Abstract
This paper provides data on the occurrence of selected human pharmaceuticals (carbamazepine, clofibric acid, diclofenac, fenofibrate, fenoprofen, gemfibrozil, ibuprofen, ketoprofen, and naproxen) including steroid hormones (17β-estradiol, 17α-ethinylestradiol, and estrone) in influents/effluents to/from the four principal wastewater treatment plants (WWTPs) serving the city of Rome (Italy), in two different sampling campaigns. Target compounds were also analyzed in the receiving River Tiber and River Aniene. Analytical determination was carried out by LC-MS/MS after sample cleanup and concentration by off-line solid-phase extraction (SPE). The aim of the study was to increase the information currently available on the presence and persistence of pharmaceuticals in Italian urban wastewaters and to evaluate the environmental impact of the pharmaceutical residues discharged through effluents into the receiving rivers. Results indicated that after the treatment processes, most of pharmaceuticals were not completely eliminated, as average removal efficiencies were in the 14–100 % wide range during both sampling periods, with higher yields in spring than in winter. Levels detected in overall samples ranged from 5 to 2,230 ng/L in influents and from 5 to 1,424 ng/L in effluents. Carbamazepine, diclofenac, ibuprofen, and gemfibrozil showed the highest persistence to removal. Concentrations in the receiving waters were about one order of magnitude lower than in effluents, with a tendency to increase progressively through the urban tract of the river. Finally, an environmental risk analysis showed that carbamazepine, gemfibrozil, and estrone can pose a high risk at the concentrations detected in effluents and a medium risk in rivers, highlighting their potential hazard for the health of the aquatic ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human pharmaceuticals are organic micropollutants widespread in the aquatic ecosystems because of their extensive use in our daily life. In European countries, there are 4,000 different pharmaceutical ingredients for human consumption (therapeutic or diagnostic purposes), and although their production and administration may vary both between countries and over time (Ternes and Joss 2006; Kummerer 2009), their consumption is increasing (Ellis 2006) and new compounds are continually being introduced. Over recent years, Italy has raised its position in the world and European pharmaceutical markets. In comparison with other EU countries, Italy was, in 2012, the third largest market, but the second country (after Germany) for pharmaceutical production volumes and number of pharmaceutical companies (Gruppo di Lavoro OsMed 2012; www.farmindustria.it 2013).
Pharmaceuticals enter the water cycle mainly through human excretion, either as parent (unchanged) compounds or as a mixture of metabolites and/or conjugated compounds. Other important sources can be from disposal of unused or expired medicines in domestic sewage and hospital wastes (Halling-Sørensen et al. 1998; Heberer 2002). Once introduced into wastewater treatment plants (WWTPs), they are not effectively removed (Vieno et al. 2007; Verlicchi et al. 2012; Martínez Bueno et al. 2012). Compounds of the same therapeutic class may have quite different chemical and physical properties resulting in different behaviors during treatment processes (tendency to remain in the dissolved phase, to adhere to flocs or particles or to undergo biodegradation) and in different removal efficiencies (Ternes and Joss 2006).
While most northern European WWTPs include tertiary wastewater treatments, in Italy, mainly primary and secondary treatments are performed, with the latter being based on conventional activated sludge, while tertiary treatments are seldom applied. Because of this incomplete elimination, WWTPs are the cause of 70–80 % of pharmaceutical occurrence in ecosystems (Ternes and Joss 2006): they have been detected in WWTP effluents, surface waters (Kolpin et al. 2002; Calamari et al. 2003; Fent et al. 2006; Gros et al. 2007; Fatta-Kassinos et al. 2011; Spongberg et al. 2011; Loos et al. 2012), seawater, and groundwater (Halling-Sørensen et al. 1998; Fent et al. 2006). The concentrations measured in WWTP effluents are generally in the ng/L to μg/L range and in the ng/L range in surface waters (Halling-Sørensen et al. 1998; Kolpin et al. 2002; Fent et al. 2006). The need to monitor pharmaceutical occurrence in water ecosystems was also recognized by the European Union in the latest revision of the Water Framework Directive (Directive 2000/60/EC); two hormones, i.e., 17α-ethinylestradiol and 17β-estradiol, and one anti-inflammatory, diclofenac, have been included in the watch list of substances (Directive 2013/39/EU 2013) to be monitored pending a possible subsequent definition of Environmental Quality Standard (EQSs). According to the current European Regulatory Guidance set by the European Medicines Agency (EMEA 2006), the approval procedure of new human pharmaceuticals requires an environmental risk assessment based on standard acute toxicity tests (to algae, Daphnia magna and fish) if the predicted (PEC) or measured (MEC) environmental concentration of the active ingredient is >10 ng/L. Despite our knowledge about the environmental occurrence of pharmaceuticals has increased, thanks to new analytical techniques capable of detecting compounds in trace quantities, there are still little data available from specific geographical regions and information about their fate in natural waters is, unfortunately, far from adequate.
Over the past decade, several studies have been conducted on the occurrence of pharmaceuticals in Italian municipal WWTPs and their removal efficiencies (Andreozzi et al. 2003; Castiglioni et al. 2005; Castiglioni et al. 2006; Zuccato et al. 2010; Ferrari et al. 2011; Al Aukidy et al. 2012; Calza et al. 2013, Repice et al. 2013; Verlicchi et al. 2014); however, such data are limited to the northern region of Italy while very few data are available for the most densely populated Italian city, Rome (central Italy), focused on steroid hormones (Johnson et al. 2000; Laganà et al. 2004) and on some pharmaceuticals in the effluents of only one out of the four principal WWTPs, in northern Rome (Andreozzi et al. 2003; Loos et al. 2012). In the light of these concerns, the aim of the present study was to investigate the occurrence of selected pharmaceutical classes in municipal wastewaters from the principal WWTPs serving the city of Rome. The main focus of this work was 12 pharmaceuticals spanning a range of therapeutic classes, in particular, five analgesics/anti-inflammatories, three lipid regulators, one antiepileptic and three (natural and synthetic) steroid hormones. The pharmaceuticals were also monitored in the receiving surface waters (i.e., the River Tiber and its tributary the River Aniene), upstream from the metropolitan area and downstream from the effluent discharge points, in order to evaluate their occurrence and impact, in terms of both single compound concentrations and mass loads, on these rivers. Two sampling periods were planned on the basis of the different hydrological regimes of the receiving waters that affect contaminant dilution. Finally, the possible ecological implications were recognized through an environmental risk analysis assessment regarding both the effluents and the receiving water bodies under investigation.
Materials and methods
Standards and chemicals
All pharmaceutical standards were purchased from Sigma-Aldrich (Steinheim, Germany). Individual stock solutions were prepared by dissolving 5 mg of each compound in 10 mL of acetonitrile and stored at −20 °C. Composite working standard solutions of the compounds were prepared by mixing suitable aliquots of the stock solutions in acetonitrile/water (45:55, v/v) and then stored at 4 °C. The isotopically labeled compounds used as internal standards were ibuprofen-d3, carbamazepine-C13C6, and estrone-2,4,16,16-d4 obtained from Sigma-Aldrich (Steinheim, Germany). Stock solutions of the internal standards were prepared in acetonitrile and stored at −20 °C. A mixture of these standards, used for internal standard calibration, was also prepared by diluting the individual stock solutions in acetonitrile/water (45:55, v/v).
For chromatographic analysis, distilled water was further purified by passing it through a Milli-Q apparatus (Millipore, Bedford, MA, USA). Methanol, acetonitrile, acetone, formic acid, and n-hexane of HPLC-grade were obtained from VWR (Radnor, PA, USA). Analytical-grade glacial acetic acid (99 %) was supplied by Carlo Erba (Milan, Italy).
Selection of pharmaceuticals and environmental sample collection
Figure 1 shows the average consumption of pharmaceuticals based on the therapeutic usage (Gruppo di Lavoro OsMed 2012), both in Italy (Fig. 1a) and Lazio (Fig. 1b) region, the region crossed by the urban stretch of the River Tiber and where the city of Rome is located (Fig. 2). The therapeutic groups most widely administered under medical prescription by primary care practices are those targeting the cardiovascular, digestive, nervous, and hormonal systems. Nevertheless, both analgesic and non-steroidal anti-inflammatory (NSAIDs) drugs rank among the most used pharmaceuticals by population (with or without any medical prescription), while antibiotics show the lowest consumption especially in Lazio region. Moreover, Fig. 3 shows the age-averaged population density of Rome in 2011 (ISTAT 2014). The figure indicates a progressive aging of the population (increase in the intermediate age range), which may partly explain the prevalence in the consumption of certain pharmaceutical classes (i.e., anti-inflammatory or lipid regulator drugs) rather than other (i.e., antibiotics, mostly consumed in childhood). Accordingly, on the basis of the main consumption by the resident population but also interest in impact on environmental health and availability of analytic methodologies, the following pharmaceuticals have been selected for the present study: carbamazepine, clofibric acid, diclofenac, fenofibrate, fenoprofen, gemfibrozil, ibuprofen, ketoprofen, naproxen, 17β-estradiol, 17α-ethinylestradiol, and estrone.
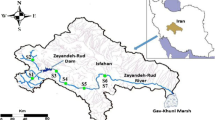
Four activated sludge treatment plants receiving sewage from the city of Rome (Italy) were sampled for this study (Fig. 2, Table 1). The WWTP-Rome North (RN) is located on the right bank of the River Tiber and collects slurry from the northern districts of the city mixed with industrial sewage. The WWTP-Rome South (RS) is situated on the left bank of the River Tiber for the treatment of sewage from the southern area of the city. The WWTP-Rome East (RE) is located on the left bank of the River Aniene, the main tributary of the River Tiber in its urban stretch, and collects domestic sewage from the eastern part of the city and also some industrial discharges. The WWTP-Rome-Ostia (RO) is located on the left bank of the River Tiber about 2 km from its mouth and collects domestic waste from coastal districts. At present, WWTP-RN and WWTP-RO include a tertiary treatment based on chlorination, but this operates only during the summer season and, in any case, not when the sampling was carried out. Table 1 summarizes the main characteristics of the WWTPs studied as well as the rivers where their effluents are discharged.
For each plant, 24-h composite untreated (influent (IN)) and treated (effluent (OUT)) wastewater samples were collected (56 samples) in pre-cleaned brown bottles. Sampling was carried out every day for a week in two seasonal periods, February and May 2011, selected on the basis of a significant difference in the flow conditions of the receiving rivers (see below).
In the laboratory, the samples were filtered through 0.7-μm glass fiber filters (Whatman, Maidstone, UK) to remove suspended matter. Filtered aqueous samples were brought to pH 3.6 with glacial acetic acid and then stored in the dark at −20 °C until analysis.
The River Tiber basin is located in central Italy, draining an area of 17,156 km2, and has an annual mean flow rate of about 240 m3/s. With a length of 409 km, the river flows through the Tuscany, Umbria, and Lazio regions and passes through the city of Rome, before flowing into the Tyrrhenian Sea. In its final stretch, the River Tiber receives a substantial amount of contamination from the waters of the tributary, the River Aniene, which in turns receives discharges from urban and industrial activities before entering the Tiber. Moreover, the River Tiber receives significant urban discharges from the metropolitan area of Rome, which officially has about 3.9 million residents and 2,224/km2 population density (ISTAT, 2014).
Grab river water samples were collected from five different sites as shown in Fig. 2: one upstream from the urban stretch of the River Tiber (site UP, 42° 05′ 13.38′ N; 12° 36′ 06.90′ E), two in the northern and southern urban stretch of the river (site RN, 41° 57′ 31.70′ N; 12° 29′ 21.54′ E; site RS, 41° 48′ 25.14′ N; 12° 25′ 4.6′ E), one near to the basin closure (site RO, 41° 44′ 42.80′ N; 12° 15′ 5.24′ E) and the last one from the River Aniene (site AN, 41° 55′ 12.86′ N; 12° 34′ 17.79′ E, mean annual flow rate 35 m3/s, length 99 km, draining area 1,414 km2); sampling was carried out in February and May 2011 in correspondence with the WWTPs sampling; the average daily discharge during sample collection was, for River Tiber, 210 and 139 m3/s in the two campaigns, respectively, while for River Aniene 31 and 25 m3/s. All the sampling sites, with the only exception of the UP site, were located about 1–2 km downstream from their respective WWTP effluents (Fig. 2).
Two liters of surface water samples were collected in means of two subsamples for each point, in the center of the stream section (20 samples totally), and put in amber glass flasks, previously cleaned with diluted hydrochloric acid and ultrapure water. All the samples were immediately refrigerated at 4 °C during the transport. In the laboratory, they were filtered through Whatman GF/F glass fiber filters (0.7-μm nominal pore size) and stored at 4 °C until analysis, which was done within few days.
Extraction procedure and instrumental analysis
The pharmaceuticals were extracted and pre-concentrated at least in duplicate from each sample by off-line solid-phase extraction (SPE), performed with an optimized method previously reported (Patrolecco et al. 2013). Briefly, 250 mL of raw wastewater, 500 mL of wastewater effluent and 1,000 mL of river sample, acidified to pH = 3.6, were processed through pre-activated Strata X cartridges at a flow rate of about 10 mL/min. Before elution, the cartridges were rinsed with 10 mL of methanol/water (5:95, v/v) followed by 10 mL of n-hexane at a flow rate of 1 mL/min. After each rinse, the cartridges were air-dried for 20 and 45 min, respectively. Twenty milliliters of acetone were used as the eluent phase at a flow rate of about 1 mL/min. The eluate was evaporated in a flask by a Rotavapor (Büchi, Flawil, Switzerland) to reach a final volume of few milliliters. The residue was evaporated under nitrogen and reconstituted with 0.5 mL of acetonitrile/water (45:55, v/v with 0.1 % formic acid). Finally, 10 μL of a 10 μg/mL standard mixture of the internal standards was added in the extract for internal standard calibration and to compensate for possible matrix effects.
Analysis was performed by liquid chromatography-ion trap mass spectrometry using a Finnigan Surveyor Plus LC System (Thermo Finnigan, San Jose, CA, USA), consisting of a quaternary pump, vacuum degasser, and autosampler. For quantitative determination, the HPLC system was interfaced to a LCQ DECA XP MAX mass spectrometer (Thermo Finnigan), which consists of an ESI interface operating in negative and positive modes and an ion trap mass analyzer (Finnigan Ion Max universal source). The software for the control of the equipment and for the acquiring and elaboration of data was Xcalibur 1.3 workstation. Instrumental parameters were optimized for each compound separately to obtain the maximum sensitive unit resolution and are listed in Table 1S (Supplementary material). The determination was carried out on the daughter ion obtained by fragmentation (MS/MS) of the isolated protonated molecule, in the positive-ion polarity, applying a spray voltage of 4.5 kV; the capillary temperature in the ion transfer tube was 300 °C; the sheath gas pressure was set to 45 psi and the auxiliary gas flow to 5 psi. The MS analysis in the negative mode was performed to quantify the deprotonated molecules of the hormones, applying a spray voltage of −3.5 kV, and the sheath gas pressure was set to 35 psi and the auxiliary gas flow to 8 psi.
Chromatographic separation of the analytes was performed using a Hypersil Gold Column (100 × 2.1 mm i.d., 5 μm) supplied by Thermo Electron Corporation (San Jose, CA). Twenty microliters of the final extract were injected by the autosampler into the LC with acetonitrile/water (v/v with 0.1 % formic acid) as the mobile phase starting at 45 % acetonitrile. At a flow rate of 300 μL/min, the gradient increased to 80 % acetonitrile in 10 min and finally decreased linearly to the initial condition in 15 min. The column temperature was maintained at 25 °C. Calibration functions were built at the following concentrations: 4; 20; 50; 100; 200; and 1,000 ng/L. Correlation coefficient values were always over 0.99. The parent mass, fragmentation products monitored, collision energies, limit of detections (LODs), limit of quantifications (LOQs), and precision data (RSD %) for both wastewater and river water are reported in Table 2S (Supplementary material). LODs were calculated accordingly to IUPAC method (IUPAC 1999). LOQ was set at the lower concentration of calibration functions and varied between 4 and 60 ng/L for surface water and between 4 and 150 ng/L for wastewater. To evaluate the matrix effect, chromatographic peak areas of each compound from the analysis of spiked wastewater and surface water extracts were compared with peak areas from matrix-free solutions spiked at the same concentration. Recoveries obtained were generally higher than 70 % for both river waters and wastewaters, with the exception of several compounds that yielded lower, but still acceptable, recoveries: clofibric acid, 63 %; ethinylestradiol, 68 %; and estradiol, 65 %.
Results and discussion
Occurrence of pharmaceuticals in the WWTPs investigated
Table 2 shows the average pharmaceutical concentrations detected in IN and OUT samples from four WWTPs in two seasonal different periods. Seven pharmaceuticals (carbamazepine, naproxen, fenoprofen, diclofenac, ibuprofen, clofibric acid, and gemfibrozil) were detected in all the WWTPs at both samplings, while ketoprofen was detected on one occasion, May 2011, in two plants and estrone only in the effluents of three out of the four WWTPs. Fenofibrate, estradiol and ethinylestradiol were never detected. Whatever the sampling time, carbamazepine, diclofenac, ibuprofen, and gemfibrozil showed the highest concentrations both in influent and effluent samples; their values in the influents were, respectively, in the overall, 110–1,519; 514–2,230; 77–564; and 113–1,489 ng/L ranges, while in the effluents, they were in the 69–886; 321–1,424; 41–184; and 56–1,032 ng/L ranges. The relevant concentrations recorded in all the WWTPs suggest that these pharmaceuticals are chronically consumed, confirming a wide use of NSAIDs, lipid regulators, and anti-epileptic drugs by the residents, especially among the middle-aged population, as previously shown in Figs. 1 and 3. Moreover, most of NSAIDs are used for diseases and symptoms which are highly prevalent among the general population and can be bought over the counter without a medical prescription in Italy.
Naproxen ranged between 20–231 and 13–80 ng/L in IN and OUT samples, respectively, fenoprofen between 10–75 and 5–41 ng/L, and clofibric acid between 7–36 and 5–19 ng/L. Ketoprofen was recorded in only two plants (WWTP-RS and WWTP-RO) at concentrations in the influent of 63–198 ng/L and in the effluent of 31–120 ng/L. Estrone was detected only in the effluents from WWTP-RN, WWTP-RS, and WWTP-RO with values in the 8–45 ng/L range, and its absence in the influents could be due to a lower method sensitivity and/or matrix effects, which can hamper the detection of some analytes in these kinds of complex waters; moreover, according to the scientific literature, during biological treatment, estrone can be generated in the sewer as a by-product of the biodegradation of natural estrogen (i.e., de-conjugation of conjugated metabolites) and subsequently released through effluents (Johnson et al. 2000).
These concentration values are consistent with previously published data on the occurrence of selected pharmaceuticals in different Italian urban wastewaters (Andreozzi et al. 2003; Laganà et al. 2004; Castiglioni et al. 2005; Al Aukidy et al. 2012), except in the case of the analgesic naproxen. Occurrence of naproxen has been reported at concentrations significantly higher than in this investigation (range of 21–5,220 ng/L in the above-cited literature against the range we measured, of 13–80 ng/L). Other studies carried out in different countries (Verlicchi et al. 2012) showed ranges of pharmaceutical variability even two or three orders of magnitude higher than those found in this study while previous data published in WWTPs serving European large city and having quite similar number of inhabitants served (Vieno et al. 2005; Gracia-Lor et al. 2012; Martínez Bueno et al. 2012) were comparable to our results.
In spring, there was a general increase in concentration values both in IN and OUT samples for all target compounds when compared with the values recorded in winter, with the only exception of ibuprofen which showed an opposite trend in WWTP-RO effluent. The higher values in spring are connected to the higher loads of drugs reaching the treatment plants in this season (see the following paragraph and Fig. 4). The average percentage removal efficiencies were estimated by comparing the concentration of each pharmaceutical in the influent and effluent wastewaters of the WWTPs under investigation and are given in Table 2. Our data show that the compounds investigated were not completely removed in all the WWTPs. The lowest removal efficiency was observed for diclofenac, followed by carbamazepine and gemfibrozil, which seemed to resist the water treatment, although their levels in the effluents were lower than in the influents (Table 2).
Comparing the removal efficiencies obtained in the two different seasons, the average removal ranged between 14 and 100 % in the winter sampling and between 19 and 93 % in the spring one (Table 2); however, no statistically significant difference was found between the two periods (t test; p > 0.05), so that a general seasonal variation was not clearly observed, but only several differences in removal efficiency for single compounds. Removal was generally higher in the spring season than in the winter one for carbamazepine, naproxen, fenoprofen, and ibuprofen, whichever WWTP was investigated; on the other hand, this did not occur for clofibric acid, diclofenac, and gemfibrozil, which were removed mainly in February. The psychiatric drug carbamazepine was quite persistent, showing removal efficiencies between 19 and 79 % in the overall plants, which is fairly in line with previous studies (Vieno et al. 2007; Zhang et al. 2008; Gonzàles-Alonso et al. 2010). With respect to the seasonal period of sampling, carbamazepine removal was, on average, 50 % in spring and 32 % in winter. Removal of the lipid regulators gemfibrozil and clofibric acid (the main metabolite of clofibrate) was in the 19–100 % range in most of the WWTPs, with a mean value in spring of 36 and 61 %, respectively, and in winter of 48 and 71 %. Fenofibrate was never detected. As regard to the anti-inflammatory class, pharmaceuticals were removed in a range between 14 % (fenoprofen) and 93 % (ibuprofen) (Table 2), with a mean value in spring (59, 60, 36, and 74 % for naproxen, fenoprofen, diclofenac, ibuprofen, respectively) generally higher than in winter (35, 45, 39, and 52 %, respectively), while ketoprofen was detected only on one occasion (May 2011) with a mean removal of 45 %. Although our study did not reveal a clear seasonal pattern in the removal of pharmaceuticals and the mechanism for their elimination in WWTPs is not precisely known, biodegradation and sorption are likely to be the main elimination processes in the WWTPs investigated, and both mechanisms are temperature-dependent. For many compounds, sorption increases with a decreasing temperature, whereas biological degradation works with a lower efficiency at a lower water temperature (Vieno et al. 2005), so that in the two periods investigated, the one or the other removal mechanism may have prevailed owing to the different seasonal temperatures and to the different physico-chemical characteristics (such as log K ow, Table 3S in Supplementary material) of the compounds. Other studies evidenced contrasting results on seasonal trend in pharmaceutical concentrations in wastewater: Collado et al. (2014) noticed a lower total pharmaceutical concentration in winter than in spring and summer samples from a municipal WWTP in Catalonia, Spain, while in a previous study Castiglioni et al. (2006), for the same classes of pharmaceuticals (namely NSAIDs, psychiatric drugs, lipid regulators, cardiovascular agents, and antibiotics), found summer WWTP influent loads about half than in winter in north Italy; Golovko et al. (2014), in a recent study conducted over 1 year in a WWTP in Czech Republic, observed a significant seasonal difference in influent and effluent pharmaceutical concentrations, with higher values in winter season. Such contrasting seasonal variations may depend upon either societal factors (production, consumption, excretion) or environmental factors (solar irradiance, precipitation, temperature, etc.) (Vieno et al. 2005; Bueno et al. 2012; Yu et al. 2013; Valcárcel et al. 2013). Substantially, our data seem to confirm what has already been reported in the literature on the incomplete elimination of most of trace organic polluting compounds, such as pharmaceuticals, in conventional WWTPs with secondary biological treatment.
Load of pharmaceuticals in WWTPs and occurrence in the receiving waters (River Tiber and River Aniene)
In order to evaluate the input of pharmaceuticals into the receiving river waters due to WWTP discharges, for each WWTP, the total load of target pharmaceuticals in influent and effluent wastewaters was calculated by multiplying the sum of the average concentrations of each pharmaceutical by the flow rates, then normalizing for the population equivalent of each plant. As shown in Fig. 4, average loads found in our survey for the different WWTPs ranged from 1.33 to 3.63 g/day/1,000 inhabitants for influents and from 0.59 to 2.15 g/day/1,000 inhabitants for effluents sampled in spring, and in winter from 0.55 to 1.89 g/day/1,000 inhabitants for influents and from 0.33 to 1.05 g/day/1,000 inhabitants for effluents. The WWTP-RS, which serves the southern district of Rome, received the highest load of target pharmaceuticals (3.63 and 1.89 g/day/1,000 inhabitants in spring and winter, respectively) and, consequently, was responsible for the highest loads in the effluents (2.15 and 1.05 g/day/1,000 inhabitants in spring and winter, respectively) that are afterward discharged into the receiving waters. Considering the average total load calculated for the overall WWTPs in the two seasons, we found a pharmaceutical load in spring about twice that in winter in both influents (2.00 vs 1.05 g/day/1,000 inhabitants in spring and winter, respectively) and effluents (1.16 vs 0.62 g/day/1,000 inhabitants in spring and winter, respectively) although, as previously discussed, the removal efficiency of each WWTP was higher in the former than in the latter season for most pharmaceuticals. This could be due to the fact that, although higher temperatures facilitate the metabolic processes occurring during biological treatment and, consequently, pharmaceuticals ought to be removed more efficiently in the warm season, concentrations in the inlets were so high that the levels remaining in effluents were still significant. This can be especially true for those pharmaceuticals most resistant to biodegradation, such as carbamazepine, diclofenac, and gemfibrozil (De Graaf et al. 2011; Grenni et al. 2013).
Average pharmaceutical concentrations detected in river waters in the two different seasons are shown in Table 3. Overall, eight out of the 12 pharmaceuticals investigated were detected in surface water although not all compounds were always found in each site. The pharmaceuticals most detected were carbamazepine, naproxen, diclofenac, ibuprofen, and gemfibrozil. Estrone, ketoprofen, and clofibric acid were found only at one or two sites along the river stretch. Finally, fenoprofen, fenofibrate, 17β-estradiol, and 17α-ethinylestradiol were never detected. The highest concentrations were found for naproxen, diclofenac, and ibuprofen (155, 120, and 112 ng/L, respectively) followed by carbamazepine and gemfibrozil (102 and 65 ng/L) in spring sampling. All the remaining compounds were detected in the low to medium ng/L range in both seasons.
Upon discharge of treated wastewaters into surface waters, concentrations of pharmaceuticals decline, mainly due to dilution, which varies depending on factors such as stream flow rate conditions and percentage of treated wastewaters in the receiving water bodies. Looking at our data set obtained in the lower flow sampling condition (spring), surface water concentrations were found to be, on average, ten times lower than the corresponding effluent concentrations, with a dilution factor, calculated on a daily average, in the River Tiber varying between about 17 (8.0 m3/s of WWTP-RS effluent mixed with 139 m3/s of river water) and about 107 (1.3 m3/s of WWTP-RO effluent mixed with 139 m3/s of river water), and one of around 6 in the River Aniene (4.3 m3/s of WWTP-RE effluent mixed with 25 m3/s of river water). The only unexpected exception was observed for naproxen at the RS and RO sites, which showed higher concentration values in river water (94 and 155 ng/L, respectively) than in the respective WWTP effluents (80 ng/L in WWTP-RS and 57 ng/L in WWTP-RO), and this occurred even in winter sampling (Tables 2 and 3); the explanation may be that there are buildings not yet connected to the sewage network, illegal local raw discharges or, possibly, resuspension from riverine settled material. Comparing our results with previous published data on Mediterranean rivers crossing metropolitan area similar sized to Rome, such as Ebro basin and other rivers in the Madrid region or such as Rhone basin in Switzerland-France regions (Gros et al. 2007; Loos et al. 2009; Gonzáles-Alonso et al. 2010; Valcárcel et al. 2013), similar concentration ranges of the same pharmaceutical classes can be found, confirming their incomplete removal from municipal wastes of densely urbanized areas. On the basis of available data, a more systematic, integrated monitoring-modeling risk assessment approach should be implemented to assess the entity of input of these pharmaceuticals and identify those that are likely to pose the greatest risk to environmental and human health.
Even though the pharmaceuticals in the river were subjected to dilution, the same spectrum of compounds was observed in both effluents and river waters, as illustrated in Fig. 5, which shows the average concentrations of the pharmaceuticals in the WWTP effluents (Fig. 5a) and in the downstream receiving waters (Fig. 5b). The UP site in River Tiber, located upstream from the metropolitan area and the WWTPs, shows low concentrations of compounds, which tend to increase progressively when the river runs through the urban area, to its mouth. These findings confirm that WWTP effluents represent the main source of pharmaceuticals in river waters, and this was evident, in our results, also from the substantial concentrations detected at site RS, located downstream from the urban area of Rome and affected not only by the WWTP-RS effluent but also by all the discharges in the upper part of the river; the same considerations can be drawn for the RO site, located at the basin closure of the River Tiber, where the highest concentrations of target compounds were found (Table 3 and Fig. 5), in spite of the higher dilution factor at this site. The dilution factor has been reported to be the main cause of the decrease in concentration of pharmaceuticals when effluents enter river waters, but other causes have to be considered, such as sorption onto solids, biodegradation, and photodegradation occurring in natural waters. For example, for diclofenac, naproxen, and ketoprofen, photodegradation has been reported to be a significant way of elimination in surface waters (Tixier et al. 2003; Andreozzi et al. 2003); despite this, they are continuously detected at high levels in the aquatic environment, due to their continuous human consumption and release by WWTP effluents (Hernando et al. 2006). In our previous study on biodegradation of two pharmaceuticals in River Tiber waters (Grenni et al. 2013), we showed that gemfibrozil was more resistant than naproxen (half-life DT50 ≥70 and 27 days, respectively) to microbial degradation, but the latter was found in higher concentrations in the river because of its pseudopersistence, linked to the spread in its use among the population. The psychiatric drug carbamazepine has been reported to be the most resistant of the investigated compounds to natural attenuation processes, as it does not biodegrade or show a tendency to adsorb or photodegrade (Andreozzi et al. 2003; Löffler et al. 2005), so that the variability in its concentration between effluents and receiving waters is most likely a consequence of hydrological conditions, dilution processes, and different mobility behaviors.
Environmental impact of selected pharmaceuticals
Residual pharmaceuticals transmitted from effluents to receiving water bodies can represent a potential risk for aquatic life (Ferrari et al. 2003; Fent et al. 2006; Isidori et al. 2007); the risk quotient (RQ) was therefore calculated from our data on both WWTP effluents and river waters, using the maximum measured environmental concentration (MEC) of each pharmaceutical and then comparing this value with its predicted no effect concentrations (PNEC). PNEC values were taken from the scientific literature, estimated for fish, daphnids, and algae (Stuer-Lauridsen et al. 2000; Ferrari et al. 2003; Tauxe-Wuersch et al. 2005; Carlsson et al. 2006; Isidori et al. 2007; Quinn et al. 2008; Lin et al. 2008) on acute toxicity (data on chronic toxicity are lacking for many pharmaceuticals) by dividing the half maximal effective concentration (EC50) values by the assessment factor 1,000 (TDG 1996). The use of EC50 to predict PNEC is widely used to estimate if levels detected would induce any adverse effect to aquatic organisms. When more than one PNEC was available, as a precaution, we chose the lowest value. On the other hand, MECs correspond to maximum levels detected for each compound in order to assess risks in the most extreme situations. Based on EMEA guidelines (EMEA 2006), if the ratio of MEC/PNEC equals or exceeds 1, then an ecological risk is suspected (Tauxe-Wuersch et al. 2005; Hernando et al. 2006; Santos et al. 2007). Common criteria for interpreting the RQ in risk assessment studies, establishing different risk levels, were also applied in the present approach: low risk is considered from 0.01 through 0.1, medium risk from 0.1 through 1 and high risk >1 (Hernando et al. 2006). Following this approach, an estimation of the aquatic risk for the target pharmaceuticals is shown in Table 4. By calculating RQ values in river waters, it could be concluded that at the concentrations found in the sampling sites surveyed, the individual pharmaceuticals could pose from no to medium environmental risk. In particular, carbamazepine, estrone, and gemfibrozil were found to be at a medium risk level in the river environment (RQ in the range 0.1–1) while in the effluents, the same compounds posed a high environmental risk (RQ >1) (Table 4). Moreover, a medium risk was found to be posed by diclofenac in WWTP effluents, while for all the other compounds, the RQ values calculated were consistently <0.1 both in effluents and river waters, corresponding to a minimal or zero risk. Carbamazepine and gemfibrozil appeared to be the more hazardous components because of their low PNEC compared to the high concentrations detected (Table 4), reflecting their potential to cause ecological effects, as reported in other studies (Zhang et al. 2008; Gonzáles-Alonso et al. 2010). It is reasonable to suppose that dilution of wastewaters, once pharmaceuticals are discharged in receiving river waters, can mitigate possible environmental hazards. In the study by Al Aukidy et al. (2012) of the Po valley (northern Italy), diclofenac, naproxen, ketoprofen, and carbamazepine were found to be low-risk pollutants; Gros et al. (2010) assessed that no significant risk was associated with the presence of pharmaceuticals in the Ebro River basin (Spain) while in an overview on the ecological risk assessment of pharmaceutical residues from literature data, Hernando et al. (2006) found carbamazepine, ibuprofen, diclofenac, naproxen, ketoprofen, and gemfibrozil to be high-risk pollutants in both WWTP effluents and surface waters. Beyond some discrepancies, all these data indicate that pharmaceuticals can be present in effluents entering the environment at concentrations high enough to have chronic effects and to pose potential risks to the aquatic environment. This can be particularly true for rivers with low dilution capacity due to low flow or intermittent regime conditions. Moreover, pharmaceuticals are present in the aquatic environment as mixtures of different therapeutic classes, which could induce toxicity to non-target organisms at concentrations at which a single compound does not show or shows only little effects.
Conclusions
The occurrence of 12 pharmaceuticals, including three steroid hormones, in four WWTPs serving the most populated Italian city, Rome, has been established, together with their concentration levels in the receiving waters, the Rivers Tiber and Aniene.
The results indicate that conventional wastewater treatment is unable to efficiently eliminate the target pharmaceuticals, showing wide ranges of removal efficiencies in both sampling seasons (spring and winter), so that most of them were detected and quantified in effluents and, consequently, found to contaminate the receiving waters. Levels in wastewaters ranged from few ng/L to several hundred ng/L in sewage effluents and up to μg/L in influents. Carbamazepine, diclofenac, ibuprofen, and gemfibrozil were the pharmaceuticals most resistant to the wastewater treatments, showing the highest concentrations in all effluents; the same pattern of concentrations was found in the receiving river waters, where level of pharmaceuticals rose continuously from the upper part of the river, located outside the urban area, to its mouth. Concentrations in the river downstream from the WWTP discharges were about one order of magnitude lower than in the respective effluents, mainly owing to dilution of wastewaters, even in the low flow sampling.
The concentration of pharmaceuticals in the environment and their evolution over time depend not only on the amount discharged from WWTPs but also on the geographical area, climate conditions, and hydrological characteristics of the receiving water bodies. Although the dilution capacity of the receiving water body can be considered of prime importance in reducing and controlling the potential ecotoxicological effects of pharmaceutical residues released into the aquatic environment, in our study, a high risk was suspected to be posed in WWTP effluents for the following compounds: carbamazepine, gemfibrozil, and estrone, while a medium risk was found for the same pharmaceuticals in river waters. For all the other compounds, concentrations found in receiving waters corresponded to a minimal or zero risk. Finally, the possible synergistic or antagonistic effects of mixtures should also be taken into account for a proper environmental risk analysis, and information on the ecological effects of metabolites and intermediates is also needed.
References
Al Aukidy M, Verlicchi P, Jelic A, Petrovic M, Barcelò D (2012) Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci Total Environ 438:15–25
Andreozzi R, Marotta R, Paxèus N (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330
Bueno MJM, Gómez MJ, Herrera S, Hernando MD, Agüera A, Fernández-Alba AR (2012) Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ Pollut 164:267–273
Calamari D, Zuccato E, Castiglioni S, Bagnati R, Fanelli R (2003) Strategic survey of therapeutic drugs in the Rivers Po and Lambro in Northern Italy. Environ Sci Technol 37:1241–1248
Calza P, Medana C, Padovano E, Giancotti V, Minero C (2013) Fate of selected pharmaceuticals in river waters. Environ Sci Pollut Res 20:2262–2270
Carlsson C, Johansson AK, Alvan G, Bergman K, Kühler T (2006) Are pharmaceuticals potent environmental pollutants? Part I: environmental risk assessments of selected active pharmaceutical ingredients. Sci Total Environ 364:67–87
Castiglioni S, Bagnati R, Calamari D, Fanelli R, Zuccato E (2005) A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. J Chrom A 1092:206–215
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2006) Removal of pharmaceuticals in sewage treatment plants in Italy. Environ Sci Technol 40:357–363
Collado N, Rodriguez-Mozaz S, Gros M, Rubirola A, Barceló D, Comas J (2014) Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ Pollut 185:202–212
De Graaf MS, Vieno NM, Kujawa-Roeleveld K, Zeeman G, Temmink H, Buisman CJN (2011) Fate of hormones and pharmaceuticals during combined anaerobic treatment and nitrogen removal by partial nitritation-anammox in vacuum collected black water. Water Res 45:375–383
Directive 2000/60/EC of the European Parliament and of the Council of October 23 establishing a framework for community action in the field of water policy (FWD), O.J. L 327, December 22, (2000).
Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy, L 226/1 (2013).
Ellis JB (2006) Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ Pollut 144:184–189
EMEA (2006) European Medicines Agency Pre-Authorization. Evaluation of medicines for human use. Committee for Medicinal Products for Human Use (CHMP), (Doc. Ref. EMEA/CHMP/SWP/4447/00), 12 pp.
Farmindustria (2013) The association of pharmaceutical companies and member of Confindustria “L’industria farmaceutica in Italia: un’analisi di confronto europeo a partire dai bilanci”. www.farmindustria.it. Accessed 12 April 2014.
Fatta-Kassinos D, Meric S, Nicolaou A (2011) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem 399:251–275
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquatic Toxicol 76:122–159
Ferrari B, Paxèus N, Lo Giudice R, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol Environ Safety 55:359–370
Ferrari F, Gallipoli A, Balderacchi M, Ulaszewska MM, Capri E, Trevisan M (2011) Exposure of the main Italian river basin to pharmaceuticals. J Toxicol 2011:1–11
Golovko O, Kumar V, Fedorova G, Randak T, Grabic R (2014) Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 111:418–426
Gonzáles-Alonso S, Catalá M, Romo Marota R, Rodríguez Gil JL, Gil de Miguel A, Valcárcel Y (2010) Pollution by psychoactive pharmaceuticals in rivers of Madrid metropolitan area (Spain). Environ Int 36:195–201
Gracia-Lor E, Sancho JV, Serrano R, Hernández F (2012) Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 87:453–462
Grenni P, Patrolecco L, Ademollo N, Tolomei A, Barra Caracciolo A (2013) Degradation of gemfibrozil and naproxen in a river water ecosystem. Microchem J 107:158–164
Gros M, Petrović M, Barceló D (2007) Wastewaters treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro River basin (Northeast Spain). Environ Toxicol Chem 26(8):1553–1562
Gros M, Petrović M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26
Gruppo di Lavoro OsMed (2012) L’uso dei farmaci in Italia. Rapporto Nazionale anno 2012, pp. 393. Agenzia Italiana del Farmaco AIFA www.agenziafarmaco.gov.it. Accessed 6 October 2014.
Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment: a review. Chemosphere 36:357–393
Heberer T (2002) Occurrence, fate and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Hernando MD, Mezcua M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Isidori M, Nardelli A, Pascarella L, Rubino M, Parrella A (2007) Toxic and genotoxic impact of fibrates and their photoproducts on non-target organisms. Environ Int 33(5):635–41
ISTAT (2014) National Institute of Statistics, Population Housing Census, www.dati.istat.it. Accessed 13 October 2014
IUPAC (1999) International Union of Pure and Applied Chemistry. Harmonized guidelines for in-house validation of methods of analysis. Technical Report 1999. http://www.iupac.org/projects/1997/501_8_97.html.
Johnson AC, Belfroid A, Di Corcia A (2000) Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci Total Environ 256:163–173
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kummerer K (2009) The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. J Environ Manage 90:2354–2366
Laganà A, Bacaloni A, De Leva I, Faberi A, Fago G, Marino A (2004) Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal Chim Acta 501:79–88
Lin AYC, Yu TH, Lin CF (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141
Löffler D, Römbke J, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Technol 39:5209–5218
Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G (2009) EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157:561–568
Loos R, Carvalho R, Comero S, António DC, Ghiani M, Lettieri T, Locoro G, Paracchini B, Tavazzi S, Gawlik BM (2012) EU Wide Monitoring Survey on wastewater treatment plant effluents, Technical Report European Commission, Joint Research Centre, Institute for Environment and Sustainability EUR 25563 EN, doi:10.2788/60663; pp. 138.
Martínez Bueno MJ, Gomez MJ, Herrera S, Hernando MD, Agüera A, Fernández-Alba AR (2012) Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: two years pilot survey monitoring. Environ Pollut 164:267–273
Patrolecco L, Ademollo N, Grenni P, Tolomei A, Barra Caracciolo A, Capri S (2013) Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem J 107:165–171
Quinn B, Gagné F, Blaise C (2008) The effects of pharmaceuticals on the regeneration of the cnidarian, Hydra attenuata. Sci Total Environ 402:62–69
Repice C, Dal Grande M, Maggi R, Pedrazzani R (2013) Licit and illicit drugs in a wastewater treatment plant in Verona, Italy. Sci Total Environ 463–464:27–34
Santos JL, Aparicio I, Alonso E (2007) Occurrence and risk assessment of pharmaceutically active compounds in wastwater treatment plants. A case study: Seville city (Spain). Environ Int 33:596–601
Spongberg WJ, Acuña J, Vargas J, Murillo M, Umaña G, Gómez E, Perez G (2011) Reconnaissance of selected PPCP compounds in Costa Rica surface waters. Water Res 45:6709–6717
Stuer-Lauridsen F, Birkved M, Hansen LP, Holten Lützhøft HC, Halling-Sørensen B (2000) Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 40:783–793
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2005) Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res 39:1761–1772
TDG (1996) Technical Guidance Document in support of Council Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) 1488/94 on risk assessment for existing substances. Office for Official Publications of the European Communities, Luxembourg
Ternes TA, Joss A (2006) Human pharmaceuticals, hormones and fragrances. The challenge of micropollutants in urban water management. IWA Publishing, London, pp 243–277
Tixier C, Singer HP, Oellers S, Müller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Valcárcel Y, Gonzáles Alonso S, Rodríguez-Gil JL, Castaño A, Montero JC, Criado- Álvarez JJ, Mirón IJ, Catalá M (2013) Seasonal variation of pharmaceutically active compounds in surface (Tagus River) and tap water (Central Spain). Environ Sci Poll Res 20(3):1396–1412
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment: a review. Sci Total Environ 429:123–155
Verlicchi P, Al Aukidy M, Jelic A, Petrović M, Barceló D (2014) Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: a case study of a catchment area in the Po valley (Italy). Sci Tot Environ 470–471:844–854
Vieno NM, Tuhkanen T, Kronberg L (2005) Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in recipient water. Environ Sci Technol 39:8220–8226
Vieno NM, Tuhkanen T, Kronberg L (2007) Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res 41(5):1001–1012
Yu Y, Wu LS, Chang AC (2013) Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci Total Environ 442:310–316
Zhang Y, Geiben SU, Gal C (2008) Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73:1151–1161
Zuccato E, Castiglioni S, Bagnati R, Melis M, Fanelli R (2010) Source, occurrence and fate of antibiotics in the Italian aquatic environment. J Hazard Mater 179:1042–1048
Acknowledgments
The authors wish to acknowledge the ACEA S.p.A. Group for providing wastewater samples and information about the treatment plants monitored. The authors express gratitude to Dr. Stefano Polesello (IRSA-CNR) for his constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leif Kronberg
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Patrolecco, L., Capri, S. & Ademollo, N. Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ Sci Pollut Res 22, 5864–5876 (2015). https://doi.org/10.1007/s11356-014-3765-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3765-z