Abstract
Brassinosteroids (BRs) are a group of steroidal phytohormone that plays an essential role in regulating various chemical and physiological processes involved in plant growth and development. Furthermore, physiological and molecular studies of BRs have revealed their potential of enhancing yield and productivity of crops by regulating variety of genes. But as per current scenario, variety of abiotic and biotic environmental stresses acts as major constraints in yield and productivity of crops. Furthermore, BRs act as nontoxic, environmentally safe steroidal compounds that has the potential in modulating plant responses against abiotic and biotic stresses. When applied exogenously at specific dose and at particular developmental stage of plant, they are known to enhance both quality and quantity of the crop plants. Moreover, BRs are also known to have antifungal, antiviral, and anti-ecdysteroidal properties, which make them potential alternate of chemical fungicide, pesticide, and herbicides. Therefore, keeping in view all these properties of BRs, the current book chapter focuses on the role of BRs in modulating enzymatic and nonenzymatic antioxidant defense mechanism of plants under abiotic and biotic stress conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Various abiotic and biotic stresses elicit threatening impact on productivity and yield of crops. Various abiotic stresses include thermal (high and low temperature), water (drought and flooding), heavy metal toxicity, salinity, and UV radiation stresses, and the biotic stress includes pathogenic stress. All these stresses have contributed in converting arable lands to unproductive barren lands, thus ultimately resulting in economic loss in agricultural field (Ahanger et al. 2018). All these stresses affect germination, growth, and various physiological processes in plants, and moreover, it has been predicted that if the similar situation continues, there will be scarcity of staple crops for human population (which is growing at an alarming rate) by 2050 (Ahanger et al. 2014). Keeping in mind, all these changes, biologists are trying to meet all these challenges by using various environmentally stable strategies like the use of biological components, drainage, water management, etc. One such strategy is the application of phytohormones, since various phytohormones play an essential role in regulating the normal and developmental processes and are also involved in combatting the effects caused due to various biotic and abiotic stresses by modulating several signaling pathways to evoke plants responses.
Among all plant hormones, brassinosteroids (BRs), a group of steroidal hormones found in lower as well as higher plants, are involved in regulating various mechanisms involved in growth and developmental processes in plants (Liu et al. 2017). BRs act as an essential regulator involved in photosynthesis, antioxidant defense system, and plant-water relation under normal as well as stress conditions, thus ultimately regulating the growth and developmental processes under normal as well as stress conditions. Furthermore, in the case of plants having mutations in BR biosynthesis, abnormal developmental phenotypes are generated, thus confirming the potencies of BRs (Sahni et al. 2016). Moreover, BR-induced ameliorating stress responses have been reported in various plants that were exposed to thermal, water, heavy metal, and pathogen stress (Hayat et al. 2010; Singh et al. 2012; Talaat et al. 2015; Zhao et al. 2016; Jasrotia and Ohri 2017a). BRs are applied exogenously to plants and are reported to have ability of mitigating different stresses in concentration-dependent manner and also on the developmental stage of plants as well as on the treated plant organs (Bao et al. 2004). So, in the current book chapter, efforts have been made to examine the potential of BRs in modulating antioxidant defense in plants growing under stress conditions. Moreover, cross talks of BRs with other phytohormones have also been summarized here.
15.2 Plant Responses to Environmental Stresses
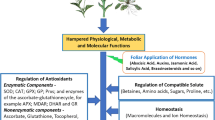
Plants respond to various environmental stresses which include abiotic factors like heavy metals, drought, wounding, salinity, changes in temperature and light, pesticides, and nutrient stress and biotic factors such as pest and pathogen attack (Gull et al. 2019). Abiotic and biotic stresses induce morphological, biochemical, molecular, and physiological changes in plants. Extreme temperature, salinity drought, and oxidative stress are often interconnected and may lead to similar cellular damage. For example, salinity and drought condition are primarily evidenced as osmotic stress, leading to disruption of ion homeostasis in the cells (Samynathan et al. 2021). Denaturation of structural and functional proteins is caused by oxidative stress, which often accompanies high temperature, drought, or salinity stress (Chaki et al. 2020). As a result, various environmental stresses often activate similar cellular responses and cell signaling pathways (Sewelam et al. 2016), such as accumulation of compatible solutes, upregulation of antioxidants, and production of stress proteins (Kosová et al. 2018; Dumont and Rivoal 2019; Hasanuzzaman et al. 2020). Plants also undergo certain biochemical adaptations which involve various changes in cell biochemistry. These changes include detoxification mechanism, synthesis of special proteins, evolution of new metabolic pathways, accumulation of the metabolites, and changes in phytohormone level (Fujita et al. 2006) (Fig. 15.1).
15.3 Biosynthesis of BRs
The pathway leading to the biosynthesis of BRs and different genes involved in BR biosynthesis has been identified in Arabidopsis as well as in rice and tomato (Divi and Krishna 2009). Initially, BR-biosynthetic pathway was established by feeding cultured cells of Catharanthus roseus and Arabidopsis seedlings with deuterium-labeled substrates followed by identification of various metabolites and reaction sequences involved in biosynthetic pathway by utilizing gas chromatography-mass spectrometry (GC-MS) (Choi et al. 1996; Fujioka et al. 2000; Noguchi et al. 2000). The precursor for the biosynthesis of brassinolide (BL), the most active BR, is campesterol (CR). Earlier, BRs were thought to be biosynthesized from two parallel pathways, namely, early C-6 and late C-6 oxidation pathways (Fujioka et al. 1998). According to these pathways, CR is first converted to campesterol (CN), then to castasterone (CS), and finally to BL (Fig. 15.2). In early C-6 oxidation pathway, CN is first converted to 6-oxocampestanol, then to cathasterone, teasterone, 3-dehydroteasterone, typhasterol, and then CS. In late C-6 oxidation pathway, CN is first hydroxylated at C-22 to form 6-deoxocathasterone and is then converted to corresponding intermediates as in early C-6 oxidation pathway but in C-6 deoxy forms. These two pathways ultimately converge at CS, which is eventually converted to BL (Zhao and Li 2012). Another branching pathway termed as CN-independent pathway, which is an early C-22 oxidation branch, has been reported (Fujioka et al. 2002). Recently, a shortcut route involving C-23 hydroxylation leading to the conversion of CR to 6-deoxytyphasterol has been described (Ohnishi et al. 2006). Experimental data on different plant species have revealed that the CN-independent and late C-6 oxidation pathways are the predominant BR-biosynthetic pathways (Zhao and Li 2012). Different genes involved in BR-biosynthesis are constitutive photomorphogenesis and dwarfism (CPD), de-etiolated-2 (DET2), and DWARF4 (DWF4) (Bartwal and Arora 2020). Constitutive expression of these genes can be modulated to regulate the endogenous levels of BR in plants (Fig. 15.2).
15.4 Role of BRs in Plant Growth and Development
BRs are steroidal phytohormones that are analogous to animal steroidal hormones. Mass spectrophotometric analysis such as UHPLC-ESI-MS/MS determined a total of around 22 natural BRs in a minute sample of plant tissue which exhibited a highly significant growth-promoting influence in plants (Tarkowska et al. 2016). They play imperative roles in divergent aspects of plant biology ranging from elongation and division of cell, root growth, photomorphogenesis, stomatal and vascular differentiation, seed germination, plant immunity, and its reproduction (Gudesblat and Russinova 2011; Vardhini and Anjum 2015; Wei and Li 2016) (Table 15.1). Besides, BRs regulate the production and oxidation of radicals and root gravitropic response and mediate plant responses to environmental cues (Krishna 2003; Bajguz and Hayat 2009; Vardhini 2019). Table 15.1 describes the physiological role of BRs in growth and development of different plant species.
15.5 Cross Talk of BRs with Other Plant Hormones
Several stress-responsive phytohormones act as a molecular regulatory element that assist sessile plants to maintain their growth plasticity and provide ability to adapt in tough environmental conditions. A cascade of interactions (occur mainly through phosphorylation/a common second messenger) that helps in regulating signaling network and persists among varied plant hormones which alter cellular dynamics is known as cross talk. This cross talk between phytohormones helps in revealing and targeting host resistance mechanisms under stress (Kohli et al. 2013; Wani et al. 2016; El-Esawi 2017; Li et al. 2021). On the basis of their action, phytohormones are grouped into two main categories: First group includes auxins (AUX), gibberellins (GA), brassinosteroids (BRs), strigolactones (SL), and cytokinins (CK) that coordinate during plant growth and development, while others play a vital role under environmental cues, namely, abscisic acid (ABA), brassinosteroids, ethylene (ET), jasmonic acid (JA), and salicylic acid (SA) (Pieterse et al. 2009; Santner et al. 2009; Denance et al. 2013; Fahad et al. 2015). Thus, BRs play a dual role in plants both under stress as well as during normal growth.
15.5.1 Interplay Between Brassinosteroids and Auxins
BRs and auxins are master hormones with coordinated effects on innumerable phases of plant growth and developmental pathways including the biosynthesis of BRs mediated by auxins (Yoshimitsu et al. 2011; Hao et al. 2013; Chaiwanon and Wang 2015). Physiological and genetic assays demonstrate opposite role of BR and AUX for controlling root growth by directing the expression of DWF4 (DWARF4) and BZR1 (BRASSINAZOLE-RESISTANT 1). On one hand, in roots where auxins enhance DWF4 expression, BRs suppress it through feedback mechanism (Yoshimitsu et al. 2011; Chaiwanon and Wang 2015). Also, during lateral root formation, BIN2 (BRASSINOSTEROID-INSENSITIVE2; a key component that mediate BR and auxin signaling during root development) plays an important role in auxin signaling, but BR signaling retards BIN2 activity which in turn couldn’t activate transcription factors BES1 (BRI1-EMS-SUPPRESSOR 1) and BZR1 leading to downstream control of plant growth and development. But BIN2 causes the phosphorylation of ARF7 and ARF19 (AUXIN RESPONSE FACTOR), thereby enhancing their DNA-binding capacity at lower levels of BR (He et al. 2002; Yin et al. 2002; Cho et al. 2014). In shoot elongation, brassinosteroid treatment downregulates transcription factor ARF genes ARF4 and ARF8 in Arabidopsis wild-type (WT) seedlings in contrast to increased level of expression in BR-deficient mutants (Jung et al. 2010); however, the overexpression of ARF8 could suppress growth of hypocotyl resulting into a weaker apical dominance (Tian et al. 2004; Peres et al. 2019) (Fig. 15.3). These outcomes clearly indicated an elaborated and a dynamic interaction of ARFs through BZR1 and BIN2 both transcriptionally and post-transcriptionally to regulate plant growth and its development via BR-auxin cross talk (Peres et al. 2019).
15.5.2 Interplay Between Brassinosteroids and Gibberellins
A cooperative and an interdependent relationship exists between BRs and GAs, with multiple layers that interact in a species, tissue, and in a dose-dependent manner. The studies revealed that the DELLA proteins (a key negative regulator of gibberellin signaling) have a suppressing effect on BZR1 transcriptional activity while interacting with BZR1/BES1 (Bai et al. 2012; Gallego-Bartolome et al. 2012; Li et al. 2012a; Peres et al. 2019). In Arabidopsis seedlings with lower levels of BR biosynthesis, hypocotyl elongation was promoted by GA or brassinazole treatment revealing the cooperative role of both hormones though it depends on the stage of growth, physiological conditions, and on the branched BR-regulated GA pathway (Gallego-Bartolome et al. 2012; Stewart Lilley et al. 2013; Unterholzner et al. 2015). BR influences GA biosynthesis not only in dicots but also in monocot plants as evident through the bioinformatics, chromatin immunoprecipitation (ChIP), and in vitro DNA binding studies, suggesting a direct binding of BZR1/BES1 to the target expression levels of two genes GA20ox, GA3ox, and GA2ox (encode enzymes in the rate-limiting step of GA production) from Arabidopsis and rice plants, respectively (Tong et al. 2014; Unterholzner et al. 2015; Peres et al. 2019). Thus, as per the postulates of the proposed model of BR-GA signaling involving the interaction between BZR1/BES1 and DELLA, BZR1/BES1 are activated post-translationally by BR to promote GA biosynthesis; further the escalated GA enhances DELLA degradation and releases the BZR1/BES1 activity. The stability of this proposed interaction is dependent upon the phosphorylation state of BZR1/BES1 proteins and the cellular localization of these processes (Ross and Quittenden 2016; Tong and Chu 2016; Unterholzner et al. 2016; Allen and Ptashnyk 2017) (Fig. 15.4). Additionally, the homeostasis between BR-GA is also affected during biotic stress where the biotic agents cause an interruption in the interplay between the hormones by producing hormonal mimicking signals for their own survival thereby disarming the immunity of their host (De Vleesschauwer et al. 2012; Peres et al. 2019).
15.5.3 Interplay Between Brassinosteroids and Cytokinins
An indirect cross talk exists between brassinosteroids and cytokinins to regulate growth and development of plants. During lateral root formation, auxin transport is employed, and at molecular level, BR induces the expression of PIN genes (auxin efflux carriers) required for the development of root primordium, while CK suppresses its establishment by downregulating the expression of PIN genes, thus disturbing the auxin accumulation (Bao et al. 2004; Benjamins and Scheres 2008; Vercruyssen et al. 2011). Enzymatic targets of BR-mediated responses such as isopentenyl transferases (IPTs) and CKXs (CK oxidases/dehydrogenases) are responsible for the biosynthesis of bioactive cytokinins as well as its inactivation respectively. For example, in Arabidopsis, CKX3 gene directs the breakdown of CKs, and its overexpression under PYK10 (a root-specific promoter) reduces the levels of CKs in roots, causing minimal leaf and root growth. Reversibly, ectopic expression of CKX3 and BRI1 showed synergistic elevation in the leaf and root growth of plants (Werner and Schmülling 2009; Werner et al. 2010; Vercruyssen et al. 2011). In the regulation of several stress responses, negative role of CKs has been observed stating the gain and loss of function of CKX and IPT. On one hand, the overexpression of CKX implicated a deficiency of CK along with an elevated tolerance for drought and salinity; the suppression of IPT resulted in reduced levels of bioactive CK with enhanced stress tolerance. This negative relation may further be attributed to much repression of CK signaling pathway and inducing ABA signaling marker genes (such as AIL1, COR47, RAB18, RD29B, and SAG29) during cross talk between ABA and CK where ABA demonstrated similar results of increased stress tolerance via exogenous application (Nishiyama et al. 2011, 2012; Peres et al. 2019). Alternately, the role of BR can also be seen in drought stress such as in transgenic rice where it depends upon the physiological state of plant. The transgenic lines with IPT driven by PSARK (a stress- and maturation-induced promoter) showed increased CK levels before the start of senescence and BR signaling genes (BRL3, BRI1, BH1, BIM1, and SERK1) and its biosynthesis genes [DWF5 and HYD1 (HYDRA1)] were also upregulated under/no stress (Peleg et al. 2011) (Fig. 15.5).
15.5.4 Interplay Between Brassinosteroids and Ethylene
Cross talk between brassinosteroid and ethylene suggested indirect controls of different facets of plant growth and development. On one side, BR negatively regulates shoot gravitropism, and ethylene promotes shoot gravitropic reorientation through the involvement of auxin signaling genes (Guo et al. 2008; Vandenbussche et al. 2013). This is mainly achieved by activating and inhibiting negative and positive auxin signaling genes such as AUX/IAA and ARF7 and AR F19, respectively. However, ethylene works antagonistically by enhancing ARF7 and ARF19 and suppresses AUX/IAA to control shoot gravitropic responses (Vandenbussche et al. 2013). In case of root gravitropic responses also, the two hormones interact in opposite ways where BR increases root gravitropism while ET retards it by revamping auxin transport in the BR and ET mutants (Buer et al. 2006; Kim et al. 2007; Vandenbussche et al. 2013). Besides it, exogenously applied BR increases ET production in Arabidopsis seedlings by upregulating the expression of its key gene ACS (1-aminocyclopropane-1-carboxylate synthase) and stability of its proteins (mainly ACS5, ACS6, and ASC9) during external and internal stimuli thereby adjusting the ethylene synthesis in the plant tissues (Hansen et al. 2009; Muday et al. 2012). During root cell elongation, BRs and ET interaction has been observed in the root hair as well as the non-hair cells. In the case of root hair cells, the targeted expression of BRI1 activates the cell elongation in all tissues; however, it is retarded in non-hair cells due to elevation in the expression of two ACS genes: ACS5 and ACS9. Consequently, ACS genes catalyze the rate-limiting step of ET synthesis by forming ACC (1-aminocyclopropane-1-carboxylate) that accumulates and enhances ethylene signaling, thereby inhibiting unidirectional cell expansion (Fridman et al. 2014; Zhu et al. 2016). On the contrary, synergistic relationship also exists between BR and ET in controlling hyponastic growth being employed by plants to cope the environmental strains. Here, ET is the main regulator and in turn is regulated by BR. C-23 hydroxylation of BR synthesis is mediated by ROT3 (ROTUNDIFOLIA3/CYP90C1), and any change in it impairs local cell expansion and inhibits BR synthesis which further lowers ethylene-induced upward leaf movement (Polko et al. 2013) (Fig. 15.6).
15.5.5 Interplay Between Brassinosteroids and Abscisic Acid
In plants, seed germination, root elongation, and even during stomatal closing, plants with defective BR signaling show enhanced sensitivity for ABA, thus showing antagonistic relationship between BRs and ABA (Steber and McCourt 2001; Zhang et al. 2009; Li et al. 2012b; Wang et al. 2020). During signaling process also, BR signaling opposes the ABA biosynthesis. This is evident through the removal of BSK5 (a positive regulator of BR signaling) which causes the induction of ABA3 and NCED3 (ABA biosynthesis-related genes) (Ha et al. 2016; Ha et al. 2018). Additionally, during BR-ABA cross talk, upstream of BIN2 kinase causes the downstream of BR receptor complex. In this, two negatively regulating ABA genes, ABI1 and ABI2, interact as well as dephosphorylate BIN2 (a negative regulator of BR signaling) to further regulate the phosphorylation of BES1. However, an in vitro ABA signal transduction mimicking showed that ABA through its receptors inhibit ABI2 which further promotes BIN2 phosphorylation (Zhang et al. 2009; Wang et al. 2018; Bulgakov and Avramenko 2020). Even under drought stress, BES1 impedes ABA induction of a drought-related transcription factor RD26 (RESPONSIVE TO DESICCATION 26) and it reciprocatively exhibits antagonism by modulating BES1-regulated transcription which hinders brassinosteroid-regulated growth (Chung et al. 2014; Ye et al. 2017). Moreover, early signaling of ABA is modified by BR as in the case of Arabidopsis, by directly affecting phosphorylation of the active ABA signaling participants such as SnRK2.2, SnRK2.3, and SnRK2.6. Here too, BIN2 kinase signaling acts as an interacting protein of SnRK2.2 causing its phosphorylation along with SnRK2.3 (Belin et al. 2006; Yoshida et al. 2010; Fujita et al. 2013; Cai et al. 2014) (Fig. 15.7).
15.5.6 Interplay Between Brassinosteroids and Salicylic Acid
The existence of the cross talk between BR and SA plays a key role in plants under a variety of environmental constraints. During biotic stress, though BR acts as an enhancer in vast range of disease resistance, this BR-mediated boosted resistance does not depend upon SA. However, the joint effect of BR and SAR (systemic acquired resistance) provides an additive protection against pathogens (Nakashita et al. 2003; Saini et al. 2015). Under biotic stress, APETALA2/ETHYLENE-RESPONSIVE FACTOR gene GhTINY2 is strongly enhanced. Its overexpression boost the plant’s tolerance, and its underexpression makes the plant susceptible to infection. This is mainly because of more SA accumulation and its signal transduction through WRKY51 (WRKY transcription factor 51). However, the overexpression of GhTINY2 retards growth, knockdown of genes induced by BRs, and upregulation of BR-repressed genes. This occurs because of its interaction with BZR1 along with restraining of the transcriptional activation of IAA19 (INDOLE-3-ACETIC ACID INDUCIBLE 19) (Xiao et al. 2021). NPR1 (NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1) regulate BR signaling genes; BIN2 and BZRI induces stress tolerance in plants, and the interplay between BR and SA may be due to the NPR1 gene which stimulates expression of the SA-related genes involved in plant defense (Divi et al. 2010; Ohri et al. 2015). Additionally, negative cross talk also exists between SA and BR signaling pathways that resulted in the immune-suppressive effect of BR. Moreover, the external application of BRs activated the master defense regulators of SA pathway such as NPR1 and OsWRKY45 (WRKY transcription factor) (De Vleesschauwer et al. 2012) (Fig. 15.8).
15.5.7 Interplay Between Brassinosteroids and Jasmonic Acid
BR modulates JA signaling and inhibits JA-dependent growth and plays a vital role in both abiotic/biotic stresses in plants. Induced OPR3 (encodes 12-oxophytodienoic acid reductase) jointly by BRs and JA signifies a potential integration node between BR action and JA synthesis (Zhang et al. 2009; Saini et al. 2015). In Arabidopsis, restoration of the sensitivity and hypersensitive reaction of JA was seen during a leaky mutation of DWF4 in coi1 mutant and a hypersensitive reaction in the wild type toward JA. But when BRs were applied exogenously, it mitigated root growth inhibition of JA because of downregulation of DWF4 in a COI1-dependent (CORONATINE INSENSITIVE1) manner by jasmonate (Ren et al. 2009; Jang et al. 2020). Jasmonate-induced anthocyanin accumulation is a hallmark of jasmonic acid-induced responses which reduces in BR-biosynthetic mutants, but the BR application helps plants accumulate anthocyanin. This occurs because of the minimal expression of MYB (transcription factor) genes PAP1 and PAP2 (PRODUCTION OF ANTHOCYANIN PIGMENT1) (Peng et al. 2011; Song et al. 2011; Wasternack and Hause 2013). In rice, thionin genes known for encoding antimicrobial peptides were greatly induced by JA but were enhanced by BR during stress (Kitanaga et al. 2006). Against insect herbivory also, BR-JA cross talk showed the defensive role of JA for anti-herbivory, while BR impedes it (Campos et al. 2009). In Oryza sativa, exogenous application of JA decreased expression of BR signaling gene, OSBRI1 and BR biosynthesis gene, and OsDWF4, during nematode infection revealing antagonistic interplay between JA and BR (Nahar et al. 2013) (Fig. 15.9).
15.5.8 Interplay Between Brassinosteroids and Strigolactones
Cross talk between BR and SL is still in its budding stage, and the inter-hormonal interaction pathways have been demonstrated recently (Faizan et al. 2020). Both BRs and SL help in regulating shoot branching in plants. This is achieved through the SL’s key signaling component E3 ubiquitin ligase MAX2 (a shoot branching inhibitor) that continuously interacts with BR’s transcription factors BZR1 and BRI1 EMS SUPPRESOR1 (BES1) by binding directly with them and causing their degradation. However, MAX2-mediated degradation of BR transcription factors is promoted by SL signaling resulting in suppressed shoot branching (Yin et al. 2002; He et al. 2005; Kim and Wang 2010; Wang et al. 2013). However, this has been contradicted in bes1-D mutant, where the role of BES1 was re-examined. The chosen phenotype with enhanced shoot branching does not show any association with the characteristic bes1-D leaf phenotype, thereby advocating that the branching defect described earlier might be wrongly referred to as a mutation in BES1 only (Bennett et al. 2016). Further, it is expected that advances in the studies of this new class of phytohormone will help in explaining the key underline players of the hormonal cross talk between BRs and SLs (Fig. 15.10).
15.6 BR-Mediated Modulation of Plant Antioxidant Defense System Under Abiotic Stress
Reactive oxygen species (ROS) are key regulatory and signaling molecules that play important role in plant growth and development. ROS, such as superoxide radical (O2..), hydrogen peroxide (H2O2), and hydroxyl radical (.OH) production, elevates when plant is exposed to certain stressed conditions. Brassinosteroids improve the scavenging mechanism of these ROS by modulating the antioxidative as well as non-antioxidative system, thus playing an essential role in plant stress tolerance (Table 15.2).
15.6.1 Thermal Stress
In present scenario, with the increase in global greenhouse effect, changes in climatic conditions lead to rise in temperature, which has become one of the major detrimental stresses amid of constantly fluctuating environmental factors (Luo and Lau 2019; Karwa et al. 2020). Transcriptomic studies reveal that thermal/heat stress causes downregulation of critical gene(s) involved in the synthesis of cell wall, carbon assimilation, transport and accumulation of starch, and many metabolic pathways (Kothari and Lachowiec 2021). Plants in the environment are inevitable to such conditions but undergo some series of mechanisms to cope up with increased temperature, namely, osmoprotectants, ion transporters, antioxidant system, late embryogenesis abundant (LEA) proteins, heat shock proteins, signaling messenger, and factors of transcriptional machinery (Rodríguez et al. 2005). Various reports are available which depict BR-induced heat tolerance in plants. Though the underlined mechanism activated by BR for providing thermal stress tolerance is still not so clear, but different studies suggest that a signaling cascade is initiated by BR application which activates and brings together the small polypeptides and proteins, such as heat shock proteins (HSPs) or stress-induced proteins, to alleviate stress conditions (Bhandari and Nailwal 2020). BRs are believable to act as immunomodulators, protecting plants from injuries of HT stress. Several reports showed that BRs increase the production of heat shock proteins (HSPs) under thermal stress, thereby protecting proteins against irreversible heat-induced damage by preventing denaturation and facilitating the refolding of damaged proteins (Chauhan et al. 2011). BRs elevate the activities of various enzymes involved in the ascorbate–glutathione (AsA-GSH) cycle and maintain the homeostatic redox potential during thermal stress in plants. BRs enhanced the expression of various genes encoding these enzymes (Zhang et al. 2014; Yadava et al. 2016; Kaur et al. 2018; Li et al. 2018; Surgun-Acar and Zemheri-Navruz 2019). Brassinosteroids alleviate the heat stress by regulating the glyoxylate and antioxidative system in the case of Ficus seedlings (Jin et al. 2015; Anwar et al. 2018). A study conducted by Sonjaron et al. (2018) revealed that 7,8-dihydro-8a-20-hydroxyecdysone (aDHECD), a mimic of brassinosteroid, improve the photosynthetic activity and carbohydrate content in rice seedlings under high temperature conditions. The mechanism of BR that contributes to plant heat stress (HS) tolerance is mediated by various essential complicated processes, namely, enhancing photosynthetic efficiency by maximizing the rate of carboxylation by Rubisco and improving the efficiency of PSII photochemistry; elevating photosynthetic pigments, stomatal conductivity, and membrane stability; activating antioxidant mechanisms; and maintaining redox homeostasis. Contrary to this, reduction in lipid peroxidation and production of ROS is observed (Hayat et al. 2010; Kaur et al. 2018; Kaya et al. 2019). Although a large number of studies demonstrated the heat stress-protective role of BR using exogenous applications, only a small number of studies are focused on molecular mechanism involved in heat stress tolerance (Ahammed et al. 2014; Zhou et al. 2014). It has been reported that a transient H2O2 production in the apoplast functions as a critical signal to mediate BR-induced heat stress tolerance in tomato (Zhou et al. 2014).
15.6.2 Heavy Metal Stress
Presently, heavy metals (HM) are regarded as major pollutants in the environment due to their toxic effect at very low concentration. “HM” is collective term, which applies to the group of metals and metalloids with greater atomic density than 4 g/cm3, or five times, greater than water (Hawkes 1997; Gjorgieva Ackova, 2018). A number of HMs include cobalt (Co), nickel (Ni), lead (Pb), silver (Ag), iron (Fe), cadmium (Cd), chromium (Cr), zinc (Zn), arsenic (As), and the platinum group elements that are present in the environment affecting all its living components (Nagajyoti et al., 2010). Plants being sessile are exposed to various stress conditions in the environment, and HM constitutes one of the major obstacles in growth and development of plants. Anthropogenic activities and improper use of fertilizers and pesticides, urbanization, industrialization, and fossil fuel combustion have led to tremendous increase in concentrations of various hazardous chemicals in agricultural soils (Chen et al. 2015; Zhao et al. 2018). Toxic effect of HMs varies according to plant species, their concentrations, and chemical nature. HM reduces the rate of photosynthesis and the required precursors for the process. Also, there is significant reduction in quality and quantity of yield cultivated in polluted area with high risk of chemical consumption by human population (Wu et al. 2017; Hasan et al. 2019).
Recently, BRs, as an alternate eco-friendly tool for improving heavy metal (HM) stress tolerance in plants, have gained momentum (Bücker-Neto et al. 2017). BRs are reported for assimilation and metabolizing capacity for these chemicals (Santos et al. 2018). Plant exposed to HMs showed varied responses at the morphological, cellular, and molecular levels, and to study the complex mechanism underlining the improved tolerance by application of BRs, various studies have been conducted by worldwide scientists showing ameliorating effect of BRs during HM stress (Nawaz et al. 2017; Santos et al. 2018; Sharma et al. 2018; Wu et al. 2019; Ahammed et al. 2020; Soares et al. 2020; Betti et al. 2021). Study conducted by Jakubowska and Janicka (2017) decipher the potent role of BRs to provide stress tolerance during cadmium stress in cucumber plants. It has been observed that the BR stress tolerance is induced by the induction of plasma membrane NADPH oxidase and H+ ATPase pump enzyme in cucumber during Cd stress. Similarly, BRs improve the tolerance against Cd in cowpea plants (Santos et al. 2018).
Exogenous application of EBL enhances the lead tolerance in both seeds and seedlings of Brassica juncea L. EBL alters the antioxidative enzyme activity and enhances the rate of ROS scavenging by their increased activity (Soares et al. 2020). Similar results have also been obtained by Wu and his coworkers (2019) in which EBL showed ameliorative effect against metalloid stress. Exogenously applied EBL (foliar spray) altered various enzymes and decreased the ROS level in wild Arabidopsis thaliana seedlings which improves stress tolerance against antimony. Similarly, EBL plays an important role in providing strength to rice plants against iron stress. Exogenous application of EBL can alter the light-capturing capacity and stomata conductance, increase thickness of epidermis of leaves, and maintain membrane integrity of leaves under Fe toxicity (Tadaiesky et al. 2021). Similarly, application of 28-Homobrassinolide improves the stress tolerance against Pb, Cd, and Zn and attenuates their toxic effect on growth and development of seedlings (Xu et al. 2019). Tolerance to high level of Zn has been reported in Solanum nigrum L by the foliar application of EBL, contributing in better physiological status and redox homeostasis in Zn-stressed seedlings (Sousa et al. 2020). BRs in combination with calcium play important role in amelioration of aluminum stress in plants (Ashraf et al. 2019). Improved root/shoot length and enhanced carotenoid, glutathione, ascorbic acid, and tocopherol content were reported with elevation in the expression catalase, peroxidase, glutathione reductase, and glutathione-S-transferase genes by EBL application during lead stress (Kohli et al. 2018). In conclusion, exogenous application of BR induce enhancement of tolerance to heavy metals is their involvement in substantial improvement in carbon metabolism, photosynthetic pigment content, antioxidative defense system, ROS scavenging capacity, glutathione content etc. (Choudhary et al. 2012; Rajewska et al. 2016). Though there are many reports available for stress tolerance properties of steroidal hormone (brassinosteroids), but there is still uncertainty about endogenous BR levels being modulated by exogenous BR under heavy metal stress.
15.6.3 Drought Stress
Water scarcity, which is one of the most deleterious of all environmental stresses, checks the growth of many crop varieties and declines the quality and quantity of crop production (Todorova et al. 2016). Severe drought stress conditions lead osmotic stress due to overproduction of reactive oxygen species (ROS) thereby reducing photosynthetic rate; revamping nitrogen and antioxidant metabolism, secondary metabolite accumulation, and mineral nutrition; and ultimately causing growth reduction in plants (Jatav et al. 2014; Ahanger et al. 2015; Ahanger et al. 2018). Various studies have reported that the exogenous application of plant growth regulators like BRs can mitigate the drought-induced adverse effects on the growth and metabolism of plants (Behnamnia 2015; Nawaz et al. 2015; Talaat et al. 2015; Ahanger et al. 2018). They enhance the antioxidative defense of plants to combat water deficiency. Exogenous application of BRs to plants suffering from drought stress causes reduction in H2O2 (hydrogen peroxide) and MDA (malondialdehyde) contents as a result of scavenging activities of antioxidative enzymes such as peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX) (Li and Feng 2011; Vayner et al. 2014; Nawaz et al. 2017). Foliar application of EBL (0.01 mg/L) can also improve the antioxidant activity and drought-induced inhibition of photosynthetic functioning in Capsicum annuum (Hu et al. 2013).
It has been observed that exogenously applied BRs increase the concentration of abscisic acid and negate the toxic effects of water stress on plants (Wang et al. 2019). Supplementation of BRs (24-EBL and 28-HBL) alleviates the toxic effects of polyethylene glycol-6000 (PEG)-induced drought stress by enhancing seed germination, seedling length, and biomass (fresh and dry weight) in Cajanus cajan by increasing abscisic acid, glycine betaine, and proline accumulation (Shahana et al. 2015). It has been observed that the application of EBL and HBL to drought-stressed pigeon pea significantly decreases H2O2 and MDA accumulation by increasing antioxidative activities of SOD, CAT, POD, APX, and GR (glutathione reductase) (Shahana et al. 2015). Earlier, both EBL and HBL treatments have been found to reverse the inhibitory effects of PEG-6000-induced water stress on radish seedlings by increasing seed germination and seedling growth associated with enhanced levels of nucleic acids and soluble proteins and decreased activities of RNase. They also maintained the membrane integrity by lowering lipid peroxidation and MDA content (Mahesh et al. 2013). Recently, Tanveer et al. (2019) discussed the potential role of EBL in improving drought stress tolerance in plants. EBL ameliorates the negative effects of water stress by increasing carbon assimilation rate, perpetuating balance between ROS and antioxidants, and accumulating solutes especially proline (Tanveer et al. 2019). It enhances photosynthesis and other leaf gas exchange traits by protecting the ultrastructure of photosynthetic pigment apparatus from degradation (Tanveer et al. 2019).
BRs mediated plant defense mechanism against oxidative stress by maintaining the expression of genes involved in encoding xyloglucan endotransglucosylase/hydrolases (XTHs) or by escalating the activity of H+-ATPase, sucrose synthase, and cellulose synthase (Clouse 2011; Nawaz et al. 2017). Application of BRs transmutes the expression of genes responsible for encoding both structural and regulatory proteins (Ahammed et al. 2020). It was studied that the overexpression of Arabidopsis BR biosynthetic gene DWF4 in Brassica napus increased seed yield, root biomass, and length and enhanced stress tolerance caused by dehydration (Sahni et al. 2016). Duan et al. (2017) cloned a BR biosynthetic gene, SoCYP85A1, from Spinacia oleracea and studied its effect on abiotic stress tolerance in tobacco. They found that overexpression of the cloned gene improved drought tolerance and resulted in longer primary root and more lateral roots in transgenic tobacco as compared to wild types by eliminating ROS and MDA accumulation and improving proline content along with antioxidant enzyme activities (Duan et al. 2017).
15.6.4 Salinity Stress
Salinity is a major abiotic stress that renders most of the agricultural lands as barren wastelands resulting in food scarcity. It affects the water absorption capacity of plants that leads to overall decrease in plant growth (Bartwal and Arora 2020). Salinity stress, often termed as physiological drought, negatively affects plant development and productivity by inducing osmotic and ionic imbalances (Ahanger and Agarwal 2017; Kaur et al. 2018; Ahammed et al. 2020). Salt stress-induced toxic effects on plants include osmotic stress, ionic toxicity, truncated nitrogen metabolism, increased production of ROS that leads to oxidative damage, retarded photosynthetic functioning, and hindrance in uptake and translocation of mineral nutrients (Ahmad et al. 2010; Iqbal et al. 2015; Ahanger and Agarwal 2017). It is well established that exogenous application of BRs can reverse the negative effects induced by saline conditions on growth and development of plants. Time and again various studies have reported the role of BRs in mitigating the toxic effects of salt stress in wide range of plants including A. thaliana, rapeseed (Brassica juncea), mustard (B. napus), eggplant (Solanum melongena), pepper (Capsicum annuum), cucumber (Cucumis sativus), maize (Zea mays), and common bean (Phaseolus vulgaris) (Yuan et al. 2012a; Yue et al. 2018; Ahammed et al. 2020).
BRs help plants to cope up with salt stress by regulating antioxidative defense system. They activate the antioxidative defense machinery by influencing or controlling the transcription and/or translation of specific genes to improve the oxidative stress tolerance potential of plants (Cheng et al. 2015; Fariduddin et al. 2014). In a study, it was found that 24-EBL application enhanced growth, soluble proteins, and antioxidant activities of Solanum tuberosum suffering from salinity stress (Khalid and Aftab 2016). In another study, it was observed that exogenous application of BR conferred tolerance and mitigated the negative effects of NaCl-induced salt stress in cucumber by improving growth, chlorophyll content, and photosynthetic efficiency; promoting the activities of antioxidative enzymes, namely, CAT, SOD, POD; and increasing proline content (Fariduddin et al. 2013). In the same plant NaCl-induced production of ROS like O2̄ and H2O2 was mitigated by the enhancement of antioxidative defense system by EBL application (Lu and Yang 2013). Supplementation of watermelon with 24-EBL increased its salt tolerance by enhancing CO2 accumulation and water use efficiency; BRs were suggested to promote photosynthesis through activation of photosynthetic enzymes like Rubisco under salt stress conditions (Cheng et al. 2015).
It is well documented that salinity stress reduces the percentage of seed germination and overall biomass of crops, but after treating stressed plants with brassinolide, an increase in germination rate as well as root and shoot length was seen, and nitrate reductase activity was also increased as compared to stressed plants (Lalotra et al. 2017). Apart from exogenous application of BRs, harmful effects of high salt content have been ameliorated, and stress tolerance has been improved by employing seed priming techniques (Ahanger et al. 2018). In an experiment Zhang and his co-workers pre-treated the seeds of Medicago sativa with EBL and then exposed them to saline conditions. They noticed that under the influence of EBL, seed germination increased and oxidative damage reduced by enhanced activities of antioxidant enzymes, namely, SOD, POD, and CAT (Zhang et al. 2007). It has been seen that BRs regulate DNA methylation that plays a pivotal role in salinity tolerance. In a study, cytosine DNA methylation was found to be decreased in Linum usitatissimum (flax) upon NaCl (150 mM) exposure; however, seed priming with 24-EBL induced total methylation and enhanced salt tolerance, suggesting its role in epigenetic modification under salinity stress (Amraee et al. 2019).
15.6.5 Other Major Abiotic Stress
Aside from the above discussed major abiotic stressors, BRs and related compounds can also play notable roles in plants to cope up with other abiotic stressors like pesticides, photoinhibition/light stress, nutrient stress, and water-logging/water-flooding stress (Ahammed et al. 2012b; Sharma et al. 2013, 2017; Xia et al. 2006; Ogweno et al. 2010; Ahanger et al. 2018; Janeczko et al. 2010; Kang et al. 2006, 2009; Liang and Liang 2009; Lu et al. 2006; Lu and Guo 2013). 24-Epibrassinolide can enhance the tolerance of Oryza sativa and Brassica juncea to stress generated by pesticide imidacloprid (IMI) by decreasing lipid peroxidation via enhanced activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APOX), guaiacol peroxidase (GPOX), glutathione reductase (GR), and monodehydroascorbate reductase (MDHAR), upregulating the expression of some genes like Fe-SOD, Mn-SOD, Cu/Zn-SOD, CAT, APOX, and GR (Sharma et al. 2013; Sharma et al. 2017). In Ca (NO3)2-exposed Cucumis sativus, EBL upregulated the ROS-scavenging metabolism of antioxidant enzymes and protected the photosynthetic membrane system (Yuan et al. 2012b). Mitigation of impacts caused by pyrene and phenanthrene toxicity in tomato has been observed as result of EBL-mediated decreased content of MDA and increased activity of CAT, APOX, GPOX, and GR (Ahammed et al. 2012a). Application of EBL decreased the lipid peroxidation and increased H2O2 metabolism via enhanced activity of GST and GSH content which were asserted to help Solanum tuberosum to counteract phenanthrene-accrued consequences (Ahammed et al. 2012b, c). In tomato exposed to phenanthrene and Cd co-contamination, decrease in lipid peroxidation and enhanced antioxidant defense system by EBL were reported by Ahammed et al. (2013a). Recently, Ahammed et al. (2013b) evidenced that EBL benefited the tomato to maintain photochemical quenching coefficient (Pq), quantum efficiency of PSII phytochemistry {(PS II)}, and photochemical efficiency of PSII (Fv/Fm) under polychlorinated biphenyls induced oxidative stress by enhancing the activities of antioxidant enzymes. Phytotoxicities of nine pesticides (abamectin, chlorpyrifos, Cuproxat, cyazofamid, Fluazifop-p-butyl, flusilazole, haloxyfop, imidacloprid, and paraquat) had been alleviated by pre-treating Cucumis sativus with EBL. EBL increased the CO2 assimilation capacity and antioxidant enzyme activity in C. sativus (Xia et al. 2006). Application of EBL under light stress in tomato (Lycopersicon esculentum) enhanced the activity SOD, CAT, APOX, and GPOX enzymes and benefited the plants to maintain photochemical quenching (qP), quantum efficiency of PSII (PS II), and net photosynthetic rate (Pn) by decreasing lipid peroxidation (Ogweno et al. 2010). Significant role of EBL in enhancing the activity of antioxidant enzymes was evidenced in plants exposed to chlorpyrifos (Xia et al. 2009). Similarly, application of 24-epiBL to Arachis hypogea mitigated the oxidative stress induced by Fe-deficiency and reduced ROS production by enhancing activity of nitrate reductase, antioxidant system, and osmolyte accumulation (Song et al. 2016). Exogenous application of EBL (soaking and foliar) reduced uptake of iron and sodium and increased uptake of magnesium, calcium, and potassium in wheat (Janeczko et al. 2010). Foliar spray of EBL in C. sativus mitigated toxic effects of excess calcium on the uptake of necessary mineral elements such as potassium, magnesium, phosphorus, and manganese (Yuan et al. 2015). BRs and related compound were evidenced to furnish tolerance to water-logging/water-flooding stress to different crops such as oilseed rape (Liang and Liang 2009), soybean (Lu et al. 2006), and cucumber (Kang et al. 2006, 2009; Lu and Guo 2013) mainly by decreasing oxidative damage via increased activities of SOD and POD (peroxidase).
15.7 BR-Mediated Modulation of Plant Antioxidant Defense System Under Biotic Stress
Under natural conditions, plants are exposed to both abiotic stress (discussed above) and biotic stress (viruses, bacteria, fungi, insects, nematodes, parasites, and weeds). Plants use inducible defense mechanism to effectively tolerate different types of stress. Induced defense mechanism of plants against biotic stress is similar to defense induced against abiotic stress (Anwar et al. 2018). BRs and related compound not only help the plants to cope up with abiotic stresses but also play an important role to enhance the tolerance against biotic stresses (Krishna 2003; Ali et al. 2007; Jager et al. 2008; Bajguz and Hayat 2009; Nawaz et al. 2017) (Table 15.3). They involve complex signaling cascade to positively regulate antioxidant defense metabolism (Belkhadir et al. 2012) and induce innate immune response to protect the cells from different biotic stresses (Wang et al. 2012). Application of brassinolide (BL) in A. thaliana infected with cucumber mosaic virus (CMV) increased the activity of antioxidant enzymes like CAT, SOD, POD, and APOX; decreased photosystem damage; and modulated expression of genes related to defense (Zhang et al. 2015). Growth and activity of antioxidant enzymes in tomato also enhanced by 28-homobrassinolide (HBL) application and resulted in reduced oxidative stress caused by nematodes (Kaur et al. 2013, 2014). In rice, induced systemic defense against nematode stress was stimulated by exogenous application of epibrassinolide (Nahar et al. 2013). At low temperature, management of Botrytis cinerea in postharvest grapes was reported by Liu et al. (2016) when EBL was applied exogenously. Similarly, Zhu et al. (2010) reported that exogenously applied brassinosteroid solution enhanced the activity of antioxidant enzymes like CAT, SOD, and POX and inhibited the development of blue mold decay caused by Penicillium expansum in harvested jujube fruit. Additionally, improved fruit quality and delayed senescence due to reduced ethylene production were observed suggesting that BR-mediated detain in fruit decay might be related with induction of disease resistance. Citrus unshiu fruit dipped in EBL manifested enhanced resistance against citrus disease due to increased activity of stress-related metabolites (Zhu et al. 2015). Reduced susceptibility against leaf blight and rice blast diseases in barley seedlings grown under hydroponic system containing EBL was also observed (Ali et al. 2014). Moreover, resistance in uzu barley lines against powdery mildew fungus Oidium sp., bacterium Pseudomonas syringae pv. Tabaci and tobacco mosaic virus (TMV) was also induced by EBL. Application of BL in tobacco and rice showed similar effects (Nakashita et al. 2003). Application of BR-containing extract of Lychnis viscaria seeds caused an enhanced resistance of tomato, cucumber, and tobacco to fungal and viral pathogens (Botrytis, Sphaerotheca fuliginea, and TMV, respectively) (Roth et al. 2000).
15.8 Conclusion
It is a well-established fact that different environmental stressors (biotic and abiotic) are responsible for negatively affecting the agricultural economy by directly reducing the productivity of different crop plants. Moreover, this decline in productivity is becoming more severe with passing times. Since, these stressors generate ROS in affected plants, this further leads to destructive effects on physiological and metabolic processes of plants. In order to overcome this ROS generation, various strategies have been developed, and one such strategy is the application of plant growth regulators, which can act as a feasible environmentally safe alternative. In this continuation phytohormones like BRs and its associated components have been reported to induce antioxidant defense system of plants under different stressful conditions. Furthermore, BRs have also the efficiency of interacting with other phytohormones under normal and stressed conditions thus making them more potent for the resistance in plants against different environmental stresses.
References
Abbas S, Latif HH, Elsherbiny EA (2013) Effect of 24-epibrassinolide on the physiological and genetic changes on two varieties of pepper under salt stress conditions. Pak J Bot 45(4):1273–1284
Ahammed GJ, Yuan HL, Ogweno JO, Zhou YH, Xia XJ, Mao, WH, Shi K, Yu, J. Q (2012a) Brassinosteroid alleviates phenanthrene and pyrene phytotoxicity by increasing detoxification activity and photosynthesis in tomato. Chemosphere 86(5):546–555
Ahammed GJ, Zhang S, Shi K, Zhou YH, Yu JQ (2012b) Brassinosteroid improves seed germination and early development of tomato seedling under phenanthrene stress. Plant Growth Regul 68(1):87–96
Ahammed GJ, Gao CJ, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2012c) Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotocol Environ Safe 80:28–36
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013a) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64(1):199–213
Ahammed GJ, Ruan YP, Zhou J, Xia XJ, Shi K, Zhou YH, Yu JQ (2013b) Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 90(11):2645–2653
Ahammed G, Xia X, Li X, Shi K, Yu J, Zhou Y (2014) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16(5):462–473
Ahammed GJ, Li X, Chen S (2020) Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul 39:1451–1464
Ahanger MA, Tyagi SR, Wani MR, Ahmad P (2014) Drought tolerance: roles of organic osmolytes, growth regulators and mineral nutrients. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment. Volume I. Springer, New York, pp 25–25
Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M (2015) Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J Plant Int 10:211–223
Ahanger MA, Agarwal RM (2017) Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol Biochem 115:449–460
Ahanger MA, Ashraf M, Bajguz A, Ahmad P (2018) Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J Plant Growth Regul 37:1007–1024
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Ibanez SG, Chinchilla D, Rathjen JP, de-Vries SC, Zipfel C (2012) Brassinosteroids inhibit pathogen-associated molecular pattern–triggered immune signaling independent of the receptor kinase BAK1. Plant Bio 109:303–308
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot 59(2):217–223
Ali SS, Kumar GS, Khan M, Doohan FM (2013) Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 103(12):1260–1267
Ali SS, Gunupuru LR, Kumar GS, Khan M, Scofield S, Nicholson P, Doohan FM (2014) Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol 14(1):1–15
Allen HR, Ptashnyk M (2017) Mathematical modelling and analysis of the brassinosteroid and gibberellin signaling pathways and their interactions. J Theor Biol 432:109–131
Alyemeni MN, Al-Quwaiz SM (2016) Effect of 28-homobrassinolide on the performance of sensitive and resistant varieties of Vigna radiata. Saudi J Biol Sci 23(6):698–705
Amraee L, Rahmani F, Abdollahi Mandoulakani B (2019) 24-Epibrassinolide alters DNA cytosine methylation of Linum usitatissimum L. under salinity stress. Plant Physiol Biochem 139:478–484
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y (2018) The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res 51:46
Ashraf S, Dixit S, Ramteke PW, Rizvi AZ (2019) Interactive role of brassinosteroids and calcium ameliorates in response to the aluminium toxicity in plants. Int J Trend Sci Res Dev 3:183–203
Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun T, Wang ZY (2012) Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol 14:810–817
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47(1):1–8
Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134:1624–1631
Bariş ÇÇ, Sağlam-Çağ S (2016) The effects of brassinosteroids on sequential leaf senescence occurring in Glycine max L. Int J Biotechnol Res 6(4):7–16
Bartwal A, Arora S (2020) Brassinosteroids: molecules with myriad roles. In: Merillon JM, Ramawat K (eds) Co-Evolution of secondary metabolites. Springer, New York
Basit F, Chen M, Ahmed T, Shahid M, Noman M, Liu J, An J, Hashem A, Fahad Al-Arjani AB, Alqarawi AA, Alsayed MF (2021) Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 10(7):1089
Behnamnia M (2015) Protective roles of brassinolide on tomato seedlings under drought stress. Int J Agri Crop Sci 8:552–559
Belin C, de Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141:1316–1327
Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J (2012) Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. PNAS 109(1):297–302
Belmonte M, Elhiti M, Ashihara H, Stasolla C (2011) Brassinolide-improved development of Brassica napus microspore-derived embryos is associated with increased activities of purine and pyrimidine salvage pathways. Planta 233:95–107
Benjamins R, Scheres B (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59:443–465
Bennett T, Liang Y, Seale M, Ward S, Müller D, Leyser O (2016) Strigolactone regulates shoot development through a core signaling pathway. Biol Open 5:1806–1820
Betti C, Della Rovere F, Piacentini D, Fattorini L, Falasca G, Altamura MM (2021) Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids. Biomol Ther 11(1):77
Bhandari S, Nailwal TK (2020) Role of brassinosteroids in mitigating abiotic stresses in plants. Biologia:1–28
Bücker-Neto L, Paiva AL, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140:1384–1396
Bukhari SAH, Wang R, Wang W, Ahmed IM, Zheng W, Cao F (2016) Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res 23:18229–18238
Bulgakov VP, Avramenko TV (2020) Linking Brassinosteroid and ABA signaling in the context of stress acclimation. Int J Mol Sci 21(14):5108
Cai Z, Liu J, Wang H, Yang C, Chen Y, Li Y, Pan S, Dong R, Tang G, Barajas-Lopez JD (2014) GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc Natl Acad Sci U S A 111:9651–9656
Campos ML, deAlmeida M, Rossi ML, Martinelli AP, Junior CGL, Figueira A et al (2009) Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J Exp Bot 60:4347–4361
Cao Y, Zhao H (2008) Protective roles of brassinolide on rice seedlings under high temperature stress. Rice Sci 15:63–68
Cardoso KPS, Silva Conceicao S, de Araujo Brito AE, da Silva Martins JT, Machado LC, Correa Costa T, dos Santos Nogueira GA, do Nascimento VR, da Silva RPP, Costa Paiva R, Correa Barbosa AV, Okumura RS, de Oliveira Neto CF (2019) Biochemical metabolism of two cultivars of cowpea treated with 24-Epibrassinolide and subjected to saline stress. Aust J Crop Sci 13(3):444–451
Carvalho RF, Monteiro CC, Caetano AC, Dourado MN, Gratão PL, Haddad CRB, Peres LEP, Azevedo RA (2013) Leaf senescence in tomato mutants as affected by irradiance and phytohormones. Biol Plant 57(4):749–757
Chaiwanon J, Wang ZY (2015) Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr Biol 25:1031–1042
Chaki M, Begara-Morales JC, Barroso JB (2020) Oxidative stress in plants. Antioxidants 9:481
Chakma SP, Chileshe SM, Thomas R, Krishna P (2021) Cotton seed priming with brassinosteroid promotes germination and seedling growth. Agron 11(3):566
Chauhan H, Khurana N, Agarwal P, Khurana P (2011) Heat shock factors in rice (Oryza sativa L.): genome-wide expression analysis during reproductive development and abiotic stress. Mol Genet Genomics 286(2):171–187
Chen BY, Wang CH, Chu QG, Tian YK, Sun JX, Xu YS (2014) Effect of exogenous brassinolide on growth and anatomical characteristics for stems and leaves of dwarf pear in vitro. Beifang Yuanyi:7–11
Chen C, Zhang H, Wang A, Lu M, Shen Z, Lian C (2015) Phenotypic plasticity accounts for most of the variation in leaf manganese concentrations in Phytolacca americana growing in manganese contaminated environments. Plant Soil 396(1–2):215–227
Cheng Y, Zhu W, Chen Y, Ito S, Asami T, Wang X (2014) Brassinosteroids control root epidermal cell fate via direct regulation of a MYB-bHLH-WD40 complex by GSK3-like kinases. E Life 3:e02525
Cheng W, Huang Y, Meng C, Zhang N, Zeng H, Ren J, Li Y, Sun Y (2015) Effect of exogenous 24-epibrassinolide on salt resistance of watermelon (Citrullus lanatus L.) under salinity stress. In: Proceedings of the 5th international conference on advanced design and manufacturing engineering. Atlantis Press
Cho H, Ryu H, Rho S, Hill K, Smith S, Audenaert D, Park J, Han S, Beeckman T, Bennett MJ et al (2014) A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat Cell Biol 16:66–76
Choi YH, Fujioka S, Harada A, Yokota T, Takatsuto S, Sakurai A (1996) A brassinolide biosynthetic pathway via 6-deoxocastasterone. Phytochemistry 43(3):593–596
Choudhary SP, Kanwar M, Bhardwaj R, Yu JQ, Tran LS (2012) Chromium stress mitigation by polyaminebrassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS One 7(3):e33210
Chung Y, Kwon SI, Choe S (2014) Antagonistic regulation of Arabidopsis growth by brassinosteroids and abiotic stresses. Mol Cells 37(11):795–803
Clouse SD (2011) Brassinosteroids. The Arabidopsis book, p 151
Cukor J, Rasakova NAM, Linda R, Linhart LUKÁÐ, Gutsch MR, Kunes I (2018) Effects of brassinosteroid application on seed germination of scots pine under standard and heat stress conditions. Balt For 24(1):60–67
Dalio R, Pinheiro HP, Sodek L, Haddad C (2013) 24-epibrassinolide restores nitrogen metabolism of pigeon pea under saline stress. Bot Stud 54(1):9
de Assis-Gomes MM, Netto AT, Campostrini E, Bressan-Smith R, Zullo MAT, Ferraz TM, Siqueira LDN, Leal NR, Núñez-Vázquez M (2013) Brassinosteroid analogue affects the senescence in two papaya genotypes submitted to drought stress. Theor Exp Plant Physiol 25(3):186–195
de Assis-Gomes MM, Pinheiro DT, Bressan-Smith R, Campostrini E (2018) Exogenous brassinosteroid application delays senescence and promotes hyponasty in Carica papaya L. leaves. Theor Exp Plant Physiol 30:193–201
De Vleesschauwer D, Van Buyten E, Satoh K, Balidion J, Mauleon R, Choi IR et al (2012) Brassinosteroids antagonize gibberellin–and salicylate-mediated root immunity in rice. Plant Physiol 158(4):1833–1846
Denance N, Sanchez-Vallet A, Goffner D, Molina A (2013) Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Front Plant Sci 4:155
Deng XG, Zhu T, Zou LJ, Han XY, Zhou X, Xi DH, Zhang DW, Lin HH (2016) Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J 85(4):478–493
Ding J, Shi K, Zhou YH, Yu JQ (2009) Effects of root and foliar applications of 24 epibrassinolide on Fusarium wilt and antioxidant metabolism in cucumber roots. HortScience 44(5):1340–1345
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol 26(3–4):131–136
Divi UK, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Bio 10:151
Dobrikova A, Vladkova R, Stanoeva D, Popova A, Velitchkova M (2013) Effects of 24-epibrassinolide pretreatment on UV-B-induced changes in the pigment content of pea leaves. C R Acad Bulg Sci 66:543–550
Domagalska MA, Sarnowska E, Nagy F, Davis SJ (2010) Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS One 5(11):e14012
Duan F, Ding J, Lee D, Lu X, Feng YQ, Song W (2017) Overexpression of SoCYP85A1, a Spinach Cytochrome p450 gene in transgenic tobacco enhances root development and drought stress tolerance. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01909
Dumont S, Rivoal J (2019) Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front Plant Sci 10:166
El-Esawi MA (2017) Introductory chapter: Hormonal regulation in plant development and stress tolerance, phytohormones—signaling mechanisms and crosstalk in plant development and stress responses. Mohamed El-Esawi, IntechOpen
El-Sayed IM, Salim RG, El-Haggar EF, El-Ziat RA, Dina M, Soliman DM (2020) Molecular characterization and positive impact of brassinosteroids and chitosan on Solidago canadensis cv. Tara characteristics. Horticulture 6:100
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S et al (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404
Fahad S, Hussain S, Saud S, Hassan S, Ihsan Z, Shah AN, Wu C, Yousaf M, Nasim W, Alharby H, Alghabari F, Huang J (2016) Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front Plant Sci 7:1250
Faizan M, Faraz A, Sami F, Siddiqui H, Yusuf M, Gruszka D, Hayat S (2020) Role of strigolactones: signaling and crosstalk with other phytohormones. Open Life Sci 15(1):217–228
Fariduddin Q, Khalil RR, Mir BA, Yusuf M, Ahmad A (2013) 24-epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Ass 185:7845–7856
Fariduddin Q, YusufM AI, Ahmad A (2014) Brassinosteroids and their role in response of plants to abiotic stresses. Biologia Plantar 58:9–17
Fedina E, Yarin A, Mukhitova F, Blufard A, Chechetkin I (2017) Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 117:25–28
Filova A, Sytar O, Krivosudska E (2013) Effects of brassinosteroid on the induction of physiological changes in Helianthus annuus L. under copper stress. Acta Univ Agric Silvic Mendel Brun 61:623–629
Filova A (2014) The responses of Helianthus annuus L. to foliar application of 28-homobrassinolide. Res J Agric Sci 46:226–235
Fridman Y, Elkouby L, Holland N, Vragović K, Elbaum R, Savaldi-Goldstein S (2014) Root growth is modulated by differential hormonal sensitivity in neighboring cells. Genes Dev 28(8):912–920
Fujioka S, Noguchi T, Yokota T, Takatsuto S, Yoshida S (1998) Brassinosteroids in Arabidopsis thaliana. Phytochemistry 48:595–599
Fujioka S, Noguchi T, Watanabe T, Takatsuto S, Yoshida S (2000) Biosynthesis of brassinosteroids in cultured cells of Catharanthus roseus. Phytochemistry 53(5):549–553
Fujioka S, Takatsuto S, Yoshida S (2002) An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiol 130(2):930–939
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9(4):436–442
Fujita Y, Yoshida T, Yamaguchi-Shinozaki K (2013) Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol Plant 147:15–27
Gallego-Bartolome J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Alabadi D, Blazquez MA (2012) Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc Natl Acad Sci U S A 109:13446–13451
Gao W, Long L, Zhu LF, Xu L, Gao WH, Sun LQ, Liu LL, Zhang XL (2013) Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol Cell Proteomics 12(12):3690–3703
Gjorgieva Ackova D (2018) Heavy metals and their general toxicity on plants. Plant Sci Today 5(1):15–19
Gudesblat GE, Russinova E (2011) Plants grow on brassinosteroids. Curr Opin Plant Biol 14:530–537
Gull A, Lone AA, Wani NUI (2019) Biotic and abiotic stresses in plants. In: de Oliveira AB (ed) Abiotic and biotic stress in plants. IntechOpen, pp 3–19
Guo D, Gao X, Li H, Zhang T, Chen G, Huang P et al (2008) EGY1 plays a role in regulation of endodermal plastid size and number that are involved in ethylene-dependent gravitropism of light-grown Arabidopsis hypocotyls. Plant Mol Biol 66:345–360
Guo HQ, Ren WB, Li P, Wu ZN, Wan DL (2014) Effect of epi-brassinosteroid and gibberellin on seed germination and seedling growth of Leymus chinensis. Caoye Kexue 31:1097–1103
Ha YM, Shang Y, Nam KH (2016) Brassinosteroids modulate ABA induced stomatal closure in Arabidopsis. J Exp Bot 67:6297–6308
Ha YM, Shang Y, Yang DM, Nam KH (2018) Brassinosteroid reduces ABA accumulation leading to the inhibition of ABA-induced stomatal closure. Biochem Biophys Res Comm 504:143–148
Hansen M, Chae HS, Kieber JJ (2009) Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J 57:606–614
Hao J, Yin Y, Fei SZ (2013) Brassinosteroid signaling network: implications on yield and stress tolerance. Plant Cell Rep 32:1017–1030
Hasan MK, Ahammed GJ, Sun SC, Li MQ, Yin HQ, Zhou J (2019) Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J Agric Food Chem 67(38):10563–10576. https://doi.org/10.1021/acs.jafc.9b02404
Hasanuzzaman M, Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112
He JX, Gendron JM, Yang Y, Li J, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A 99:10185–10190
He JX, Gendron MJ, Sun Y, Gampala SS, Gendron N, Sun CQ et al (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Sci 307:1634–1638
He J, Wang YJ, Ding HD, Ge CL (2016) Epibrassinolide confers zinc stress tolerance by regulating antioxidant enzyme responses, osmolytes, and hormonal balance in Solanum melongena seedlings. Braz J Bot 39:295–303
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
Hu Y, Xia S, Su Y, Wang H, Luo W, Su S, Xiao L (2016) Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed Res Int 2016:8231873
Hawkes SJ (1997) What is a “heavy metal”? J Chem Educ 74(11):1374
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Jager CE, Symons GM, Ross JJ, Reid JB (2008) Do brassinosteroids mediate the water stress response? Physiol Plant 133(2):417–425
Jakubowska D, Janicka M (2017) The role of brassinosteroids in the regulation of the plasma membrane H+-ATPase and NADPH oxidase under cadmium stress. Plant Sci 264:37–47
Janeczko A, Biesaga-Kościelniak J, Oklešt’ková J, Filek M, Dziurka M, Szarek-Łukaszewska G, Kościelniak J (2010) Role of 24-epibrassinolide in wheat production: physiological effects and uptake. J Agron Crop Sci 196(4):311–321
Jang G, Yoon Y, Choi YD (2020) Crosstalk with Jasmonic acid integrates multiple responses in plant development. Int J Mol Sci 21(1):305
Jasrotia S, Ohri P (2014) In vitro effect of 24-epibrassinolide on antioxidative enzymes of tomato plants during Meloidogyne incognita infection. J Environ Res Develop 9(1):188–191
Jasrotia S, Ohri P (2017a) 24-Epibrassinolide reduces stress in nematode-infected tomato (Solanum lycopersicum L.) plants cultured in vitro. In Vitro Cell Dev Biol Plant 53(6):538–545
Jasrotia S, Ohri P (2017b) Ameliorative effect of 24-Epibrassinolide on physiology of tomato plants infected with Meloidogyne incognita (Kofoid and White, 1919) Chitwood, 1949. Indian J Nematol 47(2):228–233
Jatav KS, Agarwal RM, Tomar NS, Tyagi SR (2014) Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J Indian Bot Soc 93:177–189
Jin SH, Li XQ, Wang GG, Zhu XT (2015) Brassinosteroids alleviate high temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 7:plv009. https://doi.org/10.1093/aobpla/plv009
Jiroutová P, Mikulík J, Novák O, Strnad M, Oklestkova J (2019) Brassinosteroids induce strong, dose-dependent inhibition of etiolated pea seedling growth correlated with ethylene production. Biomol Ther 9(12):849
Jung J, Lee M, Park CA (2010) Transcriptional feedback loop modulating signaling crosstalks between auxin and brassinosteroid in Arabidopsis. Mol Cells 29(5):449–456
Kang YY, Guo SR, Duan JJ, Hu XH (2006) Effects of 24-epibrassinolide on antioxidant system and anaerobic respiratory enzyme activities in cucumber roots under hypoxia stress. Plant Physiol Mol Biol 32(5):535–542
Kang YY, Guo SR, Li J, Duan JJ (2009) Effect of root applied 24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regul 57(3):259–269
Kaplan-Dalyan E, Sağlam-Çağ S (2013) The effect of epibrassinolide on senescence in horizontal sunflower (Helianthus annuus L.) seedlings. IUFS. J Biol 72(1):33–44
Karwa S, Bahuguna RN, Chaturvedi AK, Maurya S, Arya SS, Chinnusamy V, Pal M (2020) Phenotyping and characterization of heat stress tolerance at reproductive stage in rice (Oryza sativa L.). Acta Physiol Plant 42(2):1–6
Kaur R, Ohri P, Bhardwaj R (2013) Effect of 28-homobrassinolide on susceptible and resistant cultivars of tomato after nematode inoculation. Plant Growth Regul 71(3):199–205
Kaur R, Ohri P, Bhardwaj R (2014) Brassinosteroid-mediated changes in root-knot nematode susceptible and resistant tomato cultivars. Int J Pharm Bio Sci 5(4):1085–1093
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P (2018) 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep 8:8735. https://doi.org/10.1038/s41598-018-27032-w
Kaya C, Ashraf M, Wijaya L, Ahmad P (2019) The putative role of endogenous nitric oxide in brassinosteroidinduced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol Biochem 143:119–128
Khalid A, Aftab F (2016) Effect of exogenous application of 24-epibrassinolide on growth, protein contents, and antioxidant enzyme activities of in vitro-grown Solanum tuberosum L. under salt stress. In Vitro Cell Dev Biol Plant 52:81–91
Kim TW, Lee SM, Joo SH, Yun HS, Lee Y, Kaufman PB et al (2007) Elongation and gravitropic responses of Arabidopsis roots are regulated by brassinolide and IAA. Plant Cell Environ 30:679–689
Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704
Kim TW, Michniewicz M, Bergmann DC, Wang ZY (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482(7385):419–422
Kitanaga Y, Jian C, Hasegawa M, Yazaki J, Kishimoto N, Kikuchi S et al (2006) Sequential regulation of gibberellin, brassinosteroid, and jasmonic acid biosynthesis occurs in rice coleoptiles to control the transcript levels of anti-microbial thionin genes. Biosci Biotechnol Biochem 70:2410–2419
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32(7):945–957
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255(1):11–24
Kosová K, Vítámvás P, Urban MO, Prášil IT, Renaut J (2018) Plant abiotic stress proteomics: the major factors determining alterations in cellular proteome. Front Plant Sci 9:122
Kothari A, Lachowiec J (2021) Roles of brassinosteroids in mitigating heat stress damage in cereal crops. Int J Mol Sci 22(5):2706
Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regulation 22:289–297
Kumar S, Sirhindi G, Bhardwaj R, Kimar M, Arora P (2012) Role of 24-epibrassinolide in amelioration of high temperature stress through antioxidant defense system in Brassica juncea L. Plant Stress 6(1):55–58
Kuneš I, Baláš M, Linda R, Gallo J, Nováková O (2016) Effects of brassinosteroid application on seed germination of Norway spruce, Scots pine, Douglas fir and English oak. iForest 10:121–127
Lalotra S, Hemantaranjan A, Kumar S, Kant R (2017) Effect of brassinosteroid (Brassinolide) on seedling traits, morphology and metabolism in mungbean under salinity stress. Annu Res Rev Biol 12(4):1–8
Li KR, Feng CH (2011) Effects of brassinolide on drought resistance of Xanthoceras sorbifolia seedlings under water stress. Acta Physiol Plant 33:1293–1300
Li QF, Wang C, Jiang L, Li S, Sun SSM, He JX (2012a) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5:ra72
Li ZY, Xu ZS, He GY, Yang GX, Chen M, Li LC, Ma YZ (2012b) A mutation in Arabidopsis BSK5 encoding a brassinosteroid signaling kinase protein affects responses to salinity and abscisic acid. Biochem Biophy Res Comm 426:522–527
Li N, Guo SR, Shu S, Sun J (2015) Effects of exogenous 24-epibrassinolde on leaf morphology and photosynthetic characteristics of tomato seedlings under low light stress. Ying Yong Sheng Tai Xue Bao 26:847–852
Li X, Wei JP, Ahammed GJ, Zhang L, Li Y, Yan P, Zhang LP, Han WY (2018) Brassinosteroids attenuate moderate high temperature-caused decline in tea quality by enhancing Theanine biosynthesis in Camellia sinensis L. Front Plant Sci 9:1016
Li G, Hu S, Zhao X, Kumar S, Li Y, Yang J, Hou H (2021) Mechanisms of the Morphological Plasticity Induced by Phytohormones and the Environment in Plants. Int J Mol Sci 22(2):765
Liang J, Liang Y (2009) Effects of plant growth substances on water-logging resistance of oilseed rape seedling. Xinan Shifan Daxue Xuebao, Ziran Kexueban 34:58–62
Lima JV, Lobato AKS (2017) Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol Mol Biol Plants 23:59–72
Liu Q, Xi Z, Gao J, Meng Y, Lin S, Zhang Z (2016) Effects of exogenous 24-epibrassinolide to control grey mould and maintain postharvest quality of table grapes. Int J Food Sci Tech 51:1236–1243
Liu J, Zhang D, Sun X, Ding T, Lei B, Zhang C (2017) Structure activity relationship of brassinosteroids and their agricultural practical usages. Steroids 124:1–17
Lu XM, Chen Y, Gong W, Chen YM (2006) Effect of brassinolide on the seedling growth and waterlogging resistance of soybean. J Biol 23(3):37–38
Lu XM, Guo SR (2013) Effects of brassinolide on the polyamines, ATPase activity, and inorganic ion content in roots of cucumber seedlings under hypoxia stress. Chin J Ecol 32(3):611
Lu XM, Yang W (2013) Alleviation effects of brassinolide on cucumber seedlings under NaCl stress. Chin J Appl Ecol 24:1409–1414
Luo M, Lau NC (2019) Characteristics of summer heat stress in China climatology and long-term trends. Clim Dyn 53(9):5375–5388
Mahesh K, Balaraju P, Ramakrishna B, Ram Rao S (2013) Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Amer. J Plant Sci 4(12):2305–2313
Manzano S, Martinez C, Megiasm Z, Gomez P, Garrido D, Jamilena M (2011) The role of ethylene and brassinosteroids in the control of sex expression and flower development in Cucurbita pepo. Plant Growth Regul 65:213–221
Mir BA, Khan TA, Fariduddin Q (2015) 24-epibrassinolide and spermidine modulate photosynthesis and antioxidant systems in Vigna radiata under salt and zinc stress. Int J Adv Res 3:592–608
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–195
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nahar K, Kyndt T, Hause B, Höfte M, Gheysen G (2013) Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Mol Plant-Microbe Inter 26:106–115
Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33(5):887–898
Nawaz F, Ahmad R, Ashraf MY, Waraich EA, Khan SZ (2015) Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol Environ Saf 113:191–200
Nawaz F, Naeem M, Zulfiqar B, Akram A, Ashraf MY, Raheel M, Shabbir RN, Hussain RA, Anwar I, Aurangzaib M (2017) Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: a critical review. Environ Sci Pollut Res Int 24(19):15959–15975
Nazir F, Fariduddin Q, Hussain A, Khan TA (2021) Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu-triggered oxidative burst in tomato. Ecotoxicol Environ Saf 207:111081
Nergi D, Ali M (2020) The role of brassinosteroids in the regulation of senescence in Lilium orientalis. PhD thesis. http://hdl.handle.net/11343/258508
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T et al (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Nishiyama R, Le DT, Watanabe Y, Matsui A, Tanaka M, Seki M, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2012) Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS One 7:e32124
Niu JH, Anjum SA, Wang R, Li JH, Liu MR et al (2016) Exogenous application of brassinolide can alter morphological and physiological traits of Leymus chinensis (Trin.) Tzvelev under room and high temperatures. Chil J Agric Res 76:27–33
Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA (2000) Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol 124(1):201–210
Ogweno JO, Hu WH, Song XS, Shi K, Mao WH, Zhou YH, Yu JQ (2010) Photoinhibition-induced reduction in photosynthesis is alleviated by abscisic acid, cytokinin and brassinosteroid in detached tomato leaves. Plant Growth Regul 60(3):175–182
Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, Koncz C, Lafos M, Shibata K, Yokota T, Sakata K, Szekeres M, Mizutani M (2006) C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18(11):3275–3288
Ohri P, Kaur R (2011) Role of 28-Homobrassinolide on antioxidative defense system of Brassica juncea during root-knot nematode, Meloidogyne incognita, infection. Adv Appl Sci Res 3(2):120–126
Ohri P, Bhardwaj R, Kaur R (2011) Effect of 24-epibrassinolide regulated antioxidative defense system in Brassica juncea during Meloidogyne incognita infection. Annals of Plant Prot Sci 19(2):423–427
Ohri P, Bhardwaj R, Bali S, Kaur R, Jasrotia S, Khajuria A et al (2015) The common molecular players in plant hormone crosstalk and signaling. Curr Prot Pep Sci 16:369–388
Pacholczak A, Zajączkowska M, Nowakowska K (2021) The effect of brassinosteroids on rootting of stem cuttings in two barberry (Berberis thunbergii L.) cultivars. Agro 11:699
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9:747–758
Peng Z, Han C, Yuan L, Zhang K, Huang H, Ren C (2011) Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis seedlings. J Int Plant Biol 53:632–640
Peres A, Soares JS, Tavares RG, Righetto G, Zullo M, Mandava NB, Menossi M (2019) Brassinosteroids, the Sixth Class of Phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int J Mol Sci 20(2):331
Pieterse CM, Leon-Reyes A, Van Der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316
Pociecha E, Dziurka M, Oklestkova J, Janeczko A (2016) Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. Plant Growth Regul 80:127–135
Polko JK, Pierik R, van Zanten M, Tarkowská D, Strnad M, Voesenek LA, Peeters AJ (2013) Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. Journal Exp Bot 64(2):613–624
Procházka P, Štranc P, Kupka I, Štranc J, Pazderů K (2015) Forest seed treatment with brassinosteroids to increase their germination under stress conditions. J For Sci 61(7):291–296
Rajewska I, Talarek M, Bajguz A (2016) Brassinosteroids and response of plants to heavy metals action. Front Plant Sci 7:629
Ren C, Han C, Peng W, Huang Y, Peng Z, Xiong X, Zhu Q, Gao B, Xie D (2009) A leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiol 151:1412–1420
Rodríguez M, Canales E, Borrás-Hidalgo O (2005) Molecular aspects of abiotic stress in plants. Biotecnol Apl 22(1):1–10
Ross JJ, Quittenden LJ (2016) Interactions between brassinosteroids and gibberellins: synthesis or signaling? Plant Cell 28:829–832
Roth U, Friebe A, Schnabl H (2000) Resistance induction in plants by a brassinosteroid-containing extract of Lychnis viscaria L. Z Naturforsch C 55(7–8):552–559
Sadak MS, El-Awadi ME-S, Dawood MG, El-Rorkiek KGA (2020) Physiological role of brassinosteroids and cauliflower extract on quinoa plant grown under sandy soil. Asian J Appl Sci 13:68–75
Sağlam-Çağ S (2007) The effect of epibrassinolide on senescence in wheat leaves. Biotechnol Equip 21(1):63–65
Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P (2016) Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci Rep 6:28298. https://doi.org/10.1038/srep28298
Saini S, Sharma I, Pati PK (2015) Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci 6:950
Samynathan R, Kiruthikaa S, Harsha M, Ilango RVJ, Shanmugam A, Venkidasamy B, Thiruvengadam M (2021) The effect of abiotic and biotic stresses on the production of bioactive compounds in tea (Camellia sinensis (L.) O. Kuntze). Plant Gene 27:100316
Santner A, Calderon-Villalobos LI, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307
Santos LR, Batista BL, Lobato AK (2018) Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica 56(2):591–605
Savaliya DD, Mandavia CK, Mandavia MK (2013) Role of brassinolide on enzyme activities in groundnut under water deficit stress. Indian J Agric Biochem 26:92–96
Seif SN, Tafazzoli E, Talaii AR, Aboutalebi A, Abdosi V (2014) Evaluation of two grape cultivars (Vitis vinifera L.) against salinity stress and surveying the effect of methyl jasmonate and epibrassinolide on alleviation the salinity stress. Int J Biosci 5:116–125
Semida WM, Rady MM (2014) Pre-soaking in 24-epibrassinolide or salicylic acid improves seed germination, seedling growth, and anti-oxidant capacity in Phaseolus vulgaris L. grown under NaCl stress. J Hortic Sci Biotechnol 89:338–344
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci 7:187
Shahana T, Rao PA, Ram SS, Sujatha E (2015) Mitigation of drought stress by 24-epibarassinolide and 28-homobrassinolide in pigeon pea seedlings. Int J Multi Curr Res 3:905–911
Shahid MA, Balal RM, Pervez MA, Abbas T, Aqeel MA, Riaz A, Mattson NS (2015) Exogenous 24-epibrassinolide elevates the salt tolerance potential of pea (Pisum sativum L.) by improving osmotic adjustment capacity and leaf water relations. J Plant Nutr 38:1050–1072
Sharma I, Bhardwaj R, Pati PK (2013) Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pestic Biochem Phys 105(2):144–153
Sharma I, Sharma A, Pati P, Bhardwaj R (2018) Brassinosteroids reciprocates heavy metals induced oxidative stress in radish by regulating the expression of key antioxidant enzyme genes. Braz Arch Biol Technol 14:61
Sharma A, Thakur S, Kumar V, Kesavan AK, Thukral AK, Bhardwaj R (2017) 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol 17(1):1–10
Shu S, Tang Y, Yuan Y, Sun J, Zhong M, Guo S (2016) The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol Biochem 107:344–353
Singh I, Kumar U, Singh SK, Gupta C, Singh M, Kushwaha SR (2012) Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol Mol Biol Plants 18:229–236
Sirhindi G, Kumar S, Bhardwaj R, Kumar M (2009) Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plant 15(4):335–341
Slathia S, Sharma A, Sikander PC (2013) Co-application of 24-epibrassinolide and putrescine enhances salinity tolerance in Solanum lycopersicum L. by modulating stress indicators and antioxidant system. Int J Pharma BioSci 4:70–85
Soares TFSN, Dias DCFDS, Oliveira AMS, Ribeiro DM, Dias LADS (2020) Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol Env Saf 193:110296
Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Peng W, Liu Y, Peng J, Xie D (2011) The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23(3):1000–1013
Song YL, Dong YJ, Tian XY, Kong J, Bai XY, Xu LL, He ZL (2016) Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. Biol Plant 60(2):329–342
Sonjaroon W, Jutamanee K, Khamsuk O, Thussagunpanit J, Kaveeta L, Suksamrarn A (2018) Impact of brassinosteroid mimic on photosynthesis, carbohydrate content and rice seed set at reproductive stage under heat stress. Agric Nat Resour 52(3):234–240
Sousa B, Soares C, Oliveira F, Martins M, Branco-Neves S, Barbosa B, Ataíde I, Teixeira J, Azenha M, Azevedo RA, Fidalgo F (2020) Foliar application of 24-epibrassinolide improves Solanum nigrum L. tolerance to high levels of Zn without affecting its remediation potential. Chemosphere 244(125579)
Steber CM, McCourt P (2001) A role for brassinosteroids in germination in Arabidopsis. Plant Physiol 125(2):763–769
Stewart Lilley JL, Gan Y, Graham IA, Nemhauser JL (2013) The effects of DELLAs on growth change with developmental stage and brassinosteroid levels. Plant J 76:165–173
Sun S, Chen D, Li X, Qiao S, Shi C, Li C, Shen H, Wang X (2015) Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Develop Cell 34:220–228
Surgun-Acar Y, Zemheri-Navruz F (2019) 24-Epibrassinolide promotes arsenic tolerance in Arabidopsis thaliana L. by altering stress responses at biochemical and molecular level. J Plant Physiol 238:12–19
Surgun Y, Altunlu H, Türkekul S, Bürün B, Yokaş I (2015) Effects of 24-epibrassinolide on growth and some antioxidant enzymes of cotton (Gossypium hirsutum L.) cultivars under NaCl stress. J Appl Biol Sci 9:09–17
Tadaiesky LB, da Silva BR, Batista BL, Lobato AK (2021) Brassinosteroids trigger tolerance to iron toxicity in rice. Physiol Plant 3:371–387
Talaat NB, Shawky BT, Ibrahim AS (2015) Alleviation of drought induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ Exp Bot 113:47–58
Tanveer M, Shahzad B, Sharma A, Khan EA (2019) 24-Epibrassinolide application in plants: an implication for improving drought stress tolerance in plants. Plant Physiol Biochem 135:295–303
Tarkowska D, Novak O, Oklestkova J, Strnad M (2016) The determination of 22 natural brassinosteroids in a minute sample of plant tissue by UHPLC-ESI-MS/MS. Anal Bioanal Chem 408:6799–6812
Tian C, Muto H, Higuchi K, Matamura T, Tatematsu K, Koshiba T (2004) Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J 3:333–343
Todorova D, Talaat NB, Katerova Z, Alexieva V, Shawky BT (2016) Polyamines and brassinosteroids in drought stress responses and tolerance in plants. In: Ahmad P (ed) Water stress and crop plants: a sustainable approach. Wiley
Toman SS, Jasim AH, Kadhim ZK, Hassan AAH, Hamzah RM (2019) Effect of barassinolide on growth characteristics of wheat (Triticum aestivum L.) under water stress. IOP Conf Series Earth Environ Sci 388:012045
Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Jin Y, Qian Q, Chu C (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26:4376–4393
Tong H, Chu C (2016) Reply: brassinosteroid regulates gibberellin synthesis to promote cell elongation in rice: critical comments on ross and quittenden’s letter. Plant Cell 28:833–835
Trevisan S, Forestan C, Brojanigo S, Quaggiotti S, Varotto S (2020) Brassinosteroid application affects the growth and gravitropic response of maize by regulating gene expression in the roots, shoots and leaves. Plant Growth Regul 92:117–130
Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B (2015) Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell 27:2261–2272
Unterholzner SJ, Rozhon W, Poppenberger B (2016) REPLY: interaction between brassinosteroids and gibberellins: synthesis or signaling? In Arabidopsis, Both! Plant Cell 28:836–839
Upadhyaya CP, Bagri DS, Upadhyay DC (2015) Ascorbic acid and/or 24-epibrassinolide trigger physiological and biochemical responses for the salt stress mitigation in potato (Solanum tuberosum L.). Int J Appl Sci Biotech 3:655–667
Vandenbussche F, Callebert P, Zadnikova P, Benkova E, Van Der Straeten D (2013) Brassinosteroid control of shoot gravitropism interacts with ethylene and depends on auxin signaling components. Am J Bot 100:215–225
Vardhini BV, Anjum NA (2015) Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci 2:67
Vardhini BV (2019) An overview on the various physiological roles of brassinosteroids in the past decade—a mini review. Asian J Sci Technol 10(01):9320–9325
Vayner AA, Miroshnichenko NN, Kolupaev YE, Yastreb TO, Khripach VA, Sotnikov YA (2014) The influence of 24-epibrassinolide on heat resistance and productivity of millet (Panicum miliaceum) plants. Bull Kharkiv Nat Agrarian Univ Series Biol 3:35–42
Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D (2011) Combining enhanced root and shoot growth reveals crosstalk between pathways that control plant organ size in Arabidopsis. Plant Physiol 155:1339–1352
Verma A, Malik CP, Gupta VK (2012) In vitro effects of brassinosteroids on the growth and antioxidant enzyme activities in groundnut. Int Sch Res Notices 2012:356485
Vragovic K, Sela A, Friedlander-Shani L, Fridman Y, Hacham Y, Holland N, Savaldi-Goldstein S, Bartom E, Mockler TC (2015) Translatome analyses capture of opposing tissue-specific brassinosteroid signals orchestrating root meristem differentiation. Proc Natl Acad Sci U S A 112:923–928
Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46:701–724
Wang Q, Zhu Z, Ozkardesh K, Lin C (2013) Phytochromes and phytohormones: the shrinking degree of separation. Mol Plant 6:5–7
Wang R, Anjum SA, Niu J, Liu M, Li J et al (2016) Exogenous application of brassinolide ameliorate chilling stress in Leymus chinensis (Trin.) Tzvel. by modulating morphological, physiological and biochemical traits. Bangladesh J Bot 45:143–150
Wang H, Tang J, Liu J, Hu J, Liu J, Chen Y, Cai Z, Wang X (2018) Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol Plant 11(2):315–325
Wang YT, Chen ZY, Jiang Y, Duan BB, Xi ZM (2019) Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci Hortic 256:108596. https://doi.org/10.1016/j.scienta.2019.108596
Wang Q, Yu F, Xie Q (2020) Balancing growth and adaptation to stress: crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ 43:2325–2335
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. The Crop J 4(3):162–176
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111(6):1021–1058
Wei Z, Li J (2016) Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant 9:86–100
Werner T, Schmülling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12:527–538
Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-Specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and Tobacco. Plant Cell 22:3905–3920
Wu X, Yao X, Chen J, Zhu Z, Zhang H, Zha D (2014) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36(2):251–261
Wu XX, Ding HD, Chen JL, Zhu ZW, Zha DS (2015) Exogenous spray application of 24-epibrassinolide induced changes in photosynthesis and anti-oxidant defences against chilling stress in eggplant (Solanum melongena L.) seedlings. J Hortic Sci Biotechnol 90:217–225
Wu XX, Chen JL, Xu S, Zhu ZW, Zha DS (2016) Exogenous 24-epibrassinolide alleviates zinc-induced toxicity in eggplant (Solanum melongena L.) seedlings by regulating the glutathione-ascorbate-dependent detoxification pathway. J Hortic Sci Biotechnol 91:412–420
Wu Z, Liu S, Zhao J, Wang F, Du Y, Zou S, Li H, Wen D, Huang Y (2017) Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 133:1–11
Wu C, Li F, Xu H, Zeng W, Yu R, Wu X, Shen L, Liu Y, Li J (2019) The potential role of brassinosteroids (BRs) in alleviating antimony (Sb) stress in Arabidopsis thaliana. Plant Physiol Biochem 141:51–59
Xia XJ, Huang YY, Wang L, Huang LF, Yu YL, Zhou YH, Yu JQ (2006) Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic Biochem Phys 86(1):42–48
Xia XJ, Zhang Y, Wu JX, Wang JT, Zhou YH, Shi K, Yu YL, Yu JQ (2009) Brassinosteroids promote metabolism of pesticides in cucumber. J Agric Food Chem 57(18):8406–8413
Xia XJ, Gao CJ, Song LX, Zhou YH, Shi K, Yu JQ (2014) Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ 37:2036–2050
Xiao S, Hu Q, Zhang X, Si H, Liu S, Chen L, Chen K, Berne S, Yuan D, Lindsey K, Zhang X, Zhu L (2021) Orchestration of plant development and defense by indirect crosstalk of salicylic acid and brassinosteroid signaling via transcription factor GhTINY2. J Exp Bot 72(13):4721–4743
Xu Y, Zhang X, Li Q, Cheng Z, Lou H, Ge L, An H (2015) BdBRD1, a brassinosteroid C-6 oxidase homolog in Brachypodium distachyon L., is required for multiple organ development. Plant Physiol Biochem 86:91–99
Xu B, Guo Y, Yu J, Wang F, Guo J, Liu M, Chen Y, Wang G (2019) Effects of 28-homobrassinolide on the accumulation and uptake of cadmium, lead and zinc by paddy rice. Fresenius Environ Bull 28(4A):3209–3218
Xue-Xia WU, Zha DS, Zhu ZW (2011) Effects of exogenous 24-epibrassinolide on seed germination, physiological characteristics of eggplant seedlings under NaCl stress. Plant Physiol J 47:607–612
Yadava P, Kaushal J, Gautam A, Parmar H, Singh I (2016) Physiological and biochemical effects of 24-epibrassinolide on heat-stress adaptation in maize (Zea mays L.). Nat Sci 8(4):171–179
Yamagami A, Saito C, Nakazawa M, Fujioka S, Uemura T, Matsui M, Sakuta M, Shinozaki K, Osada H, Nakano A (2017) Evolutionarily conserved BIL4 suppresses the degradation of brassinosteroid receptor BRI1 and regulates cell elongation. Sci Rep 7:1–12
Yang P, Wang Y, Li J, Bian Z (2019) Effects of brassinosteroids on photosynthetic performance and nitrogen metabolism in pepper seedlings under chilling stress. Agro 9(12):839
Ye H, Liu S, Tang B, Chen J, Xie Z, Nolan TM, Jiang H, Guo H, Lin HY, Li L, Wang Y, Tong H, Zhang M, Chu C, Li Z, Aluru M, Aluru S, Schnable PS, Yin Y (2017) RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Commun 8:14573
Yin YH, Wang ZY, Mora-Garcia S, Li JM, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109:181–191
Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61:672–685
Yoshimitsu Y, Tanaka K, Fukuda W, Asami T, Yoshida S, Hayashi K, Kamiya Y, Jikumaru Y, Shigeta T, Nakamura Y et al (2011) Transcription of DWARF4 plays a crucial role in auxin regulated root elongation in addition to brassinosteroid homeostasis in Arabidopsis thaliana. PLoS One 6:e23851
Youn JH, Kang SH, Roh J, Lee JE, Yeom HS, Kim SK (2016) Brassinosteroid signaling modulates submergence-induced hyponastic growth in Arabidopsis thaliana. J Plant Biol 59:397–404
Yuan L, Shu S, Sun J, Guo S, Tezuka T (2012a) Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth Res 112(3):205–214
Yuan L, Yuan Y, Du J, Sun J, Guo S (2012b) Effects of 24-epibrassinolide on nitrogen metabolism in cucumber seedlings under Ca(NO3)2 stress. Plant Physiol Biochem 61:29–35
Yuan L, Zhu S, Shu S, Sun J, Guo S (2015) Regulation of 2, 4-epibrassinolide on mineral nutrient uptake and ion distribution in Ca(NO3)2 stressed cucumber plants. J Plant Physiol 188:29–36
Yue J, You Y, Zhang L, Fu Z, Wang J, Zhang J, Guy RD (2018) Exogenous 24-epibrassinolide alleviates effects of salt stress on chloroplasts and photosynthesis in Robinia pseudoacacia L. seedlings. J Plant Growth Regul 38(2):669–682. https://doi.org/10.1007/s00344-018-9881-0
Yusuf M, Fariduddin Q, Ahmad I, Ahmad A (2014) Brassinosteroid-mediated evaluation of antioxidant system and nitrogen metabolism in two contrasting cultivars of Vigna radiata under different levels of nickel. Physiol Mol Biol Plants:Int J Funct Plant Biol 20(4):449–460
Zhang S, Hu J, Zhang Y, Xie XJ, Knapp A (2007) Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust J Agric Res 58:811–815
Zhang SS, Cai ZY, Wang XL (2009) The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc Natl Acad Sci U S A 106:4543–4548
Zhang A, Zhang J, Zhang J, Ye N, Zhang H, Tan M, Jiang M (2011) Nitric Oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol 52(1):181–192
Zhang YP, He J, Yang SJ, Chen YY (2014) Exogenous 24-epibrassinolide ameliorates high temperature-induced inhibition of growth and photosynthesis in Cucumis melo. Biol Planta 58(2):311–318
Zhang DW, Deng XG, Fu FQ, Lin HH (2015) Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 241(4):875–885
Zhang J, Zhang Y, Khan R, Wu X, Zhou L, Xu N, Du S, Ma X (2021) Exogenous application of brassinosteroids regulates tobacco leaf size and expansion via modulation of endogenous hormones content and gene expression. Physiol Mol Biol Plants Int J Funct Plant Biol 27(4):847–860
Zhao B, Li J (2012) Regulation of brassinosteroid biosynthesis and inactivation. J Integr Plant Biol 54(10):746–759
Zhao BT, Zhu XF, Jung JH, Xuan YH (2016) Effect of brassinosteroids on ammonium uptake via regulation of ammonium transporter and N-metabolism genes in Arabidopsis. Biol Plant 60:563–571
Zhao Z, Jin R, Fang D, Wang H, Dong Y, Xu R, Jiang J (2018) Paddy cultivation signifcantly alters the forms and contents of Fe oxides in an Oxisol and increases phosphate mobility. Soil Tillage Res 184:176–180
Zheng X, Xiao Y, Tian Y, Yang S, Wang C (2020) PcDWF1, a pear brassinosteroid biosynthetic gene homologous to AtDWARF1, affected the vegetative and reproductive growth of plants. BMC Plant Biol 20:109
Zhiponova MK, Vanhoutte I, Boudolf V, Betti C, Dhondt S, Coppens F, Mylle E, Maes S, Gonzales Garcia MP, Caño-Delado AI, Inze D, Beemster GTS, De Veylder L, Russinova E (2013) Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol 197:490–502
Zhou YX, Guo XM, Lu SB, Hu YL, Hu DN et al (2013) Effect of water, nutrient and brassinolides on number of blossom, leaf nutrition and seed oil content of Camellia oleifera. Zhiwu Yingyang Yu Feiliao Xuebao. Plant Nutr Fert Sci 19:387–395
Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65(15):4371–4383
Zhu F, Yun Z, Ma Q, Gong Q, Zeng Y, Xu J, Cheng Y, Deng X (2015) Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol Tec 100:8–15
Zhu T, Deng X, Zhou X, Zhu L, Zou L, Li P, Zhang D, Lin H (2016) Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci Rep 6:35392
Zhu Z, Zhang Z, Qin G, Tian S (2010) Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol Tec 56:50–55
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, N., Kour, S., Kumar, D., Kaur, R., Khajuria, A., Ohri, P. (2022). Role of Brassinosteroids (BRs) in Modulating Antioxidative Defense Mechanism in Plants Growing Under Abiotic and Biotic Stress Conditions. In: Aftab, T., Hakeem, K.R. (eds) Antioxidant Defense in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-16-7981-0_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-7981-0_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7980-3
Online ISBN: 978-981-16-7981-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)