Abstract
Plants are continuously exposed to various environmental stresses, which cause alteration in every physiological and biochemical pathway. Plants have evolved several mechanisms that allow a plant species to tolerate/combat stress. Greater synthesis and accumulation of compatible organic osmolytes and proper mineral nutrition help plants to bring osmoregulation so that the cell water content and turgor is maintained. Under stress conditions, synthesis and accumulation of several osmolytes like free sugars, amino compounds, such as proline and glycine betaine, sugar alcohols like mannitol, and other low molecular weight metabolites are increased. Macro mineral elements such as nitrogen, phosphorus, potassium, and calcium are required for normal growth and development of plants and are known to stimulate the synthesis of osmotically active solutes. Moreover, they are actively implicated in several physiological processes including enzyme activation, transport, photosynthesis, and protein synthesis. Various phytohormones are known to have defensive roles in plants exposed to environmental stresses and their synthesis and accumulation are upregulated on exposure to environmental stress. Present review throws light on the role of organic osmolytes, mineral nutrients, and also growth regulators especially abscisic acid, ethylene, and salicylic acid in increasing the plant tolerance to drought stress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
World population is increasing at an alarming rate and is expected to reach 8.3 billion by 2030 (FAO 2010). In many densely populated developing countries of the world, expansion of arable land has become more difficult as a result of rapid urbanization, industrialization, and water scarcity (Rengasamy 2010). In future, food grain production has to be increased by 57 % so as to ensure sufficient food for the growing population (Wild 2003). In past few years, no doubt, an increase in productivity of certain major crops has been reported but repeating the same success in future for increased food production seems to be difficult.
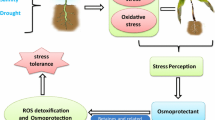
Among various abiotic stresses, drought is one of the major environmental constraints limiting crop productivity worldwide (Masoumi et al. 2010; Khamssi et al. 2011; Batlang et al. 2013). About 25 % of the world’s agricultural land is affected by drought stress (Jajarmi 2009). Changes in global climate have made this situation even more serious (Anand et al. 2003). Water shortage and soil water losses due to changes in environment and excessive land use are challenges to crop production (Xia et al. 2005). Maintaining higher plant productivity under environmental stresses is the main challenge which modern agriculture is facing (Gill and Tuteja 2010). Drought stress affects both source and sink, thereby causing reduction in yield in a time-dependent manner with respect to the severity of stress and plant developmental stage (Blum 1996). Drought stress imposes osmotic stress leading to loss of turgor and oxidative stress through production of reactive oxygen species (ROS) that results in loss of membrane integrity, protein denaturation, and oxidative damage to other biomolecules. As a consequence of such changes, inhibition of photosynthesis, metabolic dysfunction, and damage to cellular structures occurs causing growth perturbances, reducing fertility, and premature senescence (Munns and Tester 2008). Plants respond differently to water deficiency in different periods of their growth. The generative phase and the beginning of flowering are most frequently the period of the greatest sensitivity to water deficit.
2 Adaptations to Drought Stress
Distribution of plant species depend upon the prevailing environmental conditions. Tolerant plants can survive the extreme harsh environmental conditions at which the growth of sensitive ones is negatively influenced (Munns and Tester 2008). Higher tolerance to adverse environmental conditions is because of different stress response mechanisms. Plants adopt different strategies to cope with drought stress. The strategies adopted include escape, avoidance, and tolerance strategy (Levitt 1980; Chaves et al. 2002; Blum 2005; Ahmad and Sharma 2008; Rasool et al. 2013). Ephemeral plants have rapid phenological development, completing their life cycle during a period of adequate moisture and forming dormant seeds before the onset of dry seasons. Ephemerals never really experience the drought stress. In avoidance strategy, plants somehow reduce the impact of stress factor, even though the stress is present in the environment. Avoidance strategy, generally results in maintaining the favorable internal water content either by conserving water which is brought about by closing the stomata, leaf rolling, and heavy pubescence or by increasing the water uptake through development of deep root system and water spenders (Ruiz-Sanchez et al. 2007). On the other hand, in tolerance strategy, plant endures drought without undergoing injury, retaining the capacity of normal growth and development when rehydrated. In tolerance, plants mitigate the stress by maintaining high water potential through accumulation of compatible osmotic solutes. The accumulation of compatible solutes is well regarded as a basic strategy for the protection and survival of plants under abiotic stress conditions (Chen et al. 2007).
Responses of plant species to drought stress depend on several factors including duration and severity of the drought period as well as its inherent tolerance mechanisms. Severe and prolonged periods of drought stress result in oxidative damage due to the overproduction of ROS (Smirnoff 1993). Among various physiological and developmental mechanisms that a plant species adopts to tolerate periods of water deficit, accumulation of osmotically active solutes is the most commonly reported mechanism. By the accumulation of solutes, turgor and turgor-dependent processes are maintained, thereby allowing cell enlargement and plant growth during water stress and stomata to remain partially open and CO2 assimilation to continue at low water potentials that are otherwise inhibitory (Pugnaire et al. 1994).
Drought is a multidimensional stress, affecting plants at various levels of their organization (Yordanov et al. 2000). Stress-imposed effects are often manifested at phenological, morpho-physiological, biochemical, and molecular levels (Bahrani et al. 2010). Accumulation of compatible organic solutes (Da Costa and Huang 2009), changes in endogenous levels of certain phytohormones (Seki et al. 2007; Dobra et al. 2010), and overexpression of stress-responsive genes (Xiong and Yang 2003; Jaleel et al. 2006) do occur in response to stress. Most of these responses are directly triggered by the changes in water status of the cell (Chaves et al. 2003). In connection to this, plant hormones such as abscisic acid (ABA), jasmonic acid (JA), and salicylic acid (SA) are involved in a complex signal transduction network, thereby coordinating growth and development with plant responses to the changing environment (Jiang and Zhang 2002; Fujita et al. 2006; Szalai et al. 2010). In order to improve plant tolerance to stress, understanding of complete physio-biochemical responses of plant is pivotal (Jaleel et al. 2006; Ahmad and Sharma 2008; Ahmad et al. 2008a, b, 2010a).
3 Osmoregulation and Osmolytes
Osmoregulation/osmotic adjustment is the general response of plants to water stress so that solute content of the cell is increased. In order to maintain turgor and water uptake for normal growth, plants under stress need to maintain internal water potential well below that of soil which is usually acquired by increasing concentration of cell osmotica, either through uptake of solutes from soil solution or by increased synthesis of compatible solutes (Tester and Davenport 2003; Ahmad and Sharma 2008). Cytoplasm accumulates low molecular mass compounds in order to accommodate the ionic balance in the vacuoles (Zhifang and Loescher 2003). These compatible osmotic solutes do not interfere with normal metabolic reactions but rather, they replace water in these reactions (Ahmad and Sharma 2008; Koyro et al. 2012). Accumulation of these osmolytes is proportional to change in external osmolarity (within species-specific limits), thereby protecting cellular structures and maintaining osmotic balance to support continued water influx (Hasegawa et al. 2000). Majority of these compatible osmolytes are organic solutes, while some are essential ions such as K+ (Yokoi et al. 2002; Ahmad and Sharma 2008). However, the accumulation varies within the genus as well as plant species. Majority of the organic solutes accumulated are sugars (fructose, glucose, trehalose, and raffinose), sugar alcohols (glycerol and methylated inositols) (Bohnert and Jensen 1996), quaternary amino compounds (proline, glycine betaine, proline betaine, tertiary amines), and sulfonium compounds (choline O-sulfate, dimethyl sulfonium propironate) (Yokoi et al. 2002). Osmolyte accumulation is mandatory in plants for osmotic adjustment under water limiting conditions, but osmolyte accumulation mainly depends upon water status, crop growth stage, and cultivar (Shao et al. 2006). Due to accumulation of osmolytes, water status of cell and subcellular structures is maintained and membranes as well as proteins are protected from denaturing effects of osmotic stress (Ashraf and Foolad 2007).
3.1 Proline
Proline is an amino acid that plays multifunctional role in stress defense. It is actively involved in osmoregulation, scavenging of free radicals, and as a molecular chaperone for stabilizing protein structure, thus protects plant cells from damaging effects of various environmental stresses (Verbruggen and Hermans 2008; Ahmad and Sharma 2008; Szabados and Savoure 2010; Koyro et al. 2012; de Carvalho et al. 2013). Accumulation of proline in response to various environmental stresses is well documented (Ahmad 2010; Ahmad et al. 2010b, 2011, 2013; Azooz et al. 2011; Katare et al. 2012; Kim and Nam 2013). Water stress-induced increase in proline has been reported in rice (Pandey and Agarwal 1998), Medicago sativa (Slama et al. 2011), wheat (Jatav et al. 2012), and Arabidopsis (Ju et al. 2013). Besides its role in stress tolerance, accumulation of proline is possibly a useful drought injury sensor in plants (Zlatev and Stotanov 2005). Tolerant plant genotypes show large accumulation of proline which is often correlated with increased stress tolerance (Katare et al. 2012; Ahmad et al. 2012a, b). As a consequence of drought stress, the concentration of proline in plant leaves increases tenfold in leaves of Lotus japonicus (Signorelli et al. 2013).
Biosynthetic and catabolic pathways of proline determine its level (Szabados and Savoure 2010). Proline is biosynthesized from glutamate by sequential action of γ-glutamyl kinase (γ-GK), pyrroline-5-carboxylate synthetase (P5CS), pyrroline-5-carboxylate (P5C), and P5C reductase (P5CR) (Hong et al. 2000; Yamada et al. 2005). In most plant species, P5CS is encoded by two genes and P5CR is encoded by one (Armengaud 2004). Proline can also be synthesized by alternative pathway from ornithine, employing ornithine-delta-aminotransferase (d-OAT) (Miller et al. 2009). Proline synthesized in cytoplasm or chloroplasts is transported to mitochondria where it is catabolized to P5C through the sequential action of proline dehydrogenase (PDH) or proline oxidase (PROX) and P5C is then converted to glutamate by enzyme P5C dehydrogenase (P5CDH).
Proline level in plants is controlled by two important enzymes, PROX and γ-GK (Girija et al. 2002; Ahmad and Sharma 2008; Koyro et al. 2012). Increased proline accumulation during stress may be due to the activation of proline synthesis through glutamate pathway involving γ-GK, glutamyl phosphate reductases, and P5CR enzymes. During stress, increase in activity of γ-GK and decrease in activity of PROX has been reported by Jaleel et al. (2007) in Catharanthus roseus and Ahmad et al. (2010b) in Morus alba, thereby helping plants to maintain sufficient levels of proline to combat/ameliorate detrimental effects of stress.
Under stress conditions, proline synthesis is enhanced in plants and on recovery from stress, its catabolism is enhanced. It has been reported that overexpression of P5CS in Nicotiana and Petunia resulted in increased proline accumulation and enhanced salt and drought tolerance (Hong et al. 2000; Yamada et al. 2005). Rice and tobacco plants overexpressing Arabidopsis d-OAT has increased proline levels and greater tolerance to stress (Roosens et al. 2002; Qu et al. 2005).
Proline accumulation is a highly regulated process involving a set of protein kinases that is ubiquitous for stress tolerance including drought. These proteins include SNF-related protein kinases 2 (SnRK2s, i.e., SnRK 2.2, SnRK 2.3, and SnRK 2.6) which are activated on exposure to stress (Boudsocq et al. 2004). It has been reported in Arabidopsis mutants that ABA-responsive SNF-related protein kinases 2 (SnRK2)-induced ABA-dependent proline accumulation, therefore imparts more tolerance to osmotic stress (Fujii et al. 2011). Another family of SNF-related protein kinases, that enhance proline levels, is SnRK3s. These are calcineurin B-like (CBL) calcium binding proteins also known as CBL-interacting kinases (CIPKs) and overexpression of OsCIPK03 and OsCIPK12 has been reported to increase tolerance of rice to cold and drought by causing significant increase in proline (Xiang et al. 2007). In addition to the abovementioned protein kinases, Arabidopsis calcium-dependent protein kinase 6 (CDPK6) (Xu et al. 2010) and soybean calmodulin GmCAM4 (Yoo et al. 2005) have been reported to contribute positively so as to enhance proline content and stress tolerance in Arabidopsis, thereby indicating a key role for intracellular calcium signals in proline metabolism. Variety of abiotic stress responses in plants are regulated through MAPK (mitogen-activated protein kinase) cascades and it has been reported that several MAPKs are activated on exposure to various environmental stresses resulting in increased proline accumulation and tolerance as well (Kong et al. 2011; Zhang et al. 2011). Moreover, genetic manipulation of MAPK signaling pathway results in altered plant stress tolerance (Xiong and Yang 2003; Shou et al. 2004a, b). Proline accumulation can be induced by ABA as well as by other stress-related protein kinases. In addition to the abovementioned positive regulation of proline accumulation by several protein kinases, it shall be pointed out that it may also be regulated negatively, e.g., maize protein phosphatases type 2C (PP2C) regulate various processes of development and responses to environmental stress but have been reported to regulate proline accumulation negatively (Liu et al. 2009; Umezawa et al. 2010).
3.2 Glycine Betaine
Glycine betaine (GB) plays an important role in plant tolerance to stress, enzyme activity, membrane integrity, ROS detoxification, and osmotic adjustment. Glycine betaine (N,N,N-trimethylglycine), one among the quaternary ammonium compounds (glycine betaine, β-alanine betaine, choline-O-sulfate, and 3-dimethylsulfoniopropionate and proline betaine), is dipolar and exists as neutral molecule at physiological pH (Le Rudulier et al. 1984) which is known to play protective role in plants under stress (Yang et al. 2003) and its accumulation has positive associations with stress tolerance (Ashraf and Foolad 2007; Kathuria et al. 2009). GB is mainly found in chloroplasts and plays a pivotal role in protection of thylakoid membranes and other key components of photosynthetic machinery such as ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and oxygen evolving complex from stress-induced inactivation and dissociation, thereby maintaining the photosynthetic efficiency (Yokoi et al. 2002). Moreover, it stabilizes the association of the extrinsic PS II complex proteins and maintains the highly ordered state of membranes at nonphysiological temperatures and salt concentrations (Papageorgiou and Murata 1995).
Plants synthesize GB in chloroplast from either glycine or choline via two distinct pathways: (1) dehydrogenation of choline or (2) N-methylation of glycine and enzymes involved in choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) (Nye et al. 1997). Increase in glycine betaine content under stress conditions has been reported in many plants but the increase may be more pronounced in leaves than in roots, e.g., Haloxylon recurvum (Wang and Nil 2000). In most of the crop plants, concentration of naturally accumulated GB may not be sufficient enough to mitigate the deleterious effects of various environmental stresses (Subbarao et al. 2001). Accumulation of GB in response to stress has been reported in many crops, e.g., bean (Gadallah 1999), peanut (Girija et al. 2002), sorghum (Yang et al. 2003), and mustard (Ahmad 2010). But the concentrations accumulated vary with plant species, for example, sorghum accumulates manifold more GB than maize (Murata et al. 1992). Stress-tolerant species accumulate GB in high concentrations than sensitive ones (Agastian et al. 2000). Plants that accumulate low concentration of GB, exogenous application can be a useful tool to reduce the adverse effects of environmental stresses (Makela et al. 1998; Yang and Lu 2005). It has been reported that exogenous application of glycine betaine increased tolerance of tomato (Makela et al. 1998) and rice plants to salt stress (Lutts 2000). In maize, exogenous supply of glycine betaine caused considerable increase in yield, RWC, proline, and antioxidant enzyme activity but reduced lipid peroxidation under normal as well as drought conditions (Lv et al. 2007; Anjum et al. 2012). In addition, water-limited conditions increased the yield and yield components have been reported in several other crops such as rice (Rahman et al. 2002), sunflower (Iqbal et al. 2005), maize (Ali and Ashraf 2011), bean (Abou El-Yazied 2011), and Triticum aestivum (Aldesuquy et al. 2012).
However, many important crop plants like maize, potato, tomato, and eggplant lack the capability to synthesize GB in adequate amounts (Zwart et al. 2003). In such cases, both the exogenous application of GB and the introduction, via transgenes, of the GB biosynthetic pathway have become imperative to increase their tolerance to different abiotic stresses. This increased tolerance to abiotic stresses will be useful for understanding the mechanisms through which GB protects plants against abiotic stresses. So far, genetically modified plants containing transgenes for production of GB have faced the limitation of being unable to produce sufficient amounts of glycine betaine required to mitigate the stress, but applying glycine betaine exogenously to plants under stress conditions has gained more attention (Ashraf and Foolad 2007). Introduction and overexpression of choline oxidase (Cod A) gene from Arthrobacter globiformis and BADH genes from Spinach/Atriplex have been widely used for GB production in transgenic plants. Introduction of Cod A gene in Arabidopsis thaliana (Hayashi et al. 1997), Oryza sativa (Alia and Murata 1998), and Lycopersicum esculentum (Kathuria et al. 2009) and BADH gene in Triticum aestivum (Wang et al. 2010) have been reported to increase the tolerance to drought, salinity, and cold stresses by increasing the membrane integrity, enzyme activity, photosynthesis regulating ROS detoxification and also yield.
3.3 Polyamines
Polyamines are group of naturally occurring nitrogenous compounds with aliphatic structure that are implicated in several processes such as growth, development as well as responses to various environmental stresses (Ahmad et al. 2012c). Moreover, polyamines due to their hydrophilic properties are involved in the maintenance of pH and in scavenging of active oxygen compounds, therefore are considered as mediators in protective reactions against different stresses (Kovacs et al. 2010). Polyamines protect membrane from disintegration and alleviate oxidative stress (Groppa and Benavides 2008; Alcazar et al. 2011; Hussain et al. 2011; Ahmad et al. 2012c). Putrescine (PUT), spermidine (SPD), and spermine (SPM) are commonly occurring polyamines in higher plants and may exist free or covalently bound to small molecules such as phenolic compounds as well as to macromolecules such as nucleic acids and proteins in soluble-conjugated or insoluble bound forms (Kusano et al. 2007; Duan et al. 2008). In addition to these, uncommon polyamines like homospermidine, cadaverine, and canavalmine have also been reported in several biological systems including plants. At the physiological pH, polyamines usually exist as cations. This polycationic nature of polyamines is one of their important properties affecting their biological activities (Valero et al. 2002). Polyamine levels vary depending on plant species and the stress duration (Liu et al. 2008). It has been suggested that stress-tolerant plants have increased polyamine levels as compared to sensitive ones and polyamines with higher number of amino groups (SPM and SPD) are more effective in scavenging of ROS than the ones with less number of amino groups (PUT) (Kubis 2008).
Polyamines serve as messengers of stress signals (Liu et al. 2007). As a result of acid neutralizing and antioxidant capability, polyamines show antisenescence, anti-stress effects, and membrane and cell wall stabilizing abilities (Zhao and Yang 2008). Role of polyamines in modulating the defensive responses of plants to various environmental stresses is well documented (Alcazar et al. 2011).
Exogenous application of polyamines has been suggested as an effective approach for enhancing stress tolerance of crops and crop productivity as well. Exogenous application of PUT have been successfully utilized in enhancing plant tolerance to high temperature (Murkowski 2001), cold (Nayyar and Chander 2004), osmotic stress (Liu et al. 2004), salinity (Verma and Mishra 2005), drought (Zeid and Shedeed 2006), heavy metals (Wang et al. 2007), water logging (Arbona et al. 2008), and flooding (Yiu et al. 2009). Furthermore, it has been reported that genetic transformation of plants with genes that code for the enzymes involved in polyamine biosynthesis resulted in increased stress tolerance in various plant species (Liu et al. 2007). Transgenic plants overexpressing these genes show increased tolerance to multiple environmental stresses including salinity, drought, and low and high temperatures. This tolerance to multiple abiotic stresses is of practical importance as plants are often encountered by several concurrent forms of environmental stresses during their life cycle (Wi et al. 2006; Prabhavathi and Rajam 2007; Wen et al. 2008).
Plants deficient in arginine decarboxylase (ADC) and spermidine synthase (SPDS) are unable to synthesize sufficient PUT and SPM, respectively, therefore are sensitive to stress (Yamaguchi et al. 2007; Cuevas et al. 2008), whereas overexpression of ADC leads to greater synthesis of PUT and enhanced tolerance to drought (Alcazar et al. 2010; Alet et al. 2011). Tobacco plants overexpressing ornithine decarboxylase (ODC) showed increased tolerance to salt stress (Kumriaa and Rajam 2002). Moreover, it has been reported that Arabidopsis plants overexpressing SPDS showed greater tolerance to drought, salinity, and cold stress (Kasukabe et al. 2004). Scaramagli et al. (2000) reported that increase in insoluble-conjugated PUT levels was closely associated with polyethylene glycol-induced stress acclimation in potato. Liu et al. (2004) reported an increase in the polyamine levels in leaves of drought-tolerant wheat seedlings under osmotic stress, indicating the role of polyamines in facilitating osmotic stress tolerance of wheat seedlings.
4 Growth Regulators
4.1 Abscisic Acid
Growth hormones help plants to adapt to changing environments by mediating growth, development, nutrient allocation, and source/sink transitions (Peleg and Blumwald 2011). ABA, a 15-carbon sesquiterpenoid compound resembling terminal portion of some carotenoid molecules, is synthesized in chloroplast and other plastids by mevalonic acid pathway from 40-carbon precursor, zeaxanthin. Zeaxanthin epoxidase (ZEP), 9-cis-epoxycarotenoid deoxygenase (NCED), alcohol dehydrogenase (ABA2), and abscisic aldehyde oxidase (AAO3) are the main enzymes mediating its biosynthesis.
ABA is involved in many cellular processes like germination, gravitropism, and guard cell-mediated stomatal opening (Levitt 1980). ABA plays an important role in the adaptation of plants to environmental stress. Regulation of water balance and osmotic stress tolerance is a well-established function of ABA (Takahashi et al. 2000; Zhu 2002). Under stress conditions, in addition to its well-established role in closing of stomata, ABA increases the ion influx across root cell membrane and also mediates the greater synthesis and accumulation of active osmotic solutes (e.g., proline, trehalose), thereby helping in bringing osmotic adjustment (Nayyar et al. 2005). ABA accumulates under drought stress and degrades gradually upon removal of stress. Since ABA mediates so many stress responses, starting from the perception of stress signal upto changes in gene expression, which ultimately leads to increased ABA in plants (Zhang et al. 2006). Pospisilova et al. (2005) reported that ABA pretreatment further increased the endogenous ABA level in maize seedling. Presoaking seeds with ABA was reported to significantly enhance the activities of antioxidant enzymes in maize seedlings subjected to water stress (Jiang and Zhang 2002). Similarly, Boominathan et al. (2004) found that relative water content of ABA-treated plants was higher under drought stress. Moreover, exogenous application of ABA under water stress increased the grain weight in susceptible wheat cultivars (Nayyar and Walia 2004).
The role of ABA in plants exposed to drought stress has been well studied. ABA has a potential role in regulating the plant water status and growth. Increased expression of genes encoding enzymes and proteins involved in enhancing drought tolerance has also been attributed to increased ABA (Luan 2002; Zhu 2002). During stress, ABA produced in root is transported to shoot for regulating stomatal movements and leaf growth (Zhang et al. 1987; Zhang and Davies 1990a, b). The pH and ionic conditions in the xylem play an important role in this transport (Wilkinson et al. 1998; Bacon et al. 1998; Hartung et al. 2002). Source of ABA appearing in the xylem during drought has been debated. Some are of the opinion that it comes only from the root (Zhang et al. 1987), while some have reported that ABA comes from both root and leaves. Root-sourced ABA is usually involved in the initial sensing of drought to regulate the stomatal conductance so that the excess water loss may be reduced, but under severe and prolonged stress, leaf water deficit becomes unavoidable and older leaves may wilt because of weak hydraulic link or less control over stomatal conductance, increasing ABA concentration in the xylem (Zhang and Davies 1989a, b). Concentration of ABA in xylem has direct influence on leaf conductance and it has been reported that leaf conductance also responds to the flux of ABA into leaves per unit time, indicating its role in regulation of stomatal movements and due to stress-induced changes in transpiration rate, this role may be marginalized (Jarvis and Davies 1997). However, there are certain reports indicating that stomata responds to xylem ABA concentration rather than its flux, e.g., when leaf conductance has decreased considerably as a result of water stress (Jackson et al. 1995). By following the amount of ABA entering the leaves during the process of ABA-induced stomatal closure, it has been reported that changes in leaf conductance are due to xylem ABA and the rapid metabolism of this xylem-derived ABA in the leaves is very essential in order to prevent its accumulation and stomatal movements to be sensitively regulated (Jia and Zhang 1999).
ABA is important for growth and development of plants under water stress (Zhang and Davies 1990b). But increment in the concentration of xylem ABA, beyond certain limits, can restrict shoot growth (Gowing et al. 1990). However, it should be noted that shoot and root respond differently to ABA levels. Sharp et al. (2000) and Spollen et al. (2000) have reported that better growth of roots under water stress is attributed to higher amounts of ABA accumulated in the roots. Maintenance of better root growth under water deficit has positive association with drought tolerance. ABA has dual role in regulating physiology of plant, i.e., inhibitory as well as stimulatory (Finkelstein et al. 2002). Under stress conditions, when it is accumulated beyond certain limits to help plant survival, it may inhibit processes such as stomatal opening and plant size expansion for quite large time, but under normal conditions, when accumulated concentration is normal, it promotes vegetative growth (Sharp et al. 2000; Spollen et al. 2000) and post-germination development (Cheng et al. 2002). He and Cramer (1996) have reported that accumulation of ABA in lower concentrations increased salt tolerance of Brassica napus. Excess accumulation of ABA as a result of salinity has often been reported to induce inhibition of leaf expansion in different species (He and Cramer 1996; Montero et al. 1998).
ABA has an important role in signaling plant responses to drought and salt stresses, thereby triggering the expression of drought-responsive genes. As revealed from sequencing studies, among the various stress-responsive genes that are regulated by ABA, only few have been identified for having any probable physiological functions. In ABA-dependent pathway, synthesis of new proteins may or may not be required (Bray 2002). In such pathways, when the synthesis of new proteins is not required, the presence of ABA-responsive element (ABRE) at the promoter domain of ABA-responsive gene is ubiquitous which upon binding to transcription factor (TF) leads to ABA-induced gene expression, e.g., in Arabidopsis, ABA-induced expression of dehydration-responsive gene (rd29B) has two ABREs essential and two transcription factors (bZIP) (Uno et al. 2000). However, when synthesis of proteins is required for the ABA-dependent gene expression, de novo synthesis of new proteins is the prerequisite. These genes do not have any ABREs (Leung and Giraudat 1998; Bray 2002). Expression of some genes may be dependent as well as independent of ABA (Shinozaki and Yamaguchi-Shinozaki 1997), e.g., gene rd29A, which is important for water stress, has two types of regulatory cis-elements at its promoter, one ABA-dependent and the other is ABA-independent (Ingram and Bartels 1996; Leung and Giraudat 1998).
Under osmotic stress conditions, transcription levels of ABA biosynthetic genes are upregulated. Increased expression of the ZEP gene, under drought stress, has been reported in roots of Nicotiana plumbaginifolia (Audran et al. 2001) and leaves of Arabidopsis (Xiong et al. 2002). Moreover, stress-induced overexpression of NCED gene is well documented (Thompson et al. 2000; Tan et al. 2003). Under water stress conditions, accumulation of ABA is accompanied by transient increase in NCED transcript and proteins (Qin and Zeevaart 1999). Overexpression of AtNCED3 is highly induced by dehydration, although other NCED genes also contribute positively but their role is minor (Tan et al. 2003). In addition, transgenic Arabidopsis plants overexpressing NCED show greater ABA levels and increased desiccation tolerance. Similar results have been reported in transgenic tomato and Nicotiana plumbaginifolia (Thompson et al. 2000). Exogenous application of ABA has been reported to induce the expression of NCED gene in ABA-deficient mutants (Xiong et al. 2002).
4.2 Salicylic Acid
Salicylic acid (SA) is an endogenous growth regulator of phenolic nature, actively involved in plant growth, development, and several other physiological processes including germination, fruit ripening, flowering, photosynthesis, stomatal conductance, ion uptake and transport (Shakirova 2007), biogenesis of chloroplast, interaction with other organisms, and protection of plants against multiple environmental stresses such as ozone and ultraviolet radiation (Sharma et al. 1996), salinity (Borsani et al. 2001), freezing (Janda et al. 1999), herbicides (Ananieva et al. 2004), heavy metals (Ahmad et al. 2011), osmotic stress (Shi and Zhu 2008), and drought (Sadeghipour and Aghaei 2012). Salicylic acid (SA) acts as a signal involved in the expression of specific responses in plants to biotic and abiotic stresses.
SA induces systemic acquired resistance (SAR) in plants to different pathogens (Metraux 2001). SA has been reported to induce accumulation of lectins in wheat (Shakirova and Bezrukova 1997), synthesis of heat shock proteins, and activation of protein kinase in tobacco exposed to osmotic stress (Burkhanova et al. 1999; Mikolajczyk et al. 2000), suggesting the role of SA in anti-stress mechanisms. Salicylic acid (SA) has long been considered as signal molecule and is known to reduce the oxidative damage caused by salinity stress (Azooz et al. 2011; Sajid and Aftab 2012) and this ability of SA to produce a protective effect in plants under different abiotic stresses has increased the interest of researchers.
Exogenously applied salicylic acid in plants has been reported to enhance the efficiency of several developmental, physiological, and biochemical processes. It has been reported that exogenous application of SA enhances transpiration rate (Rai et al. 1986), seed germination and yield (Raskin 1992), membrane permeability (Barkosky and Einhellig 1993), growth, and photosynthesis (El-Tayeb 2005). Moreover, exogenously applied SA is involved in the defense against pathogen attack and more recently its role has been widely investigated in both biotic and abiotic stresses (Shi et al. 2006). The role of SA in inducing stress tolerance in plants is well documented, e.g., it enhances the resistance of plants against drought and salt stress (Tari et al. 2002) besides metal stress (Ahmad et al. 2011). SA has been found to induce heat stress tolerance in mustard (Dat et al. 1998), chilling tolerance in maize (Janda et al. 1999), drought tolerance in wheat (Singh and Usha 2003), heavy metal stress tolerance in barley (Metwally et al. 2003), and salinity tolerance in barley (El-Tayeb 2005). Singh and Usha (2003) have reported that under drought, application of salicylic acid to wheat increased the moisture content, total chlorophyll content, nitrate reductase activity, carboxylase activity of Rubisco, superoxide dismutase activity, and dry matter accumulation. Moreover, exogenous application of salicylic acid has been reported to maintain the stability of membranes, enhance photosynthetic rate and K+/Na+ ratio (Kaydan et al. 2007), and increases proline content and activities of antioxidant enzymes, thereby mitigating the deleterious effects of stress (Shakirova et al. 2003). Agarwal et al. (2005) have reported that under water stress conditions, application of SA to wheat enhanced the chlorophyll and relative water content while caused considerable reduction in hydrogen peroxide and lipid peroxidation.
There are certain reports indicating that exogenous application of SA does not help in mitigation of drought-induced negative effects (Waseem et al. 2006). Nevertheless, it should be noted that before applying SA, one should have a thorough knowledge about the effective means and methods of application so as to increase the efficiency of exogenously applied SA which is believed to depend on several factors including the species, developmental stage, the manner of application, and the concentration of SA as well (Borsani et al. 2001; Horvath et al. 2007; Joseph et al. 2010). Few other methods which have been reported to protect different plant species against abiotic and biotic stresses include presoaking of the seeds, addition of SA to the hydroponic solutions, tissue culture media and spraying with SA solution (Horvath et al. 2007; Sakhanokho and Kelley 2009). In recent years, tissue culture technique has been extensively utilized for screening and developing stress-tolerant plants. Under in vitro conditions, impact of varying SA concentrations on growth and induction of salt tolerance in Hibiscus plants have also been reported (Sakhanokho and Kelley 2009). Moreover, salicylic acid has an affinity to bind with the enzymes like catalase, ascorbate peroxidase, and carbonic anhydrase that are involved in metabolism of free radicals and redox homeostasis (Slaymaker et al. 2002). Any kind of imbalance in this homeostasis triggers the induction of defense responses in plants (Torres et al. 2002; Durrant and Dong 2004). Application of salicylic acid has been reported to increase the activities of the antioxidant enzymes in wheat (Agarwal et al. 2005), Brassica juncea (Yusuf et al. 2008), and broad bean (Azooz et al. 2011). Under water stress, salicylic acid-induced activity of antioxidant enzymes has also been reported in Ctenanthe setosa (Kadioglu et al. 2010). Moreover, exogenously applied salicylic acid to wheat (Shakirova et al. 2003) and Brassica juncea (Yusuf et al. 2008) under salinity and water stress, respectively, alleviated the synthesis and accumulation of proline—a good indication of increased stress tolerance.
4.3 Ethylene
In addition to its usual role in plants, ethylene is also involved in defense against a wide variety of environmental stresses (Bleecker and Kende 2000). Increased ethylene biosynthesis is triggered in plants under various environmental stresses including water stress, thereby suggesting its pivotal role in plant acclimation to stress (Gomez-Cadena et al. 1996). The effects of ethylene, whether transitory or long term, vary considerably among species (Hall and Smith 1995). Although examples of abscission of leaves in response to water deficit stress are compelling, exogenous application of ethylene or the ethylene precursor (1-aminocyclopropane-1-carboxylate) enhances leaf abscission, whereas inhibitors of ethylene synthesis (e.g., aminoethoxyvinylglycine and Ag+) reduce leaf senescence (Taiz and Zeiger 1998). Ethylene is believed to be involved in stomatal closure but seems rather contradictory. In Arabidopsis, ethylene has been reported to inhibit ABA-induced stomatal closure (Tanaka et al. 2005) because H2O2-induced stomatal closure results in loss of function in Arabidopsis mutants, therefore suggesting an important role of ethylene in guard cell ROS signaling and stomatal closure (Desikan et al. 2005).
Ethylene is biosynthesized from methionine. Two main enzymes involved in the biosynthesis of ethylene are 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase (ACO) that catalyze the conversion of S-adenosyl-l-methionine to ACC and the oxidative cleavage of ACC to ethylene, respectively (Zarembinski and Theologis 1994). Normally the activity of ACC synthase (ACS) is very low in tissues that produce less amounts of ethylene, but upon stimulation, its activity is induced rapidly so the reaction catalyzed by this enzyme is considered as rate limiting and regulatory step in induction of ethylene biosynthesis (Kende 1993). Moreover, the activity of this enzyme is also regulated by phosphorylation and dephosphorylation of proteins at the posttranslational level, thereby altering its turnover rate (Chae et al. 2003). In addition to this, induction of genes coding ACS and ACO under changing environmental conditions or endogenous cues, also enhance the ethylene biosynthesis in plants (Wang et al. 2002).
Under stress, plants produce ethylene in greater concentrations and the stress signal perceived triggers cellular responses further downstream. Protein kinases involved in regulation of ethylene synthesis under stress conditions covert these signals into cellular responses, thereby acting as important mediators of signal transduction cascade in cells (Chang and Karin 2001). Studies carried on transgenic tobacco using protein kinase and phosphatase inhibitors reveal that overexpression of NtMEK2 DD causes activation of SIPK, a tobacco MAPK, thereby resulting in increased ethylene production which coincides with the increase in ACS activity, followed by the activation of a subgroup of ACS and ACO genes, suggesting the role of MAPK in activation of ACS and posttranscriptional regulation (Kim et al. 2003).
Stress signaling cascade largely depends on transcription factors and their expression levels have direct bearing with plant adaptation to adverse environment (Schenk et al. 2000). Among several transcription factors that have been identified, ethylene response factors (ERFs) are known to be important (Zhang et al. 2009). ERFs are implicated in biotic and abiotic stress-induced transcription (Riechmann and Meyerowitz 1998; Hu et al. 2008) but among the various ERF genes, only a few are known to mediate responses to abiotic stress (El-Sharkawy et al. 2009). ERFs are DNA binding proteins that are specific to plants (Hao et al. 1998). It has been reported that ERF family genes are implicated in several stress responses like high salinity (Dubouzet et al. 2003), freezing (Yang et al. 2005), and drought (Yamaguchi-Shinozaki and Shinozaki 2006). Overexpression of the tobacco transcription factor NtERF1 leads to increased salt tolerance (Huang et al. 2004). Arabidopsis thaliana overexpressing Helianthus annus transcription factor Hahb-4 exhibits a characteristic phenotype essential for tolerance to water stress. Moreover this transcription factor is involved in ethylene-mediated signaling pathways and its expression is regulated by water availability (Manavella et al. 2006).
Particular type of ethylene-responsive transcription factor induces/enhances tolerance to stress in particular plant species. For example, expression of tomato ERF5 has been reported to impart regulation of stress responses in Arabidopsis thaliana at transcriptional level (Chuang et al. 2010). In transgenic tomato plants, overexpression of SlERF5 resulted in high tolerance to drought and salt stress which was accompanied by increased relative water content (Pan et al. 2012). Zhang et al. (2010) showed that transgenic rice overexpressing JERF3 exhibited better drought and osmotic stress tolerance which is reflected in increase in the contents of soluble sugars and proline. In addition, overexpression of JERF3 led to the upregulated expression of two OsP5CS genes in response to drought stress and also activated the expression of stress-responsive genes, including WCOR413, OsEnol, and OsSPDS2 under normal conditions.
5 Mineral Nutrients
5.1 Calcium
Calcium is one of the macronutrients required for normal growth and development of plants. It is implicated in regulating a number of fundamental cellular processes involving cytoplasmic streaming, thigmotropism, gravitropism, cell division, cell elongation, cell differentiation, cell polarity, photomorphogenesis, plant defense, and stress responses. As a divalent cation (Ca2+), it acts as an intracellular messenger in the cytosol (Marschner 1995; Nobuhiro and Mittler 2006), has structural role in the cell wall and cell membranes and as a counter cation for anions in the vacuoles (White and Broadley 2003). Calcium provides strong structural rigidity to cells by forming cross-links within the pectin polysaccharides (Easterwood 2002). In plants, Ca2+ is usually stored as calcium oxalate crystals in plastids. Better plant growth, the structural integrity of stems, and the quality of fruit produced are strongly coupled to Ca2+ availability. Calcium is also known to act as an activator of many enzymes like ATPase, phospholipases, amylase, and succinate dehydrogenase. Studies carried on Phaseolus vulgaris L. suggested that Ca2+ is associated with stomatal closure, decrease of hydraulic conductivity, sap flow, leaf dry weight, leaf K+ and Mg2+ concentrations, and inhibition of CO2 assimilation (Cabot et al. 2009).
In the absence of a stimulus, the plant cells maintain low cytosolic Ca2+ concentration (100 nM), but in response to an external stimuli including light, touch, wind, hormones, and biotic and abiotic stresses, the cytosolic concentration of calcium is rapidly elevated via an increased Ca2+ influx due to the release of Ca2+ by Ca2+ channels from endoplasmic reticulum, plasma membrane, and other cell organelles and then quickly returns to the normal level by Ca2+ efflux through Ca2+/H+ antiporter and Ca2+ pumps (Bush 1995). One of the most common signaling pathways causing increase in concentration of calcium in cytosol in response to external stimulus is the phospholipase C enzyme-mediated pathway. Enzyme phospholipase C is activated after the signal perception by cell surface receptors including G protein-coupled receptors and receptor tyrosine kinases. Activated phospholipase C hydrolyzes the membrane phospholipid PIP2 to form 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) that act as secondary messengers. DAG is also involved in the activation of protein kinase C enzyme, while IP3 after diffusing into the endoplasmic reticulum binds to receptor (IP3 receptor), a Ca2+ channel, thus releasing Ca2+ from the endoplasmic reticulum. This stimulus-specific increase in cytoplasmic calcium is called as calcium signature (Evans et al. 2001). Current evidences indicate that apart from IP3, cyclic ADP ribose (cADPR) also influences the activity of Ca2+ channels and plays an important role in elevating calcium levels in cytosol. The transduction of Ca2+ signals into various biochemical and morphological responses is a very complex and specific phenomena that is controlled by several factors. Specific signal induces a specific Ca2+ signature in different cell types (Kiegle et al. 2000). Furthermore, in plant cells, Ca2+ acts as a secondary messenger, thereby coupling a range of extracellular stimuli with intracellular responses (Sarwat et al. 2013). The specificity of eliciting appropriate physiological response is due to the temporal and spatial changes of Ca2+ and the extent of its amplitude as well (McAnish and Hetherington 1998; Allen et al. 1999), while the nature and intensity of stimulus is specified by amplitude and duration of Ca2+ transients. Ca2+ binding proteins (sensors) help in decoding and transducing the calcium signatures by activating specific targets and pathways (Shao et al. 2008b; Ahmad et al. 2012d).
Increase in the concentration of cytosolic calcium leads to the activation of various Ca2+ sensor proteins that convert these signals into a wide variety of biochemical changes. Different Ca2+ sensors that exist in higher plants include calmodulin (CaM), calcium-dependent protein kinases (CDPK), and CBL protein which play a crucial role in abiotic stress signaling in plants (Das and Pandey 2010; Ahmad et al. 2012d). Binding of Ca2+ to the sensor molecule induces conformational change in the sensor molecule and exposes the hydrophobic pockets, thereby facilitating interactions of the sensor protein with a variety of target proteins. These kinases are reported to play an important role in inhibition of autophosphorylation and enhancing substrate phosphorylation (Patil et al. 1995). Calcium and Ca2+ sensor calmodulin (CaM) regulate the expression of structural and regulatory genes by acting on transcription factors (TFs), thereby modulating their activity or Ca2+-loaded CaM may directly bind to promoter sequences to regulate gene expression, thus, indicating the role of CaM as a transcription factor. Ca2+/CaM complex may bind directly with transcription factors so as to regulate their DNA binding affinities or indirectly by associating with complex transcriptional machinery that consists of Ca2+/CaM complex, transcription factor binding protein (TFBP), and transcription factors. TFBP serves as a bridge between Ca2+/CaM and TFs, while Ca2+/CaM complex regulates gene expression by modulating the phosphorylation status of TFs through the activity of CaM binding protein kinase and protein phosphatase (Kim et al. 2009). Moreover, plant species overexpressing these protein kinases are more tolerant to drought, salinity, and cold stresses. In transgenic rice, overexpression of OsCDPK7 enhanced the induction of stress-responsive genes, resulting in increased tolerance to stress (Saijo et al. 2000). In Arabidopsis, it has been reported that under drought and salt stress conditions, expression of AtCPK10 and AtCPK11 is induced indicating their possible role in osmotic stress signaling (Urao et al. 1994). Arabidopsis plants overexpressing the CBL5 protein showed enhanced tolerance to high salt or drought stress (Cheong et al. 2010).
Ca2+ supplementation under drought stress have been reported to enhance water conservation and improve the hydrophobicity of cellular membranes while lowering its permeability through its interaction with the phosphates and proteins in cellular membranes, thus strengthening their stability (Shao et al. 2008a). Ca2+ protects membranes from hydration, improves the cohesion of cell walls, maintains protoplasm viscosity, and enhances cellular dehydration resistance. Thus, Ca2+ can stabilize plant cells and enhance drought tolerance through its direct effects on structural basis of the plant (Shao et al. 2008b; Ma et al. 2009). Schaberg et al. (2011) reported that addition of calcium increased the concentrations of amino acids, alanine and γ-aminobutyric acid (GABA) and the polyamines, putrescine (PUT) and spermidine (SPD) as well as chlorophyll content in red spruce (Picea rubens) under low temperature stress. Abdel-Basset (1998) reported that under drought stress, calcium supplementation caused significant increase in fresh weight, dry weight, chlorophyll, and relative water content, while reduced the membrane leakage in Vicia faba.
5.2 Potassium
Potassium (K) is an important macronutrient required for growth and development of plants both under normal and stress conditions (Agarwal et al. 2009). Potassium is actively involved in many basic biochemical and physiological functions such as osmoregulation, enzyme activation, and stomatal movements reducing excess uptake of ions such as Na and Fe in saline soils (Epstein and Bloom 2005; Amtmann et al. 2008; Ahmad et al. 2012c; Wang et al. 2013). Potassium is implicated in transport of inorganic anions and metabolites. Moreover, it maintains the transmembrane voltage gradients for cytoplasmic pH homeostasis (White and Karley 2010).
Potassium deficiency causes considerable reduction in leaf area, photosynthesis, and nitrogen metabolism, which ultimately result in reduced plant growth (Ebelhar and Varsa 2000). As a result of reduction in production of photoassimilates due to potassium deficiency, the sink tissues also get restricted supply of photoassimilates. Due to this reduction in partitioning of photoassimilates, the quantity as well as quality of the yield gets affected (Pettigrew and Meredith 1997; Meille and Pellerin 2004). The very first response of plants to potassium deficiency is the reduction in growth rate, and later beginning of chlorosis and necrosis in older leaves (Mengel and Kirkby 2001). Potassium deficiency induces disturbances in turgor, stomatal opening, water relations, and photosynthesis (Marschner 1995; Mengel and Kirkby 2001). Stomatal regulation largely depends upon the distribution of potassium in epidermal cells, guard cells, and leaf apoplast (Shabala et al. 2002). Supplying sufficient potassium levels has been reported to help plants to maintain higher leaf water potential, turgor potential, relative water content, and lower osmotic potential in several crop plants like wheat (Sen Gupta et al. 1989), maize (Premachandra et al. 1991), and Vigna radiata (Nandwal et al. 1998) grown under water stress.
Plants suffering from environmental stresses like drought have a larger internal requirement for K and deficiency of potassium results in overproduction of ROS (Cakmak 2005). Potassium has been reported to reduce the detrimental effects of ROS by enhancing photosynthetic electron transport while inhibiting the activity of membrane bound NAD(P)H oxidases.
Potassium promotes root growth under water stress conditions (Sangakkara et al. 1996) because potassium and magnesium enhances transport of sucrose to developing root for their normal growth and development. Moreover, it also enhances the ion uptake, as potassium itself is one among the main constituents of the phloem sap, thus maintaining the osmotica and, thereby mitigating the adverse effects of moisture stress in plants by increasing the translocation and maintaining the water balance within the plants (Jeschke et al. 1997; Walker et al. 1998). In addition to this, activity of several enzymes which are involved in drought resistance is enhanced by the supplementation of appropriate potassium and its adequate concentration in cytoplasm as well (Kant and Kafkafi 2002). Plants with appropriate K levels have enhanced membrane fluidity because potassium maintains the higher ratio of unsaturated to saturated fatty acids in membranes (Wilkinson et al. 2001). Moreover, potassium supplementation has been reported to increase the synthesis of many organic solutes like proline, free sugars, and free amino acids, under normal and water stress conditions, which contribute to osmotic adjustment. Under normal and water stress conditions, potassium-induced increase in proline has been reported in rice (Pandey et al. 2004) and wheat (Jatav et al. 2012). Potassium-induced increase in free sugars under water stress has been reported in rice by Pandey et al. (2004). In sorghum, potassium has been reported to overcome the ill effects of water stress and maintains the higher tissue water content (Umar et al. 1993). Plants accumulate osmolytes under stress conditions, thereby reducing osmotic potential and maintaining RWC (Gupta et al. 2000). Drought-induced proline accumulation and hydrolysis of macromolecules into simpler mono, disaccharides, and amino acids may lead to accumulation of osmotica.
Evidences are emerging from the molecular studies that potassium might be involved in regulating the plant stress responses (Ashley et al. 2006; Wang and Wu 2010). Low potassium status triggers several signaling cascades such as up regulation of K transporters, synthesis of ROS, and phytohormones including jasmonic acid (JA) and ethylene. In addition to up regulation of transport proteins, potassium deficiency also triggers several other responses in roots. All these strategies enable plant species to adapt with the changing environmental conditions. It has been reported that increase in ROS and phytohormone levels is accompanied by transient increase in transcripts coding potassium transporters and channels, suggesting possible regulatory role of potassium in plant stress responses (Cheong et al. 2007; Jung et al. 2009). Cheong et al. (2007) suggested that in K-deficient plants and drought-induced ABA may trigger Ca flow which acts as secondary messenger and initiates the uptake of K by roots and the regulation of stomatal guard cells. Ca signaling, which regulates leaf transpiration and root K uptake, involves membrane localized Ca sensor-interacting proteins. Jung et al. (2009) reported the increased ethylene and ROS production in K-deficient plants. This phytohormone signal is important for changes in root morphology and plant tolerance to low K supply.
6 Conclusion and Future Perspectives
Abiotic stresses cause considerable reduction in yield especially in arid and semiarid regions of the globe. Around 40–45 % of the agricultural land affected by drought stress and global climate change has made the problem even graver by causing reduction in arable land. Drought stress causes alterations in normal plant metabolism. Exposure to environmental stresses triggers the generation of ROS in different cellular organelles, especially chloroplasts and mitochondria. ROS cause disturbances in normal functioning of the cell by affecting several cellular macromolecules including DNA, lipids, proteins, carbohydrates, and their overproduction which ultimately can lead to cell death. In order to mitigate the stress, plants have evolved many adaptive mechanisms. Greater synthesis of various organic osmolytes such as proline, glycine betaine, free sugars, and polyols is considered as good indication of greater tolerance to stress. To ameliorate the damaging effects of ROS, plants are well equipped with enzymatic as well as nonenzymatic antioxidants. Positive effects of organic osmoprotectants and antioxidative defense system in combating stress-induced damage are well established. Osmolytes are known to play a role in osmotic adjustment, thereby maintaining the internal water content of cell, besides protecting subcellular structures. Moreover, synthesis of certain growth regulators is upregulated in plants under stress. Growth regulators act as signal molecules and play pivotal role in sensing and combating with the incoming stress. Proper mineral nutrition can enhance the performance of plants, both under normal and stressed conditions by preventing the oxidation of polyunsaturated fatty acids (PUFA), thereby preventing membrane leakage and excessive formation of free radicals.
The biggest challenge to plant scientists is to develop stress-tolerant plant varieties. In order to enhance the stress tolerance, researchers have to look for defined sets of markers. In connection to this, genetic manipulations in important crops are gaining pace, but it should be kept in mind that the genes incorporated should not only enhance the tolerance at certain plant growth stages but at the whole plant level as well. Genomics, proteomics, and ionomics have been contributory towards the understanding of various plant responses to abiotic stresses. These techniques will help to identify unknown links, cross talk across different stress signaling pathways that could be exploited to enhance the plant tolerance to particular abiotic stress. Keeping global climate change in mind, model plants should be developed for increasing the understanding of tolerance mechanisms and interactions with increasing concentration of CO2 so as to assess them as suitable future crop plants. At the same time, lack of thorough understanding of drought tolerance mechanisms at genetic and physiological levels and their contributions towards the stress tolerance have been a major limitation to develop drought-tolerant plants.
Our present knowledge about causes and consequences of water stress has still many dark areas and we should enhance our efforts towards these issues. Plant biotechnologists have so far been successful to some extent in developing stress-tolerant varieties but there is much more which still remains to be done.
References
Abdel-Basset R (1998) Calcium channels and membrane disorders induced by drought stress in Vicia faba plants supplemented with calcium. Acta Physiol Plant 2(2):149–153
Abou El-Yazied AA (2011) Foliar application of glycine betaine and chelated calcium improves seed production and quality of common bean (Phaseolus Vulgaris L.) under water stress conditions. Res J Agric Biol Sci 7:357–370
Agarwal S, Sairam RK, Srivastava GC, Meena RC (2005) Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol Plant 49:541–550
Agarwal RM, Tomar NS, Singh K, Sharma GL (2009) Potassium induced changes in flowering plants. In: Flower retrospect and prospect (Professor Vishwambhar Puri Birth Centenary Volume). SR Scientific Publication, Delhi, pp 158–186
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Ahmad P (2010) Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch Agron Soil Sci 56(5):575–588
Ahmad P, Sharma S (2008) Salt stress and phyto-biochemical responses of plants. Plant Soil Environ 54(3):89–99
Ahmad P, Jhon R, Sarwat M, Umar S (2008a) Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2(4):353–366
Ahmad P, Sarwat M, Sharma S (2008b) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51(3):167–173
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010a) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Ahmad P, Jaleel CA, Sharma S (2010b) Antioxidant defense system, lipid peroxidation, proline metabolizing enzymes and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ J Plant Physiol 57(4):509–517
Ahmad P, Nabi G, Ashraf M (2011) Cadmium induced oxidative damage in mustard (Brassica juncea L. Czern. and Coss.) plants can be alleviated by salicylic acid. S Afr J Bot 77:36–44
Ahmad P, Ozturk M, Gucel S (2012a) Oxidative damage and antioxidants induced by heavy metal stress in two cultivars of mustard (L) plants. Fresen Environ Bull 21(10):2953–2961
Ahmad P, Hakeem KR, Kumar A, Ashraf M, Akram NA (2012b) Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol 11(11):2694–2703
Ahmad P, Kumar A, Gupta A, Hu X, Hakeem KR, Azooz MM, Sharma S (2012c) Polyamines: role in plants under abiotic stress. In: Ashraf M, Ozturk M, Ahmad MSA, Aksoy A (eds) Crop production for agricultural improvement. Springer, Dordrecht, pp 491–512
Ahmad P, Bhardwaj R, Tuteja T (2012d) Plant signaling under abiotic stress environment. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 297–323
Ahmad P, Ashraf M, Azooz MM, Rasool S, Akram NA (2013) Potassium starvation-induced oxidative stress and antioxidant defense responses in Brassica juncea. J Plant Interact (in press). doi:10.1080/17429145.2012.747629
Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Alcazar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T (2011) Integration of polyamines in the cold acclimation response. Plant Sci 180:31–38
Aldesuquy HS, Abbas MA, Abo-Hamed SA, Elhakem AH, Alsokari SS (2012) Glycine betaine and salicylic acid induced modification in productivity of two different cultivars of wheat grown under water stress. J Stress Physiol Biochem 8(2):72–89
Alet AI, Sanchez DH, Cuevas JC (2011) Putrescine accumulation in Arabidopsis thaliana transgenic lines enhances tolerance to dehydration and freezing stress. Plant Signal Behav 6:278–286
Ali Q, Ashraf M (2011) Exogenously applied glycine betaine enhances seed and seed oil quality of maize (Zea mays L.) under water deficit conditions. Environ Exp Bot 71:249–259
Alia SA, Murata N (1998) Metabolic engineering of rice leading to biosynthesis of glycine betaine and tolerance to salt and cold. Plant Mol Biol 38:1011–1019
Allen GA, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19:735–747
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691
Anand A, Trick HN, Gill BS (2003) Stable transgene expression and random gene silencing in wheat. Plant Biotechnol J 1(4):241–251
Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161:319–328
Anjum SA, Saleem MF, Wang L, Bilal MF, Saeed A (2012) Protective role of glycine betaine in maize against drought induced lipid peroxidation by enhancing capacity of antioxidative system. Aust J Crop Sci 6(4):576–583
Arbona V, Hossain Z, Lopez-Climent MF, Perez Clemente RM, Gomez Cadenas A (2008) Antioxidant enzymatic activity is linked to water logging stress tolerance in citrus. Physiol Plant 132:452–466
Armengaud P (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450
Ashley MK, Grant M, Grabov A (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57:425–436
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Audran C, Liotenberg S, Gonneau M, North H, Frey A (2001) Localization and expression of zeaxanthin epoxidase mRNA in Arabidopsis in response to drought stress and during seed development. Aust J Plant Physiol 28:1161–1173
Azooz MM, Youssef AM, Ahmad P (2011) Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int J Plant Physiol Biochem 3(14):253–264
Bacon MA, Wilkinson S, Davies WJ (1998) pH-regulated leaf cell expansion in droughted plants is abscisic acid dependent. Plant Physiol 118:1507–1515
Bahrani MJ, Bahrami H, Haghighi AAK (2010) Effect of water stress on ten forage grasses native or introduced to Iran. Grassl Sci 56:1–5
Barkosky RR, Einhellig FA (1993) Effects of salicylic acid on plant water relationship. J Chem Ecol 19:237–347
Batlang U, Baisakh N, Ambavaram MM, Pereira A (2013) Phenotypic and physiological evaluation for drought and salinity stress responses in rice. Methods Mol Biol 956:209–225
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Blum A (1996) Crop response to drought and the interpretation of adaptation. J Plant Growth Regul 20:135–148
Blum A (2005) Drought resistance, water-use deficiency, and yield potential—are they compatible, dissonant, or mutually exclusive. Aust J Agric Res 56:1159–1168
Bohnert HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotechnol 14:89–97
Boominathan P, Shukla R, Kumar A, Manna D, Negi D, Praveen VK, Chattopathy D (2004) Long term transcript accumulation during development of dehydration adaptation in Cicer arietinum. Plant Physiol 135:1608–1620
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. J Plant Physiol 126:1024–1030
Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279:41758–41766
Bray EA (2002) Abscisic acid regulation of gene expression during water deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25:153–161
Burkhanova EA, Fedina AB, Kulaeva ON (1999) Effect of salicylic acid and (2-5)-oligo-adenylates on protein synthesis in tobacco leaves under heat shock conditions: a comparative study. Russ J Plant Physiol 46:16–22
Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46:95–122
Cabot C, Sibole Barcelo JV, Poschenrieder C (2009) Sodium-calcium interactions with growth, water, and photosynthetic parameters in salt treated beans. J Plant Nutr Soil Sci 172:637–643
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Chae HS, Faure F, Kieber JJ (2003) The eto1, eto2 and eto3 mutations and cytokinin treatment elevate ethylene biosynthesis in Arabidopsis by increasing the stability of the ACS5 protein. Plant Cell 15:545–559
Chang L, Karin M (2001) Mammalian MAP kinase signaling cascades. Nature 410:37–40
Chaves HM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot 89:907–916
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plants. Funct Plant Biol 30:239–264
Chen Z, Cuin TA, Zhou M, Twomey M, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58:4245–4255
Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, Koshiba T, Sheen J (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14:2723–2743
Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BK, Lee SC, Kudia J, Luan S (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK 23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 52:223–239
Cheong YH, Sung SJ, Kim BG, Pandey GK, Cho JS, Kim KN, Luan S (2010) Constitutive overexpression of the calcium sensor CBL5 confers osmotic or drought stress tolerance in Arabidopsis. Mol Cells 29:159–165
Chuang HW, Harnrak A, Chen YC, Hsu CM (2010) A harpin-induced ethylene-responsive factor regulates plant growth and responses to biotic and abiotic stresses. Biochem Biophys Res Commun 402:414–420
Cuevas JC, Lopez-Cobollo R, Alcazar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A (2008) Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol 148:1094–1105
Da Costa M, Huang B (2009) Physiological adaptations of perennial grasses to drought stress. In: Barrera ED, Smith WK (eds) Perspectives in biophysical plant ecophysiology. Universidad National Autonoma de Mexico, Mexico, pp 169–190. ISBN 987-0-578-00421-1
Das R, Pandey GK (2010) Expressional analysis and role of calcium regulated kinases in abiotic stress signaling. Curr Genomics 11:2–13
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermo tolerance induced by salicylic acid and heat acclimation of mustard seedlings. Plant Physiol 116:1351–1357
de Carvalho K, de Campos MK, Domingues DS, Pereira LF, Vieira LG (2013) The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol Biol Rep 40(4):3269–3279
Desikan R, Hanckok JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol 137:831–834
Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D, Gaudinova A, Havlova M, Gubis J, Vankova R (2010) Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol 167:1360–1370
Duan JJ, Li J, Guo SR, Kang YY (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short term salinity tolerance. J Plant Physiol 165:1620–1635
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice Oryza sativa L., encode transcription activators that function in drought, high salt and cold responsive gene expression. Plant J 33:751–763
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Easterwood GW (2002) Calcium’s role in plant nutrition. Fluid J 1:1–3
Ebelhar SA, Varsa EC (2000) Tillage and potassium placement effects on potassium utilization by corn and soybean. Commun Soil Sci Plant Anal 31:11–14
El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S (2009) Molecular characterization of seven genes encoding ethylene responsive transcriptional factors during plum fruit development and ripening. J Exp Bot 60:907–922
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives, 2nd edn. Sinauer Associates, Sunderland, p 400
Evans NH, McAinsh MR, Hetherington AM (2001) Calcium oscillations in higher plants. Curr Opin Plant Biol 4:415–420
FAO (2010) FAO land and plant nutrition management service. http://www.fao.org
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:15–45
Fujii H, Verslues PE, Zhu JK (2011) Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci U S A 108:1717–1722
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gadallah MAA (1999) Effects of proline and glycine betaine on Vicia faba responses to salt stress. Biol Plant 42:249–257
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Girija C, Smith BN, Swamy PM (2002) Interactive effects of sodium chloride and calcium chloride on the accumulation of proline and glycine betaine in peanut (Arachis hypogaea L). Environ Exp Bot 47:1–10
Gomez-Cadena A, Tadeo FR, Primmo-Millo ME (1996) Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol 112:401–408
Gowing DJ, Davies WJ, Jones HG (1990) A positive root-sourced signal as an indicator of soil drying in apple (Malus domestica Bork). Exp Bot 41:1535–1540
Groppa MD, Benavides MP (2008) Polyamines and abiotic stress: recent advances. Amino Acids 34:35–45
Gupta SC, Rathore AK, Sharma SN, Saini RS (2000) Response of chickpea cultivar to water stress. Indian J Plant Physiol 3:274–276
Hall MA, Smith AR (1995) Ethylene and the responses of plants to stress. Bulg J Plant 165:397–406
Hao D, Ohme-Takagi M, Sarai A (1998) Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plant. J Biol Chem 273:26857–26861
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53:27–32
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hayashi H, Alia Mustardy L, Deshnium P, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycine betaine and enhanced tolerance to salt and cold stress. Plant J 12:133–142
He T, Cramer GR (1996) Abscisic acid concentrations are correlated with leaf area reductions in two salt stressed rapid cycling Brassica species. Plant Soil 179:25–33
Hong Z, Lakkineni K, Zhang Z, Verma DP (2000) Removal of feedback inhibition of delta pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Horvath E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300
Hu Y, Zhao L, Chong K, Wang T (2008) Overexpression of OsERF1, a novel rice ERF gene, up-regulates ethylene-responsive genes expression, besides affects growth and development in Arabidopsis. J Plant Physiol 165:1717–1725
Huang ZJ, Zhang ZJ, Zhang XL, Zhang HB, Huang DF (2004) Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Lett 573:110–116
Hussain SS, Ali M, Ahmad M, Siddique KH (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:337–403
Iqbal N, Ashraf MY, Ashraf M (2005) Influence of water stress and exogenous glycine betaine on sunflower achene weight and oil percentage. Int J Environ Sci Technol 2:155–160
Jackson GE, Irvine J, Grace J, Khalil AAM (1995) Abscisic acid concentrations and fluxes in droughted conifer saplings. Plant Cell Environ 18:13–22
Jajarmi V (2009) Effect of water stress on germination indices in seven wheat cultivar. World Acad Sci Eng Technol 49:105–106
Jaleel CA, Gopi R, Manivannan P, Kishorekumar A, Sankar B, Panneerselvam R (2006) Paclobutrazol influences vegetative growth and floral characteristics of Catharanthus roseus (L) G. Don. Indian J Appl Pure Biol 21:369–372
Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R (2007) Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. S Afr J Bot 77:190–195
Janda T, Szalai G, Tari T, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L) plants. Planta 208:175–180
Jarvis AJ, Davies WJ (1997) Whole plant ABA flux and the regulation of water loss in Cedrella odorata. Plant Cell Environ 20:521–527
Jatav KS, Agarwal RM, Singh RP, Shrivastava M (2012) Growth and yield responses of wheat (Triticum aestivum L.) to suboptimal water supply and different potassium doses. J Funct Environ Bot 2(1):39–51
Jeschke WD, Kirkby EA, Peuke AD, Pate JS, Hartung W (1997) Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor oil bean (Ricinus communis L). J Exp Bot 48:75–91
Jia W, Zhang J (1999) Stomatal closure is induced rather by prevailing xylem abscisic acid than by accumulated amount of xylem derived abscisic acid. Physiol Plant 106:268–275
Jiang M, Zhang J (2002) Water stress induced abscisic acid accumulation triggers the increased generation of reactive species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Joseph B, Jini D, Sujatha S (2010) Insight into role of exogenous salicylic acid on plants growth under salt environment. Asian J Crop Sci 2(4):226–235
Ju HW, Min JH, Chung MS, Kim CS (2013) The atrzf1 mutation of the novel RING-type E3 ubiquitin ligase increases proline contents and enhances drought tolerance in Arabidopsis. Plant Sci 203–204:1–7
Jung JY, Shin R, Schachtman DP (2009) Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 91:607–621
Kadioglu A, Saruhan N, Saglam A, Terzi R, Tuba A (2010) Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul 64:27–37
Kant S, Kafkafi U (2002) Potassium and abiotic stresses in plants. In: Pasricha NS, Bansal SK (eds) Potassium for sustainable crop production. Potash Institute of India, Gurgaon, pp 233–251
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Katare DP, Nabi G, Azooz MM, Aeri V, Ahmad P (2012) Biochemical modifications and enhancement of psoralen content in salt-stressed seedlings of Psoralea corylifolia Linn. J Funct Environ Bot 2(1):65–74
Kathuria H, Giri J, Nataraja KN, Murata N, Udayakumar M, Tyagi AK (2009) Glycine betaine induced water stress tolerance in codA-expressing transgenic rice is associated with up regulation of several stress responsive genes. Plant Biotechnol J 7:512–526
Kaydan D, Yagmur M, Okut N (2007) Effects of salicylic acid on the growth and some physiological characters in salt stressed wheat (Triticum aestivum L). Tarim Bilimleri Dergisi 13(2):114–119
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44:283–307
Khamssi NN, Golezani KG, Najaphy A, Zehtab S (2011) Evaluation of grain filling rate, effective grain filling period and resistance indices under acclimation to gradual water deficit stress in chickpea cultivars. Aust J Crop Sci 5:1044–1049
Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell type specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23:267–278
Kim GB, Nam YW (2013) Δ1-pyrroline-5-carboxylate synthetase gene of Medicago truncatula plays a predominant role in stress-induced proline accumulation during symbiotic nitrogen fixation. J Plant Physiol 170(3):291–302
Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S (2003) Activation of a stress responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15:2707–2718
Kim MC, Chung WS, Yun DJ, Cho MJ (2009) Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant 2:13–21
Kong X, Pan J, Zhang M, Xing X, Zhou Y, Liu Y, Li D (2011) ZmMKK4, a novel group C mitogen-activated protein kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ 34:1291–1303
Kovacs Z, Simon-Sarkadi L, Szucs A, Kocsy G (2010) Different effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 38:623–631
Koyro HW, Ahmad P, Geissler N (2012) Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 1–28
Kubis J (2008) Exogenous spermidine differently alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water stressed cucumber leaves. J Plant Physiol 165:397–406
Kumriaa R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. J Plant Physiol 159(9):983–990
Kusano T, Yamaguchi K, Berberich T, Takahashi Y (2007) Advances in polyamine research. J Plant Res 120:345–350
Le Rudulier D, Strom AR, Danekar AM, Smith LT, Valentaine RC (1984) Molecular biology of osmoregulation. Science 224:1064–1068
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199–222
Levitt J (1980) Responses of plants to environmental stresses. Academic, New York
Liu HH, Dong BH, Zhang YY, Liu ZP, Liu YL (2004) Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T (2007) Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol 24:117–126
Liu JH, Inoue H, Moriguchi T (2008) Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environ Exp Bot 62:28–35
Liu L, Hu X, Song J, Zong X, Li D (2009) Over-expression of a Zea mays L. protein phosphatase 2C gene (ZmPP2C) in Arabidopsis thaliana decreases tolerance to salt and drought. J Plant Physiol 166:531–542
Luan S (2002) Signaling drought in guard cells. Plant Cell Environ 25:229–237
Lutts S (2000) Exogenous glycine betaine reduces sodium accumulation in salt stressed rice plants. Int Rice Res Notes 25:39–40
Lv S, Yang A, Zhang K, Wang L, Zhang J (2007) Increase of glycine betaine synthesis improves drought tolerance in cotton. Mol Breed 20:233–248
Ma YY, Song WY, Liu ZH, Zhang HM, Guo XL, Shao HB, Ni FT (2009) The dynamic changing of Ca2+ cellular localization in maize leaflets under drought stress. Cell Biol 332:35–362
Makela P, Jokinen K, Kontturi M, Peltonen Sainio P, Pehu E, Somersalo S (1998) Foliar application of glycine betaine—a novel product from sugar beet, as an approach to increase tomato yield. Ind Crops Prod 7:139–148
Manavella PA, Arce AL, Dezar CA, Bitton F, Renou JP, Crespi M, Chan RL (2006) Cross talk between ethylene and drought signaling pathways is mediated by the sunflower Hahb-4 transcription factor. Plant J 48:125–137
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, p 889
Masoumi A, Kafi M, Hamidreza Khazaei H, Davari K (2010) Effect of drought stress on water status, electrolyte leakage and enzymatic antioxidant of kochia (Kochia scoparia) under saline condition. Pak J Bot 42(5):3517–3524
McAnish MR, Hetherington AM (1998) Encoding specificity in Ca2+ signaling systems. Trends Plant Sci 3:32–36
Meille JL, Pellerin S (2004) Leaf area establishment of a maize (Zea mays L.) field crop under potassium deficiency. Plant Soil 265:75–92
Mengel K, Kirkby EA (2001) Principles of plant nutrition, 5th edn. Kluwer Academic, Dordrecht, p 849
Metraux JP (2001) Systemic acquired resistance and salicylic acid: current state of knowledge. Eur J Plant Pathol 107:13–18
Metwally A, Finkmemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mikolajczyk M, Awotunde OS, Muszynska G (2000) Osmotic stress induces rapid activation of a salicylic acid induced protein kinase and a homolog of protein kinase ASK1 in tobacco cell. Plant Cell 12:165–178
Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A (2009) Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem 284:26482–26492
Montero E, Cabot C, Poschenrieder C, Barcelo J (1998) Relative importance of osmotic-stress and ion-specific effects on ABA-mediated inhibition of leaf expansion growth in Phaseolus vulgaris. Plant Cell Environ 21:54–62
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murata N, Mohanty PS, Hayashi H, Papageorgiou GC (1992) Glycine betaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen evolving complex. FEBS Lett 296:187–189
Murkowski A (2001) Heat stress and spermidine: effect on chlorophyll fluorescence in tomato plants. Biol Plant 44:53–57
Nandwal AS, Hooda A, Datta D (1998) Effect of substrate moisture and potassium on water relations and C, N and K distribution in Vigna radiata. Biol Plant 41:149–153
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365
Nayyar H, Walia DP (2004) Genotypic variation in wheat in response to water stress and abscisic acid-induced accumulation of osmolytes in developing grains. J Agron Crop Sci 190:39–45
Nayyar H, Bains T, Kumar S (2005) Low temperature induced floral abortion in chickpea: relationship to abscisic acid and cryoprotectants in reproductive organs. Environ Exp Bot 1:39–47
Nobuhiro S, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Nye GJ, Scott P, Golbeck H, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci U S A 94:3454–3458
Pan Y, Seymour GB, Lu C, Hu Z, Chen X, Chen G (2012) An ethylene response factor (ERF5) promoting adaptation to drought and salt tolerance in tomato. Plant Cell Rep 31:349–360
Pandey R, Agarwal RM (1998) Water stress-induced changes in proline contents and nitrate reductase activity in rice under light and dark conditions. Physiol Mol Biol Plants 4:53–57
Pandey R, Agarwal RM, Jeevaratnam K, Sharma GL (2004) Osmotic stress induced alterations in rice (Oryza sativa L.) and recovery. Plant Growth Regul 42:79–87
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth Res 44:243–252
Patil S, Takezawa D, Poovaiah BW (1995) Chimeric plant calcium: calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain. Proc Natl Acad Sci U S A 92:4897–4901
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Pettigrew WT, Meredith WR (1997) Dry matter production, nutrient uptake, and growth of cotton as affected by potassium fertilization. J Plant Nutr 20:531–548
Pospisilova J, Vagner M, Malbeck J, Travnickova A, Batkova P (2005) Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol Plant 49:533–540
Prabhavathi VR, Rajam MV (2007) Polyamine accumulation in transgenic eggplant enhances tolerance to multiple stresses and fungal resistance. Plant Biotechnol 24:273–282
Premachandra GS, Saneoka H, Ogata S (1991) Cell membrane stability and leaf water relations as affected by potassium nutrition of water stressed maize. J Exp Bot 42:45–73
Pugnaire FI, Endolz LS, Pardos J (1994) Constraints by water stress on plant growth. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 247–259
Qin XQ, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci U S A 96:15354–15361
Qu LJ, Wu LQ, Fan ZM, Guo L, Li YQ, Chen ZL (2005) Overexpression of the bacterial nhaA gene in rice enhances salt and drought tolerance. Plant Sci 168:297–302
Rahman MS, Miyake H, Takeoka Y (2002) Effects of exogenous glycine betaine on growth and ultra structure of salt-stressed rice seedlings (Oryza sativa L.). Plant Prod Sci 5:33–44
Rai VK, Sharma SS, Sharma S (1986) Reversal of ABA induced stomatal closure by phenolic compounds. J Exp Bot 37:129–134
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Rasool S, Ahmad A, Siddiqi TO, Ahmad P (2013) Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant 35(4):1039–1050
Rengasamy P (2010) Soil processes affecting crop production in salt affected soils. Funct Plant Biol 37:613–620
Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379:633–646
Roosens NH, Al Bitar F, Loenders K, Angenon G, Jacobs M (2002) Overexpression of ornithine-delta-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breed 9:73–80
Ruiz-Sanchez MC, Domingo R, Perez-Pastor A (2007) Daily variations in water relations of apricot trees under different irrigation regimes. Biol Plant 51:735–740
Sadeghipour O, Aghaei P (2012) Impact of exogenous salicylic acid application on some traits of common bean (Phaseolus vulgaris L.) under water stress conditions. Int J Agric Crop Sci 4(11):685–690
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Overexpression of single calcium dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 28(3):319–327
Sajid SA, Aftab F (2012) Role of salicylic acid in amelioration of salt tolerance in potato (Solanum tuberosum L.) under in vitro conditions. Pak J Bot 44:37–42
Sakhanokho HF, Kelley RY (2009) Influence of salicylic acid on in vitro propagation and salt tolerance in Hibiscus acetosella and Hibiscus moscheutos (cv Luna Red). Afr J Biotechnol 8(8):1474–1481
Sangakkara UR, Hartwig UA, Nosberger J (1996) Soil moisture and potassium affect the performance of symbiotic nitrogen fixation in faba bean and common bean. Plant Soil 184:123–130
Sarwat M, Ahmad P, Nabi G, Hu X (2013) Ca2+ signals: the versatile decoders of environmental cues. Crit Rev Biotechnol 33(1):97–109
Scaramagli S, Biondi S, Leone A, Grub S, Torrigiani P (2000) Acclimation to low-water potential in potato cell suspension cultures leads to changes in putrescine metabolism. Plant Physiol Biochem 38:345–351
Schaberg PG, Minocha R, Long S, Halman JM, Hawley GJ, Eagar C (2011) Calcium addition at the Hubbard Brook Experimental Forest increases the capacity for stress tolerance and carbon capture in red spruce (Picea rubens) trees during the cold season. Trees 25:1053–1061
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci U S A 97:11655–11660
Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10:296–302
Sen Gupta A, Berkowitz GA, Pier PA (1989) Maintenance of photosynthesis at low leaf water potential in wheat, role of potassium status and irrigation history. Plant Physiol 89:1358–1365
Shabala S, Schimanski LJ, Koutoulis A (2002) Heterogeneity in bean leaf mesophyll tissue and ion flux profiles: leaf electrophysiological characteristics correlate with the anatomical structure. Ann Bot 89:221–226
Shakirova FM (2007) Role of hormonal system in the manifestation of growth promoting and anti-stress action of salicylic acid. In: Hayat S, Ahmad A (eds) Salicylic acid, a plant hormone. Springer, Dordrecht, p 69
Shakirova FM, Bezrukova MV (1997) Induction of wheat resistance against environmental salinization by salicylic acid. Biol Bull 24:109–112
Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR (2003) Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci 164:317–322
Shao HB, Chen XY, Chu LY, Zhao XN, Wu GY, Yong B (2006) Investigation on the relationship of proline with wheat anti drought under soil water deficits. Colloids Surf B Biointerfaces 53:113–119
Shao HB, Chu LY, Shao MA (2008a) Calcium as a versatile plant signal transducer under soil water stress. Bioessays 30:634–641
Shao HB, Song WY, Chu LY (2008b) Advances of calcium signals involved in plant anti-drought. C R Biol 331:587–596
Sharma YK, Leon J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana, the role of salicylic acid in the accumulation of defense related transcripts and induced resistance. Proc Natl Acad Sci U S A 93:5099–5104
Sharp RE, Le Noble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51:1575–1584
Shi Q, Zhu Z (2008) Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ Exp Bot 63:317–326
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115:327–334
Shou H, Bordallo P, Fan JB, Yeakley JM, Bibikova M, Sheen J, Wang K (2004a) Expression of an active tobacco mitogen-activated protein kinase enhances freezing tolerance in transgenic maize. Proc Natl Acad Sci U S A 101:3298–3303
Shou H, Bordallo P, Wang K (2004b) Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J Exp Bot 55:1013–1019
Signorelli S, Corpas FJ, Borsani O, Barroso JB, Monza J (2013) Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci 201–202:137–146
Singh B, Usha K (2003) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul 39(2):137–141
Slama I, Tayachi S, Jdey A, Rouached A, Abdelly C (2011) Differential response to water deficit stress in alfalfa (Medicago sativa) cultivars: growth, water relations, osmolyte accumulation and lipid peroxidation. Afr J Biotechnol 10(72):16250–16259
Slaymaker DH, Navarre DA, Clark D, Del-Pozo O, Martin GB, Klessig DF (2002) The tobacco salicylic acid binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci U S A 99:11640–11645
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Spollen WG, Le Noble ME, Sammuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122:967–976
Subbarao GV, Wheeler RM, Levine LH, Stutte GW (2001) Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J Plant Physiol 158:767–776
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szalai G, Horgosi S, Soos V, Majlath I, Balazs E, Janda T (2010) Salicylic acid treatment of pea seeds induces its de novo synthesis. J Plant Physiol 168:213–219
Taiz L, Zeiger E (1998) Plant physiology, 3rd edn. Panima Publishing Corporation, New Delhi, pp 528–529
Takahashi S, Katagiri T, Yamaguchi-Shinozaki K, Shinozaki K (2000) An Arabidopsis gene encoding a Ca2+-binding protein is induced by abscisic acid during dehydration. Plant Cell Physiol 41:898–903
Tan BC, Joseph LM, Deng WT, Liu LJ, Li QB (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits the abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138:2337–2343
Tari I, Csiszar J, Szalai G, Horvath F, Pecsvaradi A, Kiss G, Szepesi A, Szabo M, Erdei L (2002) Acclimation of tomato plants to salinity stress after a salicylic acid pre-treatment. Acta Biol Szeged 46:55–56
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J 23:363–374
Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and trbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99:517–522
Umar S, Rao NR, Sekhon GS (1993) Differential effects of moisture stress and potassium levels on growth and K uptake in sorghum. Indian J Plant Physiol 36(2):94–97
Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51(11):1821–1839
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci U S A 97:11632–11637
Urao T, Katagiri T, Mizoguchi T, Yamaguchi-Shinozaki K, Hayashida N, Shinozaki K (1994) Two genes that encode Ca2+ dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol Gen Genet 244:331–340
Valero D, Perez-Vicente A, Martinez-Romero D, Castillo S, Guillen F, Plum SM (2002) Storability improved after calcium and heat postharvest treatments: role of polyamines. J Food Sci 67:2571–2575
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol 162:669–677
Walker CR, Black JA, Miller J (1998) The role of cytosolic potassium and pH in the growth of barley roots. Plant Physiol 118:957–964
Wang Y, Nil N (2000) Changes in chlorophyll, ribulose biphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J Hortic Sci Biotechnol 75:623–627
Wang Y, Wu WH (2010) Plant sensing and signaling in response to K+ deficiency. Mol Plant 3:280–287
Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:131–151
Wang X, Shi G, Xu Q, Hu J (2007) Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J Plant Physiol 164:1062–1070
Wang GP, Li F, Zhang J, Zhao MR, Hui Z, Wang W (2010) Over accumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 48:30–41
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14(4):7370–7390
Waseem M, Athar H, Ashraf M (2006) Effect of salicylic acid applied through rooting medium on drought tolerance of wheat. Pak J Bot 38(4):1127–1136
Wen XP, Pang XM, Matsuda N, Kita M, Inoue H, Hao YJ (2008) Overexpression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res 17:251–263
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
White PJ, Karley AJ (2010) Potassium. In: Hell R, Mendel RR (eds) Cell biology of metals and nutrients, plant cell monographs, vol 17. Springer, Berlin, pp 199–224
Wi SJ, Kim WT, Park KY (2006) Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121
Wild A (2003) Soils, land and food: managing the land during the twenty-first century. Cambridge University Press, Cambridge, UK
Wilkinson S, Corlett JE, Oger L, Davies WJ (1998) Effects of xylem pH on transpiration from wild-type and flacca tomato leaves. A vital role for abscisic acid in preventing excessive water loss even from well watered plants. Plant Physiol 117:703–710
Wilkinson S, Clephan AL, Davies WJ (2001) Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol 126:1566–1578
Xia J, Liu MY, Jia SF (2005) Water security problem in North China: research and perspective. Pedosphere J 15:563–575
Xiang Y, Huang Y, Xiong L (2007) Characterization of stress responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 144:1416–1428
Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid inducible mitogen activated protein kinase. Plant Cell 15:745–759
Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277:8588–8596
Xu J, Tian YS, Peng RH, Xiong AS, Zhu B, Jin XF, Gao F, Fu XY, Hou XL, Yao QH (2010) AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 231:1251–1260
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y (2005) Effects of free proline accumulation in petunias under drought stress. J Exp Bot 56:1975–1981
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano TA (2007) Protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang X, Lu C (2005) Photosynthesis is improved by exogenous glycine betaine in salt stressed maize. Physiol Plant 124:343–352
Yang WJ, Rich PJ, Axtell JD, Wood KV, Bonham CC, Ejeta G, Mickelbart MV, Rhodes D (2003) Genotypic variation for glycine betaine in sorghum. Crop Sci 43:162–169
Yang Z, Tian L, Latoszek-Green M, Brown D, Wu K (2005) Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol Biol 58:585–596
Yiu JC, Juang LD, Fang DYT, Liu CW, Wu SJ (2009) Exogenous putrescine reduces flooding induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci Hortic 120:306–314
Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30:529–539
Yoo JH, Park CY, Kim JC (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280:3697–3706
Yordanov I, Velikova V, Tsone V (2000) Plant response to drought, acclimatation and stress tolerance. Photosynthetica 30:171–186
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity-induced changes in Brassica juncea. J Integr Plant Biol 50(9):1096–1102
Zarembinski TI, Theologis A (1994) Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol 26:1579–1597
Zeid IM, Shedeed ZA (2006) Response of alfalfa to putrescine treatment under drought stress. Biol Plant 50:635–640
Zhang J, Davies WJ (1989a) Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ 12:73–81
Zhang J, Davies WJ (1989b) Sequential responses of whole plant water relations towards prolonged soil drying and the mediation by xylem sap ABA concentrations in the regulation of stomatal behaviour of sunflower plants. New Phytol 113:167–174
Zhang J, Davies WJ (1990a) Changes in the concentration of ABA in xylem sap as a function of changing soil water status will account for changes in leaf conductance. Plant Cell Environ 13:277–285
Zhang J, Davies WJ (1990b) Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants. J Exp Bot 41:1125–1132
Zhang J, Schurr U, Davies WJ (1987) Control of stomatal behaviour by abscisic acid which apparently originates in roots. J Exp Bot 38:1174–1181
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97:111–119
Zhang G, Chen M, Li L, Xu Z, Chen X, Guo J, Ma Y (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60:3781–3796
Zhang H, Liu W, Wan L, Li F, Dai L, Li D, Zhang Z, Huang R (2010) Functional analyses of ethylene response factor JERF3 with the aim of improving tolerance to drought and osmotic stress in transgenic rice. Transgenic Res 19:809–818
Zhang L, Xi D, Li S, Gao Z, Zhao S, Shi J, Wu C, Guo X (2011) A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol Biol 77:17–31
Zhao H, Yang H (2008) Exogenous polyamines alleviate the lipid peroxidation induced by cadmium chloride stress in Malus hupehensis Rehd. Sci Hortic 116:442–447
Zhifang G, Loescher WH (2003) Expression of a celery mannose 6-phosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and a glucosyl-mannitol dimmer. Plant Cell Environ 26:275–283
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 53:247–273
Zlatev Z, Stotanov Z (2005) Effect of water stress on leaf water relations of young bean plants. J Cent Eur Agric 6(1):5–14
Zwart FJ, Slow S, Payne RJ, Lever M, George PM, Gerrard JA, Chambers ST (2003) Glycine betaine and glycine betaine analogues in common foods. Food Chem 83:197–204
Acknowledgment
We are highly thankful to Prof R. M. Agarwal for his valuable suggestions and Prof. Rekha Bhadauria, Head, School of Studies in Botany, Jiwaji University, Gwalior for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ahanger, M.A., Tyagi, S.R., Wani, M.R., Ahmad, P. (2014). Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In: Ahmad, P., Wani, M. (eds) Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8591-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8591-9_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8590-2
Online ISBN: 978-1-4614-8591-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)