Abstract
Brassinosteroids have been identified as polyhydroxylated steroidal plant hormones which are known for their response against stress and development processes like flowering, germination and crop production. Because of their multiple properties, brassinosteroids occupy a significant place among hormones. These naturally occurring plant hormones induce tolerance against abiotic and biotic stresses such as temperature variation (extreme cold/hot), salinity, water scarcity or drought, injury, fungal infection and metal toxicity. As a result of these stresses free radicals, like superoxide ions and peroxide are produced which cause damage to the plant system. Exogenous application of brassinosteroids at appropriate time can save plants from oxidative stresses. Brassinosteroids enhance carbon dioxide assimilation capacity, chlorophyll contents, antioxidants including ascorbate, carotenoids and proline under adverse environmental conditions. Besides inducing resistance against stresses, brassinosteroids also regulate growth, increase seed germination and ripening of fruits. It has been also noticed that the brassinazole-resistant-dependent brassinosteroid signaling up-regulates the expression of autophagy-related genes and autophagosome formation under stress. We have summarized, in this review, the information available until 2021, the impact of BRs application on plant growth and development under abiotic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All stresses experienced by the agricultural crops/plants result in decreased grain yield and reduced plant growth. Under biotic and abiotic stresses, the plants are forced to make adaptation to combat with the environmental changes which lead to changes in their physiological, metabolic and molecular functions (Cramer et al. 2011; Husen et al. 2014; Jeandroz and Lamotte 2017; Yurchenko et al. 2018; Chi et al. 2019; Wang 2020). Hormones are known to protect and improve the physical development of plants under water scarcity, soil salinity, temperature variation and metal toxicity (Kagale et al. 2007; Bajguz and Hayat 2009; Divi and Krishna 2009; Siddiqi and Husen 2017, 2019; Podlešáková et al. 2019; Heidari et al. 2020; Nolan et al. 2020) (Fig. 1).
Role of plant hormones under major abiotic stresses, their consequences and the components of plant defense system. SOD – Superoxide dismutase; CAT – Catalase; GPX – Glutathione peroxidase; GP – Guaiacol peroxidase; Prxs – Peroxiredoxins; APX – ascorbate peroxidase; MDAR – monodehydroascorbate reductase; DHAR – dehydroascorbate reductase; and GR – glutathione reductase
Brassinosteroids (BRs) and their derivatives occupy a prominent place among plant steroidal hormones owing to their multifunctional role in the development and protection of plants. Bajguz and Tretyn (2003) have reported over 69 different BRs and their derivatives in various plants. They are produced by the plants themselves under abiotic/biotic stress in order to survive and maintain normal life cycle (Hussain et al. 2020). These hormones are not toxic and hence they can be safely used to increase resistance and crop yield even under abnormal weather conditions. Grove et al. (1979) first purified the most active brassinolide (BL) from rapeseed (Brassica napus) pollen and its structure was determined by x-ray analysis. They are effective at very low concentration and widely distributed in lower as well as higher plants (Bajguz and Hayat 2009). They have been classified as per the presence of number of carbons in their structure such as C27, C28 or C29 BRs. The frequently used BRs in experimental investigation under abiotic stress in various plant species are 28-homobrassinolide (28-HBL), 24-epibrassinolide (24-EBL) and 24-epicasterone (Fig. 2 and Table 1). They alone promote the growth of plants but in combination with other growth promoting hormones the effect is enhanced manifold (Clouse et al. 1992; Sun et al. 2010; Bai et al. 2012). Plants are sensitive to stresses and respond to them quickly (Getnet et al. 2015; Embiale et al. 2016; Husen et al. 2016, 2017). Even though, BRs respond to all stresses, they are very sensitive to injury and wounds. Plants with BR deficiency exhibit stunted growth, short and dark green leaves, delayed flowering, and improper development of reproductive organs and fertilization (Sasse 2002). Adaptation against stresses increase crop yield followed by changes in plant morphology, physiology and overall development strategies (Bohnert et al. 1995). As a consequence of adaptation to stresses, many substances such as polyols, sugars, proline and betaines get accumulated (Munns and Tester 2008; Iqbal et al. 2011).
Like other BR analogs, castasterone is also widely distributed in plants. It was first isolated from the insect gall of the chestnut tree (Yokota et al. 1982). Kanwar et al. (2012) have investigated the role of BRs under heavy metal stress, for instance nickel on Brassica juncea plants. They have found that exposure to nickel accelerates the BRs biosynthesis such as castasterone, typhasterol, EBL and dolicholide in Brassica juncea.
Several attempts have been made to understand how BRs modulate overall plant growth, development and adaptation under changing environmental conditions (Bajguz and Hayat 2009; Nolan et al. 2020; Hwang et al. 2021; Kothari and Lachowiec 2021). BR biosynthesis and signaling pathways in which numerous genes are involved have also been reported (Wang et al. 2006; Guo et al. 2013; Wang et al. 2014; Belkhadir and Jaillais 2015; Nolan et al. 2017). Sahni et al. (2016) have observed that the BR-related genes are key targets for enhancing the plant productivity under abiotic and biotic stresses. Recently, Zhang et al. (2021) have reported that the exogenous EBL treatment of plants increased the leaf size and expansion by promoting the cell expansion and division via BR modulation, auxin, and gibberellin contents and the upregulation of cell growth-related genes in tobacco seedlings. It has been also reported that BR regulated the transcript expression of nitrate transporter genes to promote nitrogen uptake in maize plants. In addition to BRs biosynthetic pathway, other associated plant growth and developmental processes have also been discussed. The major objective of this review is to explore the impact of exogenous application of BRs on crops and plants under different stresses.
BRs biosynthetic pathway, plant growth and development processes
Sakurai and Fujioka et al. (1997) initially reported the BR biosynthetic pathways in vivo using Catharanthus roseus cell lines. They were analyzed by the endogenous levels of BRs in BR-deficient mutants by several researchers (Fujioka et al. 1997; Choe et al. 1999a; Choe et al. 1999b; Klahre et al. 1998). There are a number of proteins and enzymes involved in signaling process which are interdependent and are activated only in presence of BRs. Generally, BR regulated genes are involved in synthesis of hormones and plant development (Vert et al. 2005). Major work on the mechanistic pathway has been done on Arabidopsis, rice and tomato (Noguchi et al. 2000). A diagrammatic representation for BR synthesis (Fig. 3) clearly indicates two pathways (Noguchi et al. 2000; Ohnishi et al. 2006; Divi and Krishna 2009) where oxidation involving cytochrome P450 facilitates the biosynthesis. Further, Chung and Choe (2013) illustrated the BRs biosynthetic pathway in Arabidopsis, with campesterol as the key precursor of the three BR biosynthetic pathways, two derived from the conversion of campesterol to campestanol and the third one is a campestanol-independent pathway. They have reported that conversion of BRs biosynthetic pathways are mainly mediated by ROTUNDIFOLIA 3 (ROT3), CYP85A1 and CYP85A2.
The biosynthesis pathway of brassinosteroids. CPD – constitutive photomorphogenesis and dwarfism; DET2 – deetiolated2; DWF4 – DWARF4; CYP – Cytochrome P450; Br6ox – Br-6-oxidase (adopted from Divi and Krishna 2009)
BRs regulate many biological functions and development in plants. Exogenous application of BR leads to molecular changes, in order to save the plant from multiple stresses by activating antioxidant enzymes. Crops give better yield under stress perhaps due to enhanced activity of hormones, photosynthesis and gene expression in response to adverse conditions (Vert et al. 2005). As a consequence of a number of physical and chemical stresses plants are forced to produce excessive reactive oxygen species (ROS) which oxidize many essential phytochemicals in cells and damage them. In response to these damaging effects of ROS plants produce antioxidants as a secondary metabolite such as phenols, lignin and BRs. Oxidation reduction reactions occur and accumulation of ROS is prevented to a greater extent. Bartwal et al. (2013) have stated that water molecule is oxidized by photosystem II complex producing molecular oxygen which can be reduced to superoxide radical. It is true that plants produce oxygen during photosynthesis after a series of complicated chemical reactions but molecular oxygen released is never reduced to superoxide ion or superoxide radical. The following reactions show the mechanism of the formation of molecular oxygen by the splitting of water molecule.
The overall reaction can be shown as:
Hydrogen, thus produced represents the reducing power (not the free hydrogen molecule) which is used in the reduction of carbon dioxide in presence of sunlight during photosynthesis producing starch or carbohydrate. Experimentally, oxygen can be trapped in vitro but hydrogen is utilized and does not escape. If a plant produces superoxide radical/ion it will damage the system before it is removed. The O2− is commonly formed because it has s2p4 configuration and can accept two electrons into its half-filled p orbital to complete its octet. However, all these electronic transfers are made during complex formation. Free singlet oxygen or free superoxide ions are not easily available as they are highly reactive. However, enzymes and hormones minimize the damaging effect of ROS which are produced as a consequence of environmental variation. It has also been reported that during stressed condition ROS and Ca2+ are released more quickly in absence of BRs than in its presence (Gilroy et al. 2014).
Plants use defensive mechanism against stresses and activate a number of enzymes, for instance superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacolperoxidase (GP) glutathione peroxidase (GPX) (Ruley et al. 2004; Simonovicova et al. 2004; Sarker and Oba 2018; Nikoleta-Kleio et al. 2020; Kumar et al. 2021) and many non-enzymatic antioxidants (Özdemir et al. 2004; Sarker and Oba 2020). After a series of redox reactions, the ROS are converted to non-toxic compounds which are harmless to plants. BRs actually modify the pathway of enzymes when plants are exposed to stress. Li et al. (1998) have demonstrated that maize seedlings treated with BR and placed under water stress had shown an increase in SOD, APX and CAT activities. Obviously, the activity of some of the enzymes is enhanced and in others it is decreased. Oxidation and reduction occur simultaneously, but only one pathway is followed so that oxidizing species are reduced and subsequently removed as harmless substances. Similar results were obtained by Vardhini and Rao (2003) in case of sorghum. Under salinity, BR exposure to rice seedlings also exhibits an increased SOD, CAT, GR and APX activities (Nunez et al. 2003). It has been reported that EBL seed priming and optimal nitrogen supply improves salt tolerance in soybean (Soliman et al. 2020). Chakma et al. (2021) primed cotton seeds with EBL alone or in combination with other hormones and examined for germination and early seedling growth. They have noticed that the EBL promoted germination under control as well as under salinity and heat stress. However, other tested hormones were found to be ineffective under stress conditions. They have also found that the EBL promoted cotyledon opening and the development of lateral roots in germinated seedlings. Further, Liu et al. (2020a) have found that EBL at certain concentration worked as an active BR, and promoted the tolerance of canola under high-salt stress, nonetheless the same concentration was disadvantageous under low-salt stress. In a recent experiment, it was found that the BR-mediated lignin accumulation plays an important role in garlic adaption to salt stress (Kong et al. 2021). BRs generally enhance the activity of enzymes of plants under stress. Studies on Chlorella vulgaris and tomato support the above results (Bajguz and Hayat 2009; Mazorra et al. 2002). Peroxidases are calcium ion dependent enzymes because the activity depends on their equilibrium (Hu et al. 2007; Bhattacharjee 2008) which is induced by BR. They increase the yield of seeds and biomass as a whole and regulate the expression of genes.
BRs application (presoaking at 10−8 or 10−10 M) has enhanced the percentage of seed germination in Brassica juncea, Orobanche minor and Cicer arietinum seeds (Takeuchi et al. 1995; Ali et al. 2008a; Sirhindi et al. 2009). They also enhance the activity of pigments which in turn, increase the rate of photosynthesis that depends on the type and quantity of chlorophyll pigments. For instance, plants devoid of green pigments such as croton plant, showed decreased rate of photosynthesis. Thus, there is a relationship between the activity, rate of photosynthesis and the chlorophyll pigments (Gomez 2011). Bjornson et al. (2016) have shown that while BRs signaling not only causes an increase in oxylipin synthesis but it also causes changes in the jasmonates response transcription factors (Müssig et al. 2000). Under extreme stress conditions, plants slow down the transpiration by closing the stomata to prevent the loss of water. They also produce amino acids, polyols and some proteins which prevent the oxidation of cellular components such as nucleic acid (Rontein et al. 2002). BRs are activated at this stage and upregulate defensive genes (Kwak et al. 2006) nevertheless the enzymes and hormones are universally known to be more active against salinity stress. Major function of these responsive genes is to help scavenge ROS and relieve the plant from additinal stress. It has been noticed that the opening and closing of stomata in leaves and their development is controlled by BR although, abscisic acid (ABA) and auxins are also involved in this activity (Kim et al. 2012; Le et al. 2014). They have been found to suppress the development of stomata in Arabidopsis plant leaves (Kim et al. 2012; Tanaka et al. 2013) but Gudesblat et al. (2012) have observed negligible effect on the development of stomata in cotyledons. There is a close relationship between BRs and sugar signaling (Zhang and He 2015). Sugar level and expression level of BRs related genes in many plants are linked to each other. BR contents are linked to the increase/decrease of sugar concentration in Arabidopsis (Schröder et al. 2014).
Both, sugar and BR are required for flowering in Arabidopsis (Schröder et al. 2014). It has been found that BR deficient plants flower late but when they are treated with BR, flowering occurs in time (Laxmi et al. 2004). Specific type of BR (BZR1 and BZR2) transcription factors are involved in signaling (Matsoukas 2014) pathways which also interact with sugars. It has been noted that shoots are more efficiently involved in the synthesis of BR than other parts of the plant. Synthetic BRs are quite expensive, and possibly their application in agriculture to boost crop yield may not be economical. It is therefore essential to modify biosynthetic pathway to produce its own BR derivatives to improve the quality of grain and increase the crop production. Like gibberellins modification to increase rice and wheat production, BR activity can also be enhanced through modification of genes involved in the BR synthesis (Choe et al. 2001). BRs have been found to increase not only crop yield of gram but also increase florescence, plant biomass and plant length. As a result of enhanced activity of BRs, the rate of is also increased which produce more glucose/starch in plants. However, BRs accelerate photosynthesis and overall productivity in many plant species.
Clouse (2016) have reported that ABA acts as antagonist of BR in rice and inhibits their vegetative growth. Some A. thaliana mutants have been found to be insensitive to BRs, although a variety of plants respond to BR even under stress (Sasse 1991). Since BRs regulate gene expression, the mutants are rescued by their exogenous application (Evans 1988). In a detailed experiment on Cucumis sativus, Yu et al. (2004) have shown that besides other progresses and developments, absorption and complete assimilation of carbon dioxide leading to increased photosynthesis occurs when the plant was sprayed with 24-EBL. Different concentrations of BR were tested but maximum increase of 210%, in photosynthesis was observed at a concentration of 0.1 mg/L after which the rate declined which suggests that BR concentrations above 0.1 mg/L has no beneficial effect. Accordingly, an upsurge in sucrose, sugars, starch and many enzymes also occur. Similar treatment of BR on Arabidopsis mutants did not show any positive result perhaps due to defective genes. Zhu et al. (2015) have thoroughly examined the effect of BL on tomato ripening and ethylene production. Ethylene regulates fruit ripening, and any substance that catalyses the generation of ethylene can also accelerate the ripening of fruits by influencing many enzymes (Hamilton et al. 1990; Oeller et al. 1991). Application of BR to tomato fruit increased the evolution of ethylene and lycopene with a consequent lowering of chlorophyll pigments (Liu et al. 2014). BR regulates the biosynthesis of ethylene and lycopene via ACS and ACO enzymes (Barry et al. 2000; Klee and Giovannoni 2011) which help in ripening of tomato with the reduction of chlorophyll after they were plucked green and stored at 25 °C. BRs also increase the synthesis of jasmonates (Müssig et al. 2000) besides providing tolerance against biotic/abiotic stresses to plant species (Koca and Karaman 2015). Application of naturally occurring BRs also decrease the quantity of pesticides in fruits and vegetables by degrading them to harmless residues (Zhou et al. 2015).
Plant response to BRs application under abiotic stress
Application of BRs enhanced plant production during stress and abrupt environmental changes, although they are useful even under normal conditions. Any deviation from normal condition is felt by the plants and reflected from their biochemical changes which are connected to their growth and development. These responses are triggered in plants as a signal similar to reflex action in mammals. The changes in antioxidants, up-regulation and down-regulation of proteins cause changes in protein pathways. The harsh environmental conditions force the plants to make adaptations in order to survive (Smirnoff 1995; Fujita et al. 2006). BRs application in plants and their response under changing environment have been discussed under the following subheadings.
Salinity stress
Phytohormones are synthesized to enhance the yield of fruits, cereals, crops and regulate the biological function of plants (Iqbal et al. 2014) during salinity stress. BRs under normal conditions are engaged almost in all physical and chemical activities but under stress they protect the plants to maintain their normal functioning. BL treatment of Lucerne seed under salinity has been shown to enhance the dry weight and antioxidant activity (Zhang et al. 2007b). It has been observed by Tanaka et al. (2003) that BRs increased the growth of hypocotyls and cotyledonous leaf-blades in a dose-dependent manner. BRs also catalyse the efficiency of other hormones (gibberellins and auxin) and plant growth promoters (Tanaka et al. 2003). Increased salinity or water scarcity produces oxidative stress which in turn, reduces the crop production, photosynthesis, protein synthesis, respiration and overall growth of many plant species (Xiong and Zhu 2002; Hussain et al. 2013; Husen et al. 2016, 2017). Substantial quantity of ROS is produced due to salinity stress. Thus, superoxide anion O2−, hydrogen peroxide H2O2 and hydroxyl radicals OH• are generated (Mittler 2002; Masood et al. 2006). In order to prevent the damage by these species, plants produce enzymes namely SOD, APX and GRX in defense (Munns and Tester 2008) which act as antidote against them. Besides BRs, there are many more hormones which control several functions of the plant. These phytohormones are produced by rhizobacteria (Ahmad et al. 2005; Babaloa 2010) under adverse situation of salinity, high temperature or excess of water together with antibiotics to protect plants from pathogenic microbes. Phytohormones are produced to sustain the harsh environmental conditions so that the normal functioning of the plant continues. Exogenous application of BR on sorghum cultivars and sugar beet plants under stress showed positive effect in terms of increased root length, biomass and germination (Vardhini and Rao 2003). Unlike drought stress, salinity disturbs the exchange and translocation of cations and anions in the plant system. In order to reduce the concentration of the ions either the intake of salt is minimized or the common ions are exchanged with another ion which is essential for the plant. For instance, Ca2+ ions may be replaced by Mg2+ ions as their exchange is chemically favored since they both are divalent metal ions and belong to the same group of elements. Similarly, Na+ ion may be replaced by K+ ion, the concentration of which is relatively lower than that of Na+ ion. Under salinity stress, EBL treatment decreased proline accumulation to prevent the damage of seedlings (cv IR-28 rice) and thus allows normal growth (Özdemir et al. 2004). Conflicting results have been reported about accumulation of proline and enhanced activity of antioxidant enzymes in salt sensitive plants/crops (Ali et al. 2007; Hayat et al. 2007). However, degradation of proline and plant growth is the main focus of study. There is however, consensus on the ameliorating effect of EBL on salt stressed plants. These results are supported by work done on Cicer arietinum (Ali et al. 2007) Vigna radiata (Hayat et al. 2010b) and A. thaliana (Kagale et al. 2007). BR application in salt stressed rice plant increases the activity of nitrate reductase, which in turn, increases the crop production. Since ROS damage the plant metabolites, the plants modify mechanistic pathway to scavenge the ROS so that the plants grow and produce fruit/ crop in usual manner (Vardhini and Anjum 2015). It has been observed almost in all salt stressed crops that BL application improves germination and growth of seedlings (El-Khallal et al. 2009; Shahbaz and Ashraf 2007). The stress produced by a combination of two substances (copper and sodium chloride) has been reported to be mitigated by epi-BL application in two varieties of Cucumis sativus (Fariduddin et al. 2013b). Besides increasing the activity of antioxidant enzymes, the rate of photosynthesis was also enhanced. It may be a good effort to explore the combined effect of two substances on plant development but an appropriate reason for doing such experiment is required. The other metals of copper group (silver and gold) have altogether different chemical behavior which has not been tested. Application of BR analog, DI-31 showed improvement in growth in lettuce plant under salt stress (Serna et al. 2015). Salinity is known to decrease plant growth and increase ethylene production (Siddikee et al. 2012). Increased ethylene production causes more stress which reduces plant growth. The BR analog treated lettuce plant showed tolerance to salinity in terms of fresh weight (Zeng et al. 2010; Shahid et al. 2014). The rate of respiration in roots and shoots of lettuce increased under salinity stress (Zapata et al. 2007) to overcome the effect of stress. This is a defensive response of the plant. Since NaCl is completely dissociated as Na+ and Cl− ions they have higher mobility than other essential ions. They block the passage for them which cause deficiency of essential ions as a result of which the plants have to respire more rapidly than usual. BR application normalizes the respiration. K+ ion treatment of saline stressed plants showed reduction in ethylene emission (Amjad et al. 2014). The BRs increase the growth of the plant but K+ alone responds to tolerance against stress. The K+ ion is relatively larger than Na+ ion and hence its mobility is lower than that of Na+. Perhaps it can make up for the loss of essential nutrients in plants. If a combination of K+ and BR is applied to the plant/crop the rate of vegetative growth may be enhanced several fold. All adverse effects produced by NaCl stress are directly proportional to its concentration. Initially, the aerial parts of the plants show a decrease in growth, and at a reasonably higher concentration of NaCl the plant dies if appropriate measures are not taken.
Effect of NaCl and BRs concentration on peppermint (Mentha piperita) revealed that salinity is the main cause of plants to perish. In the beginning, yellowing of leaves occurs, as a result of which, quantity of chlorophyll pigments decreases which reduces the rate of photosynthesis. Weight of aerial parts of the plant significantly decreased. There was a marked reduction in essential oil production of mentha and an increase in lipid peroxidation, phenols and antioxidant enzymes with increasing NaCl concentration (Khorasaninejad et al. 2010; Çoban and Baydar 2016). Salinity causes overall reduction in growth of plants. BR application reduces the salinity stress and prevents the damage. In tomato and geranium, BR treatment enhanced their growth (Hayat et al. 2010b; Hayat et al. 2010c; Swamy and Rao 2009). Çoban and Baydar (2016) have reported that, under saline condition NaCl ionizes to produce free Na+ and Cl− ions which are deposited on the surface of cell membrane. They further reported that it decreases the pH of the cell surface as a consequence of which the protein breaks down. The acidic medium damages the plant. This proposal is hypothetical and chemically impossible. NaCl is a salt of strong acid (HCl) and a strong base (NaOH) which is completely ionized in aqueous medium. The sodium ions are always in equilibrium with chloride ions and their recombination will give neutral NaCl salt and hence the pH of the medium will never change. Ionization of NaCl and reaction of Na and Cl ions are shown below:
Assuming damage of cell membrane by lowering the pH due to free Cl ions producing HCl, is improbable. Ion leakage may be due to excessive accumulation of Na and Cl ions around the cell membrane. Excess sodium ions are toxic to all living beings because they trigger the impulses through Na+/K+ pump. Chloride ions have bleaching effect and gradually damage the chlorophyll pigments. A NaCl solution in aqueous medium is neutral and all such assumptions that it produces acidity and damages the crop are baseless. Large excess of NaCl is harmful to plants due to toxicity of Na+ ions. They are transferred from intracellular fluids to extracellular fluids through carrier proteins. Conversely, K+ ions are transferred from extracellular fluids to intracellular fluids. During salinity, excess Na+ ions are transferred to extracellular fluid which produces a charge gradient on one hand and concentration gradient on the other, across the cell membrane. This potential difference accounts for the trigger of impulses in plants and mobility of ions through osmosis. Sodium and potassium pump also maintains the volume of the cells without which volume increases uncontrollably and the cell bursts. Excess of Na ions during salinity also causes imbalance between Na and K ions which disturbs the metabolism.
It has been reported that salt stressed Oryza sativa treated with BR exhibited increased growth and development (Anuradha and Ram Rao 2003). Also, it induced the activity of nitrate reductase in salt stressed rice crop. BRs regulate the activity of antioxidant enzymes, chlorophyll pigments, rate of photosynthesis and carbohydrate metabolism to upsurge plant growth under stress. Exogenous application of BR enhances the biosynthesis of endogenous hormones and regulates signal transduction pathways to different stresses (Anwar et al. 2018). Salinity is also increased by the presence of other alkali metals (Li, Na, K) and alkaline earth metals (Be, Mg, Ca) chlorides, bicarbonates and sulfates in the soil. BRs provide tolerance against all stresses and improve the quality of fruits and grains by increasing the photosynthesis and enzyme activity (Anwar et al. 2018).
BR application has shown tolerance against salt stress in Eucalyptus urophylla. ROS is produced due to large quantity of Na ions deposited which decreases chlorophyll pigments as a consequence of which rate of photosynthesis is decreased (Kim et al. 2016). K+/Na+ pump balances the ionic concentration of these ions within the cells in plants through symport and antiport but extremely large excess of sodium ions during salt stress causes imbalance. At this point foliar application of EBR improves overall development in E. urophylla (de Oliveira et al. 2019) and reduces salt stress. K+/Na+ equilibrium was maintained which led to an increase in CAT and APX enzymes. Very recently, Liu et al. (2020b) have shown the impact of EBR/EBL application on plants under different stages of development increasing their tolerance against salt stress. It has been noticed that all concentrations of hormones are not equally effective in mitigating the influence of salinity, drought or extreme temperature variation. At low level of salinity, a certain BR concentration was effective, however with increase in salinity the same BR concentration does not work. Therefore, emphasis must be given to prevent excessive sodium ion accumulation, because it induces the ROS production. Zea mays under salinity stress, exposed to 28-HBL and 24-EBL have shown to withstand the abrasive effect of NaCl (Rattan et al. 2020). In this experiment, the quantity of saline solution added has not been mentioned which could measure the amount of salt. Further, the equilibrium between K+/Na+, antioxidant enzymes and phytochemicals were measured in maize plants. The sodium ions were decreased with a consequent increase in potassium ion concentration. In fact, it is the concentration gradient that accelerates the mobility of Na ions out and K ions inside the cell maintaining the normal functioning of K+/Na+ pump. Thus, antioxidant enzyme activity enhanced with a consequent decrease in malondialdehyde accumulation. With the removal of free radicals, the enzyme activity is enhanced which maintains the normal functioning of plants under salt stress. Treatment of soybean with EBL coupled with nitrogen, further enhances the tolerance of plant by NaCl stress (Soliman et al. 2020). Nitrogen acts as a nutrient which synergises the photosynthesis in plants.
Water/drought stress
Photosynthesis is essential for a plant to survive even under different stresses. Water, carbon dioxide, sunlight and moderate temperature are required for chlorophyll to produce carbohydrate/sugar. During drought or water scarcity the process of photosynthesis is retarded or even completely arrested. It is a general phenomenon for all plants to slow down metabolic processes. The stomata remain closed to prevent the loss of water as the growth of the plant as a whole is retarded. It has been observed that when Arabidopsis and Brassica napus seedlings were grown in very dilute solution of EBL (1 μM) and placed under artificial drought for 96 and 60 h respectively, their tolerance for drought was increased (Kagale et al. 2007). In response to EBL application some modification in the activity of antioxidant enzymes and defense genes occurs which stimulates the normal functioning of plants (Li et al. 2012). It has also been found that BR treated Cucumis sativus increases the reduction process of carbon dioxide in presence of glutathione which was indirectly involved in activating the process (Jiang et al. 2012). However, HBL treatment of mustard plant under drought enhanced CAT, POX, SOD activities and proline content. BRs and BL have also been found to increase the biomass and crop yield in soybean and mustard (Zhang et al. 2008; Fariduddin et al. 2009a). It has been reported (dos Santos Ribeiro et al. 2019) that water scarcity diminishes seed germination, biomass and root growth in soybean plants which can be ameliorated by 24-EBL application. Under water stress the oxidative damage is prevented through antioxidants (SOD, CAT, APX and POX) before it damages the plant morphology (Cruz de Carvalho 2008). Drought like condition is inversely proportional to the production of RO. The increased amount of antioxidants produced more tolerance in plants toward water deficiency. Water stressed plants showed improvement in growth after they were treated with BR. However, this effect was synergized by endogenous application of NO specifically in Capsicum annum (Kaya et al. 2019). Pereira et al. (2019) examined the role of 24-EBL on soybean plants under water deficiency and observed that quantum yield of PSII photochemistry, electron transport and net photosynthetic rates were reduced. However, exogenous application of EBR (at 100 nM) has mitigated the negative effect of water stress on the studied features. The EBR also decreased the superoxide and hydrogen peroxide and prevented cell membrane damage.
It has been noticed that the ABA controls a wide range of RAB (responsive to ABA) genes coding for the proteins concerned to the cell protection against dehydration injuries in Manihot esculenta (Feng et al. 2019). Avalbaev et al. (2020) have shown the ability of 24-EBL to stimulate additional synthesis of wheat germ agglutin under normal conditions. Severe water scarcity partially damages the aerial parts which is clearly visible. It has been reported in the case of grapevine (Vitis vinifera) under drought condition that, the production of H2O2 and superoxide radicals is enhanced with a consequent reduction in ascorbic acid and glutathione (Wang et al. 2019). After the exogenous BR application these symptoms were reversed, viz., the production of H2O2 and O2− were decreased and those of ascorbic acid and antioxidants were enhanced. It has been shown that there is a close relationship between ABA and drought stress. BRs reduce the adverse symptoms and increase the tolerance of grapevine to drought. All antioxidants and genes related to their production are activated by BR application. Activity of enzymes and proteins increased after EBL was sprayed on Echinacea purpurea under severe drought condition (Hosseinpour et al. 2020). Despite substantial increase in total protein, SOD, CAT, POX, proline and H2O2 a large reduction in plant biomass was observed. It is interesting that the substances needed for plant development were increased which suggests tolerance of E. purpurea to drought. A slight deviation from normal behavior of plant and an increase in ROS is an indication of abiotic stress.
Temperature stress
Both, extremely low and high temperature disturb the normal functioning of plants. At very high temperature the loss of water and increased permeability of plasma membrane decrease the rate of photosynthesis which is vital for all green plants. It has been found that when the plants under temperature stress are treated with EBL the proteins responsible for heat shock protect the plant as a result of which the photosynthesis efficiency is enhanced as has been found in tomato plant (Singh and Shono 2005). Plants/seedlings treated with EBL prior to exposure to high temperature for few hours (1–4 h) showed delayed adverse symptoms relative to untreated ones (Kagale et al. 2007). This is quite obvious but such short time exposure does not show any meaningful result which may be generalized because plants can recover such losses by their own immune system. High temperature and chilling cold also have damaging effect on plants which are clearly visible. For instance, reduced growth, yield (Sheehy et al. 2005) shortening and wilting of leaves, necrosis and reduction in development of reproductive organs occur (Kang and Saltveit 2002).
Photosynthesis is always hampered at abnormally low temperature, particularly when sudden change occurs (for instance, snowfall). Absorption of carbon dioxide, enzyme activities and osmosis are reduced to minimum. EBL treatment at this stage helps to recover the loss in dicots (Huang et al. 2006). However, a slight variation in temperature (20 ± 5 °C) does not show any noticeable change in treated/untreated plant (Kagale et al. 2007). BR treated wheat leaves under thermal stress (43 °C) showed normal development indicating normal protein synthesis. The untreated ones had shown a nearly threefold (Kulaeva et al. 1991) fall in the process. BRs and indole acetic acid (IAA) separately induce the plant growth under temperature stress. This effect is synergized if a combination of BRs and IAA is applied to plants. BR increases the rate of germination in seasonal crops (He et al. 1991). Some BR derivatives protect the plants even at 7 °C while others do the same at abnormally high temperature (González-Olmedo et al. 2005). BR (24 epi-BL) application shows very low tolerance to Bromus inermis development at low temperature (3–5 °C), but at high temperature (40–45 °C) the tolerance is appreciably high (González-Olmedo et al. 2005). BRs also increase the fruit yield in tomato under heat stress (Singh and Shono 2005).
Yang et al. (2019) have studied the chilling effect in BR pretreated and control pepper seedlings. Foliar spray of EBR on pepper leaves showed an increase in plant growth, rate of photosynthesis, maximum quantum efficiency and photochemical quenching coefficient. Plants also showed an increase in free amino acids and enzyme activity (glutamine synthase, nitrate reductase, glutamate synthase etc.) which enhanced nitrogen metabolism in leaves. Chilling stress produces ROS but treatment with EBR reduces the accumulation of H2O2 and superoxide anion showing increasing tolerance toward falling temperature below normal. In recent years, it has been noticed that the autophagy process is important for the degradation of dysfunctional cellular components at some stage of development under negative environmental situations (Qin et al. 2007; Liu and Bassham 2012). So far, more than thirty autophagy-related genes (ATGs) have been recognized (Yoshimoto 2012; Marshall and Vierstra 2018). In plant system, the role of autophagy has been investigated under numerous abiotic stresses (Guiboileau et al. 2013; Wang et al. 2015a, 2015b; Zhai et al. 2016). BRs worked as a positive regulator of NBR1-dependent selective autophagy in tomato plants (Chi et al. 2020). They have verified that low temperature and BRs together induced the BRASSINAZOLE-RESISTANT1 (BZR1) stability, which up-regulates ATG2, ATG6, NEIGHBOR OF BRCA1(NBR1a) and (NBR1b) expression by binding to their promoters. The upsurge in autophagy and the selective autophagy receptor NBR1 increased photoprotection via higher accumulation of functional proteins (PsbS, VDE and D1) leading to increased tolerance to cold (Fig. 4).
Projected mechanism of BZR1 induced cold tolerance by the autophagy activation in tomato plants. Cold and BRs induced BZR1 stability; and turn on the transcription of autophagy genes (ATG2, ATG6, NBR1a, and NBR1b) by their promoters binding, thereafter increasing the autophagy. Autophagy facilitates photoprotection by functional proteins accumulation namely, PsbS, VDE, and D1; and enhances the degradation of stress-damaged insoluble ubiquitinated protein aggregates through selective autophagy receptor NBR1. Arrows in the illustration showed the positive control; while the bar ends exhibit the negative control (adopted from Chi et al. 2020). BZR1 – brassinazole resistant 1; BRs – Brassinosteroids; ATG2 & ATG6 – Autophagy genes; NBR1 – neighbor of brca1; and PsbS, VDE & D1 – functional proteins
The effect of BL on rice under chilling stress has shown improvement in activity of enzymes (SOD and Peroxidases) sugars and proteins (Wang et al. 2020). Other toxic substances such as malondialdehyde were reduced. Minerals/inorganic ions like N, P and K were enhanced when rice plants were exposed to BR. In fact, these nutrients are already there but owing to low temperature their release and transportation was delayed or arrested.BR treatment induces the release of nutrients and activate the enzymes which is termed tolerance. The plants recover from cold stress when temperature becomes normal and all activities are restored even in absence of BR/EBL. However, these hormones induce the activity of antioxidants and reduce ROS under all types of stresses. Recently, Chen et al. (2021) have also suggested that BRs mediated the impact of high temperature stress on pistil activity during antithesis and increased antioxidants and suppressed ROS generation in photo-thermosensitive genetic male-sterile rice lines.
Heavy metal toxicity
Radioactive metals and toxic metals accumulated in plant parts were reduced by the application of BRs. Perhaps, metal ions forming soluble complexes with the donor groups of BRs are prevented from their deposition in cells. In tobacco seedlings, biomass accumulation was drastically reduced under cadmium stress (Ahammed et al. 2013). However, it has been noticed that the exogenous EBR application at 0.1 μM increases plant biomass by augmenting carbon dioxide assimilation capacity, chlorophyll fluorescence and photosynthetic pigment. They also suggested that the foliar application of EBR reduces cadmium uptake in roots and its translocation to tobacco leaves. In another experiment, BR (24-EBL and 24-epicasterone and 4154) treatments were found to decrease the uptake of heavy-metal (lead and cadmium) in spring wheat plants (Kroutil et al. 2010). Bukhari et al. (2016) have described that the tobacco leaf mesophyll cells (cell wall, cell membrane, and dilated thylakoid) were distorted under chromium exposure. 24-EBL application had protected the chromium-induced damage to chloroplast. In tomato seedlings, Singh and Prasad (2017) have also reported that application of 28-homobrassinoloid improved the chromium-induced decrease in growth, photosynthesis and the photochemistry of PSII. Further, Hasan et al. (2008) have reported that 28-HBL protects chickpea from cadmium toxicity by stimulating the levels of enzymatic and non-enzymatic antioxidants. Song et al. (2016) have suggested that ROS generation is increased due to heavy metal exposure and adversely influenced the overall plant metabolism, triggering oxidative injury to proteins, lipids and nucleic acids. However, Kanwar et al. (2012) have found that exposure of nickel accelerates the BRs biosynthesis such as castasterone, typhasterol, EBL and dolicholide in Brassica juncea.
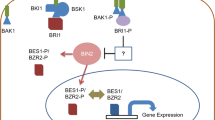
Ali et al. (2008b) have investigated the function of BRs in the reduction of aluminum toxicity in mung bean (Vigna radiata) seedlings. The seedlings were exposed to various concentrations of aluminum (0.0, 1.0 or 10.0 mM) at 1-week-old stage and were sprayed with 10−8 M of 24-EBLor 28-HBL at 14-day stage. After three weeks, carbonic anhydrase activity, chlorophyll content and the rate of photosynthesis were found to decrease. However, leaf antioxidative enzyme activities (CAT, SOD, peroxidase) and proline in leaves and roots enhanced in these seedlings. Further, foliar spray of 24-EBL or 28-HBL, in absence of aluminum strongly improved the above parameters and also accelerated their growth. Radish plant growth by foliar application of BR (24-EBL or 28-HBL at 0.5, 1.0, or 2.0 μM) in zinc toxicity alleviation has been carried out by Ramakrishna and Rao (2013). Zinc stress was found to reduce growth parameters and photosynthetic pigments but BRs exposure improved these traits. BRs application however, decreased H2O2 level, lipid peroxidation, electrolyte leakage and enhanced the water absorbing capacity of leaf under stress. Foliar application of 24-EBL was more effective than 28-EBL in zinc stress mitigation. Several other findings have also revealed that application of BRs can modify antioxidant activities in maize, mustard, radish, wheat and rice under metal stress (Sharma et al. 2007, 2010, 2011a, 2016; Ramakrishna and Rao, 2015). It has also been suggested that BRs under metal stress may inhibit lipid degradation and accelerate the antioxidative enzyme activities (Sudo et al. 2008; Soares et al. 2016). Phytochelatins (PCs) syntheses are another important mechanism of metal detoxification; and BRs are known to stimulate PCs syntheses in cells treated with lead (Rajewska et al. 2016). A possible mechanism of BRs regulation of heavy metal stress tolerance in plants is illustrated in Fig. 5.
Proposed possible mechanism (dotted lines) of BRs regulation of heavy metal tolerance in plants. APOX – ascorbate peroxidase; BAK1 – BRI1-associated kinase1; BRI1 – brassinosteroid insensitive1; CAT – catalase; DHAR – dehydroascorbate reductase; GR – glutathione reductase; GSH – glutathione; GSSG – glutathione disulphide; HM – heavy metal; K – potassium; MAPK – mitogen activated protein kinases; MDA – malondialdehyde; MDHAR – monodehydroascorbate reductase; P – phosphate; PC – phytochelatins; ROS – reactive oxygen species; V- ATPase; and vacuolar H+ − ATPase (adopted from Rajewska et al. 2016; re-drawn based on Sharma et al. 2011a)
A recent study of Brassica juncea has shown that lead (Pb) tolerance can be diminished by BR application before or after seed germination (Soares et al. 2020). If the soil is contaminated with lead it is absorbed by the plant through its roots. Of all the lead salts only lead chloride and lead nitrate are slightly soluble (not more than 1 g/100 ml in water at 25 °C) and therefore, chances of lead toxicity are rare. However, lead is known to be a cumulative poison as it accumulates in different parts of the plant and produces lesion. Exogenous application of EBL (10−8 M) was highly effective against lead stress in B. juncea. It accelerated the activity CAT and POX which prevented the damage by lead. The extent of injury depends on the concentration of the toxic metal and the stage of plant development. In a very recent study, Tadaiesky et al. (2021) have reported that EBR decreased iron toxicity in rice plants modulating the parenchyma area, contributing to the formation of an oxidative barrier and Fe immobilization at the root surface. Similarly, Guedes et al. (2021) have shown that lead produces toxicity in rice plants but EBR treatment alleviated the adverse effects of lead.
Conclusion
BRs are a group of naturally occurring plant hormones comprising of BL, castasetrone and their derivatives which regulate plant growth and development. Over 69 BRs have been isolated from different parts of plants. These steroidal hormones are timely produced and utilized by plants. They are synthesized in response to salinity, drought, extremely cold/hot temperature, injury or pathogenic attack to maintain the normal functioning of plants and enhance fruits and crop yield. In future BRs and BL would be the key hormones to increase the yield of fruits, vegetables and agri-products. Their application would also protect the plant/crops from pests, insects and physical stresses.
Abbreviations
- 24-EBL:
-
24-epibrassinolide
- 28-HBL:
-
28-homobrassinolide
- ABA:
-
Abscisic acid
- APX/APOX:
-
Ascorbate peroxidase
- ATGs:
-
Autophagy-related gene
- BL:
-
Brassinolide
- BR:
-
Brassinosteroid
- BZR1:
-
BRASSINAZOLE RESISTANT 1
- BAK1:
-
brassinosteroids associated kinase1
- BRI1:
-
brassinosteroid insensitive1
- CaCl2 :
-
Calcium chloride
- CaSO4 :
-
Calcium sulfate
- CAT:
-
Catalase
- DHAR:
-
Dehydroascorbate reductase
- GP:
-
Guaiacol peroxidase
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulphide
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- H2O2 :
-
Hydrogen peroxide
- HCl:
-
Hydrogen chloride
- HM:
-
Heavy Metal
- IAA:
-
Indole acetic acid
- NBR1:
-
next-to-BRCA1
- KCl:
-
Potassium chloride
- MDA:
-
Malondialdehyde
- MAPK:
-
Mitogen-activated protein kinases
- MDHAR:
-
Monodehydroascorbate reductase
- K:
-
Potassium
- P:
-
Phosphate
- NaCl:
-
Sodium chloride
- NaOH:
-
Sodium hydroxide
- PC:
-
Phytochelatin
- Prxs:
-
Peroxiredoxins
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
- V-ATPase:
-
vacuolar H+-ATPase
References
Abbas S, Latif HH, Elsherbiny EA (2013) Effect of 24-epibrassinolide on the physiological and genetic changes on two varieties of pepper under salt stress conditions. Pak J Bot 45:1273–1284
Aghdam MS, Asghari M, Farmani B, Mohayeji M, Moradbeygi H (2012) Impact of post harvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci Hort 144:116–120. https://doi.org/10.1016/j.scienta.2012.07.008
Aghdam MS, Mohammadkhani N (2014) Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioproc Technol 7:909–914. https://doi.org/10.1007/s11947-013-1165-x
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213. https://doi.org/10.1093/jxb/ers323
Ahmad F, Ahmad M, Khan S (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Ali Q, Athar HUR, Ashraf M (2006) Influence of exogenously applied brassinosteroids on the mineral nutrient status of two wheat cultivars grown under saline conditions. Pak J Bot 38:1621–1632
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot 59:217–223. https://doi.org/10.1016/j.envexpbot.2005.12.002
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008a) 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 72:1387–1392. https://doi.org/10.1016/j.chemosphere.2008.04.012
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008b) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159. https://doi.org/10.1016/j.envexpbot.2007.07.014
Alyemeni MN, Hayat S, Wijaya L, Anaji A (2013) Foliar application of 28-homobrassinolide mitigates salinity stress by increasing the efficiency of photosynthesis in Brassica juncea. Acta Bot Bras 27:502–505. https://doi.org/10.1590/S0102-33062013000300007
Amjad M, Akhtar J, Anwar-ul-Haq M, Yang A, Akhtar SS, Jacobsen SE (2014) Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci Hortic 172:109–116. https://doi.org/10.1016/j.scienta.2014.03.024
Anuradha S, Ram Rao SS (2003) Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul 40:29–32. https://doi.org/10.1023/A:1023080720374
Anuradha S, Rao SSR (2007) The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ 53:465–472
Anuradha S, Rao SSR (2009) Effect of 24-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 47:317–320. https://doi.org/10.1007/s11099-009-0050-3
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y (2018) The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res 51:46. https://doi.org/10.1186/s40659-018-0195-2
Arora N, Bhardwaj R, Sharma P, Arora HK (2008) Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol Plant 30:833–839. https://doi.org/10.1007/s11738-008-0188-9
Arora P, Bhardwaj R, Kanwar MK (2010) 24-Epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plant 16:285–293. https://doi.org/10.1007/s12298-010-0031-9
Avalbaev AM, Yuldashev RA, Fatkhutdinova RA, Urusov FA, Safutdinova YV, Shakirova FM (2010) The influence of 24-epibrassinolide on the hormonal status of wheat plants under sodium chloride. Appl Biochem Microbiol 46:99–102
Avalbaev A, Bezrukova M, Allagulova C, Lubyanova AR, Kudoyarova GR, Fedorova K, Maslennikova D, Yuldashev R, Shakirova F (2020) Wheat germ agglutinin is involved in the protective action of 24-epibrassinolide on the roots of wheat seedlings under drought conditions. Plant Physiol Biochem 146:420–427. https://doi.org/10.1016/j.plaphy.2019.11.038
Babaloa OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570. https://doi.org/10.1007/s10529-010-0347-0
Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY (2012) Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol 14:810–817. https://doi.org/10.1038/ncb2546
Bajguz A (2002) Brassinosteroids and lead asstimulators of phytochelatins synthesis in Chlorella vulgaris. J Plant Physiol 159:321–324. https://doi.org/10.1078/0176-1617-00654
Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62:1027–1046. doi: 1016/s0031-9422(02)00656-8
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8. https://doi.org/10.1016/j.plaphy.2008.10.002
Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D (1996) Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J9:525–535. https://doi.org/10.1046/j.1365-313X.1996.09040525.x
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32:216–232. https://doi.org/10.1007/s00344-012-9272-x
Behnamnia M, Kalantari KM, Rezanejad F (2009) Exogenous application of brassinosteroid alleviates drought-induced oxidative stress in Lycopersicon esculentum L. Gen Appl Plant Physiol 35:22–34
Belkhadir Y, Jaillais Y (2015) The molecular circuitry of brassinosteroid signaling. New Phytol 206:522–540. https://doi.org/10.1111/nph.13269
Bhattacharjee S (2008) Calcium-dependent signaling pathway in the heat-induced oxidative injury in Amaranthus lividus. Biol Plant 52:137. https://doi.org/10.1007/s10535-008-0028-1
Bjornson M, Dandekar AM, Chory J, Dehesh K (2016) Brassinosteroid's multi-modular interaction with the general stress network customizes stimulus-specific responses in Arabidopsis. Plant Sci 250:165–177. https://doi.org/10.1016/j.plantsci.2016.06.007
Bohnert HJ, Nelson DE, Jonsen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111. https://doi.org/10.1105/tpc.7.7.1099
Bukhari SA, Wang R, Wang W, Ahmed IM, Zheng W, Cao F (2016) Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res Int 23:18229–18238. https://doi.org/10.1007/s11356-016-7017-2
Chakma SP, Chileshe SM, Thomas R, Krishna P (2021) Cotton seed priming with brassinosteroid promotes germination and seedling growth. Agronomy 11:566. https://doi.org/10.3390/agronomy11030566
Chen J, Fei K, Zhang W, Wang Z, Zhang J, Yang J (2021) Brassinosteroids mediate the effect of high temperature during anthesis on the pistil activity of photo-thermosensitive genetic male-sterile rice lines. Crop J 9:109–119. https://doi.org/10.1016/j.cj.2020.07.001
Chi YH, Koo SS, Oh HT, Lee ES, Park JH, Phan KAT, Wi SD, Bae SB, Paeng SK, Chae HB, Kang CH, Kim MG, Kim W-Y, Yun D-J, Lee SY (2019) The physiological functions of universal stress proteins and their molecular mechanism to protect plants from environmental stresses. Front Plant Sci 10:750. https://doi.org/10.3389/fpls.2019.00750
Chi C, Li X, Fang P, Xia X, Shi K, Zhou Y, Zhou J, Yu J (2020) Brassinosteroids act as a positive regulator of NBR1-dependent selective autophagy in response to chilling stress in tomato. J Exp Bot 71:1092–1106. https://doi.org/10.1093/jxb/erz466
Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S, Tax FE, Feldmann KA (1999a) The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol 119:897–907 doi:https://doi.org/10.1104/pp.119.3.897
Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA (1999b) The Arabidopsis dwf7/ste1 mutant is defective in the delta7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell 11:207–221. https://doi.org/10.1105/tpc.11.2.207
Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26:573–582. https://doi.org/10.1046/j.1365-313x.2001.01055.x
Chung Y, Choe S (2013) The regulation of brassinosteroid biosynthesis in Arabidopsis. CRC Crit Rev Plant Sci 32:396–410. https://doi.org/10.1080/07352689.2013.797856
Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111:671–678. https://doi.org/10.1104/pp.111.3.671
Clouse SD (2016) Brassinosteroid/abscisic acid antagonism in balancing growth and stress. Dev Cell 38:118–120. https://doi.org/10.1016/j.devcel.2016.07.005
Çoban Ö, Baydar NG (2016) Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind Crop Prod 86:251–258. https://doi.org/10.1016/j.indcrop.2016.03.049
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:163. https://doi.org/10.1186/1471-2229-11-163
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species. Plant Signal Behav 3:156–165. https://doi.org/10.4161/psb.3.3.5536
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2013) 24-Epibrassinolide restores nitrogen metabolism of pigeon pea under saline stress. Bot Stud 54:9. https://doi.org/10.1186/1999-3110-54-9
Das T, Shukla YM, Poonia TC, Meena M, Meena MD (2013) Effects of brassinolide on physiological characteristics of rice (Oryza sativa L.) with different salinity levels. Ann Biol 29:228–231
Daur I, Tatar O (2013) Effects of gypsum and brassinolide on soil properties, and berseem (Trifolium alexandrinum L.) growth, yield and chemical composition grown on saline soil. Legume Res 36:306–311
de Oliveira VP, Lima MDR, da Silva BRS, Batista BL, da Silva Lobato AK (2019) Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J Plant Growth Regul 38:557–573. https://doi.org/10.1007/s00344-018-9870-3
Ding HD, Zhu XH, Zhu ZW, Yang SJ, Zha DS, Wu XX (2012) Amelioration of salt-induced oxidative stress in eggplant by application of 24-epibrassinolide. Biol Plant 56:767–770. https://doi.org/10.1007/s10535-012-0108-0
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol 26:131–136. https://doi.org/10.1016/j.nbt.2009.07.006
dos Santos Ribeiro DG, da Silva BRS, da Silva Lobato AK (2019) Brassinosteroids induce tolerance to water deficit in soybean seedlings: contributions linked to root anatomy and antioxidant enzymes. Acta Physiol Plant 41:82. https://doi.org/10.1007/s11738-019-2873-2
Durigan DRJ, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp to salinity. Acta Physiol. Plant 33:1887–1896. https://doi.org/10.1007/s11738-011-0732-x
Ekinci M, Yildirim E, Dursun A, Turan M (2012) Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. Hortic Sci 47:631–636. https://doi.org/10.21273/HORTSCI.47.5.631
El-Khallal SM, Hathout TA, Ashour AERA, Kerrit AAA (2009) Brassinolide and salicylic acid induced antioxidant enzymes, hormonal balance and protein profile of maize plants grown under salt stress. Res J Agric Biol Sci 5:391–402
El-Mashad A, Mohamed H (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249:625–635. https://doi.org/10.1007/s00709-011-0300-7
Embiale A, Hussein A, Husen A, Sahile S, Mohammed K (2016) Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J Agron 15:45–57. https://doi.org/10.3923/ja.2016.45.57
Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895. https://doi.org/10.1126/science.3283939
Fariduddin Q, Khanam S, Hasan SA, Ali B, Hayat S, Ahmad A (2009a) Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol Plant 31:889–897. https://doi.org/10.1007/s11738-009-0302-7
Fariduddin Q, Yusuf M, Hayat S, Ahmad A (2009b) Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ Exp Bot 66:418–424. https://doi.org/10.1016/j.envexpbot.2009.05.001
Fariduddin Q, Yusuf M, Chalkoo S, Hayat S, Ahmad A (2011) 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 49:55–64. https://doi.org/10.1007/s11099-011-0022-2
Fariduddin Q, Khalil RRAE, Mir BA, Yusuf M, Ahmad A (2013a) 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Assess 185:7845–7856. https://doi.org/10.1007/s10661-013-3139-x
Fariduddin Q, Mir BA, Yusuf M, Ahmad A (2013b) Comparative roles of brassinosteroids and polyamines in salt stress tolerance. Acta Physiol Plant 35:2037–2053. https://doi.org/10.1007/s11738-013-1263-4
Fedina EO (2013) Effect of 24-epibrassinolide on pea protein tyrosine phosphorylation after salinity action. Russ J Plant Physiol 60:351–358. https://doi.org/10.1134/S1021443713020088
Feng RJ, Ren MY, Lu LF, Peng M, Guan X, Zhou DB, Zhang MY, Qi DF, Li K, Tang W, Yun TY, Chen YF, Wang F, Zhang D, Shen Q, Liang P, Zhang YD, Xie JH (2019) Involvement of abscisic acid-responsive element-binding factors in cassava (Manihot esculenta) dehydration stress response. Sci Rep 9:12661. https://doi.org/10.1038/s41598-019-49083-3
Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A (1997) The Arabidopsis deetiolated 2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell 9:1951–1962. https://doi.org/10.1105/tpc.9.11.1951
Gao W, Long L, Zhu LF, Xu L, Gao WH, Sun LQ, Liu LL, Zhang XL (2013) Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol Cell Proteomics 12:3690–3703. https://doi.org/10.1074/mcp.M113.031013
Getnet Z, Husen A, Fetene M, Yemata G (2015) Growth, water status, physiological, biochemical and yield response of stay green sorghum {Sorghum bicolor (L.) Moench} varieties - a field trial under drought-prone area in Amhara regional state, Ethiopia. J Agron 14:188–202. https://doi.org/10.3923/ja.2015.188.202
Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19:623–630. https://doi.org/10.1016/j.tplants.2014.06.013
Gomes MMA, Netto AT, Campostrini E, Bressan-Smith R, Zullo MAT, Ferraz TM, Siqueira LN, Leal NR, Núñez-Vázquez M (2013) Brassinosteroid analogue affects the senescence in two papaya genotypes submitted to drought stress. Theor Exp Plant Phys 25:186–195
González-Olmedo JL, Cordova A, Aragon CE, Pina D, Rivas M, Rodrıguez R (2005) Effect of an analogue of brassinosteroid on FHIA-18 plantlets exposed to thermal stress. Infomusa 14:18–20
Gudesblat GE, Schneider-Pizoń J, Betti C, Mayerhofer J, Vanhoutte I, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, Russinova E (2012) SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat Cell Biol 14:548–554. https://doi.org/10.1038/ncb2471
Guedes FRCM, Maia CF, Silva BRSD, Batista BL, Alyemeni MN, Ahmad P, Lobato AKDS (2021) Exogenous 24-Epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: central roles to minimize Pb content and oxidative stress. Environ Pollut 280:116992. https://doi.org/10.1016/j.envpol.2021.116992
Guiboileau A, Avila-Ospina L, Yoshimoto K, Soulay F, Azzopardi M, Marmagne A, Lothier J, Masclaux-Daubresse C (2013) Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. New Phytol 199:683–694. https://doi.org/10.1111/nph.12307
Guo H, Li L, Aluru M, Aluru S, Yin Y (2013) Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol 16:545–553. https://doi.org/10.1016/j.pbi.2013.08.002
Hamilton AJ, Lycett GW, Grierson D (1990) Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346:284–287. https://doi.org/10.1038/346284a0
Hasan SA, Hayat S, Ahmad A (2011) Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 84:1446–1451. https://doi.org/10.1016/j.chemosphere.2011.04.047
Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ Pollut 151:60–66. https://doi.org/10.1016/j.envpol.2007.03.006
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41. https://doi.org/10.1016/j.envexpbot.2006.06.002
Hayat S, Hasan SA, Hayat Q, Ahmad A (2010a) Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma 239:3–14. https://doi.org/10.1007/s00709-009-0075-2
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010b) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112 doi: 10.1016/j.envexpbot.2010.03.004
Hayat S, Yadav S, Ali B, Ahmad A (2010c) Interactive effect of nitric oxide and brassinosteroids on photosynthesis and the antioxidant system of Lycopersicon esculentum. Russ J Plant Physiol 57:212–221. https://doi.org/10.1134/S1021443710020081
Hayat S, Alyemeni M, Hasan S (2012) Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J Biol Sci 19:325–335. https://doi.org/10.1016/j.sjbs.2012.03.005
He RY, Wang GJ, Wang XS (1991) Effect of brassinolide on growth and chilling resistance of maize seedlings, In: Cutler HG, Yokota T, Adam G(Eds), Brassinosteroids: Chemistry, Bioactivity and Applications, American Chemical Society, Washington, DC, pp. 220–230
Heidari P, Mazloomi F, Nussbaumer T, Barcaccia G (2020) Insights into the SAM synthetase gene family and its roles in tomato seedlings under abiotic stresses and hormone treatments. Plants (Basel) 9:586. https://doi.org/10.3390/plants9050586
Hosseinpour M, Ebadi A, Habibi H, Nabizadeh E, Jahanbakhsh S (2020) Enhancing enzymatic and nonenzymatic response of Echinacea purpurea by exogenous 24-epibrassinolide under drought stress. Ind Crop Prod 146:112045. https://doi.org/10.1016/j.indcrop.2019.112045
Hu X, Jiang M, Zhang J, Zhang A, Lin F, Tan M (2007) Calcium–calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol 173:27–38. https://doi.org/10.1111/j.1469-8137.2006.01888.x
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237. https://doi.org/10.1016/j.scienta.2012.11.012
Huang B, Chu CH, Chen SL, Juan HF, Chen YM (2006) A proteomics study of the mung bean epicotyl regulated by brassinosteroids under conditions of chilling stress. Cell Mol Biol Lett 11:264–278. https://doi.org/10.2478/s11658-006-0021-7
Husen A, Iqbal M, Aref IM (2016) IAA-induced alteration in growth and photosynthesis of pea (Pisum sativum L.) plants grown under salt stress. J Environ Biol 37:421–429
Husen A, Iqbal M, Aref IM (2017) Plant growth and foliar characteristics of faba bean (Vicia faba L.) as affected by indole-acetic acid under water-sufficient and water-deficient conditions. J Environ Biol 38:179–186
Husen A, Iqbal M, Aref IM (2014) Growth, water status and leaf characteristics of Brassica carinata under drought and rehydration conditions. Braz J Bot 37:217–227 doi:https://doi.org/10.1007/s40415-014-0066-1
Hussain MA, Fahad S, Sharif R, Jan MF, Mujtaba M, Ali Q, Ahmad A, Ahmad H, Amin N, Ajayo BS, Sun C, Gu L, Ahmad I, Jiang Z, Hou J (2020) Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul 92:141–156. https://doi.org/10.1007/s10725-020-00647-8
Hussain S, Khaliq A, Matloob A, Wahid MA, Afzal I (2013) Germination and growth response of three wheat cultivars to NaCl salinity. Soil Environ 32:36–43
Hwang H, Ryu H, Cho H (2021) Brassinosteroid signaling pathways interplaying with diverse signaling cues for crop enhancement. Agronomy 11:556. https://doi.org/10.3390/agronomy11030556
Iqbal N, Nazar R, Iqbal MRK, Masood A, Nafees AK (2011) Role of gibberellins in regulation of source sink relations under optimal and limiting environmental conditions. Curr Sci 100:998–1007
Iqbal N, Umar S, Khan NA, Khan MIR (2014) A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot 100:34–42. https://doi.org/10.1016/j.envexpbot.2013.12.006
Janeczko A, Koscielniak J, Pilipowicz M, Szarek-Lukaszewska G, Skoczowski A (2005) Protection of winter rape photosystem 2 by 24-epibrassinolide under cadmium stress. Photosynthetica 43:293–298. https://doi.org/10.1007/s11099-005-0048-4
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358. https://doi.org/10.1007/s10535-007-0072-2
Jeandroz S, Lamotte O (2017) Editorial: plant responses to biotic and abiotic stresses: lessons from cell signaling. Front Plant Sci 8:1772. https://doi.org/10.3389/fpls.2017.01772
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen Z, Yu JQ (2012) Cellular glutathione redox homeostasis plays an important role in the brassinosteroids induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943. https://doi.org/10.1111/j.1469-8137.2012.04111.x
Jiang YP, Huang LF, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2013) Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol Plant 148:133–145. https://doi.org/10.1111/j.1399-3054.2012.01696.x
Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroids confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225:353–364. https://doi.org/10.1007/s00425-006-0361-6
Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576. https://doi.org/10.1034/j.1399-3054.2002.1150411.x
Kang YY, Guo SR, Li J, Duan JJ (2009) Effect of root applied 24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regul 57:259–269. https://doi.org/10.1007/s10725-008-9344-x
Kanwar MK, Bhardwaj R, Arora P, Chowdhary SP, Sharma P, Kumar S (2012) Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere 86:41–49. https://doi.org/10.1016/j.chemosphere.2011.08.048
Karlidag H, Yildirim E, Turan M (2011) Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria x ananassa). Sci Hort 130:133–140. https://doi.org/10.1016/j.scienta.2011.06.025
Kaya C, Ashraf M, Wijaya L, Ahmad P (2019) The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol Biochem143:119–128 doi: https://doi.org/10.1016/j.plaphy.2019.08.024
Khorasaninejad S, Mousavi A, Soltanloo H, Hemmati K, Khalighi A (2010) The effect of salinity stress on growth parameters, essential oil yield andconstituent of peppermint (Mentha piperita L.). World ApplSci J 11:1403–1407
Kim TW, Michniewicz M, Bergmann DC, Wang ZY (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482:419–422. https://doi.org/10.1038/nature10794
Kim J, Liu Y, Zhang X, Zhao B, Childs KL (2016) Analysis of salt-induced physiological and proline changes in 46 switchgrass (Panicum virgatum) lines indicates multiple response modes. Plant Physiol Biochem 105:203–212. https://doi.org/10.1016/j.plaphy.2016.04.020
Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH (1998) The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10:1677–1690. https://doi.org/10.1105/tpc.10.10.1677
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59. https://doi.org/10.1146/annurev-genet-110410-132507
Koca N, Karaman S (2015) The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem 166:515–521. https://doi.org/10.1016/j.foodchem.2014.06.065
Kong Q, Mostafa HHA, Yang W, Wang J, Nuerawuti M, Wang Y, Song J, Zhang X, Ma L, Wang H, Li X (2021) Comparative transcriptome profiling reveals that brassinosteroid-mediated lignification plays an important role in garlic adaption to salt stress. Plant Physiol Biochem 158:34–42. https://doi.org/10.1016/j.plaphy.2020.11.033
Kothari A, Lachowiec J (2021) Roles of Brassinosteroids in mitigating heat stress damage in cereal crops. Int J Mol Sci 22:2706. https://doi.org/10.3390/ijms22052706
Kroutil M, Hejtmankova A, Lachman J (2010) Effect of spring wheat (Triticum aestivum L.) treatment with brassinosteroids on the content of cadmium and lead in plant aerial biomass and grain. Plant Soil Environ 56:43–50. https://doi.org/10.17221/148/2009-PSE
Kulaeva ON, Burkhanova EA, Fedina AB, Khokhlova VA, Bokebayeva GA, Vorbrodt HM, Adam G (1991) Effect of brassinosteroids on protein synthesis and plant-cell ultrastructure under stress conditions, in: Cutler HG, Yokota T, Adam G(Eds.), Brassinosteroids: chemistry, bioactivity and applications, American Chemical Society, Washington, DC, ACS symposium series pp. 141–155. https://doi.org/10.1021/bk-1991-0474.ch012
Kumar A, Kumar V, Dubey AK, Ansari MA, Narayan S, Meenakshi KS, Pandey V, Pande V, Sanyal I (2021) Chickpea glutaredoxin (CaGrx) gene mitigates drought and salinity stress by modulating the physiological performance and antioxidant defense mechanisms. Physiol Mol Biol Plants 27:923–944. https://doi.org/10.1007/s12298-021-00999-z
Kurepin LV, Qaderi MM, Back TG, Reid DM, Pharis RP (2008) A rapid effect of applied brassinolide on abscisic acid concentrations in Brassica napus leaf tissue subjected to short-term heat stress. Plant Growth Regul 55:165–167. https://doi.org/10.1007/s10725-008-9276-5
Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141:323–329 doi:https://doi.org/10.1104/pp.106.079004
Laxmi A, Paul LK, Peters JL, Khurana JP (2004) Arabidopsis constitutive photomorphogenic mutant bls1, displays altered brassinosteroid response and sugar sensitivity. Plant Mol Biol 56:185–201. https://doi.org/10.1007/s11103-004-2799-x
Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M, Ding ZJ, Friml J, Beeckman T, Sack F (2014) Auxin transport and activity regulate stomatal patterning and development. Nat Commun 5:3090. https://doi.org/10.1038/ncomms4090
Li L, van Staden J, Jager AK (1998) Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress. Plant Growth Regul 25:81–87. https://doi.org/10.1023/A:1010774725695
Li KR, Wang HH, Han G, Wang QJ, Fan J (2008) Effects of brassinolide on the survival, growth and drought resistance of Robinia pseudoacacia seedlings under water-stress. New Forest 35:255–266. https://doi.org/10.1007/s11056-007-9075-2
Li KR, Feng CH (2011) Effects of brassinolide on drought resistance of Xanthoceras sorbifolia seedlings under water stress. Acta Physiol Plant 33:1293–1300. https://doi.org/10.1007/s11738-010-0661-0
Li YH, Liu YJ, Xu XL, Jin M, An LZ, Zhang H (2012) Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. Biol Plant 56:192–196. https://doi.org/10.1007/s10535-012-0041-2
Li M, Ahammed GJ, Li C, Bao X, Yu J, Huang C, Yin H, Zhou J (2016) Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front Plant Sci 7:615. https://doi.org/10.3389/fpls.2016.00615
Li X, Wei JP, Ahammed GJ, Zhang L, Li Y, Yan P, Zhang LP, Han WY (2018) Brassinosteroids attenuate moderate high temperature-caused decline in tea quality by enhancing theanine biosynthesis in Camellia sinensis L. Front Plant Sci 9:1016. https://doi.org/10.3389/fpls.2018.01016
Liu H, Guo T, Zhu Y, Wang C, Kang G (2006) Effects of epi-brassinolide (epi-BR) application at anthesis on starch accumulation and activities of key enzymes in wheat grains. Acta Agron Sinica 32:924–930
Liu Y, Bassham DC (2012) Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol 63:215–237. https://doi.org/10.1146/annurev-arplant-042811-105441
Liu L, Jia C, Zhang M, Chen D, Chen S, Guo R, Wang Q (2014) Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnol J12:105–115. https://doi.org/10.1111/pbi.12121
Liu J, Yang R, Jian N, Wei L, Ye L, Wang R, Gao H, Zheng Q (2020a) Putrescine metabolism modulates the biphasic effects of brassinosteroids on canola and Arabidopsis salt tolerance. Plant Cell Environ 43:1348–1359. https://doi.org/10.1111/pce.13757
Liu J, Yang R, Jian N, Wei L, Ye L, Wang R, Gao H, Zheng Q (2020b) Putrescine metabolism modulates the biphasic effects of brassinosteroids on canola and Arabidopsis salt tolerance. Plant Cell Environ 43:1348–1359. https://doi.org/10.1111/pce.13757
Mahesh B, Parshavaneni B, Ramakrishna B, Rao SSR (2013) Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am J Plant Sci 4:2305–2313. https://doi.org/10.4236/ajps.2013.412285
Marshall RS, Vierstra RD (2018) Autophagy: the master of bulk and selective recycling. Annu Rev Plant Biol 69:173–208. https://doi.org/10.1146/annurev-arplant-042817-040606
Masood A, Shah NA, Zeeshan M, Abraham G (2006) Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Environ Exp Bot 58:216–222. https://doi.org/10.1016/j.envexpbot.2005.08.002
Matsoukas IG (2014) Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front Genet 5:218. https://doi.org/10.3389/fgene.2014.00218
Mazorra LM, Nunez M, Hechavarria M, Coll F, Sanchez-Blanco MJ (2002) Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant 45:593–596. https://doi.org/10.1023/A:1022390917656
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/s1360-1385(02)02312-9
Mousavi EA, Kalantari KM, Jafari SR (2009) Change of some osmolytes accumulation in water-stressed colza (Brassica napus L.) as affected by 24-epibrassinolide. Iranian J Sci Technol Transac A Sci 33:1–11
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Physiol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Müssig C, Biesgen C, Lisso J, Uwer U, Weiler EW, Altmann TA (2000) Novel stress inducible 12-oxophytodienoate reductase from Arabidopsis thaliana provides a potential link between brassinosteroid-action and jasmonic-acid synthesis. J Plant Physiol 157:143–152. https://doi.org/10.1016/S0176-1617(00)80184-4
Nikoleta-Kleio D, Theodoros D, Roussos PA (2020) Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci Hort 259:108812. https://doi.org/10.1016/j.scienta.2019.108812
Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA (2000) Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol 124:201–209 doi:https://doi.org/10.1104/pp.124.1.201
Nolan T, Chen J, Yin Y (2017) Cross-talk of brassinosteroid signaling in controlling growth and stress responses. Biochem J 474:2641–2661. https://doi.org/10.1042/BCJ20160633
Nolan TM, Vukasinovi N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32:295–318. https://doi.org/10.1105/tpc.19.00335
Nunez M, Mazzafera P, Mazorra LM, Siqueira WJ, Zullo MAT (2003) Influence of a brassinsteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47:67–70. https://doi.org/10.1023/A:1027380831429
Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254:437–439. https://doi.org/10.1126/science.1925603
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués N (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57. https://doi.org/10.1007/s00344-007-9030-7
Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, Koncz C, Lafos M, Shibata K, Yokota T, Sakata K, Szekeres M, Mizutani M (2006) C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18:3275–3288. https://doi.org/10.1105/tpc.106.045443
Özdemir F, Bor M, Demiral T, Turkan I (2004) Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211. https://doi.org/10.1023/B:GROW.0000026509.25995.13
Pereira YC, Rodrigues WS, Lima EJA, Santos LR, Silva MHL, Lobato AKS (2019) Brassinosteroids increase electron transport and photosynthesis in soybean plants under water deficit. Photosynthetica 57:181–191. https://doi.org/10.32615/ps.2019.029
Podlešáková K, Ugena L, Spíchal L, Doležal K, De Diego N (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48:53–65. https://doi.org/10.1016/j.nbt.2018.07.003
Qin G, Ma Z, Zhang L, Xing S, Hou X, Deng J, Liu J, Chen Z, Qu LJ, Gu H (2007) Arabidopsis AtBECLIN 1/AtAtg6/AtVps30 is essential for pollen germination and plant development. Cell Res 17:249–263. https://doi.org/10.1038/cr.2007.7
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237. https://doi.org/10.1016/j.scienta.2011.03.035
Rajewska I, Talarek M, Bajguz A (2016) Brassinosteroids and response of plants to heavy metals action. Front Plant Sci 7:629. https://doi.org/10.3389/fpls.2016.00629
Ramakrishna B, Rao SSR (2013) Preliminary studies on the involvement of glutathione metabolism and redox status against zinc toxicity in radish seedlings by 28-homobrassinolide. Environ Expt Bot 96:52–58. https://doi.org/10.1016/j.envexpbot.2013.08.003
Rattan A, Kapoor D, Kapoor N, Bhardwaj R, Sharma A (2020) Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10097-1
Rontein D, Basset G, Hanson AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metab Eng 4:49–56. https://doi.org/10.1006/mben.2001.0208
Ruley AT, Sharma NC, Sahi SV (2004) Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol Biochem 42:899–906. https://doi.org/10.1016/j.plaphy.2004.12.001
Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P (2016) Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci Rep 6:28298. https://doi.org/10.1038/srep28298
Sakurai A, Fujioka S (1997) Studies on biosynthesis of brassinosteroids. Biosci Biotechnol Biochem 61:757–762. https://doi.org/10.1271/bbb.61.757
Sarker U, Oba S (2018) Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep 8:16496. https://doi.org/10.1038/s41598-018-34944-0
Sarker U, Oba S (2020) The response of salinity stress-induced a. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front plant Sci 11:559876 doi:https://doi.org/10.3389/fpls.2020.559876
Sasse JM (1991) The case for brassinosteroids as endogenous plant hormones, In: Cutler HG, Yokota T, Adam G (eds) Brassinosteroids: chemistry, bioactivity and applications. ACS symposium series 474. American Chemical Society, Washington, DC pp. 158–166
Sasse J (2002) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288. https://doi.org/10.1007/s00344-003-0062-3
Savaliya DD, Mandavia CK, Mandavia MK (2013) Role of brassinolide on enzyme activities in groundnut under water deficit stress. Indian J Agric Biochem 26:92–96
Schröder F, Lisso J, Obata T, Erban A, Maximova E, Giavalisco P, Kopka J, Fernie AR, Willmitzer L, Müssig C (2014) Consequences of induced brassinosteroid deficiency in Arabidopsis leaves. BMC Plant Biol 14:309. https://doi.org/10.1186/s12870-014-0309-0
Serna M, Coll Y, Zapata PJ, Botella MA, Pretel MT, Amorós A (2015) A brassinosteroid analogue prevented the effect of salt stress on ethylene synthesis and polyamines in lettuce plants. Sci Hort 185:105–112. https://doi.org/10.1016/j.scienta.2015.01.005
Shahbaz M, Ashraf M (2007) Influence of exogenous application of brassinosteroids on growth and mineral nutrients of wheat (Triticum aestivum L.) under saline conditions. Pak J Bot 39:513–522
Shahbaz M, Ashraf M, Athar HUR (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64. https://doi.org/10.1007/s10725-008-9262-y
Shahid MA, Pervez MA, Balal RM, Mattson NS, Rashid A, Ahmad R, Ayyub CM, Abba T (2011) Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.). Aust J Crop Sci 5:500–510
Shahid MA, Balal RM, Pervez MA, Garcia-Sanchez F, Gimeno V, Abbas T, Mattson NS, Riaz A (2014) Treatment with 24-epibrassinolide mitigates NaCl-induced toxicity by enhancing carbohydrate metabolism, osmolyte accumulation, and antioxidant activity in Pisum sativum. Turk J Bot 38:511–525. https://doi.org/10.3906/bot-1304-45
Shang Q, Song S, Zhang Z, Guo S (2006) Exogenous brassinosteroid induced salt resistance of cucumber (Cucumis sativus L.) seedlings. Sci Agric Sinica 39:1872–1877
Sharma P, BhardwajR AN, Arora HK (2007) Effect of 28-homobrassinolide on growth, zinc metal uptake and antioxidative enzyme activities in Brassica juncea L. seedlings. Braz J Plant Physiol 19:203–210. https://doi.org/10.1590/S1677-04202007000300004
Sharma I, Pati PK, Bhardwaj R (2010) Regulation of growth and antioxidant enzyme activities by 28-homobrassinolide in seedlings of Raphanus sativus L. under cadmium stress. Indian J Biochem Biophys 47:172–177
Sharma I, Pati PK, Bhardwaj R (2011a) Effect of 28-homobrassinolide on antioxidant defence system in Raphanus sativus L. under chromium toxicity. Ecotoxicology 20:862–874. https://doi.org/10.1007/s10646-011-0650-0
Sharma I, Pati PK, Bhardwaj R (2011b) Effect of 24-epibrassinolide on oxidative stress markers induced by nickel-ion in Raphanus sativus L. Acta Physiol Plant 33:1723–1735. https://doi.org/10.1007/s11738-010-0709-1
Sharma I, Bhardwaj R, Pati PK (2013) Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pesticide Biochem Physiol 105:144–153. https://doi.org/10.1016/j.pestbp.2013.01.004
Sharma P, Kumar A, Bhardwaj R (2016) Plant steroidal hormone epibrassinolide regulate–heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ Exp Bot 122:1–9 doi: https://doi.org/10.1016/j.envexpbot.2015.08.005
Sheehy JE, Elmido A, Centeno G, Pablico P (2005) Searching for new plant for climate change. J Agric Meteorol 60:463–468. https://doi.org/10.2480/agrmet.463
Siddikee MA, Chauhan PS, Sa T (2012) Regulation of ethylene biosynthesis under salt stress in red pepper (Capsicum annuum L.) by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing halotolerant bacteria. J Plant Growth Regul 31:265–272. https://doi.org/10.1007/s00344-011-9236-6
Siddiqi KS, Husen A (2017) Plant response to strigolactones: current developments and emerging trends. Appl Soil Ecol 120:247–253. https://doi.org/10.1016/j.apsoil.2017.08.020
Siddiqi KS, Husen A (2019) Plant response to jasmonates: current developments and their role in changing environment. Bull Nat Res Cent 43:153. https://doi.org/10.1186/s42269-019-0195-6
Simonovicova M, Tamas L, Huttova J, Mistrik I (2004) Effect of aluminum on oxidative stress related enzymes activities in barley roots. Biol plant 48:261–266 ttps://doi.org/10.1023/B:BIOP.0000033454.95515.8a
Singh I, Shono M (2005) Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul 47:111–119. https://doi.org/10.1007/s10725-005-3252-0
Singh S, Prasad SM (2017) Effects of 28-homobrassinoloid on key physiological attributes of Solanum lycopersicum seedlings under cadmium stress: photosynthesis and nitrogen metabolism. Plant Growth Regul 82:161–173. https://doi.org/10.1007/s10725-017-0248-5
Sirhindi G, Kumar S, Bhardwaj R, Kumar M (2009) Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plants 15:335–341. https://doi.org/10.1007/s12298-009-0038-2
Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125. https://doi.org/10.1016/j.envexpbot.2015.09.010
Soares TFSN, Dias DCFDS, Oliveira AMS, Ribeiro DM, Dias LADS (2020) Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol Environ Saf 193:110296. https://doi.org/10.1016/j.ecoenv.2020.110296
Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M (2020) Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants 26:501–511. https://doi.org/10.1007/s12298-020-00765-7
Song S, Liu W, Guo S, Shang Q, Zhang Z (2006) Salt resistance and its mechanism of cucumber under effects of exogenous chemical activator. Yingyong Shengtai Xuebao 17:1871–1876
Song YL, Dong YJ, Tian XY, Kong J, Bai XY, Xu LL, He ZL (2016) Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. Biol Plant 60:1–14. https://doi.org/10.1007/s10535-016-0596-4
Sudo E, Itouga M, Yoshida-Hatanaka K, Ono Y, Sakakibara H (2008) Gene expression and sensitivity in response to copper stress in rice leaves. J Exp Bot 59:3465–3474. https://doi.org/10.1093/jxb/ern196
Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E, Patil S, Kim TW, Ji H, Wong WH, Rhee SY, Wang ZY (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19:765–777. https://doi.org/10.1016/j.devcel.2010.10.010
Swamy KN, Rao SSR (2009) Effect of 24-epibrassinolide on growth, photosynthesis, and essential oil content of Pelargonium graveolens (L.) Herit. Russ J Plant Physiol 56:616–620
Tabur S, Demir K (2009) Cytogenetic response of 24-epibrassinolide on the root meristem cells of barley seeds under salinity. Plant Growth Regul 58:119–123
Tadaiesky LBA, da Silva BRS, Batista BL, Lobato AKDS (2021) Brassinosteroids trigger tolerance to iron toxicity in rice. Physiol Plant 171:371–387. https://doi.org/10.1111/ppl.13230
Takeuchi Y, Omigawa Y, Ogasawara M, Yoneyyama K, Konnai M, Worsham AD (1995) Effects of brassinosteroids on conditioning and germination of clover broom rape (Orobanche minor) seeds. Plant Growth Regul 16:153–160. https://doi.org/10.1007/BF00029536
Talaat NB, Shawky BT (2013) 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant 35:729–740. https://doi.org/10.1007/s11738-012-1113-9
Tanaka K, Nakamura Y, Asami T, Yoshida S, Matsuo T, Okamoto S (2003) Physiological roles of brassinosteroids in early growth of Arabidopsis: brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J Plant Growth Regul 22:259–271. https://doi.org/10.1007/s00344-003-0119-3
Tanaka Y, Nose T, Jikumaru Y, Kamiya Y (2013) ABA inhibits entry into stomatal lineage development in Arabidopsis leaves. Plant J74:448–457. https://doi.org/10.1111/tpj.12136
Upreti KK, Murti GSR (2004) Effects of brassinosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. Biol Plant 48:407–411. https://doi.org/10.1023/B:BIOP.0000041094.13342.1b
Vardhini BV, Rao SSR (2003) Amelioration of osmotic stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul 41:25–31. https://doi.org/10.1023/A:1027303518467
Vardhini BV, Rao SSR (2005) Influence of brassinosteroids on seed germination and seedling growth of sorghum under water stress. Indian J Plant Physiol 10:381–385
Vardhini BV, Anjum NA (2015) Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci 2:67. https://doi.org/10.3389/fenvs.2014.00067
Vert G, Nemhauser JL, Geldner N, Hong F, Chory J (2005) Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol 21:177–201. https://doi.org/10.1146/annurev.cellbio.21.090704.151241
Wang ZY, Wang Q, Chong K, Wang F, Wang L, Bai M, Jia C (2006) The brassinosteroid signal transduction pathway. Cell Res 16:427–434. https://doi.org/10.1038/sj.cr.7310054
Wang B, Zhang J, Xia X, Zhang WH (2011) Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul 65:407–413. https://doi.org/10.1007/s10725-011-9595-9
Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46:701–724. https://doi.org/10.1146/annurev-genet-102209-163450
Wang W, Bai MY, Wang ZY (2014) The brassinosteroid signaling network - a paradigm of signal integration. Curr Opin Plant Biol 21:147–153. https://doi.org/10.1016/j.pbi.2014.07.012
Wang P, Sun X, Wang N, Tan DX, Ma F (2015a) Melatonin enhances the occurrence of autophagy induced by oxidative stress in Arabidopsis seedlings. J Pineal Res 58:479–489. https://doi.org/10.1111/jpi.12233
Wang Y, Cai S, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J (2015b) Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy 11:2033–2047. https://doi.org/10.1080/15548627.2015.1098798
Wang YT, Chen ZY, Jiang Y, Duan BB, Xi ZM (2019) Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci Hortic 256:108596 doi:https://doi.org/10.1016/j.scienta.2019.108596
Wang RR (2020) Chromosomal distribution of genes conferring tolerance to abiotic stresses versus that of genes controlling resistance to biotic stresses in plants. Int J Mol Sci 21:1820. https://doi.org/10.3390/ijms21051820
Wang SG, Zhao HH, Zhao LM, Gu CM, Na YG, Xie B, Cheng SH, Pan GJ (2020) Application of brassinolide alleviates cold stress at the booting stage of rice. J Integr Agri 19:975–987. https://doi.org/10.1016/S2095-3119(19)62639-0
Xing J, Wang Y, Yao Q, Zhang Y, Zhang M, Li Z (2021) Brassinosteroids modulate nitrogen physiological response and promote nitrogen uptake in maize (Zea mays L.). https://doi.org/10.1016/j.cj.2021.04.004
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139. https://doi.org/10.1046/j.1365-3040.2002.00782.x
Yang P, Wang Y, Li J, Bian Z (2019) Effects of brassinosteroids on photosynthetic performance and nitrogen metabolism in pepper seedlings under chilling stress. Agronomy 9:839. https://doi.org/10.3390/agronomy9120839
Yokota T, Arima M, Takahashi N (1982) Castasterone, a new phytosterol with plant-hormone potency, from chestnut insect gall. Tetrahedron Lett 23:1275–1278. https://doi.org/10.1016/S0040-4039(00)87081-1
Yoshimoto K (2012) Beginning to understand autophagy, an intracellular self-degradation system in plants. Plant Cell Physiol 53:1355–1365. https://doi.org/10.1093/pcp/pcs099
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143. https://doi.org/10.1093/jxb/erh124
Yuan GF, Jia C-G, Li Z, Sun B, Zhang L-P, Liu N, Wang QM (2010) Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci Hortic 126:103–108. https://doi.org/10.1016/j.scienta.2010.06.014
Yurchenko O, Kimberlin A, Mehling M, Koo AJ, Chapman KD, Mullen RT, Dyer JM (2018) Response of high leaf-oil Arabidopsis thaliana plant lines to biotic or abiotic stress. Plant Signal Behav 13:e1464361. https://doi.org/10.1080/15592324.2018.1464361
Yusuf M, Fariduddin Q, Hayat S, Hasan SA, Ahmad A (2011) Protective response of 28-homobrassinolide in cultivars of Triticum aestivum with different levels of nickel. Arch Environ Contam Toxicol 60:68–76. https://doi.org/10.1007/s00244-010-9535-0
Zafari M, Ebadi A, Sedghi M, Jahanbakhsh S, Miransari M (2020) Alleviating effect of 24- epibrassinolide on seed oil content and fatty acid composition under drought stress in safflower. J Food Comp Anal 92:103544. https://doi.org/10.1016/j.jfca.2020.103544
Zapata PJ, Botella MA, Petrel MT, Serrano M (2007) Responses of ethylenebiosynthesis to saline stress in seedlings of eight plant species. Plant Growth Regul 53:97–106. https://doi.org/10.1007/s10725-007-9207-x
Zeng H, Tang Q, Hua X (2010) Arabidopsis brassinosteroid mutants det2-1 andbin2-1 display altered salt tolerance. J Plant Growth Regul 29:44–52. https://doi.org/10.1007/s00344-009-9111-x
Zhai Y, Guo M, Wang H, Lu J, Liu J, Zhang C, Gong Z, Lu M (2016) Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front Plant Sci 7:131. https://doi.org/10.3389/fpls.2016.00131
Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agric Res 58:811–815. https://doi.org/10.1071/AR06253
Zhang MC, Zhai ZX, Tian XL, Duan LS, Li ZH (2008) Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul 56:257–264. https://doi.org/10.1007/s10725-008-9305-4
Zhang Y, He J (2010) Sugar-induced plant growth is dependent on brassinosteroids. Plant signal Behav10:e1082700 doi:https://doi.org/10.1080/15592324.2015.1082700
Zhang J, Zhang Y, Khan R, Wu X, Zhou L, Xu N, Du S, Ma X (2021) Exogenous application of brassinosteroids regulates tobacco leaf size and expansion via modulation of endogenous hormones content and gene expression. Physiol Mol Biol Plants 27:847–860. https://doi.org/10.1007/s12298-021-00971-x
Zhao Y, Liang ZY, Yang YJ (2013) Effects of exogenous brassinosteroid on cd tolerance in Solatium nigrum seedlings. Zhongguo Shengtai Nongye Xuebao 21:872–876
Zhou Y, Xia X, Yu G, Wang J, Wu J, Wang M, Yang Y, Shi K, Yu Y, Chen Z, Gan J, Yu J (2015) Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci Rep 5:9018. https://doi.org/10.1038/srep09018
Zhu T, Tan WR, Deng XG, Zheng T, Zhang DW, Lin HH (2015) Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Post Biol Technol 100:196–204. https://doi.org/10.1016/j.postharvbio.2014.09.016
Acknowledgments
The authors are thankful to the publishers for permission to adopt the figures in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqi, K.S., Husen, A. Significance of brassinosteroids and their derivatives in the development and protection of plants under abiotic stress. Biologia 76, 2837–2857 (2021). https://doi.org/10.1007/s11756-021-00853-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00853-3