Abstract
Rice crop is known to be sensitive to heat stress particularly at the flowering stage. Breeding approaches for improving heat tolerance in rice needs understanding of heat tolerance mechanisms and suitable heat tolerance donors. A study was planned for screening of rice genotypes and identification of novel heat tolerant donor(s) and physiologically characterize the component traits using contrasting set of genotypes in green house environment. Genotypes were categorized as tolerant and sensitive to heat stress using heat susceptibility index and cumulative stress response index for spikelet fertility, pollen viability, and grain yield. Among the set of genotypes screened, IET 22218 recorded high spikelet fertility (> 85%), pollen viability (> 95%) at high temperature (39–44 °C) with relative humidity (> 60–80%). This genotype recorded higher photosynthesis, canopy temperature depression, and accumulation of endogenous level of polyamines both under optimum and heat stress environments. Moreover, IET 22218 genotype recorded lower H2O2 accumulation, membrane damage and higher activity of antioxidant enzymes. Heat stress tolerance in IET 22218 was at par with heat tolerant checks, i.e., Nagina22 (N22) and Nerica L-44 (NL-44). Interestingly, IET 22218 also maintained lower chalkiness (< 34%) and higher head rice yield (> 85%) under heat stress. Based on above traits IET 22218 was selected as the novel donor for heat tolerance. The study concludes that induced polyamines and antioxidant enzymes activity in IET 22218 under stress were associated with lowering oxidative stress and maintained higher pollen viability and spikelet fertility under heat stress environment. However, more studies are recommended to understand the role of polyamines in heat stress tolerance specifically in rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is an important staple crop and feeds nearly 3.5 billion people across the globe (https://ricestat.irri.org:8080/wrsv3/entrypoint.htm, accessed on 10 April 2018). Food demand would increase with rising global population (Godfray et al. 2010) and more rice would be required to feed the majority of rice consuming population living in Asia and Africa (FAO 2014). Despite of high production demand, limiting water resources and rising temperature are posing serious threat to global rice production (Schleussner et al. 2018). Conversely, frequent occurrence of heat waves reported at regional scale in last decade had catastrophic impact on agricultural crop production across the globe (Kadam et al. 2014). The scenario A1B for future climatic change suggested that nearly, 121 million ha of irrigated global rice area will be vulnerable to rising temperature by 2100 (Teixeira et al. 2013). Rice is reported to be sensitive to heat stress at flowering/ anthesis phase (Yoshida et al. 1981; Jagadish et al. 2007, 2008; Sarsu 2018). Exposure to heat stress during reproductive stage results in impaired and poor pollen development and their germination on stigma and high sterility of spikelet in rice (Jagadish et al. 2010; Powell et al. 2012). Moreover, heat stress could alter different physiological and molecular processes that affect several growth and development process from germination to maturity (Bahuguna and Jagadish 2015). Increase in reactive oxygen species (ROS) content is one of the primary events under heat stress, which could result in peroxidation of lipids affecting the membrane integrity. However, plants have a robust antioxidant defense mechanism to maintain ROS levels below the harmful limits (Szymańska et al. 2017). Heat stress affects the photosynthetic system through change in photochemical reactions in thylakoid lamellae and carbon metabolism in the stroma of chloroplast (Wahid et al. 2007; Mathur et al. 2011). Heat stress exposure during post anthesis stage could affect reproductive success, seed set and grain filling in crop plants by altering hormonal regulation and carbon metabolism enzymes (Bahuguna and Jagadish 2015; Shi et al. 2017).
Polyamines are aliphatic polycationic compounds with low molecular weight and interact with various macromolecules DNA, proteins, or phospholipids (Tun et al. 2006; Pál et al. 2015). Role of polyamines spermidine (SPD) and spermine (SPM) providing tolerance to abiotic stresses such as heat and drought tolerance has been known in various crops (Minocha et al. 2014; Li et al. 2018) including rice (Do et al. 2013, 2014). Mechanistically, polyamines catabolism follow ROS pathway to activate antioxidant defense machinery in the plants (Liu et al. 2015). However, information related to role of polyamines and their association with heat tolerance in rice at pre- and post-flowering stage is limited. Rice diversity is the major source of genotypes having tolerance to various stresses including biotic and abiotic stresses. Moreover, a precise phenotyping and characterizing a diverse set of germplasm under natural field conditions could provide potential stress tolerant donors that can be utilized in the breeding programs. However, there is very limited number of genotypes phenotyped in detail and characterized under heat stress. N22 and Nerica L-44 are the rice genotypes reported as donors of heat stress tolerance and maintain higher spikelet fertility and pollen viability (Jagadish et al. 2007, 2010; Bahuguna et al. 2015). We selected a mini set of 36 rice genotypes and screened them under heat stress in the field. The main objectives of the study were (i) identification of potential donors with superior agronomic traits grain quality traits, (ii) to characterize the heat tolerant donor for their mechanism of tolerance, growth, yield, and quality traits.
Materials and methods
The experiment were conducted at Climatic Change Facility, Plant Physiology Division, Indian Agricultural Research Institute; New Delhi, India (28°35 N latitude, 77°12 E longitude).The climate was semi-arid with dry hot summer and mild winters. Seeds of the rice genotypes were collected from Indian Institute of Rice Research, located at Hyderabad, India.
The rice plants were raised in plastic pots during two kharif seasons of 2014–2015 and 2015–2016. In experiment I (2014–2015), screening of 36 rice genotypes (Supplementary Table 1) was done to analyze heat stress tolerance during flowering stage. For experiment II (2015–2016), contrasting set of genotypes were selected and characterized for physiological and biochemical traits and heat tolerance mechanism.
Experiment I
Experiment was conducted in white color plastic pots (14" diameter 12" height) filled with 20 kg clay-loam soil supplemented with 800 g farmyard manure mixed thoroughly and N:P:K applied as (NH4)2SO4 (0.375 g kg−1), KCl (0.075 g kg−1), and Single Superphosphate (0.075 g kg−1), respectively. Additional dose of N (0.125 g kg−1 soil) was applied at 25–30 days after transplanting. Each treatment had five pots as biological replicates. Seedlings were raised in field nursery and 21-day old seedlings were transplanted in pots and arranged randomly in the net house (4.57 m × 4.57 m × 9.14 m) under ambient environment. Pots were kept flooded (water 3–5 cm above soil surface) until two weeks before the physiological maturity. No major insect and pest events observed during the experiment.
Heat stress imposition in high temperature tunnel
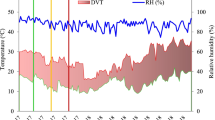
Pots were transferred in high temperature tunnel (HTT) for heat stress exposure at the heading stage of respective genotype and exposed to heat stress for at least 10 days covering the flowering and post-flowering stage of respective genotypes. After heat stress exposure, pots were shifted to ambient environment till physiological maturity. The design, structure, and control system of HTT was similar to one used earlier by Sinclair et al. (1995). The real-time data on temperature and relative humidity were monitored continuously for every 30 min time interval using MINCER obtained from NIAES, Tsukuba, Japan (Fukuoka et al. 2012). The loggers were installed at the center of the HTT and ambient at the height of 1.3 m from ground level throughout the season (Supplementary Fig. 1). However, for comparison, data represented in the figures includes the heat stress period (heading to 100% flowering) from both ambient net house and HTT and expressed as mean of the daytime (0700–1800 h) and nighttime (1800–0700 h) for both the experiments.
Experiment II
Out of 36 genotypes screened in experiment I, contrasting set of genotypes ranked for heat tolerance was selected on the basis of agronomic traits such as grain yield, spikelet fertility %, etc. using heat susceptibility indices and cumulative stress response index (CSRI) (explained in observations). The selected contrasting genotypes were further characterized for physiological and biochemical traits known for heat tolerance. Similar to experiment I, the pot experiment II was conducted using same pot size, soil type, fertilizer doses and other agronomic practices explained above. There were 10 replications (pots) for each genotype. Heat stress treatment was given from heading to 100% flowering in HTT as described for the experiment I. Flag leaf and spikelet samples were collected at 100% flowering from the respective genotypes from control (ambient) and heat stress treatment. Tissue samples were collected in liquid N2 contained falcon tubes between 0930 and 1130 h and were immediately stored in − 80 °C until further analysis. Out of ten biological replicates (plants/pot) for each genotype, five were kept for yield component analysis and non-destructive physiological observations, three for destructive biochemical samplings and two for the reproductive sampling for microscopic analysis. Plants which were used for biochemical and microscopy sampling were discarded after sampling and not used for any other observations.
Growth environment
In experiment I, the average day temperature was 5.9 °C (SD ± 2.8) higher over ambient for the treatment duration, while for experiment II, it was 4.3 °C (SD ± 2.11) higher than ambient day temperature (Supplementary Fig. 2a–d). The extent of heat stress was different (39.2–44 °C) for different genotypes based on the flowering period of the genotypes, however all the genotypes were exposed with same duration of ten days. Relative humidity (RH) during this exposure period was in range of > 60–80% in experiment 1 and 2. Vapor Pressure Deficit (VPD) in experiment 1 and 2 was in range of 1.7–2.6 kPa.
Observations
Grain yield components, spikelet fertility and in vitro pollen viability
In experiment I and II, plants were harvested at physiological maturity. Harvested samples were separated in different plant components. Panicles were sun dried in net bags while straw samples were ovens dried until constant weight was recorded. Weight of panicle and grains per plant was measured with digital analytical balance (Sartorius AG). Grains thrashed from panicles were mixed thoroughly and samples were collected randomly to estimate 1000 grain weight. Spikelet fertility (SF) percentage was calculated in both the experiments following Prasad et al. (2006) by separating filled and unfilled grain obtained from the main tiller. In vitro pollen viability analysis was done using a 2, 5-diphenyl tetrazolium bromide (MTT or Thiazolyl Blue) as described by Khatun and Flowers (1995). Thirty unopened florets were collected at the time of anthesis from plants designated for destructive samplings for each genotype across the treatments. From these florets, anthers were collected and pollens were squeezed using tweezers and were collected on the slide and incubated in MTT solution [1% MTT (w/v) + 5% sucrose (w/v)]. Pollen grains stained were recorded under a stereomicroscope (Olympus SZX7, Olympus Corp., Japan). The percent viability of pollen was estimated using of viable pollen and total pollens collected (Khatun and Flowers 1995).
Heat and cumulative stress response index
Heat susceptibility index for spikelet fertility, pollen viability, gain weight per plant and 1000 grains weight of each genotype was calculated (Fischer and Maurer 1978). The cumulative stress response index (CSRI) was calculated using the sum of response of individual component treatment (Dai et al. 1994).
Grain hulling (%) and milling (%) and quality attributes
Rice grain sample were dehusked manually by palm husker and weighted and expressed as percentage. The hulled rice was milled using polisher (Model Pearlest Kett1-8-1, Minami-Magome, Otaku, Tokyo, Japan) and an expressed as percentage. After milling the broken rice grains were isolated and the fraction was expressed as head rice recovery (Khush et al. 1978).
Grain chalkiness and amylose content
Milled grains were divided into four portions. In each portion manually separating opaque or chalky grain were isolated and weighted and was showed as per cent of total grain (within the quarter) following methods of Adu-Kwarteng et al. (2003). Amylose content (AC) was analyzed in ground rice flour following methods of Juliano (1971) and was expressed in amylose per cent. The amylose per cent was calculated by preparing the standard curve with amylose (Sigma-Aldrich).
Net photosynthesis rate and gas exchange
In experiment II, photosynthesis rate (μmol m−2 s−1), stomatal conductance (mol m−2 s−1) and rate of transpiration (mmol m−2 s−1) were recorded using Li-COR photosynthesis system (Model, LI-6400XT) between 0900 and 1130 h. CO2 level in the sample chamber was monitored by CO2 injection system (Model 6400-02B; Li-COR Inc. USA) by keeping the CO2 level of reference air nearly to 400 µmol mol–1 under a constant flow rate of 500 µmol s–1 and a saturating photosynthetic photon flux density (PPFD) of 1200 µmol m−2 s−1. Chamber block temperature was set as per ambient conditions and the RH was kept closer to 60% (Chaturvedi et al. 2017; Bahuguna et al. 2018).
Infra-Red (IR) thermal Imaging of leaf and spikelets and tissue surface temperature
In experiment II, the IR thermal images of whole plant canopies were recorded on the 7th day of heat stress exposure in between 0830 and 1130 h using Testo 890–2 Professional Infrared Camera (Testo Solutions, Inc, USA). The camera was placed on tripod at 1.0 m away from canopy and height of 1.0 m from ground and for minimizing the outside interference a black cloth was used for backing. Captured images were analyzed by IRSoft (Testo) software (Zaman-Allah et al. 2011). The tissue temperature depression was calculated as described by Ayeneh et al. (2002). During image capturing mean air temperature was 38.1 °C (SD ± 1.28) and relative humidity was 68%.
Oxidative stress (H2O2) and membrane damage (TBARS)
H2O2 in flag leaf/spikelets tissue sample was analysed spectrophotometrically as described by Alexieva et al. (2001). The content of H2O2 was expressed in μmol g−1 FW (fresh tissue weight). Thiobarbituric acid reactive substances (TBARS) in flag leaf/spikelets tissue sample were measured spectrophotometrically following Larkindale and Knight (2002) and calculated using extinction coefficient of 155 mM cm−1. Units of both H2O2 and TBARS in tissue has been shown as µmol g−1 FW.
Enzymes extraction
Flag leaf/spikelets sample (500 mg) was homogenized in pre-chilled pestle and mortar using liquid nitrogen. Homogenized mixture was transferred to 5.0 ml micro centrifuge tube (Eppendorf) containing 5.0 ml ice cold potassium phosphate buffer (0.1 M, pH 7.0) and 0.1 mM Na-ethylenediaminetetraacetic acid (Na-EDTA), (whereas in ascorbate peroxidase analysis, where 10 mM ascorbate was used in place of EDTA) and 1 mM phenylmethane sulfonyl fluoride(PMSF). The homogenate was then centrifuged at 18,400g at 4 °C for 20 min and supernatant was used as enzyme. Total soluble protein was analyzed in extract and bovine serum albumin (BSA) standard (Bradford 1976).
Enzymes assay
SOD (Superoxide dismutase) activity was assayed by monitoring based on inhibition of photochemical reduction of nitro blue tetrazolium (NBT). The reaction mixture was quantified at ʎ 560 nm and used to express as SOD unit activity mg−1 protein following Jiang and Zhang (2002).
Catalase (CAT) activity was analyzed based upon the disappearance of H2O2 at 240 nm with extinction coefficient, ε = 39.4 mM−1 cm−1 and indicated as μmol of H2O2 consumption min−1 mg−1 protein (Jiang and Zhang 2002).
Ascorbate peroxidase (APX) activity was determined as following Sharma and Dubey (2004) and indicated as µmol ascorbate oxidized (APX) min−1 mg−1 protein.
Guaiacol peroxidase (GPX) activity was determined as following de Azevedo Neto et al. (2006) and expressed as μmol of H2O2 consumption min−1 mg−1 protein.
Estimation of endogenous free polyamines
Free polyamines viz PUT, SPD and SPM were extracted and estimation by doing benzoylation and detection were performed in HPLC (Flores and Galston, 1982). Flag leaf/spikelet sample (200 mg) were homogenized in 1.0 ml of cold perchloric acid (5%, v:v) and transferred in 2.0 ml microcentrifuge tubes then this homogenate were centrifuged at 18,400g and 4 °C for 30 min. The supernatant benzoylation was done following method of Flores and Galston (1982). For benzoylation sodium hydroxide (2 M) 1.0 ml and benzoyl chlorides 10 µl were added into 500 µl of supernatant to another microcentrifuge tube (Eppendorf, 5 ml) and then they were vortex and incubated for 20 min at 37 °C. To terminate the reaction, 2.0 ml saturated solution of sodium chloride added in benzoylation mixture. Cold diethyl ether (2.0 ml) was added for extracting polyamines from benzyl polyamines. 1.0 ml of the ether phase was collected in fresh micro centrifuge tube (1.5 ml) and evaporated of ether and re-dissolved in 100 µl HPLC grade methanol (Merck) for determination of endogenous free polyamines (PUT, SPD, and SPM).
HPLC analysis was performed using Agilent 5 µm particle size reverse-phase (C18) column (size of 4.6 × 250 mm) on Agilent 1100, 20 µl of benzylated polyamines sample were injected by autosampler, under 1.0 ml/minutes flow rate of mobile phase [acetonitrile: water (52:48 v/v) (HPLC Grade, Merck)]. HPLC equipped with VWD detector at wavelength of 254 nm. Data retrieval and peak area calculation were performed using CHEM STATION for LC system (Rev B.040.3 (16) software. The concentrations of individual polyamines was calculated from the standard curve plotted using HPLC grade standard PUT, SPD, and SPM purchased from Sigma chemicals and there content were expressed on fresh weight basis (nmol g−1 FW (fresh weight).
Statistical analysis
Data obtained from both experiment were analyzed by two-way ANOVA with experiment design of completely randomized design (CRD) using SPSS 13.0 (Version 13, LEAD Technologies Inc) and the differences between cultivars, treatments and their interaction were compared. LSD mean at 5% was compare using Tukey’s Post-hoc test.
Results
Experiment I
Heat stress on pollen viability, spikelet fertility and yield attributes
In experiment I, there was a significant (P < 0.001) genotypes (G) × treatment (T) interaction for spikelet fertility. Among thirty-six phenotyped rice genotypes, US-312, PHB-71 and PR-113 recorded highest percent reduction (51–58%, respectively) in spikelet fertility over ambient grown plants, while MTU1010, IR64, IET 22218, and IET 23324 recorded least (15–17%) reduction in spikelet fertility under heat stress, as compare to ambient temperature. Heat stress tolerant checks N22 and NL-44 showed 14 and 15% reductions in spikelet fertility due to heat stress compared to ambient temperature (Table 1).
Pollen viability showed significant (P < 0.001) G × T interaction. Heat stress exposure caused significant reductions (40–51%) in pollen viability of US-312, IET 23296, PHB-71 and PR-113 under heat stress as compared to ambient temperature. Conversely, least reduction (10–11%) in pollen viability was recorded in IET 22218 and IET 23324 under heat stress, which was comparable with heat stress tolerant checks N22 and NL-44 exhibiting 11% reduction in pollen viability under heat stress as compared to ambient temperature (Table 1).
Similarly significant G × T interaction (P < 0.001) was observed for grain yield in this experiment. Heat stress reduced grain yield with maximum reduction in PR-113 (64%) followed by PHB-71(57%) and IET 23296 (53%). Conversely, four genotypes (IET 22218, MTU1010, IET 23324 and IR64) showed 13–16% reductions in grain yield due to heat stress over control and it was comparable to heat stress tolerant checks (N22 and NL-44) (Table 1). G × T interaction was observed for 1000 grain weight was also significant (P < 0.05). Maximum decline (38%) in 1000 grain weight was recorded for PR-113, while minimum in AK Dhan, N-22, and IET 22218 under heat stress as compared to ambient grown plants (Table 1).
Heat and cumulative stress response index
The heat susceptibility index (HSI) indicate the rate of change in different traits (like grain yield HSIGY, spikelet fertility HSISF, pollen viability HSIPV and for 1000 grain weight HSITGW) for each genotype in yield between the two environments (heat stress and control) in relations to the mean values of the traits for all genotypes. A greater heat stress tolerance is shown by smaller values of HSI. Supplementary Fig. 3a–c shows the most and least susceptible genotypes under heat stress based on different indices. Based on HSIGY, HSISF and HSIPV, least susceptible genotypes were N22, NL-44, MTU1010, IET 23334, IET 22218, IR64 while most susceptible genotypes identified among 36 genotypes were IET 22894, US-312, IET 23296, PHB-71 and PR-113. In case of HSITGW, there were no definite trends except in PR-113 showing maximum value of HSI (Supplementary Fig. 3d).
The CSRI was analyzed using traits like grain yield plant−1, spikelet fertility and pollen viability and 1000 grain weight under heat stress. On the basis of CSRI, out of 36 rice genotypes, CSRI for six genotypes (N22, NL-44, IET 22218, IET 23324, IR64 and MTU1010) ranged between − 37 and − 53 while for five genotypes (IET 22894, US-312, IET 23296, PHB-71 and PR-113) CSRI value ranged from − 133 to − 210 (Fig. 1). Other genotypes were identified in between these genotypes as moderately sensitive to heat stress.
Cumulative stress response index (CSRI) on the basis of yield, spikelet fertility and pollen viability of 36 rice genotypes was calculated as described by Koti et al. (2007). CSRI = ([(Trait1 in treatment-Trait1 in control)/Trait1 in control] + [(Trait2 in treatment-Trait2 in control)/Trait2 in control] + ….) × 100. Each represented data point was mean of 5 replicates with vertical bars indicate ± SEM
To understand the trait contributing maximally under heat stress, a relationship between cumulative stress response index (CSRI) with heat susceptibility Index (HSI) based on grain yield (HSIGY, A), spikelet Fertility (HSISF, B) and pollen viability (HSIPV, C), thousand grain weight (HSITGW, D) has been worked out for 36 rice genotypes (Supplementary Fig. 4). There were a significant association between HSIGY, HSISF, HSIPV and CSRI (r2 = 0.736, r2 = 0.678 and r2 = 0.716, respectively) while relationship between thousand grain weight (HSITGW) and CSRI was non-significant (r2 = 0.287) (Supplementary Fig. 4d).
Experiment II
Based on the HSI for component traits (HSIGY, HSISF, HSIPV, HSITGW) and CSRI as a cumulative stress response index in experiment I, six genotypes were identified and characterized based on physiological, biochemical, agronomical and grain quality attributes to work out the component mechanism associated with heat tolerance at reproductive stage.
Effect of heat stress on grain yield and quality
A significant (P < 0.05–0.001) interaction among genotype, treatment, as well as genotype × treatment was observed for grain weight (g hill−1) (Table 2). Heat stress exposure decreased significantly (P < 0.001) grain weight hill−1 (45 and 52%) in rice genotypes namely IET 23296 and PHB-71. Conversely, IET 22218, and IET 23324 had shown a decline of 15–19% which was at par to NL-44 (23%) and N22 (3%) grain weight hill−1 under heat stress. Significant interaction for G (P < 0.001), T (P < 0.001) as well as G × T (P < 0.001) interaction effect was also recorded for spikelet fertility (Table 2). It was reduced significantly under heat stress (10–54%) in all the rice genotypes. However, the reduction in spikelet fertility was more prominent (> 50%) in rice genotype PHB-71. Conversely, IET 22218, and IET 23324 showed lesser reduction (18%) in Spikelet fertility which was comparable to N22 (10%) and NL-44 (17%) (heat tolerant checks) under heat stress. Results for pollen viability followed the similar trend showing higher reduction (47%) in pollen viability of PHB-71 and least reduction in pollen viability of IET 22218 (16%), and IET 23324 (14%) which was comparable to N22 (3%) and NL-44 (11%) (heat tolerant checks) under heat stress (Table 2).
Significant difference (P < 0.05–0.001) in hulling, percentage of milling and recovery of head rice was recorded for genotypes × treatment (Table 2). Heat stress decreased hulling and milling percentage significantly by 10–23% for hulling and 16–33% for milling. IET 22218 (10, 16%) and IET 23324 (12, 17%) recorded lesser reduction in above attributes and followed NL-44 and N22 (10, 16%) under heat stress. Conversely, maximum percent reduction in these attributes was recorded for PHB-71 (23, 33%) (Table 2). Head rice recovery was recorded minimum (45%) in IET 23296 while, IET 22218 recorded head rice recovery of 79% which was followed IET 23324, NL-44 and N22 (60–63%) under heat stress.
Among the grain quality parameters, grain chalkiness percent increased significantly (P < 0.001) under heat stress (30–80%) as compared to ambient growth conditions. Chalky percent was higher among all the tested genotypes except IET 22218 (39%), IET 23324 (45%) following by NL-44 (30%) (Table 2). Maximum grain chalkiness of (80 and 71%) was recorded for IET 23,296 and PHB-71 across the genotypes and treatment (Table 2).
AC varied significantly (P < 0.001) for genotype and treatment, however, for G × T interaction it was non significant (Table 2). Heat stress treatment decreased significantly (P < 0.001) AC in the genotypes. IET 22218 recording significantly lesser reduction of 9% which followed NL-44 (11%) compared to control. There was significant genotypic (P < 0.001), and treatment (P < 0.001) effect for 1000 grain weight (Table 2). Heat stress showed a significant reduction (24 and 22%) in 1000 grain weight of IET 23296 and PHB-71. Conversely, there was a lesser reduction in 1000 grain weight of IET 22218 and IET 23324 (14%) which was at par to comparable to N22 (11%) and NL-44 (13%).
Characterization of component traits for heat stress tolerance
Net photosynthesis rate and gaseous exchange
A significant G, T, and G × T (P < 0.001) effect was recorded for net photosynthesis rate (PN) (Fig. 2a). PN showed significantly reduction (P < 0.001) under heat stress across the genotypes. Under heat stress treatment, two rice genotypes (IET 23296 and PHB- 71) recorded significant (P < 0.001) reductions of 42% in PN while in IET 22218 and IET 23324 minimum decline of (8 and 11%) was recorded (Fig. 2a), which was at par to heat tolerant checks NL-44 (10%) under heat stress. A significant genotypic (P < 0.001) effect was recorded for gs (Fig. 2b). Moreover, N22 recorded highest reductions (13%) in gs under heat stress as compared with ambient conditions (Fig. 2b). There was significant effect due to heat stress exposure on gs was noted for IET 22218, N22, IET 23324, and NL-44 (Fig. 2b). A significant genotypic (P < 0.001), treatment (P < 0.001) and genotype × treatment (P < 0.001) effect was recorded for transpiration rate (E). E was recorded highest in IET 23324 (16.1 mmol m−2 s−1) in the genotypes and stress treatments. However, under heat stress it was significantly increased in IET 22218 (56%), which was comparable with heat tolerant checks N22 (65%) and NL-44 (60%). While in case of PHB-71 and IET 23296 there were no significant differences in the values for E was recorded (Fig. 2c).
Effect of heat stress on gas exchange parameters (a–c) of contrasting rice genotypes under heat stress during flowering stage. Five replicated was used to represent the mean in vertical column. Bars indicate ± SEM. Tukey HSD was used to compare the means and was presented. Different letters indicate significant at 5%. PN photosynthetic rate, gS stomatal conductance, E transpiration rate, LSD least significant difference, G genotypes, T treatment, AT ambient temperature, HT heat stress. Significance level: *P < 0.05, ***P < 0.001, ns non significant
Changes in leaf and spikelets tissue temperature
Based on IR thermal imaging temperature difference between the plants grown heat stress and ambient conditions was calculated for leaf and spikelets, and termed as canopy temperature difference (CTD) in six rice genotypes. Heat stress treatment showed significant difference in flag leaf and spikelet temperature of rice genotypes (Fig. 3a–l). Maximum CTD in leaf was noted in heat tolerant checks NL-44 (5.53 °C) and N22 (5.46 °C) which was followed by IET 22218 (5.48 °C) and IET 23324 (5.12 °C). While lower CTD of 2.2 and 3.9 °C was recorded in PHB-71and IET 23296 (Fig. 3 m). Similar trend was recorded in spikelets maximum cooling was presented in N22 (4.62 °C) followed by IET 23324, NL-44, and IET 22218 (4.37, 3.40, and 2.82 °C), respectively. On the other hand, the CTD for PHB-71 and IET 23296 varied from 1.4 and 1.56 °C (Fig. 3n).
Surface temperature of flag leaf (m) and spikelets (n) of contrasting rice genotypes exposed to heat stress using Testo 890-2- Professional Infrared Camera (Testo Solutions, Inc, USA) distance of 1 m from canopy. Tissue surface temperature depression was calculated, air temperature at the time of measurement was 38.1ºC (SD ± 1.28) recorded using MINCER data logger. Five replicated was used to represent the mean vertical bar. Bars indicate ± SEM. Each filled circles shows difference between air temperature and tissue surface temperature. Bars indicate ± SE. Thermal images under ambient and heat stress conditions a, g IET 22218; b, h IET 23324; c, i N22; d, j NL-44; e, k IET 23,296; f, l PHB-71. AT ambient temperature, HT heat stress, CTD canopy temperature depression
Change in oxidative stress and membrane stability
Effect of heat stress on membrane lipid peroxidation (TBARS) and oxidative stress (H2O2 accumulation) in flag leaf and spikelet tissues are given in Table 3. There was significant variation for H2O2 and TBARS across G (P < 0.001) and T (P < 0.001) and G × T (P < 0.01 to 0.001) was recorded. Highest H2O2 content was recorded in flag leaf (4.99 µmol g−1 FW) of N22 and in spikelets (1.78 µmol g−1 FW) of IET 23296 while lowest in flag leaf (1.34 µmol g−1 FW), and in spikelets (1.12 µmol g−1 FW) of NL-44 across the genotypes and treatment (Table 3). IET 22218 maintained lower content of H2O2 (1.78 µmol g−1 FW) in flag leaf which was followed NL-44 and N22 under heat stress.
Maximum TBARS accumulation was noted in spikelets of IET 23296 (9.65 µmol g−1 FW) whereas lowest in IET 22218 (2.3 µmol g−1 FW) across the genotypes and treatment while in flag leaf it was highest in IET 23296 (14.37 µmol g−1 FW) and lowest in N22 and IET 22218 (6.36 µmol g−1 FW) across the treatment (Table 3). In general, heat stress significantly (P < 0.001) increased the H2O2 and TBARS content of flag leaf as well as spikelet tissues of rice genotypes. Under heat stress exposure, IET 22218, IET 23324, N22, and NL-44 recorded lower H2O2 (4–13%) and TBARS (5–20%) accumulation in flag leaf and in spikelet (3–7% for H2O2 and 3–22% for TBARS) than IET 23296 and PHB-71 showing higher accumulation of H2O2 (58 and 57%) and TBARS (88 and 123%) in flag leaf and in spikelet (43 and 25% for H2O2 and 105 and 41% for TBARS) compared to their respective controls (Table 3).
Change in antioxidant enzymes under heat stress
There was significant genotypic G difference (P < 0.05–0.001) for SOD, APX, catalase CAT, and GPX in the flag leaf and spikelets tissues (Fig. 4 and Supplementary Table 2). In the leaf tissue, the maximum activity of SOD was recorded in N22, CAT in IET 23296, APX in IET 22218 and NL-44, and GPX in IET 23324 while least activity of SOD, CAT, and APX in PHB-71 and GPX in IET 23296 across the genotypes and treatment (Fig. 4a). In case of spikelet tissues, the maximum SOD activity was recorded in NL-44, CAT in IET 23324, APX in IET 22218 and NL-44, and GPX in IET 23324 across the genotypes and treatment while least activity of enzyme viz. SOD, CAT, APX and GPX in IET 23296 across the genotypes and treatment (Fig. 4b). Heat stress caused significant increase in flag leaf and spikelet enzyme activity of SOD (30–50% and 15–32%), APX (44–62% and 30–49%) and GPX (20–60% and 24–64%) in of IET 22218, IET 23324, NL-44 and N22 (Fig. 4a, b). Conversely, PHB-71 and IET 23296 showed lesser enhancement in the antioxidant enzyme activity in flag leaf and spikelet tissues (Fig. 4a, b).
Change in activity of antioxidant enzymes viz. SOD, CAT, APX and GPX in the flag leaf (a) and spikelet (b) tissue of rice genotypes. All activity of enzymatic across all the tissue was analyzed and represent in unit for SOD, µmol H2O2 for CAT and GPX) and µmol ascorbate oxidized (APX) min−1 mg−1 protein. Each data point represents the relative change between heat stress and ambient temperature. Horizontal bar represents ± SEM. LSD value at 5% for evaluation are given in Supplementary Table 2
Endogenous polyamine induction under heat stress
Endogenous free polyamines (PUT, SPD and SPM) showed significant interaction (P < 0.05 to 0.001) G, T and G × T effect for spikelets and flag leaf (Fig. 5a–f). In case of leaf tissues, maximum PUT concentration (866.3 nmoles g−1 FW) was recorded in NL-44 while minimum (43.9 nmoles g−1 FW) in PHB-71 across the genotypes and treatment (Fig. 5a). IET 22218 (702.1 nmoles g−1 FW) recorded significantly accumulation of PUT in follow to N22 and NL-44 under heat stress. While in spikelet, maximum PUT concentration (591.3 nmoles g−1 FW) was recorded in IET 22218 while minimum (37.5 nmoles g−1 FW) in PHB-71 across the genotypes and treatment (Fig. 5d). Similarly in leaf tissue SPD and SPM concentration was highest in NL-44 (114.9 and 1639.7 nmoles g−1 FW) and lowest in PHB-71 (48.7 and 56.3 nmoles g−1 FW) across the genotypes and treatment (Fig. 5b, c). In spikelet, IET 22218 recorded highest SPD and SPM concentration (466 and 951.5 nmoles g−1 FW) and lowest in PHB-71 (45.6 and 55.7 nmoles g−1 FW) across the genotypes and treatment (Fig. 5e, f). In general, under heat stress, accumulation of PUT, SPD and SPM was increased in genotypes IET 22218, IET 23324, N22 and NL-44 in flag leaf as well as spikelet tissues compared to respective control. While genotypes IET 23296 and PHB-71 have shown either no change or significant decline in PUT, SPD and SPM concentration of leaf as well as spikelet tissues due to heat stress.
Changes in endogenous content of polyamines in flag leaf (a–c) and spikelet (d–f) tissues of rice genotypes under heat stress. Five replicated was used to represent the mean vertical column. Bars indicate ± SEM. Tukey HSD was used to compare the means and was presented. Different letters indicates significant at 5%. PUT putrescine, SPD spermidine, SPM spermine. LSD least significant difference, G genotypes, T treatment. Significance level: *P < 0.05, **P < 0.01, ***P < 0.001, ns non significant
Discussion
Heat stress exposure in rice at flowering stage is known to decreased grain yield in rice (Jagadish et al. 2015). Despite of increase in global mean temperature, heat spikes at regional level coinciding with critical growth stages could be detrimental to rice yield and quality attributes (Krishnan et al. 2011; Lyman et al. 2013). Breeding efforts for heat tolerance in rice are hampered majorly due to limited understanding of heat stress tolerance mechanism and unavailability of adequate number of donor genotypes (Challinor et al. 2014). N22 is well known check for heat stress tolerance (Jagadish et al. 2007). However, undesired poor agronomic traits of N22 make it poor choice for the breeding programs (Bahuguna et al. 2015). Nerica L-44 has been shown as an excellent source for heat stress tolerance at vegetative as well as reproductive stage, having excellent agronomic traits (Bahuguna et al. 2015; Chaturvedi et al. 2017). There is need to find out genetic diversity for heat tolerance in rice and identification of donors is warranted to support breeding programs for heat stress tolerance. This study was planned to phenotype/ screen rice genotypes and characterize them for heat stress tolerance.
Screening of rice genotypes under heat stress
A mini set of rice genotypes was screened to find out potential heat tolerance donor(s). Heat susceptibility index and cumulative stress response index revealed some genotypes that could maintain high spikelet fertility (> 75%), pollen viability (> 85%) and grain yield under heat exposure (39.2–44 °C) at the flowering and early grain filling stage (Table 1; Supplementary Fig. 3). In this study, we have identified two potential donors as IET 22218 and IET 23324, which recorded spikelet fertility (> 75%) and pollen viability (> 85%) at par with known checks N22, Nerica L-44 and MTU1010 (Fig. 1; Supplementary Fig. 3). Two top performing genotypes (IET 22218, IET 23324) were selected for the further characterization under heat stress at flowering stage along with heat tolerant checks and two least performing genotypes.
Yield components and grain quality under heat stress
Exposure to heat stress at flowering stage caused significant reduction in spikelet fertility (10–54%) grain yield (3–52%), and 1000 grain weight (13–25%) in sensitive cultivars across the experiment I and II (Tables 1, 2; Supplementary Fig. 3). Spikelet fertility is considered as most prominent trait that gets affected under heat stress environment. Pollen viability and their germination on stigma, are the major factors affecting in spikelet fertility when exposed to heat stress during anthesis (Jagadish et al. 2010; Bahuguna et al. 2015; Zhang et al. 2016). High percent of spikelet fertility (> 75%) even under heat exposure for 7 consecutive days confirms reproductive resilience of new donor genotypes to long-term heat stress (40 °C). These results were in line with high percent of pollen viability (85–90%) in the genotypes showing higher spikelet fertility (Tables 1 and 2). Higher spikelet sterility is major cause of yield reduction in rice. Genotypes showing high spikelet fertility recorded higher grain yield and 1000 grain weight across the experiments and treatments. Both IET 22218 and IET 23324 recorded higher grain yield and 1000 grain weight at par with high yielding variety (IR64) across the treatments. Besides grain yield, rice grain quality is crucial factor to determine the rice market prize (Lyman et al. 2013). Heat stress (day/night) can affect rice grain quality by affecting starch accumulation in grain resulting in lower brown and milled rice percent, high proportion of chalky grains resulting in poor head rice recovery and lower amylose content (Counce et al. 2005; Dong et al. 2014; Bahuguna et al. 2015, 2017). Genotypes IET 22218 and IET 23324 recorded higher milling outcome showing with higher percent of hulled rice, milled rice, and head rice recovery. Moreover, IET 22218 showed significantly lower % chalkiness as compared to sensitive cultivars and at par to heat tolerant checks (Table 2).
Net photosynthesis rate and gas exchange traits under heat stress
Photosynthesis is the most important process, which gets affected under heat stress. Sensitivity of photosystem II and membrane damage by high ROS generation are the most prominent routes that reduce photosynthesis (Szymańska et al. 2017). In this study, photosynthesis reduced significantly due to heat stress exposure particularly in the sensitive genotypes (Fig. 2a). However, stomatal conductance and transpiration rate showed contrasting response as compare to photosynthetic rate across the genotypes. Transpiration rate was significantly higher in IET 22218 and IET 23324 including heat tolerant checks N22 and Nerica L-44 (Fig. 2c). It has been reported that, under adequate water supply, plants avoid rise in tissue temperature by increased evapotranspiration resulting in significant lower tissue temperature as compared to ambient air temperature. We could observe a significant difference in thermal cooling capacity between sensitive and tolerant genotypes (Fig. 3). Tolerant genotypes IET 22218 recorded higher CTD values as compared to sensitive ones. CTD is an important parameter to determine the level of thermal avoidance in a plant (Zhang et al. 2016; Ayeneh et al. 2002). IET 22218 and IET 23324 showed significantly higher degree of thermal avoidance in flag leaf and spikelets as compared to sensitive cultivars and at par with heat tolerant checks N22 and Nerica L-44 (Fig. 3m, n).
Change in oxidative stress and antioxidative enzymes under heat stress
Reduction in rate of photosynthesis due to heat stress can be attributed to heat stress-induced damage to photosynthetic machinery by higher ROS generation. We have observed higher accumulation of H2O2, which is a prominent ROS species and signature molecule for oxidative stress. Sensitive cultivars recorded many fold increase in tissue H2O2 level. Consequently, higher TBARS content in the corresponding plants could relate heat stress-induced damage in membranes and other cellular components. Interestingly, IET 22218 and IET 23324 recorded significantly lower levels of H2O2 and TBARS content in flag leaf and spikelets under heat stress as compared to sensitive genotypes (Table 3). Lower accumulation of H2O2 and TBARS in IET 22218 and IET 23324 and heat tolerant checks N22 and Nerica L-44 could be attributed to induced higher activity of antioxidant enzymes. Tolerant checks and newly identified genotypes IET 22218 and IET 23324 recorded higher activity of ascorbate peroxidase (APX) and guiacol peroxidase (GPX) compared to sensitive genotypes across the flag leaf and spikelets (Fig. 4). Both APX and GPX are involved in scavenging H2O2 from the system (Das and Roychoudhury 2014) and directly related to the heat tolerance in several crops (Wahid et al. 2007). Some of the transgenic plants overexpressing APX and GPX genes has been shown to have heat tolerance when compared with wild type plants (Milla et al. 2003; Shi et al. 2001). Conversely, lower catalase activity recorded in IET 22218, IET 23324 and heat tolerant checks plausibly favored salicylic acid induced pathway for heat tolerance, which operates by down regulation of salicylic acid binding proteins (catalase) to maintain desired H2O2 levels to activate systemic defense signaling throughout the plants and activating antioxidant defense machinery (Conrath et al. 1995; Dat et al.1998).
Induced polyamines accumulation under heat stress
Polyamines with low molecular weight aliphatic nitrogenous bases contain two or more amino groups (Pál et al. 2015). Polyamines are central to the defense signaling involved under various types of abiotic stress including heat stress in several crop species (Minocha et al. 2014; Pál et al. 2015). Accumulation of putrescine, spermine, and spermidine has been shown to activate defense pathway via H2O2 signaling when spermidine and spermine breaks down by enzymes PAO (polyamine oxidase) under stress condition. Initial accumulation of putrescine required reaching a threshold level before activating the accumulation of spermidine and spermine. Earlier studies have demonstrated that accumulation of putrescine alone did not correlate well with the tolerance level of the plant. However, higher accumulation of both spermidine and spermine has been associated with better tolerance of plants to heat stress or any other abiotic stress conditions (Liu et al. 2004; Do et al. 2014; Ikbal et al. 2014). Interestingly, we have observed that IET 22218, IET 23324 and heat tolerant checks recorded significantly higher accumulation of putrescine, spermidine, and spermine under heat stress across the flag leaf and spikelet tissues as compared to the plants grown under ambient temperature. In contrast, sensitive genotypes recorded lower levels of all three polyamine molecules across the treatments (Fig. 5). Accumulation of polyamines under heat stress has been associated with higher photosynthesis, augmented antioxidant system and osmotic adjustment capability (Tian et al. 2012; Tang et al. 2018). Ability of polyamines to activate antioxidant enzymes could help in scavenging accumulated ROS in the tissues and prevent membrane lipid peroxidation and help to stabilize the membrane structure under stress environment (Singh 2015; Ouyang et al. 2017; Chen et al. 2019).
Conclusion
This study led to identification of two novel rice donors for heat stress tolerance on the basis higher spikelet fertility, pollen viability as well grain yield and quality. One of the donor (IET 22218) performed better than reported heat tolerant checks, i.e., N22 and NL-44 for above traits under heat stress environment. The study suggests that higher photosynthesis, canopy temperature depression, antioxidant enzymes activity and accumulation of spermidine and spermine were the component traits that could be explored to dissect the tolerance mechanism in the identified donors. These donors can be used in future rice breeding programs by focusing on components traits along with acceptable agronomic traits and yield potential under optimum and stress environments. High grain chalkiness score under heat stress reduces head rice yield and other quality traits. Low chalkiness score under heat stress was found in above tolerant rice cultivars in this study and could be used as source for improving grain yield and quality of elite cultivars. This study also highlights the possible involvement of polyamines in heat tolerance in rice and suggests further investigation to explore the mechanism of polyamine mediated pathway contributing to heat stress tolerance.
Author contribution statement
The authors have made the following declaration about their contributions. Conceived and designed the experiments: AKC, RNB and MP. Performed the experiments: SK, SM, AKC, and RNB. Analyzed and interpreted the data: SK, AKC and RNB. Performed statistical analysis: AKC, RNB, and SK. Drafted the manuscript: AKC, RNB, SK SS, VC and MP. Edited and finalized the manuscript: RNB, AKC, and MP.
References
Adu-Kwarteng EE, Oduro WO, Manful JT (2003) Rice grain quality: a comparison of local varieties with new varieties under study in Ghana. Food Control 14:507–514
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ayeneh A, Ginkel V, Reynolds MP, Ammar K (2002) Comparison of leaf, spike, peduncle and canopy temperature depression in wheat under heat stress. Field Crops Res 79:173–184
Bahuguna RN, Jagadish KSV (2015) Temperature regulation of plant phenological development. Environ Exp Bot 111:83–90
Bahuguna RN, Solis CA, Shi W, Jagadish KSV (2017) Post-flowering night respiration and altered sink activity account for high night temperature-induced grain yield and quality loss in rice (Oryza sativa L.). Physiol Plant 159:59–73
Bahuguna RN, Tamilselvan A, Muthurajan R, Solis CA, Jagadish SVK (2018) Mild preflowering drought priming improves stress defences, assimilation and sink strength in rice under severe terminal drought. Fun Plant Biol 45:827–839
Bahuguna RNJ, Pal J, Shah M, Lawas D, Lovely MF, Khetarpal S, Jagadish SVK (2015) Physiological and biochemical characterization of NERICA-L-44: a novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol Plant 154:543–559
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Blum A, Shpiler L, Golan G, Mayer J (1989) Yield stability and canopy temperature of wheat genotypes under drought-stress. Field Crops Res 22(4):289–296
Challinor AJ, Watson J, Lobell DB, Howden SM, Smith DR, Chhetri N (2014) A meta-analysis of crop yield under climate change and adaptation. Nat Clim Change 4:287
Chaturvedi AK, Bahuguna RN, Shah D, Pal M, Jagadish SVK (2017) High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink-strength in rice. Sci Rep 7:8227
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1945
Conrath U, Chen Z, Ricigliano JR, Klessig DF (1995) Two inducers of plant defense responses, 2, 6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc Nat Acad Sci 92(16):7143–7147
Counce PA, Bryant RJ, Bergman CJ, Bautista RC, Wang YJ, Siebenmorgen TJ, Moldenhauer KAK, Meullenet JFC (2005) Rice milling quality, grain dimensions, and starch branching as affected by high night temperatures. Cereal Chem 82:645–648
Dai Q, Shaobing P, Chavez AQ, Vergara BS (1994) Intraspecific responses of 188 rice cultivars to enhanced UV-B radiation. Environ Exp Bot 34:433–442
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116(4):1351–1357
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Do PT, Degenkolbe T, Erban A, Heyer AG, Kopka J et al (2013) Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 8(4):e60325. https://doi.org/10.1371/journal.pone.0060325
Do PT, Drechsel O, Heyer AG, Hincha DK, Zuther E (2014) Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: a comparison with responses to drought. Front Plant Sci 5:182. https://doi.org/10.3389/fpls.2014.00182
Dong W, Chen J, Wang L, Tian Y, Zhang B, Lai Y, Meng Y, Qian C, Guo J (2014) Impacts of night time post-anthesis warming on rice productivity and grain quality in East China. The Crop J 2:63–69
FAO (2014) (Food and Agriculture Organization). http://www.fao.org/faostat/en/?#home. Accessed 5 Jan 2018
Fischer R, Maurer R (1978) Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust J Agril Res 29:897–912
Flores HE, Galston AW (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69:701–706
Fukuoka M, Yoshimoto M, Hasegawa T (2012) MINCER: A novel instrument for monitoring the micrometeorology of rice canopies. J Agril Meteorol 68:135–147
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Ikbal FE, Hernández JA, Barba-Espín G, Koussa T, Aziz A, Faize M, Diaz-Vivancos P (2014) Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J Plant Physiol 171:779–788
Jagadish SVK, Cairns J, Lafitte R, Wheeler TR, Price AH, Craufurd PQ (2010) Genetic analysis of heat tolerance at anthesis in rice. Crop Sci 50:1633–1641
Jagadish SVK, Craufurd PQ, Wheeler TR (2008) Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci 48(3):1140–1146
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). J Exp Bot 58:1627–1635
Jagadish SVK, Murty MV, Quick WP (2015) Rice responses to rising temperatures—challenges, perspectives and future directions. Plant Cell Environ 38:1686–1698
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Juliano BO (1971) A simplified assay for milled-rice amylose. Cereal Sci Today 16:334–360
Kadam NN, Xiao G, Melgar RJ, Bahuguna RN, Quinones C, Tamilselvan A, Prasad PVV, Jagadish KS (2014) Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. Adv Agron 127:111–156
Khatun S, Flowers TJ (1995) Effects of salinity on seed set in rice. Plant, Cell Environ 18:61–67
Khush GS, Paule CM, Dela Cruz NM (1978) Rice grain quality evaluation and improvement at IRRI. In: Proceedings of the workshop on chemical aspects of rice grain quality. Los Banos, Philippines, International Rice Research Institute 1979.
Koti S, Reddy KR, Kakani VG, Zhao D, Gao W (2007) Effects of carbon dioxide, temperature and ultraviolet-B radiation and their interactions on soybean (Glycine max L.) growth and development. Environ & Exp Bot 60(1):1–10
Krishnan P, Ramakrishnan B, Reddy KR, Reddy VR (2011) High-temperature effects on rice growth, yield, and grain quality. Adv Agron 111:87–206
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Li L, Gu W, Li J, Li C, Xie T, Qu D, Meng Y, Li C, Wei S (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55
Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL (2004) Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827
Lyman NB, Jagadish SVK, Nalley LL, Dixon BL, Siebenmorgen T (2013) Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 8:e72157
Mathur S, Jajoo A, Mehta P, Bharti S (2011) Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum L.). Plant Biol 13:1–6
Milla MAR, Maurer A, Huete AR, Gustafson JP (2003) Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. The Plant J 36:602–615
Minocha R, Majumdar R, Minocha SC (2014) Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci 5:175–175
Ouyang J, Song C, Chen D (2017) Research progress on heat-tolerance mechanism and transports of polyamines in plant. Mol Plant Breed 15:3286–3294
Pál M, Szalai G, Janda T (2015) Speculation: Polyamines are important in abiotic stress signaling. Plant Sci 237:16–23
Powell NJ, Ravash X, Edlington R, Rudy JD (2012) Yield stability for cereals in a changing climate. Funct Plant Biol 39:539–552
Prasad PVV, Boote KJ, Allen LH Jr, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res 95:398–411
Sarsu F (2018) Screening protocols for heat tolerance in rice at the seedling and reproductiv stages, In: Pre-field screening protocols for heat-tolerant mutants in rice. Springer, Cham, pp 9–24. https://doi.org/10.1007/978-3-319-77338-4_2
Schleussner CF, Deryng D, Müller C, Elliott J, Saeed F, Folberth C, Liu W, Wang X, Pugh TAM, Thiery W, Seneviratne SI, Rogelj J (2018) Crop productivity changes in 1.5 °C and 2 °C worlds under climate sensitivity uncertainty. Environ Res Lett 13:064007
Sharma P, Dubey RS (2004) Ascorbate peroxidase from rice seedlings: properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci 167:541–550
Shi W, Yin X, Struik PC, Solis C, Xie F, Schmidt RC, Huang M, Zou Y, Ye C, Jagadish SVK (2017) High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J Exp Bot 68:5233–5245
Shi WM, Muramoto Y, Ueda A, Takabe T (2001) Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 273:23–27
Sinclair TR, Allen LH, Drake GM (1995) Temperature gradient chambers for research on global environment change: II. Design for plot studies. Biotronics: reports of Biotron Institute. Kyushu Univ 24:99–108
Singh MP (2015) Role of putrescine in growth, development and abiotic stress tolerance of rice (Oryza sativa L.) (Doctoral dissertation, Division of Plant Physiology, Indian Agricultural Research Institute, New Delhi)
Szymańska R, Ślesak I, Orzechowska A, Kruk J (2017) Physiological and biochemical responses to high light and temperature stress in plants. Environ Exp Bot 139:165–177
Tang S, Zhang H, Li L, Liu X, Chen L, Chen W, Ding Y (2018) Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct Plant Biol 45(9):911–921
Teixeira EI, Fischer G, Van Velthuizen H, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agril For Meteorol 170:206–215
Tian J, Wang LP, Yang YJ, Sun J, Guo SR (2012) Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperatures. J Am Soc Hort Sci 137:11–19
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, Scherer GFE (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Yoshida S, Satake T, Mackill DS (1981) High-temperature stress in rice [study conducted at IRRI, Philippines]. IRRI Research Paper Series (Philippines)
Zaman-Allah MJ, David M, Vadez V (2011) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Funct Plant Biol 38:270–281
Zhang CX, Fu GF, Yang XQ, Yang YJ, Zhao X, Chen TT, Zhang XF, Jin QY, Tao LX (2016) Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202:394–408
Acknowledgement
Authors thank to S.R.Voleti and P.R.Rao, IIRR, Rajendranagar, Hyderabad Telangana, 500030, India for providing seed material under ACRIP project,
Funding
Authors acknowledge the financial grant received from Indian Council of Agricultural Research (ICAR), New Delhi, India through National Innovations on Climate Resilient Agriculture (NICRA-IARI) Project Grant No.12–115. RNB acknowledges the financial support from DST Fast Track Young Scientist Grant (YSS/2015/000523) 2015–2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests related to publication of this paper.
Additional information
Communicated by J. Huang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karwa, S., Bahuguna, R.N., Chaturvedi, A.K. et al. Phenotyping and characterization of heat stress tolerance at reproductive stage in rice (Oryza sativa L.). Acta Physiol Plant 42, 29 (2020). https://doi.org/10.1007/s11738-020-3016-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3016-5