Abstract
Soil salinity is one of the most severe factors limiting growth and physiological response in Vigna sinensis plants. Plant salt stress tolerance requires the activation of complex metabolic activities including antioxidative pathways, especially reactive oxygen species and scavenging systems within the cells which can contribute to continued growth under water stress. The present investigation was carried out to study the role of brassinolide in enhancing tolerance of cowpea plants to salt stress (NaCl). Treatment with 0.05 ppm brassinolide as foliar spray mitigated salt stress by inducing enzyme activities responsible for antioxidation, e.g., superoxide dismutase, peroxidase, polyphenol oxidase, and detoxification as well as by elevating contents of ascorbic acid, tocopherol, and glutathione. On the other hand, total soluble proteins decreased with increasing NaCl concentrations in comparison with control plants. However, lipid peroxidation increased with increasing concentrations of NaCl. In addition to, the high concentrations of NaCl (100 and 150 mM) decreased total phenol of cowpea plants as being compared with control plants. SDS-PAGE of protein revealed that NaCl treatments alone or in combination with 0.05 ppm brassinolide were associated with the disappearance of some bands or appearance of unique ones in cowpea plants. Electrophoretic studies of α-esterase, β-esterase, polyphenol oxidase, peroxidase, acid phosphatase, and superoxide dismutase isoenzymes showed wide variations in their intensities and densities among all treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is still one of the major environmental factors that limit crop production. Egypt is one of the countries that suffer from severe salinity problems. For example, 33% of the cultivated land is salinized due to low precipitation (<25 mM annual rainfall) and irrigation with saline water.
As a result of the human activities of the last decades, soil salinity and drought are serious problems in both agricultural and natural ecosystems. About 20% of the cultivated area and nearly half of all irrigated lands in the world are affected by salinity and/or drought. Salt stress causes a lot of deteriorative internal physiological and biochemical changes including the accumulation of low weight solutes, such as proline, polyols, amino acids, proteins, and betaine commonly referred to as compatible solutes and regulatory mechanism for ion transport (Agarwal and Pandey 2004). Moreover, salinity leads to oxidative stress in plants due to production of reactive oxygen species (ROS) such as superoxide radical (O −2 ), hydrogen peroxide (H2O2), and hydroxyl radical (OH−).These ROS produced in the cell and interact with a number of vital cellular molecules and metabolites thereby leading to a number of destructive processes causing cellular damage (Ashraf 2009). Excess of ROS trigger phytotoxic reactions such as lipid peroxidation, protein degradation, and DNA mutation. Some plant species have the potentiality to evolve mechanisms to protect their cellular and subcellular systems from the effects of these reactive oxygen radicals by enhancing enzymes such as superoxide dismutase, catalase, peroxidase, glutathione reductase, polyphenol oxidase, and non-enzymic ascorbate and glutathione (Agarwal and Pandey 2004).
Brassinosteriods (BRs) are a group of naturally occurring plant steroidal compounds with wide ranging biological activity that offer the unique possibility of increasing crop yield through both changing plant metabolism and protect plants from environmental stresses (Arora et al. 2008). BRs are recognized as regulators of transcription and translation thereby improving the level of proteins. Several BRs mainly brassinolide have been evaluated in the field and have produced significant yield increases in various crops. Application of BRs improved tolerance against salt in rice (Özdemir et al. 2004) and wheat (Sairam et al. 2005).
The aim of the present study was to assess the effect of different concentrations of NaCl on physiological and biochemical attributes of cowpea plants and to alleviate the effect of NaCl by addition of plant growth regulation (brassinolide).
Materials and methods
Plant material
The experimental plant used in this investigation was pure strain of cowpea (Vigna sinensis) seeds were kindly obtained from the Agricultural Research Center in Giza Egypt.
Treatments
Seeds were surface sterilized with 0.5% (v/v) sodium hypochlorite. The washed seeds were sown in pots (25 cm in diameter) containing equal amounts of homogeneous soil. Each treatment contains three pots, and ten seeds were sown in each pot. The pots were divided into three groups. The first group was irrigated with pure water to severe as controls. The second group was irrigated with four concentrations of sodium chloride (25, 50, 100, and 150 mM). The last group was irrigated with the four concentrations of sodium chloride (25, 50, 100, and 150 mM) and then sprayed twice with 0.05 ppm brassinolide after 25 days (when each plant was carrying three compound leaves) and 32 days from sowing. At 45 days from sowing, the plants were collected to determine certain morphological characters in addition to the total phenol, lipid peroxidation, total soluble protein, non-enzymatic and enzymatic antioxidants, protein electrophoresis, and isoenzyme.
Determination of total phenols
Levels of soluble phenols in cowpea leaves were determined in accordance with Dihazi et al. (2003). The absorbance of the developed blue color was read at 725 nm. Tannic acid was used as standard and the amount of soluble phenols was expressed as milligrams of tannic acid per gram of dry weight.
Lipid peroxidation
Lipid peroxidation was determined by estimating the malondialdehyde content following the method of Heath and Packer (1968). The absorbance of the resulting supernatant was recorded at 532 and 600 nm. The nonspecific absorbance at 600 nm was subtracted from the 532 nm absorbance. The absorbance coefficient of malondialdehyde was calculated by using the extinction coefficient of 155 mM–1 cm–1.
Determination of total soluble protein
The leaves were grinded in sodium phosphate buffer at pH 6.5. The total soluble protein content in the supernatant was determined according to Lowry et al. (1951).
Assay of non-enzymatic antioxidant
Ascorbic acid contents
Content of ascorbic acid was estimated according to Mukherjee and Choudhuri (1983). The absorbance was recorded at 530 nm.
Tocopherols contents
The absorbance of α-tocopherol was recorded at 520 nm against ethanol as a blank (Philip et al. 1954). The content of α-tocopherol in the extracts was calculated from the regression equation of the standard curve.
Glutathione contents
The measurement of total non-protein SH group was carried out following the method of Cakmak and Marschner (1992).
Extraction and assay of enzymatic antioxidant
The leaves were grinded in sodium phosphate buffer at pH 6.5 for superoxide dismutase (SOD), peroxidase (POX), and polyphenol oxidase (PPO). The supernatant was used to measure the activity of the enzymes.
POX and PPO were assayed following the method of Kar and Mishra (1976). The samples were read at 430 nm, and the enzyme activity was expressed as the change in the optical density/g of fresh weight/hour.
SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium chloride (NBT), using a reaction mixture consisting of 1 M Na2CO3, 200 mM methionine, 2.25 mM NBT, 3 mM EDTA, 60 mM riboflavin, and 0.1 M phosphate buffer (pH 7.8). Absorbance was read at 560 nm (Beauchamp and Fridovich 1971).
Protein electrophoresis
Gel electrophoresis
SDS-PAGE was carried out with gel slabs according to the method of Laemmli (1970). Protein subunit bands were stained with Coomassie blue R-250 by standard techniques. The gel was scanned using Gel pro-Analyzer.
Isoenzymes
α-Esterase and β-esterase
Esterase isozymes were detected on 12% native polyacrylamide gels. The gel was stained for esterase activity by incubation at 37°C in a solution of 100 mg α-naphthyl acetate or β-naphthyl acetate (as a substrate) and 100 mg fast blue RR salt in 200 ml of 0.1 M phosphate buffer pH 6.5 (Scandalios 1964).
Acid phosphatase
After electrophoresis, the gels were soaked in 100 ml of 50 mM Na-acetate buffer at pH 5 containing 100 mg Fast blue BB salt, 100 mg α-naphthyl phosphate, 100 mg MgCl2 and 100 mg MnCl2 (Wendel and Weeden 1989).
Peroxidase
Detection of peoxidase isozymes on gels was done by method of Larsen and Benson (1970). Five drops of hydrogen peroxide (30%) were added to the medium mixture just before staining. Gels were incubated at room temperature in the staining solution (0.25 g benzidine dehydrochloride, two drops of glacial acetic acid, and then completed the solution to 100 ml by distilled water) till bands appeared.
Polyphenol oxidase
The gel incubated in staining solution (100 ml of 0.1 M Sodium acetate buffer at pH 6.8, 15 mg Catechol and 50 mg Sulfanilic acid) at 30°C until positively stained bands appear. Wash stained gel in water and stored in 50% glycerol (Sato and Hasegawa 1976).
Superoxide dismutase
Detection of superoxide dismutase isozymes on gels was done using the method of Weisiger and Fridovich (1973). Incubate with gel at room temperature under a neon tube. SOD appears as light bands on a blue background after 1 or 2 h.
Statistical analysis
The data were statistically analyzed using F test and LSD at 5% and 1% levels of probability according to SAS-Programme (1982).
Results and discussion
Changes in morphological criteria
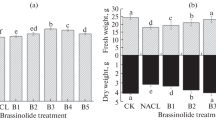
The results presented in Table 1 showed that the high concentrations of salinity (100 and 150 mM) caused high significant decrease in shoot and root lengths, number of leaves, leaves area, and fresh and dry weights of shoots and roots while the low concentrations of salinity (25 mM) caused high significant increase in all morphological criteria. The increase in fresh weight of the shoot system may be due to the ability of the plant to increase the size of its sap vacuoles, which allows for the collection of a lot of water, and this in turn dissolves salt ions that have accumulated and leads to the subsequent increase in fresh weight (Munns 2002). The elongation of the stem when treated with low concentrations of salts may induce osmotic adjustment activity in the plants which may improve growth.
On the other hand, treatment with 0.05 ppm brassinolide alleviates the harmful effect of salinity stress. These results are in agreement with Al-Maskri et al. (2010) who found that number of leaves, plant fresh weight, shoot fresh weight, shoot dry weight, shoot dry matter percentage, root fresh weight, root dry weight, root dry weight percentage, leaf area, and leaf area index were significantly affected by salinity levels (50 and 100 mM) in lettuce (Lactuca sativa L.). Also, Eleiwa et al. (2011) who found that foliar application of BRs on wheat plants increased yield and yield attributes of treated plants and significantly overcome the depressive effect of saline irrigation water at all levels on crop productivity and photosynthetic pigments. In addition, BRs play an essential role in plant growth and development and have been implicated in many physiological responses (Bajguz and Hayat 2009).
Such decline in shoot and root length in response to salinity stress might be due to either decrease in cell elongation resulting from the inhibiting effect of water shortage on growth promoting hormones which, in turn, led to a decrease in each of cell turgor, cell volume and eventually cell growth (Banon et al. 2006), and/or due to blocking up of xylem and phloem vessels, thus, hindering any translocation through (Lavisalo and Schuber 1998). Moreover, the decline in both fresh and dry weight of shoots and roots of cowpea reveals the influence of water in stimulating and regulating the photosynthetic enzymes and growth promoting hormones, which thus influence dry matter production (Monti et al. 2006).
Changes in total phenols content
The results presented in Table 2 revealed that total phenols content were significantly accumulated in leaves of cowpea irrigated with 25 and 50 mM NaCl. These increases might be due to the increase in their biosynthesis. The increase in phenol levels have been reported in a number of plants grown under salinity stress condition (Ksouri et al. 2007). However, the exposure of cowpea plants to 150 mM NaCl reduces the accumulation of these compounds.
The application of brassinolide at concentration 0.05 ppm to cowpea leaves induced stimulatory effects on the production and accumulation of phenols at all levels of NaCl. The maximum phenols accumulation was displayed in leaves at the lowest level of NaCl (25 mM).
Total phenols play a significant role in the regulation of plant metabolic process and over all plant growth. In addition, phenols act as free radical scavengers as well as substrates for many antioxidant enzymes (Martin-Tanguy 2001). The increase in the accumulation of the phenolic compounds in stressed cowpea leaves might be due to their increase in their biosynthesis.
Changes of lipid peroxidation product (TBARS)
Data recorded in Table 2 revealed that, thiobarbituric acid reactive substances (TBARS) content was highly significantly increased at all levels of salinity. These results are in agreement with Astorga and Meléndez (2010) who observed lipid peroxidation increased with 100, 150, and 200 mM of sodium chloride in Paulownia imperialis.
Treatment with brassinolide reduced the levels of TBARS in leaves of salt-stressed plants. The present observations are in consistence with those obtained by (Özdemir et al. 2004; Arora et al. 2008), who observed that brassinosteroids treatment lowered the lipid peroxidation in the seedlings of rice (Oryza sativa) and Zea mays. The increase in lipid peroxidation may be due to the incapability of antioxidants to buffer and scavenge all the active oxygen species resulted from the oxidative salt stress. It is assumed that BRs act as secondary messengers for the induction of antioxidant defenses in stressed plants (Khripach et al. 2000); thus, based on our results in MDA measurement, it is very possible that brassinolide effectively scavenged ROS by increasing the activity of antioxidant enzyme systems.
Changes in soluble protein content
The effect of various concentrations of NaCl in the absence and presence of brassinolide on the total soluble protein of cowpea leaves is presented in Table 2. The results indicated that the high concentrations of NaCl (100 and 150 mM) caused high significant decrease in the total soluble proteins content of cowpea leaves below those of untreated ones. These results are in agreement with Lobato et al. (2008) in Vigna unguiculata. In addition to, protein degradation in a saline environment might be due to the decrease in protein synthesis, accelerated proteolysis, decrease in the availability of amino acid and denaturation of enzymes involved in protein synthesis. A decrease in protein content is a common phenomenon in salinity stress. The reason for this is that the amino acid of proteins will react with active radical and will be degraded (Wagner et al. 2004).
On the other hand, the low concentration of NaCl (25 mM) caused as opposite pattern of change. These results are in close agreement with the findings of Abd El-Samed et al. (2004) who reported that the soluble protein in shoots of maize plant cv. 324 increased with increasing salinity.
Application of brassinolide at all salinity levels caused high significant increase in the total soluble protein content in leaves of cowpea plants. In general the inhibition of total protein contents by salt stress was partially alleviated by brassinolide application. These results are in agreement with Arora et al. (2008) who found that enhanced levels of proteins were observed under the influence of BRs indicating the synthesis of stress-protective proteins in the maize seedlings
Changes in antioxidant compounds
Tocopherol content
Data presented in Table 2 revealed that the salt stress (100 and 150 mM NaCl) caused high significant increase in tocopherol contents in leaves of cowpea plants as compared with control. These results are in agreement with Sklodowska et al. (2009) who reported that treatment of tomato plants with 150 mM NaCl increased tocopherol content. Application of brassinolide caused high significant increase in tocopherol content in leaves of cowpea plants at all levels of NaCl.
Exogenous application of BRs modified antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, ascorbate peroxidase and non-enzymatic antioxidants, such as ascorbic acid, tocopherols, carotenoids, glutathione, etc. in plants under different stress conditions. Vitamins C, E, and glutathione react directly or via enzyme catalysis with OH, H2O2, or O2 (Özdemir et al. 2004).
Ascorbic acid content
It is evident that salinity stimulated the accumulation of ascorbic acid (ASA) as compared with that of the unsalinized control (Table 2). These results are in harmony with those obtained by Sarwat and El-Sherif (2007) who reported that ascorbic acid content tends to increase with increasing salinity level in all cultivares of barley plant. Generally, application of brassinolide significantly increased the accumulation of ASA in leaves of cowpea plants exposed to all concentrations of NaCl. Many compounds are being used to cope with the toxic effects of salinity including ascorbic acid (Khafagy et al. 2009). Ascorbic acid can directly scavenge superoxide, hydroxyl radicals and singlet oxygen and reduce H2O2 to water via ascorbate peroxidase reaction (Noctor and Foyer 1998).
Total reduced glutathione content
Glutathione content (GSH) level was highly significantly increased in leaves of cowpea plants irrigated with 25 and 50 mM NaCl. The increase in GSH level in leaves of saline-stressed cowpea plants might be due to the increased GSH synthesis, decreased GSH degradation and the increase in the transport of GSH. Similar reports were offered by Szalai et al. (2009).
However, a reverse pattern was obtained in cowpea leaves exposed to 100 and 150 mM as compared with those of unsalinized control. With this respect Hernandez et al. (2000), suggested that glutathione synthesis could be restricted by higher NaCl concentrations. Moreover, the reduction in GSH at high NaCl does probably due to the limitation of NADPH as substrate during the reduction.
At the mean time, treatment with brassinolide markedly enhances the accumulation of GSH in stressed cowpea leaves compared with their correlative controls grown at 25, 50, and 100 mM NaCl. These results are in line with those obtained by Ali and Abdel-Fattah (2006) who reported that treatment with brassinosteroid caused increased in glutathione content and enhanced salt tolerance of Phaseolus vulgaris and Hordeum vulgaris. On the other hand, high significant decreased in GSH levels in cowpea leaves grown in 150 mM NaCl as compared with control.
Glutathione is involved in several physiological processes in plants under both optimal and stress conditions. It participates in both direct and the indirect control of ROS levels. In addition, GSH removes lipid peroxides. GSH also interacts with ROS redox molecules and plant hormones thereby; GSH takes part in the regulation of growth, development, the cell cycle, gene expression, and protein activity due to its effect on the redox state of cells (Shao et al. 2008). The high GSH in cowpea leaves may be attributing to the increase in its synthesis or decrease in its degradation.
Changes in antioxidant enzymes activities
Polyphenol oxidase activity
The activity of polyphenol oxidase in salinized cowpea leaves was highly significantly increased as compared with that of non salinized control. The greatest activity of PPO was measured in cowpea leaves exposed to 25 mM NaCl as compared with all the investigated treatments. Similar results have been obtained by Agarwal and Pandey (2004) in stressed Senna seedlings. It seems possible that PPO may play a role in the defense against salt stress. The application of brassinolide increased PPO activity as compared with those salinized cowpea leaves.
Peroxidase activity
The activity of peroxidase in cowpea leaves at all levels of NaCl was found to be significantly increased as compared with that of the nonsalinized seedlings. The greatest POX was measured in leaves exposed to 150 mM NaCl. The increase in POX activity by salinity stress is established by Cavalcanti et al. (2007). The application of brassinolide highly significantly increases the POX activity as being compared with those of corresponding controls.
Superoxide dismutase activity
Salt stress resulted in a considerable increase in the activity of SOD of cowpea plants. The greatest activity of SOD was displayed at the highest level of NaCl (150 mM) as being compared with the unstressed control plants. The increase in SOD activity in leaves of salt-stressed cowpea might be due to the activation of preexisting SOD or due to synthesis of new SOD under salt conditions. These results are in accordance with Sairam et al. (2005) who showed that total SOD activity is increased in salt-stressed wheat plants, but it was higher at 100 mM NaCl than at 200 mM NaCl.
The treatment of cowpea leaves with brassinolide caused high significant increase in the activity of SOD of stressed plants as compared with their respective controls. These results are in agreement with Arora et al. (2008) who found that the level of SOD is increased by the application of 28-homoBL to overcome the stress generated by NaCl and to boost the resistance capacity of plants. The higher level of these enzymes suggests a possible role of homoBL in amelioration of oxidative stress generated by salt stress. Brassinolide may confer tolerance to salt stress by increasing the activities of antioxidative enzymes and/or by reducing the uptake of salts and activated the antioxidative enzymes. Similar conclusion was offered by Özdemir et al. (2004) in O. sativa and Arora et al. (2008) in Z. mays.
The activity of enzymes studied (PPO, POX, and SOD) increased when the plants are sprayed with BR. The reason for the increase in the activity of these enzymes may be the effects of BR on expression of biosynthetic genes of these enzymes that resulted in increased oxidation of harmful substrates. These results, accompanied with the decrease in lipid peroxidation contents, probably represent a decline in ROS and an indicator of removal of stressful conditions by antioxidant enzymes activated by BR.
Protein electrophoretic pattern
In the present work (Table 3; Fig. 1), three types of modifications are observed in the protein patterns of cowpea leaves, some protein bands were disappeared, other proteins were selectively increased and synthesis of new set of protein was induced. Some of these responses were observed under brassinolide and salinity treatments, while others were induced by either brassinolide or salinity.
Electrophoretic banding patterns of cowpea leaves in response to treatment with different concentrations of NaCl alone or in combination with brassinolide. 1, Control; 2, 0.05 ppm brassinolide; 3, 25 mM NaCl; 4, 50 mM NaCl; 5, 100 mM NaCl; 6, 150 mM NaCl; 7, 25 mM NaCl + 0.05 ppm brassinolide; 8, 50 mM NaCl + 0.05 ppm brassinolide; 9, 100 mM NaCl + 0.05 ppm brassinolide; 10, 150 mM NaCl + 0.05 ppm brassinolide
The protein patterns of cowpea leaves under all treatments comprise three major bands having molecular weights of (246, 24, and 21 kDa). Three protein bands of molecular weights (154, 85, and 31 kDa) were de novo synthesized in cowpea leaves grown under salinity stress alone or in combination with brassinolide.
In addition, application of brassinolide at concentration (0.05 ppm) induced the synthesis of three new protein bands having the molecular weights of 43, 36, and 22 kDa in salinized cowpea leaves.
Brassinolide and salinity stress induced a considerable variation in the protein patterns of cowpea leaves. This variation has been manifested as the novel expression of some polypeptide; the absence of others and overexpression of a third-class polypeptides.
Several of new proteins which are synthesized in response to interaction between environmental stress and growth substances applied have been reported as stress protein in plants. Many of these proteins were suggested to protect the cell against the adverse effect of salt stress. Changes in protein synthesis under salinity and growth regulators (brassinolide) treatments may be due to changes in the efficiency of mRNA translation or the regulation of RNA transcription transport and stability
The protein band which has molecular weight 52 kDa was de novo synthesized in salinized cowpea leaves. It has been suggested that these proteins have an osmoprotection function or protected cellular structures. In this respect, Eman et al. (1992) showed the appearance of excess bands in leaf samples of Z. mays under salt stress. These findings indicated that these bands were salt inducible and could be involved in plant adaptation for growth under stress condition. Also, salt stress lead to difference in gene expressions where alterations in protein could be due to alteration in regulation of transcription, mRNA processing, or due to altered rates of protein degradation.
The total number of bands in leaves of cowpea treated with 100 mM NaCl was decreased as being compared with the respective controls. These results indicated that the decrease in the protein level in salt-stressed plants might be attributed to a decrease in protein synthesis, the decrease availability of amino acids and the denaturation of enzymes involved in amino acid and protein synthesis.
Also, salinity stress caused disappearance of some bands in cowpea leaves. One possible explanation for complete disappearance of some proteins under salt stress is that the gene (S) responsible for certain proteins might be completely suppressed as a result of stress. Therefore, the developed tissues had lost their ability to synthesize these proteins. It is also possible that the gene (S) had not been completely suppressed but inhibited as the result of stress, and complete recovery of the inhibition was not achieved. This may apply to the protein that stained less densely under stress (ELObeidy et al. 2001).
Protein profile of cowpea leaves indicate that BR may regulate the expression of salt-stress inducible proteins as well as induced de novo synthesis of specific polypeptides, which are anticipated to play an active role in salt resistance. Also, involvement of growth regulators in the induction of alteration in protein patterns was attributed to their role in controlling cell division in the apical meristems by regulating certain genes namely prolifera or cyclins. BR induced changes in protein profile of wheat (El-Khallal and Nafie 2000) and Vicia faba (Zaky 2000) in response to water and salt stress, respectively.
α-Esterase isoenzyme
α-Esterase electrophoretic patterns are illustrated in Fig. 2a. Four bands were exhibited with different intensities and densities among all treatments. Band no. 1 and 2 which have R f 0.045 and 0.13 were present in all treatments (common bands). The other two bands were present in some treatments and absent in the others (polymorphic). The high concentrations of salinity alone (50, 100, and 150 mM) or in combination of brassinolide increased the activity of esterase isoenzymes. These results are in agreement with Hassanein (1999), who found that salinity increase esterase isoenzymes and the highest numbers of esterase isoenzymes were detected under the highest NaCl concentration.
Electrophoretic patterns of α-esterase (a), β-esterase (b), polyphenol oxidase (c), peroxidase (d), acid phosphatase, (e) superoxide dismutase, and (f) isoenzyme of cowpea leaves in response to treatment with different concentrations of NaCl alone or in combination with brassinolide. 1, Control; 2, 0.05 ppm brassinolide; 3, 25 mM NaCl; 4, 50 mM NaCl; 5, 100 mM NaCl; 6, 150 mM NaCl; 7, 25 mM NaCl + 0.05 ppm brassinolide; 8, 50 mM NaCl + 0.05 ppm brassinolide; 9, 100 mM NaCl + 0.05 ppm brassinolide; 10, 150 mM NaCl + 0.05 ppm brassinolide
β-Esterase isoenzyme
β-Esterase electrophoretic patterns are illustrated in Fig. 2b. Three bands with different intensities and densities were observed among the profiles of all treatments. One band was presented in all treatments (monomorphic bands) at R f 0.29. The other two bands were presented in some treatments and absent in the others. The band which has R f 0.11 become very intensified at all treatments as compared with control treatment. These results are similar to Mohmamed (2005) who found that under salt stressed, 150 mM NaCl caused enhancement of the esterase isozyme bands in shoots of maize plants similar pattern was observed in roots.
Polyphenol oxidase isoenzymes
Polyphenol oxidase electrophoretic patterns are illustrated in Fig. 2c. Seven bands with different intensities and densities were observed among the profiles of all treatments. Five bands were presented in all treatments (monomorphic bands) at R f 0.058, 0.082, 0.15, and 0.22. The other two bands were presented in some treatments and absent in the others (polymorphic bands). The bands which have R f 0.34 and 0.52 were presented in salinized cowpea (50, 100, and 150 mM) and in salinized cowpea (25 and 50 mM in combination with brassinolide). These results are in agreement with El-Sayed et al. (2007) who found that Salinity stress induced two PPO bands in wheat Sakha 93 cultivar at both of 0.9% and 1.2% NaCl.
Peroxidase isoenzyme
Expression of the peroxidase isoenzyme was detected in cowpea leaves treated with different concentrations of NaCl alone or in combination with growth regulator (0.05 ppm brassinolide) using 12% native PAGE (Fig. 2d). The results showed that two bands were exhibited at R f 0.031 and 0.051 with different densities and intensities in untreated and salt treated plants.
The band with R f 0.031 was high intensities and densities in salinized cowpea leaves alone (50, 100, and 150 mM) and in combination with brassinolide. These results indicated that salt stress increased the accumulation of the peroxidase enzyme and that the encoding gene (S) was accelerated in response to salt stress. These results are in harmony with the findings of El-Baz et al. (2003) who used peroxidase isoenzyme as marker for salt stress tolerance in cucumber plants and they found that the profile of peroxidase isoenzyme was modified during salt stress conditions. This behavior may be due to its ability to tolerate salt stress or due to the effect of salt stress which may cause some shift in gene expression.
The activity of peroxidase increased when cowpea sprayed with brassinolide. The reason for the increase in the activity of peroxidase may be the effect of brassinolide on expression of biosynthetic genes of the enzymes that resulted in increased oxidation of harmful substrates, as reported by Shahbaz et al. (2008). High peroxidase isoenzyme activity in cowpea leaves of salt stress reflect the changed mechanical properties of the cell wall which in turn could be related to salt adoption process.
Acid phosphatase isoenzyme
Isoenzyme profiles of acid phosphatase in cowpea leaves grown under NaCl alone or in combination with brassinolide are shown in Fig. 2e. It is evident that one common band was observed under control as well as salinity and brassinolide treatments.
This band becomes much intensified in salinized cowpea leaves alone (100 and 150 mM) and in combination with brassinolide at 150 mM NaCl as compared with control treatment. These results are in agreement with the findings of Mohamed (2005) who reported that the induction of new isoenzymes and the change in the acid phosphatase isoenzyme profile is considered to play an important role in the cellular defense against oxidative stress, caused by salt stress.
Superoxide dismutase isoenzyme
SOD electrophoretic patterns are illustrated in Fig. 2f. A total of three bands were observed among the profile of all treatments. These three bands were present in some treatments and absent in the others (polymorphic) with substantial differences in their intensities and densities. The two bands which have R f 0.08 and 0.68 were present in all treatments (monomorphic bands) while the other band which has R f 0.53 was present in salinized cowpea leaves (25, 50, 100, and 150 mM). The band which has R f 0.68 become very intensified in all treatments as compared with control treatments. SOD activity might play a role in the molecular mechanism of plant cell tolerance to salt stress (Olmos et al. 1994).
Conclusions
Soil salinity is an enormous production problem for vegetable crops as saline conditions are known to suppress plant growth under irrigation. Salt stress affects all the major processes such as growth, yields, photosynthesis, protein synthesis, and lipid metabolism. The conclusion of the present study is that brassinolide (plant growth regulator) can be used to alleviate the harmful effect of salt stress. Therefore, the use of brassinolide can help to solve the production problems caused by high salinity.
Abbreviations
- ROS:
-
Reactive oxygen species
- POX:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- PPO:
-
Polyphenol oxidases
- GR:
-
Glutathione reductase
References
Abd El-Samad HM, Shaddad MA, Mahmmod MD (2004) Mechanism of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul 44:165–174
Agarwal S, Pandey V (2004) Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol Plant 48:555–560
Ali AA, Abdel-Fattah RI (2006) Osmolytes antioxidant behavior in Phaseolus vulgaris and Hordeum vulgare with brassinosteroid under salt stress. J Agro 5(1):167–174
Al-Maskril A, Al-Kharusi L, Al-Miqbali H, Khan M (2010) Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Inter J Agric Bio 12:377–380
Arora N, Bhradwaj R, Sharma P, Kumar H (2008) Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activites in seedlings of Zea mays L.under salinity stress. Acta Physiol Plant 30:833–839
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotech Adva 27:84–93
Astorga GIA, Meléndez LA (2010) Salinity effects on protein content, lipid peroxidation, pigments, and proline in Paulownia imperialis (Siebold & Zuccarini) and Paulownia fortunei (Seemann & Hemsley) grown in vitro. Electro J Biotech 13(5)
Bajguz A, Hayat S (2009) Effect of brassinosteroids on the plant responses to environmental stresses. Plant Physio Bioch 47:1–8
Banon SJ, Ochoa J, Franco JA, Alarcon JJ, Sanchez-Blanco MJ (2006) Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ Exp Bot 56:36–43
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gel. Anal Bio Chem 44:276–287
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cavalcanti FR, Lima JPMS, Ferreira-Silva SL, Viegas RA, Silveira JAG (2007) Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J Plant Physiol 164:591–600
Dihazi AD, Jaitt F, Zouine J, Hassni ME, Hardami IE (2003) Effect of salicylic acid on phenolic compounds related to data palm resistance to Fusarium oxysporum sp. Albedimis. Phytopath Medit 423:9–16
El-Baz FK, Mohamed AA, Aly AA (2003) Development of biochemical markers for salt stress tolerance in cucumber plants. Pak J Bio Sci 6(1):16–22
Eleiwa ME, Bafeel SO, Ibrahim SA (2011) Influence of brassinosteroids on wheat plant (Triticum aestivum l.) production under salinity stress conditions. I- growth parameters and photosynthetic pigments. Aust J Basic Appl Sci 5(5):58–65
El-Khallal SM, Nafie EM (2000) Alleviation of drought damage for two cultivars of wheat seedlings by application of growth regulators brassinolide and uniconazole. Egypt J Physiol Sci 24(2–3):297–317
ELObeidy AA, Fayek MA, Mohmoud RA (2001) Quantifying salt tolerance responses of some banana genotypes in vitro. In: Shayji YA, Siolhu JS, Saleem M, and Guerinik K (eds) 1st international conference on: “Biotechnology Applications for the Arid Regions”. State of Kuwait, Kuwait. pp 135–165
El-Sayed OE, Rizkalla AA, Sabri SRS (2007) In vitro mutagenesis for gGenetic improvement of salinity tolerance in wheat. Res J Agri Biol Sci 4(5):377–383
Eman FM, Abdel-Tawab FM, Tayel AA, Baheildin A, El-Enany MA (1992) Biochemical genetic markers for salt tolerance in maize (Zea mays). Annals Agric Sci Ain Shams Univ Cairo 37:147–157
Hassanein AM (1999) Alteration in protein and esterase peanut in response to salinity stress. Bio Plant 42:241–248
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Bioch Bioph 125(1):189–198
Hernandez JA, Jimenez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenol oxidase activity during leaf senescence. Plant Phsiol 57:315–319
Khafagy MA, Arafa AA, El-Banna MF (2009) Glycinebetaine and ascorbic acid can alleviate the harmful effects of NaCl salinity in sweet pepper. Aust J Crop Sci 3:257–267
Khripach VA, Zhabinskii VN, De Groot AE (2000) Twenty years of brassinosteroids: steroidal hormones warrant better crops for the XXI Century. Ann Bot 86:441–447
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physio Bioch 45(3–4):244–249
Laemmli UK (1970) Cleavage of structural proteins during assembly of head bacteriophage T4. Nature 227:680–685
Larsen AL, Benson WC (1970) Variety specific variants of oxidative enzymes from soybean seeds. Crop Sci 10:493–495
Lavisolo C, Schuber A (1998) Effects of water stress on vessel size xylem hydraulic conductivity in Vitis vinifera L. J Exp Bot 49(321):693–700
Lobato AKS, Oliveira Neto CF, Costa RCL, Santos Filho BG, Cruz FJR, Laughinghouse IV (2008) Biochemical and physiological behavior of Vigna unguiculata (L.) Walp. under water stress during the vegetative phase. Asian J Plant Sci 7:44–49
Lowry OH, Rosembrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Bio Chem 193(1):267–275
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Mohamed AA (2005) Two-dimensional electrophoresis of soluble proteins and profile of some isozymes isolated from maize plant in response to NaCl. Res J Agri Bio Sci 1(1):38–44
Monti A, Amaducci MT, Pritoni G, Verturi G (2006) Variation in carbon isotope discrimination during growth and at different organs in sugar beet (Beta vulgaris L.). Field Crops Res 98:157–163
Mukherjee SP, Choudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Olmos E, Hernández JA, Sevilla F, Hellín E (1994) Induction of several antioxidant enzymes in the selection of a salt-tolerant cell line of Pisum sativum. J Plant Physiol 144:594–598
Özdemir F, Bor M, Demiral T, Turkan I (2004) Effects of 24- epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42(3):203–211
Philip B, Bernard L, William H (1954) Vitamins and deficiency diseases. In: Practical physiological chemistry. McGraw-Hill, New York. pp 1272–1274
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–89
Sarwat MI, El-Sherif MH (2007) Increasing salt tolerance in some barley genotypes (Hordeum vulgare) by using kinetin and benzyladenin. World J Agri Sci 3(5):617–629
SAS-Programme (1982) SAS user,s Guide Statistics SAS Institute, INC, Raleiegh. NC. 584
Sato M, Hasegawa M (1976) The latency of spinach chloroplast phenolase. Phytochemistry 15:61
Scandalios JG (1964) Tissue specific isozyme variation in maize. Heredity 55:281–285
Shahbaz M, Ashraf M, Athar H (2008) Dose exogenous application of 24 epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.). Plant Growth Regul 55:51–64
Shao L, Cui J, Young LT, Wang JF (2008) The effect of mood stabilizer lithium on expression and activity of glutathione s-transferase isoenzymes. Neuroscience 151:518–524
Skłodowska M, Gapińska M, Gajewska E, Gabara B (2009) Tocopherol content and enzymatic antioxidant activities in chloroplasts from NaCl-stressed tomato plants. Acta Physio Plant 31(2):392–400
Szalai G, Kellős T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28(1):66–80
Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Wursch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306:1183–1185
Weisiger RA, Fridovich I (1973) Superoxide dismutase: organelle specificity. J Biol Chem 248:3582
Wendel JF, Weeden JF (1989) Visualization and interpretation of plant isozymes. In: Soltis DE and Soltis PS (eds) Isozymes in plant biology. Chapman & Hall, London. p 18
Zaky LM (2000) Effects of brassinolide on growth, metabolite content and yield of vicia faba. Egypt J Biotechnol 8:255–275
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Friedrich Bentrup
Rights and permissions
About this article
Cite this article
El-Mashad, A.A.A., Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249, 625–635 (2012). https://doi.org/10.1007/s00709-011-0300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0300-7