Abstract
The effect of salinity (100 mM NaCl) on ethylene metabolism in the early phase of vegetative development of several plant species has been investigated. The effects of saline treatment on shoot and root growth, ranged in sensitivity with respect to species: pepper (Capsicum annum L. cv Pairal) > tomato (Lycopersicon esculentum Mill. cv Malpica) > broccoli (Brassica oleraceae L. var. Italica Plenk. cv Marathon F1) ≅ lettuce (Lactuca sativa var. longifolia Lam. cv Inverna) ≅ melon (Cucumis melo L. cv Ruano F1, Roche type) > bean (Phaseolus vulgaris L. cv. Gator Green 15) ≅ spinach (Spinacia oleracea L. cv Boeing) > beetroot (Beta vulgaris L. var. crassa (Alef.) J. Helm. cv Detroit). After saline treatment, ethylene production increased 4.2-fold in pepper shoots. Significant increases were also found in shoots of tomato, broccoli and bean. In contrast, salinity decreased shoot ethylene production rate in melon, spinach, and beetroot. In roots, the general effect of salinity was a decrease in ethylene production, especially in broccoli and bean, except in tomato root, in which a sharp increase in ethylene production occurred. In general, saline treatment increased total ACC concentration in both shoot and root in most of the plant species examined, which was related to plant sensitivity to salinity. For example, pepper shoot was the most sensitive to saline treatment, showing the highest fresh weight inhibition and the highest increase in total ACC concentration (8.5-fold), while, beetroot was less affected by salinity and showed no effect on total ACC concentration in response to saline treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of stresses, such as wounding, mechanical damage, water deficit, air pollution, nutrient deficiency, mineral toxicity and salinity, are known to increase ethylene production in plant tissues (Wang et al. 1990; Abeles et al. 1992; Morgan and Drew 1997). The term “stress ethylene” refers to the accelerated biosynthesis of this hormone induced by environmental or biological stresses (Abeles et al. 1992). The pathway of ethylene biosynthesis in higher plants starts with the conversion of methionine to S-adenosyl-methionine (SAM), which is converted to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC-synthase (ACS) and in turn, ACC is converted to ethylene by ACC-oxidase (ACO). It is known that both, ACS and ACO occur as a multigene family and each gene is regulated independently by specific stresses (Barry et al. 1996; Bleecker and Kende 2000; Ge et al. 2000). Moreover, SAM is also involved in some stress responses, since transcripts from its multiple gene family are differentially expressed in elicitor-mediated stress responses, including salt stress (Espartero et al. 1994). In addition, some of the very rapid effects of stress on ethylene biosynthesis may initially occur by activating an existing pool of ACS, through its phosphorylation (Felix et al. 1994). Finally, ACC may be conjugated to N-malonyl ACC (MACC) or 1-γ-l-glutamy-ACC (GACC), and their rapid synthesis may reduce the availability of ACC to be converted to ethylene (Bleecker and Kende 2000).
The effect of salt stress on ethylene production is still unclear. In Arabidopsis seedlings, subjected to two levels of salinity, ethylene production increased, in proportion to the concentration of NaCl applied (Hall and Smith 1995). Accordingly, in tomato and cucumber, increasing salt concentration in the rooting zone gradually increased leaf ethylene production rates, which appeared to promote leaf senescence (Feng and Barker 1992; Helmy et al. 1994), while in Carrizo citrange, increased ethylene production induced leaf abscission (Gómez-Cadenas et al. 1998, 2003; Arbona et al. 2003a). NaCl treatment has also been shown to increase ethylene synthesis in leaves of a number of rice cultivars, which was linked to the salt-sensitivity of the cultivars (Lutts et al. 1996). A similar rise in ethylene production was also found in salt-adapted callus of sunflower, in which ethylene production was related to stress tolerance (Alvarez et al. 2003). In contrast, salinity did not enhance ethylene production in maize (Cramer 1992). It remains unclear if the increased rate of ethylene production, as a consequence of salt stress, actually causes some of the symptoms of stress or induces acclimation processes (Petruzzelli et al. 2000).
In previous work, we have found that salinity induced an increase in ethylene biosynthesis in several cultivars of lettuce during the germination phase as well as in pepper, broccoli and beetroot, while a decrease was found in melon, spinach and tomato (Zapata et al. 2003, 2004). The aim of this paper was to evaluate the effect of salinity on ethylene metabolism in several plant species, with different sensitivity to salinity, in the early phase of vegetative development. This comparative study is intended to establish if modification in ethylene metabolism could be related to plant sensitivity to saline stress.
Materials and methods
Plant material and experimental conditions
Experiments were carried out with eight plant species: Spinach (Spinacia oleracea L. cv Boeing), lettuce (Lactuca sativa var. longifolia Lam. cv Inverna), melon (Cucumis melo L. cv Ruano F1, Roche type), pepper (Capsicum annum L. cv Pairal), broccoli (Brassica oleraceae L. var. Italica Plenk. cv Marathon F1), beetroot (Beta vulgaris L. var. crassa (Alef.) J. Helm. cv Detroit), bean (Phaseolus vulgaris L. cv. Gator Green 15) and tomato (Lycopersicon esculentum Mill. cv Malpica).
Seeds were sterilized with 5% (v/v) sodium hypochlorite solution for 5 min and washed thoroughly with distilled water. Seeds were germinated in trays containing vermiculite moistened with a solution of 0.5 mM Ca2SO4, covered with perforated aluminum paper and maintained in a germination chamber. Distilled water was added when necessary. The germination chamber was kept in the dark at a temperature of 20°C (for spinach, bean, lettuce, broccoli, and beetroot) and at 25°C (for melon, pepper, and tomato). After germination, when cotyledons had fully emerged (after 3 days tomato, broccoli and lettuce, and after 4 days spinach, bean, pepper, beetroot, and melon), the seedlings were transferred to a growth chamber programmed at 20/16°C for spinach, bean, lettuce, broccoli, and beetroot, and 28/22°C for melon, pepper and tomato. The light/dark cycle was 16/8 h, and the relative humidity was 60/80%. The photon flux density was 450 μmol m−2 s−1. After 10 days, the seedlings were transferred to 13-l pots containing aerated half strength Hoagland solution. The pH was adjusted daily to 5.5–6.0 and the volume of the solutions was maintained by the addition of deionized water. The nutrient solution was renewed every 3 days. Treatments were control (NaCl 1 mM) and a saline (NaCl 100 mM). Saline solution was progressively increased up to 33, 66 and 100 mM, on days 5, 6 and 7, respectively, in order to avoid osmotic shock. From day 7 after transplanting saline treatment was maintained to NaCl 100 mM. For each plant species 6 pots (40 × 50 cm) with 13 l of nutrient solution and 48 plants per pot were used (three replicates per treatment). On days 5 (before saline treatment), 8 and 13 after transplanting, five plants from each pot were taken, which were combined and divided into root and shoot, in order to have an homogenous sample of root and shoot from each replicate, in which fresh weight, respiration rate, ethylene production rate and free, conjugated and total ACC concentration were determined.
Analytical measurements
Fresh weight was recorded for shoots and roots of five plants for each replicate and expressed as g per plant. Results are the mean ± SE of three replicates, for each plant species and treatment.
Immediately after fresh weight determination, the shoots or roots of each replicate were placed in glass jars of 500 and 120 mL, respectively, which were hermetically sealed for 1 h with a rubber stopper, in order to quantify ethylene production and respiration rates at 20°C and under light conditions similar to those of the growth chamber. In order to avoid stress by dehydration, which might affect ethylene and/or respiration rates, shoots were placed inside the glass jar in a 20 ml glass containing distilled water (Arbona et al. 2003b) and roots on a sheet of Watman No. 1 filter paper moistened with 5 ml of distilled water (Zapata et al. 2003). Ethylene concentration was determined three times in each jars, by extracting three samples of 1 ml of the head space atmosphere, in which ethylene concentration was determined using a Hewlett Packard 5890 Series II gas chromatograph, equipped with a flame ionization detector, and a 3-m stainless steel column with an inner diameter of 3.5 mm containing activated alumina of 80/100 mesh. Column temperature was 90°C and injector and detector temperature 150°C. Ethylene production rate was expressed in nl g−1 h−1 and results are the mean ± SE of the three replicates (data of each replicate was the mean of three measurements) for each plant specie and treatment (n = 3). Additionally, three samples of 1 ml of head space atmosphere were taken to determine respiration rate, monitoring the CO2 concentration in a Shimadzu 14-A gas chromatograph with a thermal conductivity detector (TCD). Column temperature was 50°C. Respiration rate was expressed as μg g−1 h−1 and results are the mean ± SE of three replicates (n = 3) for each plant specie and treatment (data of each replicate were the mean of three measurements). Subsequently, shoots or roots of each replicate were frozen and ground in liquid N2, and used to determine free, conjugated and total ACC.
Total ACC (free and conjugated) was extracted as previously described (Zapata et al. 2004). Shoot or root tissue was macerated in a mortar with a pestle in 0.2 M trichloroacetic acid (1:3 w/v) and centrifuged at 7,000g for 10 min. The supernatant was used to determine free-ACC concentration by chemical conversion of ACC to ethylene. Conjugated ACC in the supernatant was hydrolyzed to free ACC with 2 N HCl, which was then quantified as described above. In both cases, repeated measurements were made in triplicate. A relative calibration procedure was used to determine the amount of ACC in samples using the standard curve of ACC from Sigma-Aldrich (Poole, England). Conjugated ACC was calculated as the difference between total ACC and free ACC. Results were expressed as nmol g−1 FW and are the mean ± SE of the three replicates (data of each replicate were the mean of three measurements).
Statistics
All data were statistically analyzed by ANOVA (Table 1) and a Student t-test was performed for each species to assess significant (P < 0.05) differences between the controls and the saline treatments.
Results
Fresh weight
Plant fresh weight 13 days after transplanting was significantly different among plant species (Table 1). Saline treated plants showed a significantly lower fresh shoot and root weights compared control plants, although the effect was higher in the shoot than in root for all species (Table 1). In addition, significant differences among species in fresh weight reduction were found (Fig. 1). Pepper was most affected by saline treatment, with reductions of 62% and 50% in fresh weight of shoots and roots, respectively, after 8 days of saline treatment. Tomato showed reductions of 49% and 46%. Broccoli, lettuce, melon, bean, and spinach showed saline reductions in shoot fresh weight around 40%, while root fresh weight ranged from 31% in broccoli to 11% in spinach. Beetroot was least affected by salinity, with a 20% reduction of shoot fresh weight and with no significant effect on root fresh weight. The influence of saline treatment, on reducing fresh weight, was apparent after only 3 days of treatment (day 8 after transplanting) in shoots of pepper and tomato (data not shown). In addition, in pepper, symptoms of saline toxicity, such as chlorosis in young leaves, were observed after 8 days of treatment.
Fresh weight of shoot and roots of seedlings grown under control and saline treated conditions 13 d after transplanting, when plants had been subject to 6 days with a full strength (100 mM) saline treatment. Results are the mean ± SE of three replicates of five plants (n = 3). Number on bars show increase or decrease in saline treated plant with respect to control ones
Respiration rate
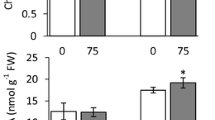
Respiration rates of shoots and roots of control plants remained unchanged or decreased during development, while the effect of saline treatment was more evident as the exposure period under saline conditions increased (data not shown). After 8 days of treatment respiration rate was significantly higher (Table 1) in tomato, broccoli and bean plants (450–470 μg CO2 g−1 h−1) than in the other plant species (210–290 μg CO2 g−1 h−1). The effect of saline treatment on respiration rate was different among species (Table 1) and between shoots and roots. Thus, in both the shoot and root of broccoli, bean and melon, respiration rate decreased as a consequence of saline treatment, while it increased in shoots and roots of lettuce. In tomato, shoots respiration rate was higher in control plants than in saline-treated shoots, while the opposite effect was found in roots. Respiration rate was not affected by salinity in pepper and spinach, either in the shoot or the root, however in beetroot no effect was observed in the shoot while a decrease occurred in roots (Fig. 2).
Ethylene production rate
Differences in ethylene production and ACC concentration between control and treated plants increased from day 3 to day 8 during saline treatment (data not shown). By day 8, shoot ethylene production rate in plants grown under control conditions was significantly different among species (Table 1), with values of 1.53 ± 0.14 nl g−1 h−1 in pepper; between 2.5 and 4.5 nl g−1 h−1 in tomato, broccoli, bean, spinach and beetroot; with the highest rate of 10–11 nl g−1 h−1, in lettuce and melon. However, the highest root ethylene production rates under control conditions were found in lettuce, broccoli and bean (around 13 nl g−1 h−1), with the lowest in pepper, spinach, melon, and beetroot (between 2 and 4 nl g−1 h−1). The effect of saline treatment on shoot and root ethylene production rate differed among species (Table 1). The saline treatment increased (4.2-fold) ethylene production rate in pepper shoots, while lower but significant increases were found in shoots of tomato, broccoli, and bean. However, salinity decreased shoot ethylene production rates in melon, spinach and beetroot. In roots, generally salinity reduced ethylene production, especially in broccoli and bean. Only in pepper root was there no significant effect of saline treatment. By contrast, in tomato root, a sharp increase in ethylene production was found under saline conditions (Fig. 3).
Ethylene production rate of shoot and roots of control and saline-treated plants 13 days after transplanting, when plants had been subject to 6 days of 100 mM saline treatment. Results are the mean ± SE of three replicates (n = 3). Number on bars show fold-increase or decrease in saline treated plant with respect to control ones
Free, conjugated and total ACC
The concentration of free ACC in shoots of plants grown under control conditions ranged from 0.27 ± 0.01 nmol g−1 FW in bean to 1.78 ± 0.17 nmol g−1 FW in broccoli, while in roots, generally, higher concentrations were found, ranging from 0.37 ± 0.03 nmol g−1 FW in melon to 3.18 ± 0.31 nmol g−1 FW in tomato (Fig. 4). The effect of saline treatment was dependent on species and plant organ (Table 1). Thus, in shoots of pepper, tomato, and broccoli significant increases of 8.8-, 2.7-, and 9.3-fold, respectively, were found in saline plants when compared to their controls, while in spinach shoot free ACC concentration decreased by 30%. No significant effect of saline treatment was found in the other species. The effect of saline treatment was lower in roots than in shoots, with only a 1.5-fold increase in roots of tomato and bean and a significant decrease in beetroot being observed.
Free ACC concentration in shoot and roots of control and saline-treated plants 13 days after transplanting, when plants had been subject to 6 days of 100 mM saline treatment. Results are the mean ± SE of three replicates (n = 3). Number on bars show fold-increase or decrease in saline treated plant with respect to control ones
The highest total ACC concentration under control conditions was found in tomato, with 6.34 ± 0.56 and 19.30 ± 0.69 nmol g−1 FW in shoot and root, respectively. In general, saline treatment induced a significant increase in total ACC concentration in shoots and roots of most species (Table 1), except in beetroot; the highest increases being found in the shoots of pepper, broccoli and tomato, with 8.5-, 7.7-, and 4.3-fold increases recorded, respectively, and in roots of broccoli, lettuce, and tomato, with 5.7-, 3.6-, and 2.8-fold increases, respectively. It was also apparent that under saline conditions the highest concentrations of total ACC were found in both shoots and roots of tomato, 27.54 ± 1.31 and 53.50 ± 4.08 nmol g−1 FW, respectively (Fig. 5). Tomato also contained the highest concentration of conjugated ACC, in both shoots and roots under control and saline conditions (Fig. 6), with the percentage of ACC in the conjugated form being >90–95%, while conjugated ACC was very low in broccoli (<3%), particularly in root tissues of control plants, although a sharp increase was found under saline conditions.
Total ACC concentration in shoot and roots of control and saline-treated plants 13 days after transplanting, when plants had been subject to 6 days of 100 mM saline treatment. Results are the mean ± SE of three replicates (n = 3). Number on bars show fold-increase or decrease in saline treated plant with respect to control ones
Conjugated ACC concentration in shoot and roots of control and saline-treated plants 13 days after transplanting, when plants had been subject to 6 days of 100 mM saline treatment. Results are the mean ± SE of three replicates (n = 3). Number on bars show fold-increase or decrease in saline treated plant with respect to control ones
Discussion
Salinity limits vegetative and reproductive growth of plants by inducing severe physiological dysfunction and causing widespread direct and indirect effects, such as reduced rate of leaf emergence, reduced rate of leaf and root elongation, reduced final leaf size and/or number of lateral shoots, altered flower time and reduced seed production (Shannon et al. 1994). In laboratory experiments involving salinity it is difficult to avoid giving plants an osmotic shock, with recovery being dependent on saline concentration (Munns 2002). However, Rodríguez et al. (1997) showed that saline treatment, at concentrations up to 100 mM NaCl had no osmotic effect on maize roots if saline concentration was increased gradually. In our study salinity was gradually increased from day 5 to day 7 after transplanting and then, the effect of saline treatment in the studied species was due to salt stress rather than to osmotic shock.
Salt tolerance is complex and difficult to quantify, as it varies with numerous environmental factors, such as soil type, fertility, method of irrigation and climate, as well as with various plant factors, such as species and cultivar, and growth stage (Kozlowski 1997). In this work, environmental conditions and saline treatments were similar for all species, and in turn the different effects of saline treatment among species were due to plant factors alone. Thus taking into account the effect of saline treatment on shoot and root growth, the species studied can be ranked from the most to the least saline sensitive as follows: pepper > tomato > broccoli ≅ lettuce ≅ melon > bean ≅ spinach > beetroot. However, during germination tomato was shown to be the most sensitive to saline conditions, followed by broccoli, lettuce and melon, while pepper, spinach and beetroot were only slightly affected by salinity (Zapata et al. 2004). Thus, the effect of saline stress in a particular species changes depending on its development stage, as has been shown in tomato (Foolad 1999), melon (Botia et al. 1998) and barley (Lynch et al. 1982).
Different responses in shoot and root respiration rate have been observed in different species. Shoot respiration rate decreased as a consequence of saline treatment in tomato, broccoli, melon, and bean. However, it has been shown that salinity increased respiration rate in shoots of pepino (Kai et al. 1999), alfalfa (Khavari-Nejad and Chaparzadeh 1998), and bean (Hamada and El-Enany 1994), as well as in wheat seedlings (Kasai et al. 1999). In roots, salinity has been shown to decrease respiration rate in citrange (Karstens et al. 1993), according to our results in broccoli, bean and beetroot, while an increase occurred in mango (Schmutz and Ludders 1999), Opuntia (Gersani et al. 1993) and wheat (Bouraoui et al. 1998), as well as in this experiment in tomato and lettuce. Therefore, it cannot be established a clear relationship between increase in respiration rate and plant saline sensitivity. Nevertheless, this effect can also depend on the exposure time to saline treatment. In this regard, it has been reported that respiration rate in peas was not affected after 2 days of saline treatment (Fedina et al. 1993) but increased with longer periods of exposure (Hamada and El-Enany 1994).
In general, saline treatment increased total ACC concentration in both shoots and roots in most species, which was related to plant sensitivity to salinity. Thus, in this experiment, pepper shoots were the most sensitive to saline treatment, showing the highest fresh weight reduction (even toxicity symptoms were observed), and the highest increase in total ACC concentration (8.5-fold). To the contrary, beetroot was the least affected by salinity and showed no effect on total ACC concentration with saline treatment. Thus, salinity increased ACS activity, the effect being correlated to plant saline sensitivity. Moreover, SAM-synthase has been reported to be also induced by salinity in tomato (Sánchez-Aguayo et al. 2004), which increases the pool of SAM to be converted into ACC and ethylene. Increases in ACC concentration have also been found in leaves of the saline-sensitive Troyer and Carrizo citrange; the increase being progressive over time and proportional to the magnitude of salt stress, while no changes were observed in the saline-tolerant Cleopatra mandarin (Bar et al. 1998; Gómez-Cadenas et al. 1998; Arbona et al. 2003a, b). Similar results have been found in wheat seedlings (Khan 2003) and petioles and roots of tomato; in the latter the effect being dependent on genotype and salinity concentration (El-Ikil et al. 2002). In these reports the increases in ACC concentration were followed by rises in ethylene production rate, as in the present study, in both shoots and roots of pepper and tomato. However, in broccoli, in spite of the large rise in total ACC concentration, in both shoots and roots, as a consequence of saline treatment (7.7- and 5.7-fold, respectively), only a slight increase in shoot ethylene production was found, while a sharp decrease occurred in root. Under saline conditions in broccoli shoots, 90% of the total ACC was free ACC and, therefore, an effect of saline treatment decreasing ACO activity might be responsible for low ethylene production. Accordingly, salinity has been shown to reduce ACO activity in alfalfa seedlings as well as the synthesis de novo of ACO through a negative regulation of ACO transcription (Li and Ni 2001). Thereafter, free ACC concentration in roots of broccoli was not affected by saline treatment, but the rise in total ACC was diverted to conjugated ACC, and then, the inhibition of ethylene production under saline conditions may be due to a stimulation of the MACC- and/or GACC-synthases (Bleecker and Kende 2000). This increase in the percentage of conjugated ACC might also be responsible for the low ethylene production under saline conditions in shoots of spinach and melon and roots of bean and melon.
In conclusion, it is proposed that saline stress increased total ACC concentration, the magnitude of this effect being dependent on plant sensitivity to salinity. However, other enzymes controlling ethylene biosynthesis, such as ACO and MACC- and GACC-synthases, may also be affected and in turn, ethylene increases as a consequence of saline treatments may not occur in some species with similar sensitivities to salinity. In addition, the increase in ACC concentration may occur initially in roots and then be transported to shoots. This transport would be rapid in pepper plant, since total ACC only accumulated in the shoot, while it was slower in tomato, broccoli and lettuce, in which a high accumulation of total ACC in roots was present 8 days after saline treatment.
References
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology, 2nd edn. Academic Press, San Diego
Alvarez I, Tomaro ML, Benavides MP (2003) Changes in polyamines, proline and ethylene in sunflower calluses treated with NaCl. Plant Cell Tissue Organ Cult 74:51–59
Arbona V, Flors V, Jacas J, García-Agustín P, Gómez-Cadenas A (2003a) Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive Citrus rootstock, to different levels of salinity. Plant Cell Physiol 44:388–394
Arbona V, Foó ML, Escrig P, Marco AJ, Jacas JA, Gomez-Cadenas A (2003b) Influence of abscisic acid and other plant growth regulators on citrus defense mechanism to salt stress. Spanish J Agric Res 1:59–65
Bar Y, Apelbaum A, Kafkafi U, Goren R (1998) Ethylene association with chloride stress in citrus plants. Sci Hort 73:99–109
Barry CS, Blume B, Bouzayenm M, Cooper W, Hamilton AJ, Grierson D (1996) Differential expression of the 1-amino-cyclopropane-1-carboxylate oxidase gene family of tomato. Plant J 9:525–535
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Ann Rev Cell Develop Biol 16:1–18
Botia P, Carvajal M, Cerdá A, Martínez V (1998) Response of eight Cucumis melo cultivars to salinity germination and early vegetative growth. Agronomie 18:503–513
Bouraoui N, Grignon C, Zid E (1998) Effect of NaCl on root growth and root respiration in triticale (Triticosecale Wittmack). Cahiers Agric 7:372–376
Cramer GR 1992 Kinetics of maize leaf elongation. III. Silver thiosulfate increases the yield threshold of salt-stressed plants, but ethylene is not involved. Plant Physiol 100:1044–1047
El-Iklil Y, Karrou M, Mrabet R, Benichou M (2002) Effets du stress salin sur la variation de certains métabolites chez Lycopersicon esculentum et Lycopersicon sheesmanii. Can J Plant Sci 82:177–183
Espartero J, Pintor-Toro JA, Pardo JM (1994) Differential accumulation of S-adenosylmethionine synthetase transcript in response to salt stress. Plant Mol Biol 25:217–227
Fedina IS, Tsonev TS, Guleva EI (1993) The effect of pre-treatment with proline on the responses of Pisum sativum to salt stress. Photosynthetica 29:521–527
Felix G, Regenass M, Spanu P, Boller T (1994) The protein phosphatase inhibitor calyculin A mimes elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with (33P)phosphate. Proc Natl Acad Sci 91:952–956
Feng J, Barker AV (1992) Ethylene evolution and ammonium accumulation by tomato plants under water and salinity stresses. Part. II. J Plant Nutr 15:2471–2490
Foolad MR (1999) Comparison of salt tolerance during seed germination and vegetative growth in tomato by QTL mapping. Genome 42:727–734
Ge L, Liu JZ, Wong WS, Hsiao WLW, Chong K, Xu ZK, Yang SF, Kung SD, Li N (2000) Identification of a novel multiple environmental factor-responsive 1-aminocyclopropane-1-carboxylate synthase gene, NT-ACS2, from tobacco. Plant Cell Environ 23:1169–1182
Gersani M, Graham EA, Nobel PS (1993) Growth responses of individual roots of Opuntia ficus-indica to salinity. Plant Cell Env 16:827–834
Gómez-Cadenas A, Tadeo FR, Primo-Millo E, Talón M (1998) Involvement of abscisic acid and ethylene in the responses of citrus seedlings to salt shock. Physiol Plant 103:475–484
Gómez-Cadenas A, Arbona V, Jacas J, Primo-Millo E, Talon M (2003) Abscisic acid reduces leaf abscission and increases salt tolerance in Citrus plants. J Plant Growth Regulat 21:234–240
Hall MA, Smith AR (1995) Ethylene and the response of plants to stress. Bulg J Plant Physiol 21:71–79
Hamada AM, El-Enany AE (1994) Effect of NaCl salinity on growth, pigment and mineral contents, and gas exchange of broad bean and pea plants. Biol Plant 36:75–81
Helmy YH, El-Abd SO, Abou-Hadid AF, El-Beltagy U, El-Betagy AS (1994) Ethylene production from tomato and cucumber plants under saline conditions. Egypt J Hortic 21:153–160
Kai C, Qian HG, Keutgen N, Janssens MJJ, Lenz F, Chen K, Hu GQ (1999) Effects of NaCl salinity and CO2 enrichment on pepino (Solanum muricatum Ait.). II. Leaf photosynthetic properties and gas exchange. Sci Hortic 81:43–56
Karstens GS, Ebert G, Ludders P (1993) Long-term and short-term effects of salinity on root respiration, photosynthesis and transpiration of Citrus rootstocks. Angewandte Botanik 67:1–2, 3–8
Kasai K, Mori N, Nakamura C (1999) Changes in the respiratory pathways during germination and early seedling growth of common wheat under normal and NaCl-stressed conditions. Cereal Res Comm 26:217–224
Khan NA (2003) NaCl-induced chlorophyll synthesis and associated changes in ethylene evolution and antioxidative enzyme activities in wheat. Biol Plant 47:437–440
Khavari-Nejad RA, Chaparzadeh N (1998) The effects of NaCl and CaCl2 on photosynthesis and growth of alfalfa plants. Photosynthetica 35:461–466
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol Monogr No 1:1–29
Li ZG, Ni JD (2001) Studies on inhibition mechanism of germination by ethylene in salt-stressed alfalfa seeds. Chin J Appl Environ Biol 7:24–28
Lutts S, Kinet JM, Bouharmont J (1996) Ethylene production by leaves of rice (Oryza sativa L.) in relation to salinity tolerance and exogenous putrescine application. Plant Sci 116:15–25
Lynch J, Epstein E, Läuchli A (1982) Na+/K+ relationship in salt-stressed barley. In: Scaife A (ed) Proceedings of the ninth international plant nutrition colloquium, Commonwealth Agricultural Bureau, Farnham Royal, Bucks, Warwick, England, pp 347–352
Morgan PW, Drew MC (1997) Ethylene and plant responses to stress. Physiol Plant 100:620–630
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Env, 25:239–250
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211:144–149
Rodríguez HG, Roberts JKM, Jordan WR, Drew MC (1997) Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiol 113:881–893
Sánchez-Aguayo I, Rodríguez-Galán JM, García R, Torreblanca J, Pardo JM (2004) Salt stress enhances xylem development and expression of S-adenosyl-l-methionine synthase in lignifying tissues of tomato plants. Planta 220:278–285
Schmutz U, Ludders P (1999) Physiological descriptors for salt stress susceptibility in Mangifera (mango) plant genetic resources. Plant Genetic Res Newslett 118:7–11
Shannon MC, Grieve CM, Francois LE (1994) Whole-plant response to salinity. In: Wilkinson RD (ed) Plant environment reaction. Marcel Dekker, New York, pp 199–244
Wang CY, Wang CY, Wellburn AR (1990) Role of ethylene under stress conditions. In: Alscher R, Cumming J (eds) Stress responses in plants: adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 147–173
Zapata PJ, Serrano M, Pretel MT, Amorós A, Botella MA (2003) Changes in ethylene evolution and polyamine profiles of seedlings of nine cultivars of Lactuca sativa L. in response to salt stress during germination. Plant Sci 164:557–563
Zapata PJ, Serrano M, Pretel MT, Amorós A, Botella MA (2004) Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci 167:781–788
Acknowledgements
This work was supported by the Conselleria de Agricultura, Pesca y Alimentación de la Generalitat Valenciana-Spain. Proyect GV-CAPA00-14. We thank Michael Jordan for the English correction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zapata, P.J., Botella, M.Á., Pretel, M.T. et al. Responses of ethylene biosynthesis to saline stress in seedlings of eight plant species. Plant Growth Regul 53, 97–106 (2007). https://doi.org/10.1007/s10725-007-9207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-007-9207-x