Abstract
Egy1 was isolated as an ethylene-dependent gravitropism-deficient Arabidopsis mutant. Molecular studies reveal that EGY1 gene encodes a 59-kDa plastid-targeted metalloprotease. It is actively expressed in hypocotyl tissue and targets to endodermal and cortex plastid. Its protein level is up-regulated by both ethylene and light. CAB protein accumulation and chlorophyll level is severely reduced in hypocotyls and endodermal cells, respectively. Sucrose is able to restore the severely reduced starch and lipid contents as well as the deficient endodermal plastid size found in light-grown egy1 hypocotyls yet it fails to rescue the reduced plastid number and chlorophyll level in egy1 endodermal cells. The loss-of-function egy1 mutation results in a smaller size (1.9 ± 0.3 μm in diameter) and less number (5 ± 1) of plastids in endodermal cells, which are nearly 50% of the wild-type. EGY1 is specially required for the development of full-size endodermal plastid in seedlings that are grown on sucrose-free media under light. It plays a direct role in controlling the light-induced chlorophyll production, grana formation and plastid replication in endodermal cell. However, it plays an indirect role in regulation of endodermal plastid size. It is likely that the ethylene-dependent gravitropism-deficient phenotype of egy1 hypocotyls may result from the smaller size and less number of endodermal plastids. Gravicurvature assays performed on ethylene-insensitive mutants, etr1-1, etr2-1, ers2-1, ein4-1 and ein2-5, have clearly demonstrated the necessary role for ethylene in vigorous gravitropism of light-grown hypocotyls. The degree of ethylene-dependent gravicurvature is positively correlated with the combined state of endodermal plastid mass and number. Neither ethylene nor EGY1-regulated full-size endodermal plastid is sufficient for promotion of vigorous hypocotyl gravitropism. Presence of 4 full-size plastids per endodermal cell together with ethylene pretreatment of hypocotyls becomes sufficient to trigger vigorous gravicurvature in light-grown seedlings. A model is therefore proposed to address the role of EGY1 in regulation of endodermal plastid size and number as well as the stimulatory effect of ethylene on hypocotyl gravitropism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gravitropism is a complex physiological process, which involves graviperception, signal formation/transduction and differential growth (Chen et al. 2002; Morita and Tasaka 2004). The sensing of the altered gravity vector direction by a displaced plant stem (or hypocotyl) is presumably achieved by the interaction of sedimenting amylopalst with transvacular membrane, cytoskeleton and plasmamembrane (Sack 1991; Kiss and Edelmann 1999; Baluska and Hasenstein 1997; Yoder et al. 2001; Saito et al. 2005; Morita et al. 2006). Alternative to this organelle interaction-triggered gravity-sensing model is a “gravitational pressure model”, in which the gravireceptor lies at the junction between the plasmamembrane and cell wall, and a subtle change in compression pressure resulting from the whole protoplast reorientation within the displaced organs may generate a mechanical signal (Wayne and Staves 1997; Telewski 2006). This internal mechano-signal is then converted into numerous downstream intermediary signals such as protons, calcium, potassium, inositol 1, 4, 5-triphosphate, protein phosphor-relay, auxin and probably ethylene (Wheeler and Salisbury 1980; Estelle 1996; Sinclair and Trewavas 1997; Friedman et al. 1998; Fasano et al. 2001; Kato et al. 2002; Perera et al. 2006). Finally, the initial gravity signals are translated into the differential growth response, which is then mediated by asymmetric auxin distribution via lateral auxin transport (Chen et al. 2002; Friml et al. 2002; Paciorek et al. 2005).
In stem and stem-like organ, starch-filled endodermal plastid (amyloplast) is considered as a statolith (gravity sensor, Morita and Tasaka 2004). Little is known about the nuclear genes that are involved in regulation of the biogenesis and replication of the starch-filled plastid in endodermal cell in hypocotyls. Arabidopsis pgm1 (plastidic PhosphoGlucoMutase 1) mutant may be the first of its type (Caspar and Pickard 1989; Kiss et al. 1989). This type of mutant contains starch-less plastids in the whole plant and has reduced gravitropism in stem and stem-like organ (Kiss et al. 1997). Arc (Accumulation and Replication of Chloroplast) mutants are another class of plastid-related gravisensing mutants. These mutants have two enlarged amyloplasts in each of the gravity-sensing cells (endodermis, Roberston et al. 1995; Yamamoto et al. 2002). As a result, both inflorescence stem and hypocotyls of these mutants exhibit reduced gravitropism as pgm1 does.
To elucidate the molecular mechanisms underlying the gravity signaling in stems and stem-like organ, genetic screens have been deployed to identify Arabidopsis mutants defective in either hypocotyls or inflorescence stem gravitropism (Fukaki et al. 1998; Wysocka-Diller et al. 2000; Tasaka et al. 2001). This approach has been proven to be very effective and has uncovered numerous interesting gravity signaling components in shoot organ (Tasaka et al. 2001). One of the examples is the identification of a C2H2-type zinc finger protein, SGR5, which is expressed in gravity-sensing endodermal cells and involved in early events of gravitropism in Arabidopsis inflorescence stems (Morita et al. 2006).
Ethylene is a major plant hormone (Fluhr and Mattoo 1996). Its biosynthesis can be induced by a variety of environmental factors (Ge et al. 2000). Ethylene is involved in gravitropism of both plant stems (Zobel 1973; Wheeler and Salisbury 1980; Lu et al. 1999, 2001) and roots (Guisinger and Kiss 1999). It has a stimulatory effect on hyponasty of petiole growth (vertical orientation of petiole, Millenaar et al. 2005). However, it plays an intricate dual role in gravitropism of hypocotyls (Kiss et al. 1999; Madlung et al. 1999; Lu et al. 2002; Edelmann 2002). Generally speaking, in dark-grown seedlings, ethylene reduces the hypocotyl gravitropic response (Kiss et al. 1999), whereas in the light-grown seedlings, a prolonged ethylene treatment promotes the hypocotyl gravitropism (Liscum and Hangarter 1993; Golan et al. 1996; Lu et al. 2002). Up to now, studies on genetic mutants related to ethylene-dependent gravitropism of light-grown hypocotyls have rarely been reported. Egy1 (ethylene-dependent gravitropism-deficient and yellow green 1) was initially isolated as a dual phenotype mutant (Chen et al. 2005). One of the defective phenotypes is the loss of vigorous ethylene-dependent gravitropism in light-grown hypocotyls. Molecular characterization of the EGY1 gene has revealed that it encodes a 59 kDa plastid-targeted membrane-associated ATP-independent metalloprotease, and it is homologous to a member of SREBP Site 2 proteases that regulates lipid biosynthesis in mammalian cell (Brown and Goldstein 1997). Especially, EGY1 protein level is up-regulated by ethylene in leaf tissue. In this manuscript, we report a pivotal role played by the ethylene-enhanced EGY1 gene in regulation of plastid size and number as well as chlorophyll content in endodermal cell and further investigated the synergistic role played by both ethylene and EGY1-regulated endodermal plastids in regulation of full gravicurvature in light-grown hypocotyls. It is believed that egy1 may be a new type of plastid-related gravity-sensing mutant and the loss-of-function egy1 gene suppresses ethylene-dependent hypocotyl gravicurvature via alteration of the endodermal plastid size and number. Both ethylene signaling and EGY1-regulated full-size endodermal plastids are required for vigorous gravitropism of light-grown hypocotyls.
Materials and methods
Plant materials and growth conditions
The wild-type Arabidopsis thaliana ecotypes and mutants, ein4-1, egy1-2 (Col-0 background, SALK_134931), var1-1, phyA/B and pgm1, were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). Egy1-1 mutant was isolated by Chen et al. (2005). Cry1/2, ein2-5, etr1-1, etr1-3, ers2-1 and etr2-1 were gifts of Drs. Chentao Lin, Joseph Ecker and Elliot Meyerowitz, respectively. Arabidopsis seedlings used for gravitropism assays are grown under light (8 μmol photons m−2 s−1), unless otherwise mentioned, either on water or 0.2 × MS salt-soaked paper towers which are defined as the low nutrient medium (LNM). Soil used for growing Arabidopsis has been described previously (Lu et al. 2001, 2002).
Hypocotyl gravitropism assay and length measurement
Light-grown hypocotyl gravtiropism assay followed the method described by Lu et al. (2002). The P-value of the test was calculated using Student’s t-distribution.
Recombinant binary vectors and transformation
A 2.3-kb EGY1 gene promoter was first amplified by PCR with a pair of primers: 5′-CGC GGA TCC GGA ACA AAC AAG TTG GCA CAT-3′ (BamHI site underlined) and 5′-TCC CCA TGG GAG TGG AGA GAG-3′ (NcoI site underlined). The resulting EGY1 promoter was digested with Bam HI and Nco I and inserted into a binary vector pCAMBIA1301 and fused to the 5′ end of a β-glucuronidase (GUS) reporter gene. Pro 35S -GUS (uidA) was introduced into the wild type Arabidopsis via the floral dips (Clough and Bent 1998). With a similar approach, the EGY1-GFP fusion protein encoded in the Pro 35S -EGY1-GFP construct was introduced into egy1-1. Transgenic seedlings were selected by 50 mg/l hygromycin.
Histological GUS staining and confocal microscopic examination
GUS staining followed a modified method described by Wang et al. (2005).
Lipid, fatty acid, starch and glucose measurements
Hypocotyl segment of each 7-day-old light-grown seedling was excised out with a razor blade one by one. Analysis of lipid content and fatty acid composition plant was performed on both the wild-type and egy1-2 hypocotyls according to the method described by Xiao et al. (2005). Starch and glucose analysis of hypocotyl segments followed a method described by Pucher et al. (1948). Potato starch was used as the positive control. Glucose content was determined by a modified dinitrosalicylic acid (DNSA) method (Canizares-Maclas et al. 2001).
Iodine-staining of gravity-sensing cells
Seedlings were stained with iodine according to a previous reported method (Fukaki et al. 1998). The plastid size and number in gravity-sensing and cortex cells were measured under a light microscope (Zeiss, Model Axiophot) with 100-fold enlargement setting.
Transmission electron microscopy examination
Ethylene-pretreated hypocotyls were first fixed with 3% glutaraldehyde (in pH 7.0 potassium phosphate buffer) overnight. After being rinsed with potassium phosphate buffer (pH 7.0) twice, hypocotyls were post-fixed with 2% osminum tetroxide overnight. Hypocotyls were dehydrated in 50%, 70%, 85%, 95% and 100% ethanol, respectively. They were then embedded in spurr at 68°C for 24 h. Seventy nm ultra-thin cross sections were cut by Reichert ultracuts (Leica) and stained with 1% uranyl acetate and lead citrate. These sections were observed under 100 kv on a JEM 100cxII transmission electron microscope (Chen et al. 2005).
Antibodies and protein gel blot analysis
Antibodies against EGY1 were raised as previously described (Chen et al. 2005). Purchased were antibodies raised against Lhca1 (Santa Cruz Biotechnology, Cat SC-12691), the mouse anti-α-tubulin (chicken microtubules) monoclonal antibody (Sigma, Cat T6199) and, finally, the goat anti-rabbit IgG secondary antibodies conjugated with horseradish peroxidase (BioRad).
Results
Egy1 reduces vigorous ethylene-dependent gravitropism of light-grown hypocotyls
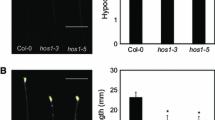
Unlike the etiolated Arabidopsis hypocotyls, the green hypocotyls do not have a vigorous gravitropic response (Fig. 1A, B; Caspar and Pickard 1989; Kiss et al. 1999). Especially, as the light-grown seedlings grow older, the ability of Arabidopsis hypocotyls to undergo gravicurvature gradually diminishes (Fig. 1D). At day 7 after germination, they are only able to bend up to 10° or less regardless of whether they are grown in soil or on a sucrose-free media (or called LNM, low nutrient media, Fig. 1A, B). The seedlings grown in soil appear to loose the gravitropism at earlier stages than those on LNM (Fig. 1A–D). However, ethylene exposure counteracts the stage effect and enhances the hypocotyl gravicurvature of 5–7 days old seedlings (Fig. 1). Such a stimulatory effect by ethylene is independent of the growth media examined. The loss-of-function egy1 mutant defective in hypocotyl gravitropism was originally screened out from ethylene-pretreated seedlings grown LNM (Chen et al. 2005). Gravicurvature kinetics study (Fig. 1E) indicates that the wild-type hypocotyls are able to bend up to 51 ± 1° within 8−12 h of gravistimulation, whereas egy1 seedlings only bend up to 5° or less within 48 h of gravistimulation. Egy1 seedlings are overall shorter than those of the wild-type and they are especially so when grown on LNM (Fig. 1C, D lower panels). Both ethylene pretreatment and gravistimulation promote the elongation of the two hypocotyls (Fig. 1F), suggesting that the loss of gravitropic response in egy1 does not result from the cease of elongation.
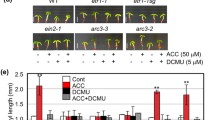
The ethylene-dependent hypocotyl gravitropism of wild-type and egy1 is influenced by the growth medium and the developmental stage of hypocotyls. (A) and (B) are 7-day-old light-grown wild-type (Col-0) and egy1-2 seedlings grown in soil and on low nutrient medium (LNM), respectively. Seedlings were pretreated either with air or 10 ppm ethylene before being gravistimulated for 48 h in dark. Ethylene was removed during hypocotyl gravicurvature assays. Arrows indicate the direction of gravity vector before (g1) and after (g2) reorientation of hypocotyls. Bar = 1 mm. (C) and (D) show the gravicurvature and hypocotyl length of light-grown seedlings at different growth stages. The 5–7 days old seedlings were subjected to gravicurvature assays and length measurements after they had been pretreated either with air (A) or 10 ppm ethylene (E) for 48 h. Photos were taken at 12-h of gravistimulation. Ethylene was removed during gravistimulation. Gravicurvature and lengths of these hypocotyls were measured as described previously (Lu et al. 2002). (E) The gravicurvature kinetics of 7-day-old light-grown wild-type (Col-0) and egy1-2 seedlings were measured in dark for 48 h of gravistimulation. Both seedlings were pretreated with 10 ppm ethylene for 48 h before being subjected to the gravicurvature assay. Error bars represent standard error (n ≥ 30). (F) The effect of 12-h gravistimulation and 10 ppm ethylene on the hypocotyl elongation in 7-day-old light-grown seedlings, which were grown on LNM. The untreated control is considered as 100%. GS, gravistimulation
During these developmental stages, the wild-type and egy1 hypocotyls continue to elongate (Fig. 1C, D). Ethylene promotes this growth response (Fig. 1C, D). The soil-grown seedlings are, in general, 30–37% longer than those on LNM. The shorter LNM-grown seedlings appear to be more responsive to ethylene’s stimulation than those soil-grown ones (Fig. 1C, D). Thus, egy1 mutation suppresses both elongation and gravicurvature of light-grown hypocotyls, whereas ethylene promotes both responses.
Egy1 may affect the gravity-sensing amyloplast during ethylene-dependent gravicurvature
To determine which aspect of ethylene-dependent gravicurvature egy1 may influence, we first examined the gravicurvature phenotypes of light- and ethylene-signaling mutants, an amyloplast-deficient mutant and a pigment mutant (Fig. 2) together with egy1. All 3 wild-type ecotypes were included as the control (Fig. 2) because of the different genetic backgrounds of these mutants. Var1-1 is a pigment mutant (Sakamoto et al. 2002). It was selected for the gravicurvature assay because it also has a defective metalloprotease like egy1 does. Light signaling is able to integrate with gravity signaling to regulate plant tropic response (Hangarter 1997; Behringer and Lomax 1999). Two double mutants, cry1/2 and phyA/B, were therefore included in this experiment to address whether egy1 behaves like a light signaling mutant. Figure 2 shows the gravitropism results of these mutants. All these mutants exhibit the wild-type phenotype in the assay, suggesting that egy1 belongs to a mutant class different from var1, cry1/2 and phyA/B. Interestingly, when the amyloplast-deficient mutant pgm1 and ethylene-insensitive mutants, ein2-5, etr1-1, etr1-3, etr2-1, ers2-1 and ein4-1, were assayed for ethylene-dependent gravicurvature, both groups of mutants exhibit a reduced gravicurvature as egy1 does (Fig. 2). Since the etiolated egy1 seedlings display a normal “triple response” phenotype upon ethylene treatment (data not shown), it is less likely that EGY1 acts as an upstream ethylene signaling component. Thus, egy1 may affect amyloplast formation and function downstream of ethylene receptors and EIN2. At the same time, the data on ethylene-sensing mutants confirm that ethylene is required for vigorous gravicurvature in light-grown seedlings. As the hypocotyl lengths are not significantly different among egy1, pgm1, ethylene insensitive mutants and wild-type seedlings (Fig. 2), the observed gravitropism defects of these mutants do not result from the cease of elongation.
The ethylene-dependent gravitropism-deficient phenotype of egy1 is similar to those of starch-deficient and ethylene-insensitive mutants. The upper and lower panels show the ethylene-dependent hypocotyl gravicurvature and the length of 7-day-old wild-type Arabidopsis seedlings (Col-0, WS, Ler) and mutants, egy1-2, var1-1 (leaf-VARiegated, Sakamoto et al. 2002), cry1/2 (CRYptochrome, Thum et al. 2001), phyA/B (PHYtochrome, Schepens et al. 2004), pgm1 (plastidic PhosphoGlucoMutase 1, Caspar and Pickard 1989), etr1-1, etr1-3, etr2-1 (Ethylene Response), ers2-1 (Ethylene Response Sensor, Hua and Meyerowitz 1998), ein4-1 and ein2-5 (Ethylene INsensitive, Guzman and Ecker 1990) following 12 h of gravistimulation (Lu et al. 2002). All these seedlings had been pretreated with 10 ppm ethylene for 48 h. Ethylene was removed during the gravicurvature assays. Stars represent the T-test result between egy1 and WT/other mutant lines. ** represents P < 0.01. Error bars represent standard error (n ≥ 30). Seedlings were grown on LNM
EGY1 is actively expressed in light-grown hypocotyl and targets to endodermal plastid
EGY1 metalloprotease is known to regulate chloroplast development in leaves (Chen et al. 2005). As various plastids are interrelated (Thomson and Whatley 1980), it is likely that egy1 mutation affects the development of endodermal plastid. To confirm our speculation, we studied gene expression of EGY1 in various tissues using GUS-staining (Data not shown). The GUS activities were detected by the histochemical GUS-staining in transgenic seedlings of Arabidopsis transformed with a Pro EGY1-GUS (uidA) construct. GUS-staining indicates that EGY1 is actively expressed in cotyledons of both etiolated and light-grown seedlings (Fig. 3a, b). Similarly, it is expressed in both etiolated and light-grown hypocotyls but more active in light-grown ones (Figs. 3a, b, 5). Although GUS activity increases in light-grown hypocotyls, it is undetectable in the root tips of both etiolated and light-grown seedlings (Fig. 3c, d), suggesting that EGY1 is not involved in the biogenesis of columella amyloplast.
Histochemical study of light-enhanced EGY1 gene expression in seedling. The blue color is the histochemical GUS-staining of the Pro EGY1 -GUS (uidA) transgenic seedlings. Photo (a) and (c) are 3.5-day-old etiolated seedlings and their root tips (a: bar = 1 mm; c: bar = 20 μm). Photo (b) and (d) are 4-day-old light-grown seedlings and their root tips (b: bar = 1 mm; d: bar = 20 μm)
To address if EGY1 metalloprotease targets to endodermal plastid in hypocotyls, a recombinant construct containing Pro 35S -EGY1-GFP fusion gene cassette was introduced into egy1. Stable transgenic plants over-expressing EGY1-GFP fusion protein driven by a 35S promoter was able to complement egy1 phenotype. Confocal light microscopy examination reveals that EGY1-GFP fusion protein co-localizes with the auto-fluorescence of chlorophyll in the endodermal plastid (Fig. 4A), suggesting that EGY1 protein is indeed localized to endodermal plastid. The EGY1-GFP fluorescence was also observed to co-localize with the chlorophyll auto-fluorescence of plastids in cortex of light-grown hypocotyls. Neither the chlorophyll auto-fluorescence nor the EGY1-GFP fluorescence was found to associate with amyloplasts in columella cells of the same transgenic plant (Fig. 4B). The control GFP was found in cytosol and nucleus of both shoot and root (Fig. 4). Taken together, these data suggest that EGY1 should play a role in endodermal instead of columella plastid biogenesis. This conclusion is consistent with the fact that the promoter activity of EGY1 in root tip is undetectable.
Subcellular localization of EGY1 protein in hypocotyl gravity-sensing cells. (A) shows the GFP green fluorescence in endodermal cells of transgenic hypocotyls expressing EGY1-GFP fusion gene and GFP gene. Image of GFP fluorescence (a) and (e) and chlorophyll auto-fluorescence of plastid (b) and (f) is shown in green and red, respectively. Panel (c) and (g) are overlapping images of (a) + (b) and (e) + (f), respectively. Panels (d) and (h) are black and white images of endodermal cells. Panels (a)–(d) are images of the endodermal cells of EGY1-GFP fusion gene-transformed plants, whereas (e)–(h) are images of the endodermal cells of GFP gene-transformed plants. Hypocotyls of 7-day-old light-grown transgenic seedlings were examined by a confocal laser microscope. Bars = 20 μm, En, endodermal cell. Chl, chlorophyll auto-fluorescence. (B) shows the GFP green fluorescence in columella cells of transgenic root tips. Panels (a)–(c) are images of EGY1-GFP fusion protein in the transgenic Arabidopsis root tips and panels (d)–(f) are the images of control GFP protein expressed in Arabidopsis root tips. Roots of 7-day-old seedlings were examined with a confocal laser microscope. Image of GFP fluorescence and chlorophyll auto-fluorescence of amyloplasts are shown in panel (a), (d) and (b), (e), respectively. (c) and (f) are the bright field images of root tips (Bars = 20 μm, Co, columella cells. Chl, chlorophyll auto-fluorescence)
EGY1 plays a direct and an indirect role in regulation of endodermal plastid number and size, respectively
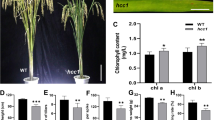
To study how egy1 may influence plastid biogenesis, three major constituents of plastids, sugar, starch and lipid, were investigated for the light-grown hypocotyls (Table 1). The glucose and starch contents of egy1 hypocotyls is 39% and 58% of that of the wild-type, respectively, while the lipid content in egy1 hypocotyls is 61% of that of the wild-type. These results demonstrate that egy1 indeed affects major constituents of hypocotyl plastids. Subsequently, we investigated the role of EGY1 in plastidic gene expression (Fig. 5). It was found that EGY1 protein is absent in egy1 hypocotyls and enhanced by both light and ethylene in this tissue (Fig. 5). As egy1 mutation suppresses the light-induced accumulation of Lhca1 protein (CAB, Fig. 5), the functional EGY1 metalloprotease is therefore required for the light-induced accumulation of CAB protein in hypocotyl plastids. Comparative examination of chlorophyll content in both the wild-type and egy1 endodermal plastid, it was found that chlorophyll auto-fluorescence is absent from egy1 endodermal plastid regardless of ethylene and sucrose treatments (Fig. 6). It is evident that neither sugar nor ethylene alone could rescue the defect in chlorophyll biosynthesis in egy1. Based on our results, it is clear that EGY1 is involved in plastidic gene expression in hypocotyl tissue and regulates chlorophyll biosynthesis in endodermal cell. It may also play an indirect role in sugar, starch and lipid biosynthesis. However, it is not clear how EGY1 mediates chlorophyll a/b binding (CAB) protein accumulation and chlorophyll biosynthesis at molecular level.
EGY1 is involved in the regulation of plastidic gene expression in light-grown hypocotyls. (A) shows protein gel blot analysis of EGY1 metalloprotease (59 kDa) and chlorophyll a/b binding protein (CAB, 20 kDa) in 7-day-old wild-type and egy1-2 hypocotyls. D, L, E and S represent protein samples from hypocotyls of dark-grown, light-grown, 10 ppm ethylene pretreatment and 1% sucrose-pretreatment, respectively. Tubulin (TUB, 50 kDa) is used as the protein loading control. (B) is the measurement of the protein band density for EGY1 and CAB. The amount of protein defined in arbitrary unit is normalized against that of TUB. Both the EGY1 protein level in the dark-grown wild-type hypocotyl tissue and the CAB protein level in sucrose-treated egy1 hypocotyl tissue were defined as one unit. Error bars represent the standard errors
Chlorophyll auto-fluorescence in WT and egy1 plastids. The chlorophyll auto-fluorescence of WT and egy1 plastids in hypocotyl endodermal cells were examined under confocal microscope. Images of chlorophyll bright field (a, c, e, g, i, k, m, o) and corresponding chlorophyll auto-fluorescence (b, d, f, h, j, l, n, p) are, respectively, shown. Hypocotyls were from 7-day-old light-grown seedlings under different treatment conditions. The concentration of sucrose and ethylene used in pretreatments is 1% and 10 ppm, respectively. Bars = 2 μm. Chl, chlorophyll auto-fluorescence; BF, bright field. Arrows indicate the endodermal plastids
The other dramatic effects that egy1 mutation has are on the plastid size and number of the gravity-sensing cells (endodermis, Figs. 7, 8, Table 2). Both of them are severely reduced to nearly a half of that of the wild-type. In contrast, the number and size of cortex plastids in egy1 hypocotyl is 93% and 71% of that of the wild-type, respectively (Table 3). Particularly interesting is that the plastid number in cotyledons of the wild-type and egy1 is 150 and 170, respectively. Thus, EGY1 plays a direct role in controlling the endodermal plastid replication. Alternatively, it may be required for maintenance of a normal number of plastids in endodermal cells.
EGY1-regulated plastid size and number in gravity-sensing cells. (A) and (B) show the iodine-stained endodermal plastids of the wild-type and egy1-2 hypocotyls. Seven-day-old light-grown seedlings were grown in the absence (a, b, e, f, i, j, m, n) and the presence (c, d, g, h, k, l, o, p) of 1% sucrose and either without (a–d, i–l) or with (e–h, m–p) ethylene pretreatment before being subjected to iodine-staining assay. Bars = 8 μm. En, endodermal cell. Arrows indicate endodermal plastids. (B) are the photos of closer-looks of amyloplasts in various hypocotyls treated either with ethylene or sucrose or both
TEM analysis of WT and egy1 plastids. The TEM photos of both endodermal (a, b, e, f) and cortex plastids (c, d, g, h) in both the wild-type and egy1-2 hypocotyls were taken from light-grown seedlings grown in the absence (a–d) and the presence (e–h) of 1% sucrose. Both groups of seedlings were 7-day-old and pretreated with 10 ppm ethylene. Bars = 1 μm
Because egy1 influences chloroplast biogenesis in leaf tissue (Chen et al. 2005), the defective size of egy1 endodermal plastid was speculated to result from a lack of sugar supply from leaves where photosynthetic assimilates are produced. It is therefore possible that egy1 influences endodermal plastid size and number indirectly via reducing the sugar supply from leaves and cotyledons. To test our speculation, egy1 mutant seedlings were treated with 1% sucrose as it was done on starch-deficient Arabidopsis mutants (Guisinger and Kiss 1999). As a result, the glucose, starch and lipid contents in hypocotyls were dramatically enhanced (Table 1). The size of endodermal plastid increases nearly 100% upon sucrose treatment and develops into fuller amyloplasts in egy1 according to the iodine-staining (Table 2, Fig. 7) and transmission electron microscopy results (Fig. 8). The endodermal plastid in sucrose-treated egy1 hypocotyls fails to develop into stacked thylokoids (grana) and interconnected lamella system (Fig. 8f) even though the plastid size increases tremendously (nearly doubled). Similarly, sucrose cannot rescue the reduced plastid number in egy1 endodermis, suggesting that the functional EGY1 gene plays a direct role in controlling the number and grana development of endodermal plastid but has an indirect role in controlling endodermal plastid size. EGY1 protein level is down-regulated by sucrose treatment (Fig. 5), suggesting a negative feedback regulation of EGY1 gene expression by sugar in light-grown hypocotyls. Since sucrose increases the size of cortex plastid only about 25% (Table 3, Figs. 8d, h) and has no major impact on the number of cortex plastid in ethylene-treated and light-grown hypocotyls (Table 3), it is believed that the endodermal plastid is one of the primary subcellular targets for EGY1 metalloprotease. In another word, EGY1 may have higher impact on the biogenesis and replication of endodermal plastid than, for example, columella plastid.
On the contrary to the effect of sugar, ethylene pretreatment of light-grown egy1 seedlings led to a further loss of an endodermal plastid (Table 2), which is consistent with a decrease in overall lipid and starch level observed in ethylene-treated egy1 hypocotyls (Table 1). Ethylene pretreatment is unable to rescue the reduced number of plastid in egy1 and nor does it promote an increase in endodermal plastid size in light-grown hypocotyls (Table 2, Fig. 7). To our surprise, the etiolated hypocotyls of the wild-type and egy1, which are grown on sucrose-free medium, contain 4–5 endodermal amyloplasts with a diameter of 4.1 ± 0.3 and 4.0 ± 0.2 μm, respectively. The endodermal amyloplast size and number are similar to what were found in sucrose-treated light-grown egy1 hypocotyls. It is therefore that EGY1 is required for the formation of full-size endodermal plastid in light-grown rather than etiolated hypocotyls and it regulates the light-induced plastid number increase in endodermal cell.
The EGY1-regulated full-size amyloplasts and ethylene signaling are required for full gravicurvature in light-grown hypocotyls
To determine whether the egy1-mediated smaller size endodermal plastids are responsible for the deficient ethylene-dependent gravicurvature, the gravicurvature kinetics of the wild-type, egy1, pgm1, ein2-5 and etr1-3 hypocotyls were measured within 24 h of gravistimulation in either presence or absence of sucrose and ethylene (Fig. 9). Without ethylene pretreatment, hypocotyls of all 5 genotypes bend up to 4° or less. Following an ethylene pretreatment, the wild-type and pgm1 hypocotyls curve up to 48 ± 3° and 24 ± 3°, respectively, while egy1, ein2-5 and etr1-3 bend up to 4° or less. Sucrose promotes the gravicurvature of the wild-type Arabidopsis hypocotyls slightly (around 14 ± 2°), while other four genotypes of hypocotyls curve up to 7° or less (Fig. 9A). When these hypocotyls are pretreated with ethylene and sucrose simultaneously, the wild-type, egy1, pgm1, ein2-5 and etr1-3 hypocotyls bend up to 43 ± 2, 44 ± 2, 15 ± 2, 5 ± 1 and 7 ± 1°, respectively (Fig. 9A). Sucrose treatment is able to rescue both endodermal plastid size and ethylene-dependent hypocotyl gravitropism simultaneously in egy1. Under these growth and treatment conditions, all five genotypes of hypocotyls are able to elongate and their growth rates are much higher in the presence of sucrose (Fig. 9B). Conceivably, sucrose plays a predominant role in promoting elongation of hypocotyls, while ethylene is necessary for light-grown hypocotyls to exhibit vigorous gravitropism (Fig. 10).
Correlation of the endodermal plastid size and number with the ethylene-dependent hypocotyl gravitropism. (A) and (B) show the gravicurvature kinetics and the growth rate of 7-day-old light-grown wild-type, egy1-2, ein2-5, pgm1 and etr1-3 hypocotyls, respectively. The concentration of sucrose and ethylene used in pretreatments is 1% and 10 ppm, respectively. Seedlings were grown under light for 5 days on a media supplemented either with or without sucrose. A half of seedlings grown on each growth condition were further pretreated either with air or ethylene for additional 48 h followed by 24 h of gravistimulation (90° of re-orientation). Error bars represent the standard error (n ≥ 30)
A model showing the role of EGY1 in regulation of endodermal plastid size and number and the effect of ethylene in the gravitropism of light-grown hypocotyls. Dashed arrows stand for gravity signaling while solid arrows the positive regulatory effect. Thicker line stands for relatively stronger effect
On the basis of both iodine-staining and TEM studies as well as gravicurvature assays (Table 2, Figs. 7–9), 4–5 smaller size (1.9 ± 0.3 μm) plastids are associated with the compromised ethylene-dependent gravitropic response in hypocotyls, while a similar number (4 ± 1) of larger size amyloplasts with a diameter of 3.7 ± 0.5 μm in an endodermal cell are correlated with vigorous gravicurvature following an ethylene pretreatment (Figs. 7–9). Particularly interesting is that pgm1 mutant, which has 10 endodermal plastids of intermediate sizes (3 μm in diameter), also exhibits an intermediate gravicurvature (Fig. 9). It is therefore believed that the combined state of endodermal plastids (size and number) is correlated with the degree of ethylene-dependent gravicurvature. The light-grown ethylene-insensitive mutants, ein2-5 and etr1-3, do not show any vigorous gravicurvature under both ethylene and sucrose treatments (Fig. 9), indicating that ethylene signaling is necessary for gravitropic process. Egy1 suppresses the ethylene-dependent hypocotyl gravitropic response via blocking the formation of normal size and number of endodermal plastids in light-grown hypocotyls (Figs. 1, 7–9, Table 2). Sucrose treatment increases the size of endodermal plastids, while ethylene pretreatment potentiates the light-grown hypocotyls to curve up. Taken together, it is concluded that both ethylene and EGY1-regulated endodermal plastids are required to induce the vigorous hypocotyl gravitropism in light-grown seedlings (Figs. 1, 7–9).
To test the conclusion that the EGY1-regulated endodermal amyloplast is required for ethylene-dependent gravicurvature, we have applied a starch biosynthesis inhibitor LiCl (Masuda et al. 2001) to light-grown egy1 seedlings on the sucrose-containing medium. As expected, the starch-staining of endodermal plastid was seriously reduced and the plastid’s size was 69% of that of the untreated egy1 hypocotyls grown sucrose-containing medium. In contrast, the plastids’ size in cortex cells was not affected. This reduced endodermal plastid size and loss of iodine-staining resulting from the LiCl treatment is tightly correlated with the reduced ethylene-dependent gravicurvature of egy1 seedlings. These results support the conclusion that (1) EGY1 plays an indirect role in regulation of endodermal plastid biogenesis, (2) a certain number of full-size endodermal plastids are required for ethylene-dependent gravicurvature in light-grown hypocotyls and (3) EGY1 is required for the formation of the full-size endodermal plastid in the light-grown hypocotyls.
Discussion
One of the defective phenotypes of egy1 is the loss of vigorous ethylene-dependent gravitropism in light-grown hypocotyls. The central issue of the present studies is to elucidate how the loss-of-function egy1 influences the conditional hypocotyl gravitropism. To achieve that end, the initial objective was to define a stimulatory role for ethylene in hypocotyl gravitropism of light-grown seedlings (Figs. 1, 2, 7–9), which is necessary for vigorous hypocotyl gravitropism in seedlings grown in both soil and LNM. Because ethylene biosynthesis is constitutively active in the light-grown seedlings (Wang et al. 2005), it is believed that a basal level of ethylene biosynthesis (0.3 nL g−1 h−1, Lu et al. 2002) may be responsible for potentiating the gravicompetence of the wild-type young seedlings so that they can bend up to a certain degrees without a long-term higher level of ethylene treatment (Fig. 1D). As seedlings grow older, the requirement for ethylene becomes stringent (the sensitivity to ethylene decreases presumably). A long-term ethylene pretreatment, which further enhances ACC synthase gene expression and ethylene biosynthesis rate in light-grown hypocotyls (Lu et al. 2002; Wang et al. 2005), is therefore necessary for this organ to acquire the ability to undergo vigorous gravicurvature.
We speculated that ethylene may enhance the sensitivities of endodermal cells to gravity signals triggered by sedimenting amyloplasts. Alternatively, it may stimulate actual gravicurvature by having a cross-talk with auxin signaling (Swarup et al. 2002). This positive effect of ethylene on gravitropism is supported by the observation that ethylene insensitive mutants etr1-1, etr2-1, ers2-1 and ein4-1 failed to undergo vigorous hypocotyl gravicurvature even when these mutant seedlings are 5 days old and ethylene-pretreated (data not shown). Apparently, the role of ethylene in promoting vigorous hypocotyl gravicurvature is independent to both seedling development stage and EGY1-mediated plastid biogenesis and replication. The stimulatory effect of ethylene on the white light-grown hypocotyls, as demonstrated in our experiments, is consistent with the previously reported on the red light-grown Arabidopsis seedlings (Golan et al. 1996).
Once it is established that ethylene has a positive effect of on hypocotyl gravitropism of various seedling stages and under different growth conditions (Figs. 1, 2), we applied the gravitropism assay on a number of mutant hypocotyls (Fig. 2) including a starch-deficient Arabidopsis mutant, pgm1. The rapid assay results (Fig. 2), in addition to what was reported for a role for EGY1 in leaf chloroplast biogenesis (Chen et al. 2005), allowed us to speculate that EGY1 plays an important role in regulation of endodermal plastid biogenesis and replication. Our studies show that EGY1 is expressed in hypocotyl tissue including the gravity-sensing endodermal cells (Figs. 3–5) and that it targets to both endodermal plastids (Fig. 4) and those plastids in cortex cells. Lack of GFP fluorescence in root tip of EGY1-GFP transgenic plant serves as a nice control to suggest that GFP fluorescence observed on endodermal plastids in EGY1-GFP transgenic hypocotyls does not result from the constitutive activities of CaMV 35S promoter (see section “Materials and methods”). Given that the enhancement of EGY1 protein level by ethylene and light does not double the endodermal plastid size while depletion of EGY1 gene product in egy1 reduces the endodermal plastid number, it is likely that EGY1 plays a major and direct role in regulation of endodermal plastid number instead of size. Sucrose is able to suppress the EGY1 protein level in the wild-type light-grown hypocotyls yet it increases the endodermal plastid size in the hypocotyls. It is therefore likely that the function of EGY1 is quite potent and a lower level of EGY1 protein is sufficient to regulate biogenesis and replication of endodermal plastid in light-grown hypocotyls. This observation is consistent with the enzymatic nature of EGY1 (a metalloprotease, Chen et al. 2005). The specific role of EGY1 in regulation of endodermal rather than columella plastid size and number is supported by the following results: (1) EGY1 promoter activity is undetectable in columella cells (Fig. 3); (2) normal size amyloplasts are observed in egy1 columella cells by iodine-staining (data not shown) and (3) EGY1-GFP fusion protein is not present in columella cell (Fig. 4B). Thus, EGY1 does not regulate columella amyloplast biogenesis and replication. As EGY metalloprotease family has three members, EGY1, EGY2 and EGY3 (Chen et al. 2005), it is possible that one or two members of this gene family may be involved in regulation of one particular type of plastid.
The loss-of-function egy1 gene results in a severe reduction in the CAB protein accumulation in leaf chloroplast of different stages of plants grown under light, the direct consequence of which should be a reduction in the photosynthesis rate and photosynthetic assimilate accumulation in leaf tissue. The reduced glucose may result in lower-level starch and lipid contents and smaller-size endodermal plastids in egy1 hypocotyls (Tables 1, 2) because an insufficient amount of photosynthetic products are transported to hypocotyls from leaf and cotyledon (Turgeon 2006). Supplementary sucrose increases glucose content 7.4 fold in egy1 hypocotyls (Table 1) and it compensates the starch and lipid deficiency in hypocotyls. The increased starch and lipid content may in turn rescue the defect in endodermal plastid size (Figs. 7–9 and Tables 1, 2). EGY1 is therefore indirectly involved in regulation of starch and lipid biosynthesis.
Generally speaking, a protein target for EGY1 metalloprotease should accumulates to a higher level if EGY1 becomes defective. However, CAB protein accumulation is lower in egy1 hypocotyls. CAB protein is therefore not a direct candidate substrate for EGY1 metalloprotease. The direct substrates for EGY1 are currently under investigation via a functional proteomics approach. It is likely that EGY1 may function in lipid metabolic pathways because EGY1 metalloprotease family is known to be closely related to a sterol-regulatory element binding protein site 2 protease (SREBP S2P, Chen et al. 2005), which regulates SREBP-dependent lipid biosynthesis pathway (Brown and Goldstein 1997). The ratio of C18 to C16 fatty acid in egy1 mutant was observed to be consistently lower than that of the wild-type regardless of treatments applied (Table 1). It is possible that EGY1 gene plays a positive role in elongation of C16 to C18 fatty acid. Ethylene treatment enhances EGY1 metalloprotease level in wild-type hypocotyls (Fig. 5), whereas it has little positive effect on the overall lipid content in these hypocotyls (Table 1). In fact, ethylene further decreases overall lipid content in egy1 (Table 1). These results suggest that EGY1 regulates overall lipid biosynthesis indirectly in hypocotyls, and it may influence lipid level through alteration of photosynthetic product supply. Since there are 3 EGY family members, regulation of lipid biosynthesis may require a coordinated action of all three members.
The etiolated wild-type hypocotyls have five amyloplasts with a diameter of 4 μm per endodermal cell. Upon light-induction, endodermal amyloplast number increases to 10 (Table 2). Sucrose rescues endodermal amyloplast size but number in egy1 (Fig. 7–9, Table 2), which strongly suggests that the functional EGY1 is required for the light-induced endodermal plastid replication and biogenesis. Lack of chlorophyll in the endodermal plastid of sucrose-treated egy1 supports this conclusion (Fig. 6, 10). Alternatively, it is also possible that it controls the number of amyloplasts present in each endodermal cell via an unknown mechanism. Ethylene treatment further reduces the endodermal plastid number to four in egy1 (Table 2). Sucrose treatment rescues the formation of endoermal plastid yet it fails to increase the number of plastid back to five. These data suggest that four full-size endodermal plastids are sufficient to initiate vigorous ethylene-dependent gravitropism response in hypocotyls upon gravistimulation. However, it is not known about the minimum number of full-size endodermal plastids in hypocotyls that is needed to initiate such a response. Arc6 and arc12 are two Arabidopsis mutants (Roberston et al. 1995) having two super large size plastids (about 18 μm in diameter, Yamamoto et al. 2002) within an endodermal cell. These two mutants appear to have abnormal hypocotyl gravitropism (Yamamoto et al. 2002). Further study of ethylene-dependent gravitropism of these two arc mutants in the future may help address the important issue raised here. Because molecular studies have revealed that ARC6 gene encodes a chloroplast-targeted DnaJ-Iike protein that is localized to the plastid envelope membrane and ARC gene was hypothesized to play a role in the assembly and/or stabilization of the plastid-dividing FtsZ ring during plastid division (Vitha et al. 2003), it is possible that ARC gene may function downstream of EGY1.
In light of the results obtained from this study and earlier reports (Roberston et al. 1995; Yamamoto et al. 2002), it is hypothesized that both the gravity perception sites (equal to the number of plastid) and a threshold of signal intensity (the effective interaction surface between the transvacular membrane and endodermal plastids) act together to establish a threshold for gravity-sensing in endodermal cells. This theory is consistent with the conclusion that the mass translocation of plastids toward lower end of the cell involves the interactions with the transvacular membrane to elicit gravity signals (Saito et al. 2005). According to this model, one role for EGY1 is to establish sufficient number of gravity perception sites in light-grown hypocotyls (Fig. 6, 10 and Table 2), while another is to provide sufficient photosynthetic products for starch and lipid biosynthesis that leads to formation of the full-size statoliths in endodermal cells i.e. to enhance the gravity signal intensity during gravicurvature.
Egy1 is a unique mutant of its kind. Based on the results presented here, the functions of EGY1 are clearly different from what have been reported for ARC, SGR5 and PGM1 in regulation of gravity-sensing in hypocotyls. The role of EGY1 in regulation of endodermal plastid number in hypocotyls is unequivocal. Moreover, the indirect role for EGY1 in regulation of endodermal plastid size has been further confirmed by a LiCl treatment on egy1 hypocotyls in the presence of sucrose. LiCl is known to inhibit the activity of phosphoglucomutase (pgm, Masuda et al. 2001) and blocks the formation of amyloplasts. The direct consequence of this treatment is a reduced ethylene-dependent gravitropism observed in hypocotyls. Future experiments will focus on a genetic approach by making an egy1pgm1 double mutant that supposes to serve the function of blocking endodermal amyloplast formation in hypocotyls. Expression of a functional EGY1 under the control of an endodermal cell-specific SCR (SCARECROW) promoter (DiLaurenzio et al. 1996; Morita et al. 2006) should also further support the role of EGY1 in regulation of endodermal amyloplasts.
One interesting phenomenon observed in this study is the intermediate ethylene-dependent gravicurvature displayed by younger egy1 hypocotyls and 7-day-old pgm1 hypocotyls (Figs. 1, 9). Ethylene seems to be able to compensate, to some extent, the reduced number or size of endodermal plastids in light-grown hypocotyls of these mutants. The “gravitational pressure model” (Wayne and Staves 1997) could be used to address the phenomenon observed here. MacCleery and Kiss (1999) once reported that plastid sedimentation in Arabidopsis root is not required for the gravisensitivity even though it contributes to it. Thus, both plastid-based and whole protoplast-based gravity-sensing mechanisms may operate in younger hypocotyls. Only when the seedlings grow older, the endodermal plastid-based gravity-sensing becomes dominant. Nevertheless, in addition to ethylene’s stimulatory effect on hypocotyl gravicurvature, the normal size endodermal plastid regulated by EGY1 do contribute to vigorous gravicurvature in light-grown hypocotyls. The effects of endodermal plastid and ethylene are synergistic in achieving a full gravicurvature in light-grown hypocotyls (Figs. 1, 7–10). This conclusion is consistent with results obtained from hypergravity experiments on starch-deficient Arabidopsis mutants (Fitzelle and Kiss 2001).
References
Baluska F, Hasenstein KH (1997) Root cytoskeleton: its role in perception of and response to gravity. Planta 203(suppl):S69–S78
Behringer FJ, Lomax TL (1999) Genetic analysis of the roles of phytochromes A and B1 in the reversed gravitropic response of the lz-2 tomato mutant. Plant Cell Environ 22:551–558
Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340
Canizares-Maclas P, Hernandez-Garciadiego L, Gomez-Ruiz H (2001) An automated flow injection analysis procedure for the determination of reducing sugars by DNSA method. J Food Sci 66:407–411
Caspar T, Pickard BG (1989) Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta 177:185–197
Chen R, Guan C, Boonsirichai K, Masson PH (2002) Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol 49:305–317
Chen G, Bi YR, Li N (2005) EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. Plant J 41:364–375
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
DiLaurenzio L, WysockaDiller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86(3):423–433
Edelmann HG (2002) Ethylene perception generates gravicompetence in gravi-incompetent leaves of rye seedlings. J Exp Bot 53:1825–1828
Estelle M (1996) Plant tropisms: the ins and outs of auxin. Curr Biol 6:1589–1591
Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13:907–921
Fitzelle KJ, Kiss JZ (2001) Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J Exp Bot 52:265–275
Fluhr R, Mattoo AK (1996) Ethylene: Biosynthesis and perception. Crit Rev Plant Sci 12:479–523
Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, Philosoph-Hadas S (1998) Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol 118:483–492
Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415:806–809
Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14:425–430
Ge L, Liu JZ, Wong WS, Hsiao WLW, Chong K, Xu ZK, Yang SF, Kung SD, Li N (2000) Identification of a novel multiple environmental factor-responsive 1-aminocyclopropane-1-carboxylate synthase gene, NT-ACS2, from tobacco. Plant Cell Environ 23:1169–1182
Golan A, Tepper M, Soudry E, Horwitz BA, Gepstein S (1996) Cytokinin, acting through ethylene, restores gravitropism to Arabidopsis seedlings grown under red light. Plant Physiol 112:901–904
Guisinger MM, Kiss JZ (1999) The influence of microgravity and spaceflight on columella cell ultrastructure in starch-deficient mutants of Arabidopsis. Am J Bot 86(10):1357–1366
Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2:513–523
Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20:796–800
Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271
Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14:33–46
Kiss JZ, Edelmann RE (1999) Spaceflight experiments with Arabidopsis starch-deficient mutants support a statolith-based model for graviperception. Adv Space Res 24:755–762
Kiss JZ, Hertel R, Sack FD (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177:198–206
Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS (1997) Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol 38:518–525
Kiss JZ, Edelmann RE, Wood PC (1999) Gravitropism of hypocotyls of wild-type and starch-deficient Arabidopsis seedlings in spaceflight studies. Planta 209:96–103
Liscum E, Hangarter RP (1993) Genetic evidence that the red-absorbing form of phytochrome b modulates gravitropism in Arabidopsis thaliana. Plant Physiol 103:15–19
Lu BW, Chan WK, Zhang H, Li N (1999) Ethylene as a dual function modulator of the negative gravitropism of arabidopsis inflorescence stem. Plant Biol, July 24–28, Baltimore, MD, USA
Lu BW, Pei LK, Chan WK, Zhang H, Zhu G, Li JY, Li N (2001) The dual effects of ethylene on the negative gravicurvature of arabidopsis inflorescence, an intriguing action model for the plant hormone ethylene. Chin Sci Bull 46:279–283
Lu BW, Yu HY, Pei LK, Wong MY, Li N (2002) Prolonged exposure to ethylene stimulates the negative gravitropic responses of Arabidopsis inflorescence stems and hypocotyls. Funct Plant Biol 29:987–997
MacCleery SA, Kiss JZ (1999) Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol 120(1):183–192
Madlung A, Behringer FJ, Lomax TL (1999) Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiol 120(3):897–906
Masuda CA, Xavier MA, Mattos KA, Galina A, Montero-Lomeli M (2001) Phosphoglucomutase is an in vivo lithium target in yeast. J Biol Chem 276:37794–37801
Millenaar FF, Cox MC, van Berkel YE, Welschen RA, Pierik R, Voesenek LA, Peeters AJ (2005) Ethylene-induced differential growth of petioles in Arabidopsis. Analyzing natural variation, response kinetics, and regulation. Plant Physiol 137:998–1008
Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7:712–718
Morita MT, Sakaguchi K, Kiyose S, Taira K, Kato T, Nakamura M, Tasaka M (2006) A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J 47:619–628
Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, Friml J (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435:1251–1256
Perera IY, Hung CY, Brady S, Muday GK, Boss WF (2006) A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol 140:746–760
Pucher GW, Leavenworth CS, Vickery HB (1948) Determination of starch in plant tissues. Anal Chem 20:850–853
Robertson EJ, Pyke KA, Leech RM (1995) Arc6, an extreme chloroplast division mutant of Arabidopsis also alters proplastid proliferation and morphology in shoot and root apices. J Cell Sci 108:2937–2944
Sack FD (1991) Plant gravity sensing. Int Rev Cytol 127:193–252
Saito C, Morita MT, Kato T, Tasaka M (2005) Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17:548–558
Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M, Sodmergen (2002) The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells 7:769–780
Schepens I, Duek P, Fankhauser C (2004) Phytochrome-mediated light signalling in Arabidopsis. Curr Opin Plant Biol 7:564–569
Sinclair W, Trewavas AJ (1997) Calcium in gravitropism. A re-examination. Planta 203(suppl 1):S85–S90
Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49:411–426
Tasaka M, Kato T, Fukaki H (2001) Genetic regulation of gravitropism in higher plants. Int Rev Cytol 206:135–154
Telewski FW (2006) A unified hypothesis of mechanoperception in plants. Am J Bot 93:1466–1476
Thomson WW, Whatley JM (1980). Development of non-green plastids. Annu Rev Plant Physiol 31:375–394
Thum KE, Kim M, Christopher DA, Mullet JE (2001) Cryptochrome 1, cryptochrome 2, and phytochrome a co-activate the chloroplast psbD blue light-responsive promoter. Plant Cell 13:2747–2460
Turgeon R (2006) Phloem loading: how leaves gain their independence. Bioscience 56:15–24
Vitha S, Froehlich JE, Koksharova O, Pyke KA, van Erp H, Osteryoung KW (2003) ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell 15:1918–1933
Wang NN, Shih MC, Li N (2005) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56:909–920
Wayne R, Staves MP (1997) A down-to-earth model of gravisensing. Gravit Space Biol Bull 10(2):57–64
Wheeler RM, Salisbury FB (1980) Gravitropism in plant stems may require ethylene. Science 209:1126–1127
Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN (2000) Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127:595–603
Xiao Y, Huang Y, Chen ZY (2005) Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr 94:544–550
Yamamoto K, Pyke KA, Kiss JZ (2002) Reduced gravitropism in inflorescence stems and hypocotyls, but not roots, of Arabidopsis mutants with large plastids. Plant Physiol 114:627–636
Yoder TL, Zheng HQ, Todd P, Staehelin LA (2001) Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol 125:1045–1060
Zobel RW (1973) Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol 52:385–389
Acknowledgements
Authors like to express sincere thanks to Drs. Chentao LIN, Joseph Ecker and Elliot Meyerowitz for providing Arabidopsis mutants. The authors also like to extend their gratitude to Drs. Zhenyu CHEN and Ying XIAO for offering the GC columns and the useful instructions in analysis of lipid and many thanks to Ms. Kaying MA for her help in management of GC machine. This work was supported by grants, DAG04/05.SC08, DAG05/06.SC09, HKUST6105/01M, HKUST6276/03M and HKUST6413/06M awarded to Ning LI from the Research Grant Council of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, D., Gao, X., Li, H. et al. EGY1 plays a role in regulation of endodermal plastid size and number that are involved in ethylene-dependent gravitropism of light-grown Arabidopsis hypocotyls. Plant Mol Biol 66, 345–360 (2008). https://doi.org/10.1007/s11103-007-9273-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-007-9273-5