBC treatment system allows microorganisms to grow on the surface of closely spaced parallel discs mounted on a rotating shaft where biodegradation takes place (Spellman 1999). Compared with attached growth systems, suspended growth systems were more efficient in removing steroid estrogens (Ye et al. 2012). Steroid estrogens in this stage are removed from aqueous phase by sorption onto the micro-flocs and subsequently biodegraded by bacteria (Khanal et al. 2006; Zeng et al. 2008). The presence of bacteria will utilize steroid estrogens as carbon sources for metabolism (Silva et al. 2012; Manickum and John 2014). Hence, the biodegradation mechanism during secondary treatment unit plays a major role in steroid estrogen removal. Biodegradation is more rapid and complete under aerobic conditions through catabolic pathways. Studies show that both sludge retention times (SRT) and HRT have appeared to be especially important parameters in removing steroid estrogens from secondary treatment systems (Hamid and Eskicioglu 2012, Manickum and John 2014). There is a positive association between SRT and HRT in the treatment systems and the removal efficiency of steroid estrogens (Clara et al. 2005; Mohagheghian et al. 2014). A longer retention time denotes more degradation time, which results in a higher removal efficiency of steroid estrogens (Esperanza et al. 2007; Ye et al. 2012; Mohagheghian et al. 2014). Studies observed that a minimum of 10 to 12.5 days retention time is required for the growth of microorganisms to decompose steroid estrogens (Ye et al. 2012; Manickum and John 2014). This may attribute to long retention time, such as one greater than 10 days, allows enrichment of slowly growing bacteria and establishment of a more diverse ecological community (Racz and Goel 2009).
Tertiary treatment, also known as advanced treatment, improves the secondary effluent quality by nitrogen removal, chlorination, and ultraviolet (UV) disinfection. From nitrogen removal perspectives, Servos et al. (2005) emphasized that both nitrifying and denitrifying activities in tertiary treatment were able to degrade natural steroid estrogens, while synthetic estrogen can only be degraded in nitrifying condition. Andersen et al. (2003) demonstrated that steroid estrogens have better removal efficiency with higher nitrifying activity performed in tertiary treatment, which could exceed 98% for natural steroid estrogens and above 90% for synthetic estrogen. On the other hand, both chlorination and UV disinfection processes involved in tertiary treatment were able to further oxidize the residual steroids (Liu et al. 2012). Chlorination has better steroid estrogen removal compared with UV disinfection (Liu et al. 2012; Ye et al. 2012). Activated sludge systems followed by chlorination in tertiary treatment had an average steroid estrogen removal of 95% (Pessoa et al. 2014), whereas activated sludge systems with UV disinfection only had an average steroid estrogen removal rate of 49% (Huang et al. 2014).
Table 1 shows the inlet concentration and removal efficiency of steroid estrogen. So far, main investigated areas of these compounds were more focused on various types of secondary treatment systems in WWTPs. The variety types of secondary treatment systems are conventional activated sludge (CAS), aerated lagoon (AL), oxidation ditch (OD), sequencing batch reactor (SBR), extended aeration (EA), and membrane bioreactor (MBR). Based on Sperling (2006), CAS treatment system involves an aeration tank and secondary sedimentation tank where oxygen is supplied by mechanical aerators or by diffused air to promote the biological oxidation of wastewater, while AL treatment system is a treatment pond where oxygen is supplied by mechanical aerators. The OD treatment system, SBR treatment system, and EA treatment system are categorized as mechanical plant without media. The OD is a modified activated sludge treatment system that consists of a ring- or oval-shaped channel equipped with mechanical aerator, which utilizes long SRT to remove biodegradable organics. The SBR treatment system treats wastewater from anaerobic digesters or mechanical biological treatment system in batches, where oxygen is bubbled through the mixture of wastewater and activated sludge to reduce the organic matter. The difference between SBR treatment system and CAS treatment system is that the SBR tank carries out the function of equalization aeration and sedimentation concurrently rather than in the conventional space sequence of continuous flow system. The EA, also a modified activated sludge treatment system, is robust and can withstand surges in hydraulic or organic load, which required oxygen for biodegradation by agitation or submerged diffusers. Mittal (2011) mentioned that MBR treatment system is the combination of a membrane process such as microfiltration or ultrafiltration with a suspended growth bioreactor. The difference between MBR treatment system and CAS treatment system is that the MBR treatment system separates the bio-solids by means of a polymetric membrane based on microfiltration or ultrafiltration unit, which is against the gravity settling process in the CAS treatment system.
Lagana et al. (2004) reported that the mean inlet concentration in CAS treatment system was 35.0 ng/L for E1, 25.0 ng/L for E2, and 31.0 ng/L for E3, with removal rate of 54.3–96.8%, showing that the concentrations in effluent were substantially lower than influent but were not completely removed. Cui et al. (2006) indicated that CAS treatment system had mean inlet concentration of 80.0 ng/L for E1, 85.0 ng/L for E2, 73.0 ng/L for E3, and 155.0 ng/L for EE2. The removal rate was 79% for E1, 73% for E2, 85% for E3, and 67% for EE2. Since the studied WWTP treated wastewater from an oral contraceptive-producing factory, the inlet concentrations were higher than municipal WWTPs and thus difficult to degrade steroid estrogens by microorganisms because of complexity and anti-biodegradation of wastewater due to presence of steroids as compared to municipal WWTPs. Lishman et al. (2006) observed the mean inlet concentration of 29.5 ng/L for E1 and 8.3 ng/L for E2 in CAS treatment system. The removal rate for E1 was 80% but not calculated for E2 due to non-quantifiable in the effluent. Zorita et al. (2009) determined that the inlet concentration in five WWTPs was at the range of 3.0–70.0 ng/L for E1 and 2.5–9.2 ng/L for E2. The removal rate for E1 was 78.0% but also no E2 detected in the effluent. Koh et al. (2007) also quantified the steroid estrogens in CAS treatment system, with inlet concentration of 15.0 ng/L for E1, 5.0 ng/L for E2, 50.0 ng/L for E3, and removal rate of 80 to 98%. The inlet concentration of EE2 was 1.2 ng/L, but the removal efficiency was merely 17%, due to recalcitrant EE2 which is more persistent to biodegradation. Besides, Zhang et al. (2011) demonstrated the inlet concentration of CAS treatment system was at the range of 10.2–34.9 ng/L for E1, 46.6–93.0 ng/L for E2, and 49.8–216.9 ng/L for E3. The relative high concentration of E3 observed in influent may due to rapid deconjugation of the conjugated E3 metabolites. Despite E3 was most abundant in influent, it could be removed efficiently by treatment system with mean removal rate of 94%. Behera et al. (2011) determined relative high E3 mean inlet concentration in CAS treatment system (415 ng/L) as compared to E1 (47.0 ng/L) and E2 (4.0 ng/L), with removal rate of 87.1–100%. Sim et al. (2011) also reported relative high E3 mean inlet concentration in CAS treatment system (379.0 ng/L) as compared to E1 (29.0 ng/L), but with removal rate of 45.6% for E3 and 34.5% for E1. Martin et al. (2012) quantified the concentration of 90.0–830.0 ng/L of E3 and 70.0–180.0 ng/L of EE2 in WWTP influent, having removal efficiency of 26.0% for E3 and 47.0% for EE2. The relative low log K ow value of E3 (2.8) and EE2 (4.2) showed that they are hydrophobic organic compounds which have low dissolution in wastewater, but favor sorption in elimination. Moreover, Tan et al. (2007) reported that CAS treatment system had inlet concentration of 13.1 ng/L for E1, 16.6 ng/L for E2, and 110.0 ng/L for E3 with removal efficiency of −219.8% for E1, 90.4% for E2, and 100% for E3. Atkinson et al. (2012) also observed negative removal efficiency of E1 in CAS treatment system, having inlet concentration of 35.0–104.0 ng/L and effluent concentration of 11.2–370.0 ng/L, respectively. The occurrence of negative removal efficiency is likely attributed to both decoupling of estrogen sulfates and estrogen glucuronide conjugates by E. coli enzyme β-glucuronidase, and oxidation of E2 to E1. Furthermore, Chang et al. (2011) reported that inlet concentration of CAS treatment system from seven WWTPs was at the range of 6.5–19.1 ng/L for E1 and 0.9–3.8 ng/L for E2, with removal rate of 55.0–98.3% for E1 and 55.0–92.1% for E2, respectively. Table 1 shows the fifth WWTP has relative low removal efficiency, probably interpreted by huge portion of domestic wastewater given that all other WWTPs have additional industrial influence. Furthermore, Manickum and John (2014) quantified the concentrations of steroid estrogens in CAS treatment system with reported inlet concentration of 84.0 ng/L for E1, 119.0 ng/L for E2, 5.0 ng/L for E3, and 30.0 ng/L for EE2. The results show higher levels of E2 compared to E1 were attributed to a widespread use of the hormonal contraceptive pill by women in this area, which typically contains E2 and EE2. The removal rate of 72.6–90.0% indicated steroid estrogens can be effectively removed during secondary biological treatment stage, whereby microbial degradation and adsorption has played a major role. Mohagheghian et al. (2014) also highlighted CAS treatment system had removal rate of 68.2–80.4% given that the inlet concentration of steroid estrogens range from 3.0 to 11.4 ng/L. The study determined that seasonal and temperature changes could alter the removal rate, where increase in temperature usually leads to better removal efficiency as the metabolic rate of microorganisms in the biological treatment plant increases.
In perspectives of CAS treatment system, Esperanza et al. (2007) reported steroid estrogen mean inlet concentration in aerobic condition ranged from 14.1 to 40.7 ng/L, whereas in anaerobic condition were at the range of 22.1 to 55.9 ng/L. The performance of aerobic digester for removal or biodegradation of steroid estrogens was better than anaerobic digester (Lee and Liu 2002). Conversely, Mnif et al. (2010) observed anaerobic condition had estrogenic activity of 291.0 ng-EEQ/L in influent that exhibits 88% removal efficiency, which is higher than their aerobic counterpart that has inlet concentration of 400.0 ng-EEQ/L and removal efficiency of 26%. This could be due to longer retention time of anaerobic CAS treatment system than aerobic CAS treatment system. Apart from that, Zhang and Zhou (2008) indicated that E1 and EE2 removal rate elevated in CAS with UV disinfection treatment system, given that the inlet concentration is 20.0–60.0 ng/L for E1 and 26.0–51.0 ng/L for E2 with removal rate of 78–92% for E1 and 69–90% for E2, respectively. Muz et al. (2012) also demonstrated that CAS with nitrification treatment system had removal efficiency of 43.1%, which is higher than CAS without nitrification treatment system (23.9%). Besides, Surujlal-Naicker and Bux (2013) observed the steroid estrogen inlet concentration for CAS with nitrification treatment system and AL treatment system was at the range of 0.5–103.6 and 0.1–47.5 ng/L, respectively. The removal rate was relatively high for CAS with nitrification treatment system (80.4 to 86.5%) as compared to AL treatment system (55.1 to 73.4%), due to the availability of advanced treatment. In addition, Pessoa et al. (2014) reported the mean inlet concentration for CAS with chlorination treatment system and AL treatment system was 566.0 ng/L for E1, 143.0 ng/L for E2, and 421.0 ng/L for EE2. The relative high steroid estrogen inlet concentration was possibly caused by high temperatures (25–29 °C) and low precipitation (<27 mm) which result in low dilution effect. The removal rate for CAS with chlorination treatment system is 84–100% which are relatively high comparing to AL treatment system (30–99%), most probably attributed to biological degradation, abiotic removal by chlorine, and adsorption onto solids. Hashimoto et al. (2007) compared the steroid estrogen concentration between CAS treatment system and OD treatment system. The CAS treatment system had inlet concentration of 29.0 ng/L for E1, 12.0 ng/L for E2, and 164.0 ng/L for E3 with removal rate of −55.9 to 99.5%, while the OD treatment system had inlet concentration of 20.0 ng/L for E1, 9.2.0 ng/L for E2, and 120.0 ng/L for E3 with removal rate of 83.4 to 98.9%. These outputs showed OD treatment system has better steroid estrogen removal efficiency comparing with CAS treatment system. The negative removal rate of E1 in CAS treatment system indicated E1 concentrations in effluents were higher than influents. This may be attributed to biological conversion of E2 to E1, and the degradation of E1 is less than that of E2.
Apart from that, Ye et al. (2012) quantified the steroid estrogen concentration from four types of WWTP treatment systems, namely OD, OD with ultraviolet, OD with chlorination, and SBR. The inlet concentration was at the range of 42.2–110.7 ng/L for E1, 7.4–32.7 ng/L for E2, 108.7–845.6 ng/L for E3, and 8.6–44.6 ng/L for EE2. This is probably due to more urinary E1 and E3 excreted from premenopausal women than E2 and EE2. The outputs of this study showed SBR treatment system is able to remove more than 82% of steroid estrogens, yet it is less efficient than OD treatment system with advance treatment, namely, chlorination and UV disinfection. The poor performance of SBR treatment system may also be attributed to short hydraulic retention time. Huang et al. (2014) also reported the mean inlet concentration of steroid estrogens was 126.8 ng/L for E1, 31.0 ng/L for E2, 48.8 ng/L for E3, and 13.4 ng/L for EE2 in four various types of WWTP treatment systems, such as OD with UV, CAS with UV, EA with UV, and MBR with UV. The removal rate was at the range of 75.4 to 94.7% because of UV disinfection availability, denoting that advanced treatment is the contributing factor for effective steroid estrogen removal. Among these four types of WWTP treatment systems, MBR treatment system has a removal rate of over 88%, followed by CAS treatment system, MBR treatment system, and OD treatment system. The MBR treatment system has better removal efficiency most probably interpreted by longer retention time or sorption of steroid estrogens on colloidal and suspended particles as well as biodegradation. Furthermore, Servos et al. (2005) determined that the mean E1 and E2 inlet concentration in 17 variety types of WWTP treatment system was 49.0 and 15.6 ng/L, respectively. The removal rate in this study was at the range of 45.8 to 97.8 ng/L for E1 and at the range of 18.5 to 98.8 ng/L for E2. There was an increase of E1 concentrations observed in effluent most probably due to the oxidation of E2 to E1 by the microorganisms under aerobic conditions or the stability of estrone conjugates, especially sulfate conjugates (Braga et al. 2005; Nelson et al. 2007; Atkinson et al. 2012). The outputs of this study showed TF treatment system has negative removal efficiency of E1 and E2 when compared with activated sludge treatment systems such as CAS, EA, AL, OD, MBR, and SBRs. Ternes et al. (1999), Khanal et al. (2006), and Kumar et al. (2011) highlighted that TF treatment system is less effective than activated sludge treatment at removing steroid estrogens from wastewater. TF is an attached growth treatment system, therefore less effective than suspended growth treatment system in removing steroid estrogens. This maybe because suspended growth treatment systems are more porous, allowing more intra-particle mass transfer and resulting in greater sorption than attached growth treatment systems (Racz and Goel 2009). Combalbert and Hernandez-Raquet (2010) also reviewed low steroid estrogen elimination by TF treatment system, with mean removal efficiencies of 30% for E1 and 70% for E2. The poor removal of steroid estrogens by TF treatment system may cause the saturation of steroid estrogens on the biofilm and lower hydraulic retention time compared to the activated sludge system (Chimchirian et al. 2007). Kumar et al. (2011) also reported that the mean inlet concentration in 11 WWTPs was 51 ng/L for E1, 43 ng/L for E2, 54 ng/L for E3, and 2 ng/L for EE2, with removal rate of 80% for E1, 70% for E2, 100% for E3, and 75% for EE2. These outputs showed that all glucuronide conjugate excretes by humans were transformed to free estrogens before released to receiving river.
From the perspectives of estrogenic activity, Svenson et al. (2003) make comparison between CAS treatment system and TF treatment system. The estrogenic activity in influent was 5.0–29.8 ng-EEQ/L with removal rate of 68.0–94.0% for CAS treatment system and 3.1–22.4 ng-EEQ/L with removal rate of 33.6–74.8% for TF treatment system. The results showed TF treatment system was less effective than CAS treatment system in removing steroid estrogens. Jugan et al. (2009) also determined CAS treatment system (51.7 ng-EEQ/L in influent) had removal efficiency of 95.2% which is better than TF treatment system (62.3%, given 29.6 ng-EEQ/L in influent). Based on Ma et al. (2007), the estrogenic activity (EEQ) in WWTP influent is 0.3–1.7 ng-EEQ/L, where the effluents displayed much lower estrogenic effects with removal rate of 74–87%. Besides, Yang et al. (2011) indicated the EEQ in WWTP influent ranged from 47.7–80.1 ng-EEQ/L with removal rate of 62.3–83.6%, denoting estrogenic activity in wastewater had been reduced significantly after CAS treatment system. Ra et al. (2011) also observed the EEQ in WWTP influent at the range of 10.8–27.6 ng-EEQ/L was sharply decreased after the final treatment, with removal rate of 75.8–86.5%. Diniz et al. (2010) reported that the estrogenic activity of CAS with UV treatment system was at the range of 8.4–52.8 ng-EEQ/L. The removal rate is not calculated due to non-detection of steroid estrogens in effluent. A probable interpretation for non-detection was the low sample volume collected (500 mL) for analysis, which may not have been enough to detect the low levels of steroid estrogens that are usually present in WWTP effluent. Moreover, Liu et al. (2012) reported that the EEQ in influent of two WWTPs, namely, OD with UV treatment system and CAS with chlorination treatment system, was at the range of 41.5–60.2 ng-EEQ/L. The removal rate for OD with UV treatment system was 46.9–77.9%, whereas for CAS with chlorination treatment system was 46.0–94.7%. By comparing among these two types of treatment systems, the steroid estrogens were significantly eliminated in aqueous phase after OD treatment due to their sorption onto sludge and degradation by microorganisms. The steroid estrogens however could further eliminate through advanced treatment, namely, chlorination and UV disinfection, because the hypochlorous acid (HOCl) in the chlorination process and hydroxyl radical in UV treatment could oxidize the phenol moiety in steroid estrogens. The chlorination has better oxidation performance than UV treatment as chlorination involves chlorine substitution reactions followed by dehydration or cleavage of the C9–C10 bond.
Regulatory aspects of steroid estrogens
To date, international governments, namely, the European Union, the USA, Canada, and Japan, are in the process of establishing testing approaches and regulatory frameworks to assess the risks in the endocrine systems of human and aquatic organisms (Hecker and Hollert 2011). Table 2 summarizes the regulations or approaches implemented in respective countries. In general, the European Union has established most of the regulatory aspects related to steroid estrogens. The European Union regulated the use of estrogenic compounds in agriculture and aquaculture by the Directive 96/22/EC (Noppe et al. 2008). This was later amended by the Directive 2003/74/EC to reduce the circumstances at which E2 was administered under strict veterinary control and can only be used for purposes other than growth promotion, such as animal’s uterus disease, uterus induction, and treatment of fetus maceration. In December 2000, the Directive 2000/60/EC, also known as the Water Framework Directive (WFD), included E2 and EE2 in the list of priority substances for surface water quality (Guedes-Alonso et al. 2014), to achieve good chemical and ecological status for surface and groundwater bodies as well as prevention of deterioration (Cunha et al. 2016). In the Directive 2000/60/EC, the annual average value of environmental quality standards was proposed, stating that E2 concentrations must not exceed 4 × 10−4 μg/L and EE2 concentrations must not exceed 3.5 × 10−5 μg/L for inland surface waters like rivers, lakes, and heavily modified or artificial water bodies. The Priority Substances Directive, also known as the Environmental Quality Standards Directive 2008/105/EC, had amended the Directive 2000/60/EC, which set environmental quality standards for the substances in surface waters (river, lake, transitional, and coastal) and confirmed their designation as priority or priority hazardous substances. The Environmental Quality Standards Directive 2008/105/EC was later amended by the Directive 2013/39/EU under the European WFD. The Directive 2013/39/EU had included E2 and EE2 in the “Watch List”, which monitors mechanisms in order to collect high-quality Union-wide monitoring data for the purpose of supporting future prioritization practices. Despite the European Union’s established legislation, steroid estrogens are still not regulated in drinking water for human consumption. However, the USA has included steroid estrogens in the Contaminant Candidate List for human drinking water consumption, even if they are not included in the list of quality control for water bodies (Cunha et al. 2016). Moreover, the US National Environmental Policy Act required the Center for Drug Evaluation and Research, which is under the Food and Drug Administration, to assess the environmental impacts resulting from humans who took approved pharmaceutical estrogens (Laurenson et al. 2014). The US Environmental Protection Agency established a two-tier Endocrine Disruptor Screening Program, which involved in vitro and in vivo assays to identify estrogenic compounds’ potential association with endocrine systems in Tier 1 and then developed dose–response relationships in animal models in Tier 2 (Hecker and Hollert 2011). Furthermore, Environment Canada has proposed Wastewater Systems Effluent Regulations in the Canada Gazette, with Part I in 2010, to minimize the discharge of steroid estrogens, with WWTP effluents utilizing secondary or equivalent treatments (Hamid and Eskicioglu 2012). In Asia, only Japan has established a screening program, namely, the Strategic Program on Endocrine Disruptors (SPEED), in 1998 to monitor the endocrine-disrupting effects of chemicals to the environment and organisms. SPEED was then actively involved in the Joint Working Group on Endocrine Disrupters Testing and Assessment (EDTA) sponsored by the Organization for Economic Cooperation and Development (Hecker and Hollert 2011).
Conclusions
WWTPs are a significant source of steroid estrogen into the receiving environment. The sources of steroid estrogens in WWTPs originated from municipal, pharmaceutical industries, and hospitals. Humans excrete both natural (E1, E2, E3) and synthetic (EE2) steroid estrogens which are discharged into the environment due to incomplete removal during wastewater treatment. There are three treatment units involved in a typical wastewater treatment system, namely, primary, secondary, and tertiary treatments. Studies showed that both sorption and biodegradation are the main treatment mechanisms for steroid estrogens in WWTPs. Primary treatment involves sorption of steroid estrogens onto primary sludge whereas secondary treatment comprises sorption of steroid estrogens onto micro-flocs and subsequent biodegradation by microbes. Tertiary treatment further improves the secondary effluent quality by involving nitrification, chlorination, or UV disinfection. As for the fate of steroid estrogens from removal efficiency perspectives, suspended growth activated sludge treatment systems have a better removal efficiency of up to 100% compared to attached growth trickling filter treatment systems (up to 75%). Moreover, the removal efficiency of advance treatment systems could exceed 90%. Steroid estrogen removal rate is relatively high for WWTPs that involve both secondary and tertiary treatments. Relevant regulatory aspects on steroid estrogens are mostly established in the countries of the European Union. In Asia, only Japan has established a screening program and is actively involved in the endocrine disruptor testing and assessment group. It is then essential to sustain international effort for developing, validating, and updating the current testing approaches and regulatory frameworks for effective steroid estrogen removal.
References
Andersen, H. R., Hansen, M., Kjolholt, J., Stuer-Lauridsen, F., Ternes, T., & Halling-Sorensen, B. (2005). Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment. Chemosphere, 61, 139–146.
Andersen, H., Siegrist, H., Halling-Sorensen, B., & Ternes, T. A. (2003). Fate of estrogens in a municipal sewage treatment plant. Environmental Sciences and Technology, 37(18), 4021–4026.
Atkinson, S. K., Marlatt, V. L., Kimpe, L. E., Lean, D. R. S., Trudeau, V. L., & Blais, J. M. (2012). The occurrence of steroidal estrogens in south eastern Ontario wastewater treatment plants. Science of the Total Environment, 430, 119–125.
Avbersek, M., Somen, J., & Heath, E. (2011). Dynamics of steroid estrogen daily concentrations in hospital effluent and connected waste water treatment plant. Journal of Environmental Monitoring, 13, 2221.
Aziz, H.A., Mojiri, A., Ghobahi, Y., & Zarbakhsh, M. (2014). Wastewater engineering: advanced wastewater treatment systems. International Journal of Scientific Research Books, IJSR Publications, ISSN, 2322–4657.
Behera, S. K., Kim, H. W., Oh, J. E., & Park, H. S. (2011). Occurrence and removal of antibiotics, hormones, and several other pharmaceuticals in wastewater treatment plants of the largest industry city of Korea. The Science of the Total Environment, 409, 4351–4360.
Braga, O., Smythe, G. A., Schafer, A. I., & Feitz, A. J. (2005). Fate of steroid estrogens in Australian inland and coastal wastewater treatment plants. Environmental Science & Technology, 39, 3351–3358.
Cajthaml, T., Kresinova, Z., Svobodova, K., Sigler, K., & Rezanka, T. (2009). Microbial transformation of synthetic estrogen 17α-ethinylestradiol. Environmental Pollution, 157, 3325–3335.
Campbell, C. G., Borglin, S. E., Green, F. B., Grayson, A., Wozei, E., & Stringfellow, W. T. (2006). Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: a review. Chemosphere, 65, 1265–1280.
Chang, H., Wan, Y., Wu, S., Fan, Z., & Hu, J. (2011). Occurrence of androgens and progestrogens in wastewater treatment plants and receiving river waters: comparison to estrogens. Water Research, 45, 732–740.
Chimchirian, R. F., Suri, R. P., & Fu, H. (2007). Free synthetic and natural estrogen hormones in influent and effluent of three municipal wastewater treatment plants. Water Environment Research, 79(9), 969–974.
Clara, M., Kreuzinger, N., Strenn, B., Gans, O., & Kroiss, H. (2005). The solids retention time—a suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Research, 39, 97–106.
Clara, M., Strenn, B., Saracevic, E., & Kreuzinger, N. (2004). Adsorption of bisphenol-A, 17β-estradiole and 17α-ethinylestradiole to sewage sludge. Chemosphere, 56, 843–851.
Combalbert, S., & Hernandez-Raquet, G. (2010). Occurrence, fate, and biodegradation of estrogens in sewage and manure. Applied Microbial and Biotechnology, 86, 1671–1692.
Cui, C. W., Ji, S. L., & Ren, H. Y. (2006). Determination of steroid estrogens in wastewater treatment plant of a contraceptives producing factory. Environmental Monitoring and Assessment, 121, 409–419.
Cunha, D. L., Silva, S. M. C., Bila, D. M., Mota, O. J. L., Novaes, S. P., & Larentis, A. L. (2016). Regulation of the synthetic estrogen 17α-ethinylestradiol in water bodies in Europe, the United States, and Brazil Cad Saude Publica. Rio de Janeiro., 32(3), 56715.
Diniz, M. S., Mauricio, R., Petrovic, M., Alda, M. J. L., Amaral, L., Peres, I., Barcelo, D., & Santana, F. (2010). Assessing the estrogenic potency in a Portuguese wastewater treatment plant using an integrated approach. Journal of Environmental Sciences, 22(10), 1613–1622.
Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000.
Directive 2008/105/EC of the European Parliament and of the Council of 16th December 2008.
Directive 2013/39/EU of the European Parliament and of the Council of 12th August 2013.
Esperanza, M., Suidan, M. T., Marfil-Vega, R., Gonzalez, C., Sorial, G. A., McCauley, P., & Brenner, R. (2007). Fate of sex hormones in two pilot scale municipal wastewater treatment plants: conventional treatment. Chemosphere, 66, 1535–1544.
Guedes-Alonso, R., Montesdeoca-Esponda, S., Sosa-Ferrera, Z., & Santana-Rodriguez, J. J. (2014). Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems. Trends in Environmental Analytical Chemistry, 3, 14–27.
Hamid, H., & Eskicioglu, C. (2012). Fate of estrogenic hormones in wastewater and sludge treatment: a review of properties and analytical detection techniques in sludge matrix. Water Research, 46, 5813–5833.
Hashimoto, T., Onda, K., Nakamura, Y., Tada, K., Miya, A., & Murakami, T. (2007). Comparison of natural estrogen removal efficiency in the conventional activated sludge process and the oxidation ditch process. Water Research, 41, 2117–2126.
Hecker, M., & Hollert, H. (2011). Endocrine disruptor screening: regulatory perspectives and needs. Environmental Sciences Europe, 23, 15.
Huang, B., Li, X., Sun, W., Ren, D., Li, X., Li, X., Liu, Y., Li, Q., & Pan, X. (2014). Occurrence, removal, and fate of progestrogens, androgens, estrogens, and phenols in six sewage treatment plants around Dianchi Lake in China. Environmental Science and Pollution Research, 21, 12898–12908.
Johnson, A. C., Belfroid, A., & Corcia, A. D. (2000). Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Science of the Total Environment, 256, 163–173.
Johnson, A. C., & Sumpter, J. P. (2001). Removal of endocrine-disrupting chemicals in activated sludge treatment works. Environmental Science and Technology, 35(24), 4697–4703.
Jugan, M. L., Oziol, L., Bimbot, M., Huteau, V., Tamisier-Karolak, S., Blondeau, J. P., & Levi, Y. (2009). In vitro assessment of thyroid and estrogenic endocrine disruptors in wastewater treatment plants, rivers, and drinking water supplies in the greater Paris area (France). Science of the Total Environment, 407, 3579–3587.
Jurado, A., Vazquez-Sune, E., Carrera, J., Alda, M. L., Pujades, E., & Barcelo, D. (2012). Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in European context. Science of the Total Environment, 440, 82–94.
Khanal, S. K., Xie, B., Thompson, M. L., Sung, S., Ong, S. K., & Leeuwen, J. (2006). Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environmental Science and Technology, 40(21), 6536–6546.
Koh, Y. K. K., Chiu, T. Y., Boobis, A., Cartmell, E., Lester, J. N., & Scrimshaw, M. D. (2007). Determination of steroid estrogens in wastewater by high performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1173, 81–87.
Koh, Y. K. K., Chiu, T. Y., Boobis, A., Cartmell, E., Scrimshawm, M. D., & Lester, J. N. (2008). Treatment and removal strategies for estrogens from wastewater. Environmental Technology, 29, 245–267.
Kolodziej, E. P., Harter, T., & Sedlak, D. L. (2004). Dairy wastewater, aquaculture, and spawning fish as sources of steroid hormones in the aquatic environment. Environmental Science and Technology, 38(22), 6377–6384.
Kumar, V., Nakada, N., Yasojima, M., Yamashita, N., Johnson, A. C., & Tanaka, H. (2011). The arrival and discharge of conjugated estrogens from a range of different sewage treatment plants in the UK. Chemosphere, 82, 1124–1128.
Kuster, M., Lopez de Alda, M. J., Hernando, M. D., Petrovic, M., Martin-Alonso, J., & Barcelo, D. (2008). Analysis and occurrence of pharmaceuticals, estrogens, progestrogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). Journal of Hydrology, 358, 112–123.
Lagana, A., Bacaloni, A., Leva, I., Faberi, A., Fago, G., & Marino, A. (2004). Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Analytica Chimica Acta, 501, 79–88.
Lai, K. M., Johnson, K. L., Scrimshw, M. D., & Lester, J. N. (2000). Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environmental Science and Technology, 34, 3890–4000.
Laurenson, J.P., Bloom, R.A, Page, S., & Sadrieh, N. (2014). Ethinyl estradiol and other human pharmaceutical estrogens in environment: a review of recent risk assessment data. American Association of Pharmaceutical Scientists, 16(2), 299–310.
Layton, A. C., Gregory, B. W., Seward, J. R., Schultz, T. W., & Sayler, G. S. (2000). Mineralization of steroidal hormones by biosolids in wastewater treatment systems in Tennessee U.S.A. Environmental Science and Technology, 34, 3925–3931.
Lee, H. B., & Liu, D. (2002). Degradation of 17β-estradiol and its metabolites by sewage bacteria. Water Air Soil Pollution, 134, 353–368.
Leusch, F. D. L., Chapman, H. F., Heuvel, M. R., Tan, B. L. L., Gooneratne, S. R., & Tremblay, L. A. (2006). Bioassay-derived androgenic and estrogenic activity in municipal sewage in Australia and New Zealand. Ecotoxicology and Environmental Safety, 65, 403–411.
Li, Y.M., Zeng, Q.L., & Yang, S.J. (2011). Removal and fate of estrogens in an anaerobic-anoxic-oxic activated sludge system. Water Science and Technology, 51–56.
Lishman, L., Smyth, S. A., Sarafin, K., Kleywegt, S., Toito, J., Peart, T., Lee, B., Servos, M., Beland, M., & Seto, P. (2006). Occurrence and reductions of pharmaceuticals and personnel care products and estrogens by municipal wastewater treatment plants in Ontario, Canada. Science of the Total Environment, 367, 544–558.
Liu, Z. H., Ogejo, J. A., Pruden, A., & Knowlton, K. F. (2011). Occurrence, fate and removal of synthetic oral contraceptives (SOCs) in the natural environment: a review. Science of the Total Environment, 409, 5149–5161.
Liu, S., Ying, G. G., Zhao, J. L., Zhou, L. J., Yang, B., Chen, Z. F., & Lai, H. J. (2012). Occurrence and fate of androgens, estrogens, glucocorticoids and progestagens in two different types of municipal wastewater treatment plants. Journal of Environmental Monitoring, 14, 482.
Luo, Y. L., Guo, W. S., Ngo, H. H., Nghiem, L. D., Hai, F. I., Zhang, J., Liang, S., & Wang, X. C. (2014). A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of the Total Environment, 473-474, 619–641.
Ma, M., Rao, K., & Wang, Z. (2007). Occurrence of estrogenic effect in sewage and industrial wastewaters in Beijing, China. Environmental Pollution, 147, 331–336.
Manickum, T., & John, W. (2014). Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa). Science of the Total Environment, 468-469, 584–597.
Manickum, T., & John, W. (2015). The current preference for the immune-analytical ELISA method for quantification of steroid hormones (endocrine-disruptor compounds) in wastewater in South Africa. Analytical and Bioanalytical Chemistry, 407, 4949–4970.
Martin, J., Camacho-Munoz, D., Santos, J. L., Aparicio, I., & Alonso, E. (2012). Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and exotoxicological impact of wastewater discharges and sludge disposal. Journal of Hazardous Materials, 239-240, 40–47.
McAdam, E. J., Bagnall, J. P., Koh, Y. K. K., Chiu, T. Y., Pollard, S., Scrimshaw, M. D., Lester, J. N., & Cartmell, E. (2010). Removal of steroid estrogens in carbonaceous and nitrifying activated sludge processes. Chemosphere, 81, 1–6.
Mes, T., Zeeman, G., & Lettinga, G. (2005). Occurrence and fate of estrone, 17β-estradiol and 17α-ethinylestradiol in STPs for domestic wastewater. Environmental Science and Technology, 4, 275–311.
Mittal, A. (2011). Biological wastewater treatment. Water Today, 32–44.
Mnif, W., Dagnino, S., Escande, A., Pillon, A., Fenet, H., Gomez, E., Casellas, C., Duchesne, M. J., Hernandez-Raquet, G., Cavailles, V., Balaguer, P., & Bartegi, A. (2010). Biological analysis of endocrine disrupting compounds in Tunisian sewage treatment plants. Archives of Environmental Contamination and Toxicology, 59, 1–2.
Mohagheghian, A., Nabizadeh, R., Mesdghinia, A., Rastkari, N., Mahvi, A. H., Alimohammadi, M., Yunesian, M., Ahmadkhaniha, R., & Nazmara, S. (2014). Distribution of estrogenic steroids in municipal wastewater treatment plants in Tehran, Iran. Journal of Environmental Health Science and Engineering, 12, 97.
Muz, M., Sonmez, M. S., Komesli, O. T., Barirdere, S., & Gokcay, C. F. (2012). Determination of selected natural hormones and endocrine disrupting compounds in domestic wastewater treatment plants by liquid chromatography electrospray ionization tandem mass spectrometry after solid phase extraction. Analyst, 137, 884.
Nelson, J., Bishay, F., Roodselaar, A., Ikonomou, M., & Law, F. C. P. (2007). The use of in vitro bioassays to quantify endocrine disrupting chemicals in municipal wastewater treatment plant effluents. Science of the Total Environment, 374, 80–90.
Noppe, H., Bizec, B. L., Verheyden, K., & Brabander, H. F. (2008). Novel analytical methods for the determination of steroid hormones in edible matrices. Analytica Chimica Acta, 611, 1–16.
Pal, A., Gin, K. Y. H., Lin, A. Y. C., & Reinhard, M. (2010). Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Science of the Total Environment, 408, 6062–6069.
Pereira, R. O., Postigo, C., Alda, M. L., Daniel, L. A., & Barcelo, D. (2011). Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere, 82, 789–799.
Pessoa, G., Souza, N. D., Vidal, C., Alves, J. A. C., Firmino, P. I. M., Nascimento, R. F., & Santos, A. B. D. (2014). Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Science of the Total Environment, 490, 288–295.
Ra, J. S., Lee, S. H., Lee, J., Kim, H. Y., Lim, B. J., Kim, S. H., & Kim, S. D. (2011). Occurrence of estrogenic chemicals in South Korea surface waters and municipal wastewaters. Journal of Environmental Monitoring, 13, 101.
Racz, L. A., & Goel, R. K. (2009). Fate and removal of estrogens in municipal wastewater. Journal of Environmental Monitoring, 12, 58–70.
Ren, Y. X., Nakano, K., Nomura, M., Chiba, N., & Nishimura, O. (2007). Effects of bacterial activity on estrogen removal in nitrifying activated sludge. Water Research, 41, 3089–3096.
Sarmah, A. K., Northcott, G. L., Leusch, F. D. L., & Tremblay, L. A. (2006). A survey of endocrine disrupting chemicals (EDCs) in municipal sewage and animal waste effluents in the Waikato region of New Zealand. Science of the Total. Environment, 355, 135–144.
Servos, M. R., Bennie, D. T., Burnison, B. K., Jurkovic, A., Mclnnis, R., Neheli, T., Schnell, A., Seto, P., Smyth, S. A., & Ternes, T. A. (2005). Distribution of estrogens, 17β-estradiol and estrone, in Canadian municipal wastewater treatment plants. Science of the Total Environment, 336, 155–170.
Silva, C. P., Otero, M., & Esteves, V. (2012). Process for the elimination of estrogenic steroid hormones from water: a review. Environmental Pollution, 165, 38–58.
Sim, W. J., Lee, J. W., Shin, S. K., Song, K. B., & Oh, J. E. (2011). Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants. Chemosphere, 82, 1448–1453.
Soto, A. M., Calabro, J. M., Prechtl, N. V., Yau, A. Y., Orlando, E. F., Daxenberger, A., Kolok, A. S., Guillete, L. J., Bizec, B., Lange, I. G., & Sonnenschein, C. (2004). Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in Eastern Nebraska, USA. Environmental Health Perspectives, 112(3), 346–352.
Spellman, F.R. (1999). Spellman’s standard handbooks for wastewater operators. 1.
Sperling, M. (2006). Wastewater characteristics, treatment and disposal. Biological Wastewater Treatment Series. 1.
Surujlal-Naicker, S., & Bux, F. (2013). Application of radio-immunoassays to assess the fate of estrogen EDCs in full scale wastewater treatment plants. Journal of Environmental Science and Health, 48, 37–47.
Svenson, A., Allard, A. S., & Ek, M. (2003). Removal of estrogenicity in Swedish municipal sewage treatment plants. Water Research, 37, 4433–4443.
Swart, N., & Pool, E. (2007). Rapid detection of selected steroid hormones from sewage effluents using an ELISA in Kuils River water catchment area, South Africa. Journal of Immunoassay and Immunochemistry, 28(4), 395–408.
Tan, B. L. L., Hawker, D. W., Muller, J. F., Leusch, F. D. L., Tremblay, L. A., & Chapman, H. F. (2007). Comprehensive study of endocrine disrupting compounds using grab and passive sampling at selected wastewater treatment plants in South East Queensland, Australia. Environment International, 33, 654–669.
Ternes, T. A., Kreckel, P., & Mueller, J. (1999). Behaviour and occurrence of estrogens in municipal sewage treatment plants—II. Aerobic batch experiments with activated sludge. Science of the Total Environment, 225, 91–99.
Xu, N., Xu, Y. F., Xu, S., Li, J., & Tao, H. C. (2012). Removal of estrogens in municipal wastewater treatment plants: a Chinese perspective. Environmental Pollution, 165, 215–224.
Yang, M., Wang, K., Shen, Y., & Wu, M. (2011). Evaluation of estrogenic activity in surface water and municipal wastewater in Shanghai, China. Bulletin of Environmental Contamination and Toxicology, 87, 215–219.
Ye, X., Guo, X., Cui, X., Zhang, X., Zhang, H., Wang, M. K., Qiu, L., & Chen, S. (2012). Occurrence and removal of endocrine disrupting chemicals in wastewater treatment plants in the Three Gorges Reservoir area, Chongqing, China. Journal of Environmental Monitoring, 14, 2204.
Ying, G. G., Kookana, R. S., & Ru, Y. J. (2002). Occurrence and fate of hormone steroids in the environment. Environmental International, 28, 545–551.
Yu, C. P., Deeb, R. A., & Chu, K. H. (2013). Microbial degradation of steroidal estrogens. Chemosphere, 9, 1225–1235.
Zeng, Q. L., Li, Y. M., Gu, G. W., Zhao, J. M., Zhang, C. J., & Luan, J. F. (2008). Sorption and biodegradation of 17β-estradiol by acclimated aerobic activated sludge and isolation of the bacterial strain. Environmental Engineering Science, 26(4), 783–790.
Zhang, Z., Feng, Y., Gao, P., Wang, C., & Ren, N. (2011). Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. Journal of Environmental Monitoring, 13, 1366.
Zhang, Y., & Zhou, J. L. (2008). Occurrence and removal of endocrine disrupting chemicals in wastewater. Chemosphere, 73, 848–853.
Zhou, Y., Zha, J., Xu, Y., Lei, B., & Wang, Z. (2012). Occurrence of six steroid estrogens from different effluents in Beijing, China. Environmental Monitoring Assessment, 184, 1719–1729.
Zorita, S., Martensson, L., & Mathiasson, L. (2009). Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Science of the Total Environment, 407, 2760–2770.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ting, Y., Praveena, S.M. Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: a mini review. Environ Monit Assess 189, 178 (2017). https://doi.org/10.1007/s10661-017-5890-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5890-x