Abstract
Main conclusion
Our study demonstrated that CMV resistance was upregulated by brassinosteroids (BRs) treatment, and BR signaling was needed for this BRs-induced CMV tolerance.
Plant steroid hormones, brassinosteroids (BRs), play essential roles in variety of plant developmental processes and adaptation to various biotic and abiotic stresses. BR signal through plasma membrane-localized receptor and other components to modulate several transcription factors that modulate thousands of target genes including certain stress-responsive genes. To study the effects of BRs on plant virus defense and how BRs induce plant virus stress tolerance, we manipulated the BRs levels in Arabidopsis thaliana and found that BRs levels were positively correlated with the tolerance to Cucumber mosaic virus (CMV). We also showed that BRs treatment alleviated photosystem damage, enhanced antioxidant enzymes activity and induced defense-associated genes expression under CMV stress in Arabidopsis. To see whether BR signaling is essential for the plant virus defense response, we made use of BR signaling mutants (a weak allele of the BRs receptor mutant bri1-5 and constitutive BRs response mutant bes1-D). Compared with wild-type Arabidopsis plants, bri1-5 displayed reversed tolerance to CMV, but the resistance was enhanced in bes1-D. Together our results suggest that BRs can induce plant virus defense response through BR signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are exposed to a diversity of biotic and abiotic stresses throughout their life cycles and need to constantly regulate their developmental and physiological processes to respond to various internal and external stimuli (Zhang et al. 2009; Ning et al. 2010). Agricultural crops worldwide survey from a vast array of pathogens including bacteria, fungi, and viruses which cause tremendous losses in yield and quality of produce (Culbreath et al. 2003; Rodoni 2009). Multiple methods have been developed to enhance the plant resistance to viruses. One of the fundamental areas for biotechnological advances in plant improvement is boosting plants internal defense mechanism to survive adverse environmental cues.
Brassinosteroids (BRs) are a group of plant steroid hormones which mainly regulate plant growth and developmental processes such as cell expansion and division, senescence, vascular development. Loss-of-function mutants such as bri1-5 display dwarf phenotypes with reduced cell elongation (Clouse et al. 1996; Li et al. 1996; Szekeres et al. 1996). By contrast, gain-of-function mutants such as bes1-D have long hypocotyls, leaf petioles, curly leaves, and early leaf senescence (Yin et al. 2002). In plants, BR signal through receptor kinase BRI1 (BRASSINOSTEROID-INSENSITIVE1), co-receptor BAK1 (BRI1-ASSOCIATED RECEPTOR KINASE 1), and several other signaling components to control BES1 (BRI1 EMS SUPRESSOR 1) and BZR1 (BRASSINAZOLE RESISTANT 1) family transcription factors (Zhu et al. 2013). Global gene expression studies demonstrated that BRs can regulate up to 4,000–5,000 genes at different growth stages, with about half induced and half repressed by BRs (Guo et al. 2013). ChIP-Chip analyses indicated that BES1 and BZR1 transcription factors likely account for a majority of the BR-regulated gene expression, as BES1 and BZR1 have thousands of target genes and many of them are regulated by BRs (Sun et al. 2010; Yu et al. 2011).
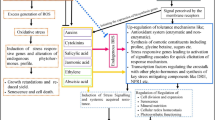
Although the BR signaling cascades for plant growth and development have been firmly established, the mechanism by which BRs increase tolerance is still unclear. Previous reports showed that exogenous applications of BRs are widely used to improve stress tolerance in plants (Acharya and Assmann 2009) and the application of BRs has become a routine stress management protocol (Divi and Krishna 2009; Peleg and Blumwald 2011; Hayat et al. 2012). Treatment of cucumber (Cucumis sativus) with BRs has been reported to enhance tolerance to photo-oxidative and cold stresses and is accompanied by H2O2 accumulation and systemic induction of genes associated with stress responses, such as MAPK1 and MAPK2 (Xia et al. 2009, 2011). Similarly, application of BRs to radish (Raphanus sativus) improves tolerance to Cu and Cr stress owing to the upregulation of a protective antioxidant system and the modulation of endogenous abscisic acid, auxin and polyamine profiles (Choudhary et al. 2010, 2011). Recently, there is increasing evidence that BRs could induce the expression of genes encoding both structural and regulatory proteins such as antioxidants and heat shock proteins (HSPs) (Kagale et al. 2007; Xia et al. 2009; Cui et al. 2011). These results confirmed the roles of BRs in stress responses.
It has been demonstrated that BRs induces resistance of tobacco, rice and cucumber to bacterial, fungal and viral pathogens, establishing a role for BRs in both monocot and dicot plant defense mechanisms. However, signaling cascade mediating BR’s action in plant resistance against viruses remains largely unclear. In this study, we demonstrated that application of BRs offers a unique possibility of enhancing plant virus defense response through activating several key antioxidant enzymes and inducing several resistance genes. Furthermore, we also tested the resistance to Cucumber mosaic virus (CMV, genus Cucumovirus, family Bromoviridae), which is highly virulent on Arabidopsis, in bri1-5 and bes1-D and found that compared to wild type, CMV stress tolerance is weaker in bri1-5 and stronger in bes1-D. All of these data illustrated that BR signaling is needed for BR-induced resistant to plant virus. Our results also demonstrated that BR signaling might induce several resistance genes in response to CMV infection.
Materials and methods
Plant material and growth conditions
All Arabidopsis thaliana seedlings were grown under 8/16 h light/dark cycles of medium light (100 μmol m−2 s−1) for 4 weeks. Arabidopsis thaliana seeds of Col-0, WS-2, BR-insensitive mutant bri1-5 (WS-2 background) and gain-of-function mutant bes1-D (Col-0 background), were given by Dr Yanhai Yin (Iowa State University, Ames, IA, USA).
Chemical treatments and pathogen inoculation
Brassinolide (BL, the most active BR) and brassinazole (BRZ, a specific inhibitor of BR biosynthesis) were purchased from Wako Pure Chemical Industries, ltd (Chuo-Ku, Osaka, Japan) and Santa Cruz Biotechnology, inc (Dallas, TX, USA), respectively. The hormone and inhibitor solutions were prepared in water containing 0.02 % (v/v) Tween 20. For chemical treatment of plants, the 20-day-old seedlings were pretreated by foliar spraying with 1 μM BL or 1 μM BRZ. Distilled water containing 0.02 % (v/v) Tween 20 was used as a control treatment. All experiments were repeated with similar results.
In infection experiments, the chemicals were sprayed 24 h before virus inoculation. Virus isolates of Cucumber mosaic virus (CMV) were acquired from Horticulture Institute, Sichuan Academy of Agricultural Sciences, China. The inoculation with virus was carried out as described previously (Xi et al. 2007; Shang et al. 2011). In brief, virus RNA at a concentration of 1 µg/ml in 10 mM Hepes buffer (pH 7.0) was rubbed onto Arabidopsis leaves with cheese cloth. Hepes buffer without virus RNA only was rubbed onto the leaves with cheese cloth as the mock treatment.
Oxidative damage estimation
Electrolyte leakage was measured as previously described (Xu et al. 2012). After measuring the conductivity of the fresh leaves, the leaves were boiled for 15 min to achieve 100 % electrolyte leakage. Lipid peroxidation was estimated by measuring the thiobarbituric acid-reactive substances (TBARS) as previously described (Xu et al. 2012). The lipid peroxides were expressed as TBARS content.
Cell death determination
Tissue staining with trypan blue (1.25 mg/ml, Sigma) to show that the cell death was performed as described previously (He et al. 2007). At least three leaves were used for each staining.
Determination of antioxidant enzymes
For the enzyme assays, 0.3 g of leaf were ground with 3 mL ice-cold 25 mM Hepes buffer (pH 7.8) containing 0.2 mM EDTA, 2 mM ascorbate and 2 % polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 4 °C for 20 min at 12,000g and the resulting supernatants were used for the determination of enzymatic activity (Wang et al. 2011). Superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD) activities were assayed as described previously (Wang et al. 2011).
Analysis of chlorophyll fluorescence
Chlorophyll fluorescence was determined with an imaging pulse amplitude modulated fluorometer (IMAG-MINI; Heinz Walz, Effeltrich, Germany). For measurement of F v/F m, plants were dark adapted for 30 min. Minimal fluorescence (F o) was measured during the weak measuring pulses, and maximal fluorescence (F m) was measured by 0.8 s pulse of light at about 4,000 μmol m−2 s−1. An actinic light source was then applied to obtain steady-state fluorescence yield (F s), after which a second saturation pulse was applied for 0.7 s to obtain light-adapted maximum fluorescence (F m ′). F v /F m, фPSII were calculated as F m − F o/F m, (F m ′ − F s)/F m ′, respectively (Genty et al. 1989; Bilger and Björkman 1990).
Total RNA extraction and quantitative RT-PCR
Total RNA was extracted from leaves according to a previously described method (Zhang et al. 2010). RNA contents were calculated by measuring the absorbance value taken at 260 nm. The cDNA was amplified using SYBR Premix Ex Taq (TaKaRa). All gene expression levels were measured by quantitative real-time PCR (qRT-PCR). The C t (threshold cycle), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence was first detected, was used as a measure for the starting copy numbers of the target gene. Three technical replicates were performed for each experiment. PP2AA3 (encoding protein phosphatase 2A subunit A3, At1g13320) and ACTIN1 genes were used as internal controls. All primers are shown in Supplementary Table S1.
Protein extraction and western blotting analysis
Plant tissues collected from systemically infected leaves were obtained. Total proteins were extracted with extraction buffer (50 mM Tris–HCl, 5 % mercaptoethanol, 10 % glycerol, 4 % SDS, 4 M urea, pH 6.8) in an ice bath. The resulting slurries were centrifuged at 8,000g for 10 min at 4 °C, and the supernatants were stored at −80 °C. Protein concentrations were determined by the Bradford method using bovine serum albumin as a standard (Bradford 1976). Western blot analysis was carried out using CMV coat protein-specific antisera.
Statistical analysis
Means of three biological replicates were measured. Student’s t test was used for comparison between different treatments. A difference was considered to be statistically significant when P < 0.05.
Results
BRs increase CMV tolerance in Arabidopsis thaliana
To investigate the role of BRs in CMV tolerance, Arabidopsis thaliana leaves were pretreated with BL, water and BRZ before CMV infection and then the CMV replication and accumulation levels were detected by qRT-PCR (Fig. 1a) and Western blotting analysis (Fig. 1b) at 3, 6 and 9 days postinoculation (dpi). When the disease symptom appeared, CMV coat protein was easily detected in uninoculated leaves, which indicated that CMV had already spread and replicated largely in systemic leaves. As shown in Fig. 1, the accumulation level of CMV was less and detectable earlier in BL-pretreated plants as compared with water-pretreated plants (Fig. 1; supplementary Fig. S1). However, the viral replication level was significantly higher in BRZ-pretreated plants than water-pretreated plants. These results confirmed the role of BRs in innate immunity.
Virus replication and expression levels of water, 1 μM BL or 1 μM BRZ-pretreated Col-0 infected with CMV. a Western blotting analysis of coat protein accumulation of CMV at 3, 6 and 9 dpi. The systemic leaves were used for detection. Rubisco proteins were used as loading controls and were stained by Ponceau S. Experiments were repeated three times with similar results. b qRT-PCR analysis of replication levels of CMV at 3, 6 and 9 dpi. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Electrolyte leakage and TBARS content can indicate the degree of damage in plants caused by stresses. To confirm the effects of virus infection on membrane integrity, TBARS content and electrolyte leakage were determined after virus infections. As shown in Fig. 2, BRZ-pretreated plants had higher oxidative damages (Fig. 2a, b) and more cell-death occurrence than water-treated plants (Fig. 2c). By contrast, oxidative damages and cell-death occurrence in BL-pretreated plants were lower than those in water-treated plants. These results suggested that virus infection reduced the stability and integrity of the plasma membrane, and BRs were important for plant response to CMV.
TBARS content (a), electrolyte leakage (b) and cell death (c) of water, 1 μM BL or 1 μM BRZ-pretreated Col-0 infected with CMV. Leaf samples were harvested at 6 dpi with CMV inoculation. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
BRs alleviate photosystem damage under CMV stress
Most viruses have harmful effects on various biological processes in plants, including the photosystem II (PSII). Chlorophyll fluorescence measurements showed that the PSII photochemical efficiency was decreased by viral infection (Hodgson et al. 1989; Balachandran et al. 1994; Rahoutei et al. 2000; Guo et al. 2005). F v/F m and фPSII are indicators of PSII photochemistry. To analyze the effects on photosynthetic efficiency, we compared the photochemical efficiency of PSII in the dark-adapted state. As shown in Fig. 3a and b, compared with water-treated plants, CMV-induced reduction of F v/F m was less in BL-pretreated plants but greater in BRZ-pretreated plants. The фPSII results showed a similar trend (Fig. 3c, d). Taken together, these results indicated that BRs played a protective role in plant photosystem against CMV infection.
Chlorophyll fluorescence parameters of water, 1 μM BL or 1 μM BRZ-pretreated Col-0 infected with CMV at 6 dpi. Images of Fv/Fm (a), фPSII (c). The false color code depicted at the bottom of the image ranged from 0 (black) to 1.0 (purple). Five plants were used for each treatment and the picture of one representative plant is shown. b and d Average values for the respective chlorophyll fluorescence images. Bars represent mean and standard deviation of values obtained from five biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Involvement of antioxidant system in BRs-induced CMV defense
Then, we investigated whether antioxidant systems were involved in the BRs-induced CMV defense response. We analyzed antioxidants which protect cells from oxidative damage by scavenging reactive oxygen species (ROS). In this work, the enzyme activity of several antioxidative enzymes, such as SOD, POD, CAT and APX, was detected. As shown in Fig. 4, all antioxidant enzymes activity increased in BL-pretreated plants. The activities of these enzymes, however, were little changed in BRZ-pretreated plants. Viral infection increased the activity of SOD, POD, CAT and APX. Again, the increasing amplitude of all these antioxidant enzymes activity were higher in BL-pretreated plants, compared with water or BRZ-pretreated plants. All these results indicated that BRs-induced CMV defense was related to antioxidant system.
Antioxidant enzymes activities of water, 1 μM BL or 1 μM BRZ-pretreated Col-0 infected with CMV. Leaf samples were harvested at 6 dpi with CMV inoculation, and the activities of antioxidant enzymes were analyzed. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Defense-associated genes expression in response to BRs Levels
To analyze the underlying molecular mechanisms for BR-induced stress tolerance to CMV, we examined the effects of BRs levels on expression of several genes involved in the defense response. Transcripts of two transcription factors WRKY30 (Peng et al. 2012), WRKY33 (Zheng et al. 2006; Lippok et al. 2007; Birkenbihl et al. 2012), pathogen-responsive mitogen-activated protein kinases MAPK3 and MAPK6 (Mao et al. 2011), salicylic acid (SA) responsible resistance protein PR1 and PR2 (Spoel et al. 2003, 2009; Durrant and Dong 2004), ROS-related resistance protein glutathione S-transferase (GST) (Uquillas et al. 2004), and Phenylalanine ammonia-lyase (PAL) (Dixon and Paiva 1995; MacDonald and D’Cunha 2007) in which activity respond to various stresses, were detected. As shown in Fig. 5, transcripts of all these resistance genes were significantly increased in BL-treated plants and almost had little change in BRZ-pretreated plants compared with water-treated plants. In CMV infection plants, compared with water-pretreated plants, expression of all defense genes was upregulated obviously upon treatment with BL but downregulated after BRZ treatment. Among the eight genes, reductions of PR1, PR2, MAPK3, MAPK6 and WRKY30 expression by BRZ were more obviously compared with water-pretreated plants.
Defense gene expression levels in Col-0 were detected by qRT-PCR analysis at 6 dpi. Water pretreatment with purified water, BL pretreatment with 1 μM BL, BRZ pretreatment with 1 μM BRZ. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
BR signaling pathway plays an essential role in the BRs-enhanced CMV stress tolerance
To determine the possible role of BR signaling pathway in the BRs-enhanced CMV stress tolerance, the BR-insensitive mutant bri1-5 and gain-of-function mutant bes1-D were used to analyze their possible roles in antiviral defenses. Our results suggested that bri1-5 was more susceptible to CMV infection than the wild-type plants WS-2. bri1-5 showed more serious symptoms [higher oxidative damages (Fig. 6a, b), more cell-death occurrence (Fig. 6c), and more compromised in PSII photochemistry (Fig. 7)] than the wild-type plants after virus infection. Furthermore, expression of most resistance genes (Fig. 8) and antioxidant enzymes activity (Fig. 10) cannot be induced after the infections in bri1-5, thus resulting in more virus accumulation as compared with WS-2 after inoculation (Fig. 9). However, bes1-D adapted to virus infections better, which showed a lighter symptom and had less cell-death occurrence (Fig. 6). Resistance gene transcripts (Fig. 8) and antioxidant enzymes activity (Fig. 10) were significantly higher in bes1-D compared with those in Col-0. Moreover, the accumulation levels of CMV were both delayed and decreased in bes1-D plants as compared with the wild-type plants (Fig. 9). These data further suggest that BR signaling pathway plays an important role in plant systemic resistance against CMV.
TBARS content (a), electrolyte leakage (b) and cell death (c) of Col-0, bes1-D, WS-2, bri1-5 plants at 6 dpi with CMV inoculation. bri1-5 is in the WS-2 background. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Chlorophyll fluorescence parameters of Col-0, bes1-D, WS-2, bri1-5 plants at 6 dpi with CMV inoculation. Images of Fv/Fm (a), фPSII (c). The false color code depicted at the bottom of the image ranged from 0 (black) to 1.0 (purple). Five plants were used for each treatment and the picture of one representative plant is shown. b, d Average values for the respective chlorophyll fluorescence images. Bars represent mean and standard deviation of values obtained from five biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Resistance gene expression levels of Col-0, bes1-D, WS-2, bri1-5 plants after CMV inoculation. Gene expression levels were detected by qRT-PCR analysis at 6 dpi. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Virus replication and expression levels of Col-0, bes1-D, WS-2, bri1-5 after CMV inoculation. a Western blotting analysis of coat protein accumulation of CMV at 3, 6 and 9 dpi with CMV inoculation. The systemic leaves were used for detection. Rubisco proteins were used as loading controls and were stained by Ponceau S. Experiments were repeated three times with similar results. b qRT-PCR analysis of CMV replication levels at 3, 6 and 9 dpi with CMV inoculation. Bars represent mean and standard deviation of values obtained from three biological replicates. Significant differences (P < 0.05) are denoted by different lowercase letters
Discussion
Effects of BRs on plant growth and developmental processes such as cell expansion and division, senescence, vascular development, photomorphogenesis and environment stress responses were well studied. But exploration of BRs in plant virus resistance is rarely reported. Plant viral disease is one of the most important factors affecting yield and quality of agricultural products (Rodoni 2009). It is urgent to seek new effective ways to control plant viruses. In this study, we showed that BRs could be used in control of plant viruses.
Our experiments showed that BL treatment increased but BRZ treatment reduced CMV resistance in Arabidopsis (Fig. 1). This result confirmed a positive of BRs in virus tolerance. Previous studies indicated BRs-enhanced generation of H2O2 and induced antioxidative enzymes activity (Xia et al. 2009, 2011). In our study, we found BRs elevated antioxidative enzymes activities (Fig. 4) and defense-associated genes expression (Fig. 5), which were involved in the CMV tolerance. These observations indicated that BRs might participate in variety of physiological functions that activate defense system and enable plants to acquire resistance. Systemic acquired resistance is characterized by micro-oxidative bursts, accumulation of SA, and expression of pathogenesis-related (PR) genes such as PR1 and PR2 in systemic tissues (Kwon et al. 2009). The present study showed that the expression of PR1 and PR2 was induced after BRs treatment, which was accompanied by the activation of systemic resistance to CMV.
BRs are known to regulate numbers of genes. Among them, there are a cluster of genes involved in stress resistance. Interestingly, we found that a transcription factor WRKY30 is induced by BL treatment (Fig. 5). Previous study indicated constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice (Peng et al. 2012). Recent ChIP-chip studies have indicated that WRKY30 was induced by BL and was a direct target of BES1 and BZR1 (Sun et al. 2010; Yu et al. 2011). So we speculated BR signaling regulate defense-associated genes through some transcription factors such as WRKY30.
BR signal through receptor kinase BRI1, co-receptor BAK1 and other components to modulate BES1 and BZR1 family transcription factors modulate thousands of target genes including certain stress-responsive genes. Previous studies indicated that BR signaling components play essential roles in innate immunity, while most studies focused on the role of BAK1. Indeed, besides its role in BR signaling, BAK1 is also involved in the regulation of microbe-induced cell death (Kemmerling et al. 2007), and interacts with various pattern recognition receptors (PRRs), including the flagellin receptor FLS2, to drive Pathogen-triggered immunity (PTI) (Albrecht et al. 2012; Belkhadir et al. 2012). In rice, OsSERK1, an ortholog of BAK1, displayed a positive regulator in disease resistance (Hu et al. 2005). We previously reported that BR signaling positive regulators BAK1 and BKK1 are essential for plants in CMV resistance (Yang et al. 2010). Here, we reported BR signaling receptor BRI1 and transcription factor BES1, which were involved in plant growth regulation, played positive roles in virus resistance. In our study, we found loss-of-function of BRI1 (in bri1-5) reduced CMV resistance but active-of-function of BES1 (in bes1-D) enhanced CMV tolerance. Furthermore, activities of antioxidative enzymes and transcripts of defense-related genes were downregulated in bri-5 but upregulated in bes1-D (Figs. 9, 10). So the activated defense system by BR signaling may contribute to virus resistance in plants. In addition, whether other BR signaling-regulated pathways which were downstream of BRI1 are involved in BRs-induced virus resistance need further research.
In summary, our results demonstrated CMV resistance was upregulated by BRs treatment. And BR signaling was needed for this BRs-induced CMV tolerance. Our studies, therefore, offered a hypothesis that BR signaling control virus resistance through regulating antioxidative enzymes and some defense-related genes, such as WRKY30. Though the mechanism needs further study, our study contributes to the understanding of signaling cascades mediated by BRs in response to virus.
Author contribution
D.-W. Zhang and H.-H Lin conceived the study and designed the experiments. D.- W. Zhang, X.-G Deng and F.-Q Fu performed most of the experiments. D.-W. Zhang, X.-G. Deng and H.-H Lin wrote the paper.
Abbreviations
- BR:
-
Brassinosteroid
- BL:
-
Brassinolide
- BRZ:
-
Brassinazole
- BRI1:
-
BRASSINOSTEROID-INSENSITIVE1
- BES1:
-
BRI1 EMS SUPRESSOR 1
- CMV:
-
Cucumber mosaic virus
- ROS:
-
Reactive oxygen species
- Dpi:
-
Days postinoculation
References
Acharya BR, Assmann SM (2009) Hormone interactions in stomatal function. Plant Mol Biol Rep 69:451–462
Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Rathjen JP, Chinchilla D, Vries SC, Zipfel C (2012) Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc Natl Acad Sci USA 109:303–308
Balachandran S, Osmond CB, Makino A (1994) Effects of two strains of tobacco mosaic virus on photosynthetic characteristics and nitrogen partitioning in leaves of Nicotiana tabacum cv Xanthi during photoacclimation under two nitrogen nutrition regimes. Plant Physiol 104:1043–1050
Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J (2012) Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. P Natl Acad Sci USA 109:297–302
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Birkenbihl RP, Diezel C, Somssich IE (2012) Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol 159:266–285
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choudhary SP, Bhardwaj R, Gupta BD, Dutt P, Gupta RK, Biondi S, Kanwar M (2010) Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol Plant 140:280–296
Choudhary SP, Kanwar M, Bhardwaj R, Gupta B, Gupta R (2011) Epibrassinolide ameliorates Cr(VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 84:592–600
Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111:671–678
Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen Z, Yu JQ (2011) Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ 34:347–358
Culbreath A, Todd J, Brown S (2003) Epidemiology and management of tomato spotted wilt in peanut. Annu Rev Phytopathol 41:53–75
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. N Biotechnol 26:131–136
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Durrant W, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA Gen Subj 990:87–92
Guo DP, Guo YP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ (2005) Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci 168:57–63
Guo H, Li L, Aluru M, Aluru S, Yin Y (2013) Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol 16:545–553
Hayat S, Maheshwari P, Wani AS, Irfan M, Alyemeni MN, Ahmad A (2012) Comparative effect of 28 homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol Biochem 53:61–68
He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17:1109–1115
Hodgson RA, Beachy RN, Pakrasi HB (1989) Selective inhibition of photosystem II in spinach by tobacco mosaic virus: an effect of the viral coat protein. FEBS Lett 245:267–270
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225:353–364
Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C (2007) The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17:1116–1122
Kwon SJ, Jin HC, Lee S, Nam MH, Chung JH, Kwon SI, Ryu CM, Park OK (2009) GDSL lipase-like 1 regulates systemic resistance associated with ethylene signaling in Arabidopsis. Plant J 58:235–245
Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272:398–401
Lippok B, Birkenbihl RP, Rivory G, Brümmer J, Schmelzer E, Logemann E, Somssich IE (2007) Expression of AtWRKY33 encoding a pathogen-or PAMP-responsive WRKY transcription factor is regulated by a composite DNA motif containing W box elements. Mol Plant Microbe Interact 20:420–429
MacDonald MJ, D’Cunha GB (2007) A modern view of phenylalanine ammonia lyase. Int J Biochem Cell B 85:273–282
Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23:1639–1653
Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Peng X, Hu Y, Tang X, Zhou P, Deng X, Wang H, Guo Z (2012) Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta 236:1485–1498
Rahoutei J, García-Luque I, Barón M (2000) Inhibition of photosynthesis by viral infection: effect on PSII structure and function. Physiol Plant 110:286–292
Rodoni B (2009) The role of plant biosecurity in preventing and controlling emerging plant virus disease epidemics. Virus Res 141:150–157
Shang J, Xi DH, Xu F, Wang SD, Cao S, Xu MY, Zhao PP, Wang JH, Jia SD, Zhang ZW (2011) A broad-spectrum, efficient and non-transgenic approach to control plant viruses by application of salicylic acid and jasmonic acid. Planta 233:299–308
Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K (2003) NPR1 modulates cross-talk between salicylate-and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770
Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X (2009) Proteasome-mediated turnover of the transcription co-activator NPR1 plays dual roles in regulating plant immunity. Cell 137:860
Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19:765–777
Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85:171–182
Uquillas C, Letelier I, Blanco F, Jordana X, Holuigue L (2004) NPR1-independent activation of immediate early salicylic acid-responsive genes in Arabidopsis. Mol Plant Microbe Interact 17:34–42
Wang SD, Zhu F, Yuan S, Yang H, Xu F, Shang J, Xu MY, Jia SD, Zhang ZW, Wang JH, Xi DH, Lin HH (2011) The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 234:171–181
Xi D, Feng H, Lan L, Du J, Wang J, Zhang Z, Xue L, Xu W, Lin H (2007) Characterization of synergy between Cucumber mosaic virus and Tobacco necrosis virus in Nicotiana benthamiana. J Phytopathol 155:570–573
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Xia XJ, Zhou YH, Ding J, Shi K, Asami T, Chen Z, Yu JQ (2011) Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol 191:706–720
Xu F, Zhang DW, Zhu F, Tang H, Lv X, Cheng J, Xie HF, Lin HH (2012) A novel role for cyanide in the control of cucumber (Cucumis sativus L.) seedlings response to environmental stress. Plant Cell Environ 35:1983–1997
Yang H, Gou X, He K, Xi D, Du J, Lin H, Li J (2010) BAK1 and BKK1 in Arabidopsis thaliana confer reduced susceptibility to turnip crinkle virus. Eur J Plant Pathol 127:149–156
Yin Y, Wang ZY, Mora Garcia S, Li J, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109:181–191
Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65:634–646
Zhang S, Cai Z, Wang X (2009) The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc Natl Acad Sci USA 106:4543–4548
Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH (2010) Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 33:2121–2131
Zheng Z, Qamar SA, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48:592–605
Zhu JY, Sae Seaw J, Wang ZY (2013) Brassinosteroid signalling. Development 140:1615–1620
Acknowledgments
We thank Dr Yanhai Yin (Iowa State University, Ames, USA) for providing WS-2, bri1-5 and bes1-D seedlings. This study was supported by the National Natural Science Foundation of China (91417305, 31470342 and 31400211), the National Basic Research Program of China (973 Program) (2015CB150100) and the Doctoral Foundation of the Ministry of Education (20120181130008, 20110181110059).
Author information
Authors and Affiliations
Corresponding author
Additional information
D.-W. Zhang and X.-G. Deng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, DW., Deng, XG., Fu, FQ. et al. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 241, 875–885 (2015). https://doi.org/10.1007/s00425-014-2218-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2218-8