Abstract
Background, aim, and scope
Many pollutants have received significant attention due to their potential estrogenic effect and are classified as endocrine disrupting compounds (EDCs). Because of possible ecological effects and increased attention for water reuse schemes, it is important to increase our understanding of the EDC removal capacities of various wastewater treatment systems. However, there has so far been little research on the fate and behavior of EDCs in stabilization pond systems for wastewater treatment, which represent an important class of wastewater treatment systems in developing countries because of their cost-effectiveness. The aim of this work is to study the fate and behavior of EDCs in algae and duckweed ponds. Because the synthetic hormone 17α-ethinylestradiol (EE2) and the natural hormones estrone (E1), as well as 17β-estradiol (E2), have been detected in effluents of sewage treatment plants and been suggested as the major compounds responsible for endocrine disruption in domestic sewage; E1, E2, and EE2 were therefore chosen as target chemicals in this current work.
Materials and methods
Both batch tests and continuous-flow tests were carried out to investigate the sorption and biodegradation of estrogens in algae and duckweed pond systems. The applied duckweed was a Lemna species. The applied algae was a mixture of pure cultures of six different algae genera, i.e., Anabaena cylindrica, Chlorococcus, Spirulina platensis, Chlorella, Scenedesmus quadricauda, and Anaebena var. Synthetic wastewater were used in all tests. The concentrations of estrogens were measured with three different enzyme-linked immunosorbent assay kits specific for E1, E2, or EE2. When the concentrations of estrogens in water samples were below the lowest quantitative analysis range (0.05 µg/l), preconcentration of the water samples were performed by means of solid phase extraction (SPE) with C18 cartridges.
Results
The 6-day batch tests show that the presence of algae or duckweed accelerated the removal of the three estrogens from the synthetic wastewater. More estrogens were removed in the tests with duckweed than in tests with algae or with wastewater. In the sorption tests, a swift sorption of the three estrogens was observed when the estrogens were contacted with duckweed or algae, while the estrogen concentrations in tap water kept unchanged during the 3-h sorption tests. The mass balances indicated that only about 5% of the estrogens were bound to the algae sediment or duckweed at the end of the 6-day tests. Results of the continuous-flow tests revealed that the algae and duckweed ponds effectively removed E1, E2, and EE2 even at nanograms per liter level. Interconversion of E1 and E2 occurred both in batch and continuous-flow tests. E2 could be readily transformed to E1, especially in the tests with algae.
Discussion
Different processes like sorption, biodegradation and photolytic degradation might play an important role in the removal of estrogens from the aquatic phase. The 3-h sorption tests support the importance of sorption for estrogen removal, in which a rapid initial sorption was observed over the first 2 min for E1/E2/EE2 to both duckweed and algae. In the 6-day batch tests, estrogens were sorbed by algae or duckweed during the early stage when algae and duckweed were contacted with the synthetic wastewater and the sorbed estrogens were further biodegraded by the microorganisms developed in the wastewater. The persistent estrogen concentrations in tap water, however, implied that no sorption, biodegradation, or photolytic degradation occurred in tap water under the specific experimental conditions. Under aerobic or anoxic conditions, E2 could be first oxidized to E1, which is further oxidized to unknown metabolites and finally to CO2 and water. Under anaerobic conditions, E1 can also be reduced to E2. However, the interconversion might be much more complex especially in the tests with algae because both aerobic and anaerobic conditions occurred in these tests due to the variation of the dissolved oxygen concentration induced by the light regime.
Conclusions
This study shows that estrogens, E1, E2, and EE2, can be effectively removed from the continuous-flow algae and duckweed ponds even when their concentrations are at nanograms per liter level. The presence of algae and duckweed accelerate the removal of estrogens from the synthetic wastewater because estrogens can be quickly sorbed on duckweed or algae. The sorbed estrogens are subsequently degraded by microorganisms, algae, or duckweed in the wastewater treatment system. E1 and E2 are interconvertible in both duckweed and algae pond systems. E2 can be readily transformed to E1, especially in the tests with algae.
Recommendation and perspectives
Based on the tests performed so far, one can conclude that both sorption and biodegradation are important to the estrogens removal from stabilization pond systems for wastewater treatment. Further research using, e.g., radioimmunoassay is needed to investigate the biodegradation pathway of estrogens in algae and duckweed ponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
The problem of endocrine disrupting chemicals (EDCs) has emerged as a major environmental and human health issue, generating a vast amount of attention among scientific communities worldwide and considerable media interest (Birkett and Lester 2003). EDCs include certain types of pesticides, plasticizers, and other industry-related materials as well as natural compounds such as human hormones and their breakdown products (Arditsoglou and Voutsa 2008; Sibel et al. 2005). Extensive studies carried out recently in the Netherlands, Germany, and other European countries showed that almost all selected (xeno-)estrogens are present at higher or lower concentrations in the aquatic environment (Johnson et al. 2005) and effluent discharged into rivers carry an estrogenic potential (Höhne and Püttmann 2008; Keiter et al. 2006).
Among the EDCs, the natural hormones estrone (E1) and 17β-estradiol (E2), as well as the synthetic hormone 17α-ethinylestradiol (EE2), were detected in effluents of sewage treatment plants. These three estrogens have been suggested as the major compounds responsible for endocrine disruption in domestic sewage (Onda et al. 2002). The estrogenic potencies of these three estrogens are three or more orders of magnitude higher than those of most EDCs, such as nonylphenol and bisphenol A (Shi et al. 2004). EE2 showed the highest estrogenic potency of the three estrogens mentioned in in vitro tests (de Mes et al. 2005), and their potencies can be expressed as EE2 > E2 > E1 (Larsson et al. 1999). As the release of these three estrogens from humans cannot be discontinued, the estrogens must be removed by treatment (Suzuki and Maruyama 2006). Because of possible ecological effects and increased attention for water reuse schemes, it is important to increase our understanding of the EDC removal capacities of various wastewater treatment systems.
Different studies around the world agree on average removal rates for estrogens in sewage treatment plants of around 80% for both E2 and EE2 and 65% for E1 (Johnson and Williams 2004). However, there has so far been little research on the fate and behavior of EDCs in stabilization pond systems for wastewater treatment, which represent an important class of wastewater treatment systems in developing countries because of their cost-effectiveness (Gijzen 2001). Waste stabilization ponds, such as algae ponds and duckweed ponds, are low-cost wastewater treatment systems producing high-quality effluents that allow water reuse in irrigation (Zimmo et al. 2004). Different authors have proposed the use of duckweed ponds for the efficient and low-cost treatment of domestic wastewater at urban or rural levels (Dalu and Ndamba 2003).
The objective of this work was to study the fate and behavior of EDCs in algae and duckweed ponds. For this purpose, E1, E2, and EE2 were chosen as target chemicals. Both batch tests and continuous-flow tests were carried out to investigate their removal in algae and duckweed pond systems.
2 Materials and methods
2.1 Inocula
The applied duckweed was a Lemna species, a genus of free-floating aquatic plants from the duckweed family. It was collected from a canal in Delft (The Netherlands). Pure cultures of six different algae genera, i.e., Anabaena cylindrica, Chlorococcus, Spirulina platensis, Chlorella, Scenedesmus quadricauda, and Anaebena var were incubated individually at 20°C. The six algae genera were then mixed to make up the algae inoculum for the algae pond systems.
2.2 Synthetic wastewater
Estrone (E1, purity >98%), 17ß-estradiol (E2, purity >98%), and 17α-ethinylestradiol (EE2, purity >98%) were purchased from Sigma Chemical Industries Let. (Germany) and were used without further purification. Due to their low aqueous solubility, E1, E2, and EE2 solutions were prepared by dissolving them in pure methanol followed by the addition of demineralized water. The estrogen concentration in the stock solution is 10 mg/l. The composition of the stock solution with respect to solvents was 10% methanol and 90% water (v/v). The stock solution was further diluted with demineralized water to 100 µg/l as working solution.
Synthetic wastewater was prepared to simulate the characteristics of effluent from anaerobic pond, which is normally used as the pre-treatment of an integrated duckweed or algae pond system. The total chemical oxygen demand (COD) was around 100 mg/l. Total nitrogen was in the range of 30–40 mg/l while phosphorus was adjusted to 3.6–3.8 mg/l. The synthetic wastewater was prepared in tap water. Macronutrients and micronutrients were added as indicated in Table 1. The prepared estrogen working solutions were mixed with the prepared synthetic wastewater to obtain a known initial concentration. The maximum contribution of the methanol to the carbon concentration (C) in the synthetic wastewater was 3 mg C/l (at estrogen concentration of 1 µg/l).
2.3 Batch tests
2.3.1 E1/E2/EE2 removal tests
The batch tests were carried out to study the degradation of E1, E2, and EE2 with higher concentrations (about 1 µg/l) in synthetic wastewater, wastewater seeded with duckweed, and wastewater seeded with algae. Tests with tap water were used as control. Duckweed [5 g fresh weight, which was equal to a duckweed density of 700 g (fresh weight) per m2 in full-scale duckweed ponds] was seeded to each beaker containing 1 l wastewater; while for the algae, about 100 mg of total suspended solids (TSS) was seeded to 1 l wastewater, to simulated TSS concentration about 90 mg/l in a full-scale algae pond system for wastewater treatment (van der Steen et al. 1999). All the beakers were incubated at room temperature (20°C) and exposed to a 12-h light and dark regime by illumination with high-pressure mercury lamps. The light intensity was 100 μE/m2/s, which provided sufficient light simulating natural condition. There was no agitation or shaking during the 6-day batch tests. Tests were performed in duplicate. Each replicate was analyzed in duplicate, and the average of the results is presented. Around 10 ml water samples were taken from each beaker at the pre-set time intervals of 0, 0.125, 0.75, 1, 3, and 6 days and were then centrifuged at 3,600 rpm for 10 min. The supernatant was decanted to measure the concentration of each estrogen.
2.3.2 Batch tests of sorption
To distinguish between biodegradation and physical sorption processes, short-term batch tests were carried out to study the sorption of E2, EE2 on duckweed, or algae. The sorption behavior was investigated by mixing E2 or EE2 (1 µg/l ) with 1 l of tap water seeded with 5,000 mg fresh duckweed (TSS about 380 mg) or 128 mg/l algae (TSS). The mixture was shaken horizontally (90 strokes per min) over 3 h. Tap water was used as control in these tests. All the tests were performed in duplicate. Water samples were taken after 0, 2, 5, 20, 60, and 180 min contact time to measure the estrogen concentration in the water phase.
2.3.3 Batch tests of mass balance
The mass balance of E1, E2, and EE2 in wastewater seeded with algae or duckweed was also carried out with similar batch tests as described in section 2.3.1. Initial and final estrogen concentrations in the water phase were measured. In order to measure the concentration of each estrogen in the solid phase, all biomass (duckweed or algae) was harvested and mixed with 100 ml pure methanol and further shaken horizontally (90 strokes per min) over 16 h in the dark. The suspension was centrifuged at 3,600 rpm for 10 min. The supernatant was decanted and extracted by solid phase extraction (SPE) with C18 cartridges (500 mg/6 mL, Mallinckrodt Baker, Inc., The Netherlands) and further analyzed with the enzyme-linked immunosorbent assay (ELISA) kits. The sorption of E1, E2, and EE2 was calculated from its concentration in the supernatant solution.
2.4 Continuous-flow setup
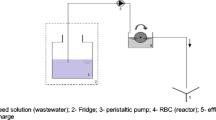
In order to investigate the fate of the three steroids in duckweed and algae pond systems, continuous-flow tests were performed, in which both the behavior of the three estrogens in the water and solid phase were studied. The setup (Fig. 1), designed as a plug flow reactor (Körner et al. 2003), consisted of two series of glass aquaria operated in parallel under the same conditions. Both series consisted of three aquaria (\( L \times B \times H = 50 \times 29 \times 25\,{\hbox{c}}{{\hbox{m}}^3} \) each). One series was operated as duckweed pond while another series as algae pond. The setup was exposed to the same illumination regime as indicated above. Wastewater dosed with E1, or E2, or EE2 was pumped into the parallel pond systems. One hundred grams fresh weight of duckweed was put into each duckweed pond, while algae ponds were started with seeding 200 ml of algae inoculum as described in section 2.1 to each pond. After the seeding, the setup was kept running for 30 days for the startup.
The continuous-flow setup was operated at a hydraulic retention time (HRT) of 15 days, which is a typical HRT applied to full-scale duckweed pond systems or algae pond systems. Duckweed was harvested every 4 days to maintain the 700 g/m2 fresh weight density as recommended by Skillicorn et al. (1993). No sediment was discharged from either the duckweed or algae ponds. Algae in the reactor can stay in free suspension or settle to the bottom of the aquaria as particles. Because algae rarely settle well, some losses of algae with the effluent could not be avoided. However, there was no significant change of algae concentration in the experimental setup during the running period because of net growth of algae.
To simulate the common estrogen concentration in real sewage, concentrations of nanograms per liter level were used in these continuous-flow tests. Two cycles of HRT after the startup, grab samples were collected from the influent wastewater and the discharged points of each pond (see Fig. 1). Water samples were concentrated by SPE with C18 cartridges after filtration with a glass–fiber filter GF/C (Ø47 mm, Whatman Group). One hundred microliters of the eluate was further analyzed with the ELISA kits. Because E1 and E2 might be interconvertible, both E1 and E2 were measured, when either E1 or E2 was dosed to the continuous systems.
2.5 Desorption tests
To distinguish between sorption and biodegradation in the continuous-flow tests, desorption tests with duckweed or sediment taken from each pond of the continuous-flow systems were preformed. To do so, known amounts of duckweed or sediment were mixed with 100 ml pure methanol and shaken horizontally for 16 h in the dark. The suspension was centrifuged and further extracted in the same manner as described above in the mass balance tests to detect the estrogen concentrations. The dry weight (DW) of the duckweed and algae sediment was also measured after filtration.
2.6 Analytical techniques
COD and TSS were measured according to standard methods. Dissolved oxygen, pH, and temperature were measured in situ with electrodes.
The concentrations of estrogens were measured with three different ELISA kits specific for E1, E2, or EE2 (EnviroChemical, Ltd., Japan). ELISA is a rapid, simple and cost-effective analytical method (Farre et al. 2006; Goda et al. 2004; Hintemann et al. 2006; Hirobe et al. 2006; Huang and Sedlak 2001; Suzuki and Maruyama 2006), which are used recently by many authors to detect EDCs at nanograms per liter level in environmental and biological samples (Farre et al. 2006; Goda et al. 2004; Hintemann et al. 2006; Hirobe et al. 2006; Huang and Sedlak 2001; Suzuki and Maruyama 2006).
The ELISA measurement is highly reproducible with a coefficient of variation generally below 10%. This method has a limit of detection around 1 ng/l, while the quantitative analysis ranges are 0.05–5.00 µg/l (E1), 0.05–1.00 µg/l (E2), and 0.05–3.00 µg/l (EE2). When raw wastewater samples were spiked at 1 µg/l of E1, E2, or EE2 and immediately analyzed with the ELISA kits, the average recoveries were 96.2% (E1), 99% (E2), and 100% (EE2). The monoclonal antibody of the ELISA kits exclusively binds with one kind of specific estrogen and does not show cross-reaction with other hormones or chemicals of similar structures (from the user]s guide).
The assays were in accordance with the instructions of the manufacturer. Absorbance was measured at 450 nm for each standard solution and sample with a microplate reader (Anthos Labtec, Austria).
3 Results
3.1 E1, E2, and EE2 removal in batch tests
The three estrogens followed similar trends of removal under the experimental conditions (Fig. 2). More estrogens were removed in the tests with duckweed than in tests with algae or with wastewater. Within 6 days, more than 95% of the E1, E2, and EE2 in the wastewater seeded with duckweed were removed, while only about 50% of the E1, E2, or EE2 was degraded in the synthetic wastewater. By contrast, the concentrations all of the three estrogens in tap water remained almost unchanged over 6 days. However, in terms of the sorbed concentration in tests with algae and duckweed, 1 g algae [dry weight, DW)] could sorb more estrogen than 1 g DW duckweed. For example, the sorbed concentration of E1 by algae was 6.1 µg/g (DW algae), while it was 2.34 µg/g (DW duckweed).
Estrogens were also more quickly removed in the experiments with duckweed than in the tests with wastewater (see Fig. 2). After 1 day, more than 80% of the estrogens were removed in the tests with duckweed. However, only less than 20% of estrogens were removed in the synthetic wastewater during the same period.
3.2 Sorption of E2 and EE2 on duckweed and algae
E2 and EE2 in wastewater can be removed from the liquid phase by sorption when estrogens are contacted with duckweed or algae (Fig. 3). The estrogen concentrations in tap water kept unchanged during the 3 h sorption tests. Only about 35% of E2 and 25% of EE2 were removed in the tests with algae, while more than 80% of E2 and EE2 were removed from the water phase in the tests with duckweed. For the tests with duckweed, three sorption phases can be distinguished, i.e., a rapid sorption between 0 and 20 min, followed by a period of slower sorption up to 60 min and then a steady decrease in sorption. However, it was somewhat different in the tests with algae: the equilibrium is reached after 20 min (see Fig. 3). Therefore, only rapid sorption took place in the tests with algae.
3.3 Mass balance of E1, E2, and EE2 in batch tests
Duckweed removed more estrogens from the system than algae (Fig. 4). In the tests with duckweed, 79% of E1, 80% of E2, and 86% of EE2 were removed from the system, while only 52% of E1, 54% of E2, and 56% of EE2 were removed in the tests with algae. It was also found that E1 and E2 were interconvertible in the mass balance tests. Both E1 and E2 were detected in the water and solid phase when E1 or E2 was added to the system. Tests with algae showed the highest transformation potential, in which about 17% of the dosed E2 was converted into E1 (see Fig. 4d).
3.4 Fate of E1, E2, and EE2 in continuous-flow systems
3.4.1 E1, E2, and EE2 removal in water phase
E1, E2, and EE2 dosed into the influent can be removed effectively when wastewater was flowing through the two pond systems (Figs. 5, 6, and 7), even when the estrogen concentrations were at the common level of nanograms per liter in the wastewater. The duckweed pond system showed a bit higher efficiency to remove estrogens than the algae pond system. The first pond removed more estrogens than the following two tanks, both in the algae and duckweed system. For example, about 76.8% and 85.4% of the E1 was removed in the first algae and duckweed pond, while about 7.1% and 8.9% of the E1 was removed by the subsequent two algae or duckweed ponds (see Figs. 5a and 6a).
3.4.2 E1 and E2 in the solid phase
Both E1 and E2 were desorbed from the collected algae sediments, and the harvested duckweed (Fig. 8) when either E1 or E2 was dosed to the influent of the continuous-flow setup. More estrogen was desorbed from the collected algae sediments than from the harvested duckweed. For example, about 41.4 ng E1+E2 were desorbed from 1 g DW of the algae sediments collected from algae tank3, while only 8 ng E1+ E2 were desorbed from one gram DW of the harvested duckweed from duckweed tank3 (when E2 dosed, see Fig. 8b).
4 Discussion
In this study, removal of three kinds of estrogens from algae and duckweed wastewater ponds was assessed. The continuous-flow tests showed that about 83.9% (Fig. 5a) - 95.4% (Fig. 6b) estrogens were removed from water phase in algae ponds and duckweed ponds. From the results, one can expect similar estrogen removal efficiencies in full-scale pond systems because the experimental conditions used in this study reflect well the real-world operation conditions of such pond systems. In practice, algae ponds are usually designed to have a total retention time of approximately 10–20 days, with a TSS concentration in the effluent of about 90 mg/l (van der Steen et al. 1999). In duckweed ponds, frequent harvesting of the duckweed is necessary to maintaine the plant densities at optimum levels which is recommended among 400 and 800 g of fresh weight per square meter (Skillicorn et al. 1993). In some laboratory experiments and full-scale duckweed ponds, the HRT is about 20 days or longer (Al-Nozaily et al. 2000; Zimmo et al. 2003, 2004). Indeed, some factors, such as HRT, TSS, duckweed densities, and the duckweed harvesting procedure in the present study, were chosen prudentially to simulate the real operation conditions of algae and duckweed pond system.
There are no other studies concerning estrogen removal from wastewater in algae pond and duckweed pond systems, but removal rate values observed here can be compared with values reported for activated sludge wastewater treatment systems in literature. Estrogen removal efficiencies, for example, 61–69% for E1, 86–96% for E2 (D’Ascenzo et al. 2003; Onda et al. 2003) and 71% for EE2 (Johnson et al. 2000), varied among sewage treatment plants depending on the plant design and efficiency. The estrogen removal efficiencies in algae ponds and duckweed ponds obtained in this study are comparable to that of conventional activated sludge system (Table 2), or are even somewhat higher than those of activated sludge systems as reported in the literature.
Concerning the removal mechanisms, it has been generally accepted that different processes like sorption, biodegradation, and photolytic degradation might play an important role in the removal of estrogens from wastewater in activated sludge system (de Mes et al. 2005). Although there might be some differences due to plant uptake and metabolism, one can still speculate that these processes probably played the same role on the removal of estrogens from wastewater treated in algae ponds or duckweed ponds. Figure 2 shows that only sorption and degradation processes occurred under the specific experimental conditions in this study because the photooxidative processes were shown to be not quantitatively relevant in the control experiment with tap water.
The nonpolar and hydrophobic nature of these estrogens makes them sorb easily onto particulates. Figure 2 illustrates indeed that estrogens were sorbed during the early stage of contacting with algae and duckweed. For example, in the tests with duckweed, up to 46% of E1, 22% of E2, and 43% of EE2 were removed rapidly within 3 h of the 6 days static batch tests. The importance of sorption for the estrogen removal was further supported by the 3 h sorption tests, in which a rapid initial sorption was observed over the first 2 min (see Fig. 3). The observation of rapid sorption is in agreement with sorption tests of five different estrogens on activated sludge, in which E2 was found to be rapidly removed from the liquid phase over the first 10 min (Ren et al. 2007).
Estrogens in wastewater were not only sorbed on activated sludge but were also degraded by microorganisms (Johnson et al. 2005). Similarly, this study also reveals the importance of biodegradation for the estrogen removal. From Fig. 2, one can find that the combination of biodegradation and sorption leads to higher estrogen removal rates in wastewater seeded with duckweed or algae than in wastewater only. One might also find a difference in estrogen concentrations between day 1 and day 6 in the synthetic wastewater (see Fig. 2). This might be due to the development of microorganism at the later stage of the 6-day batch tests. A phenomenon observed during the batch tests was the increase of turbidity, implying the development of microorganisms in flocs, which could further enhance the sorption or biodegradation of the estrogens in the synthetic wastewater. Figure 8 shows that only a small part of the estrogens were desorbed from duckweed in the desorption tests, suggesting the importance of biodegradation on the estrogen removal. What should be stressed here is the possibility of estrogen removal by duckweed uptake. As reported by Janeczko and Skoczowski (2005), E1 and E2 were detected in 68 plant species, implying that the estrogens are taken up by plants. While in this study, the roles of plant uptake to the estrogen removal still need further investigation.
It has been reported that under aerobic or anoxic conditions, E2 could be first oxidized to E1, further oxidized to unknown metabolites, and finally to CO2 and water. Under anaerobic conditions, E1 could be reduced to E2 (Joss et al. 2004; Ternes et al. 1999). This reversible interconversion was also confirmed in this study (see Figs. 4, 5, 6, and 8). While the interconversion might be more complex especially in the tests with algae because of both aerobic and anaerobic conditions in algae ponds. In algae ponds, the dissolved oxygen (DO) concentrations measured during daytime are much higher (more than 10 mg/l) due to the photosynthesis by algae, while the DO is completely consumed at night. In the tests with duckweed, the situation is somewhat different. The DO concentrations were always lower (0–2 mg/l) because the dense cover of duckweed may have reduced the direct oxygen transfer from the atmosphere (Moorhead and Reddy 1988). Even though DO concentrations are always lower than 2.0 mg/L in duckweed ponds as measured in this study, the interconversion can also be detected in both the mass balance (see Fig. 4) and the continuous tests (see Fig. 6). The general trend in the conversion rates is that the rates of E2 to E1 are higher than the rates of E1 to E2 probably because E2 is the most labile of the steroid estrogens investigated (Andersen et al. 2005). Indeed, in this study, among the remained estrogen in the water phase of test with E2 dosed, E1 took up about 50% of the total estrogen concentration in all three algae ponds, as well as in the second and third duckweed pond (see Figs. 5b and 6b), implying the high conversion rate from E2 to E1.
5 Conclusions
-
Estrogens E1, E2, and EE2 can be effectively removed from the continuous-flow algae and duckweed ponds even when their concentrations are at nanograms per liter level.
-
The presence of algae and duckweed accelerate the removal of estrogens from the synthetic wastewater because estrogens can be quickly sorbed on the duckweed or algae. The sorbed estrogens are subsequently degraded by microorganisms and algae or duckweed in the wastewater treatment system.
-
Duckweed ponds show a bit higher efficiency to remove estrogens than algae ponds.
-
E1 and E2 are interconvertible in both duckweed and algae pond systems. E2 can be readily transformed to E1, especially in the tests with algae.
6 Recommendations and perspectives
This study showed that both sorption and biodegradation are important to the estrogens removal from algae or duckweed pond systems for wastewater treatment. Further research using, e.g., radioimmunoassay is needed to investigate the biodegradation pathway of estrogens in algae and duckweed ponds.
References
Al-Nozaily F, Alaerts G, Veenstra S (2000) Performance of duckweed-covered sewage lagoons—I. Oxygen balance and COD removal. Water Res 34:2727–2733
Andersen HR, Hansen M, Kjolholt J, Stuer-Lauridsen F, Ternes T, Halling-Sorensen B (2005) Assessment of the importance of sorption for steroid estrogens removal during activated sludge treatment. Chemosphere 61(1):139–146
Arditsoglou A, Voutsa D (2008) Determination of phenolic and steroid endocrine disrupting compounds in environmental matrices. Env Sci Pollut Res 15(3):228–236
Birkett JW, Lester JN (2003) Endocrine disrupters in wastewater and sludge treatment processes. CRC Press LLC, Florida
Dalu JM, Ndamba J (2003) Duckweed based wastewater stabilization ponds for wastewater treatment (a low cost technology for small urban areas in Zimbabwe). Phys Chem Earth, Parts A/B/C 28(20–27):1147–1160
D’Ascenzo G, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, Samperi R (2003) Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci Total Environ 302:199–209
de Mes T, Zeeman G, Lettinga G (2005) Occurrence and fate of estrone, 17b-estradiol and 17a-ethynylestradiol in STPs for domestic wastewater. Rev Environ Sci Bio/Technology 4:275–311
Farre M, Brix R, Kuster M, Rubio F, Goda Y, de Alda MJL, Barcelo D (2006) Evaluation of commercial immunoassays for the detection of estrogens in water by comparison with high-performance liquid chromatography tandem mass spectrometry HPLC-MS/MS (QqQ). Anal Bioanal Chem 385(6):1001–1011
Gijzen HJ (2001) Low cost wastewater treatment and potentials for re-use: a cleaner production approach to wastewater management. International Symposium on Low-Cost Wastewater Treatment and Re-use, Cairo, Egypt
Goda Y, Kobayashi A, Fujimoto S, Toyoda Y, Miyagawa K-I, Ike M, Fujita M (2004) Development of enzyme-linked immunosorbent assay for detection of alkylphenol polyethoxylates and their biodegradation products. Water Res 38:4323–4330
Hintemann T, Schneider C, Ler HFS, Schneider RJ (2006) Field study using two immunoassays for the determination of estradiol and ethinylestradiol in the aquatic environment. Water Res 40:2287–2294
Hirobe M, Goda Y, Okayasu Y, Tomita J, Takigami H, Ike M, Tanaka H (2006) The use of enzyme-linked immunosorbent assays (ELISA) for the determination of pollutants in environmental and industrial wastes. Water Sci Technol 54(11–12):1–9
Höhne C, Püttmann W (2008) Occurrence and temporal variations of the xenoestrogens bisphenol A, 4-tert-octylphenol, and tech. 4-nonylphenol in two German wastewater treatment plants. Environ Sci Pollut Res 15:405–416
Huang CH, Sedlak DL (2001) Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environ Toxicol Chem 20(1):133–139
Janeczko A, Skoczowski A (2005) Mammalian sex hormones in plants. Folia Histochem Cytobiol 43(2):71–79
Johnson AC, Williams RJ (2004) A model to estimate influent and effluent concentrations of estradiol, estrone and ethinylestradiol at sewage treatment works. Environ Sci Technol 38:3649–3658
Johnson A, Belfroid A, Di Corcia A (2000) Estimating steroid estrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci Total Environ 256:163–173
Johnson AC, Aerni HR, Gerritsen A, Gibert M, Giger W, Hylland K, Jurgens M, Nakari T, Pickering A, Suter MJF, Svenson A, Wettstein FE (2005) Comparing steroid estrogen, and nonylphenol content across a range of European sewage plants with different treatment and management practices. Water Res 39(1):47–58
Joss A, Andersen H, Ternes T, Richle PR, Siegrist H (2004) Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions: consequences for plant optimization. Environ Sci Technol 38:3047–3055
Keiter S, Rastall A, Kosmehl T, Wurm K, Erdinger L, Braunbeck T, Hollert H (2006) Ecotoxicological assessment of sediment, suspended matter and water samples in the upper Danube River. Environ Sci Pollut Res 13(5):308–319
Körner S, Vermaatb JE, Veenstrac S (2003) The capacity of Duckweed to treat wastewater: ecological considerations for a sound design. J Environ Qual 32(5):1583–1590
Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Pettersson M, Berg AH, Olsson PE, Forlin L (1999) Ethinyloestradiol—an undesired fish contraceptive? Aquat Toxicol 45(2–3):91–97
Moorhead KK, Reddy KR (1988) Oxygen transport through selected aquatic macrophytes. J Environ Qual 17:138–142
Onda K, Yang SY, Miya A, Tanaka T (2002) Evaluation of estrogen-like activity on sewage treatment processes using recombinant yeast. Water Sci Technol 46(11–12):367–373
Onda K, Nakamura Y, Takatoh C, Miya A, Katsu Y (2003) The behavior of estrogenic substances in the biological treatment process of sewage. Water Sci Technol 47(9):109–116
Ren Y-X, Kazunori N, Nobuo C (2007) Osamu Nishimura, a thermodynamic analysis on adsorption of estrogens in activated sludge process. Water Res 41:2341–2348
Shi J, Fujisawa S, Nakai S, Hosomi M (2004) Biodegradation of natural and synthetic estrogens by nitrifying activated sludge and ammonia-oxidizing bacterium Nitrosomonas europaea. Water Res 38:2323–2330
Sibel I, Oktay E, Aydin A (2005) Degradation of 17β-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques. J Hazard Mater B126:54–62
Skillicorn P, Spira S, Journey W (1993) Duckweed agriculture. The World Bank, Washington
Suzuki Y, Maruyama T (2006) Fate of natural estrogens in batch mixing experiments using municipal sewage and activated sludge. Water Res 40:1061–1069
Ternes T, Kreckel P, Mueller J (1999) Behaviour and occurrence of estrogens in municipal sewage treatment plants II. Aerobic batch experiments with activated sludge. Sci Total Environ 225(1–2):91–99
van der Steen P, Brenner A, van Buuren J, Oro G (1999) Post-treatment of UASB reactor effluent in an integrated duckweed and stabilization pond system. Water Res 33(3):615–620
Zimmo OR, van der Steen NP, Gijzen HJ (2003) Comparison of ammonia volatilisation rates in algae and duckweed-based waste stabilisation ponds treating domestic wastewater. Water Res 37(19):4587–4594
Zimmo OR, van der Steen NP, Gijzen HJ (2004) Nitrogen mass balance across pilot-scale algae and duckweed-based wastewater stabilisation ponds. Water Res 38:913–920
Acknowledgements
This work was supported by the State Key Lab of Urban Water Resource and Environment (HIT, 2008QN05), SWITCH project (EU FP6, contract No.018530) and the National High Technology Research and Development Program of China (2006AA06Z303). Many thanks to Fred Kruis, Frank Wiegman, Don van Galen, Peter Heerings, and Lyzette Robbemont for supporting the determination of estrogens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Shi, W., Wang, L., Rousseau, D.P.L. et al. Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems. Environ Sci Pollut Res 17, 824–833 (2010). https://doi.org/10.1007/s11356-010-0301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0301-7






 Remained in the water;
Remained in the water;  sorbed on duckweed or algae;
sorbed on duckweed or algae;  E1 (or E2) converted to E2 (or E1);
E1 (or E2) converted to E2 (or E1);  The converted estrogen sorbed on duckweed or algae)
The converted estrogen sorbed on duckweed or algae)
 ; filled triangle, removal rate)
; filled triangle, removal rate)
 ; filled triangle removal rate)
; filled triangle removal rate)
 ; filled triangle, removal rate in algae ponds and multiplication symbol, removal rate in duckweed ponds)
; filled triangle, removal rate in algae ponds and multiplication symbol, removal rate in duckweed ponds)
 )
)