Abstract
The effects of 0.1 μM 24-epibrassinolide (EBL) on plant growth (plant height, leaf area, fresh weight, and dry weight), chlorophyll content, photosynthetic characteristics, antioxidant enzymes, and chloroplast ultrastructure were investigated using cucumber seedlings (Cucumis sativus L. cv. Jinyou No. 4) with 80 mM Ca(NO3)2 to induce stress. The presence of Ca(NO3)2 caused significant reductions in net photosynthetic rate (P N), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) of leaves. In addition, Ca(NO3)2 markedly reduced the chlorophyll content and inhibited photochemical activity, including the actual photochemical efficiency (ΦPSII). In contrast, EBL increased the chlorophyll content, especially chlorophyll b, and minimized the harmful effects on photosynthesis caused by the Ca(NO3)2. The application of EBL to the plants subjected to Ca(NO3)2-enhanced photochemical activity. EBL protected the photosynthetic membrane system from oxidative damage due to up-regulating the capacity of the antioxidant systems. Microscopic analyses revealed that Ca(NO3)2 affected the structure of the photosynthetic apparatus and membrane system and induced damage of granal thylakoid layers, while EBL recovered the typical shape of chloroplasts and promoted the formation of grana. Taken together, EBL compensated for damage/losses by Ca(NO3)2 due to the regulation of photosynthetic characteristics and the antioxidant system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 800 million hectares of land are affected by salt throughout the world (FAO 2012). The majority of these soils are naturally saline, but recently a significant proportion of cultivated land is also being influenced by secondary salinization. Globally, 32 million out of 1.5 billion hectares of cultivated land in a dryland (2 %) are influenced considerably by secondary salinity, a soil condition characterized by high concentrations of soluble salts; and 45 million of the 230 million hectares of irrigated land (~20 %) are salt affected (FAO 2012). In China, greenhouse cultivation is the most common vegetables production in farmlands. Secondary salinization of soil is primarily attributable to over-irrigation, intensive farming, lack of rain and concentration of salt through intense evaporation (Ashraf 1994; Liang et al. 2005; Yu et al. 2005). Secondary salinization is an important limitation to crop production in greenhouses in China (Yu et al. 2005).
In China, 40–75 % of the total salt contained in the 0–20 cm layer is concentrated in the soil surface layer (0–5 cm depth). The anions in soil due to secondary salinization are \({ {\text{NO}}_{3}}^{ - } \), Cl−, \( {{\text{SO}}_{4}}^{2- } \), and \( {{\text{HCO}}_{3}}^{ - } \) and the cations are Na+, K+, Ca2+, and Mg2+. Among these ions, \( {{\text{NO}}_{3}}^{ - } \) and Ca2+ are usually dominant. More specifically, Ca2+ accounts for over 60 % of total cations and \( {{\text{NO}}_{3}}^{ - } \) accounts for ~67–76 % of the total anions (Wu 2001; Li et al. 2004). According to Tong and Chen (1991), the high level of Ca(NO3)2 accumulation was one of the main reasons for soil salinization. Excessive Ca(NO3)2 caused severe oxidative damage and metabolic disorder, suggesting reductions in biomass production in cucumber plants (Sun et al. 2009; Jin et al. 2010) and tomato plants (Zhang et al. 2008). Extensive or definitive research to describe the negative effects of excessive Ca(NO3)2 has not been conducted.
Brassinosteroids (BRs), a phytohormone, induce physiological and morphological responses in higher plants. When subjected to stress from heavy metal, thermal, pesticide or NaCl, 24-epibrassinolide (EBL) were found to enhance total chlorophyll content (Janeczko et al. 2007; Ali et al. 2008a), stomatal conductance (Ali et al. 2008a; Hu et al. 2010), the capacity of antioxidant system (Xia et al. 2006) and to protect the quantum yield of PSII (Ogweno et al. 2008) in plants. Exogenous EBL, however, showed no significant effect on chlorophyll content or photochemical efficiency of PSII in wheat plants and pepper plants subjected to NaCl stress (Qayyum et al. 2007; Ali et al. 2008b; Houimli et al. 2008).
Little information is currently available in literature regarding the effects of excess Ca(NO3)2 on the photosynthetic characteristics or the possible alleviation mechanisms of exogenous EBL on the negative effects of Ca(NO3)2. The purpose of this study is to clarify whether exogenous EBL alleviates the negative growth suppression effect of Ca(NO3)2 due to changes in photosynthetic characteristics, antioxidant capacity, and the chloroplast ultrastructure in Cucumis sativus L.
Materials and methods
Plants
Cucumber (Cucumis sativus L., cv. ‘Jinyou No. 4’) seeds were obtained from Tianjin Kernel Cucumber Research Institute, China. The seeds were germinated on two layers of wet filter paper in Petri dishes in the dark for ~24 h at 29 ± 1 °C. The germinated seeds were sown in sand and moved to a plant growth chamber (Ningbo Jiangnan Instrument Factory, Ningbo, China) at Nanjing Agricultural University, Nanjing, China. The growth chamber was kept at 28 ± 1 °C (day) and 19 ± 1 °C (night) with relative humidity (RH) of 50–60 %. The maximum photosynthetic photo flux density (PPFD) was ~1,200 μmol m−2 s−1. When the second leaves were fully expanded, 12 seedlings were transplanted into plastic containers containing half strength Hoagland solution (Zhang et al. 2009). The nutrient solution was aerated using an air pump at an interval of 20 min to maintain the dissolved oxygen concentration at 8.0 ± 0.2 mg l−1 during this experiment.
Experimental design
The Ca(NO3)2 concentration in the nutrient solution was increased at a rate of 40 mM per day until a final concentration of 80 mM was attained. The concentrations of Ca(NO3)2 and EBL were based on the results of our previous experiment (data not shown). To investigate the effects of EBL (Sigma-Aldrich, USA) sprayed on whole plants grown hydroponically, the cucumber seedlings were divided into four groups. Since the EBL stock solution contained ethanol as a solvent, the nutrient solutions (including distilled water as the control) were prepared with the same ethanol level as the stock solution. After pre-culturing for 3 days, the seedlings were treated as follows:
-
(a) Cont, 0 mM Ca(NO3)2 + 0 μM EBL;
-
(b) CB, 0 mM Ca(NO3)2 + 0.1 μM EBL;
-
(c) N, 80 mM Ca(NO3)2 + 0 μM EBL;
-
(d) NB, 80 mM Ca(NO3)2 + 0.1 μM EBL.
The seedlings were sprayed with EBL every 2 days and the nutrient solutions were renewed every 2 days. At 9 days of treatment, photosynthesis and the chlorophyll fluorescence of the plants were measured. At 10 days of treatment, the seedlings were sampled for the determination of morphological parameters, chlorophyll content, oxidative damage, and the activities of antioxidant enzymes. The seedlings were organized in a complete randomized block design.
Morphological analyses
After the shoot length of each plant was measured, the area of the third expanded leaf (from the top) was measured with an Expression 1680 scanner (Epson, Sydney, Australia) and image analysis software (WinRHIZO, Regent Instruments. Inc., Quebec, Canada). After all the leaves were washed with distilled water and topical moisture removed, their fresh weights were measured; subsequently the dry weights were obtained after drying at 75 °C for 72 h.
Gas exchange parameters
The net photosynthetic rate (P N), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of the fully expanded leaves, which were the third leaves from the top, were measured using a portable photosynthesis system (LI-6400, LI-COR Inc., USA). Leaf temperatures were maintained at 25 °C. RH in the assimilation chamber was maintained at 70 %, the external CO2 concentration remained at 380 ± 10 μmol mol−1 and the light intensity was consistent at 1,000 μmol photons m−2 s−1.
Chlorophyll fluorescence parameters
Chlorophyll fluorescence of the third fully expanded leaf was measured with a portable fluorometer (PAM 2100, Walz, Germany) after dark adaptation for 30 min according to the method of Kooten and Snel (1990). The maximum quantum yield of PSII (Fv/Fm) was determined after dark adaptation for 30 min. The initial Chl fluorescence yield (Fo) was determined in low-modulated measuring light (<0.1 μmol m−2 s−1) and a 0.8-s pulse of saturating white light (8,000 μmol m−2 s−1) was applied to obtain the maximum fluorescence yield (Fm). To determine the minimal fluorescence level in a leaf during the illumination (Fo′), black cloth to keep out light was rapidly installed to cover the leaf and the leaf-clip holder in the presence of far-red light to oxidize the PSII centers fully. Immediately after blockage of light, leaf fluorescence dropped to the Fo′ level and rose again within several seconds. The steady-state fluorescence level (Fs) and the maximum fluorescence level (Fm′) during the exposure to light were also measured. Fluorescence levels were used to calculate: (1) the maximum quantum efficiency of PSII, Fv/Fm = (Fm − Fo)/Fm; (2) the photochemical fluorescence quenching coefficient, qP = (Fm′ − Fs)/(Fm′ − Fo′); (3) the non-photochemical quenching, qN = (Fm − Fm′)/(Fm′ − Fo′); and (4) the actual efficiency of PSII, ΦPSII = (Fm′ − Fs)/Fm′.
Chlorophyll content
The chlorophyll was extracted from the third fully expanded leaf with a mixture containing acetone, ethanol, and water (4.5:4.5:1, v/v/v). The chlorophyll content was measured on a fresh weight basis according to a modified version of the method of Strain and Svec (1966).
Free radical production and lipid peroxidation
The content of malondialdehyde (MDA) was measured according to the method of Heath and Packer (1968). The concentration was calculated from the value of A 532, and non-specific turbidity at the measurements was canceled by subtracting the value of A 600.
The superoxide formation rate was measured according to a modified version of the method of Elstner and Heupel (1976). Each leaf sample (1.0 g) was homogenized in 4 mL of 65 mM potassium phosphate buffer (pH 7.8) and centrifuged at 5,000g for 10 min. The reaction solution contained 0.9 mL of 65 mM potassium phosphate buffer (pH 7.8), 0.1 mL of 10 mM hydroxylamine hydrochloride, and 1 mL of the supernatant. After the solution was incubated at 25 °C for 20 min, 17 mM sulfanilic acid, and 7 mM α-naphthylamine were added into the solution. The measurement was performed at A 530. The \( {{\text{O}}_{ 2}}^{\, \bullet -} \) concentration was calculated from a standard curve of NaNO2.
H2O2 content in cucumber leaves was estimated according to the method of Patterson et al. (1984). The assay was based on the absorbance change of the titanium peroxide complex at A 415.
Activities of antioxidant enzymes
Ascorbate peroxidase (APX) (EC 1.11.1.1) was assayed according to the method of Nakano and Asada (1981). The reaction mixture contained 50 mM phosphate buffer (pH 6.0), 0.1 μM ethylenediaminetetraacetic acid (EDTA), 0.5 mM ascorbate, 1.0 mM H2O2, and 50 μL enzyme fraction.
The superoxide dismutase (SOD) (EC 1.15.1.1) activity was assayed by the method of Giannopolitis and Ries (1977). The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 0.1 μM EDTA, 13 mM methionine, 75 μM NBT (nitro-blue tetrazolium), 2 μM riboflavin, and 50 μL enzyme fraction.
The guaiacol peroxidase (GPX) (EC 1.11.1.7) activity was determined according to the method of Herzog and Fahimi (1973). The reaction mixture contained 50 mM phosphate buffer (pH 6.0), 25 mM guaiacol, 10 mM H2O2, and 0.1 mL enzyme fraction.
The catalase (CAT) (EC 1.11.1.6) activity was measured according to the method of Aebi (1984). The reaction mixture contained 25 mM sodium phosphate buffer (pH 7.0), 10 mM H2O2, and 0.1 mL enzyme fraction.
Ultrastructure of chloroplasts
The ultrastructure of the photosynthetic apparatus in chloroplasts was analyzed using electron micrographs. The leaves were cut into pieces of ~1 mm2. The cut leaves were then immersed in a solution containing 3 % glutaraldehyde and 1 % formaldehyde in a 0.1 M phosphate buffer (pH 7.4) for 2 h (primary fixation), then immersed in 2 % osmic acid in the same buffer for 2 h (second fixation). After dehydration in acetone and embedding in Durcupan ACM (Fluka), the resulting leaves were cut to obtain ultra-thin sections, stained with uranium acetate and lead citrate in series and examined using a HITACHI transmission electron microscope (Carl Zeiss, Göttingen, Germany) at an accelerating voltage of 80 kV.
Statistical analysis
The data were statistically analyzed with SAS software (SAS Institute, Cary, NC, USA) using Duncan’s multiple range test at the P < 0.05 level of significance.
Results
Morphological parameters
Under control condition, EBL alone (CB) enhanced the leaf area and dry weight of plants, as compared to the control (Table 1). Ca(NO3)2 stress (N) significantly inhibited the height, leaf area, fresh weight, and dry weight of the plants to 58.8, 33.2, 46.2, and 60.7 %, respectively. However, EBL in the NB solution alleviated the growth inhibition caused by Ca(NO3)2 (N) (Table 1).
Chlorophyll content
The EBL (CB) significantly increased the contents of Chl a, Chl b and Chl a + b in the cucumber leaves (Fig. 1a–c), but did not affect the Chl a/b ratio (Fig. 1d). The contents of Chl a, Chl b and Chl a + b decreased with the salt treatment (N) by 19.8, 17.7, and 19.3 %, respectively, compared to the control. However, the Chl a/b ratio did not significantly change from the control. In the case of NB, the contents of the Chl a, Chl b, and Chl a + b were significantly increased, whereas the ratio of Chl a/b showed a tendency to decline (Fig. 1).
Effects of Ca(NO3)2 and/or EBL on Chl a, Chl b, Chl a + b and Chl a/b in cucumber plants. Values represent the mean ± SE. (n = 3). Letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests. Cont, control; CB, 0.1 μM EBL sprayed on leaves; N, 80 mM Ca(NO3)2 added into nutrient solution; NB, both EBL and Ca(NO3)2 treatments
Gas exchange parameters
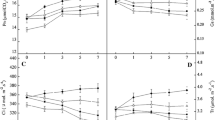
In the cucumber plants treated with EBL alone (CB), the gas exchange parameters indicated a significant increase in the P N (Fig. 2a), but showed no significant influence on the Gs (Fig. 2b), Ci (Fig. 2c) or Tr (Fig. 2d). P N in the plants subjected to Ca(NO3)2 stress (N) showed significantly lower values than the control plants; 11.0 and 19.3 μmol CO2 m−2 s−1, respectively. The Gs, Ci, and Tr also decreased by the Ca(NO3)2 treatment (N). The EBL in the NB solution enhanced the values of photosynthetic parameters that were suppressed by Ca(NO3)2, although they remained below the levels measured in the control plants.
Effects of Ca(NO3)2 and/or EBL on net photosynthetic rate (P N), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) in cucumber plants. Values represent the mean ± SE (n = 3). Letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests. Cont, control; CB, 0.1 μM EBL sprayed on leaves; N, 80 mM Ca(NO3)2 added into nutrient solution; NB, both EBL and Ca(NO3)2 treatments
Chlorophyll fluorescence parameters
EBL alone (CB) showed no effect on the Chl fluorescence parameters, as compared to the control. Ca(NO3)2 (N) significantly decreased the ΦPSII and qP in cucumber plants. However, both of them were significantly enhanced by EBL in the NB solution (Table 2).
Chl fluorescence analysis showed differences in the dissipation of excitation energy among the treatments (Table 2). The qN was significantly decreased in the plants subjected to Ca(NO3)2 and the EBL in the NB solution enhanced it. Ca(NO3)2 (N) slightly reduced the Fv/Fm ratio, while the EBL in the NB solution alleviated this decline.
MDA content, \( {{\text{O}}_{ 2}}^{ \bullet -} \) formation rate, and H2O2 content
EBL (CB) showed no effect on the MDA content (Fig. 3a) and \( {{\text{O}}_{ 2}}^{\, \bullet -} \) formation rate (Fig. 3b). In contrast, it significantly decreased the H2O2 content (Fig. 3c). Ca(NO3)2 (N) significantly increased the MDA content, \({ {\text{O}}_{ 2}}^{\, \bullet -} \) formation rate and H2O2 content, and showed the highest levels among all treatments. However, EBL in the NB solution induced low levels of the MDA content, \({{ {\text{O}}_{ 2}}}^{\, \bullet -} \) formation rate and H2O2 content to 76.9, 72.4, and 80.8 %, respectively, as compared to the salt-stressed plants (N).
Effects of Ca(NO3)2 and/or EBL on MDA content, \({ {\text{O}}_{ 2}}^{ - } \) formation rate and H2O2 content in leaf of cucumber plants. Values represent the mean ± SE (n = 3). Letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests. Cont, control; CB, 0.1 μM EBL sprayed on leaves; N, 80 mM Ca(NO3)2 added into nutrient solution; NB, both EBL and Ca(NO3)2 treatments
Activities of antioxidant enzymes
EBL in the CB solution significantly enhanced the activities of APX (Fig. 4a) and GPX (Fig. 4c). In contrast, the activities of SOD (Fig. 4b) and CAT (Fig. 4d) were not influenced by EBL in the CB solution. The Ca(NO3)2 stress (N) significantly reduced the activities of APX, SOD, GPX and CAT to be 80.8, 80.2, 86.3, and 63.5 %, respectively, as compared to the control. Whereas, EBL in the NB solution greatly enhanced the activities of APX, SOD, GPX, and CAT, as compared to the activities of the enzymes in the plants (N) with salt stress (Fig. 4).
Effects of Ca(NO3)2 and/or EBL on APX, SOD, GPX, and CAT activities in leaf of cucumber plants. Values represent the mean ± SE (n = 3). Letters indicate significant differences at P < 0.05 according to Duncan’s multiple range tests. Cont, control; CB, 0.1 μM EBL sprayed on leaves; N, 80 mM Ca(NO3)2 added into nutrient solution; NB, both EBL and Ca(NO3)2 treatments
Ultrastructure of the photosynthetic apparatus
Under control condition, the chloroplasts showed thylakoids with grana (Fig. 5). The ultrastructure of the photosynthetic apparatus seemed to show no difference between the control plants and plants treated with EBL alone (CB). However, Ca(NO3)2 (N) caused remarkable structural changes. Specifically, internal lamellae of the stromal thylakoids were damaged to induce partly granal thylakoid layers to loosen. Furthermore, the chloroplast envelopes (membranes) were damaged by salt (N) and became indistinct. EBL in the NB solution apparently enhanced recovery of the chloroplast shape in that the stromal lamellae seemed to form formally the grana ultrastructure and the chloroplasts were observed to make distinct envelopes (membranes).
Discussion
According to Ali et al. (2008a) and Hayat et al. (2010), BRs can ameliorate the detrimental effect of NaCl stress on P N, Gs, Ci, and enhance the antioxidant system in plants. Current literature provides little evidence for the effects of EBL on plants under Ca(NO3)2 stress. In this study, the exogenous EBL enhanced plant growth and photosynthetic characteristics, alleviated oxidative damage and supported the recovery of chloroplast ultrastructure in plants when subjected to Ca(NO3)2 stress. These phenomena appear to result in physiological/metabolic alteration due to EBL as described below.
EBL in the CB solution caused a significant increase in the P N in cucumber plants. The levels of chlorophyll content (Fig. 1) enhanced by EBL seemed to contribute to an increase in the P N (Fig. 2). Moreover, this phenomenon may result in activation and/or induction of enzymes in chloroplasts by EBL. EBL also enhances the capacity of CO2 assimilation in the Calvin cycle (Yu et al. 2004; Xia et al. 2009).
EBL in the NB solution significantly enhanced the P N and induced a higher biomass accumulation, as compared to plants stressed with salt (Table 1). The enhancement of P N may be attributed to activation of stomatal and/or non-stomatal ability, because the enhancement also seemed to activate Gs and Ci, as shown in Fig. 2. In this work, Ca(NO3)2 stress (N) caused reduction of P N, Gs and Ci in cucumber plants (Fig. 2a–c). These phenomena appeared to be due to a deterioration of stomatal condition caused by Ca(NO3)2. The transpiration stream has a very important function with the transport of nutrients, such as inorganic elements in soil, from the soil to the roots (Lambers et al. 2008). Therefore, Tr reduced by salt (N) (Fig. 2d) seemed to decrease the absorption and utilization of water and nutrients in cucumber. In contrast, EBL in the NB solution caused significant increases in the Gs, Ci, and Tr, enhancing the photosynthesis as shown in Fig. 2. This phenomenon suggested that the influence by EBL on photosynthesis was more pronounced in plants stressed by Ca(NO3)2 than in the control. Understanding the causes of this differential response will require further investigation.
In this work, EBL in the NB solution significantly increased the Chl a and Chl b contents, but it decreased the ratio of Chl a/b (Fig. 1). These results suggested that the increases in the contents of chlorophyll by EBL in the NB solution are different between Chl b and Chl a. Specifically, in the salt-stressed plants, EBL caused a greater increase in Chl b than in Chl a. Horn et al. (2007) demonstrated that Chl b combined with the LHC more slowly and more tightly than Chl a. Moreover, they proposed that the Chl b-combining form finally stabilized the conformation of the LHC. The present study found that Ca(NO3)2 treatment darkened the green color of the leaves, which indicated that the total of chlorophyll content, on an area basis, was higher than in the control plants (data not shown). However, the chlorophyll content on a fresh weight basis in the salt-treated cucumber plants was lower than in the control (Fig. 1), suggesting that Ca(NO3)2 promoted the thickening of the leaves.
EBL in the NB solution played a role in the protection of PSII against excess energy through enhancing the thermal dissipation of the excitation energy (Table 2). ΦPSII, decreased by Ca(NO3)2 stress, was involved with changes in the photochemical conversion efficiency of PSII, which was regarded as injury to PSII complexes and the possible photoinhibition of photosynthesis. The decreases in the ΦPSII and qP by Ca(NO3)2 stress (N) (Table 2) suggested that the electron transport capacity in PSII was reduced. Salinity was also known to decrease qN, indicating that the radiant energy dissipation process was affected by Ca(NO3)2 stress. Furthermore, the Fv/Fm decreased by Ca(NO3)2 stress seemed to damage the reaction centers. EBL in the NB solution enhanced the ΦPSII, qP, and qN, and alleviated the decline of Fv/Fm. In addition, EBL might accelerate the repair process of pre-D1, release the photoinhibition of PSII and enhance thermal dissipation, thus avoiding the damage caused by excess energy excitation in the reaction centers. This can be illustrated as an increased qN (Table 2).
According to Mittler (2002), EBL treatment effectively protected plants from the oxidative stress induced by salt through enhancing the capacity of the antioxidant system. ROS can trigger peroxidative reactions and cause major damage to essential macromolecules, such as proteins involved in various enzymes of photosynthesis and in the systems of photosynthetic membranes (Foyer et al. 1994). Yabuta et al. (2002) reported that the overexpression of tAPX cDNA enhanced the level of thylakoid membrane-bound APX to maintain a higher ascorbic acid content which was engaged in maintaining cellular redox homeostasis under stresses. In this work, Ca(NO3)2 stress induced the accumulation of ROS (Fig. 3) and decreased the antioxidant enzyme activities (Fig. 4) in the cucumber leaves. The inactivation of SOD appeared to be due to excess Ca2+, which might inhibit the absorption of coupling factors such as Fe, Mn, Cu, or Zn ions. The mechanism of the repression of the antioxidant enzyme genes or the transcription/translation of the enzyme proteins by excess Ca2+ remains to be investigated. The addition of EBL significantly increased the activities of APX, SOD, GPX, and CAT in the salt-stressed plants. These phenomena seemed to decrease the levels of ROS by the EBL (Fig. 3). EBL also regulates the expression of the peroxidase-encoding genes, ATP2 and ATP24a, in Arabidopsis (Goda et al. 2002). Therefore, the enhanced activities of antioxidant enzymes seemed to play an important role in order to scavenge the ROS (Fig. 3b, c). As a result, the activation of the enzymes will probably reduce the inhibitory effect of H2O2 on the synthesis of pre-D1 and protect the photosynthetic apparatus.
The activated behavior of antioxidant system in this study (Fig. 4) suggested that EBL acted to normalize the shape of thylakoid, promoted the formation of thylakoid grana and maintained the integral chloroplast envelopes in the leaves of cucumber plants treated with Ca(NO3)2. According to Parida et al. (2003), Sam et al. (2003), and Yamane et al. (2003), NaCl stress induced extreme damage on the ultrastructure of chloroplasts and thylakoids. However, few studies are reported on the harmful effects of Ca(NO3)2 on the ultrastructure of the photosynthetic apparatus in higher plants. The microscopic analysis in this study revealed that Ca(NO3)2 stress caused the blurred/loose shapes of the part of internal lamellae thylakoids as shown in Fig. 5, although most of granal thylakoids were still maintained. The chloroplasts and thylakoids envelope were shown to be degraded to varying degrees by the oxidative stress (cf. the higher MDA content in Fig 3). The thylakoid plays an important role in the function of light absorption, electron transport, and energy conversion. Moreover, its structure also plays a functionally important role in the photosynthetic apparatus (Ioannidis and Kotzabasis 2007). Therefore, the oxidative damage of the thylakoids layers is considered to cause the blockage of electron transport, which leads to the decline of the photosynthetic rate. The results in this study suggested that EBL in the NB solution relieved this structural damage by protecting the photosynthetic membrane system from oxidative stress. Thylakoids and chloroplasts in cucumber plants by EBL were maintained their typical shapes similar to those of the control.
In conclusion, Ca(NO3)2 suppressed plant height, leaf area, biomass and physiological actions of cucumber plants. Ca(NO3)2 caused oxidative damage to the photosynthetic apparatus and resulted in the decline of the net photosynthetic rate and a slight change in chlorophyll fluorescence. The EBL positively regulated the content of the photosynthetic pigments, enhancing the capacity of the antioxidant system and protecting the reaction centers from photoinhibition. These results indicated that EBL played an important role in the relief of the harmful effect by Ca(NO3)2 and promotion of plant growth. Although the application of EBL (CB) under normal conditions also promoted plant growth and enhanced net photosynthetic rate and capacity of antioxidant system, the effects of EBL in plants under the salt-stressed condition were more prominent than those under the condition without salt. The mechanism of these phenomena by EBL remains obscure. These issues are currently being investigated from viewpoints of biochemistry and molecular biology.
Abbreviations
- APX:
-
Ascorbate peroxidase
- BRs:
-
Brassinosteroids
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- EBL:
-
24-Epibrassinolide
- Fv/Fm:
-
Maximal quantum yield of PSII photochemistry
- GPX:
-
Guaiacol peroxidase
- LHC:
-
Light-harvesting complex
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- ΦPSII:
-
The actual efficiency of PSII
- qN:
-
Non-photochemical quenching
- qP:
-
Coefficient for photochemical quenching
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008a) 24-Epibrassinolide protects against the stress generated by saline and nickel in Brassica juncea. Chemosphere 72:1387–1392. doi:10.1016/j.chemosphere.2008.04.012
Ali Q, Athar HR, Ashraf M (2008b) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116. doi:10.1007/s10725-008-9290-7
Ashraf M (1994) Breeding for salinity tolerance in plants. Crit Rev Plant Sci 13:17–42. doi:10.1080/07352689409701906
Elstner EF, Heupel A (1976) Inhibition of nitrate formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
FAO (2012) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plantarum 92:696–717. doi:10.1111/j.1399-3054.1994.tb03042.x
Giannopolitis CN, Ries SK (1977) Superoxide dismutase in higher plants. Plant Physiol 59:309–314
Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130:1319–1334. doi:10.1104/pp.011254
Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112. doi:10.1016/j.envexpbot.2010.03.004
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetic and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Herzog V, Fahimi H (1973) Determination of the activity of peroxidase. Anal Biochem 55:554–562
Horn R, Grundmann G, Paulsen H (2007) Consecutive binding of chlorophylls a and b during the assembly in vitro of light-harvesting chlorophyll-a/b protein (LHCII b). J Mol Biol 366:1045–1054. doi:10.1016/j.jmb.2006.11.069
Houimli SM, Denden M, El Hadj SB (2008) Induction of salt tolerance in pepper (Capsicum annuum) by 24-epibrassinolide. Eur Asia J Bio Sci 2:83–90
Hu WH, Wu Y, Zeng JZ, He L, Zeng QM (2010) Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 48(4):537–544. doi:10.1007/s11099-101-0071-y
Ioannidis NE, Kotzabasis K (2007) Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim Biophys Acta 1767:1372–1382. doi:10.1016/j.bbabio.2007.10.002
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effect of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358. doi:10.1007/s10535-007-0072-2
Jin CY, Sun J, Guo SR (2010) Effects of exogenous spermidine on growth and active oxygen metabolism in cucumber seedlings under Ca(NO3)2 stress. Acta Bot Boreal 30(8):1627–1633
Kooten O, Snel J (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150. doi:10.1007/BF00033156
Lambers H, Chapin FS, Pons TL (2008) Plant water relations. In: Lambers H, Chapin FS, Pons TL (eds) Plant physiological ecology. Springer, New York, pp 163–223. doi:10.1007/978-0-387-78341-3_5
Li DP, Wu ZJ, Liang CH, Chen LJ (2004) Characteristics and regulation of greenhouse soil environment. Chin J Ecol 23:192–197
Liang YC, Si J, Nikolic M, Peng Y, Chen W, Jiang Y (2005) Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol Biochem 37:1185–1195. doi:10.1016/j.soilbio.2004.11.017
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. doi:10.1016/S1360-1385(02)02312-9
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:679–690
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogues S (2008) Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57. doi:10.1007/s00344-007-9030-7
Parida AK, Das AB, Mittra B (2003) Effects of NaCl stress on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parvifolra chloroplasts. Photosynthetica 41:191–200. doi:10.1023/B:PHOT.0000011951.37231.69
Patterson BD, Macrae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plants extracts using titanium (IV). Ann Biochem 139(2):487–492. doi:10.1016/0003-2697(84)90039-3
Qayyum B, Shahbaz M, Akram NA (2007) Interactive effect of foliar application of 24-epibrassinolide and root zone salinity on morpho-physiological attributes of wheat (Triticum aestivum L.). Int J Agric Biol 9:584–589
Sam O, Ramírez C, Coronado MJ, Testillano PS, Risueño MC (2003) Changes in tomato leaves induced by NaCl stress: leaf organization and cell ultrastructure. Biol Plantarum 47:361–366. doi:10.1023/B:BIOP.0000023878.58899.88
Strain HH, Svec WA (1966) Extraction, separation, estimation and isolation of the chlorophylls. In: Vernon LP, Seeley GR (eds) The chlorophylls. Academic Press, New York, pp 21–66
Sun YD, Luo WR, Li XZ, Qi AG (2009) Effects of Ca(NO3)2 stress on the growth and physiological indexes of cucumber seedlings. Environ Sci Info Appl Technol 268–271. doi:10.1109/ESIAT.2009.110
Tong YW, Chen DF (1991) Study on the cause and control of secondary saline soils in greenhouse. Acta Hortic Sin 18:159–162
Wu XG (2001) Causes and preventive measures of salt accumulation on face soil in vegetable greenhouse. J Zhejiang Wanli Univ 14:19–21
Xia XJ, Huang YY, Wang L, Huang LF, Yu YL, Zhou YH, Yu JQ (2006) Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pest Biochem Phys 86:42–48. doi:10.1016/j.pestbp.2006.01.005
Xia XJ, Huang LF, Zhou YH, Mao WH, Shi K, Wu JX, Asami T, Chen ZX, Yu JQ (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196. doi:10.1007/s00425-009-1016-1
Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J 32:915–925. doi:10.1046/j.1365-313X.2002.01476.x
Yamane K, Kawasaki M, Taniguchi M, Miyake H (2003) Differential effect of NaCl and polyethylene glycol on the ultrastructure of chloroplasts in rice seedlings. J Plant Physiol 160:573–575. doi:10.1078/0176-1617-00948
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143. doi:10.1093/jxb/erh124
Yu HY, Li TX, Zhou JM (2005) Secondary salinization of greenhouse soil and its effects on soil properties. Soils 37(6):581–586
Zhang GW, Liu ZL, Zhou JG, Zhu YL (2008) Effects of Ca(NO3)2 stress on oxidative damage, antioxidant enzymes activities and polyamine contents in roots of grafted and non-grafted tomato plants. Plant Growth Regul 56:7–19. doi:10.1007/s10725-008-9281-8
Zhang RH, Li J, Guo SR, Tezuka T (2009) Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth Res 100:155–162. doi:10.1007/s11120-009-9441-3
Acknowledgments
This work was funded by National Basic Research Program of China (973 334 Program, No. 2009CB119000), National Natural Science Foundation of China (No. 30900995; No. 335 31071831), and the Priority Academic Program Development of Jiangsu Higher Education Institutions 336 (PAPD), and supported by the China Earmarked Fund for Modern Agro-industry 337 Technology Research System (CARS-25-C-03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lingyun Yuan and Sheng Shu contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Yuan, L., Shu, S., Sun, J. et al. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth Res 112, 205–214 (2012). https://doi.org/10.1007/s11120-012-9774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9774-1