Abstract
Juvenile zebrafish (Danio rerio) were exposed to different dilutions (0, 0.67, 2.5, 10, and 50%) of effluent water from a Swedish pulp mill that previously has been reported to be androgenic to fish. Exposure was performed between days 10–38 days post-hatch. Fish were sampled for whole-body vitellogenin concentrations at day 38 post-hatch and for histological examination of gonads at day 60 post-hatch. In fish exposed to the highest concentration of pulp mill effluent, elevated concentrations of vitellogenin were measured. The androgenicity of the pulp mill water was confirmed by the increased number of males recorded at 60 days post-hatch. Image analysis of testes indicated stimulation of spennato genesis. Intersex fish were observed in all exposure groups. An androgenic activity equivalent to 5.6 ng/L dihydroxytestosterone was measured using the yeast androgen screen (YAS) assay. The present study demonstrates that both androgenic and estrogenic effects can be detected when exposing zebrafish during the juvenile period to complex mixtures of chemicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Reproduction disorders have been reported from a number of species of wild fish. The cause is often unknown, but a number of possible factors have been proposed, including pollutants, nutrients, and other different abiotic and biotic factors. During the last decade, the role of endocrine-disrupting chemicals (EDCs) has been in focus. A variety of anthropogenic chemicals have been shown to act as EDCs, including high-volume products such as phthalates, bisphenol A, and alkylphenols (Sonnenschein and Soto 1998; Tyler et al. 1998; Vos et al. 2000). Most attention has been drawn to chemicals acting through the same mechanisms as endogenous estrogens. However, in recent years also other endocrine-disrupting chemicals including androgens have been discussed. Masculinisation in wild populations of fish has been described. In Florida streams receiving paper mill effluents, female mosquito fish (Gambusia affinis) developed an elongated anal fin resembling the male gonopodium (Howell et al. 1980; Cody and Bortone 1997; Bortone and Cody 1999; Jenkins et al. 2001). In the vicinity of a Swedish pulp mill, male-biased sex ratios in offspring of eelpout (Zoarces viviparus) have been observed (Larsson et al. 2000; Larsson and Förlin 2002). In female three-spined sticklebacks (Gasterosteus aculeatus), effluent water from the same Swedish pulp mill induced the male-specific nest-building protein spiggin (Katsiadaki et al. 2002). Androgenic effects of pulp and paper mill effluents have been shown in receptor-based in vitro assays (Svenson and Allard 2004).
Also other effects have been described in fish exposed to pulp and paper mill effluents, such as reduced sex hormone levels, increased or decreased vitellogenin levels, reduced gonad size, and delayed sexual maturation (Tremblay and Van Der Kraak 1999; Mellanen et al. 1999; Karels et al. 2001; van den Heuvel and Ellis 2002; Denslow et al. 2004).
Extrapolation between laboratory fish and observations on wild fish species may be an important tool for evaluation of individual chemicals and complex mixture of chemicals, such as sewage effluents. The zebrafish (Danio rerio), together with the Japanese medaka (Oryzia latipes) and the fathead minnow (Pimephales promelas), is considered a model test species for risk assessment of EDCs (OECD 1999; 2000; 2004). Endpoints that are used to evaluate EDCs include vitellogenin, gonad differentiation, sex ratios, and reproduction success. Exposure of zebrafish to model compounds, such as 17α-ethinylestradiol and 17α-methyltestosterone, results in feminisation and masculinisation, respectively (Örn et al. 2003). The zebrafish is considered as an undifferentiated gonochoristic species, with all individuals initially developing undifferentiated ovaries. In fish becoming males, the oocytes degenerate, testicular stromal tissue develops, and the gonad proceeds into a phenotypic testis. This phenomenon was termed juvenile hemiaphroditism when described by Takahashi (1977). However, differences in the period of gonad transformation have been observed. The disappearance of oocytes in the transforniing gonad has been reported to take place between 3–4 weeks post-hatch (Takahashi 1977; Uchida et al. 2002). In the study by Maack and Segner (2003), the period was more extended and occurred approximately between 5–7 wph. Our own observation was that this transformation period mainly occurred between 4–5 wph (Örn et al. 2003). In non-exposed juvenile zebrafish, whole-body vitellogenin concentrations have been measured to be low in fish at the age of 25, 32, and 39 dph (Andersen et al. 2003). However, somewhat increased Vtg levels were measured in fish sampled at 46 dph, indicating the start of vitellogenin production in some individuals (Andersen et al. 2003). In the present study, juvenile zebrafish were exposed during four weeks to effluent water from a Swedish pulp mill. The exposure period was 10–38 days post-hatch, i.e., before and during the transformation period of the gonad (Örn et al. 2003).
The aim of the study was to evaluate if vitellogenin measurement and gonad development in zebrafish are suitable endpoints in tests with chemically complex mixtures, such as pulp mill effluents. The aim was also to see if the earlier observations of androgenicity to fish (Larsson et al. 2000; Larsson and Förlin 2002; Katsiadaki et al. 2002) could be confirmed in zebrafish.
Materials and Methods
Sampling and Extraction of Pulp Mill Effluent
The investigated mill is a kraft pulp mill situated in Sweden. Bleaching of the pulp is based on totally chlorine-free (TCF) processing. In February 2001; effluent water was sampled after the final activated sludge treatment step. The water was sampled in 25-L polyethylene containers, immediately frozen and stored at −20°C. Extraction of samples of effluent pulp mill water was performed using solid phase extraction (Körner et al. 1999). A 500-ml volume of water was passed through solid phase columns containing 200 mg hydroxylated polystyrene-divinylbenzene (ENV+, Sorbent AB, Sweden). Particles in the water were removed by a 20-μm porous filter connected to the solid phase column. Lipophilic compounds sorbed onto the columns were eluted with acetone. Dimethylsulfoxide (50 μl) was added, and the acetone was evaporated in a gentle stream of nitrogen. The extraction samples were stored at −20°C.
Yeast Androgen Screen Assay
The assay, based on recombinant yeast containing the human androgen receptor gene, was performed in 96-well microtitre plates using a published procedure (Sohoni and Sumpter 1998). Each plate contained negative controls of growth medium, a series of 12 concentrations of dihydrotestosterone (DHT) as a positive control and the effluent extract in 12 dilutions. The assays were run in triplicates. The plates were incubated at 32°C for 3 days and the absorbance was read on an automatic plate recorder (Spectracount, Packard) at 570 nm. The absorbance results of the positive control DHT were used to calculate the EC50 of the dose-response curves. The results of the effluent extract were evaluated similarly using dilution factors as concentrations. The EC50 value of the extract was compared with the results of DHT, and recalculated into ng/L DHT equivalents.
General Experimental Conditions
Adult zebrafish were bought from a local supplier. Fish were adapted to laboratory conditions for 1 month in charcoal-filtered tap water, kept at 25°C and at a light:dark cycle of 12:12 h. Standardised water (ISO 7346-1,1996) was used throughout the experimental study and prepared from deionised water with the addition of CaCl2·2H2O (117.6 mg 1−1), MgSO4·7H2O (49.3 mg 1−1), NaHCO3 (25.9 mg 1−1), and KC1 (2.3 mg 1−1). Frozen effluent pulp mill water was thawed overnight at room temperature prior to use. Zebrafish larvae were fed Sera micron (Sera®), live Artemia nauplii, and powdered freeze-dried red grubs (Nutrafin®) three times daily. Juvenile and adult zebrafish were fed Sera Vipan (Sera®) and freeze-dried red grubs two times daily.
Partial Life-Cycle Exposure
Female (n = 10) and male (n = 10) zebrafish were placed together in stainless steel reproduction funnels. Eggs were collected 2 hours after onset of light in the morning, and transferred into 250-ml glass beakers containing standardised water. After 24 hours, fertilised eggs were transferred into a 20-L aquarium. At 10 days post-hatch (dph), the larvae were randomly divided into different exposure groups, and each group was kept in 10-L glass aquaria. The aquaria contained standardised water (controls) or effluent pulp mill water diluted with standardised water at concentrations of 0.67, 2.5, 10, and 50% (v/v). For each exposure group, as well as control fish, triplicate aquaria were used. Each aquarium contained 50 individual fish. The water was renewed with 50% of the exposure volume three times per week. The fish were exposed from 10 to 38 dph. At 38 dph, five fish were sampled from each replicate, frozen in liquid nitrogen, and analysed for whole-body vitellogenin concentrations. The remaining fish were kept in standardised water and sampled at 60 dph. After anaesthetising in MS222, the fish were fixed in phosphate-buffered formalin and processed for histological evaluation.
Vitellogenin Analysis
Samples for measurement of whole-body vitellogenin concentrations were sent on dry ice to the Institute of Biology, South Danish University, Odense, Denmark, and analysed using a direct non-competitive sandwich ELISA described by Holbech et al. (2001).
Histology
After fixation and dehydration, groups of 7–10 individuals were embedded in paraffin blocks, Longitudinal sections were cut from the ventral side, stained by HE (haematoxylin-eosin), and each section was evaluated under light microscopy (LM) with focus on sex ratios and histological abnormalities of the gonad, e.g., intersex.
Image Analysis
In the HE-stained sections, the maturity of the gonads was evaluated using image analysis. Digitized images of the gonads were obtained with a Nikon Digital Camera DXM 1200 connected to a Nikon Eclipse E600 microscope. On each section, several images were taken to include the whole gonad section area in the analyses. For each female, the total section area of the ovaries, as well as the area of immature oocytes up to the perinucleolar stage were measured. For each male, the total section area of the testes, as well as the area of spermatozoa, were measured. On each image, the section area of the gonads as manually marked and selected for measurement by the use of image analysis software (Easy Image Analysis 2000; Tekno Optic AB, Stockholm, Sweden). The area of the densely stained immature oocytes and spermatozoa was then obtained by thresholding RGB colour values. Images of the ovaries were also thresholded for measurement of background/non-tissue area, due to technical artifacts such as cracks in sections. After subtraction of background area, calculations were made of the total section area of the gonads, area of immature oocytes or spermatozoa, and the percentage of the area of immature oocytes or spermatozoa in each fish.
Statistics
Vitellogenin concentrations in exposed fish were compared with controls using the Kruskal-Wallis (non-parametric Anova) test followed by the post-hoc test Bonferronni/Dunn. The sex ratios, i.e., the number of males and females, and intersex ratios, i.e., the number of intersex fish per total number offish in each exposed group, were compared with the controls using the Fisher’s exact test. Measurements of areas on gonads using image analysis were compared between exposed groups and controls using one-way Anova. All tests were made using StatView for Windows 5.0.1. The significance level was set at 95% (p < 0.05). The symbols *, **, and *** refer to p < 0.05, p < 0.01, and p < 0.001, respectively.
Results
Yeast Androgen Screen Assay
In vitro assay of extracts of the pulp mill effluent (PME) showed a dose-dependent increase in androgenicity. At higher doses, however, a decline was observed, due to cell growth inhibition of components co-extracted from the effluent. The dose-response curve at lower doses was evaluated using a non-linear curve fit and an EC50 was calculated. The effluent water displayed an in vitro androgenic activity of 5.6 (limits of one standard deviation 4.1–7.6) ng dihydrotestosterone equivalents L−1.
Partial Life-Cycle Test
The mortalities in controls and fish exposed to 0.67, 2.5, and 10% PME were approximately 30%. Exposure to 50% PME resulted in 50% mortality. There were no significant differences between the vitellogenin concentrations of the PME replicates. The replicates were, therefore, combined into single dilution groups and further tested. No significant differences in vitellogenin concentrations were measured in fish exposed to 0.67, 2.5, and 10% PME compared with controls (Fig. 1) Mean whole-body vitellogenin concentrations in controls, 0.67, 2.5, and 10% PME were 86.7, 122, 73.4, and 136 ng/g fish, respectively. In fish exposed to 50% PME, significantly (p < 0.0001) higher vitellogenin concentrations were measured. The mean concentration was 2,400 ng/g fish.
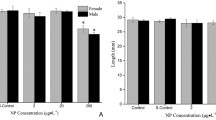
There were no statistical differences in sex ratios between the replicates of the PME dilutions (Fig. 2). The replicates were, therefore, combined into single dilution groups and further tested. The histological evaluation revealed that the mean percentage of males in the control group was 49%. In groups exposed to 0.67, 2.5, and 10% PME, the mean percentages of males were 54, 55, and 57%, respectively. A significantly (p < 0.05) higher number of males (mean 63%) was recorded after exposure to 50% PME.
A low non-significant ratio offish with intersex gonads was detected in all groups exposed to pulp mill effluent (Fig. 2). Generally, the intersex gonads was characterised by the presence of one or a few oocytes surrounded by testicular tissue (Fig. 3). However, in one fish exposed to 50% PME, one gonad was divided into ovarian and testicular tissue parts, containing both eggs at the vitellogenic stage and mature spermatozoa (Fig. 4).
Image analysis revealed no differences between the groups in measured areas of the gonads, neither in females nor in males. In females, the mean areas of the ovaries ranged between 576,000–808,000 μm2. The mean areas of immature oocytes (up to the perinucleolar stage) ranged between 168,000–236,000 μm2. The mean percentages of immature oocytes of the ovary sections increased from 49% in controls up to 60% in fish exposed to 10% PME (Fig. 5), although not significantly. In male fish, the mean areas of testes ranged between 113,000–148,000 μm2. The mean areas of spermatozoa ranged between 4,400–5,800 μm2. A trend in increase in the percentage of spermatozoa was measured in exposed fish (Fig. 6), ranging from 2.4% in controls to 3.3% in fish exposed to 50% PME.
Discussion
In the present study, exposure of juvenile zebrafish to effluent water from a Swedish pulp mill revealed both androgenic and estrogenic effects. However, we also encountered a rather high overall mortality, including non-exposed fish, which is why sex-dependent mortality cannot be ruled out. This mortality might be due to a number of reasons, such as nutrition, stocking density, or handling and change of environment for fish at the early start of exposure. Toxicity to pulp mill effluent (PME) has been observed in yeast cells (Svenson and Allard 2004) and might explain the higher mortality observed for fish exposed to 50% PME. The androgenic activity in the pulp mill effluent was 5.6 ng/L DHT-equivalents, as measured with the YAS assay. Similar results were obtained in a previous study of pulp and paper mill extracts, although interference with cell growth inhibition was encountered (Svenson and Allard 2004). Using the same assay, Thomas et al. (2002) reported androgenic activity between <2 and 9 ng/L DHT-equivalents in seven different UK estuary surface waters. In one of those, receiving sewage effluent discharge, 4-androstenedione and its metabolite 5α-androstanedione were identified as the major contributors to the androgenic activity (Thomas et al. 2002). In a recent paper by Larsson et al. (2006), chemically fractionated extracts of effluent water from the present pulp mill were tested in a competitive androgen receptor binding assay from Atlantic croaker (Micropogonias undulates). The primary effluent contained 96 ng DHT equivalents/L, whereas the final effluent 6 ng/L, which is equal that of the present YAS assay. Further chemical analyses revealed 35 androgen receptor ligands in different fractions, although the agents have not been folly identified, supporting the fact that masculinisation observed in effluent exposed fish is caused by androgens present in the pulp mill (Larsson et al. 2006).
The male-biased sex ratios observed in the present study are in accordance with previous findings. In 1998; Larsson et al. (2000) measured male-biased eelpout offspring in fish sampled in the vicinity of the same Swedish pulp mill. In a further study on historical samples of eelpout, Larsson and Fo¨rlin (2002) reported male-biased sex ratios at the same location in the years 1991; 1998, and 2000. In 1999; normalised sex ratios were measured. This recovery was related to a 17-day shut down at the mill, coinciding with the period of gonadal differentiation in eelpout embryos (Larsson and Förlin, 2002). In laboratory experiments, Larsson et al. (2002) reported enhanced coloration of female guppies exposed for 42 days to a 10% dilution of the effluent, indicating an androgenic response. The androgenicity of the pulp mill effluent has also been confirmed using female three-spined sticklebacks. Increased epithelial cell height and increased production of the glue protein spiggin in the kidneys are androgen-regulated processes normally occurring in male sticklebacks during reproduction. These androgenic effects were detected in females after exposure to 10% pulp mill effluent for six weeks (Katsiadaki et al. 2002). In the present study, the measurements of the spermatozoa area of the gonads might indicate stimulation of spermatogenesis. In a previous study, we measured increased testis area and increased percentage area of spermatozoa in zebrafish exposed to 50 ng/L of the androgen 17β-trenbolone (Örn et al. 2006). Similar findings were also observed in zebrafish exposed to concentrations ≥100 ng/L of 17α-methyltestosterone (Örn et al. 2003). In juvenile male sea bass (Dicentrarchus labrax), implantation of testosterone resulted in accelerated gonadal differentiation and to some extent also stimulated spermatogenesis Zanuy et al. (1999).
A well-known example of masculinization of wild fish is that of mosquito fish (Gambusia affinis) in streams in Florida, USA. In Fenholloway River, receiving paper mill effluents, female mosquitofish have been reported to develop an elongated anal fin resembling the male gonopodium (Howell et al. 1980; Cody and Bortone 1997; Bortone and Cody 1999; Jenkins et al. 2001). The cause to this masculinisation is still unknown, although the presence of androgens in the river has been verified. The androgenic hormone androstenedione has been identified both in the water of Fenholloway River at a concentration of 0.14 nM and in the sediment at a concentration of 2.4 nM (Jenkins et al. 2001; Jenkins et al. 2003). Other non-identified androgens are known to be present in the river water (Parks et al. 2001; Durban et al. 2002). Sediment samples from the Fenholloway River have been measured to contain relatively high concentrations (155 nM) of progesterone suggested to be derived from microbial degradation of phytosteroids (Jenkins et al. 2003). Masculinisation of female mosquitofish has been observed after exposure to phytosteroids that have been metabolically converted (Denton et al. 1985; Howell and Denton 1989). The androgens androstenedione and androstadienedione can be produced in vitro from progesterone by the bacterium Mycobacterium smegmatis (Jenkins et al. 2004).
Masculinised female mosquitofish from Fenholloway river have been measured to have higher ovarian and brain aromatase activity than fish from a reference site (Orlando et al. 2002). Exposures to high doses of androgenic hormones are known to cause feminisation of fish by aromatisation of the androgenic hormone into an estrogenic hormone (Rinchard et al. 1999; Piferrer et al. 1993). Exposure of fathead minnows to bleached sulfite mill effluent caused changes in secondary sex characteristics (Parrot et al. 2003). Masculinisation of female fish was observed at lower effluent exposure concentrations, while feminisation of males was observed at higher concentrations (Parrot et al. 2003).
The increased production of vitellogenin in the zebrafish in the present study might be due to aromatisation of androgens present in the pulp mill effluent. Vitellogenin induction in fish have previously been reported after exposure to single androgenic hormones, e.g., androstenedione (Shilling and Williams 2001) and 17α-methyltestosterone (Ankley et al. 2001; Zerulla et al. 2002; Hornung et al. 2004), as well as to complex pulp or paper mill effluents (Soimasou et al 1998; Mellanen et al. 1999; Tremblay and Van Der Kraak 1999; van den Heuvel and Ellis 2002). However, other chemicals must be taken into consideration also. The increased Vtg production might be caused by direct effects of estrogenic phytosteroids. Pulp mill effluents are known to contain a large number of different phytosterols. Increased vitellogenin production has been measured after exposure to various phytosterols, such as b-sitosterol, genistein, biochanin A, equol, and coumestrol (Pelissero et al. 1991; Mellanen et al. 1996; Tremblay and Van der Kraak 1999; Latonnelle et al. 2002).
In the present study, intersex fish were observed in all exposure groups. The occurrence of intersex in exposed fish can be due to disturbances in gonad development by direct estrogenic and androgenic effects of phytosteroids present in the pulp mill effluent. Intersex might also be caused by sudden changes in endogenous sex hormone concentrations during gonadal development due to aromatisation of xeno-androgens. In a previous study, we observed intersex after exposure of juvenile zebrafish to 1 μg/L of 17α-methyltestosterone, as well as elevated levels of vitellogenin compared with lower doses (Örn et al. 2003). The presence of aromatisible androgenic compounds in the pulp mill effluent could explain the male-biased sex ratios, the increased vitellogenin production, and the occurrence of zebrafish with intersex.
Vitellogenin measurement and gonad development were shown to be suitable endpoints for evaluation of a chemically complex water. The androgenicity of the effluent was confirmed by the increased number of males and with the YAS assay. However, the elevated Vtg levels also revealed the estrogenic potency of the effluent. This highlights the importance of combining endpoints with effects at different biological levels when evaluating unknown chemicals or complex mixtures.
References
Andersen L, Holbech H, Gessbo A, Norrgren L Petersen GI (2003) Effects of exposure to 17alpha-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 134:365–374
Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA (2001) Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem 20(6):1276–1290
Bortone SA, Cody RP (1999) Morphological masculinization in poeciliid females from a paper mill effluent receiving tributary of the St. Johns river, Florida, USA. Bull Environ Contam Toxicol 63:150–156
Cody RP, Bortone SA (1997) Masculinization of mosquitofish as an indicator of exposure to kraft mill effluent. Bull Environ Contain Toxicol 58:429–436
Denslow ND, Kocerha J, Sepulveda MS, Gross T, Holm SE (2004) Gene expression fingerprints of largemouth bass (Micropterus salmoides) exposed to pulp and paper mill effluents. Mut Res 552:19–34
Denton TE, Howell WM, Allison JJ, McCollum J, Marks B (1985) Masculinisation of female mosquitofish by exposure to plant sterols and Mycobacterium smegmatis. Bull Environ Contain Toxicol 35(5):627–632
Durhan EJ, Lambright C, Wilson V, Butterworth BC, Kuehl OW, Orlando EF, Gray Jr LJ, Gray LE, Ankley GT (2002) Evaluation of androstenedione as an androgenic component of river water downstream of a pulp and paper mill effluent. Environ Toxicol Chem 21(9):1973–1976
Holbech H, Andersen L, Petersen GI, Korsgaard B, Pedersen KL, Bjerregaard P (2001) Development of an ELIS A for vitellogenin in whole body homogenate of zebrafish (Danio rerio). Comp Biochem Phys Part C 130:119–131
Hornung MW, Jensen KM, Korte JJ, Kahl MD, Durban EJ, Denny JS, Henry TR, Ankley GT (2004) Mechanistic basis for estrogenic effects in fathead minnow (Pimephales promelas) following exposure to the androgen 17a-methyltestosterone: conversion of 17a-methyltestosterone to 17a-methylestradiol. Aquat Toxicol 66:15–23
Howell WM, Denton TE (1989) Gonopodial morphogenesis in female mosquitofish, Gambusia afffinis, masculinized by exposure to degradation products from plant sterols. J Fish Res Board Can 32:795–796
Howell WM, Black DA, Bortone SA (1980) Abnonnal expression of secondary sex characters in a population of mosquitofish, Gambusia affinis holbrooki: evidence for environmentally induced masculinization. Copeia 1980(4):43–51
International Organization for Standardization, ISO 7346-1:1996 Water quality: Determination of the acute lethal toxicity of substances to a freshwater fish [Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)], Part 1: Static method
Jenkins R, Angus RA, McNatt H, Howell WM, Kemppainen JA, Kirk M, Wilson EM (2001) Identification of androstenedione in a river containing paper mill effluent. Environ Toxicol Chem 20(6):1325–1331
Jenkins RL, Wilson EM, Angus RA, Howell WM, Kirk M (2003) Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol Sci 73:53–59
Jenkins RL, Wilson EM, Angus RA, Howell WM, Kirk M, Moore R, Nance M, Brown A (2004) Production of androgens by microbial transformation of progesterone in vitro: a model for androgen production in rivers receiving paper mill effluent. Environ Health Perspect 112(15):1508–1511
Karels A, Markkula E, Oikari A (2001) Reproductive, biochemical, physiological, and population responses in perch (Perca fluviatilis L.) and roach (Rutilus rutilus L.) downstream of two elemental chlorine-free pulp and paper mills. Environ Toxicol Chem 20(7):1517–1527
Katsiadaki I, Scott AP, Hurst MR, Matthiessen P, Mayer I (2002) Detection of environmental androgens: a novel method based on enzyme-linked immunosorbent assay of spiggin, the stickleback (Gasterosteus aculeatus) glue protein. Environ Toxicol Chem 21(9):1946–1954
Körner W, Hanf V, Schuller W, Kempter C, Metzger J, Hagenmaier H (1999) Development of a sensitive E-screen assay for quantitative analysis of estrogenic activity in municipal sewage plant effluents. Sci Total Environ 225(1-2):33–48
Larsson DGJ, Förlin L (2002) Male-biased sex ratios of fish embryos near a pulp mill: temporary recovery after a short-term shutdown. Environ Health Perspect 110(8):739–742
Larsson DGJ, Hällman H, Förlin L (2000) More male fish near a pulp mill. Environ Toxicol Chem 19(12):2911–2917
Larsson DGJ, Kinnberg K, Sturve J, Stephensen E, Skön M, Förlin L (2002) Studies of masculinization, detoxification, and oxidative stress responses in guppies (Poecilia reticulata) exposed to effluent from a pulp mill. Ecotoxicol Environ Saf 52:13–20
Larsson DGJ, Adolfsson-Erici M, Thomas P (2006) Characterization of putative ligands for a fish gonadal androgen receptor in a pulp mill effluent. Environ Toxicol Chem 25(2):419–427
Latonnelle K, Le Menn F, Kaushik SJ, Bennetau-Pelissero C (2002) Effects of dietary phytoestrogens in vivo and in vitro in rainbow trout and Siberian sturgeon: interests and limits of the in vitro studies of interspecies differences. Gen Comp Endocrin 126(1):39–51
Maack G, Segner H (2003) Morphological development of the gonads in zebrafish. J Fish Biol 62:895–906
Mellanen P, Petanen T, Lehtimaki J, Makela S, Bylund G, Holmbom B, Mannila E, Oikari A, Santti R (1996) Wood-derived estrogens: studies in vitro with breast cancer cell lines and in vivo in trout. Toxicol Appl Pharmacol 136(2):381–388
Mellanen P, Soimasou M, Holmbom B, Oikari A, Santti R (1999) Expression of the vitellogenin gene in the liver of juvenile whitefish (Coregonus lavaretus) exposed to effluents from pulp and paper mills. Ecotoxicol Environ Saf 43:133–137
OECD (Organisation for Economic Cooperation and Development) (1999) Report of the OECD expert consultation on testing in fish-EDF1, London, October 1998. Paris: OECD
OECD (Organisation for Economic Cooperation and Development) (2000) Report of the OECD expert consultation on testing in fish-EDF2, Tokyo, March 2000. Paris: OECD
OECD (Organisation for Economic Cooperation and Development) (2004) OECD draft report of the initial work towards the validation of the fish screening assay for the detection of endocrine active substances: Phase 1A. Paris: OECD
Orlando EF, Davis WP, Guillette LJ Jr (2002) Aromatase activity in the ovary and brain of the eastern mosquitofish (Gambusia holbrooki) exposed to paper mill effluent. Environ Health Perspect 110(3):429–433
Örn S, Holbech H, Madsen TH, Norrgren L, Petersen GI (2003). Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat Toxicol 65:397–411
Örn S, Yamani S, Norrgren L (2006) Comparison of vitellogenin induction, sex ratio and gonad morphology between zebrafish and Japanese medaka after exposure to 17α-ethinylestradiol and 17ß -trenbolone. Arch Environ Contain Toxicol, accepted for publication
Parks LG, Lambright CS, Orlando EF, Guillette LJ, Ankley GT, Gray LE Jr (2001) Masculinization of female mosquitofish in kraft mill effluent-contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol Sci 62:257–267
Parrot JL, Wood CS, Boutoto P, Dunn S (2003) Changes in growth and secondary sex characteristics of fathead minnows exposed to bleached sulphite mill effluent. Environ Toxicol Chem 22(12):2908–2915
Pelissero C, Bennetau B, Babin P, Le Menn F, Dunogues J (1991) The estrogenic activity of certain phytoestrogens in the Siberian sturgeon (Acipenser baeri). J Steroid Biochem Mol Biol 38(3):293–299
Piferrer F, Baker IJ, Donaldson EM (1993) Effectrs of natural, synthetic, aromatizable, and non-aromatizable androgens in inducing male sex differentiation in genotypic female chinook salmon (Oncorhynchus tshawytscha). Gen comp Endocrinol 91:59–65
Rinchard J, Dabrowski K, Garcia-Abiado MA, Ottobre J (1999) Uptake and depletion of plasma 17alpha-methyltestosterone during induction of masculinization in muskellunge, Esox masquinongy: effect on plasma steroids and sex reversal. J Steroids 64(8):518–525
Shilling AD, Williams DE (2000) The non-aromatizible androgen, dihydrotestosterone, induces antiestrogenic responses in the rainbow trout. J Steroid Biochem Mol Biol 74(4):187–194
Sohoni P, Sumpter JP (1998) Several environmental oestrogens are also anti-androgens. J Endocrinol 158(3):327–339
Soimasou MR, Karels AE, Leppanen H, Santti R, Oikari AO (1998) Biomarker responses in whitefish (Coregonus lavaretus) experimentally exposed in a large lake receiving effluents from pulp and paper industry. Arch Environ Contain Toxicol 34(1):69–80
Sonnenschein C, Soto AM (1998) An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 65(1-6):143–150
Svenson A, Allard A-S (2004) In vitro androgenicity in pulp and paper mill effluents. Environ Toxicol 19(5):510–517
Takahashi H (1977) Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ 28(2):57–65
Thomas KV, Hurst MR, Matthiessen P, McHugh M, Smith A, Waldock MJ (2002) An assessment of in vitro androgenic activity and the identification of environmental androgens in United Kingdom estuaries. Environ Toxicol Chem 21(7):1456–1461
Tremblay L, van der Kraak G (1999) Comparison between the effects of the phytosterol ß-sitosterol and pulp and paper mill effluents on sexually immature rainbow trout. Environ Toxicol Chem 18(2):329–336
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28(4):319–361
Uchida D, Yamashita M, Kitano T, Iguchi T (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718
van den Heuvel MR, Ellis RJ (2002) Timing of exposure to a pulp and paper effluent influences the manifestation of reproductive effects in rainbow trout. Environ Toxicol Chem 21(11):2338–2347
Vos JG, Dybing E, Greim HA, Ladefoged O, Lambré C, Tarazona JV, Brandt I, Vethaak DA (2000) Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situtation. Crit Rev Toxicol 30(1):71–133
Zanuy S, Carrillo M, Mateos J, Trudeau V, Kah O (1999) Effects of sustained administration of testosterone in pre-pubertal sea bass (Dicentrarchus labrax L.). Aquaculture 177:21–35
Zeralla M, Lange R, Steger-Hartmann T, Panter G, Hutchinson T, Dietrich DR (2002) Morphological sex reversal upon short-term exposure to endocrine modulators in juvenile fathead minnow (Pimephales promelas). Toxicol Lett 131(1-2):51–63
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Örn, S., Svenson, A., Viktor, T. et al. Male-Biased Sex Ratios and Vitellogenin Induction in Zebrafish Exposed to Effluent Water from a Swedish Pulp Mill. Arch Environ Contam Toxicol 51, 445–451 (2006). https://doi.org/10.1007/s00244-005-0199-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0199-0