Abstract
Heavy metal accumulation due to environmental pollution, especially in agricultural ecosystem can cause serious deterioration of crop yield and quality. In present study we assessed the effect of exogenous 28-homobrassinoloid (HBL; 10−8 M) on growth, photosynthesis, indices of chlorophyll a fluorescence and nitrogen metabolism in Solanum lycopersicum seedlings grown under two doses (Cd1: 3 mg kg−1 sand and Cd2: 9 mg kg−1 sand) of cadmium. Accumulation of Cd in root tissues was considerably higher than shoot hence, Cd declined the growth, pigment contents, and photosynthetic O2 yield in its concentration dependent manner. Chlorophyll a fluorescence due to Cd stress was negatively affected as shown by decreased QA − reoxidation kinetics: φP0, ψ0, φE0 and PI_ABS and increased energy flux parameters: ABS/RC, TR0/RC, ET0/RC and DI0/RC. HBL application under Cd stress improved the photochemistry of photosystem II (PS II) by affecting these parameters positively. Treatment of Cd in test seedlings resulted into significant decrease in nitrate reductase, nitrite reductase, glutamine synthetase and glutamate synthase activities, and induced enhancing effect on ammonium content and glutamate dehydrogenase activity. Exogenous HBL treatment alleviated the negative effect of Cd on growth, photosynthesis, contents of protein, carbohydrate and inorganic nitrogen and nitrogen assimilating enzymes. The data indicate that exogenous HBL protects the test seedlings during the early growth phase against Cd phytotoxicity by regulating Cd accumulation in tissues and two key metabolic processes; photosynthesis and nitrogen metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The agricultural activities in the catchment area of cities are highly affected as the farmers apply the contaminated water for irrigation which is discharged from sewage and factories (Sharma et al. 2009), and due to such practices vegetables accumulate high amount of toxic heavy metals. Cadmium (Cd) contamination in agricultural soil is one of the serious problems as crops cultivated under Cd contaminated sites accumulate considerable amount of this metal, hence reduced growth and crop productivity (Gill et al. 2011; Singh et al. 2016). Cd concentration in crop field soil in Varanasi, India was reported in the range of 0.90–5.65 mg Cd kg−1 soil (Sharma et al. 2006) while in peri-urban area of Titagarh, India which is a metal contaminated site showing several folds higher concentration of Cd i.e. 22.20–51.00 mg Cd kg−1 soil (Gupta et al. 2008; Sharma et al. 2009). The toxic effects of Cd on plant metabolism such as inhibition in photosynthesis, pigment biosynthesis (Durand et al. 2010; Cao et al. 2015), uptake of nutrients (Sun and Shen 2007), and increased oxidative stress (Ahammed et al. 2013; Singh and Prasad 2014) may lead to decreased crop productivity. Cd in leaf tissues may affect chlorophyll fluorescence kinetics, hence it causes damage to structural and functional attributes of photosynthetic apparatus (Strasser et al. 2000; Li et al. 2015).
Nitrogen is one of the important nutrients which play a crucial role in maintaining the growth and development of plants even under stressful condition. Inorganic nitrogen such as nitrate (NO3 −) is readily taken up by the plants and assimilated into ammonia (NH4 +) and finally into amino acids (Gajewska and Sklodowska 2009). Reduction of NO3 − to NH4 + involves nitrate reductase (NR) and nitrite reductase (NiR) while ammonium assimilation is usually performed by GS–GOGAT pathway involving glutamine synthetase (GS), glutamate oxoglutarate aminotransferase (GOGAT) enzymes. However, under stressful condition ammonium assimilation is performed by an alternate pathway involving glutamate dehydrogenase (GDH) enzyme (Ogawa et al. 2000). It has been reported that glutamate dehydrogenase activity was moderately increased in nodules and roots following the Cd (50 µM) treatment, and an impressive decrease in enzyme activity of glutamine synthetase/glutamate synthase in soybean plant was also noticed (Balestrasse et al. 2003). Although nitrogen plays an essential role in plant metabolism, scant information is available regarding the influence of Cd on nitrogen metabolism.
In recent years, various strategies are being develop to minimize the adverse effects of heavy metal and one such technique is exogenous application of plant hormones (Ahammed et al. 2013; Singh and Prasad 2014; Soaresa et al. 2016). Brassinosteroids (BRs) are plant specific steroidal hormones with strong growth promoting properties and induce resistance in plants against various biotic and abiotic stresses (Ali et al. 2008; Ahammed et al. 2014; Zhou et al. 2014; Divi et al. 2016; Soaresa et al. 2016). More than 70 BRs analogs have been identified throughout the plant kingdom, and among them Brassinolide (BL), 24-epibrassinolide, (24-EBL) and 28-homobrassinolide (28-HBL) are being applied exogenously in recent studies (Rao et al. 2002). 28-HBL (10−6 and 10−11 M) has been reported to combat the impact of salt stress in Brassica juncea (Hayat et al. 2007) and heavy metal (Ni, Cd) stress in Triticum aestivum, Beta vulgaris, Abelmoschus esculentus and Brassica oleracea (Singh and Prasad 2011; Yusuf et al. 2011).
Considering the importance of BRs, in present study an effort has been made to investigate the impact of foliar application of 28-homobrassinoloid (HBL) on growth, photosynthesis and nitrogen metabolism of Solanum lycopersicum L. (tomato) growing under Cd stress. The study becomes significant as the tomato, a common vegetable being cultivated on the contaminated site in the catchment area of the city. Such practices not only affect the yield of tomato but also cause risk to human health. Application of BRs may improve the growth performance of test seedlings by affecting the Cd uptake and improving vital metabolic activities such as photosynthesis and nitrogen metabolism.
Materials and methods
Growth conditions and cadmium treatment
Healthy seeds of Solanum lycopersicum (tomato; var. NS-2535) were procured from local market of Allahabad city, India and surface sterilized with 2% (v/v) sodium hypochlorite solution followed by repeated washing with sterilized distilled water. Wet seeds were wrapped in sterilized moistened muslin cloth and left overnight for germination. The germinated seeds were sown in plastic pots containing 150 mg of sterilized sand and kept in darkness at 26 ± 1 °C. After 2 days of sowing, seedlings were transferred in plant growth chamber (CDR model GRW-300 DGe, Athens, Greece) under photosynthetically active radiation (PAR) of 250 µmol photons m−2 s−1 with 16:8 h day–night regime and 65 ± 5% relative humidity at 26 ± 1 °C. On the basis of screening experiments with varying concentrations (1.5–15 mg Cd kg−1 sand) of Cd the two doses i.e. Cd1 (3 mg Cd kg−1 sand) and Cd2 (9 mg Cd kg−1 sand) were selected for present study and mixed into the sand before seed sowing. After the emergence of primary leaf (5–6 days after sowing), the seedlings were irrigated with Hoagland and Arnon’s (1950) half strength nutrient medium and with sterilized double distilled water on alternate day.
Treatment pattern of hormone (HBL)
A stock solution of HBL (10−4 M) was prepared by dissolving required quantity of the HBL (Sigma Aldrich, USA) in 2 ml of ethanol in a flask (100 ml) and final volume was maintained by using double distilled water (DDW). The desired concentration of HBL (10 nM) was prepared by the dilution of stock solution with DDW. The seedlings were sprayed with HBL after 15 days of sowing. The stock solution was added with Tween-20 (0.1%, v/v) as leaf surfactant. The experimental set up included six combinations: control (without Cd as well as HBL treatment), Cd1, Cd2, HBL, Cd1 + HBL and Cd2 + HBL. The control plants were also sprayed with distilled water containing same amount of Tween-20 without HBL. After 15 days of HBL treatment, seedlings were harvested and various parameters were analyzed immediately. There were three replicates for each treatment and each pot contained four seedlings.
Estimation of growth and cadmium accumulation
The seedlings from each set were uprooted and washed gently with water to ensure the removal of adhering sand particle from roots and were blotted to remove the surface water. Seedlings were weighed to record fresh weight by using single pan digital balance (Model, CA- 223, Contech, India). For analysis of Cd contents in root and shoot tissues, sample of HBL treated and untreated seedlings were carried out according to Allen et al. (1986). Samples of each set were digested in tri acid mixture (HNO3, H2SO4 and HClO4 in 5:1:1 ratio, v/v) at 80 °C until a transparent solution was obtained. After cooling, the digested sample was filtered using Whatman No. 42 filter paper. The content of Cd in digested samples was estimated by atomic absorption spectrometer (iCE 3000 Series, model-3500 AAS, Thermo scientific, UK), fitted with specific lamp of particular metal using appropriate drift blank.
Estimation of pigment contents and photosynthesis
Photosynthetic pigments i.e. chlorophylls (Chl a and Chl b) and carotenoids (Car) contents were extracted in 80% (v/v) acetone from fresh leaves (20 mg) until the pellets became colourless. The absorbance of the resulting solutions was recorded at 663.2, 646.5 and 470 nM spectrophotometrically (Shimadzu double beam UV–Visible spectrophotometer-1700, Japan). The amount of Chl and Car were calculated by the equations of Lichtenthaler (1987).
Photosynthetic oxygen yield and respiration rates in leaf discs were estimated by Clark type oxygen electrode (Digital Oxygen System, Model-10, Rank Brothers, UK) in presence of 3 ml of 50 mM HEPES–NaOH buffer (pH 7.6) containing 20 mM NaHCO3 as described by Kurra–Hotta et al. (1987). Fresh leaves (50 mg) of treated and untreated samples were sliced into 1 mM wide strips in a Petri dish containing 1 ml of 0.5 mM CaSO4. The sliced leaf discs were transferred into the temperature controlled air tight reaction vessel of oxygen electrode at 25 °C, and O2 consumption (respiration) in darkness and evolution (photosynthesis) under the saturating light intensity of 400 µmol photons m−2 s−1 (PAR; photosynthetically active radiation) were recorded.
Measurement of chlorophyll a fluorescence transient
For the photosynthetic performance, chlorophyll a fluorescence measurements were taken in 30 min dark adapted intact leaves of control and Cd treated seedlings using hand held leaf fluorometer (FluorPen FP 100, Photon System Instrument, Czech Republic). The fluorescence parameters: size and number of active reaction centre of photosynthetic apparatus (Fv/F0), efficiency of water splitting complex (F0/Fv),the quantum yield of primary photochemistry (Fv/Fm or φP0), yield of electron transport per trapped exciton (Ψ0 or Psi_0), quantum yield of electron transport (φE0 or Phi_E0), performance index of PS II (PIABS), the energy fluxes for absorption of photon per active reaction center (RC) (ABS/RC), trapped energy flux per active RC (TR0/RC), electron transport flux per active RC (ET0/RC) and energy dissipation flux per active RC (DI0/RC) were determined according to Strasser et al. (2000).
Estimation of leaf carbohydrate and protein contents
The total carbohydrate content was estimated as per the method of Dubois et al. (1956). For this, 10 mg dried leaf from each sample was homogenized in 2.5 N HCl and then digested in a boiling water bath for 1 h. Reaction mixture consisted of 50 µl of sample, 1 ml of 5% phenol and 5 ml of concentrated H2SO4. The assay mixture was incubated at 25 °C for 20 min. The intensity of the characteristic straw color was determined by reading its absorbance at 490 nM and the total carbohydrate content was calculated from the standard curve prepared with graded solution of glucose. For the estimation of total protein content, fresh leaf from control and treated seedlings was homogenized in 50 mM potassium phosphate buffer. The aliquots were centrifuged at 10,000×g for 15 min at 4 °C. Protein contents of the extract were determined following the method of Bradford (1976).
Estimation of inorganic nitrogen contents in leaves
For nitrate, nitrite and ammonium contents estimation, fresh leaves were homogenized in distilled water, boiled for 15 min and then filtered using Whatman No. 1 filter paper. Nitrate content was estimated using the method of Cataldo et al. (1975) based on nitration of salicylic acid under acidic condition. Nitrate content was calculated using a calibration curve prepared for KNO3 and expressed in µmol NO3 − g−1 FW and the nitrite content was determined according the method of Snell and Snell (1949) and calculated using a standard calibration curve prepared for NaNO2 and expressed in µmol NO2 − g−1 FW. Ammonium content was determined using the Nessler reagent as described by Molins-Legua et al. (2006). The reaction mixture consisted of 0.1 ml supernatant, 0.01 ml 10% K–Na tartrate, 2.4 ml distilled water, and 0.1 ml Nessler reagent (Fluka). After 5 min the absorbance was measured at 425 nM. Ammonium content was calculated using a standard calibration curve prepared for NH4Cl and expressed in µmol NH4 + g−1 FW.
Assay of nitrate assimilating enzymes
Nitrate assimilating enzymes: nitrate reductase (NR; EC 1.6.6.1) and nitrite reductase (NiR; EC 1.7.7.1) activities were assayed by the modified method described by Debouba et al. (2006). For estimation of NR and NiR activities, fresh leaves were homogenized in an ice–cold mortar using 0.1 M potassium phosphate buffer (pH 7.5) containing 5 mM cysteine, 2 mM EDTA, and 0.5% PVP. After centrifugation (20,000×g for 20 min at 4 °C), the supernatant was used for the determination of enzyme activity. The reaction mixture for NR activity consisted of 0.1 M potassium phosphate buffer (pH 7.5) containing 5 mM EDTA, 7 mM KNO3, 0.14 mM NADH, and enzyme extract. The reaction was started by addition of NADH. After 30 min of incubation at 28 °C, the reaction was stopped by addition of 0.5 M zinc acetate and then centrifuged. The nitrite formed was determined spectrophotometrically after diazotation with 1% sulfanilamide and 0.01% naphthylene diamine dihydrochloride (NEDD).
The activity of NiR was measured as reduction in the amount of NO2 − in the reaction mixture. The reaction mixture consisted of 0.1 M potassium phosphate buffer (pH 6.8), 0.4 mM NaNO2, 2.3 mM methyl viologen, enzyme extract, and 4.3 mM sodium dithionite in 100 mM NaHCO3, which started the reaction. After 30 min of incubation, the reaction was stopped by vortexing and boiling for 1 min and nitrite that remained in the reaction mixture were determined at 540 nM after diatoziation with sulfanilamide and NEDD.
Assay of ammonia assimilating enzymes
Glutamine synthetase (GS; EC 6.3.1.2) activity was measured by the method of Lillo (1984). The absorbance of reaction mixture was read at 540 nm and the enzyme activity was calculated by using standard curve prepared with γ–glutamylhydroxamic acid and enzyme activity is expressed as A540 (g FW)−1 h−1. Glutamine 2-oxoglutarate aminotransferase also called as glutamate synthase (NADH-GOGAT; EC 1.4.1.14) activity was determined by the method of Singh and Srivastava (1986). The enzyme activity was calculated from the standard curve prepared with NADH. Enzyme activity is expressed as µmol NADH oxidized (g FW)−1 h−1. Glutamate dehydrogenase (GDH; EC 1.4.1.2) activity (aminating) was assayed by the method of Singh and Srivastava (1983). Activity of glutamate dehydrogenase is expressed in terms of µmol NADH oxidized (g FW)−1 h−1.
Statistical analysis
The experimental set up included six combinations: Control (without Cd as well as HBL treatment), Cd1, Cd2, HBL, Cd1 + HBL and Cd2 + HBL. Total three independent experiments were performed with three replicates of each and the results presented in figures and tables are the means ± standard error (SE) of the average values obtained from the three replicates of the individual experiments (n = 3). All the data were statistically evaluated by analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) was applied for mean separation for significant differences among treatments at P < 0.05 significance level. SPSS-16 software was used for DMRT. Correlations were done to show the impact of Cd and foliar application of homobrassinoloid (HBL) on different parameters of S. lycopersicum seedlings exposed to cadmium using Pearson’s correlation coefficient (r).
Results
Effect on growth and Cd accumulation
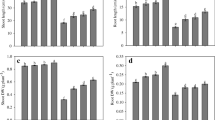
Seedlings under cadmium stress exhibited a significant reduction in fresh weight (Fig. 1), and the decrease was 8 and 16% with Cd1 and Cd2 doses (without HBL), respectively. Foliar application of homobrassinoloid (HBL) in Cd untreated seedlings (control) enhanced the fresh weight by 13% over the value of control (without Cd and/or HBL treatment). Further, the HBL treatments in Cd1 stressed seedlings alleviated the toxic effect on growth and in Cd2 treated seedlings though there was significant alleviation but the values were still less than that of control.
Impact of exogenous homobrassinoloid (HBL) on growth of S. lycopersicum seedlings exposed to cadmium (Cd1: 3 mg Cd Kg−1 and Cd2: 9 mg Cd Kg−1 sand) stress. Data are means ± standard error of three replicates. Values with different letters show significant differences at P < 0.05 significance level between treatments according to the Duncan’s multiple range test
Accumulation of Cd in the roots and shoots of test seedlings was found to increase with increasing concentration of Cd in sand, and its content in Cd2 treated seedlings was increased by 15% in root and 10% in shoot over the respective values recorded under Cd1 treatment (Fig. 2). The Cd accumulation in root was 3.8-folds greater than shoot under Cd1 treatment while in case of Cd2 dose this ratio was 3.96-folds. HBL application caused significant (P < 0.05) reduction in Cd accumulation in tissues showing reduction of 25 and 33% in Cd content of root and 47 and 39% of shoot in test seedlings exposed to Cd1 and Cd2 stress, respectively. Cadmium content was not detected in control as well as HBL treated (without Cd) seedlings.
Impact of exogenous homobrassinoloid (HBL) on Cd accumulation in S. lycopersicum seedlings exposed to cadmium (Cd1: 3 mg Cd and Cd2: 9 mg Cd Kg−1 sand) stress (nd not detected). Data are means ± standard error of three replicates. Values with different letters show significant differences at P < 0.05 significance level between treatments according to the Duncan’s multiple range test
Effect on pigment contents, photosynthesis and respiration
Data pertaining to the photosynthetic pigments i.e. Chl a, Chl b and Car, and photosynthetic O2 yield and dark respiratory rate are presented in Table 1. Supply of Cd to the seedlings had an adverse impact on the pigment contents showing a decrease of 3 and 5% in Chl a, 15 and 19% in Chl b and 7 and 13% in Car under Cd1 and Cd2 treatments, respectively. When Cd untreated seedlings were applied with HBL the photosynthetic pigment contents were greater than control (without Cd and/or HBL treatment). Further, in Cd treated seedlings foliar application of HBL though caused significant rise in Chl a, Chl b and Car contents but the values were still less than that of control.
The rate of photosynthetic oxygen yield in S. lycopersicum seedlings grown under Cd1 and Cd2 treatments showed significant (P < 0.05) reduction as the decrease was 10 and 24%, respectively over the values of control. The HBL application alone showed stimulation in the rate of oxygen yield, and along with Cd doses, it ameliorated Cd induced toxicity showing a decrease of 3 and 17% in rate of photosynthetic oxygen yield in Cd1 and Cd2 treated seedlings, respectively. Unlike this, respiratory oxygen uptake rate was increased by 7 and 16% over the value of control when compared to Cd1 and Cd2 stressed seedlings, respectively (Table 1). Under HBL treatment on Cd stressed seedlings showed a declining trend in respiratory rate however, it was still greater than that of control.
Effect on chlorophyll a fluorescence transient
In order to understand the impact of Cd on photosynthetic activity in presence and absence of HBL, the changes in photochemistry of photosystem II (PS II) was analyzed using JIP-test (Figs. 3, 4). The values related to changes in chlorophyll a fluorescence kinetics parameters i.e. size and number of active reaction centres in the photosynthetic apparatus (Fv/F0), quantum yield of primary photochemistry (Fv/Fm or φP0), yield of electron transport per trapped exciton (Ψ0 or Psi_0), the quantum yield of electron transport (φE0) and performance index of PS II (PIABS) were found to decrease in Cd stressed seedlings, while the values showing the efficiency of water splitting complex (F0/Fv) exhibited an enhancement under similar condition (Fig. 3). Exogenous HBL caused significant increase in the values of FV/F0, FV/Fm, Ψ0, ϕE0 and PIABS in Cd1 and Cd2 treated seedlings while the values related with the F0/FV were found to decline. Furthermore, the treatment of Cd increase the energy fluxes parameters: ABS, TR0, ET0 and DI0 per RC significantly hence indicated the decreased proportion of active reaction centre (RC) in seedlings under tested stress. On the other hand, the application of HBL lowered the values of energy flux parameters in Cd treated seedlings indicating an enhancement in active RC even under Cd stress (Fig. 4).
Impact of exogenous homobrassinoloid (HBL) on maximum quantum yield of primary photochemistry (Phi_P0), yield of electron transport per trapped exciton (Psi_0), quantum yield of electron transport (Phi_E0) and performance index of PS II (PIABS) of S. lycopersicum seedlings exposed to cadmium (Cd1 and Cd2) stress. Data are means ± standard error of three replicates. Values with different letters show significant differences at P < 0.05 significance level between treatments according to the Duncan’s multiple range test
Impact of exogenous homobrassinoloid (HBL) on absorption of energy flux per active reaction centre (ABS/RC), electron transport flux per active RC (ET0/RC), trapped energy flux per active RC (TR0/RC) and energy dissipation flux per active RC (DI0/RC) in S. lycopersicum seedlings exposed to cadmium (Cd1 and Cd2) stress. Data are means ± standard error of three replicates. Values with different letters show significant differences at P < 0.05 significance level between treatments according to the Duncan’s multiple range test
Effect on leaf carbohydrate and protein contents
Data pertaining to the carbohydrate and protein contents have been presented in Table 2. Seedlings grown under Cd stress showed significant reduction in carbohydrate and protein contents as the decrease was 3 and 12% in carbohydrate and 12 and 32% in protein contents under Cd1 and Cd2 treatments, respectively (Table 2). Exogenous HBL on test seedlings induced appreciable rise in protein and carbohydrate contents in Cd unstressed seedlings, and a significant increase in these contents was also noticed in Cd stressed seedlings but the contents were still lower than that recorded in control (without Cd and HBL).
Effect on inorganic nitrogen contents
Effects of HBL on nitrate, nitrite and ammonium contents in Cd treated and untreated seedlings are presented in Table 2. Results revealed that Cd stress caused significant decrease in leaf NO3 − and NO2 − contents in the test seedlings showing a reduction of 15 and 38% in NO3 − and 13 and 32% under Cd1 and Cd2 treatment, respectively. Following HBL application there was significant rise in the levels of NO3 − and NO2 − but the amount was still lower than that observe in control (Table 2). Unlike NO3 − and NO2 − contents, both the doses of Cd were found to increase the NH4 + content by 19% in Cd1 and 26% in Cd2 treated seedlings. Under similar condition, exogenous application of HBL resulted in significant decrease in NH4 + content, but it was still higher than that of control.
Effect on nitrate and ammonia assimilating enzymes
The results pertaining to nitrate and ammonia assimilating enzymes i.e. NR, NiR, GS, GOGAT and GDH activities are shown in Fig. 5. Cadmium at Cd1 and Cd2 doses declined NR activity by 12 and 25% and the corresponding decrease in NiR was 6 and 13%, respectively as compared to control. Foliar application of HBL ameliorated the inhibitory effect caused by both the doses of Cd on NR and NiR activities significantly (P < 0.05).
Impact of exogenous homobrassinoloid (HBL) on nitrate reductase (NR), nitrite reductase (NiR), glutamine synthase (GS), glutamate synthase (GOGAT) and glutamate dehydrogenase (GDH) activities in S. lycopersicum seedlings exposed to cadmium (Cd1 and Cd2) stress. Data are means ± standard error of three replicates. Values with different letters show significant differences at P < 0.05 significance level between treatments according to the Duncan’s multiple range test
The activity of GS and GOGAT under Cd treatments was decreased considerably. Cadmium at Cd1 and Cd2 doses declined GS activity by 19 and 34% and the corresponding decrease in GOGAT was 20 and 33%, respectively as compare to control. Contrary to GS and GOGAT, GDH activity showed stimulation under Cd treatment in test seedlings. Cd1 and Cd2 doses increased GDH activity by 18 and 29%, respectively as compare to control (Fig. 5e). Exogenous HBL caused alleviating effect on Cd induced inhibition in GS and GOGAT activities in the test seedlings. Further, exogenous HBL treatment on Cd (Cd1 and Cd2) treated seedlings the GDH activity was reduced as compare to the respective values recorded under Cd1 and Cd2 treatment without HBL however, it was still higher than that of control (without Cd and HBL exposure).
Correlation analysis was performed between the effects of Cd and HBL alone as well as in combination on growth, photosynthesis, respiration and nitrogen assimilating enzymes of S. lycopersicum seedlings (Table 3). In the presence of Cd, growth (r = −0.929; P < 0.01) and photosynthesis (r = −0.961; P < 0.01) showed negative correlation and the respiration rate (r = 0.832; P < 0.01) was positively correlated while upon HBL + Cd treatment the growth (r = −0.396; ns) and respiration rate (r = 0.506; P < 0.05) exhibited similar correlation but the level of significance was less. Furthermore, the nitrogen metabolic enzymes such as NR (r = −0.966; P < 0.01), NiR (r = −0.885; P < 0.01), GS (r = −0.979; P < 0.01) and GOGAT (r = −0.979; P < 0.01) were also showing negative correlations and GDH (r = 0.962; P < 0.01) was positively correlated with the Cd alone while together with HBL the correlation was similar but the level of significance was found to be decreased for NR (r = −0.544; P < 0.05) and NiR (r = −0.672; P < 0.05) only (Table 3).
Discussion
The present study was performed to analyze the mechanisms of the beneficial effect of 28-homobrassinoloid (HBL) on S. lycopersicum seedlings exposed to two levels (Cd1 and Cd2) of cadmium. The toxicity of Cd increased with progressive rise in Cd concentration in sand culture. Such reduction in growth could be ascribed mainly to decrease in (i) pigment contents and photosynthetic O2 yield, (ii) performance of photosystem II (PS II) due to considerable accumulation of Cd in test seedlings (Fig. 2). In agreement with our result Hasan et al. (2011) also noticed the decrease in growth parameters of S. lycopersicum under increasing concentration of Cd. The foliar application of HBL significantly (P < 0.05) alleviated Cd toxicity in seedlings and it appeared that exogenous HBL played important role in counteracting the inhibitory effects of Cd stress on growth. The results are in agreement with those recorded by Ahammed et al. (2013) where brassinosteroids (BRs) modified the Cd toxicity in tomato plants. Further, the appreciable increase in the growth of seedlings owing to application of HBL could be correlated with increased photosynthetic activity as evidence by photosynthetic oxygen yield and chlorophyll fluorescence indices. Accumulation of Cd in roots at both the doses was considerably high (3.81- to 3.98-folds) as compared to shoots which indicates lower rate of translocation from roots to shoots. HBL application though caused substantial reduction in Cd accumulation in roots and shoots; however, the translocation ratio of Cd in roots to shoots was not affected. Our results are in agreement with earlier work where Cd accumulation was about seven times greater in roots than shoots in tomato plants and in presence of other plant hormone 24-epibrassinolide, a significant reduction in Cd accumulation was noticed (Ahammed et al. 2013). The significant rise in growth performance of Cd exposed seedlings following HBL application could be explained on the basis of decreased Cd accumulation (Fig. 2). The damaging effect on Chl and Car contents in S. lycopersicum seedlings may directly be linked with Cd induced inhibitory effect on enzymes involved in pigment biosynthesis as reported in barley leaves (Sabater and Rodriquez 1978). In contrast to this, the appreciable rise in Chl and Car contents by HBL application in Cd treated seedlings may be correlated with stimulating effect on pigments biosynthesis and down regulation of pigment degradation process. Similarly, Hayat et al. (2007) also reported that 28-HBL (10−8 M) increased chlorophyll pigment concentration in Brassica juncea plant pre-treated with NaCl.
In order to understand the Cd induced impact on photosynthesis, photosynthetic oxygen yield and chlorophyll fluorescence indices were analyzed in HBL treated and untreated S. lycopersicum seedlings. Photosynthetic oxygen yield was found to decline with Cd concentration dependent manner which may be correlated with the direct effect of Cd on oxygen evolving complex and different components of light reactions (Prasad and Zeeshan 2005). Besides this, the decrease in oxygen evolution might have resulted due to substantial damaging effect on light harvesting pigments. The dark respiration rate significantly increased with the increasing Cd concentrations which could be correlated with the supply of ATP needed to carry on the basic metabolism of plants (Prasad and Zeeshan 2005). Furthermore, Cd is known to induce uncoupling effect; hence enhanced respiratory oxygen uptake was noticed (Tiwari et al. 2002).
Photosynthetic O2 evolution in plants is directly correlated with photochemical activity of PS II thus the chlorophyll a fluorescence measurement which is emerged as a non-invasive and powerful technique to elucidate the damaging alterations in photosynthetic organization (PS II) in stressed plants (Govindjee 1995). The maximum primary yield (Fv/Fm) of photochemistry of PS II was decreased by Cd. The reduction in Fv/Fm signifies towards the inability of PS II in reducing the primary acceptor (QA) under Cd stress, while decline in size and number of active photosynthetic centres (Fv/F0) is indicating either a decreased rate of photochemistry (as primary electron acceptor pool becomes increasingly oxidized), or a reduction of the pool size of QA associated with PS II activity (Krause and Weiss 1991). Considerable decrease in Fv/Fm (φP0), Ψ0, φE0 and PIABS under Cd stress indicated an inhibition of downstream of QA − electron transfer. Hence, high concentration of Cd may inhibit the electron transport from QA − to QB. Similarly, significantly decreased performance index (PIABS) of test seedlings exposed to Cd was due to a decrease in the values of Fv/F0, Fv/Fm (φP0), Ψ0 and φE0. Furthermore, under Cd stress increased energy flux parameters such as ABS, TR0, ET0 and DI0 per RC in PS II may due to (i) decreased number of active reaction centers (Singh and Prasad 2015) and (ii) decreased photosynthetic pigments and photosynthesis. However, in Cd stressed seedlings HBL application exhibited an ameliorating effect on Cd induced toxicity on PS II photochemistry as evidence by less decrease in the values related with fluorescence kinetic parameters (i.e. φP0, Ψ0, φE0 and PIABS) and a slower rise in energy flux (ABS, TR0, ET0 and DI0) parameters. This effect was more prominent in Cd1 stressed seedlings under HBL application.
Protein and nitrogen contents may be considered as important indicators to assess the growth performance of plants under stress conditions. Protein synthesis is closely related to the production of new tissue and considered as a principal sink for nitrogen compounds. Cd at both the doses decreased leaf protein content in test plant (Table 2). Our results are in agreement with the earlier findings where Balestrasse et al. (2003) in Glycine max had correlated decrease in protein content under Cd stress as a result of enhanced protein degradation due to increased protease activity and/or decreased protein synthesis. Furthermore, the reduction in protein content under Cd stress might be associated with the possible decrease in amino acid content as a result of significant inhibition in GS and GOGAT activities in test plant (Fig. 5), and similar explanation has also been given for soybean by Balestrasse et al. (2003) and for Solanum nigrum and Solanum torvum by Xu et al. (2012) under Cd stress. However, with 28-HBL treatment in Cd stressed seedlings significant rise in the protein content over the values recorded under Cd stress only may be explained on the basis of more amino acid influx through GS-GOGAT pathway. Our results are in agreement with the earlier finding where Cd and Hg stressed Raphanus sativus showed significant rise in protein level following HBL application (Sharma et al. 2014).
Nitrogen is an integral part of proteins, nucleic acids and other important molecules like chlorophylls that determines growth and development of plants. The first step of nitrogen metabolism is catalyzed by cytosolic NR activity that converts NO3 − into NO2 − and subsequently NO2 − is converted into NH4 + by the action of chloroplastic NiR activity (Ogawa et al. 2000). In this study, the decrease in NO3 − contents in leaf tissues could also be correlated with (i) Cd-induced possible decrease in transpiration which may result into appreciable decrease in NO3 − translocation from root to shoot through xylem, (ii) excess generation of ROS induced by Cd which increased the membrane leakage in cells and decrease the nitrate absorption by root cells (Lu et al. 2009). Further, the reduction in NO2 − content may directly be linked with reduced NO3 − contents and also due to significant inhibition of NR activity. Contrary to this, increase in NH4 + under Cd stress in concentration dependent manner is probably due to (i) enhanced proteolysis activity and (ii) hindrance in ammonia assimilation process. On the other hand, Cd stressed seedlings upon HBL treatment exhibited significant rise in the nitrite contents even under Cd stress that may also be correlated with HBL induced increased activity of NR in leaf tissues of test seedlings. Cd stressed seedlings with 28-HBL treatment caused significant reduction in NH4 + content that could occur due to faster utilization of NH4 + to generate amino acid through increased GS and GOGAT pathway.
Furthermore, marked decrease was recorded in NR and NiR activities in test seedlings under Cd stress and this decrease might have occurred due to (i) reduced NO3 − uptake by roots or low NO3 − translocation through the xylem and subsequently low availability to plants or limitation in reducing capacity (Kumar and Joshi 2008), (ii) increased production of ROS that may alter the active site of enzymes and/or on enzyme synthesis (Balakumar and Paliwal 1998) and (iii) Cd might have down regulated the genes related to NR enzymes. Furthermore, in the present study, HBL (10−8 M) improved nitrogen metabolism, i.e. activity of nitrate reductase that may be because of BR and ABA act antagonistically and co-regulate various developmental process related to nitrogen metabolism (Chan and Gresshoff 2009).
In plants, NH4 + may be gradually formed at different metabolic reaction and its high level is deleterious to plant cell. Therefore, plants have developed a mechanisms via the GS/GOGAT cycle or GDH (an alternative routes for assimilating ammonium may be triggered when GS activity is impaired) pathways to minimize damage by ammonia (Britto and Kronzucker 2002). The NH4 + produced by NiR activity is then incorporated into an organic form primarily by GS/GOGAT cycle (Hodges 2002). Our results indicated that the decreased GS and GOGAT activities in seedlings seemed to be connected with the disturbance in NH4 + assimilation process as indicated by decreased protein and nitrogen contents, and increased NH4 + content under Cd stress. On the contrary, we have reported a marked increase in GDH activity under Cd stress and this increase in GDH activity might have compensated decreased GS and GOGAT activities. However, under similar treatments it seems that increased GDH activity might not be sufficient to sustain NH4 + assimilation as indicated by increased NH4 + content and decreased growth under Cd stress. It has been reported that GDH activity did not participate in primary NH4 + assimilation process in plants (Masclaux-Daubresse et al. 2006) however, under stress increase in GDH activity may play an important role in relieving pressure of NH4 + accumulation and in the production of glutamate for synthesis of protective compounds (Skopelitis et al. 2006; Gajewska and Sklodowska 2009). Moreover, application of HBL together with Cd showed lower GDH activity in comparison to the activity in Cd alone treated seedlings. The results suggest that seedlings grown under Cd toxicity when sprayed with HBL have improved primary route of NH4 + assimilation i.e. GS/GOGAT pathway than in Cd alone treated seedlings. Further, the alleviation in Cd phytotoxicity by the exogenous application of HBL is mostly due to increased levels of protein and carbohydrate contents and sustained nitrogen metabolism compared to Cd treatments alone. The beneficial aspects of HBL are also justified by correlating the studied parameters with both the treatments using Pearson correlation analysis. It showed that when the HBL was applied with Cd then the reduction in growth of seedlings was non-significant (Table 3) whereas only with Cd it was highly significant (P < 0.01) that exhibited ameliorative role of HBL in the reduction of Cd toxicity on plant growth.
Conclusion
This study indicated that exogenous application of HBL caused differential changes in S. lycopersicum seedlings under Cd phytotoxicity. Cd decreases the growth, photosynthetic pigments, photosynthesis and nitrogen metabolism, which was accompanied by significant increase in accumulation of Cd in tissues. Foliar application of 10−8 M HBL in seedlings together with Cd improved the growth, photosynthesis and nitrogen metabolism along with the reduction in Cd accumulation compared to seedlings treated with Cd alone. Thus, results suggest that application of HBL has practical importance in agricultural systems, as it improves the yield as well as quality of crops by reducing the Cd toxicity and by regulating physiological performance of plant. It also minimizes risk to human health by decreasing the metal accumulation in vegetable crops significantly.
Abbreviations
- ABS/RC:
-
Energy fluxes for absorption of photon per active reaction centre
- DI0/RC:
-
Energy dissipation flux per active reaction centre
- ET0/RC:
-
Electron transport flux per active reaction centre
- Fv/F0 :
-
Size and number of active reaction centres in photosynthetic apparatus
- F0/Fv :
-
Efficiency of water splitting complex
- Fv/Fm (φP0):
-
Maximum quantum efficiency of PS II photochemistry
- GS:
-
Glutamine synthatase
- GOGAT:
-
Glutamate synthase
- GDH:
-
Glutamate dehydrogenase
- NR:
-
Nitrate reductase
- NiR:
-
Nitrite reductase
- Psi_0 or Ψ0 :
-
Yield of electron transport per trapped exciton
- Phi_E0 or φE0 :
-
Quantum yield of electron transport
- RC:
-
Reaction centre
- TR0/RC:
-
Trapped energy flux per active reaction centre
References
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Ahammed G, Xia X, Li X, Shi K, Yu J, Zhou Y (2014) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16:462–473
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) 24-Epibrassinolide Protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 72:1387–1392
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds), Methods Plant Ecol. Blackwell Scientific Publication, Oxford, 285–344
Balakumar T, Paliwal K (1998) Action sites of UV-B radiation in the nitrate assimilation pathway of crop plants. In: Biologic effects of light. Springer, Kluwer Academic Publishers, Berlin, pp 57–59
Balestrasse KB, Benavides MP, Gallego SM, Tomaro ML (2003) Effect of cadmium stress on nitrogen metabolism in nodules and roots of soybean plants. Funct Plant Biol 30:57–64
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britto D, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Cao FB, Cai Y, Liu L, Zhang M, He XY, Zhang GP, Wu FB (2015) Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul 75:715–723
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Anal 6:71–80
Chan PK, Gresshoff PM (2009) Roles of plant hormones in legume nodulation. In: Horst W, Doelle Edgar J, DaSilva (eds) Encyclopedia of Life Support Systems (EOLSS): biotechnology. EOLSS Publishers, Oxford
Debouba M, Gouia H, Suzuki A, Ghorbel MH (2006) NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato Lycopersicon esculentum seedlings. J Plant Physiol 163:1247–1258
Divi UK, Rahman T, Krishna P (2016) Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol J 14:419–432
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Durand TC, Sergeant K, Planchon S, Carpin S, Label P, Morabito D, Hausman JF, Renaut J (2010) Acute metal stress in Populus tremula xP. alba (717–1B4 genotype): leaf and cambial proteome changes induced by Cd2+. Proteomics 10:349–368
Gajewska E, Sklodowska M (2009) Nickel-induced changes in nitrogen metabolism in wheat shoots. J Plant Physiol 166:1034–1044
Gill SS, Khan NA, Anjum NA, Tuteja N (2011) Amelioration of cadmium stress in crop plants by nutrients management: morphological, physiological and biochemical aspects. In: Anjum NA, Lopez–Lauri F (ed) Plant nutrition and abiotic stress tolerance III. Plant Stress 5:1–23
Govindjee (1995) Sixty–three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Gupta S, Nayek S, Saha RN, Satpati S (2008) Assessment of heavy metal accumulation in macrophyte, agricultural soil and crop plants adjacent to discharge zone of sponge iron factory. Environ Geol 55:731–739
Hasan SA, Hayat S, Ahmad A (2011) Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 84:1446–1451
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347: 1–39
Hodges M (2002) Enzyme redundancy and importance of 2-oxoglutarate in plant ammonium assimilation. J Exp Bot 53:905–916
Krause GH, Weiss E (1991) Chlorophyll fluorescence and photosynthesis: the basic. Annu Rev Plant Physiol 42:313–349
Kumar S, Joshi UN (2008) Nitrogen metabolism as affected by hexavalent chromium in sorghum (Sorghum bicolor L.). Environ Exp Bot 64:135–144
Kurra-Hotta M, Satoh K, Katoh S (1987) Relationship between photosynthesis and Chl content during leaf senescence of rice seedlings. Plant Cell Physiol 28:1321–1329
Li S, Yang W, Yang T, Chen Y, Nia W (2015) Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi: a cadmium accumulating plant. Int J Phytoremed 17:85–92
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lillo C (1984) Diurnal variations of nitrite reductase, glutamine synthetase, glutamate synthase, alanine amino transferase and aspartate amino transferase in barley leaves. Physiol Plant 61:214–218
Lu Y, Duan B, Zhang X (2009) Differences in growth and physiological traits of Populus cathayana populations as affected by enhanced UV-B radiation and exogenous ABA. Environ Exp Bot 66:100–109
Masclaux-Daubresse C, Reisdorf-Cren M, Pageau K, Lelandias M, Grandjean J, Valadier MH, Feraud M, Jouglet T, Suzuki A (2006) Glutamine synthetase, glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink source nitrogen cycle in tobacco. Plant Physiol 140:444–456
Molins-Legua C, Meseguer-Lloret S, Moliner-Martinez Y, Campıns-Falco P (2006) A guide for selecting the most appropriate method for ammonium determination in water analysis. Trends Anal Chem 25:282–290
Ogawa K, Soutome R, Hiroyama K, Hagio T, Ida S, Nakagawa H (2000) Co-regulation of nitrate reductase and nitrite reductase in cultured spinach cells. J Plant Physiol 157:299–306
Prasad SM, Zeeshan M (2005) UV-B radiation and cadmium induced changes in growth, photosynthesis, and antioxidant enzymes of cyanobacterium Plectonema boryanum. Biologia Plant 49:229–236
Rao SSR, Vardhini BV, Sujatha E, Anuradha S (2002) Brassinosteroids – a new class of phytohormones. Curr Sci 82 (10):1239–1245
Sabater B, Rodriquez MT (1978) Control of chlorophyll degradation in detached leaves of barley and oat through effect of kinetin on chlorophyllase levels. Physiologia Plant 43:274–276
Sharma RK, Agrawal M, Marshall FM (2006) Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull Environ Contam Toxicol 77:312–318
Sharma RK, Agrawal M, Marshall F (2009) Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food Chem Toxicol 47:583–591
Sharma N, Hundal GS, Sharma I, Bhardwaj R (2014) 28-Homobrassinolide Alters Protein Content and Activities of Glutathione-S-Transferase and Polyphenol Oxidase in Raphanus Sativus L. Plants Under Heavy Metal Stress. Toxicol Int 21(1):44–50
Singh A, Prasad SM (2011) Reduction of heavy metal load in food chain: technology assessment. Rev Environ Sci Biotechnol 10:199–214
Singh S, Prasad SM (2014) Growth, photosynthesis and oxidative responses of Solanum melongena L. seedlings to cadmium stress: mechanism of toxicity amelioration by kinetin. Sci Hortic 176:1–10
Singh S, Prasad SM (2015) IAA alleviates Cd toxicity on growth, photosynthesis and oxidative damages in eggplant seedlings. Plant Growth Regul 77(1):87–98
Singh RP, Srivastava HS (1983) Regulation of glutamate dehydrogenase activity by amino acids in maize seedlings. Physiol Plant 57:549–554
Singh RP, Srivastava HS (1986) Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol Plant 66:413–416
Singh S, Singh A, Bashri G, Prasad SM (2016) Impact of Cd stress on cellular functioning and its amelioration by phytohormones: an overview on regulatory network. Plant Growth Regul 80(3):253–263
Skopelitis DS, Paranychianakis NV, Paschalidis KA (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18:2767–2781
Snell FD, Snell CT (1949) Colorimetric methods of analysis, vol 3. Van Nostrand, New York, NY, pp 804–805
Soaresa C, Alexandra de Sousa A, Pinto A, Azenha M, Teixeira J, Antunes Azevedoc R, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterise and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing Photosynthesis: Mechanisms, Regulation and Adaptation. Taylor & Francis, London, pp 445–483
Sun JY, Shen ZG (2007) Effects of Cd stress on photosynthetic characteristics and nutrient uptake of cabbages with different Cd-tolerance. Chin J App Ecol 18:2605–2610
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Xu J, Sun J, Du L, Liu X (2012) Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol 196:110–124
Yusuf M, Fariduddin Q, Hayat S, Hasan SA, Ahmad A (2011) Protective responses of 28 homobrssinolide in cultivars of Triticum aestivum with different levels of nickel. Arch Environ Contam Toxicol 60:68–76
Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65:4371–4383
Acknowledgements
We are very grateful to University of Allahabad, Allahabad for providing necessary facilities. The University Grants Commission, New Delhi is thankfully acknowledged for providing financial assistant to Prof. S.M. Prasad as PI [Project No.: 41–460/2012(SR)] and to Shikha Singh as project fellow to carry out this work. I must acknowledge Prof. Anoop Chaturvedi, Head of the Statistic Department, and Dr. Anita Singh (DST Young Scientist) Department of Botany, University of Allahabad, Allahabad for their kind suggestion regarding statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Singh, S., Prasad, S.M. Effects of 28-homobrassinoloid on key physiological attributes of Solanum lycopersicum seedlings under cadmium stress: Photosynthesis and nitrogen metabolism. Plant Growth Regul 82, 161–173 (2017). https://doi.org/10.1007/s10725-017-0248-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0248-5