Abstract
In mammalian systems, there are six families of steroid hormones that can be classified on both a chemical and a biological basis. They are the estrogens, progesterone, androgens, mineralocorticoids, glucocorticoids, and vitamin D. These steroid hormones play a critical role in numerous physiological and pathophysiological processes and consequently garnered substantial research interest over the last century. The vast majority of circulating steroids in mammals come from the endocrine activity of the gonads and adrenal glands, which metabolize the lipid cholesterol to generate the steroid repertoire. Numerous investigations spanning decades have painstakingly elucidated the molecular enzymes and reactions of steroidogenesis distributed throughout the mitochondrial and microsomal compartments of steroidogenic cells. This chapter deals with the biosynthetic pathways, release, and transport of the major classes of steroid hormones in mammals. In particular, steroidogenesis is discussed as a single process that is repeated in each gland with cell type-specific variations on a single theme. Moreover, the homeostatic mechanisms that regulate the secretion or release of the steroid hormones and precursor hormone by feedback loops or by biological rhythms have been also discussed. Finally, the role of steroid-specific plasma transport proteins and the local inactivation of the excess of active steroids inside the cells are reported in order to obtain a clear picture on how the concentrations of active steroid hormones are regulated.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Cholesterol
- Steroidogenic enzymes
- Steroid hormones
- Steroid-binding proteins
- Steroidogenic glands
- Non-steroidogenic tissues
Introduction
Steroids play a critical role in numerous physiological and pathophysiological processes and consequently garnered substantial research interest over the last century. The first steroid hormone, estrone, was isolated in 1929 at a time before the characteristic ring structure of the steroid nucleus had been elucidated (Miller 1988). Today well over 230 naturally occurring steroids have been isolated and chemically characterized. In addition, an uncountable number of steroids and steroid analogs have been chemically synthesized and evaluated for their pharmacological properties. The basis of these recent developments is to be found in the papers authored by Professor Adolf O.R. Windaus (1876–1959) a German chemist who defined the structural determination of cholesterol. Professor Windaus was awarded with Nobel Prize in Chemistry in 1928 “for the services rendered through his research into the constitution of the sterols and their connection with the vitamins” (Miller 1988).
Steroids have a complicated structure of fused rings, which can be subjected to a wide array of modifications by the introduction of hydroxyl or carbonyl substituents and unsaturation (double or triple bonds). In addition, heteroatoms such as nitrogen or sulfur can replace the ring carbons, and halogens and sulfhydryl or amino groups may replace steroid hydroxyl moieties. Furthermore, the ring size can be expanded or contracted by the addition or removal of carbon atoms. An important structural feature of any steroid is recognition of the presence of asymmetric carbon atoms and designation in the formal nomenclature of the structural isomer that is present. Steroids are derived from a phenanthrene ring structure to which a pentane ring has been attached; this yields in the completely hydrogenated form, cyclopentanoperhydrophenanthrene, or the sterane ring structure (Fig. 1). The three six-carbon cyclohexane rings are designated A, B, and C rings, and the five-carbon cyclopentane ring is denoted as the D ring. The six-carbon atoms of a cyclohexane ring are not fixed rigidly in space, but are capable of interchanging through turning and twisting between several structural arrangements in space (chair and boat conformations). The approach of steroid conformational analysis has been of great value to the organic chemistry as a tool to predict or understand the course of synthetic organic chemical reactions. It is also known that conformational considerations play an increasingly useful role in the understanding of steroid hormone–receptor interactions (Fieser and Fieser 1959).
The diversity of steroid structures ranges from insect steroid hormones (ecdysone) to the world of plant growth regulators (brassinolides). In mammalian systems, there are six families of steroid hormones that can be classified on both a chemical (structure) and a biological (hormonal) basis (see Table 1). They are the estrogens, progestins, androgens, mineralocorticoids, glucocorticoids, and vitamin D. In addition, the bile acids are structurally related to cholesterol and thus could constitute a seventh member of the steroid family.
This chapter deals with the biosynthetic pathways, release, and transport of the major classes of steroid hormones in mammals.
Steroid Biosynthesis
Steroidogenesis entails processes by which cholesterol is converted to biologically active steroid hormones. Historically, steroid hormone synthesis only occurred in the steroidogenic glands (i.e., adrenal glands, gonads, and placenta). A significant number of studies have now challenged this view by demonstrating that several organs, including the brain, adipose tissue, and intestine, are capable of producing steroid hormones. These are called non-steroidogenic or intracrine tissues. Intracrine tissues do not have the ability to transform cholesterol into active steroid hormones, but depending on enzymes that are expressed in the tissues, active steroids are produced from various steroid precursors (Luu-The 2013). Whereas most endocrine texts discuss adrenal, ovarian, testicular, placental, and other steroidogenic processes in a gland-specific fashion, according to Walter L. Miller’s view, steroidogenesis is better understood as a single process that is repeated in each gland with cell type-specific variations on a single theme (Miller and Auchus 2011). Thus, in this paragraph, an overview of cholesterol uptake and steroidogenic enzymes will precede the description of the synthesis of specific hormones in both steroidogenic and non-steroidogenic tissues.
Cholesterol
All of mammalian steroids are biologically derived from cholesterol. The cholesterol is the most prevalent steroid in all animals and has multiple physiological roles that include its structural presence in all membranes. Cholesterol also is the starting point in the biosynthesis of all steroid hormones, vitamin D and its steroid hormone daughter metabolite, 1α,25-dihydroxy vitamin D (1α,25(OH)2D), and the bile acids. The level of the total body cholesterol is determined by a complex interplay of dietary available cholesterol, de novo synthesis of cholesterol, and excretion of cholesterol and bile salts. The liver and intestine together account for more than 60% of the body’s daily biosynthesis of this sterol from acetate via a complex pathway primarily found in the endoplasmic reticulum, but most steroidogenic cholesterol is derived from circulating lipoproteins (Chang et al. 2006). High-density lipoproteins (HDLs) may be taken up via scavenger receptor B1 (SR-B1), and low-density lipoproteins (LDLs) are taken up by receptor-mediated endocytosis via LDL receptors. LDL can suppress the rate-limiting enzyme in cholesterol synthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase. Although rodents preferentially use the HDL/SR-B1 pathway, the principal human source to obtain steroidogenic cholesterol is receptor-mediated endocytosis of LDL. After circulating LDL is internalized by receptor-mediated endocytosis, the resulting endocytic vesicles fuse with lysosomes, where the LDL proteins are degraded by proteolysis, liberating the cholesteryl esters, which are then hydrolyzed to “free” cholesterol by lysosomal acid lipase (Horton et al. 2002; Brown et al. 1979; Kraemer 2007). However, cholesterol is never truly free, as its solubility is only about 20 μmol/L, so that the term “free cholesterol” refers to cholesterol that is bound to proteins or membranes, but lacks a covalently linked group. Free cholesterol may be used by the cell or stored in lipid droplets following reesterification by acyl coenzyme A-cholesterol-acyltransferase. Similarly, HDL cholesteryl esters that enter the cell via SR-B1 are elaborated by hormone-sensitive neutral lipase, following which the free cholesterol may also be used or reesterified for storage. Intracellular cholesterol transport may be vesicular (mediated by membrane fusion) or non-vesicular (bound to proteins) (Chang et al. 2006). Both vesicular and non-vesicular cholesterol transport occur in steroidogenic cells, but non-vesicular transport involving high-affinity cholesterol-binding steroidogenic acute regulatory protein (StAR)-related lipid transfer (START) domain proteins appears to be the principal means of cholesterol transport from lipid droplets to the outer mitochondrial membrane (OMM). Movement of cholesterol from the OMM to the inner mitochondrial membrane (IMM) requires a multi-protein complex on the OMM (Chang et al. 2006).
Steroidogenic Enzymes
Numerous investigations have elucidated the molecular enzymes and reactions of steroidogenesis distributed throughout the mitochondrial and microsomal compartments of steroidogenic cells (Miller and Auchus 2011). Six P450 enzymes participate in steroidogenesis, and at least three more participate in the processing of vitamin D; five of these are found in mitochondria. The first step of steroidogenesis occurs in the mitochondria, where the cytochrome P450 side-chain cleavage enzyme (P450scc, CYP11A1 gene) cleaves the aliphatic tail of cholesterol. The final product of this first reaction common to all steroidogenic pathways is the pregnenolone (Miller and Auchus 2011). The expression of the CYP11A1 gene and, thus, the pregnenolone synthesis render a cell “steroidogenic.” P450scc and its cofactors are localized on the matrix face of the inner mitochondrial membrane. Although P450scc is known to be the rate-limiting enzyme for adrenal and gonadal steroid hormones, it is not the catalytic process of the cholesterol side-chain cleavage enzyme that is rate limiting. Rather, the presence and properties of the StAR transporter at the OMM facilitate the movement of cholesterol across the OMM to the IMM site of the P450scc. Indeed, StAR facilitates the actions of cholesterol side-chain cleavage that result in the production of mineralocorticoids and glucocorticoids, in the adrenals, and of estrogens or androgens in the gonads (Stocco et al. 2005). The most convincing evidence for the essential nature of StAR is that mutations of the StAR gene can result in a defect associated with the disease known as lipoid congenital adrenal hyperplasia characterized by a deficiency of both adrenal and gonadal steroid hormones (Stocco et al. 2005; Riegelhaupt et al. 2010).
Once pregnenolone is produced from cholesterol, it may undergo 17α-hydroxylation to 17OH-pregnenolone that ultimately leads in the adrenal cortex to the synthesis of aldosterone and cortisol, in the ovarian theca and granulosa cells to progesterone, and in the testes into testosterone. 3β-hydroxysteroid dehydrogenase (3βHSD) converts pregnenolone to progesterone. The hydroxysteroid dehydrogenase, 290–380 amino acids (35–45 kDa), may be found both in the mitochondria and in the endoplasmic reticulum; it utilizes the nicotinamide adenine dinucleotide (NADH/NAD+ or NADPH/NADP+) as electron acceptors or electron donors. Although rodents contain multiple 3βHSD isoforms, the human genome has only two active genes and several pseudogenes. The type 1 enzyme catalyzes 3βHSD activity in the placenta, breast, liver, brain, and some other tissues. This isoform is required for placental progesterone production during pregnancy. In contrast, the type 2 enzyme (3βHSD2) is the principal isoform in the adrenals and gonads. Alternatively, pregnenolone may exit the mitochondrion and become the substrate for P450c17 in the endoplasmic reticulum. Pregnenolone appears to exit the mitochondrion unaided; no transport protein has been found, and physiologic evidence does not suggest the presence of such a transporter (Miller and Auchus 2011).
The subsequent steps in steroid biosynthesis (see next paragraphs) are synthesized by a family of homologous oxidative enzymes (~57 human enzymes) collectively known as the cytochrome P450 hydroxylases. Each individual P450 enzyme is composed of about 500 amino acids and has a single heme (protoporphyrin ring with a single chelated Fe2+ group). The cytochrome moiety is structurally analogous to the hemoprotein cytochromes of the electron transport chain present in mitochondria that are dedicated to the production of ATP. All contain some kind of covalently bound protoporphyrin ring coordinately bound to one atom of iron, which can be reversibly oxidized and reduced. As a class, most of these P450 enzymes are subject to inhibition by the presence of carbon monoxide. Cytochrome P450 steroid enzymes are known to be present in the liver, adrenal cortex, ovary, testis, kidney, placenta, lungs, intestinal mucosa, and selected regions of the brain. Each P450 hydroxylase has a substrate-binding domain that is comparable in its ability to define substrate specificity to that of the ligand-binding domains of steroid receptors and plasma transport proteins for steroid hormones. Thus, the three-dimensional structure of the substrate-binding domain of a P450 hydroxylase determines which of the some 22–27 carbons of the substrate will acquire a new hydroxyl group (Miller and Auchus 2011).
Catalysis by P450scc and other mitochondrial P450 enzymes requires two electron-transfer intermediates, ferredoxin reductase and ferredoxin. Ferredoxin, which has ~116 amino acids (14 kDa), is a sulfur/iron electron shuttle protein in the mitochondrial electron transport process associated with steroid hydroxylation. The ferredoxin protein acts as a shuttle, accepting electrons from ferredoxin oxidoreductase, which then diffuses in the mitochondrial matrix to a P450 hydroxylase where it donates a pair of electrons. Ferredoxin oxidoreductase is an inner mitochondrial membrane-bound flavoprotein with a molecular weight of 51,100. It is responsible for the transfer of electrons from NADPH to ferredoxin and is widely expressed in many human tissues (Miller and Auchus 2011).
Glucocorticoid and Mineralocorticoid Hormone Synthesis
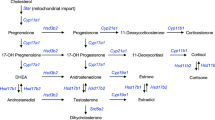
The naturally occurring cortisol is the most prevalent member of the family of glucocorticoids and binds tightly to the glucocorticoid receptor, but in vitro it has a high affinity for mineralocorticoid receptor (MR) (Cooper and Stewart 2009). Whenever the blood concentration of cortisol falls below the normal circulating concentration of 6–20 μg/100 mL, additional cortisol will be produced. The daily secretory rate of cortisol is 10–20 mg/day. In addition, when an individual experiences significant levels of stress, there will be an increased production of cortisol reaching 200 μg/100 mL. Cortisol’s most important action is to increase and maintain blood glucose levels via the biochemical process of gluconeogenesis in the combined actions of muscle cells, fat cells, and liver cells. Mineralocorticoids are a class of steroid hormones that regulate salt and water balances. Aldosterone is the primary mineralocorticoid. Mineralocorticoids promote sodium and potassium transport, usually followed by changes in water balance. Cells of the adrenal zona fasciculata and zona reticularis synthesize and secrete the glucocorticoid cortisol (Fig. 2). The adrenal zona glomerulosa cells preferentially synthesize and secrete aldosterone (Fig. 3) (Ehrhart-Bornstein et al. 1998).
Major steroidogenic pathways in the adrenal zona fasciculata. This zona express P450c17, so pregnenolone is hydroxylated to 17β-hydroxypregnenolone (or progesterone to 17-hydroxyprogesterone). 3βHSD2 and P450c17 generate 17-hydroxyprogesterone, the preferred substrate for P450c21, yielding 11-deoxycortisol. P450c11β, which is unique to the zona fasciculata, completes the synthesis of cortisol. Corticosterone is normally a minor product (dashed arrows) derived from a parallel pathway without the action of P450c17. The kidney, as well as other non-steroidogenic tissues , expresses 11βHSD1, which interconverts hormonally active glucocorticoids such as cortisol and corticosterone in their inactive counterpart cortisone and dehydrocorticosterone
Major steroidogenic pathways in the adrenal zona glomerulosa. The conversion of cholesterol to pregnenolone by P450scc is common to all three zones. 3βHSD2 converts pregnenolone to progesterone. P450c17 is absent, but P450c21 produces deoxycorticosterone, which is a substrate for P45011AS. P45011AS catalyzes 11-hydroxylations, which completes aldosterone synthesis
In these adrenal zones, progesterone is converted to 11-deoxycorticosterone by 21-hydroxylase (P450c21), which catalyzes the 21-hydroxylation of both glucocorticoids and mineralocorticoids. The final steps in the synthesis of both glucocorticoids and mineralocorticoids again take place in the mitochondria, where two proteins that share 93% sequence identity, 11β-hydroxylase (P450c11β, CYP11B1) and aldosterone synthase (P450c11AS, CYP11B2), reside (Figs. 2 and 3). P450c11β catalyzes the 11β-hydroxylation of 11-deoxycortisol to cortisol, and P450c11AS catalyzes the 11β-hydroxylation, 18-hydroxylation, and 18-methyl oxidation to convert deoxycorticosterone to aldosterone.
The interconversion of active cortisol and hormonally inactive glucocorticoids such as cortisone and dehydrocorticosterone is mediated by the two isozymes of 11β-hydroxysteroid dehydrogenase (11βHSD) (Fig. 2). Both enzymes are hydrophobic, membrane-bound proteins that bind cortisol/cortisone and corticosterone/11-dehydrocorticosterone, but otherwise their properties and physiological roles differ substantially. The type 1 enzyme (11βHSD1) is a dimer of 34 kDa subunits expressed mainly in glucocorticoid-responsive tissues such as the liver, testis, lung, fat, and kidney proximal tubule. The type 1 enzyme catalyzes both the oxidation of cortisol to cortisone using NADP+ as cofactor and the reduction of cortisone to cortisol using NADPH cofactor. Thus, the net flux of steroid driven by 11βHSD1 depends on the relative concentrations of available NADPH and NADP+, which usually favors reduction in cells. The 41 kDa type 2 enzyme (11βHSD2) has only 21% sequence identity with 11βHSD1 and catalyzes only the oxidation of cortisol to cortisone using NAD+; whether or not 11βHSD2 catalyzes reductive reactions remains undemonstrated. 11βHSD2 is expressed in mineralocorticoid-responsive tissues and thus serves to prevent cortisol from overwhelming renal or placenta mineralocorticoid receptors. The placenta also has abundant NADP+ favoring the oxidative action of 11βHSD1, so that in placenta both enzymes protect the fetus from high maternal concentrations of cortisol (Cooper and Stewart 2009; Miller and Auchus 2011). In vitro, cortisol has a high affinity for mineralocorticoid receptor (MR), but its inactivation to cortisone (which is unable to bind the MR) enables aldosterone to bind to the MR. The expression of 11βHSD1 is constitutive in a range of tissues including the liver, white adipose tissue, bone, and the central nervous system. However, it also has inducible expression in many other tissues including fibroblasts, skeletal and smooth muscle, and immune cells (Cooper and Stewart 2009).
Comparison of the subcellular localization of the various hydroxylase enzymes with the sequence of steroid movement through a metabolic pathway indicates the important role of cellular compartmentalization. Thus, in the conversion of cholesterol into cortisol in the adrenal cortex, the steroid must move sequentially from the mitochondria (side-chain cleavage) to the endoplasmic reticulum (17α- and 21-hydroxylation) and then back to the mitochondria (11β-hydroxylation). Histologic and electron microscopic examination of steroidogenic cells suggests that domains of the endoplasmic reticulum containing the steroidogenic P450 enzymes come close to the OMM during hormonally induced steroidogenesis, forming a steroidogenic complex, so that the movement of steroidal intermediates from the mitochondrion to the endoplasmic reticulum involves very small distances (Miller and Auchus 2011; Miller 2013).
Androgen Hormone Synthesis
Testosterone is the principal male androgen (300–1100 ng/dl plasma). An important biologically active metabolite of testosterone, produced in certain target tissues, is 5α-dihydrotestosterone. The biological actions of androgens can be divided into those directed toward the development and maintenance of the male reproductive system and those that have anabolic effects on several other organs including skeletal muscle and brain.
In the adrenal gland, both 17α-dihydroxypregnenolone and 17α-dihydroxyprogesterone can be converted to the 19-carbon androgen precursors dehydroepiandrosterone (DHEA) and androstenedione by the 17,20-lyase activity of P450c17 (Fig. 4). The rate of the lyase reaction can be increased more than tenfold by cytochrome b5 (b5) which promotes the electron transfer for the lyase reaction. Cytochrome b5 is a small (12–17 kDa) hemoprotein found as a membrane-bound protein in the liver and as a soluble protein lacking the C-terminal membrane anchor in erythrocytes. The adrenal zona reticularis expresses large amounts of P450c17 and cytochrome b5, maximizing 17,20-lyase activity, so that DHEA is produced, much of which is sulfated to DHEAS by cytosolic sulfotransferase (SULT2A1) enzyme. The adrenal zona reticularis produces abundant DHEA. As DHEA accumulates, small amounts are converted to androstenedione, and very small amounts of this androstenedione are converted to testosterone (Auchus and Rainey 2004). Up to 30 mg of DHEA is secreted daily by the adrenal cortex, and the blood levels of this prohormone and its sulfate derivative (DHEAS) are high. DHEA is considered a weak androgen that can be converted to testosterone and androstenediol or to estrogens in other steroidogenic (ovary, testis) and non-steroidogenic tissues (adipose tissue, brain) (Auchus and Rainey 2004; Miller and Auchus 2011). A group of dehydrogenases catalyzes the conversions DHEA to androstenedione and testosterone, estrone and estradiol, and others. These enzymes are collectively known as the 17β-hydroxysteroid dehydrogenases (17βHSDs), sometimes also termed 17-oxidoreductases or 17-ketosteroid reductases. There are at least 14 human 17βHSD isoforms, which vary widely in size, structure, substrate specificity, cofactor utilization, and physiological functions. The most important in normal steroidogenesis are 17βHSD1, 17βHSD3, and 17βHSD5. The reactions in which these enzymes are involved are reported in Figs. 4 and 5.
Major steroidogenic pathways of DHEA and testosterone synthesis. (a) The adrenal zona reticularis has large amounts of P450c17 and cytochrome b5 (b5) but little 3βHSD2, so that pregnenolone is sequentially oxidized to 17-hydroxypregnenolone and then DHEA. The adrenal zona fasciculata contains little b5, minimizing the 17,20-lyase activity of P450c17, and little DHEA is produced from 17β-hydroxypregnenolone. SULT2A1 sulfates DHEA, and resulting DHEAS is released to circulation for non-steroidogenic tissues. Testosterone and estradiol synthesis are minor pathways (dashed arrows). (b) In testicular Leydig cells, cholesterol is converted to DHEA by the same enzymes using the same cofactors as in the adrenal zona reticularis. Leydig cells contain abundant 17βHSD3, so that Leydig cells efficiently produce testosterone, via androstenedione and/or androstenediol. Estradiol synthesis is a minor pathway (dashed arrows) in Leydig cells
Major steroidogenic pathways of ovarian steroid hormones. The ovarian theca cells express StAR, P450scc, and P450c17 and hence produce C19 androgens. Theca cells do not express aromatase (P450aro); hence, androstenedione and testosterone must return to the granulosa cells, which contain abundant aromatase and 17βHSD1, completing the synthesis of estradiol (the two cell model of ovarian steroidogenesis). In the luteal phase, 3βHSD2 in the corpus luteum metabolizes nascent pregnenolone to progesterone, the final product. Minor pathways are shown with dashed arrows
In the testis, the cleavage of cholesterol side chain is confined to the mitochondria of the Leydig cells in which the role of the StAR protein is the same as in other steroidogenic cells. DHEA produced in the testis is not sulfated but is readily converted to androstenedione and then testosterone. Testicular testosterone synthesis, as reflected in plasma hormone levels, changes throughout the life of a normal human male. Testosterone production has two peaks during the second trimester in utero and another during the 6 months after birth. The relative quiescence of the androgen synthetic pathway persists throughout childhood until the beginning of the pubertal period. Plasma testosterone rises to adult levels by the end of puberty and begins to decline in middle age (andropause) (Auchus and Rainey 2004; Miller and Auchus 2011). In many target tissues, unmodified testosterone interacts with the androgen receptor (AR) to bring about the appropriate biological responses. In others, not limited to the prostate and hair follicles, testosterone is reduced at the 5α position to form 5α-dihydrotesterone (DHT), which has a greater affinity for the AR than testosterone; this is sometimes referred to as the amplification pathway. In some tissues, exemplified by the bone and brain, the active derivative of testosterone is estradiol, produced locally by aromatase, which then interacts with the estrogen receptors (ERs). This has been referred to as the diversification pathway (Miller and Auchus 2011).
The enzyme responsible for the conversion of testosterone to DHT is a Δ4-3-ketosteroid-5α-oxidoreductase (5α-reductase) that requires NADPH as a cofactor (Fig. 4). In rodents and humans, there are two forms of 5α-reductase. The two reductases are encoded by separate genes and share about 50% amino acid homology. The two 5α-reductase subtypes are important beyond the context of male genital differentiation and androgen action because both enzymes reduce a variety of steroids in degradative pathways. Progesterone, 17-dihydroxyprogesterone, and related C21 steroids are excellent substrates for both 5α-reductases, particularly the type 1; cortisol, cortisone, corticosterone, and related compounds are also good substrates. Such 5α-reduced steroids may be metabolized further and conjugated for excretion in the urine (Russell and Wilson 1994).
Aromatization of testosterone to estradiol by P450 aromatase (see below) occurs in several tissues of the adult male, including the adipose, testis (Sertoli cells and Leydig cells), brain, bone, breast, liver, and blood vessels. In these tissues androstenedione can also be aromatized, yielding the weak estrogen estrone, which can then be metabolized to estradiol through reduction of the 17-keto group by 17β-hydroxysteroid dehydrogenase (Fig. 4) (Normington and Russell 1992).
Studies of fetal androgen biosynthesis and mechanisms of virilization in the tammar wallaby have revealed the presence of a novel, alternative, so-called backdoor pathway that leads from 17-hydroxyprogesterone to DHT without going through androstenedione or testosterone as intermediate steroids. This pathway is initiated when either progesterone or 17-hydroxyprogesterone is reduced by 5α-reductase. This pathway is an alternative, backdoor pathway to DHT, by which DHT is produced without utilizing DHEA, androstenedione, and testosterone as intermediates. Consequently, the presence of 5α-reductases in steroidogenic and non-steroidogenic cells does not preclude the production of C19 steroids, but rather paradoxically enhances the production of DHT. Originally described in marsupials, the backdoor pathway is relevant to human steroidogenesis (Miller and Auchus 2011). Human enzymes catalyze all of the reactions required to complete this alternative route to DHT, and good evidence documents production of 5α-reduced androgens by the fetal adrenal, at least in some pathological states.
Estrogen and Progesterone Hormone Synthesis
The two most important steroid hormones of the adult female are 17β-estradiol (estradiol) and progesterone. In addition, two metabolites of estradiol, estrone (E1) and estriol (E3), circulate at high levels at certain phases of menstrual cycle and during pregnancy. E1 and E3 have been thought to be the inactive metabolites of estradiol, but E3 has significant effects on the immune system, and a closer examination of the physiological functions of these two steroids is warranted. As for androgens, the biological actions of E2 can be divided into those directed toward the development and maintenance of the female reproductive system and those that have effects on several other organs including the cardiovascular system, metabolism, and brain.
The naturally occurring estrogens are typically 18-carbon steroids that have an aromatic A ring with a phenolic hydroxyl; the naturally occurring progestin, progesterone, has 21 carbons, with another one additional oxygenation (oxo) on both C-3 and C-20. Ovarian estrogen synthesis, as reflected in plasma hormone levels, pulsates every month, during different phases of the menstrual cycle, and changes throughout the life of a normal human female. The peak of estrogen synthetic pathway during the 6 months after birth is followed by the relative quiescence throughout childhood until the beginning of the pubertal period. Plasma estrogen pulsates at adult levels by the end of puberty and begins to decline in middle age (menopause). The amounts of estradiol, estrone, and progesterone produced by the ovary and circulating during different phases of the menstrual cycle are shown in Table 2.
The enzymatic steps of estradiol synthesis are partitioned between the granulosa and theca cells of the ovary, which surround the oocyte and form a follicle (Fig. 5). The cells of the theca interna express cholesterol side-chain cleavage activity (P450scc) and StAR (Tian et al. 2015). Pregnenolone is converted to androstenedione by the removal of carbons 20 and 21. Androstenedione diffuses across the basement membrane of the follicle into the follicular fluid from which it is taken up by granulosa cells. The endoplasmic reticulum of granulosa cells expresses P450 aromatase that converts androstenedione to estrone (Tian et al. 2015). All circulating estrone and estradiol are produced by the aromatization of androgens, including those derived from adrenal and placental steroidogenesis. In addition, P450 aromatase is expressed in non-steroidogenic tissues , especially fat and bone. A single gene on chromosome 15q21.1 encodes P450 aromatase. This gene contains five different transcriptional start sites with individual promoters that permit the tissue-specific regulation of its expression in diverse tissues. Estradiol is further synthesized by the action of 17βHSD1 (see previous paragraph) that converts estrone to estradiol (Fig. 5). This enzyme is also expressed in the liver and placenta where it reduces 16α-hydroxyestrone to estriol (E3), the characteristic estrogen of pregnancy. As a whole, in order for sufficient amounts of estrogen to be synthesized for maturation and ovulation of the follicle, both granulosa cells and theca cells must be functional.
Following ovulation, the major steroid produced by the luteinized cells of the corpus luteum is progesterone, although estrogen continues to be synthesized and secreted as well (Fig. 5).
Vitamin D Synthesis
Vitamin D and its metabolites are not technically steroids in the strict chemical sense, as the B ring of cholesterol is opened (secosteroid). Nevertheless, these sterols are derived from cholesterol, assume shapes that are very similar to steroids, and bind to a nuclear receptor (Norman 1998; Miller and Auchus 2011).
In the human skin, ultraviolet radiation at 270–290 nm directly cleaves the 9–10 carbon–carbon bonds of the cholesterol B ring, converting 7-dehydro-cholesterol to cholecalciferol (vitamin D3) (Norman 1998). Plants and yeast produce ergocalciferol (vitamin D2), which has essentially the same properties as cholecalciferol. Both calciferols are biologically inactive prohormones that are then activated, and subsequently inactivated, by mitochondrial P450 enzymes. The initial step in the activation of vitamin D is its hepatic 25-hydroxylation to 25(OH)D, which may be catalyzed by 25-hydroxylase (CYP2R1). The active, hormonal form of vitamin D, 1,25(OH)2D (calcitriol), is produced in the kidney proximal tubule by the hydroxylation of 25(OH)D by the mitochondrial 1α-hydroxylase, P450c1α, encoded by the CYP27B1 gene. 1α-hydroxylation is the rate-limiting step in the activation of vitamin D. These chemical transformations can occur in the absence of further ultraviolet exposure. The resulting vitamin D3 is then transported in the general circulatory system by the 50 kDa vitamin D-binding protein (DBP). 1,25(OH)2D in the circulation derives primarily from the kidney, but 1α-hydroxylase activity is also found in keratinocytes, macrophages, osteoblasts, and placenta (Norman 1998; Miller and Auchus 2011).
Control of Steroid Hormone Synthesis and Release
The production and/or secretion of most hormones are regulated by highly specific homeostatic mechanisms. The secretion or release of the hormone is normally related to the requirement for the biological response(s) generated by the specific hormone. Because of their hydrophobic nature, steroid hormones and precursors can leave the steroidogenic cell easily and are not stored. Thus, steroidogenesis (excluding vitamin D metabolites) is regulated primarily at the first step in their synthesis (the cleavage of the side chain of cholesterol) and at the level of steroidogenic enzyme gene expression and activity (Miller and Auchus 2011).
Once the biological response has been generated, the secretion of the hormone is restrained to prevent an overresponse. Thus, a characteristic feature of most endocrine systems is the existence of a feedback loop that limits or regulates the secretion of the hormone. Two general categories of endocrine feedback systems have been described (Fig. 6): those in which the function achieved by the hormone directly feeds back upon the endocrine gland that secretes the hormone (positive or negative loops) and those involving the inputs from internal and external environments to the central nervous system (CNS) and hypothalamus (generally negative long loops). This latter category of endocrine feedback is organized into endocrine axes, which contain three levels of hormonal output. The highest level of hormonal output is neurohormonal and relies on the release of neurohormones, called releasing hormones, from hypothalamic nuclei into the portal vessels between the hypothalamus and the pituitary gland. The cells of the adenohypophysis make up the intermediate level of an endocrine axis releasing the tropic or stimulating hormones, which, in turn, stimulate the peripheral endocrine glands/cells (including steroidogenic cells) to produce the final, biologically active hormone (Fig. 6). Throughout the hypothalamus/pituitary/gland axes, the CNS exerts the control of hormone synthesis and release, and it realizes the integration of body functions (Molina 2010). Another contributor to the biological availability of steroid hormones that deserve mention here is the control exerted by biological rhythms on steroid hormone production (Lin et al. 2015).
Feedback regulation of hormone synthesis. Two general categories of endocrine feedback systems have been described: those in which the function achieved by the hormone directly feeds back upon the endocrine gland that secretes the hormone (a) and those involving the central nervous system (CNS) and hypothalamus (b)
Hypothalamus/Pituitary/Adrenal (HPA) Axis and Cortisol Synthesis and Release
The endocrine axis that controls the cortisol synthesis from the steroidogenic cells of adrenal zona fasciculata begins from the release of corticotropin-releasing hormone (CRH) from the hypothalamic neurons. CRH, a peptide of 41 amino acids, binds to its cognate receptors (a G protein-coupled receptor associated to a Gs/cAMP/PKA signaling pathway) on corticotrope cells to the pituitary. CRH acutely stimulates the release of adrenocorticotropin hormone (ACTH) into the circulatory system. ACTH, a 39-amino acid peptide, binds to the melanocortin-2 receptor (MC2R) located on the cells in the zona fasciculata of adrenal cortex (Gaffey et al. 2016). Within minutes upon ACTH binds to MC2R, cellular cholesterol is rapidly mobilized and transported to mitochondria. ACTH rapidly increases the expression and the activity of StAR through PKA-dependent phosphorylation resulting in the increase of pregnenolone levels. Over a period of several hours, ACTH increases the transcription of the gene encoding the steroidogenic enzymes (e.g., P450scc and the 11β-hydroxylase) and their coenzymes crucial to the production of cortisol as well as the expression of LDL receptor, thus increasing cholesterol uptake and its utilization to produce high level of cortisol (Miller and Auchus 2011).
The cortisol released into bloodstream inhibits both the release of CRH from hypothalamic neurons and ACTH secretion from pituitary corticotrope cells. There is a long negative feedback loop of cortisol, which is initiated by cortisol release from the adrenal zona fasciculata that then travels through the entire circulatory system to engage with the glucocorticoid receptor (GR) in target cells. Some cortisol will cross the blood–brain barrier and send a negative signal to both the brain cortex and the hippocampus, so that the hypothalamus diminishes the CRH signal sent from the hypothalamus to the pituitary. This then lowers the rate of secretion of ACTH by the pituitary. The overall feedback inhibition of ACTH is implemented very rapidly. The feedback on the hippocampus may shut off further electrical activity responsible for the release of CRH and the subsequent release of ACTH. These actions are mediated by GR located in these cells that operate transcriptionally (Wood 2013).
The release of CRH, and hence of ACTH, is pulsatile with about 7–15 episodes per day. The stimulation of cortisol release occurs within 15 min of the surge of ACTH. An important feature in the release of cortisol is that in addition to being pulsatile, it follows a circadian rhythm, with a peak in early morning and a nadir in late afternoon (see paragraph in section “Regulatory Feedback of Vitamin D Synthesis and Release”). However, many types of stress, neurogenic (e.g., emotional like fear), pathological (e.g., infection), and metabolic (e.g., hypoglycemia), overtake both the regulation exerted by circadian rhythm and the negative feedback from cortisol levels driving to the extensive secretion of ACTH, which will further elevate the circulating concentrations of cortisol. This means that hypothalamus could reset the “set point” of the HPA axis in response to stress (Lin et al. 2015; Gaffey et al. 2016).
Stress is anything that throws the body out of homeostatic balance – for example, an injury, an illness, or exposure to extreme heat or cold. Stress thus occurs when the body is exposed to a “stressor,” which threatens homeostasis, and the “stress response” is the attempt of the body to counteract the stressor and reestablish homeostasis (allostasis). There are two key aspects of stress response. On the one hand, the body responds to short-term stressor by releasing epinephrine (and norepinephrine) from the adrenal medulla, as well as cortisol secretion from the adrenal cortex that increases heart rate, blood pressure, and glucose availability. These mediators promote adaptation to an acute stressor, as well as to simple acts like getting out of bed in the morning or climbing a flight of stairs. On the other hand, chronic elevation of stressors, e.g., intensive cold, prolonged loud noise, serious injury, burns, surgery, and significant changes in the environment that chronically increased heart rate and blood pressure, can cause pathophysiological changes, for example, in the cardiovascular system. These stressful circumstances necessitate the response adaptation, which is not well granted by a single mediator. Rather, the combination of multiple mediators (e.g., vasopressin, CRH, cortisol) addresses the specific aspects of a stressor that culminate in the breakdown of glycogen necessary to enable escape or survive the “fight or flight” or provide “nervous energy.” Both types of stress responses usually occur simultaneously; one change in operation does not preclude the utilization of the other pathway. There are, however, certain conditions that can cause the pathways to operate separately. The sympathetic system generating epinephrine and norepinephrine is activated when the organism attempts to escape from or deal with the environmental challenge or the fight or flight. On the other hand, the HPA axis, ending in cortisol release from the adrenal gland, is also operative when the individual becomes immobile, passive, and depressed. A chronic emotional reaction of passivity and defeat to a stressful situation can produce dire consequences, as the adrenal hypertrophy and levels of cortisol continue to increase. This can generate a Cushingoid-like bodily reaction in which visceral fat accumulates and blood pressure becomes elevated, and arteriosclerosis and type 2 diabetes eventually develop. Sequential episodes of elevated glucocorticoids cause sufficient repression of glucose uptake in peripheral cells to involve insulin release from the β-cells of the pancreas (Gaffey et al. 2016).
The Control of Mineralocorticoid and Androgen Release from Adrenals
In contrast to glucocorticoids, which are under exclusive neuroendocrine regulation by HPA, aldosterone synthesis and release in the adrenal zona glomerulosa are predominantly regulated by angiotensin II and extracellular K+ and, to a lesser extent, by ACTH. Aldosterone is part of the renin-angiotensin-aldosterone system, which is responsible for preserving circulatory homeostasis in response to a loss of salt and water. Renin is an enzyme secreted by the granular cells associated with the Bowman’s capsule of the kidney’s nephron in response to a drop in blood pressure and/or a decrease in blood Na+ concentration. Renin’s substrate is the blood protein angiotensinogen (57 kDa) secreted by the liver and then localized within the capillaries of the lungs. The hormone angiotensin II is an octapeptide produced by the angiotensin converting enzyme (ACE) acting on the precursor, angiotensin I, the product of renin activity. Angiotensin II is a hormone that acts on the zona glomerulosa of the adrenal cortex where it stimulates the production and secretion of aldosterone (Molina 2010). Aldosterone binds to its receptor (MR) in the kidney’s collecting duct where it increases the reabsorption of both Na+ and water and, also, increases secretion of H+ and K+ into the urine, thus leading to an increased blood volume, which increases blood pressure until it has returned to normal. Although both angiotensin II and K+ stimulate aldosterone release by increasing intracellular Ca2+ concentrations, they achieve this result through different mechanisms (Molina 2010). Angiotensin II binds to G protein-coupled receptor resulting in activation of phospholipase C, which results in the production of two second messengers: diacylglycerol and inositol 1,4,5-trisphosphate which, respectively, activate protein kinase C activity and stimulate the release of Ca2+ from the endoplasmic reticulum stores. K+, on the other hand, mediates an influx of extracellular Ca2+ via voltage-gated L- and T-type Ca2+ channels. The surge of intracellular Ca2+ concentration increases calcium/calmodulin activity in the zona glomerulosa that promotes transcription of genes for steroidogenic enzymes, especially the gene encoding P450scc, thus increasing the amounts of the steroidogenic enzymes involved in aldosterone synthesis (Clyne et al. 1997; Bassett et al. 2004).
The control and regulation of the release of adrenal androgens (DHEA) depends on ACTH. However, it is known that adrenal secretion of DHEA increases in children at the age of 6–8 years (adrenarche) and peak between the ages of 20 and 30 years. Thereafter, serum levels of DHEA decrease markedly during the aging process. This is not paralleled by a similar decrease in ACTH or cortisol production (Wood 2013).
Hypothalamus/Pituitary/Testis Axis and Testosterone Synthesis and Release
The hypothalamic decapeptide gonadotrophin-releasing hormone (GnRH) released by secretory granules of the GnRH hypothalamic neurons is required for the male and female reproductive function. In the absence of GnRH, secretion of the two pituitary gonadotrophic hormones is either completely (luteinizing hormone, LH) or greatly (follicle-stimulating hormone, FSH) diminished.
The secretion of GnRH is characterized by its pulsatile nature. The amplitude and frequency of GnRH pulses are restrained from the age of 4 to 6 months until the onset of puberty, at which time the increase in both amplitude and frequency of GnRH (and therefore gonadotrophin) secretion is the hallmark of the onset of reproductive maturation. Furthermore, the two gonadotrophins show pulse frequency discrimination, with LH responding to faster frequencies of the GnRH pulse and FSH to slower frequencies. The pulse generator for GnRH secretion is now thought to lie in neurons of the preoptic area that contain a triad of neuropeptides, kisspeptin, neurokinin B, and dynorphin, which project into the cell bodies and terminals of GnRH neurons of the hypothalamus. Although kisspeptin is thought to be an important component of the GnRH pulse generator, the exact mechanism by which this occurs and the role of other neuropeptides such as neurokinin B are still under intense study (Plant 2008; Molina 2010; Chevrier et al. 2011).
Although the first steps of hypothalamus/pituitary/gonad axis are similar between sexes, the effects exerted by pituitary tropic hormones are different in male and in female. The testicular target of LH, the Leydig cells, serves two principal functions: (a) they are the site of production of testosterone, producing, in adult males, approximately 7 mg daily for systemic transport to distal target tissues, and (b) they have paracrine interactions with the immediately adjacent seminiferous tubules to support spermatogenesis. LH-mediated stimulation of testosterone synthesis and secretion is initiated by the binding of LH to specific receptors on the plasma membranes of the Leydig cell. An increased level of cAMP within the Leydig cell activates PKA (cyclic AMP-dependent protein kinase) which, through phosphorylation of specific transcription factors, induces the synthesis of StAR. Under prolonged stimulation, LH increases the expression and activities of other enzymes in the pathway from pregnenolone to testosterone (Miller and Auchus 2011). In the adult male, FSH in conjunction with testosterone acts on the Sertoli cells of the seminiferous tubule to initiate sperm production. In humans, FSH is required for normal spermatogenesis throughout adult life. FSH binds to its specific G protein-coupled receptor on the Sertoli cell to increase, through a cAMP-dependent mechanism, the synthesis of specific proteins, including the androgen-binding protein (ABP) and inhibin. ABP is thought to function to concentrate androgens in the seminiferous tubules and deliver the steroid hormone to developing spermatocytes and spermatids (Molina 2010). ABP is now known to be a homolog of sex hormone-binding globulin, SHBG, the serum-binding protein for androgens and estrogens (see next chapter).
Testosterone can exert negative feedback on the axis through three possible levels: the kisspeptin neurons of the arcuate nucleus, which regulate the output of GnRH neurons, the GnRH neurons themselves, and the pituitary gonadotrope cells. The relative contribution of each of these components to overall LH and FSH secretion varies with species, but each probably contributes to the negative feedback effect of testosterone in humans. Although kisspeptin neurons contain both androgen (AR) and estrogen (ERs) receptors, testosterone represses kisspeptin expression and GnRH secretion via AR activity. In the pituitary, testosterone decreases the expression and release of LH and FSH only after the aromatization of testosterone to estrogen. Thus, in contrast to the situation in kisspeptin neurons, pituitary estrogen and ERs play an important role in the negative feedback that controls testosterone synthesis (Plant 2008; Molina 2010; Chevrier et al. 2011).
The adult human male produces approximately 45 μg of estradiol per day, mostly from aromatization of testosterone in the adipose tissue, bone, brain, breast, blood vessels, liver, and both the Sertoli and Leydig cells of the testes. The aromatization of testosterone is a critical step in its action in several tissues. In bone, estrogen mediates the closure of the epiphyseal plate during puberty and decreases bone mineral resorption; in the spermatozoa, where it mediates the cell motility; in prostate, where ERs are necessary for water resorbing and gland maturation; and in the brain where estrogen participates in the negative feedback inhibition of testosterone on GnRH secretion. Estradiol produced from testosterone affects other areas of brain affecting mood and cognitive function. In a small number of cases of inactivating mutations of aromatase in men, observations have included tall stature, low bone mineral density, and changes in carbohydrate and lipid metabolism (Miller and Auchus 2011).
Hypothalamus/Pituitary/Ovary Axis and Synthesis and Release of Estrogen and Progesterone
Reproductive function in females is pulsatile being characterized by cycles of follicle development, ovulation, and preparation of the uterine endometrium for implantation of the blastocyst resulting from a fertilized egg (Plant 2008). The hormones that constitute the hypothalamic-pituitary-ovarian axis orchestrate and synchronize these events. There are two differences that distinguish this system from its male counterpart: (1) there are two distinct phases of the cycle, follicular and luteal; (2) at one brief specific point in the cycle, the pituitary and hypothalamic centers respond.
During the majority of the follicular phase (first half) of the cycle, the theca cells are the target of LH, while the granulosa cells are the FSH target. The type of receptor each cell expresses determines this responsiveness. The cells of the theca interna express receptors for LH, the response to which is an increase in steroid acute regulatory protein (StAR) and the cleavage of the side chain of cholesterol. Androstenedione diffuses across the basement membrane of the follicle into the follicular fluid from which it is taken up by granulosa cells. These cells express the FSH receptor which, when activated by FSH, brings about, through adenyl cyclase activation, the synthesis of aromatase that converts androstenedione to estrone. Action of 17β-hydroxysteroid dehydrogenase (17-ketosteroid reductase; HSD17B1) converts estrone to estradiol. Thus, unlike the male, both gonadotrophins must be secreted in appropriate amounts to assure estrogen synthesis. Following ovulation, when theca and granulosa cells of the follicle have differentiated into the corpus luteum, LH from the pituitary is required for the production of progesterone, which is necessary for the growth of the uterine endometrium (Plant 2008; Miller and Auchus 2011).
The sex steroid hormones, estrogens and progesterone, control gonadotrophin secretion. Both estradiol and progesterone exert negative feedback inhibition on GnRH secretion by the hypothalamus as well as by direct inhibition of gonadotrophin secretion at the pituitary (Plant 2008). The latter involves alterations in the expression of the genes required for LH and FSH synthesis as well as modulation of the sensitivity of pituitary gonadotrophs to GnRH. The negative feedback effect of estrogen predominates during the follicular phase of the reproductive cycle and that of progesterone, synthesized in large amounts by the corpus luteum, predominates during the luteal phase. However, the surge of estrogen hormones, typical of ovulation, exerts a positive feedback on GnRH and LH secretion further increasing estrogen synthesis in the ovary. A group of kisspeptin-1 neurons in the hypothalamic anteroventral periventricular nucleus mediates this estrogen-positive stimulus. These cells respond to the rapid rise in estrogens produced by the maturing follicle with increased Kiss-1 secretion and stimulation of GnRH secretion that drives the midcycle LH surge. The positive feedback of estrogen on LH (and FSH) secretion is also exerted at the pituitary gland and involves enhancement of the sensitivity to GnRH. The relative role of the pituitary, as opposed to changes in GnRH secretion, in mediating the ovulatory LH surge varies with species. In primates, including humans, the pituitary appears to be the predominant site of this regulatory event. The molecular mechanism of the switch from negative to positive feedback by estrogen is not understood, but it is probably related to the different effects exerted by different estrogen concentrations on the levels of their cognate receptors (Molina 2010).
Androgens play important roles in the reproductive functions of females. Indeed, testosterone and androstenedione are the substrates for aromatase and obligatory intermediates in the production of estradiol and estrone, respectively. Adrenal androgens play a critical role in female puberty bringing about the changes in pubic and axillary hair (adrenarche). Excessive exposure to androgens during uterine life can bring about masculinization of a female fetus, depending on the timing and extent of the exposure. Excess androgens at any point in adult life can have masculinizing effects on the female, manifested as excess hair growth, voice changes, and changes in body composition (Miller and Auchus 2011).
Regulatory Feedback of Vitamin D Synthesis and Release
The regulation of vitamin D synthesis and release is a good example of endocrine feedback systems in which the function achieved by the hormone directly feeds back upon the endocrine gland that secretes the hormone (positive or negative loops). Indeed, the production of 1,25(OH)2D by 1α-hydroxylase in the kidney is a tightly regulated process and is a central factor in the feedback regulation of calcium homeostasis. The production of the active form of vitamin D [1,25(OH)2D or calcitriol] is under negative feedback regulation by plasma Ca2+ levels. A rise in plasma Ca2+ levels inhibits the hydroxylation at C-1 and favors hydroxylation at C-24, leading to the synthesis of an inactive metabolite of vitamin D (24,25(OH)2D). In addition, the parathyroid hormone, released from parathyroid glands, stimulates the activity of kidney 1α-hydroxylase, favoring an increase in synthesis of the active form of vitamin D. Vitamin D, as well as high Ca2+ levels, suppresses the activity of 1α-hydroxylase, decreasing its own synthesis and favoring the synthesis of 24,25(OH)2D. Vitamin D increases intestinal Ca2+ absorption and suppresses the synthesis and release of parathyroid hormone from the parathyroid glands completing the endocrine feedback system (Khundmiri et al. 2016).
Hormonal Rhythms
The profound environmental changes brought about by the rotation of the Earth around its axis allowed the evolution of endogenous timekeepers that enable an organism to reliably predict the time of day and adjust behavior and physiology accordingly. Not surprisingly, large aspects of our endocrine system, including hormone synthesis and release, are tightly connected to the circadian clock. In 1970s it was discovered that information about the external light–dark cycle provide photic data to both classical retinal photoreceptors – cone and rod cells – as well as to melanopsin-containing retinal ganglion cells. Through the retino-hypothalamic tract (RHT), this information is passed to an anatomical entity underlining the mammalian circadian, the suprachiasmatic nucleus (SCN). The SCN is a bilaterally paired structure with high cell body density located adjacent to the third ventricle and directly atop the optic chiasm. The current model suggests that the central mechanism of the mammalian molecular clock is composed of a set of clock genes intertwined with a delayed interlocking transcriptional–translational feedback loop, coupled to several auxiliary mechanisms reinforcing robustness and stability. The functional molecular clockwork does not exist only in SCN neurons, but (almost) every single cell in the brain and periphery is capable of oscillating in a circadian manner. Molecular clock rhythms have been shown even in cultured cells, such as immortalized fibroblast cells, which display robust oscillations of clock gene expression. One major function of the SCN is to synchronize internal biological processes to external time cues. To do this, SCN innervates other regions of the brain, in particular the hypothalamic nuclei which are important integrating centers for energy homeostasis and control of steroid hormone synthesis. Indeed, cortisol represents the best-studied steroid hormone that is subject to direct and dominant regulation by the circadian clock (Lin et al. 2015; Barclay et al. 2012).
Blood levels of cortisol display a robust circadian rhythm. The circadian rise of cortisol is phase-locked to the time of awakening, peaking at few hours before the onset of the active phase, i.e., the early morning for diurnal animals such as humans and the evening for nocturnal animals such as rodents. This cortisol rise promotes arousal and boosts performance during the early active phase. Importantly, cortisol rhythms persist under constant environmental conditions, suggesting that the endogenous circadian clock drives them. Surgical ablation of the SCN completely abolishes the circadian rhythm of cortisol in blood, indicating that the SCN is the origin of cortisol rhythmicity. Well before the discovery of clock genes or peripheral clocks, it was shown that adrenal glands when isolated and cultured in vitro display a robust circadian rhythm of metabolism and steroid secretion. In line with this, researchers have provided evidence that a local adrenocortical clock imposes a circadian gating mechanism altering ACTH sensitivity during the course of the day. Thus, while the SCN is indispensable for the circadian rhythm of cortisol secretion, the adrenal clock provides an additional level of control to modulate the proficiency of cortisol production across the circadian cycle, and further clocks along the HPA axis may be involved. Adrenalectomy shortens re-entrainment in the SCN, lung, and kidney following phase shifts, suggesting that GCs may serve to stabilize the phase of peripheral clocks against external noise. In the case of jetlag-induced circadian desynchronization, it was shown that manipulation of the cortisol rhythm could speed up or slow down activity adaptation to the new light–dark cycles, depending on the intervention time (Lin et al. 2015; Barclay et al. 2012).
Circadian rhythms, which do influence GnRH and gonadotrophin secretion in other mammalian species, do not have a strong influence in humans, but sleep itself appears to have direct effects on the nature of LH secretion, which vary with the reproductive status of the individual (Lin et al. 2015; Barclay et al. 2012).
Steroid Secretion and Transport
Steroid hormones, like other hormones, are chemical messengers that send a signal within a physiological system from point A (secretion) to point B (biological action). Steroid hormones are synthesized, but not stored, within specific endocrine cells that could be associated with an anatomically defined endocrine gland.
Upon the receipt of an appropriate physiological signal, which may take the form of either a change in the concentration of some component in the blood (e.g., another hormone, Ca2+, stressors) or a neural signal, the hormones are released into the circulation. They are transported in the bloodstream to one or more target cells, which are defined as targets by the presence of the specific high-affinity receptors, members of nuclear receptor superfamily located either on the membrane or within the cell (endocrine system) (Fig. 7) (Molina 2010; Norman and Henry 2015).
A type of hormonal communication system does not involve the circulatory system at all. In paracrine systems, hormones secreted from the steroidogenic cells interact with their cognate receptors in neighboring cells, which are reached by diffusion (Fig. 7). As with endocrine system, the nearby target cells may be all the same type or may differ from each other. Several, if not all, of the steroid hormones act by paracrine in addition to endocrine mechanisms. For example, in the testis, testosterone not only is released into the blood from the interstitial cells in which it is produced but also diffuses to nearby seminiferous tubules to support the production of sperm (see previous paragraphs).
Finally, some cells both produce the same hormone and respond to it. This type of system is referred to as autocrine for all class of hormones excluding steroid hormones. The appearance during evolution of the repertoire of cell-specific steroidogenic enzymes (see previous chapter) permits to produce steroid hormones intracellularly according to the local needs without biologically significant release of active sex steroids in the circulation. This type of system, specific for steroid hormones, is referred to as intracrine (Fig. 7). Examples of intracrine system involve androgen/estrogen synthesis in non-steroidogenic tissues during andropause and menopause. All tissues, except the endometrium, possess the intracrine enzymes able to transform DHEA into androgens and/or estrogens. Humans, along with other primates, are unique among animal species in having adrenals that secrete large amounts of the inactive precursor steroid DHEA, which is converted at various levels into active androgens and/or estrogens in specific peripheral tissues according to the mechanisms of intracrinology. It is very important to mention that an essential aspect of intracrinology is that the active sex steroids are not only made locally but that they are also inactivated locally at exactly the same site where synthesis takes place. In fact, the sex steroids made from DHEA in peripheral tissues are essentially released outside the cells as inactive compounds. DHEA of adrenal, ovarian, or exogenous (e.g., drugs) origin is distributed by the general circulation to all tissues indiscriminately. The transformation of DHEA into estrogens/androgens, however, is tissue specific, ranging from none in the endometrium to various cell-specific levels in the other tissues of the human body. Most importantly, approximately 95% of the active estrogens and androgens are inactivated locally before being released in the blood as inactive metabolites, thus avoiding inappropriate exposure of the other tissues (Luu-The 2013; Labrie 2015). As a whole, the intracrine process that is typical for steroid hormones binding to nuclear receptors is thus equivalent to autocrine and paracrine processes activated by hormones binding to transmembrane receptors. Although the terms autocrine and paracrine are used for both nuclear and transmembrane receptors, it is important to make the distinction, especially because nuclear steroid receptors could be also found extrinsically linked to the plasma membrane and act in an extranuclear manner (Molina 2010; Norman and Henry 2015).
Steroid-Binding Proteins
Most steroid hormones have limited solubility in plasma due to their intrinsic hydrophobic nature; accordingly, steroid hormones are largely (99%) bound to specific plasma transport proteins, which are synthesized in the liver. All steroid hormones, except one, have their cognate plasma-binding protein. The exception is aldosterone; 50% of aldosterone is believed to circulate as the free steroid in the plasma compartment. Each transport protein has a specific ligand-binding domain for its cognate hormone, which displays little amino acid sequence homology with the ligand binding of the cognate receptors. The Kd of a steroid hormone for its plasma transport is always “looser,” e.g., 1–100 × 10−8 M, than that of the Kd of the nuclear receptor. Thus, the tighter binding of the steroid hormone to its target receptor when it has arrived at a target tissue allows the hormone to be concentrated inside the target cell. The current view is that it is the “free” form of steroid hormones and not the complex of the hormone with its plasma transport proteins that interacts with receptors in or on the target cells to begin the sequence of steps that result in the generation of a biological response (Molina 2010; Norman and Henry 2015).
In the plasma compartment, the steroid hormones move through the circulatory system bound to their partner transport protein. However, an important issue concerns the details of the mode of delivery of steroid hormones to their target cells. Because the “free” form of the steroid hormone is believed to be the form of steroid that moves across the outer plasma membrane of a target cell, it has been postulated that the steroid ligand dissociates from its plasma transport protein and then diffuses first through the capillary wall and then through the outer wall membrane of target cells. However, it is apparent that the endothelial wall of capillaries contains fenestrations. Thus, it is also possible for the plasma steroid transport protein (with bound steroid hormone) to exit the capillary bed via a fenestration and move to be immediately adjacent to the outer cell membrane of the appropriate target cell for the steroid hormone in question. Here the steroid hormone will dissociate from the transport protein, diffuse through the plasma membrane, and then bind to an unoccupied partner steroid receptor.
A corticosteroid-binding globulin (CBG) is present in blood. This protein is also referred to as transcortin. It is synthesized in the liver and exported to the circulation. This protein binds cortisol with relatively high affinity (binding constant ≅ 108 M−1; the dissociation constant for the reaction, CBG + cortisol ↔ CBG – cortisol, is about 10−8 M cortisol). Because of the affinity of the protein for cortisol, most of the hormone circulates in the bound form as reflected in the equilibrium, which favors the complex: CBG + cortisol ↔ CBG-cortisol.
There is only a small amount of the free steroid hormone in a target cell. Nevertheless, at the target cell, the free steroid enters the cell plasma membrane, probably by a free diffusion process. The driving force behind the movement of free hormone in the target cell appears, in part, to be proportionate to the number of unoccupied cortisol hormone-specific nuclear receptor molecules that have moved from the nucleus of the target cell out to the cytoplasm that still have empty ligand-binding sites (unoccupied receptors). The affinity of the nuclear receptor for cortisol is similar (20 nM) to that of the circulating CBG-cortisol complex. Steroid hormones cycle into and out of a target cell, and the number of unoccupied nuclear receptors determines the proportion of molecules retained in the cell.
Of the total testosterone in the circulation, 0.5–3% is free (not bound to protein), 54–68% is bound to albumin with relatively low affinity, and the remainder is bound to sex hormone-binding globulin, SHBG, with high affinity. SHBG, synthesized in the liver, is a dimeric glycosylated protein with a molecular mass of 84 kDa. SHBG has a preference for steroids with a 17β-hydroxyl [Kd ≅ (1–5) × 10–10 M], so it binds testosterone, DHT, and estradiol, but not, for example, progesterone or cortisol. Thus, it serves as the specific transport protein for both testosterone and estradiol. Plasma levels of SHBG are twofold greater in nonpregnant women than in men. The synthesis, and therefore the plasma concentration, of SHBG is increased in pregnancy and hyperthyroidism and is decreased by androgens, glucocorticoids, insulin, and growth hormone.
The plasma contains the vitamin D-binding protein (DBP) that is utilized to transport vitamin D secosterols. DBP resembles the corticosteroid-binding globulin, which carries glucocorticoids, and the steroid hormone-binding globulin. DBP is a slightly acidic (pH = 5.2) monomeric glycoprotein of 53,000 Da, which is synthesized and secreted by the liver as a major plasma constituent.
DBP is a multifunctional protein in that it binds both vitamin D and its metabolites. One molecule of DBP has only one ligand-binding domain-binding site for secosterols of the vitamin D family. Thus, while a single DBP can carry only one ligand, the relatively high concentration of DBP molecules present in the blood compartment permits the DBP population as a whole to bind the hydrophobic parent, vitamin D3, as well as the hydrophobic daughter metabolites, 25(OH)D3, 1α,25(OH)2D3, and 24,25(OH)D3. Since the total plasma concentration of vitamin D sterols is only ~0.2 μM, while DBP circulates at 9–13 μM, under normal circumstances only a very small proportion of the sterol-binding sites on DBP are occupied.
For some endocrine systems, the concentration of the plasma transport protein can be subject to physiological regulation; that is, the concentration of plasma transport proteins can be either increased or decreased. Thus, changes for plasma transport proteins can alter the amount of free hormone in the blood, as well as affect the total amount of hormone in the blood. This role of the binding proteins in the availability of steroid hormones can be of considerable physiological relevance in clinical situations (Molina 2010; Norman and Henry 2015).
Steroid Hormone Metabolism
The effective concentration of a hormone is determined by the rates of its production, delivery to the target tissue, and degradation. The excess of steroids that are not bound to the receptors will be inactivated into the target cell, and hormone metabolites will be secreted in the circulation. In addition, the local inactivation of the excess of active steroids inside the cells is an important manner to regulate active steroid concentration (Molina 2010; Norman and Henry 2015).
The hydrophobic steroid hormones and the vitamin D are filtered by the kidney and generally reabsorbed. About 1% of the cortisol produced daily ends up in the urine. Steroid hormones are ordinarily handled by metabolizing them to inactive and to water-soluble forms that are more effectively eliminated. The free steroid fraction is accessible to the metabolic inactivation. The inactivation is accomplished by converting hydroxyl groups to keto groups, reducing double bounds, and conjugating the steroids with glucuronide and sulfate groups. These processes occur in the liver through phase I and phase II biotransformation reactions. Over 50 different steroid metabolites have been described.
Cortisol is reversibly inactivated by conversion to cortisone and to tetrahydrocortisol and tetrahydrocortisone in the liver and kidney. These metabolites are referred to as 17-hydroxycorticosteroids, and their determination in 24 h urine collections is used to assess the status of adrenal steroid production. As discussed before, localized tissue metabolism contributes to modulation of the cortisol biological effects by the isoforms of the enzymes 11βHSD1 and 11βHSD2.
Aldosterone is metabolized in the liver to tetrahydroglucuronide derivative and excreted in the urine. A fraction of aldosterone is metabolized to aldosterone 18-glucuronide, which can be hydrolyzed back to free aldosterone under low pH conditions.
In the liver, testosterone undergoes 5β-reduction, followed by 3α-reduction and reduction of the 17-keto group. DHT undergoes 3α- and 17β-reduction. Both reduced catabolites are then conjugated with glucuronic acid or, to a lesser extent, sulfate, released back into the circulation and removed from the body in the urine or bile.
Estrogens are metabolized by sulfation or glucuronidation, and the conjugates are excreted into the urine. Estrogen can also be metabolized through hydroxylation and subsequent methylation to form catechol and methoxy estrogens.
1,25(OH)2D may be inactivated by the principal hepatic drug-metabolizing enzyme, microsomal CYP3A4, or by its 24-hydroxylation by vitamin D 24-hydroxylase (P450c24), encoded by the CYP24A1 gene. This mitochondrial enzyme can catalyze the 24-hydroxylation of 25(OH)D3 to 24,25(OH)2D3 and of 1,25(OH)2D3 to 1,24,25(OH)3D3, primarily in the kidney and intestine, thus inactivating vitamin D (Norman and Henry 2015).
Conclusions
Steroid hormones regulate a wide variety of developmental and physiological processes from fetal life to adulthood. Important functions of these hormones include regulation of the mammalian stress response, electrolyte and fluid homeostasis, and the development and maintenance of both primary and secondary sexual characteristics. Despite efforts to correlate steroid structures with their activities, this area was not understood until the various steroid hormone receptors were identified and cloned. Thus, the contemporary definition of each class of steroid is based on the nuclear receptor(s) to which it binds, rather than on the chemical structure of the steroid. In addition, disorders of steroid hormone synthesis were formerly thought to be confined to rare genetic lesions; consequently, more study has been devoted to steroid hormone action than to steroid hormone synthesis. Work in the past 30 years has identified the steroidogenic enzymes and their genes, reinvigorating studies of steroid biosynthesis by discoveries of roles for altered regulation of steroidogenesis in common disorders such as hypertension and the polycystic ovary syndrome and by discoveries of steroid-modifying enzymes in target tissues that mediate some forms of apparent tissue specificity of hormone action.
Summary
Steroidogenesis entails processes by which cholesterol is converted to biologically active steroid hormones. Six classes of steroid hormones, all of which are indispensable for mammalian life, are made from cholesterol via complex biosynthetic pathways that are initiated by specialized, tissue-specific enzymes found in mitochondria and endoplasmic reticulum. These hormones include glucocorticoids (cortisol, corticosterone) and mineralocorticoids (aldosterone) produced in the adrenal cortex; estrogens (estradiol), progestins (progesterone), and androgens (testosterone, dihydrotestosterone) produced in the gonads; and calciferols (1,25-dihydroxy vitamin D [1,25OH2D3]) produced in the kidney. Historically, steroid hormone synthesis only occurred in the steroidogenic glands (i.e., adrenal glands, gonads, and placenta). A significant number of studies have now challenged this view by demonstrating that several organs, including the brain, adipose tissue, and intestine, are capable of producing steroid hormones. These are called non-steroidogenic or intracrine tissues.
The production and/or secretion of most hormones are regulated by the homeostatic mechanisms, which operate in that particular endocrine system. The secretion or release of the hormone is normally related to the requirement for the biological response(s) generated by the specific hormone. Because of their hydrophobic nature, steroid hormones and precursors can leave the steroidogenic cell easily and are not stored. Thus, steroidogenesis is regulated primarily at the first step in their synthesis (the cleavage of the side chain of cholesterol) and at the level of steroidogenic enzyme gene expression and activity. Once the biological response has been generated, the secretion of the hormone is restrained to prevent an overresponse. Thus, a characteristic feature of most endocrine systems is the existence of a feedback loop (either direct or multistep) that limits or regulates the secretion of the hormone. Another contributor to the biological availability of steroid hormones is the control exerted by biological rhythms on steroid hormone production.
Steroid hormones are synthesized, but not stored, within specific endocrine cells from which they are easily secreted in the plasma. However, most steroid hormones have limited solubility in plasma due to their intrinsic hydrophobic nature; accordingly, steroid hormones are largely bound to specific plasma transport proteins, which are synthesized in the liver. All steroid hormones, except aldosterone, have their cognate plasma-binding protein.
The excess of steroids that are not released or bound to the receptors will be inactivated into the cell, and hormone metabolites will be secreted in the circulation. This local inactivation of the excess of active steroids inside the cells is an important manner to regulate active steroid concentration.
As a whole, the key determinants of the steroid hormone response are the presence of a response system (receptors and signaling transduction pathways in target cells) and hormone blood concentration. The effective concentration of a steroid hormone is determined by the rates at which the steroid is biosynthesized and enters the body pools, the “tightness” of binding of the steroid to its plasma carrier protein, and the rate at which the steroid is biologically inactivated by catabolism and removed from body pools.
Cross-References
References
Auchus RJ, Rainey WE. Adrenarche–physiology, biochemistry and human disease. Clin Endocrinol (Oxf). 2004;60:288.
Barclay JL, Tsang AH, Oster H. Interaction of central and peripheral clocks in physiological regulation. Prog Brain Res. 2012;199:163.
Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004;217:67.
Brown MS, Kovanen PT, Goldstein JL. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Rec Prog Horm Res. 1979;35:215.
Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking and esterification. Annu Rev Cell Dev Biol. 2006;22:129.
Chevrier L, Guimiot F, de Roux N. GnRH receptor mutations in isolated gonadotropic deficiency. Mol Cell Endocrinol. 2011;346:21.
Clyne CD, Zhang Y, Slutsker L, Mathis JM, White PC, Rainey WE. Angiotensin II and potassium regulate human CYP11B2 transcription through common cis-elements. Mol Endocrinol. 1997;11:638.
Cooper MS, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J Clin Endocrinol Metab. 2009;94:4645.
Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101.
Fieser LF, Fieser M. Steroids. New York: Reinhold Publishing Corp; 1959. p. 1–945.
Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neurosci Biobehav Rev. 2016;68:928.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125.
Khundmiri SJ, Murray RD, Lederer E. PTH and Vitamin D. Compr Physiol. 2016;6:561.
Kraemer FB. Adrenal cholesterol utilization. Mol Cell Endocrinol. 2007;42:265–6.
Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol. 2015;145:133.
Lin XW, Blum ID, Storch KF. Clocks within the Master Gland: Hypophyseal Rhythms and Their Physiological Significance. J Biol Rhythms. 2015;30:263.
Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J Steroid Biochem Mol Biol. 2013;137:176.
Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295.
Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62.
Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81.
Molina PE. Endocrine physiology. 3rd ed. New York: McGraw-Hill Company; 2010. p. 1–303.
Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-1100 hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108.
Norman AW, Henry HL. Hormones. 3rd ed. London: Elsevier; 2015. p. 1–413.
Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5-reductase enzymes. Evidence for distinct physiological functions. J Biol Chem. 1992;267:19548.
Plant TM. Hypothalamic control of the pituitary–gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol. 2008;20:719.
Riegelhaupt JJ, Waase MP, Garbarino J, Cruz DE, Breslow JL. Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J Lipid Res. 2010;51:1134.
Russell DW, Wilson JD. Steroid 5-reductase: two genes/two enzymes. Ann Rev Biochem. 1994;63:25.
Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647.
Tian Y, Shen W, Lai Z, Shi L, Yang S, Ding T, Wang S, Luo A. Isolation and identification of ovarian theca-interstitial cells and granulose cells of immature female mice. Cell Biol Int. 2015;39:584.
Wood CE. Development and programming of the hypothalamus-pituitary-adrenal axis. Clin Obstet Gynecol. 2013;56:610.
Further Reading
d’Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology (Bethesda). 2010;25:207–17.
Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol. 2017;165:18–37. pii: S0960-0760(16)30055-3.
Rahmani B, Ghasemi R, Dargahi L, Ahmadiani A, Haeri A. Neurosteroids; potential underpinning roles in maintaining homeostasis. Gen Comp Endocrinol. 2016;225:242–50.
Tsang AH, Barclay JL, Oster H. Interactions between endocrine and circadian systems. J Mol Endocrinol. 2013;52:R1–16.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this entry
Cite this entry
Acconcia, F., Marino, M. (2018). Steroid Hormones: Synthesis, Secretion, and Transport. In: Belfiore, A., LeRoith, D. (eds) Principles of Endocrinology and Hormone Action. Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-44675-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-44675-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44674-5
Online ISBN: 978-3-319-44675-2
eBook Packages: MedicineReference Module Medicine