Abstract
As major endocrine disruptors, natural estrogens such as 17β-estradiol (E2) have been found with adverse effects on animals and humans. How to control E2 pollution as well as that of other estrogens in the environment is a worldwide concern. A novel E2-degrading bacterium (strain JX-2) was isolated from the activated sludge of a sewage treatment plant and was identified as Rhodococcus sp. Strain JX-2 grew well and metabolized up to 94 % of the substrate E2 added (30 mg L−1) within 7 days at 30 °C. The optimal environmental conditions for E2 degradation by JX-2 were pH 7.0 and 30 °C. Strain JX-2 was immobilized in sodium alginate. The optimal conditions for strain JX-2 immobilization were 4 % sodium alginate, 1:1 bacteria/sodium alginate ratio, 5 % CaCl2⋅2H2O, and 6 h crosslinking time. The degradation performance of immobilized strain JX-2 was apparently superior to that of the free strain, particularly under pH <6.0 or >8.0 either below 20 or above 35 °C. Immobilized strain JX-2 removed E2 in natural sewage and cow dung with removal efficiency of more than 64 and 81 %, respectively. This is the first report of utilizing immobilized bacteria to remove estrogens in sewage and livestock manure. The results suggest that strain JX-2 could be used to remove E2 from the environment efficiently.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Endocrine-disrupting compounds (EDCs) are of global concerns because exposure to these compounds causes detrimental impacts on freshwater organisms, ecosystem sustainability, and human health (Tyler et al. 1998; Vos et al. 2000). Estrogens are a major group of EDCs of public interest. They are subdivided into natural and synthetic estrogens. The former includes estrone (E1), 17β-estradiol (E2), and estriol (E3), and the latter includes 17α-ethynylestradiol (EE2). Both natural and synthetic estrogens are the main sources of estrogenic activity in treated wastewater (Johnson and Williams 2004; Solé et al. 2000). It has been documented that estrogens in treated sewage water are responsible for feminizing male fish and sexual disruption in several aquatic animals (Folmar et al. 1996; Jobling et al. 2002). As the most common estrogen, E2 of 0.1–1 ng L−1 may cause reproductive disorders in aquatic animals (Purdom et al. 1994). E2 is more harmful to humans, as even extremely low effective concentrations of estrogens in the environment can decrease endogenous gonadal sex hormone secretion and sperm counts, cause reproductive organ abnormalities, and increase the incidence of cancers (Crisp et al. 1997; Kavlock et al. 1996).
Estrogens are produced naturally by humans and animals and are discharged daily in urine and feces and thus are present in domestic sewage water. They are commonly observed in influents and effluents of sewage treatment plants (STPs) in many countries (Baronti et al. 2000; Belfroid et al. 1999; Desbrow et al. 1998; Kuch and Ballschmiter 2001; Snyder et al. 1999; Ternes et al. 1999). E2 in influents of Japanese STPs are determined with concentrations of 30–90 ng L−1 in autumn and 20–94 ng L−1 in summer (Nasu et al. 2001). E2 in effluent samples was observed at concentrations of 3.2–55 ng L−1 in summer and 2.8–30 ng L−1 in autumn (Tabata et al. 2001). Zhou et al. (2012) detected E1, E2, EE2, and E3 in influents of a STP in Beijing, China, at concentrations of 120, 7.2, 16.2, and 120 ng L−1, respectively. At the same time, E1 and EE2 concentrations in effluents were 4.3 and 11.4 ng L−1. However, E2 and E3 were undetectable. Thus, STPs are considered as a main estrogen source in the environment. Livestock farming is another source of estrogens, and large concentrations of estrogens are found in livestock waste. Zheng et al. (2007) detected estrogen levels of 2103.0 ± 123.0 ng L−1 in fresh cow dung from a dairy farm in the USA by gas chromatography/mass spectroscopy. Andaluri et al. (2012) analyzed municipal biosolids, poultry manure, and cow manure for E2 using liquid chromatography/mass spectroscopy (LC-MS) and found levels of 229.7, 149.8, and 16.6 μg kg−1, respectively. Eliminating estrogens from sewage and cow dung has become a concern, given the severity of estrogen pollution worldwide.
Many studies have focused on sorption, membrane filtration, advanced oxidation processes, and composting techniques to remove estrogens from sewage and animal manure (Le et al. 2013; Silva et al. 2012; Zeng et al. 2013). In fact, bioremediation techniques (i.e., the use of microorganisms in environmental decontamination) are cheaper, more efficient, and environmentally friendly than physiochemical methods (Yang et al. 2009). The degradation of estrogens by isolated bacterial strains has been characterized in the last decades. Several human intestinal bacteria and oral microorganisms are found capable of transforming E2 to E1 and vice versa (Järvenpää et al. 1980; Kornman and Loesche 1982; Ojanotko-Harri et al. 1991). An E2-degrading bacterium (Novosphingobium tardaugens sp. nov.) was isolated by Fujii et al. (2002) from activated sludge. Since then, many other estrogen-degrading bacteria have been obtained from sludge by Weber et al. (2005), Yoshimoto et al. (2004), and Yu et al. (2007), while Shi et al. (2002) reported an EE2-degrading microorganism (Fusarium proliferatum) obtained from cowshed samples.
A challenge in the bacteria application as environmental biodegradation agents is that they can compete with indigenous microorganisms or that they are less adapted to the ambient environmental conditions (Li et al. 2007). Introduced bacteria are washed out and diluted in open water systems, leading to decreases in their concentrations and inefficient bioremediation (Rahman et al. 2006). Thus, bacteria have been immobilized onto a carrier matrix as a suspension (Gentili et al. 2006). Sodium alginate is a common carrier material with good mass transfer performance, and it is nontoxic to the environment and can be degraded by soil microorganisms (Covarrubias et al. 2012). Once the carrier is protected, the E2 biodegradation is enhanced after immobilizing the cells. For instance, immobilized Pseudomonas putida CCRC14365 degrades 1000 mg L−1 phenol, while 600 mg L−1 phenol is degraded by free cells (Chung et al. 2003). However, few scientific studies have been reported on the immobilization and application of E2-degrading bacteria. Furthermore, it is unclear whether sodium alginate is suitable as a carrier material in sewage and animal manure.
In this study, a novel E2-degrading bacterium JX-2, identified as Rhodococcus sp., was obtained from the activated sludge of a STP. The environmental conditions for E2 degradation by this strain were optimized. Strain JX-2 was immobilized and applied to treat E2 in sewage and cow dung. Results of this investigation provide a potential novel method to clean up estrogens in contaminated environments using bacteria-immobilized agents.
2 Materials and Methods
2.1 Chemicals and Media
E2 (>98 % purity) was bought from Sigma-Aldrich (St. Louis, MO, USA). The chemical characteristics (molecular weight, solubility in water, octanol-water partition coefficient, and vapor pressure) of E2 are 272.38 g mol−1, 5.4–13.3 mg L−1, logKow 3.8–4.0, and 3 × 10−8 kPa, respectively. All solvents used were of pure analytical grade. A highly concentrated stock solution of E2 was prepared in acetone (1000 mg L−1 acetone).
The mineral salt medium (MSM), referred to Sun et al. (2014), contained 1.50 g L−1 (NH4)2SO4, 1.91 g L−1 K2HPO4⋅3H2O, 0.50 g L−1 KH2PO4, 0.20 g L−1 MgSO4⋅7H2O, and 1 mL trace element solution (0.1 mg L−1 CoCl2⋅6H2O, 0.425 mg L−1 MnCl2⋅4H2O, 0.05 mg L−1 ZnCl2, 0.01 mg L−1 NiCl2⋅6H2O, 0.015 mg L−1 CuSO4⋅5H2O, 0.01 mg L−1 Na2MoO4⋅2H2O, 0.01 mg L−1 Na2SeO4⋅2H2O).
The MSM-E2 medium was MSM supplemented with E2 at concentrations of 5–50 mg L−1. The liquid MSM-E2 medium was obtained as follows: E2 (1000 mg L−1 in acetone) was added into the sterile flasks, followed by the addition of MSM after the acetone volatilized. To obtain the solid MSM-E2 medium plates, E2 (1000 mg L−1 in acetone) was mixed with the melting MSM, and the medium was poured into the plates, and the acetone was volatilized with sterile air. Added to the MSM-E2 was 0.5 g L−1 of glucose as the carbon source unless otherwise specified to maintain a better bacterial growth.
The Luria-Bertani (LB) medium contained tryptone, yeast extract, and NaCl at concentrations of 10.0, 5.0, and 10.0 g L−1, respectively. Solid medium plates were prepared by adding 18 g L−1 agar into the above liquid medium.
2.2 Isolation of the E2-Degrading Strain JX-2
The E2-degrading strain was isolated from activated sludge collected from a STP in Lin’an, China. Ten milliliters of seed sludge was inoculated into a 250-mL conical flask containing 100 mL MSM with 10 mg L−1 E2 and incubated at 30 °C for 7 days in a rotary shaker at 150 rpm. Ten milliliters of enriched E2-degrading culture was transferred four times to 100 mL fresh MSM with E2 concentrations increasing from 15 to 30 mg L−1. During the enrichment procedure, no glucose was added in the MSM-E2. The final transferred aliquot of the enriched culture was tested for E2 degradation by high-performance liquid chromatography (HPLC). Serial dilutions were processed and spread onto MSM plates containing 30 mg L−1 of E2. The plates were incubated for 5 days at 30 °C. The bacterial colonies were purified on the plates by streaking three times, and morphologically distinct colonies were obtained and tested for the E2 degradation ability. An E2-degrading bacterium was isolated and named JX-2.
E2 in MSM was detected by HPLC according to the method described by Xu et al. (2014) and Li et al. (2015). Briefly, an equal volume of methanol was added into the flasks to solve residual E2; the mixture solution was ultrasonicated for 30 min and then filtered with 0.22-μm filters (polytetrafluoroethylene, PTFE). E2 residue in the flask was measured using an HPLC machine (Shimadzu LC-20AT, Japan), fitted with an ultraviolet detector and an Inertsil ODS-SP-C18 column (5 μm, 150 × 4.6 mm) using acetonitrile/water (70/30, v/v) in the mobile phase at a flow rate of 1 mL min−1. Chromatography was performed at 40 °C. E2 was detected at 280 nm, and the injection volume was 20 μL. The biodegradation rate was calculated by comparing the peak area with that of an E2 standard according to the E2 standard curve.
The main metabolites of E2 produced by strain JX-2 were detected with LC-MS (Agilent Technologies, 1200RRLC/6410B, Japan). Metabolites were extracted from 20 mL of 5-day MSM-E2-JX2 culture supernatant after centrifugation at 17,000g for 10 min. The supernatant was dried by lyophilization and dissolved in 2 mL methanol. The metabolites were analyzed by LC-MS, ionized by electrospray with a negative polarity. MSM-E2 without strain JX2 was analyzed as control.
2.3 Identification of Strain JX-2
Transmission electron microscopy was utilized for morphological observations of strain JX-2. The physiological and biochemical characteristics of strain JX-2 were obtained according to a bacterial identification manual (Garrity et al. 2004). For 16S ribosomal RNA (rRNA) gene analysis, the genomic DNA of strain JX-2 was extracted using a DNA Extraction Kit (Tiangen, China), and 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the genomic DNA as a template and the bacterial universal primers 16S rRNA-27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 16S rRNA-1492R (5′-TACCTTGTTACGACTT-3′) (Byers et al. 1998). The PCR amplification products were sequenced by the Nanjing GenScript Biotechnology Company (Nanjing, China). The 16S rRNA gene sequence was compared with sequences in the GenBank database using the NCBI Blast program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). A phylogenetic tree was constructed from the evolutionary distances using the neighbor-joining method. The tree topologies were evaluated by performing a bootstrap analysis of 1000 data sets with the MEGA5.05 package.
2.4 Immobilization of Strain JX-2
The sodium alginate embedding conditions were optimized by the orthogonal test. A four-factor, three-level orthogonal table L9 (3)4 was designed. Four main factors were evaluated, including three levels of the mass fraction of sodium alginate (A1-3 %, A2-4 %, A3-5 %), bacteria/sodium alginate ratio (B1-1:1, B2-1:2, B3-2:1), mass fraction of CaCl2⋅2H2O (C1-3 %, C2-4 %, C3-5 %), and crosslinking time (D1-4 h, D2-6 h, D3-8 h). The optimal embedding conditions were determined by evaluating the E2 degradation efficiency.
An inoculum of strain JX-2 was prepared by cultivating bacteria in LB medium for 24 h at 30 °C and 150 rpm on a rotary shaker. The bacterial cells were centrifuged at 8000 rpm for 5 min, washed twice, and resuspended in sterile MSM to an optical density at 600 nm (OD600) of 1.0. Sodium alginate (SA) was dissolved and sterilized by autoclaving at 121 °C for 20 min. The cell suspension was mixed with SA at the ratios shown in Table 1. The mixture was immediately dropped into sterile CaCl2⋅2H2O solution and crosslinked at 4 °C to obtain a 0.02–0.04-g pellet that was 3–4 mm in diameter. The CaCl2⋅2H2O solution was washed out with sterile MSM, and the pellets were transferred to MSM-E2 for batch experiments.
2.5 Degradation of E2 by Strain JX-2 in Liquid MSM-E2
An inoculum of strain JX-2 was prepared as described above. The cells were inoculated at 5 % (v/v) into 20 mL MSM containing 30 mg L−1 of E2 (pH 7.0) in a 50-mL flask and incubated at 30 °C and 150 rpm on a rotary shaker for all experiments. The growth and E2-degrading ability of JX-2 were determined after cell inoculation into MSM-E2. The effects of the incubation pH (4.0–10.0), temperature (15–40 °C), initial E2 concentration (5–50 mg L−1), and inoculum size (1–25 %) on E2 biodegradation by JX-2 were experimented. MSM-E2 containing inactivated JX-2 served as control. All experiments were performed in triplicate. The viable bacterial populations in the conical flasks were obtained by plate counts. E2 concentration was analyzed using HPLC as above. The control consisted of the identical mixtures but with inactivated cells.
The E2 degradation rate was determined as follows:
2.6 Degradation of E2 by Immobilized Strain JX-2 in Liquid MSM-E2
The immobilized bacteria of strain JX-2 was prepared as described in Section 2.4, and E2 degradation by immobilized strain JX-2 in liquid MSM-E2 was performed as outlined in Section 2.5. The MSM-E2 containing an equal concentration of the free strain JX-2 served as a positive control and MSM-E2 containing an equal quantity of sterile beads as the negative control (blank control).
2.7 Degradation of E2 by Immobilized Strain JX-2 in Sewage and Cow Dung
The immobilized bacteria of strain JX-2 was prepared as described in Section 2.4. Three sewage samples were collected near different discharging positions from a lake which received raw domestic sewage and became too polluted in Nanjing, China. For each sample, the surface water (within 0.5 m depth) was collected randomly from five different sites within a radius of 10 m2 with a water sampler, mixed together in a glass bottle, and then brought back to the lab immediately for E2 detection. The E2 concentrations in the three sewage samples were 55.59, 39.78, and 43.35 ng L−1, respectively. The immobilized bacteria pellets (100 g) were introduced into 1 L sewage and incubated at 30 °C for 7 days on a rotary shaker at 150 rpm. The residual E2 concentration was determined, and the E2 removal efficiency was calculated the same as the calculation of E2 degradation rate in Section 2.5. Sewage without the immobilized bacteria pellets was used as control.
Four cow dung samples were collected from a dairy farm in Nanjing, China. Each fresh cow dung sample was collected randomly from five different sites within a radius of 25 m2, mixed in a plastic cask, and then brought back to the lab immediately for E2 detection. The E2 concentrations in the four cow dung samples were 146.31, 148.84, 158.70, and 174.01 μg kg−1, respectively. The immobilized bacteria pellets (50 g) were introduced into 100 g freeze-dried cow dung. Sterile water was added every day to maintain 60–70 % water content. The mixture was stirred every 12 h and incubated at 30 °C for 7 days. The residual E2 concentration was determined, and the E2 removal efficiency was calculated as above. Cow dung without immobilized bacteria pellets was used as control.
2.8 E2 Analysis in Sewage and Cow Manure
E2 in sewage was detected as described by Li et al. (2015). Briefly, a certain volume of sewage was passed through a C18 column (200 mg/6 mL) at a flow rate of 5 mL L−1. The column was eluted with 5 mL ultrapure water, and then 15 mL methanol was added. The eluate was collected in fractions and dried under nitrogen gas, and the extract was resuspended in methanol to 2 mL. After filtration through a 0.22-μm filter, E2 was detected using a HPLC system fitted with a fluorescence detector (FLD) and an Intertsil ODS-SP-C18 column (5 μm, 150 × 4.6 mm). Methanol/acetonitrile/water (20/30/50, v/v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1. HPLC was performed at 40 °C, and the injection volume was 20 μL. The fluorescence excitation and emission wavelengths were 280 and 310 nm, respectively.
E2 in cow manure was detected as described by Li et al. (2015). Briefly, 15 mL ethyl acetate was added to freeze-dried cow manure. Tubes were closed with a Teflon-liner cap, and the cow manure was extracted by ultrasonication for 1 h. Samples were centrifuged for 10 min at 4000 rpm to separate the manure from the aqueous solution, and 5 mL supernatant was dehydrated by percolation through a column containing Na2SO4 anhydride. The column was eluted with 10 mL 1:1 (v/v) ethyl acetate and methanol. After that, the E2 in the eluate was collected in fractions, dried under nitrogen gas, and resuspended in methanol for HPLC detection as described for the detection of E2 in sewage.
2.9 Statistical Analysis
All data were processed using Microsoft Office Excel 2010 (Microsoft, Inc. Redmond, WA, USA) and SPSS ver. 13.0 software (SPSS Inc., Chicago, IL, USA). All experiments were carried out in triplicate. Data are presented as the mean ± standard deviation. A p value <0.05 was considered significant.
3 Results
3.1 Isolation and Identification of Strain JX-2
Strain JX-2 with E2-degrading ability was isolated from the activated sludge of a STP. The LC-MS analysis of the metabolites of E2 by strain JX-2 is shown in S1 in the Electronic Supplementary Materials. From the metabolite peaks of the two figures, we can infer that the potential main metabolite of E2 by strain JX-2 should be E1. However, no other metabolites of E2 were identified with this method.
Strain JX-2 grew well on MSM plates containing 30 mg L−1 of E2. It is a short rod-shaped, Gram-positive aerobic bacterium without flagellum (S2 in Electronic Supplementary Materials). The methyl red test and Voges-Proskauer test were negative, and the indole test was positive. Strain JX-2 was positive for nitrate reductase, citrate, and catalase but negative for phenylalanine dehydrogenase. This strain grew well in the medium containing glucose and fructose but could not hydrolyze starch or gelatin.
The 16S rRNA gene sequence of strain JX-2 had been submitted to GenBank, and its accession number was KX867463. A BLAST analysis of the 16S rRNA gene sequence from strain JX-2 against the sequences in GenBank showed that strain JX-2 was >99 % identical to the strains of Rhodococcus sp. A phylogenetic tree including strain JX-2 and related species is given in Fig. 1. JX-2 was identified as a strain of Rhodococcus sp. based on its morphology, physiological and biochemical characteristics, and 16S rRNA gene sequence.
3.2 Immobilization of Strain JX-2
The sodium alginate embedding conditions were optimized by the orthogonal test, according to the E2 degradation rate. As shown in Table 1, nine treatments (T1–T9) consisting of three different levels of mass fraction of sodium alginate, bacteria/sodium alginate ratio, the mass fraction of CaCl2⋅2H2O, and crosslinking time were performed, and the E2 degradation rate of each treatment was listed.
When the mass fraction of sodium alginate was 3 %, and the other three factors were different (T1, T2, and T3), the E2 degradation rate was 84.21, 86.75, and 88.57 %, respectively, and the average E2 degradation rate of T1, T2, and T3 was 86.51 % (shown as k 1 in Table 1). When the mass fraction of sodium alginate was 4 and 5 %, the average E2 degradation rate (shown as k 2 and k 3 in Table 1) was 91.25 and 85.83 %. Among the three values representing the average E2 degradation rate, k 2 (91.25 %) was the maximum, indicating that the optimal mass fraction of sodium alginate was A2-4 %. Similarly, as shown in Table 1, the optimal bacteria/sodium alginate ratio, mass fraction of CaCl2⋅2H2O, and crosslinking time were B1-1:1, C3-5 %, and D2-6 h, respectively.
Overall, according to the maximum average E2 degradation rate, the optimal design scheme to prepare the immobilized bacteria was A2B1C3D2 (Table 1): 4 % sodium alginate, 1:1 bacteria/sodium alginate ratio, 5 % CaCl2⋅2H2O, and 6 h crosslinking time. Under this optimal embedding condition, pellets containing strain JX-2 with 3–4 mm diameter were prepared (S3 in the Electronic Supplementary Materials).
3.3 Degradation of E2 by Strain JX-2 in Liquid MSM-E2
High concentrations of E2 (100–1000 mg L−1) have been used for enrichment and isolation of E2-degrading bacteria (Fujii et al. 2002; Yoshimoto et al. 2004). In contrast, Yu et al. (2007) employed an extremely low concentration (∼3 mg L−1) of estrogens for enrichment and isolation procedure. Combining the two former studies, we employed intermediate E2 levels (10–30 mg L−1) for the isolation of E2-degrading bacterial strain and E2 degradation tests. The dynamics of E2 degradation were investigated in liquid MSM-E2 with JX-2 (Fig. 2) using an initial E2 concentration of 30 mg L−1; 94 % of E2 was degraded by strain JX-2 within 7 days. Direct counts of colony-forming units (CFUs) on MSM-E2 plates displayed a small increase in the number of cells on day 1; afterward, the cell counts increased markedly as E2 was utilized from days 1 to 5. After a 5-day incubation, the E2 concentration decreased further, and this resulted in a increase in the number of bacterial cells. The initial and 5-day cell counts were 6.92 and 7.85 (log CFU mL−1), respectively. Strain JX-2 degraded 94 % of the E2 in 7 days.
The conditions necessary to maintain a high rate of E2 degradation by strain JX-2 were optimized. Figure 3a shows the influence of pH on the E2 degradation rate by strain JX-2. Strain JX-2 did not grow normally at pH <4.0 or >10.0, and the degradation rate was <40 %. The E2 degradation rate by strain JX-2 was >88 % after 7 days at pH 6.0–8.0, whereas a lower degradation rate was evident when the initial pH was higher than 8.0 or lower than 6.0. The optimal pH for E2 degradation by strain JX-2 was 7.0, at which the degradation rate was 93 %. Figure 3b shows that increased temperatures of 15–30 °C enhanced bacterial growth and increased the E2 degradation rate. The optimal temperature for E2 degradation by strain JX-2 was 30 °C, at which the E2 degradation rate was 92 %. However, temperatures >30 °C resulted in inhibition of growth and degradation.
Figure 3c, d shows the influence of the initial E2 concentration and inoculum density on the E2 degradation rate by strain JX-2. When the initial E2 concentration was 5–50 mg L−1, the degradation rate after 7 days was >80 % (Fig. 3c). E2 degradation rate increased first and decreased after that with the increase of E2 concentration from 5 to 50 mg L−1. When the E2 concentration was 30 mg L−1, the best degradation rate seen was 92.6 %. Figure 3d gives the effect of inoculum size on the E2 degradation rate by strain JX-2. The E2 degradation rate after 7 days of incubation at 1 % initial inoculum density was 67.7 %, the lowest rate of all. It was difficult for the bacteria to thrive in a relatively short period with a lower initial inoculum size. Increasing the initial inoculum size to 5 % increased the degradation rate to >91 %. As the inoculum size was increased continually, the E2 degradation rate was maintained at 90–94 %.
Overall, the optimal pH value was 7.0, and the optimal temperature was 30 °C. Strain JX-2 had good environmental adaptability and grew well under a high E2 concentration (up to 50 mg L−1), showing a high E2 degradation rate. The optimal inoculum size to achieve effective degradation without excess bacterial suspension was 5 %.
3.4 Degradation of E2 by Immobilized Strain JX-2 in Liquid MSM-E2
Figure 4 shows E2 degradation by the free and immobilized JX-2 strains under the same conditions. The degradation abilities of the free and immobilized strains were nearly the same (>80 %) under pH 6.0–8.0 and 20–35 °C temperatures (Fig. 4a, b). However, the degradation ability of the immobilized strain was much better than that of the free strain under pH <6.0 or >8.0 either below 20 or above 35 °C. This situation was not true for the E2 concentration or inoculum size (Fig. 4c, d), as the free and immobilized strains showed almost the same E2 degradation rates under all E2 concentrations and inoculum sizes. Figure 4 indicates that the immobilized bacteria maintained high degradation efficiency even if the environmental conditions were not suitable for growth. These results indicated that the immobilized strain JX-2 would be more advantageous to degrade E2 in extreme environments.
As shown in Fig. 4, the immobilized carrier of the blank control also removed E2 because of its adsorption capacity. The removal contribution by adsorption of E2 in the solution was small, generally 5–10 %. It was notable that although the degradation data of the free strain shown in Fig. 4 slightly differed from those in Fig. 3, because the two experiments were conducted separately and some environmental conditions were different, the two groups of degradation data showed a similar tendency.
3.5 Removal of E2 in Sewage and Cow Dung by Immobilized Strain JX-2
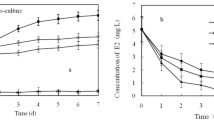
The immobilized strain JX-2 could efficiently remove E2 in natural sewage samples and cow dung samples. As shown in Table 2, more than half of the E2 in natural sewage samples was eliminated after the treatment by immobilized strain JX-2. The E2 removal efficiency in sewage sample 1 and sewage sample 2 was 73.5 and 64.4 %, respectively. However, the E2 concentration in sewage sample 3 could not be determined, because it was lower than the detection limit (0.186 μg L−1), and almost all of the E2 was removed.
Similar results were also obtained in natural cow dung samples. As shown in Table 2, more than 81 % of the E2 in natural cow dung samples was eliminated after treatment by immobilized strain JX-2. The E2 removal efficiency in four dung samples was 87.1, 84.2, 86.8, and 81.3 %, respectively.
Thus, the immobilized strain JX-2 could not only efficiently degrade high concentrations of E2 in the laboratory but also rapidly remove low levels of E2 in the natural environments.
4 Discussion
Microbial biodegradation of estrogens has drawn considerable attention in the last decades. In this study, an E2-degrading bacterial strain (Rhodococcus sp. JX-2) was isolated from the activated sludge in a STP. Strain JX-2 could grow and degrade E2 well under a wide range of environmental conditions. The E2 degradation ability of strain JX-2 was stronger than that of previously isolated E2-degrading bacteria. For example, Novosphingobium sp. ARI-1, isolated by Fujii et al. (2002), degraded 5 mg E2 in 30 mL medium in 20 days. Strain JX-2 also showed a high tolerance to E2, as a high degradation efficiency was detected at an E2 concentration of 50 mg L−1. Also, the E2-degrading bacteria isolated by Yu et al. (2007) and Jiang et al. (2010) only degraded 3 and 1 mg L−1 E2, respectively. The immobilized strain JX-2 adapted to harsh environments was produced under optimal conditions and used to remove E2 in sewage and cow dung. The results suggested that the immobilized JX-2 could efficiently remove E2 from sewage and cow dung and would be a potential candidate for E2 elimination in other environmental samples. Furthermore, this is the first report utilizing immobilized bacterial strain to remove estrogens from environmental samples.
Previous reports also showed that E2 was highly susceptible to microbial biodegradation, with transformation to E1 as the first step (Pauwels et al. 2008; Yu et al. 2007). Fourteen phylogenetically diverse E2-degrading bacteria were isolated from activated sludge by Yu et al. (2007), but only three strains showed an ability to degrade E1. In this investigation, no other metabolites were detected by LC-MS analysis except E1. This confirms that E1 was the main microbial metabolite of E2 in the degradation by Rhodococcus sp. JX-2. The widespread ability of JX-2 to transform E2 into E1 is important, as this reduces total estrogenic activity. More effort will be made to isolate E1-degrading bacteria because cooperation between the E2-degrading bacterium JX-2 and an E1-degrading bacterium would promote biodegradation of estrogens and eliminate estrogenic activity in the environments.
The embedding immobilization method has been widely used to overcome some microbial degradation problems (Lau et al. 1997). Agar, gelatin, sodium alginate, polyvinyl alcohol, and acrylamide gel are typically used as embedding carriers. Agar is rarely used due to its low mechanical strength, and polyacrylamide gel must be improved as a carrier because of the exothermic reaction of the gel crosslinking process and toxicity of the crosslinking agent. Furthermore, the cells often lose their activity during immobilization. The compact internal structure of gelatin is not conducive to mass transfer, and it does not help improve the degradation efficiency. Polyvinyl alcohol is an appropriate immobilization carrier, but the boric acid crosslinking agent is toxic, so it should be used judiciously. In comparison to the characteristics of the five cell immobilization carriers, sodium alginate was the most suitable embedding medium due to its high mechanical stability, good mass transfer, lower toxicity, and feasibility for use during entrapment (Selimoglu and Elibol 2010).
Other researchers also investigated the effects of the sodium alginate concentration, bead diameter, initial pH, and temperature on cell growth and lactic acid production, and the optimal fermentation conditions were determined. Idris and Suzana (2006) reported that the maximum lactic acid concentration was achieved after 56 h of fermentation when 2.0 % sodium alginate concentration was adopted. Maximum lactic acid production was also found using a 1.0-mm bead diameter at an initial pH of 6.5 and 37 °C. In our experiment, 2.0 % sodium alginate was too low to allow bead formation, and its mechanical strength was poor; thus, it was not suitable for use. To obtain beads with a high degradation rate and high mechanical strength, 4.0 % sodium alginate concentration was chosen. In general, as the bead diameter decreases, degradation increases. However, strain JX-2 had high degradation ability, and a 3–4-mm bead diameter was selected to ensure a high degradation rate.
The immobilized bacteria had a better E2 degradation ability than that of the free strains and showed a wider range of reaction temperatures and pH values. This is because the carrier played a vital role in protecting the bacteria from inhibitors in the external environments to achieve an efficient degradation effect (Quan et al. 2004). The degradation performance of the immobilized strain was better than that of the free strain when the E2 concentration was >50 mg L−1, indicating that the immobilized strain had a better tolerance to the high concentration of E2, while the degradation ability of the free strains was suppressed in a similar condition. The carrier prevented direct contact between the bacteria and high concentration of E2, thus buffering biodegradation by the immobilized bacteria (Duan 2012; Quan et al. 2004). Although the immobilized carrier had adsorption capacity, the removal contribution by adsorbing E2 in the solution was subtle between 5 and 10 %. Many studies have utilized immobilized bacteria to degrade organic pollutants in the environment and achieved a better degradation effect than those using free bacterial cells. For example, a strain of Acinetobacter sp. was isolated and immobilized by Ahmad et al. (2012). The immobilized cells degraded phenol completely within 108, 216, and 240 h at 1100, 1500, and 1900 mg L−1 phenol concentrations, respectively, while free cells took 240 h to degrade 1,00 mg L−1 phenol completely, and its phenol-degrading activity was inhibited at concentrations >1300 mg L−1. The immobilized bacteria displayed a higher degradation rate at higher pollutant concentrations compared with free cells.
Even though the sewage and cow dung sample components were very complex, the experimental results showed that the immobilized JX-2 had a positive degradation effect. This is because the immobilized carrier protected the strain JX-2 from inhibitors in the external environments. Carriers resist vicious competition of indigenous microorganisms in the environment, weaken phage phagocytosis, and mitigate damage by toxic substances and high osmotic pressure. Thus, the immobilized bacteria have a significant application potential. At present, embedding is usually achieved by immobilization for wastewater treatment, including microbial denitrogenation treatment (Vanotti and Hunt 2000), removing refractory organic matter such as pyridine (Lee et al. 1994), and printing and dye wastewater treatment (Chang et al. 2001). The 2,4-dichlorophenol-degrading bacterium Bacillus insolitus was isolated from a mixed culture by Wang et al. (2000), and immobilized B. insolitus showed greater removal of 2,4-dichlorophenol than that of B. insolitus in suspension at initial 2,4-dichlorophenol concentrations of 10–50 mg L−1. However, no study has used an immobilized strain to remove estrogens in sewage or livestock manure. Therefore, the immobilized bacterium presented here has a high potential for domestic wastewater and livestock manure treatment.
Several aspects, including the ability to degrade other organics and estrogenic compounds in wastewater and livestock manure and the degrading genes involved in estrogen degradation by strain JX-2, are currently under elucidation to fully capitalize on the degradation ability of strain JX-2. Furthermore, environmental antibiotic pollution is becoming worse in several countries. Thus, the effects of antibiotics on degradation by strain JX-2 will also be a focus in the future. Moreover, although the immobilized JX-2 performed perfect E2-degrading ability under the tested temperatures ranging from 20 to 35 °C, its E2-degrading performance under below 15 °C was still unclear. In conventional practice, when the environmental temperature was low, the enzyme activities involved in the degradation of organic contaminants would be restrained, and the metabolism process would be decelerated. Therefore, the practical application of immobilized JX-2 for E2 treatment in environments should be carried out in the season whose mean environmental temperature was above 20 °C.
5 Conclusion
The immobilized bacteria with the capacity to degrade estrogens have significant implications for eliminating estrogens in environments. In this study, an effective E2-degrading bacterium (Rhodococcus sp. JX-2) was isolated from the activated sludge of a STP. Strain JX-2 could degrade more than 90 % of E2 (30 mg L−1) in MSM within 7 days under environmental conditions. Additionally, after immobilization, strain JX-2 showed better environmental adaptability and could efficiently remove E2 in natural sewage and cow dung. Although the molecular mechanisms involved in E2 degradation by JX-2 and the practical application of immobilized JX-2 for E2 removal in field study still require further exploration, the potential use of immobilized JX-2 for E2 removal in natural environments is strongly supported by the research outlined here. This is the first report regarding the utilization of immobilized bacteria to degrade estrogens in the environment, and the results provide a new perspective and technique for environmental estrogen pollution control.
References
Ahmad, S. A., Shamaan, N. A., Arif, N. M., Koon, G. B., Shukor, M. Y. A., & Syed, M. A. (2012). Enhanced phenol degradation by immobilized Acinetobacter sp. strain AQ5NOL 1. World Journal of Microbiology and Biotechnology, 28, 347–352.
Andaluri, G., Suri, R. P. S., & Kumar, K. (2012). Occurrence of estrogen hormones in biosolids, animal manure and mushroom compost. Environmental Monitoring and Assessment, 184, 1197–1205.
Baronti, C., Curini, R., D’Ascenzo, G., Di Corcia, A., Gentili, A., & Samperi, R. (2000). Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environmental Science & Technology, 34, 5059–5066.
Belfroid, A. C., Van der Horst, A., Vethaak, A. D., Schafer, A. J., Rijs, G., Wegener, J., & Cofino, W. P. (1999). Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands. Science of the Total Environment, 225, 101–108.
Byers, H. K., Stackebrandt, E., Hayward, C., & Blackall, L. L. (1998). Molecular investigation of a microbial mat associated with the Great Artesian basin. FEMS Microbiology Ecology, 25, 391–403.
Chang, J. S., Chou, C., & Chen, S. Y. (2001). Decolorization of azo dyes with immobilized Pseudomonas luteola. Process Biochemistry, 36, 757–763.
Chung, T. P., Tseng, H. Y., & Juang, R. S. (2003). Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochemistry, 38, 1497–1507.
Covarrubias, S. A., De-Bashan, L. E., Moreno, M., & Bashan, Y. (2012). Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Applied Microbiology and Biotechnology, 93, 2669–2680.
Crisp, T. M., Clegg, E. D., Cooper, R. L., Anderson, D. G., Baetcke, K. P., Hoffmann, J. L., Morrow, M. S., Rodier, D. J., Schaeffer, J. E., & Touart, L. W. (1997). Special report on environmental endocrine disruption: an effects assessment and analysis. Washington: Environmental Protection Agency.
Desbrow, C., Routledge, E. J., Brighty, G. C., Sumpter, J. P., & Waldock, M. (1998). Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environmental Science & Technology, 32, 1549–1558.
Duan, H. M. (2012). Biodegradation characteristics of chlorpyrifos by immobilized bacteria. Chinese Journal of Eco-Agriculture, 20, 1636–1642.
Folmar, L. C., Denslow, N. D., Rao, V., Chow, M., Crain, D. A., Enblom, J., Marcino, J., & Guillette, L. J. (1996). Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environmental Health Perspectives, 104, 1096–1101.
Fujii, K., Kikuchi, S., Satomi, M., Ushio-Sata, N., & Morita, N. (2002). Degradation of 17 beta-estradiol by a gram-negative bacterium isolated from activated sludge in a sewage treatment plant in Tokyo. Japan Applied and Environmental Microbiology, 68, 2057–2060.
Garrity, G. M., Bell, J. A., & Lilburn, T. G. (2004). Taxonomic outline of the prokaryotes. Bergey’s manual of systematic bacteriology. New York: Springer.
Gentili, A. R., Cubitto, M. A., Ferrero, M., & Rodriguez, M. S. (2006). Bioremediation of crude oil polluted seawater by a hydrocarbon-degrading bacterial strain immobilized on chitin and chitosan flakes. International Biodeterioration and Biodegradration, 57, 222–228.
Idris, A., & Suzana, W. (2006). Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochemistry, 41, 1117–1123.
Järvenpää, P., Kosunen, T., Fotsis, T., & Adlercreutz, H. (1980). In vitro metabolism of estrogens by isolated intestinal micro-organisms and by human faecal microflora. Journal of Steroid Biochemistry, 13, 345–349.
Jiang, L., Yang, J., & Chen, J. (2010). Isolation and characteristics of 17 beta-estradiol-degrading Bacillus spp. strains from activated sludge. Biodegradation, 21, 729–736.
Jobling, S., Beresford, N., Nolan, M., Rodgers-Gray, T., Brighty, G. C., Sumpter, J. P., & Tyler, C. R. (2002). Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biology of Reproduction, 66, 272–281.
Johnson, A. C., & Williams, R. J. (2004). A model to estimate influent and effluent concentrations of estradiol, estrone, and ethinylestradiol at sewage treatment works. Environmental Science & Technology, 38, 3649–3658.
Kavlock, R. J., Daston, G. P., DeRosa, C., FennerCrisp, P., Gray, L. E., Kaattari, S., Lucier, G., Luster, M., Mac, M. J., Maczka, C., Miller, R., Moore, J., Rolland, R., Scott, G., Sheehan, D. M., Sinks, T., & Tilson, H. A. (1996). Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the US EPA-sponsored workshop. Environmental Health Perspectives, 1044, 715–740.
Kornman, K. S., & Loesche, W. J. (1982). Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infection and Immunity, 35, 256–263.
Kuch, H. M., & Ballschmiter, K. (2001). Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the picogram per liter range. Environmental Science & Technology, 35, 3201–3206.
Lau, P. S., Tam, N., & Wong, Y. S. (1997). Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environmental Technology, 18, 945–951.
Le, T. A. H., Clemens, J., & Nguyen, T. H. (2013). Performance of different composting techniques in reducing oestrogens content in manure from livestock in a Vietnamese setting. Environmental Monitoring and Assessment, 185, 415–423.
Lee, S. T., Rhee, S. K., & Lee, G. M. (1994). Biodegradation of pyridine by freely suspended and immobilized Pimelobacter sp. Applied Microbiology and Biotechnology, 41, 652–657.
Li, H., Zhang, Y., Irina, K., Xu, H., & Mang, C. (2007). Dynamic changes in microbial activity and community structure during biodegradation of petroleum compounds: a laboratory experiment. Journal of Environmental Sciences (China), 19, 1003–1013.
Li, X., Ling, W. T., Liu, J. X., Sun, M. X., Gao, Y. Z., & Liu, J. (2015). Immobilization of estrogen-degrading bacteria to remove the 17β-estradiol and diethylstilbestrol from polluted water and cow dung. Environmental Sciences, 36, 2581–2590 (in Chinese).
Nasu, M., Goto, M., Kato, H., Oshima, Y., & Tanaka, H. (2001). Study on endocrine disrupting chemicals in wastewater treatment plants. Water Science and Technology, 43, 101–108.
Ojanotko-Harri, A., Laine, M., & Tenovuo, J. (1991). Metabolism of 17β-estradiol by oral Streptococcus mutans, Streptococcus sanguis, Bacillus cereus and Candida albicans. Oral Microbiology and Immunology, 6, 126–128.
Pauwels, B., Wille, K., Noppe, H., De Brabander, H., van de Wiele, T., Verstraete, W., & Boon, N. (2008). 17 Alpha-ethinylestradiol cometabolism by bacteria degrading estrone, 17 beta-estradiol and estriol. Biodegradation, 19, 683–693.
Purdom, C. E., Hardiman, P. A., Bye, V. V. J., Eno, N. C., Tyler, C. R., & Sumpter, J. P. (1994). Estrogenic effects of effluents from sewage treatment works. Chemistry and Ecology, 8, 275–285.
Quan, X. C., Shi, H. C., Zhang, Y. M., Wang, H. L., & Qian, Y. (2004). Biodegradation of 2,4-dichlorophenol and phenol in an airlift inner-loop bioreactor immobilized with Achromobacter sp. Separation and Purification Technology, 34, 97–103.
Rahman, R. N. Z. A., Ghazali, F. M., Salleh, A. B., & Basri, M. (2006). Biodegradation of hydrocarbon contamination by immobilized bacterial cells. Journal of Microbiology, 44, 354–359.
Selimoglu, S. M., & Elibol, M. (2010). Alginate as an immobilization material for MAb production via encapsulated hybridoma cells. Critical Reviews in Biotechnology, 30, 145–159.
Shi, J. H., Suzuki, Y., Lee, B. D., Nakai, S., & Hosomi, M. (2002). Isolation and characterization of the ethynylestradiol-biodegrading microorganism Fusarium proliferatum strain HNS-1. Water Science and Technology, 45, 175–179.
Silva, C. P., Otero, M., & Esteves, V. (2012). Processes for the elimination of estrogenic steroid hormones from water: a review. Environmental Pollution, 165, 38–58.
Snyder, S. A., Keith, T. L., Verbrugge, D. A., Snyder, E. M., Gross, T. S., Kannan, K., & Giesy, J. P. (1999). Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environmental Science & Technology, 33, 2814–2820.
Solé, M., de Alda, M., Castillo, M., Porte, C., Ladegaard-Pedersen, K., & Barceló, D. (2000). Estrogenicity determination in sewage treatment plants and surface waters from the Catalonian area (NE Spain). Environmental Science & Technology, 34, 5076–5083.
Sun, K., Liu, J., Jin, L., & Gao, Y. (2014). Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant and Soil, 374, 251–262.
Tabata, A., Kashiwada, S., Ohnishi, Y., Ishikawa, H., Miyamoto, N., Itoh, M., & Magara, Y. (2001). Estrogenic influences of estradiol-17 beta, p-nonylphenol and bisphenol-A on Japanese Medaka (Oryzias latipes) at detected environmental concentrations. Water Science and Technology, 43, 109–116.
Ternes, T. A., Stumpf, M., Mueller, J., Haberer, K., Wilken, R. D., & Servos, M. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Science of the Total Environment, 225, 81–90.
Tyler, C. R., Jobling, S., & Sumpter, J. P. (1998). Endocrine disruption in wildlife: a critical review of the evidence. Critical Reviews in Toxicology, 28, 319–361.
Vanotti, M. B., & Hunt, P. G. (2000). Nitrification treatment of swine wastewater with acclimated nitrifying sludge immobilized in polymer pellets. Transactions of ASAE, 43, 405–413.
Vos, J. G., Dybing, E., Greim, H. A., Ladefoged, O., Lambre, C., Tarazona, J. V., Brandt, I., & Vethaak, A. D. (2000). Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Critical Reviews in Toxicology, 30, 71–133.
Wang, C. C., Lee, C. M., & Kuan, C. H. (2000). Removal of 2, 4-dichlorophenol by suspended and immobilized Bacillus insolitus. Chemosphere, 41, 447–452.
Weber, S., Leuschner, P., Kampfer, P., Dott, W., & Hollender, J. (2005). Degradation of estradiol and ethinyl estradiol by activated sludge and by a defined mixed culture. Applied Microbiology and Biotechnology, 67, 106–112.
Xu, R. F., Sun, M. X., Liu, J., Wang, H., Li, X., Zhu, X. Z., & Ling, W. T. (2014). Isolation, characteristics, and performance of a diethylstilbestrol-degrading bacteria strain Serratia sp. Environmental Sciences, 35, 328–333 (in Chinese).
Yang, S., Jin, H., Wei, Z., He, R., Ji, Y., Li, X., & Yu, S. (2009). Bioremediation of oil spills in cold environments: a review. Pedosphere, 19, 371–381.
Yoshimoto, T., Nagai, F., Fujimoto, J., Watanabe, K., Mizukoshi, H., Makino, T., Kimura, K., Saino, H., Sawada, H., & Omura, H. (2004). Degradation of estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Applied and Environmental Microbiology, 70, 5283–5289.
Yu, C., Roh, H., & Chu, K. (2007). 17 Beta-estradiol-degrading bacteria isolated from activated sludge. Environmental Science & Technology, 41, 486–492.
Zeng, Q., Li, Y., & Yang, S. (2013). Sludge retention time as a suitable operational parameter to remove both estrogen and nutrients in an anaerobic-anoxic-aerobic activated sludge system. Environmental Engineering Science, 30, 161–169 (in Chinese).
Zheng, W., Yates, S. R., & Bradford, S. A. (2007). Analysis of steroid hormones in a typical dairy waste disposal system. Environmental Science & Technology, 42, 530–535.
Zhou, Y., Zha, J., Xu, Y., Lei, B., & Wang, Z. (2012). Occurrences of six steroid estrogens from different effluents in Beijing. China Environmental Monitoring Assessment, 184, 1719–1729.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51278252, 21477056), Research Project of Environmental Protection in Jiangsu Province, China (2015023), and Jiangsu Provincial Key Research and Development Plan, China (BE2015682).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Jingxian Liu and Juan Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, J., Liu, J., Xu, D. et al. Isolation, Immobilization, and Degradation Performance of the 17β-Estradiol-Degrading Bacterium Rhodococcus sp. JX-2. Water Air Soil Pollut 227, 422 (2016). https://doi.org/10.1007/s11270-016-3122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3122-6