Abstract
The present study deals with the effects of 24-epibrassinolide on growth, lipid peroxidation, antioxidative enzyme activities, non-enzymatic antioxidants and protein content in 30 days old leaves of Brassica juncea (var. PBR 91) under zinc metal stress in field conditions. Surface sterilized seeds of B. juncea were given pre-soaking treatments of 24-EBL (10−10, 10−8 and 10−6 M) for 8 h. Different concentrations of zinc metal in the form of ZnSO4.7H2O (0, 0.5, 1.0, 1.5 and 2.0 mM) were added in the soil kept in experimental pots. Seeds soaked in 24-EBL for 8 h were sown in the earthern pots containing different concentrations of Zn metal. After 30 days of sowing, the plants were analyzed for growth parameters in terms of shoot length and number of leaves. Thereafter, leaves were excised and content of proteins, non-enzymatic antioxidants, malondialdehyde (MDA) and the activities of antioxidative enzymes (superoxide dismutase (SOD) (EC 1.15.1.1) catalase (CAT) (EC 1.11.1.6), ascorbate peroxidase (APOX) (EC 1.11.1.11), guaiacol peroxidase (POD) (EC 1.11.1.7) glutathione reductase (GR) (EC 1.6.4.2), monodehydroascorbate reductase (MDHAR) (EC 1.1.5.4) and dehydroascorbate reductase (DHAR) (EC 1.8.5.1)) were analyzed. It was observed that the growth of plants was inhibited under Zn metal stress. However, 24-EBL seed-presoaking treatment improved the plant growth in terms of increase in shoot length. 24-EBL also mitigated the toxicity of Zn metal by increasing the number of leaves. The activities of antioxidative enzymes (SOD, CAT, POD, GR, APOX, MDHAR and DHAR) and contents of proteins and glutathione were also enhanced in leaves of plants treated with 24-EBL alone, 10−8 M concentration being the most effective. The activities of antioxidative enzymes also increased in leaves of B. juncea plants by the application 24-EBL supplemented Zn metal solutions. Similarly, the content of proteins and glutathione increased considerably in leaves of B. juncea plants treated with 24-EBL, whereas the level of MDA content decreased in 24-EBL treated plants as compared to untreated control plants thereby revealing stress-protective properties of the brassinolide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brassica juncea L., an amphidiploid species, is grown as an oilseed crop in India. Although, oilseed Brassicas are grown over 15% arable land in India but their productivity is considerably hindered by various biotic and abiotic stresses (Shah 2002), like drought, chilling, pesticide and heavy metals etc. The soil in which plants grow may contain phytotoxic levels of the heavy metals including Cr, Cu, Hg, Ni and Zn etc. Zinc is a vital component of many important enzymes, a structural stabilizer for proteins, membrane and DNA-binding proteins (Zn-fingers) (Vallee and Auld 1992). Zn toxicity is indicated by a decrease in growth and development, metabolic activity and an induction of oxidative damage in various plant species (Panda et al. 2003). Although considered tolerant, uptake of these heavy metals in excess to nutritional requirements by plants may initiate a variety of metabolic responses which can cause damage at the cellular level or possibly lead to wider phytotoxic responses (Vangronsveld and Clijsters 1994). Due to this phytotoxic response, the plant stimulates the formation of reactive oxygen species (ROS) at various sites of respiratory and photosynthetic electron transport chain (Arora et al. 2002) and thus creates oxidative stress in cellular systems. The ROS such as hydrogen peroxide, superoxide radical and hydroxyl radical are highly reactive and induce lipid peroxidation, thereby affecting the structural integrity and permeability of cellular membranes. ROS can also cause protein denaturation and DNA damage (Savoure et al. 1997). To prevent accumulation of these reactive molecules, plants have developed a highly efficient antioxidative defense system, including low-molecular-weight antioxidants, such as ascorbate and glutathione and protective enzymes, such as SOD, CAT and the enzymes of the ascorbate-glutathione cycle. A common adaptive response of plants to oxidative stress is the increase of antioxidative compounds, as well as the increase in the activity and/or expression of one or more antioxidant enzymes (Hernandez et al. 2000). Several hormones have been implicated in modulating plant responses to oxidative stress, including ethylene (Vahala et al. 2003), salicylic acid (SA) (Metwally et al. 2003), abscisic acid (Kovtun et al. 2000) and brassinosteroids (BRs) (Cao et al. 2005).
Brassinosteroids (BRs) are potent plant growth regulators of steroidal nature, of which first compound isolated from a natural source was brassinolide. They are widely distributed in the plant kingdom and are active at very low concentrations ranging from nanomolar to micromolar. These are involved in multiple plant growth and development processes, such as cell elongation, vascular development, senescence, photomorphogenesis, flowering time control, and stress responses. An important feature of BRs is their ability to increase not only the yield, but also the quality of crops (Prusakova et al. 1999a). Many results suggest that BRs are required for optimal productivity and resistance to unfavourable influences of the environmental stresses (Khripach et al. 2000). The potential applications of BRs in agriculture and horticulture are based not only on their ability to increase crop yield, but also to ameliorate stress. Although innumerable works have confirmed the potential of the plant hormones to synergistically improve crop performance under normal conditions, very little light has been thrown on the influence of BRs under heavy metal stress. Keeping in mind the stress-ameliorative properties of BRs, the purpose of the present study was to increase our understanding of the effects of 24-epibrassinolide (24-EBL) on antioxidative defense system of Brassica juncea L. plants under zinc metal stress.
Materials & methods
The seeds of B. juncea L. cv. PBR 91 (certified) used in the present experiment were obtained from the Department of Plant Breeding, Punjab Agriculture University, Ludhiana, India. They were surface sterilized with 0.01% HgCl2 followed by three rinses in double distilled water. These surface sterilized seeds were soaked for 8 h in different concentrations of 24-EBL (0, 10−10, 10−8 and 10−6 M). The earthern pots to be used for the experiment were arranged in triplicates in the Botanical Garden of the University. Different concentrations of zinc metal in the form of ZnSO4.7H2O (0, 0.5, 1.0, 1.5 and 2.0 mM) were added in the pots containing approximately 5 Kg soil per pot. The soil used for the present study was prepared using garden soil, silt and cow dung manure in the ratio of 2: 1: 1. The seeds treated (8 h) with 24-EBL were sown (12 seeds) in the earthern pots (24 cm diameter) up to 3–4 cm deep that contained different concentrations of Zn metal. The earthern pots were kept in natural seasonal conditions. Irrigation was applied every 2 days to achieve soil water field capacity level. Thirty plants from three replicate (10 plants from each of the three replicates) were analyzed for shoot length and number of leaves per plant after 30 days of sowing. Thereafter, the leaves were excised from each plant.
For estimation of antioxidative enzyme activities and protein content, 0.5 g leaves of 30-day old B. juncea L. plants were homogenized in 5.0 ml of 100 mM potassium phosphate buffer (pH, 7.0). The homogenate was centrifuged at 4°C for 20 min at 15,000 g. The supernatant was used for assays of the activities of SOD, POD, CAT, GR, APOX, MDHAR and DHAR.
Antioxidative enzymes assays
Superoxide dismutase (EC 1. 15. 1. 1)
The activity of SOD was estimated according to Kono (1978) by monitoring its potential to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) dye by superoxide radicals, which are produced by the autooxidation of hydroxylamine hydrochloride
Catalase (EC 1. 11. 1. 6)
Catalase activity was determined as per the method of Aebi (1974).
Guaiacol peroxidase (EC 1. 11. 1. 7)
The activity of peroxidase was estimated according to the method proposed by Putter (1974).
Ascorbate peroxidase (EC 1. 11. 1. 11)
The activity of ascorbate peroxidase was estimated according to the method proposed by Nakano and Asada (1981).
Glutathione reductase (EC 1. 6. 4. 2)
Glutathione reductase activity was measured using the method given by Carlberg and Mannervik (1975).
Monodehydroascorbate reductase (EC 1.1.5.4)
Monodehydroascorbate reductase activity was determined according to the method proposed by Hossain et al. (1984).
Dehydroascorbate reductase (EC 1.8.5.1)
Activity of dehydroascorbate reductase was measured following the method given by Dalton et al. (1986).
Non-enzymatic antioxidant
Glutathione (GSH)
The reduced glutathione content was determined by the method proposed by Sedlak and Lindsay (1968).
Lipid peroxidation
Lipid peroxidation was measured in terms of malondialdehyde (MDA) content using the method of Heath and Packer (1968).
Protein estimation
Protein content was determined following the method of Lowry et al. (1951).
Results
-
1.
Growth parameters
30 DAS (Days After Sowing)
Morphological parameters
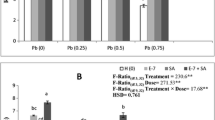
The observation made on various morphological parameters revealed that the treatment of 24-EBL to plants under Zn stress reduced the toxicity of metal by showing improved growth. The effects of seed presoaking treatments of 24-EBL on morphological parameters (shoot length and number of leaves) under Zn metal stress in field experiment are presented in Fig. 1(a).The studies revealed that the shoot length was reduced considerably from 6.1 cm in untreated control plants to 2.9 cm under increasing concentrations of Zn stress. However, 24-EBL treatment markedly increased the shoot length at 10−10, 10−8 and 10−6 M concentrations. Supplementation of Zn metal solution with 24-EBL considerably reduced the inhibitory effect of Zn on plant growth. The shoot length of plants treated with 10−6 M of 24-EBL supplemented with 0.5 mM of Zn metal solution (7.82 cm) was maximum in comparison to metal treated plants (5.68 cm), whereas the number of leaves did not reveal significant changes with Zn treatment as compared to untreated and unstressed control (or distilled water treated) leaves of B. juncea. The number of leaves of plants revealed very less decrease as the concentration of metal increased and was observed in case of plants treated with 1.0 mM of Cr (4.33) when compared to untreated and unstressed control plants (4.66). However, treatment with 24-EBL further improved the number of leaves at 10−10, 10−8 and 10−6 M alone and in combination with Zn metal (Fig. 1a).
-
2.
Biochemical studies
-
I.
Protein content and lipid peroxidation:
Protein content: Different concentrations of zinc viz., Zn 0.5, Zn 1.0, Zn 1.5 and Zn 2.0 mM lowered the protein content (24.11 mg/g FW in Zn 0.5) compared to untreated control (42.42 mg/g FW) in leaves of 30 days old B. juncea plants (Fig. 1b). But, seed-presoaking treatment with 24-EBL considerably increased protein content in B. juncea leaves under Zn metal stress. Plants treated with 10−8 M of 24-EBL under the stress of 2.0 mM Zn depicted maximum protein content (81.24 mg/g FW) in comparison to metal treated plants (32.07 mg/g FW).
Lipid peroxidation: The leaves of B. juncea showed an increase in MDA (malondialdehyde) content with increasing concentrations of Zn metal (Fig. 1b). The increase in MDA content was maximum at 2.0 mM (27.62 μmol/g FW) in comparison to untreated control (4.19 μmol/g FW). However, 24-EBL treatment lowered the MDA content in leaves of B. juncea plants. 10−6 M concentration of 24-epiBL caused a maximum decrease in MDA content (2.27 μmol/g FW). Minimum content of MDA (3.76 μmol/g FW) was observed in 10−6 M of 24-EBL supplemented with 2.0 mM of Zn solution as compared to 2.0 mM of Zn metal treated plants (27.62 μmol/g FW).
-
II.
Antioxidant
Glutathione content
The glutathione content of leaves of 30 days old plants increased considerably in all treatments of 24-EBL in comparison to untreated control (Fig. 1b). It was maximum in leaves of plants treated with 10−8 M of 24-EBL (0.26 mg/g FW) when compared to untreated control plants (0.11 mg/g FW). It was remarkably higher (0.47 mg/g FW) in the leaves of plants treated with 10−6 M of 24-EBL supplemented with 2.0 mM of Zn when compared to Zn 2.0 metal treated plants (0.33 mg/g FW).
-
III.
Antioxidative enzyme activities
The activities of antioxidative enzymes (SOD, CAT, POD, GR, APOX, MDHAR and DHAR) were also enhanced in leaves of plants treated with 24-EBL alone, 10−8 M concentration being the most effective. The activities of antioxidative enzymes were also enhanced in leaves of B. juncea by the application 24-EBL supplemented Zn solutions.
Enzymes involved in detoxification of oxygen radical
-
(a)
Detoxification of superoxide (O −2 ) radical
SOD activity got enhanced from 5.75 mol UA/mg protein in untreated control leaves of B. juncea plants to 8.13 mol UA/mg protein in Zn metal treated plants (2.0 mM). 24-EBL alone further boosted the SOD activity, with maximum rise at 10−8 M concentration of 24-EBL. The activity of SOD revealed considerable increase at 10−6 M of 24-EBL supplemented with Zn2.0 solution (13.58 mol UA/mg protein) when compared to Zn2.0 concentration (8.13 mol UA/mg protein) treated plants.
-
(b)
Detoxification of hydrogen peroxide
CAT and POD: The activities of CAT and POD did not increase significantly under zinc metal stress (Figs. 1b & c for CAT and POD respectively). 10−10 M of 24-EBL showed maximum activity of CAT (24.59 mol UA/mg protein) whereas 10−8 M showed highest POD activity (0.048 m mol UA/mg protein) in comparison to untreated control (5.22 mol UA/mg protein and 0.041 m mol UA/mg protein respectively for CAT and POD). The activities of these hydrogen peroxide detoxifying enzymes were enhanced by 24-EBL under Zn metal stress. Maximum increase in activity of CAT (22.0 mol UA/mg protein) and POD (0.11 m mol UA/mg protein) was observed in leaves of plants treated with 10−10 and10−8 M respectively of 24-EBL in combination with 2.0 and 1.5 mM of Zn respectively as compared to Zn2.0 for CAT (5.93 mol UA/mg protein) and Zn1.5 for POD (0.06 m mol UA/mg protein).
-
(c)
Ascorbate-glutathione cycle
APOX and GR: During the present investigation, there was an increase in the activity of APOX but there wasn’t considerable increase in the activity of GR with increasing concentrations of Zn metal (Fig. 1c). The APOX activity got increased from 0.43 m mol UA/mg protein (untreated control) to 1.08 m mol UA/mg protein (2.0 mM of Zn metal stress). Whereas GR activity merely revealed an increase from 0.141 m mol UA/mg protein in untreated control plants to 0.147 m mol UA/mg protein in 2.0 mM of Zn metal treated plants. Seed presoaking treatment at a concentration of 10−6 M of 24-EBL showed an increment in the APOX activity (2.49 m mol UA/mg protein) whereas 10−8 M of 24-EBL treatment increased the activity of GR (0.16 m mol UA/mg protein) in comparison to untreated control (0.43 m mol UA/mg protein and 0.14 m mol UA/mg protein for APOX and GR). To overcome the stress, maximum activity of APOX was observed at 10−8 M of 24-EBL in combination with 2.0 mM of Zn metal solution (1.089 m mol UA/mg protein) whereas maximum GR activity was observed at 10−8 M of 24-EBL in combination with 1.5 mM of Zn solution (0.306 m mol UA/mg protein) as compared to leaves of 2.0 mM of Zn metal treated plants (1.081 m mol UA/mg protein) for APOX and 1.5 mM of Zn (0.132 m mol UA/mg protein) for GR.
MDHAR and DHAR: The results of the studies carried out to ascertain the involvement of other enzymes (MDHAR and DHAR) of the ascorbate-glutathione cycle in detoxification of toxic oxygen species are depicted in Fig. 1d. The activity of MDHAR did not reveal significant increase whereas DHAR activity was enhanced considerably over the control leaves under zinc metal stress. MDHAR activity revealed a mere increase from 0.294 m mol UA/mg protein (untreated control) to 0.33 m mol UA/mg protein (Zn2.0) whereas DHAR activity showed a tremendous increase from 0.207 m mol UA/mg protein (untreated control) to 0.459 m mol UA/mg protein (Zn2.0). MDHAR and DHAR activity further got enhanced by the treatment of 24-EBL alone, with maximum rise at 10−10 M concentration of 24-EBL (0.38 mol UA/mg protein for MDHAR and 0.42 mol UA/mg protein for DHAR). MDHAR activity revealed a maximum increase at 10−6 M of 24-EBL in combination with 2.0 mM concentration of Zn solution (0.69 mol UA/mg protein) whereas DHAR activity showed a maximum increase at 10−10 M of 24-EBL in combination with 1.5 mM Zn solution (0.45 mol UA/mg protein) as compared to Zn2.0 (0.33 mol UAmg protein) and Zn1.5 (0.27 mol UA/mg protein) metal treated plants respectively.
-
I.
a Effect of 24-EBL on shoot length and number of leaves of 30-days old leaves of B. juncea under Zn metal stress. Bars represent the SE (n = 3) and asterisks indicate statistically significant differences from control treatments at P < 0.05. b Effect of 24-EBL on protein content, glutathione content, malondialdehyde content and specific activity of catalase on 30-days old leaves of B. juncea L. under Zn metal stress. Bars represent the SE (n = 3) and asterisks indicate statistically significant differences from control treatments at P < 0.05. c Effect of 24-EBL on specific activities of superoxide dismutase, guaiacol peroxidase, ascorbate peroxidase and glutathione reductase on 30-days old leaves of B. juncea L. under Zn metal stress. Bars represent the SE (n = 3) and asterisks indicate statistically significant differences from control treatments at P < 0.05. d Effect of 24-EBL on specific activities of monodehydroascorbate reductase and dehydroascorbate reductase on 30-days old leaves of B. juncea L. under Zn metal stress. Bars represent the SE (n = 3) and asterisks indicate statistically significant differences from control treatments at P < 0.05
Discussion
India ranks second in the world with respect to production of Brassicas (Afroz et al. 2005) and supplies about 7% of the world’s edible oil (Khan et al. 2002). However, Indian mustard production still remains inadequate to meet even the daily requirement of its people (Khan et al. 2002). This low economic yield can be attributed to the crop’s susceptibility to a number of biotic and abiotic stresses, among which of alarming concern is the heavy metal. The role of an agronomist is, therefore, to manipulate the crop in order to counteract the influence of this stress, and boost performance even under metal stressed conditions. In this regard, attention has now been paid to the use of plant growth regulators, such as gibberellic acid (GA3), cytokinins, salicylic acid, BRs etc. which are known to be prominently concerned in the regulation of plant responses. BRs are endogenous plant polyhydroxy-steroids eliciting remarkable growth-promoting and developmental effects at nanomolar to micromolar concentrations (Ali et al. 2008; Hasan et al. 2008). They are involved in regulatory processes, which are more specific to plant growth, including photomorphogenesis and skotomorphogenesis (Cevahir et al. 2008; Li and Jin 2007; Vert and Chory 2006). The enthusiasm of BR research stemmed from exciting early observations showing that brassinolide treatment significantly improved growth rate, yield, stress tolerance, and disease resistance of the major crops, ornamentals, vegetables and trees (Szekeres et al. 1996). In the present study, it was observed that application of 24-EBL improved the growth of 30-d old Zn-stressed B. juncea plants (Fig. 1a). Plant growth is dependent upon the synthesis of nucleic acids and proteins. BRs participate in the growth of plant tissue in the processes of transcription and translation. Bajguz (2000) demonstrated that the activation of the growth of plant tissue and higher levels of RNA and DNA polymerase is manifested by the increase of the DNA, RNA and protein contents. Enhancement of these enzyme activities may be the result of regulation of gene expression by BRs and may be concerned directly, or indirectly, with growth promotion induced by BRs. Earlier studies also indicated stress-protective properties of BRs. Ali et al. (2007) also observed that 24-EBL and 28-HBL improved the growth of Al-stressed mung bean seedlings by increasing the rate of photosynthesis and activity of carbonic anhydrase. The studies on BRs report that they regulate cell elongation and divisional activities by activating the cell wall loosening enzymes, which increase the synthesis of cell wall and membrane materials (Khripach et al. 2000). The cell wall loosening enzymes get activated by H+- ATPases which acidifies the apoplast. The involvement of BRs in enhancing the growth of seedlings might be taking place through activation of H+-ATPase (Cerana et al. 1983; Haubrick and Assmann 2006). Similarly, Vardhini and Rao (2003) reported that BRs application resulted in enhancement of seedling growth, which was evident in terms of seedling length, seedling fresh and dry weights of all the three varieties of sorghum (Sorghum vulgare) viz. CSH-14 and ICSV-745 (susceptible to water stress) and M-35-1 (resistant towater stress) under osmotic stress.
24-EBL further improved the plant growth by increasing the protein content and by decreasing the levels of lipid peroxidation under Zn metal stress (Fig. 1b). BRs are found to affect the transcription and translation processes of specific genes related to stress tolerance (Bajguz 2000; Dhaubhadel et al. 2002; Kagale et al. 2007). Kartal et al. (2009) reported that 28-HBL treatment significantly increased protein content in barley seedlings. Kulaeva et al. (1991) reported that BRs can protect cereal leaf cells from heat shock stress. Pre-treatment with both 22S, 23S-homoBL and 24-EBL activated total protein synthesis and de novo synthesis of different polypeptides when wheat leaves were heat-shocked by subjecting them to 40°C as well as normal temperatures. In addition, 22S, 23S-homoBL stimulated the formation of heat-shock granules in the cytoplasm and increased thermo tolerance of total protein synthesis under heat shock. Our earlier studies also reported that BRs confer tolerance against heavy metals (Ni, Cu, Mn) either by reducing their uptake or by activating the antioxidative enzymes in case of B. juncea, B. campestris and Zea mays (Kaur and Bhardwaj 2003; Sharma and Bhardwaj 2007; Arora et al. 2008; Bhardwaj et al. 2008). As membrane destruction results from ROS induced oxidative damage (Mittler 2002), the 24-EBL treated plants might be scavenging ROS more effectively than the plants treated with metal alone. The observations are in consistence with the results of Ozdemeir et al. (2004), who reported that lipid peroxidation level induced by NaCl was significantly lowered in rice seedlings when treated with 24-EBL. In the present study, it was also observed that the root length got decreased in plants treated with 24-epibrassinolide (24-EBL) which in turn might have reduced the protein content. 24-EBL has also been found to retard root growth in maize, mungbean, wheat and soybean (Roddick and Ikekawa 1992; Hunter 2001). The alteration of root growth may be an expression of the cascade of the altered pattern of biochemical reactions either by directly involving the genes or through extra-genetic route (Hayat and Ahmad 2003). The greater increase in malondialdehyde (MDA) content at 10−10 M of 24-EBL treated plants may be a reflection of changes in the activities of antioxidative enzymes. It might be possible that different mechanisms may co-operate in order to strengthen the antioxidative defense system of plants (Rizhsky et al. 2002).
In the present study, there was an increase in glutathione content by the application of 24-EBL in leaves of 30-d old B. juncea plants (Fig. 1b). The results are in coherence with Bajguz (2000). He reported that BRs and its derivatives at a concentration of 10−4 mol/L under lead metal stress in Chlorella vulgaris acted as a stimulator of phytochelatins which are derived from glutathione pool thus proving the role of glutathione in lead metal detoxification.
The present investigation also reveals that treatment of 24-EBL significantly enhanced the activities of all antioxidative enzymes (e.g. SOD, CAT, POD, APOX, GR, MDHAR and DHAR) when compared to untreated plants (treated with Zn alone) (Fig. 1c and d). The increase in activities of these antioxidative enzymes is a general response to ROS produced by various biotic and abiotic stresses including heavy metal stress (Arora et al. 2002). Among ROS, superoxide radical (O2 .−) is dismutated by SOD into H2O2 and is further removed by CAT in the peroxisomes or by APOX of the ascorbate-glutathione antioxidant cycle in the chloroplast or by membrane bounded POD (Foyer et al. 1997). GR activity maintains the pool of glutathione in the reduced state, which in turn reduces dehydroscorbate to ascorbate through the ascorbate-glutathione cycle (Noctor and Foyer 1998). Besides, SOD, CAT and POD represent the endogenous defense of plant cells. These enzymes are present in different isoforms in numerous cell compartments and their expression is genetically controlled and regulated both by developmental (as in the present study i.e. 30 days after sowing) and environmental stimuli and also according to the necessity for removing ROS (reactive oxygen species) produced in cells (Mittler et al. 2004). Apart from this, the regulation of number of processes such as alteration of antioxidative enzyme activities, assimilate partitioning etc. varies with the type of plant, the cultivar used for study, the type of hormones and the mode of application (Fariduddin et al. 2008).
It is widely accepted that ROS are responsible for various stress-induced damages to macromolecules and ultimately to cellular structures (Halliwell and Gutteridge 1999). Consequently, the role of antioxidative enzymes, such as SOD, CAT, POD, APOX, GR, MDHAR and DHAR becomes very important.
In the present study it was observed that application of 24-EBL to stressed plants further boosted and strengthened the antioxidative defense system of the plant by stimulating the activities of various antioxidative enzymes viz. SOD, CAT, POD, APOX, GR, MDHAR and DHAR. Earlier reports also showed that exogenous application of BRs modified antioxidant enzyme activity (Arora et al. 2008; Ali et al. 2007; Hayat et al. 2009). Nunez et al. (2003) observed higher activity of antioxidative enzymes in rice, grown under salinity and supplemented with polyhydroxylated spirostanic brassinosteroid analogue (BB-16). 24-EBL also ameliorated the cadmium toxicity in B. juncea and Cicer arietinum plants by increasing the activities of peroxidase, catalase and superoxide dismutase (Hayat et al. 2007; Hasan et al. 2008). The present study therefore reveals the anti-stress properties of BRs in the B. juncea, an important oilseed crop of edible and medicinal importance, exposed to heavy metal (Zn) stress. 24-EBL improved the vigor in terms of increased shoot length and number of leaves, decreased lipid peroxidation, increased protein and glutathione content and enhanced activities of Asada-Halliwell pathway regulated key antioxidative enzymes.
Abbreviations
- ANOVA:
-
Analysis of variance
- APOX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- Cont.:
-
Control
- DHAR:
-
Dehydroascorbate reductase
- 24-EBL:
-
24-epibrassinolide
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- 28-HBL:
-
28-homobrassinolide
- MDHAR:
-
Monodehydroascorbate reductase
- POD:
-
Guaiacol peroxidase
- ROS:
-
Reactive Oxygen Species
- SA:
-
Specific activity
- SOD:
-
Superoxide dismutase
- UA:
-
Unit activity
- Zn:
-
Zinc
- Zn0.5 :
-
0.5 mM of Zn
- Zn1.0 :
-
1.0 mM of Zn
- Zn1.5 :
-
1.5 mM of Zn
- Zn2.0 :
-
2.0 mM of Zn
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhan, pp 673–684
Afroz SM, Firoz S, Hayat MH, Siddiqui (2005) Exogenous application of gibberellic acid counteracts the ill effect of sodium chloride in mustard. Turk J Biol 29:233–236
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum). Environ Exp Bot 59:217–223
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiate L. Wilczek). Environ Exp Bot 62:153–159
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Arora N, Bhardwaj R, Sharma P, Arora HK, Arora P (2008) Amelioration of zinc toxicity by 28-homobrassinolide in Zea mays L. Can J Pure Applied Sci 2(3):503–509
Bajguz A (2000) Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiol Biochem 38(3):209–215
Bhardwaj R, Sharma P, Arora HK, Arora N (2008) 28-Homobrassinolide regulated Mn-uptake and growth of Brassica juncea L. Can J Pure Applied Sci 2(1):149–154
Cao S, Xu Q, Cao Y, Quian K, An K, Zhu Y, Bineng H, Zhao H, Kuai B (2005) Loss of function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123:57–66
Carlberg I, Mannervik B (1975) Purification of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Cerana R, Bonetti A, Marre MT, Romani G, Lado P (1983) Effects of a brassinosteroid on growth and electrogenic proton extrusion in Azuki bean epicotyls (Vigna angularis). Physiol Plant 59:23–27
Cevahir G, Yentur S, Eryilmaz F, Yilmazer N (2008) Influence of brassinosteroids on pigment content of Glycine max L. (Soybean) grown in dark and light. J Appl Biol Sci 2(1):23–28
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83:3811–3815
Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29(6):681–691
Fariduddin Q, Hasan SA, Ali B, Hayat S, Ahmad A (2008) Effect of modes of application of 28-homobrassinolide on mung bean. Turk J Biol 32:17–21
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, Oxford
Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environ Poll 151:60–66
Haubrick LL, Assmann SM (2006) Brassinosteroids and plant function: some clues, more puzzles. Plant Cell Environ 29:446–457
Hayat S, Ahmad A (2003) 28-Homobrassinolide induced changes favoured germinability of wheat grains. Bulg J Plant Physiol 29(1–2):55–62
Hayat S, Ali B, Hassan SA, Ahmad A (2007) Brassinosteroids enhanced antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60(1):33–41
Hayat S, Ali B, Hassan SA, Hayat Q, Ahmad A (2009) Brassinosteroids protect Lycopersicon esculentum from cadmium toxicity applied as shotgun approach. Protoplasma. doi:10.1007/s00709-009-0075-2
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stiochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernandez JA, Jimenez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ 23:853–862
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hunter WJ (2001) Influence of root-applied epibrassinolide and carbenoxolone on the nodulation and growth of soybean (Glycine max L.) seedlings. J Agron Crop Sci 186(4):217–221
Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroids confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225:353–364
Kartal G, Temel A, Arican E, Gozukirmizi N (2009) Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regul 58:261–267
Kaur S, Bhardwaj R (2003) Brassinosteroids regulated heavy metals uptake in Brassica campestris L. Annual Meeting of the American Society of Plant Biologists. Plant Biol. 628. Honolulu
Khan NA, Ansari HR, Khan M, Mir R, Samiullah (2002) Effect of phytohormones on growth and yield of Indian mustard. Indian J Plant Physiol 7:75–78
Khripach VA, Zhabinskii VN, de-Groot AE (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Kono Y (1978) Generation of Superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated MAPK cascade in plants. Proc Natl Acad Sci 97:2940–2945
Kulaeva ON, Burkhanova EA, Fedina AB, Khokhlova VA, Bokebayeva GA, Vorbrodt HM, Adam G (1991) Effect of brassinosteroids on protein synthesis and plant-cell ultrastructure under stress conditions. In: Cutler HG, Yokota T, Adam G (eds) Brassinosteroids: chemistry, bioactivity and applications. American Chemical Society symposium series 474. American Chemical Society, Washington, pp 141–155
Li J, Jin H (2007) Regulation of brassinosteroid signalling. Trends Plant Sci 12:37–41
Lowry OH, Resbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler RS, Vanderauwera M, Gollery F, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Nunez M, Mazzafera P, Mazorra LM, Siqueira WJ, Zullo MAT (2003) Influence of brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47:67–70
Ozdemeir F, Bor M, Demiral T, Turkan I (2004) Effects of 24- epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211
Panda SK, Chaudhury I, Khan MH (2003) Heavy metals induce lipid peroxidation and affects antioxidants in wheat leaves. Biol Plant 46:289–294
Prusakova LD, Ezhov MN, Salnikov AI (1999a) The use of emistim,epibrassinolide and uniconazole to overcome quality difference of buckwheat grains. Agrarian Russia: 41–44
Putter J (1974) Peroxidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhan, pp 685–690
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inzé D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32:329–342
Roddick JG, Ikekawa N (1992) Modification of root and shoot development in monocotyledon and dicotyledon seedlings by 24-epibrassinolide. J Pl Physiol 140:70–74
Savoure A, Hua XJ, Bertauche N, Van Montagu M, Verbruggen N (1997) Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol Gen Genet 254:104–109
Sedlak J, Lindsay RH (1968) Estimation of total, protein bound and non-protein sulphydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Shah SH (2002) Effects of salt stress on mustard as affected by gibberellic acid application. Gen Appl Plant Physiol 33(1–2):97–106
Sharma P, Bhardwaj R (2007) Effects of 24-Epibrassinolide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol Plant 29:259–263
Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85:171–182
Vahala J, Ruonala R, Reinanen M, Tuomenen H, Kangasjarvi J (2003) Ethylene insensitivity modulates ozone induced cell death in Birch. Plant Physiol 132:185–195
Vallee BL, Auld DS (1992) Active zinc binding sites of zinc metalloenzymes. Matrix Suppl 1:5–19
Vangronsveld JH, Clijsters (1994) Toxic effects of metals. In: Farago ME (ed) Plants and the chemical elements. Biochemistry, uptake, tolerance and toxicity. VCH Publishers, Weinheim, pp 150–177
Vardhini BV, Rao SSR (2003) Amelioration of osmotic stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul 41:25–31
Vert G, Chory J (2006) Downstream nuclear events in brassinosteroid signaling. Nature 441:96–100
Acknowledgements
Financial assistance from University Grants Commission, New Delhi, India is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arora, P., Bhardwaj, R. & Kumar Kanwar, M. 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress. Physiol Mol Biol Plants 16, 285–293 (2010). https://doi.org/10.1007/s12298-010-0031-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-010-0031-9