Abstract
The concentration of 17β-estradiol (E2) was measured through stages of wastewater treatment at a central Illinois wastewater treatment plant (WWTP). E2 concentration was quantified using a competitive enzyme-linked immunosorbent assay (ELISA). The concentration of E2 was compared with demographic effects of a university; physical parameters of the wastewater (dissolved oxygen, pH, and temperature); and daily influent and effluent flow rates. Effluent concentrations ranged from 0 to 25.3 ng L−1 with an average discharge of 3.6 ng L−1. E2 concentration was shown to increase at the start of each university semester; however, this trend was not observed in the summer sessions. Low influent and effluent flow rates, which correspond to increased water retention time at the WWTP, were correlated to increased removal efficiency of E2, where low flow was linked to 91 % removal efficiency and high flow with 58 % removal efficiency. This study concludes that E2 was being discharged at concentrations known to cause ecological risk, and that the demographic changes in a university student body had a significant effect on E2 concentration throughout the treatment process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
17β-estradiol, often abbreviated as E2 because it has two hydroxyl groups in its molecular structure, is an essential form of estrogen in the body (Combalbert and Hernandez-Raquet 2010). E2 is one of three forms of estrogen naturally produced by all vertebrate organisms. Low-dose concentrations (1–10 ng L−1) of this chemical has been shown to disrupt normal reproductive function in fish (Cripe et al. 2009). E2 is also linked to many diseases in humans (Braga et al. 2005; Shappell 2006; Singh et al. 2003). Treatment of our wastewaters containing steroid estrogens and other contaminants of concern is the first line of defense in reducing these chemicals from our environment because human excretion is thought to be the primary source of elevated concentrations of E2 in our environment (Combalbert and Hernandez-Raquet 2010). The focus of this study is to determine how effective an activated sludge wastewater treatment plant is at reducing levels of E2 from raw sewage, if demographic changes in a university student body have an effect on concentrations of E2 throughout the treatment process and whether physical parameters (pH, dissolved oxygen, temperature) influenced the concentration of E2 throughout the treatment process.
Specifically, estrogens and related compounds are used for a wide array of therapeutic purposes including menopausal therapy, osteoporosis, endometrial diseases, prostate cancer, breast cancer, and heart disease (Wright-Walters et al. 2007). Moreover, estrogenic compounds derived from plant compounds such as isoflavones (e.g., soy concentrate found in protein bars and shakes) and polyphenols (found in many health food supplements) are used in high concentrations to enrich foods (Adlercreutz 2002; Farre et al. 2007; Liu et al. 2001). As a result, the load of estrogenic chemicals that we are exposed to and subsequently excrete into our waters is continuously increasing (Jones-Lepp et al. 2009). Aquatic organisms such as fish and amphibians often serve as primary biological indicators (Hutchinson et al. 2005) with E2 contamination first being observed in fish species living in lotic systems downstream of municipal wastewater treatment plants (WWTP; Jobling et al. 2003; Jobling et al. 2005).
Over the last 10 years, chronic exposure to E2 has repeatedly been observed to cause intersex gonads in male fish, increased plasma vitellogenin, reduced egg and sperm production, a change in physiological behavior, lower quality gametes, and the complete feminization of male fish (Woods and Kumar 2011; Hutchinson et al. 2005; Jobling et al. 2003; Rankouhi et al. 2004). The concentration of E2 required to cause intersex gonads in male fish has been shown to be as little as 1 ng L−1 with vitellogenin onset as low as 5 ng L−1 (Jobling et al. 2003, 2005). The exposure of E2 and the observed risks to aquatic organismal health has raised concerns as to what damage and/or health-related conditions are being expressed in humans and terrestrial wildlife as a result of similar exposures.
Children and immature wildlife are at the greatest risk to elevated environmental E2 concentrations. In humans, studies have shown that E2 exposure in pre-pubertal and pubertal children may lead to excessively rapid growth as well as early onset of puberty in females and late onset of puberty in males (ATSDR 2007). In post-pubertal developmental stages, environmental estrogens have the ability to induce testicular (Giannandrea et al. 2013) and ovarian cancer (Kang et al. 2013) as well as stimulate endometriosis, heart disease, osteoporosis, and Alzheimer’s disease (Wright-Walters et al. 2007).
The concern over steroid estrogens in our waterways has become more apparent in the last 10 years with improvements in technology that can detect lower estrogen concentrations. Today, steroid estrogens in water and wastewater can be easily detected to the level of a part per trillion (ng L−1) using enzyme-linked immunosorbent assay (ELISA; Farre et al. 2007; Shappell 2006), enabling researchers to ask questions regarding the effects of low-level exposures. The US Environmental Protection Agency (USEPA) is currently determining how to regulate E2. This hormone is currently on the USEPA’s Contaminant Candidate List (CCL3; USEPA 2014), which is a list of contaminants that are currently not subject to any proposed or promulgated national primary drinking water regulations, that are known or anticipated to occur in public water systems, and which may require regulation under the Safe Drinking Water Act (USEPA 2009). Information generated from this study will have direct application to filling the data gaps associated with the USEPA’s CCL3 listing. The World Health Organization (WHO) also recognizes E2 as a known chemical which is found in drinking water in countries around the world (WHO 2011).
The primary concern of steroid estrogens in the environment is linked to their “endocrine disrupting ability” (Coleman et al. 2007; Wu et al. 2011; Shappell 2006; Howell 2005; Huang and Sedlak 2001). There is a body of literature that has shown that endocrine disrupting chemicals have the ability to greatly alter the health and reproduction of a diversity of animal life (Dodwell and Vergote 2005; Caldwell et al. 2010). These taxa can also be looked at as a warning of potential dangers to humans (i.e., a canary in a coal mine). For example, E2 in our drinking water could affect male fertility by interfering with sperm production (Braga et al. 2005). The first step to reducing endocrine-active compounds in our waterways is to determine how our current wastewater treatment processes and associated environmental parameters affect the bioavailability of E2. Steroid estrogens not removed during wastewater treatment will be released into our environment. The efficiency of wastewater treatment processes need to be quantified and better understood in terms of the ability to remove contaminants of concern.
The purpose of this study is to determine how effective a traditional activated sludge WWTP is at removing E2. Activated sludge is the process by which sewage and industrial wastewaters are treated using air and a “biological floc” composed of bacteria and protozoa. This is one of the most common methods employed by WWTPs to clean sewage (Tong et al. 1980). The study is unique in the fact that it characterizes the effectiveness of all wastewater treatment stages as well as coupling specific parameters, specifically pH, dissolved oxygen (DO), temperature, and influent and effluent flow rates, which may aid in the reduction/uptake of E2, while taking demographic effects into account. Other research investigating the abundance of steroid estrogens in sewage treatment have focused on novel methods for E2 reduction, including the use of advanced oxidative techniques or the incorporation of nano/ultra filtration methods (Coleman et al. 2007; Yoon and Westerhoff 2004). There have also been various studies that compare how effective sewage treatment facilities employing completely different methods of treatment (activated sludge vs. membrane bioreactor vs. other advanced treatment techniques) compare in regards to estrogen reduction (Huang and Sedlak 2001; Wu et al. 2011). This project strictly focuses on methods currently employed in over 95 % of WWTPs within the USA and aims to quantify and describe how the various treatment stages influence E2 removal or transformation.
The objectives of the project are to: (1) quantify concentrations of E2 through multiple stages of wastewater treatment (influent, mixed liquor, effluent); (2) determine if any physical parameters (pH, DO, temperature) influence the concentration of E2 in these different stages; (3) identify if Eastern Illinois University (EIU)’s student body impacts E2 concentration. The null hypotheses tested were (1) Ho1: there is no difference in E2 concentration based on treatment stage; (2) Ho2: E2 concentration is independent of pH, DO, and temperature in the wastewater; (3) Ho3: E2 concentration is independent of the student body population. In addition to testing these hypotheses, concentrations of E2 found in the final effluent stage are compared to “action levels” (i.e., allowable concentrations) established by different international (WHO), federal (USEPA has proposed possible action levels), and state agencies (Illinois and other states with action levels) to create hazard indices. If the ratio of our observed concentration (C) to action levels (A) exceeds 1 (i.e., C/A > 1) then this indicates risk to human and aquatic life. This is a standard risk assessment measure (Suter 2006).
Materials and methods
Sample collection and preparation
Water samples were collected two times per week (once a week/one time per weekend between 07:30 a.m. and 10:00 a.m. CST) at the Charleston, IL WWTP. Samples were collected in 250-mL Nalgene plastic bottles from multiple stages of waste water treatment (influent, mixed liquor, effluent) yielding a total of 12 samples per week (52 weeks = 624 samples). The physical parameters (temperature, dissolved oxygen, and pH) were recorded at time of sampling using a HACH multi-probe meter when the meter was available. The collected samples were filtered using Whatman 50-μm glass microfiber filter pads to remove particles that would interfere with subsequent analytical procedures. Filtered samples were then placed in 100-mL glass tubes and frozen at −25 °C for analysis at a later date.
17-β estradiol (E2) quantification
Analyses of E2 concentration were performed using the Abraxis magnetic particle ELISA 17β-estradiol Kit (PN 580002; http://www.abraxiskits.com/estrogen-test-kits/). ELISA for E2 quantification is well established in wastewater analysis and has been validated using GCMS and HPLC (Farre et al. 2007; Huang and Sedlak 2001). The kit protocol for the 17β-estradiol ELISA was strictly followed. Immediately prior to analysis, all samples were homogenized on a shaker table at 250 rpm for 8 h. For every ELISA (94 samples; five calibration standards and one 10 ng L−1 spike), at least a 15 % sample replication was employed to ensure quality assurance/quality control (QA/QC). At the end of the study, a 20 % replication was achieved for all samples, yielding a coefficient of determination (R 2) of 0.63 (Fig. 1). The detection range of the kit was 0.5–25 ng L−1. Two samples fell below detection. Those above-mentioned detection were diluted with reverse osmosis water either at a 2:1 or 3:1 dilution to assure proper quantification. Diluted samples were properly numerically adjusted to be included in the dataset. Samples were quantified using the Abraxis Photometric Analyzer II, with a calibration R 2 of no less than 0.990 nor a replication percent coefficient of variation above 10 %. The Abraxis 17β-estradiol Assay detects 17β-estradiol specifically with little cross-reactivity with other hormones tested. The user guide provides a specificity table for data on several other steroid hormones.
Statistical analysis
All data were archived in Microsoft Excel and statistical analyses were performed using the “R” statistical package version 3.1.2 (R Core Team 2014). A general linear model was used to determine if mean E2 concentration varied by stage of treatment, EIU semester term, and year of sample. To address the effect of flow on E2, two additional general linear models were run. The first included stage of treatment and EIU semester term as factors and flow-in and flow-out [in million gallons per day (MGD)] as covariates. The second included stage of treatment and EIU semester term as factors and the difference between flow-in and flow-out as a covariate. The last model analyzed mean E2 concentration as a function of stage of treatment, EIU semester term, DO, pH, and temperature. One-way ANOVAs with pairwise comparison tests utilizing a Holm correction (Holm 1979) for E2 concentration by term were performed to identify which treatment stages differed within semester terms for E2 concentration, DO concentration, temperature, and pH. All linear models were subjected to a model selection procedure based upon the Akaike Information Criterion (Burnham and Anderson 2002). To determine the daily load of E2 entering and leaving the treatment process, flow data (MGD) were converted to millions of liters per day (MLD), which was then multiplied by the concentration of E2 present in the influent and effluent samples collected on the days that flow was measured. These data were then used to create a mass balance of E2 through the process of waste water treatment. Removal efficiency was calculated by dividing the difference between influent and effluent concentration/load by the influent value and multiplying by 100. Flow rates were considered either high or low. High flow is considered a daily average flow of over 11 MLD and low flow is daily flow under 11 MLD.
Study site (Charleston, IL waste water treatment plant)
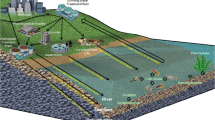
The city of Charleston is located in Coles County in east-central Illinois and lies within the Embarrass River drainage basin. The city operates an activated sludge wastewater treatment facility with a capacity of 3.3 million gallons per day (MGD) (12.49 MLD) design average flow (DAF) and 6.0 MGD (22.71 MLD) design maximum flow (DMF), which includes preliminary, primary and secondary treatments (Fig. 2). The facility also utilizes both aerobic and anaerobic digestion and produces approximately 400 dT (dry tons) of anaerobically treated sludge annually. The city operates a combined sewer collections system consisting of approximately 240 km of sewers sized from 10- to 107-cm pipe with 11 lift stations within eight drainage basins. This system serves a population of 21,100 (US Census 2010), which includes the faculty, staff, and student body of Eastern Illinois University.
Results
17β-estradiol was found in concentrations ranging between LOD = 25.3 ng L−1 in the effluent of the Charleston WWTP with a mean discharge concentration of E2 at 3.6 ng L−1 (Fig. 3). Overall, the Charleston WWTP was shown to have daily average removal efficiency for E2 at 64 % with a range between 0 and 99 %. E2 concentration differed by semester term in the influent, mixed liquor, and effluent stages (Table 1, Figs. 3 and 4). These concentrations also decreased throughout the wastewater treatment stages (Table 1, Figs. 3 and 4). The general linear model also yielded a main effect of year and interactions between treatment stage and EIU semester term and between treatment stage and year of sample (Table 1). Thus, E2 concentration varied significantly by semester term, year of sample, and stage of treatment (F 20, 519 = 24.37, p = 6.18 × 10−62, R 2 = 0.4843), but stage of treatment (37.2 %), semester term (22.0 %), and their interaction (36.7 %) accounted for most of the explained variation compared to year of sample (1.3 %) and the interaction of stage of treatment and year of sample (2.7 %).
17β-estradiol concentration of Charleston, IL WWTP treatment stages over a 1-year period (Jan.–Dec. 2013). Samples from 2012 and 2014 were omitted for presentation purposes. SP1 spring semester first half, SP2 spring semester second half, SU- all summer sessions, FA1 fall semester first half, FA2 fall semester second half
Mean 17β-estradiol concentration (ng L−1) comparison of the Charleston, IL WWTP by treatment stage (influent, mixed liquor, effluent) within EIU semesters. Each time block represents half of a semester (spring early spring semester first half, spring late spring semester second half, Fall early fall semester first half, fall late fall semester second half), except summer (summer all summer sessions). Letters (A/B/C) are based upon all pairwise comparisons with a Holm (1979) correction of the probabilities. Letters that are identical represent no significant difference between stages
Daily influent and effluent flow shows a seasonal pattern (Fig. 5) with lower flows during the fall terms. Influent flow varies from a high of 22.71 MLD to a low of 5.44 MLD and effluent flow varies from a high of 21.18 MLD to a low of 1.04 MLD. The total mass of E2 shows a decrease from influent to effluent similar to the decrease in E2 concentration (Fig. 6). The seasonal pattern of E2 concentration and mass are very similar for both influent (Fig. 7) and effluent (Fig. 8) samples. The spikes in E2 mass are made relatively smaller in the fall relative to spring and summer due to lower fall flows in comparison to the spikes in concentration for both influent (Fig. 7) and effluent (Fig. 8) samples. A general linear model analyzing E2 concentration as a function of factors for semester term and stage of treatment with influent and effluent flow as covariates (F 24, 515 = 19.90, p = 1.49 × 10−58, R 2 = 0.4812; Table 2) showed large effects due to stage of treatment (37.7 % of explained variation), semester term (18.7 % of explained variation) and their interaction (39.1 % of explained variation) and smaller effects for the interaction of semester term and influent flow (2.3 % of explained variation), and the interaction of semester term and effluent flow (2.0 % of explained variation) and the main effects of influent (0.13 % of explained variation) and effluent (0.11 % of explained variation) flow. A general linear model analyzing E2 concentration as a function of factors for semester term, year of sample, and stage of treatment and utilizing the difference between influent and effluent flow as a covariate (F 19, 520 = 24.86, p = 6.64 × 10−61, R 2 = 0.4760; Table 3) shows qualitatively the same results. Sample year was dropped from both of these models as it was not an influential parameter in the previous model.
Charleston, IL WWTP influent and effluent daily flow totals in million gallons per day (MGD) for samples collected from January 2013 through December 2013. EIU semester terms are labeled; SP1 spring semester first half, SP2 spring semester second half, SU all summer sessions, FA1 fall semester first half, FA2 fall semester second half
Total 17β-estradiol loading (mg day−1) of the Charleston, IL WWTP influent and effluent. Samples were collected from January 2013 through December 2013. EIU semester terms are labeled; SP1 spring semester first half, SP2 spring semester second half, SU all summer sessions, FA1 fall semester first half, FA2 fall semester second half
Charleston, IL WWTP influent E2 concentration (ng L−1), influent daily flow total (million liters per day MLD) and influent daily total of E2 loading (mg per day). Samples were collected from January 2013 through December 2013. EIU semester terms are labeled; SP1 spring semester first half, SP2 spring semester second half, SU all summer sessions, FA1 fall semester first half, FA2 fall semester second half
Charleston, IL WWTP effluent E2 concentration (ng L−1), effluent daily flow total (million liters per day MLD) and effluent daily total of E2 loading (mg per day). Samples were collected from January 2013 through December 2013. EIU semester terms are labeled; SP1 spring semester first half, SP2 spring semester second half, SU all summer sessions, FA1 fall semester first half, FA2 fall semester second half
High influent concentrations of E2 were associated with low flow, especially during the months of August and September (Figs. 3 and 7). However, how low daily flow influences the spikes in E2 concentration during the months of January and February could not be modeled because the retention time is unknown. The daily load of E2 entering and leaving the plant (effluent) follows similar trends to that of concentration of E2 (Figs. 7 and 8). E2 removal efficiency was influenced by flow rate. Average load of E2 removed per day was 72 %; however, the average removal efficiency for E2 was 91 % for periods of low flow rate and 58 % for high flow rates.
The physical parameters of the slurry also vary as a function of the stage of treatment and semester term. For dissolved oxygen (DO), the saturated model was the final model selected (Stage: F 2, 381 = 44.64, p = 3.82 × 10−18; Term: F 4, 381 = 3.17, p = 0.0141; Interaction: F 8, 381 = 2.06, p = 0.0393). The overall linear model fit was F 14, 381 = 8.39, p = 8.21 × 10−16, R 2 = 0.2357. For temperature, the main-effects model was the final model selected (Stage: F 2, 389 = 25.26, p = 4.84 × 10−11; Term: F 4, 389 = 407.95, p = 1.05 × 10−137). The overall linear model fit was F 6,389 = 279.80, p = 9.60 × 10−138, R 2 = 0.8119. For pH, the saturated model was the final model selected (Stage: F 2, 381 = 2170.26, p = 5.67 × 10−209; Term: F 4,381 = 27.96, p = 2.26 × 10−20; Interaction: F 8,381 = 5.11, p = 4.62 × 10−6). The overall linear model fit was: F 14,381 = 323.9, p = 1.21 × 10−201, R 2 = 0.9225.
A final general linear model was used to determine if E2 concentration was a function of treatment stage, EIU semester term, DO, pH, and temperature (Table 4: F 47,334 = 10.20, p = 1.82 × 10−41, R 2 = 0.5893). Sample year was dropped from this model as it was not an influential parameter in previous models. Stage of treatment (50.9 %), semester term (17.3 %), and their interaction (6.9 %) accounted for most of the explained variation compared to year of sample (1.3 %) and the interaction of stage of treatment and year of sample (2.7 %). The physical parameters only entered the model as two- or three-way interactions with stage of treatment and semester term (Table 4).
Discussion
Adverse effects to ecological health are often reported with respect to fish populations downstream of WWTP effluents (Jobling et al. 2005; Nash et al. 2004; Routledge and Sheahan 1998; Thorpe and Hutchinson 2001; Wise et al. 2011; Woods and Kumar 2011). When exposed to estrogens or estrogen-like compounds (xenoestrogens) at low-dose concentrations (≥1 ng L−1), fish populations have shown a disruption to normal reproductive health including inter-sexing of male gonads, complete feminization of male fish, a change in plasma vitellogenin, reduced egg and sperm production, lower quality gametes, and a change in normal physiological behavior (Jobling et al. 2003, 2005; Metcalfe et al. 2001; Thorpe et al. 2001; Wise et al. 2011). E2 was found in the Charleston WWTP discharge at concentrations greater than 1 ng L−1 with a range of 0.5–25.3 ng L−1 and a mean of 3.6 ng L−1. One of the objectives of this study was to provide hazard indices for E2 discharging from the Charleston WWTP. However, neither the state of Illinois, USEPA, nor the World Health Organization provides action level standards in either ecological or environmental health guidelines (USEPA CCL3; WHO 2011). Despite this, it is clear that the E2 concentrations being released as effluent in this study are higher than concentrations that have been shown to result in ecological insult. The UK has proposed predicted-no-effect-concentrations (PNEC) of 1 ng L−1 for E2 (Young et al. 2004); therefore, the hazard index for any sample can simply be divided by “1,” with any index >1.0 indicating risk.
The onset of adverse reproductive effects to fish populations can be seen within a range of 1–10 ng L−1 (Cripe et al. 2009; Gunnarsson et al. 2007; Jobling et al. 2005 and Jobling et al. 2003; Kawamura et al. 2002; Kidd et al. 2007; Metcalfe and Metcalfe 2001; Nash et al. 2004; Rankouhi et al. 2004; Razmara et al. 2008; Routledge and Sheahan 1998; Seki et al. 2006; Thorpe and Hutchinson 2001; Thorpe 2000; Velu and Ramanathan 2011; Woods and Kumar 2011) from exposure to E2, which indicates a risk to ecological health in receiving waters of the Charleston WWTP. The mean concentration of E2 that is discharged throughout the duration of this study indicates acute exposure of E2 to receiving waters of the Charleston WWTP. Ecological risk often serves as the forerunner to establishing environmental risk guidelines for contaminant exposure (Suter 2006); however, at this time, there are no regulations or limits regarding E2 concentration in drinking or surface water, although it is recognized as a chemical of concern by the World Health Organization, the EPA, and other government agencies (USEPA CCL3, WHO 2011).
Human excretion and activity is a significant source of estrogens in the environment, thereby making wastewater treatment the first line of defense for reducing the concentration of these chemicals (Nash et al. 2004; Routledge and Sheahan 1998; Thorpe and Hutchinson 2001). Activated sludge has become the standard of wastewater treatment in the USA and is typically incorporated at most levels of sewage treatment (treatment larger than lagoon level). Manipulation of physical parameters could possibly influence efficiency for removing contaminants of concern in our wastewaters; however, this is unlikely to occur because these parameters, such as DO, pH, and temperature often need to be maintained within a regulatory range set by a governing agency (e.g., USEPA) to ensure healthy waters. Although pH was shown to have a significant interaction effect in one of the general linear models, the range (7.1–7.4) is most likely not biochemically meaningful. Many other studies have focused on new or advanced technologies to reduce pharmaceutical and endocrine disrupting chemical concentrations, with very few aiming to improve the efficiency for removal of endocrine disrupting chemicals in wastewater treatment processes already in practice (Coleman et al. 2007; Wu et al. 2011). It appears that the most influential parameter that could be manipulated for plants similar to the one in this study would be retention time.
The demographic effect of a city with a student population making up approximately one half of the city’s population was observed. Mean E2 concentration was shown to be highly influenced by semester term, stage of treatment, and the interaction of term and stage (Table 2). The student population, specifically the young female population within childbearing years, is likely to increase the concentration of estrogenic compounds in the water supply via excretion of metabolized oral contraceptives and natural estrogen (Wise et al. 2011; Wright-Walters et al. 2007). Specifically, menstruating females can release up to 3.5 μg day−1 as opposed to post-menopausal women and males at 2.3 and 1.6 μg day−1, respectively (Wise et al. 2011). This is supported by the increased influent concentrations and loading values of E2 during the initial weeks of each semester (Fig. 1). EIU’s dining services may also be a contributing factor of estrogens received at the WWTP through food waste, and may have an added influence of altering the physical parameters of the water due to a highly carbonaceous waste stream. This effect was not measured in the design of this study, but should be looked at in future research examining the influence of industry on municipal wastewater.
The patterns of daily E2 load of the WWTP influent and effluent closely followed that of E2 concentration. However, the flow rate alone does not account for all of the fluctuations in E2 concentration. Daily flow is the determining factor in the retention time of water within the treatment process (high flow = short water retention time, low flow = long retention time). During low daily flow periods, water can be retained in the treatment process for up to 24 h, while high daily flow periods can lead to a retention time as little as 8 h (Author: Collard, Superintendent, Charleston WWTP). Other studies have shown that treatment time (retention time at a WWTP) is a determining factor in the efficiency of removing estrogens through common waste water treatment processes (Braga et al. 2005).
Daily flow rate was shown to be closely associated with the efficiency at which the treatment process removed E2 from the received water streams. The WWTP had an average removal efficiency of 72 % for daily load of E2 with a range between 0 and 99 %. The months of August and September received the lowest daily average flow recorded at the WWTP; however, this period exhibited the highest average removal efficiency for daily E2 load (91 %). When compared to February and March, which had the highest daily average flow, the removal efficiency of E2 removal dropped to an average of 58 %. This suggests that E2 removal efficiency during wastewater treatment is linked to retention time at the plant. Flow rate dictates the retention time of water at the WWTP, thus flow rate is a critical factor for E2 removal efficiency throughout the treatment process.
The decomposition process throughout wastewater treatment has the potential to change the speciation of many chemicals of concern in our watersheds (Braga et al. 2005). Previous studies have shown effective removal of E2 through wastewater treatment; however, estrone (E1) has been found to increase in concentration throughout the treatment process (Braga et al. 2005). E2 is suspected to breakdown into E1 throughout the aerobic wastewater treatment processes (Weber et al. 2005). The effect of this conversion and the abundance of E1 during wastewater treatment were beyond the scope for this study, but is something that should be considered in future studies. However, studies which have compared biodegradation between the steroids have demonstrated a rank order of decreasing persistence: 17α-ethinylestradiol > E1 > E2. D’Haese et al. (2000) tested 17β-estradiol and 17α-ethinylestradiol in the ISO 9888 test (a standardized batch test that examines aerobic biodegradability of organic compounds) and showed that E2 biodegraded where 17α-ethinylestradiol was persistent. Other experiments in which 30 ng L−1 of 17β-estradiol was added to sewage effluent indicated that after 1 day, significant biodegradation had occurred (65 % removal) with E1 as the resulting metabolite. After 3 days, neither E2 nor E1 was detected. By comparison, the degradation of 17α-ethinylestradiol differed compared to natural estrogens: sewage effluent spiked at 100 ng L−1, removal occurred slower with an adaptation phase of 7 days and 20 % remaining after 21 days. Interestingly, changes in temperature of approximately 15 °C had a statistically significant effect on the rate of mineralization and removal of E2 (increased rate with increasing temperature), although no such effect was seen for 17α-ethinylestradiol (Young et al. 2004).
References
Adlercreutz, H. (2002). Phytoestrogens and cancer. Journal of Steroid Biochemistry and Molecular Biology, 83(1–5), 113–118.
ATSDR. (2007). Sunnyside area groundwater contamination. US department of health and human services. Public Health Service: Division of Health Assessment and Consultation. Atlanta, Georgia 30333
Braga, O., Smythe, G., Schafer, I., & Feitz, J. (2005). Steroid estrogens in primary and tertiary wastewater treatment plants. Water science and technology : a journal of the international association on water pollution research, 52(8), 273–278.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: a practical information-theoretic approach (2nd ed.). NY: Springer Verlag.
Caldwell, D. J., Mastrocco, F., Nowak, E., Johnston, J., Yekel, H., Pfeiffer, D., Hoyt, M., DuPlessie, B. M., & Anderson, P. D. (2010). An assessment of potential exposure and risk from estrogens in drinking water. Environmental Health Perspectives, 118(3), 343.
Coleman, H. M., Vimonses, V., Leslie, G., & Amal, R. (2007). Removal of contaminants of concern in water using advanced oxidation techniques. Water Science & Technology, 55(12), 301–306.
Combalbert, S., & Hernandez-Raquet, G. (2010). Occurrence, fate, and biodegradation of estrogens in sewage and manure. Applied Microbiology and Biotechnology, 86(6), 1671–1692.
Core Team, R. (2014). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Cripe, G. M., Hemmer, B. L., Goodman, L. R., Fournie, J. W., Raimondo, S., Vennari, J. C., Danner, R. L., Smith, K., Manfredonia, B. R., Kulaw, D. H., & Hemmer, M. J. (2009). Multigenerational exposure of the estuarine Sheepshead Minnow (Cyprinodon variegates) to 17b-estradiol: organism level effects over three generations. Journal of Environmental Toxicology, 28(11), 2397–2408.
D’Haese, I., Kozak, A., Huysmans A., Van Malderen, V., Dhooge, W., Weemases, M. and Verstraete, W. (2000) Pharmaceuticals in the environment: focus on the estrogen 17α-ethinyloestradiol. In: Proceedings of International Seminar on Pharmaceuticals in the Environment. March 9th 2000, Brussels, Tecnological Institute.
Dodwell, D., & Vergote, I. (2005). A comparison of fulvestrant and the third-generation aromatase inhibitors in the second-line treatment of postmenopausal women with advanced breast cancer. Cancer Treatment Reviews, 31(4), 274–282.
Farre, M., Kuster, M., Brix, R., Rubio, F., Lopez de Alda, M. J., & Barcelo, D. (2007). Comparative study of estradiol enzyme-linked immunosorbent assay kit, liquid chromatography-tandem mass spectrometry, and ultra performance liquid chromatography-quadrupole time of flight mass spectrometry for part-per trillion analysis of estrogens in water samples. Journal of Chromatography A, 1160, 166–175.
Giannandrea, F., Paoli, D., Figà-Talamanca, I., Lombardo, F., Lenzi, A., & Gandini, L. (2013). Effect of endogenous and exogenous hormones on testicular cancer: the epidemiological evidence. The International Journal of Developmental Biology, 57, 255–263. http://doi.org/10.1387/ijdb.130015fg.
Gunnarsson, L., Kristiansson, E., Förlin, L., Nerman, O., & Larsson, D. G. J. (2007). Sensitive and robust gene expression changes in fish exposed to estrogen—a microarray approach. BMC Genomics, 8, 149.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70.
Howell, A. (2005). New developments in the treatment of postmenopausal breast cancer. Trends in Endocrinology & Metabolism, 16(9), 420–428.
Huang, C. H., & Sedlak, D. L. (2001). Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environmental Toxicology and Chemistry / SETAC, 20(1), 133–139.
Hutchinson, T. H., Ankley, G. T., Segner, H., & Tyler, C. R. (2005). Screening and testing for endocrine disruption in fish—biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environmental Health Perspectives, 114(S-1), 106–114.
Jobling, S., Casey, D., Rodgers-Gray, T., Oehlmann, J., Schulte-Oehlmann, U., Pawlowski, S., Baunbeck, T., Turner, A. P., & Tyler, C. R. (2003). Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquatic Toxicology (Amsterdam, Netherlands), 65(2), 205–220.
Jobling, S., Williams, R., Johnson, A., Taylor, A., Gross-Sorokin, M., Nolan, M., Tyler, C. R., van Aerle, R., Santos, E., & Brightly, G. (2005). Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environmental Health Perspectives, 114(S-1), 32–39.
Jones-Lepp, T. L., Alvarez, D., Englert, B., & Batt, A. (2009). Pharmaceuticals and hormones in the environment. Encyclopedia of Analytical Chemistry. doi:10.1002/9780470027318.a9059.
Kang, N.-H., Hwang, K. A., Lee, H. R., Choi, D. W., & Choi, K.-C. (2013). Resveratrol regulates the cell viability promoted by 17β-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor α and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 59, 373–379. http://doi.org/10.1016/j.fct.2013.06.029.
Kawamura, T., Sakai, S., Omura, S., Hori-e, R., Kawahara, T., Kinoshita, M., & Yamashita, I. (2002). Estrogen inhibits development of yolk veins and causes blood clotting in transgenic medaka fish overexpressing estrogen receptor. Zoological Science, 19(12), 1355–1361.
Kidd, K. A., Blanchfield, P. J., Mills, K. H., Palace, V. P., Evans, R. E., Lazorchak, J. M., & Flick, R. W. (2007). Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences of the United States of America, 104(21), 8897–8901.
Liu, J., Burdette, J. E., Xu, H., Gu, C., van Breemen, R. B., Bhat, K. P., Booth, N., Constantinou, A. I., Pezzuto, J. M., Fong, H. H., Farnsworth, N. R., & Bolton, J. L. (2001). Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. Journal of Agricultural and Food Chemistry, 49(5), 2472–2479.
Metcalfe, C., & Metcalfe, T. (2001). Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environmental Toxicology and Chemistry, 20(2), 297–308.
Nash, J. P., Kime, D. E., Van der Ven, L. T. M., Wester, P. W., Brion, F., Maack, G., Stahlschmidt-Allner, P., & Tyler, C. R. (2004). Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environmental Health Perspectives, 112(17), 1725–1733.
Rankouhi, T. R., Sanderson, J. T., Van Holsteijn, I., Van Leeuwen, C., Vethaaka, D., & Van den Berg, M. (2004). Effects of natural and synthetic estrogens and various environmental contaminants on vitellogenesis in fish primary hepatocytes: comparison of bream (Abramis brama) and carp (Cyprinus carpio). Toxicological Sciences : an Official Journal of the Society of Toxicology, 81(1), 90–102.
Razmara, A., Sunday, L., Stirone, C., Wang, X. B., Krause, D. N., Duckles, S. P., & Procaccio, V. (2008). Mitochondrial effects of estrogen are mediated by estrogen receptor in brain endothelial cells. Journal of Pharmacology and Experimental Therapeutics, 325(3), 782–790.
Routledge, E., & Sheahan, D. (1998). Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environmental Science and Technology, 32(11), 1559–1565.
Seki, M., Fujishima, S., Nozaka, T., Maeda, M., & Kobayashi, K. (2006). Comparison of response to 17 beta-estradiol and 17 beta-trenbolone among three small fish species. Environmental Toxicology and Chemistry / SETAC, 25(10), 2742–2752.
Shappell, N. W. (2006). Estrogenic activity in the environment: municipal wastewater effluent, river, ponds, and wetlands. Journal of Environmental Quality, 35(1), 122–132.
Singh, B., Bhat, T. K., & Singh, B. (2003). Potential therapeutic applications of some antinutritional plant secondary metabolites. Journal of Agricultural and Food Chemistry, 51(19), 5579–5597.
Suter, G., II. (2006). Ecological risk assessment (2nd ed.). Boca Raton: CRC Press.
Thorpe, K. (2000). Development of an in vivo screening assay for estrogenic chemicals using juvenile rainbow trout (Oncorhynchus mykiss). Environmental Science and Technology, 19(11), 2812–2820.
Thorpe, K., & Hutchinson, T. (2001). Assessing the biological potency of binary mixtures of environmental estrogens using vitellogenin induction in juvenile rainbow trout (Oncorhynchus mykiss). Environmental Science and Technology, 35(12), 2476–2481.
Tong, R. M., Beck, M. B., & Latten, A. (1980). Fuzzy control of the activated sludge wastewater treatment process. Automatica, 16(6), 695–701.
USEPA. (2009). Development of estimated quantitation levels for the second six-year review of national primary drinking water regulations (56pp, 674K) October 2009 EPA 815-B-09-005.
USEPA. (2014). Contaminant candidate list (CCL3). EPA-HQ-OW-2007-1189 at Regulations.gov.
Velu, M., & Ramanathan, V. (2011). Impact of 17β estradiol on catfish, Heteropneustes fossilis (Bloch.). Recent Research in Science & Technology, 3(7), 63–68.
Weber, S., Leuschner, P., Kmpfer, P., Dott, W., & Hollender, J. (2005). Degradation of estradiol and ethinyl estradiol by activated sludge and by a defined mixed cluture. Applied Microbiology and Biotechnology, 67(1), 106–112.
WHO. (2011). Pharmaceuticals in drinking water. World health organization, WHO/HSE/WSH/11.05.
Wise, A., O’Brien, K., & Woodruff, T. (2011). Are oral contraceptives a significant contributor to the estrogenicity of drinking water? Environmental Science and Technology, 45(1), 51–60.
Woods, M., & Kumar, A. (2011). Vitellogenin induction by 17β-estradiol and 17α-ethynylestradiol in male Murray rainbowfish (Melanotaenia fluviatilis). Environmental Toxicology and Chemistry / SETAC, 30(11), 2620–2627.
Wright-Walters M., Volz C, Assessment E. (2007). Municipal wastewater concentrations of pharmaceutical and xeno-estrogens: wildlife and human health implications. Proceedings of the 2007 national conference on environmental science and technology, (1), 1–9.
Wu, C., Xue, W., Zhou, H., Huang, X., & Wen, X. (2011). Removal of endocrine disrupting chemicals in a large scale membrane bioreactor plant combined with anaerobic-anoxic-oxic process for municipal wastewater reclamation. Water Science and Technology : A Journal of the International Association on Water Pollution Research, 64(7), 1511–1518.
Yoon Y., Westerhoff P. (2004). Removal of 17Î2 estradiol and fluoranthene by nanofiltration and ultrafiltration. Journal of environmental toxicology, (December), 1460–1468.
Young, W.F., Whitehouse, P., Johnson, I. and Sorokin, N. (2004). Proposed predicted-no-effect-concentrations (PNECs) for natural and synthetic steroid oestrogens in surface waters. Environmental Agency Technical Report P2-T04/1 WRc-NSF Report No.: EA5098. Bristol, UK.
Acknowledgments
We would like to thank the staff, students, and faculty of Eastern Illinois University as well as the Charleston, IL Wastewater Treatment Plant staff for their professional support. We would also like to thank the Charleston, IL Wastewater Treatment Plant and Eastern Illinois University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heffron, K.T., Gaines, K.F., Novak, J.M. et al. 17β-Estradiol influent and effluent concentrations in wastewater: demographic influences and the risk to environmental health. Environ Monit Assess 188, 288 (2016). https://doi.org/10.1007/s10661-016-5292-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5292-5