Abstract
Osteoporosis may affect young individuals, albeit infrequently. In childhood, bone mass increases, reaching its peak between the second and third decades; then, after a period of stability, it gradually declines. Several conditions, including genetic disorders, chronic diseases, and some medications, can have an impact on bone homeostasis. Diagnosis in young patients is based on the criteria defined by the International Society for Clinical Densitometry (ISCD), published in 2013. High risk factors should be identified and monitored. Often simple interventions aimed to eliminate the underlying cause, to minimize the negative bone effects linked to drugs, or to increase calcium and vitamin D intake can protect bone mass. However, in selected cases, pharmacological treatment should be considered. Bisphosphonates remain the main therapeutic agent for children with significant skeletal fragility and are also useful in a large number of other bone conditions. Denosumab, an anti-RANKL antibody, could become a potential alternative treatment. Clinical trials to evaluate the long-term effects and safety of denosumab in children are ongoing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although more commonly associated with aging, osteoporosis may affect younger individuals (children and adolescents). |
A range of risk factors include non-modifiable factors (gender, ethnicity) as well as potentially modifiable factors (hypovitaminosis D, poor nutrition, previous fracture, immobility, inflammatory states, physical activity and delayed puberty). |
Bisphosphonates are the main therapeutic agents for children with significant skeletal fragility and are also used in a number of other conditions. |
Clinical trials of the anti-RANKL monoclonal antibody therapy denosumab (currently approved in adults) are ongoing to determine its long-term efficacy and safety in pediatric patients. |
1 Introduction
Bone is a complex and highly dynamic tissue, consisting of organic and inorganic components, characterized by a continuous structural remodeling of synthesis and destruction influenced by different intrinsic and extrinsic factors such as genetics, hormones, diet, and mechanical loading. In childhood, bone mass increases, reaching its peak between the second and third decades then, after a period of stability, it gradually declines. The word osteoporosis literally signifies ‘porous bone’. In this condition, impaired bone formation and/or excessive bone loss, as well as microarchitecture deterioration, reduce the mechanical bone behavior, increasing the risk of fracture.
Osteoporosis is usually the result of the aging process that compromises the regenerative bone potential, predisposing to a negative balance [1]. However, several other conditions, including genetic disorders, chronic diseases, and some medications, may negatively affect bone homeostasis. Therefore, although infrequently, osteoporosis can also occur in childhood, often as a secondary form, sometimes as an idiopathic one [2, 3].
The diagnosis in young patients is based on the criteria defined in the revised pediatric position paper by the International Society for Clinical Densitometry (ISCD), published in 2013 [4]. Both a clinically significant fracture history and a bone density deficiency are required, thus limiting over-diagnosis and treatments based on dual-energy X-ray absorptiometry (DXA) measurements alone. High-risk children, even if they are asymptomatic, need to be monitored for bone health to prevent fractures and related complications. Often simple interventions aimed to eliminate the underlying cause, to minimize the negative bone effects linked to drugs, or to increase calcium and vitamin D intake can protect bone from deterioration. However, in selected cases bone-sparing medications should be considered [5].
In the present review, we report the definition of osteoporosis in the pediatric setting, review the underlying etiopathogenesis and diagnosis, and focus on treatment strategies.
2 Etiology
Several risk factors with different mechanisms of action can have an impact on bone mass acquisition and bone microarchitecture during childhood, leading to juvenile osteoporosis (JO). Among them, gender and ethnicity [6] are the most relevant non-modifiable risk factors, whereas hypovitaminosis D [7], poor nutrition, previous fractures, immobility, inflammatory states, physical activity, and delayed puberty should be taken into account as modifiable risk factors [8].
JO is divided into primary and secondary forms. In primary JO, genetic conditions compromising skeletal maturation represent the main cause of bone fragility [9]. Osteogenesis imperfecta (OI), a heterogeneous group of connective tissue disorders, is the most common inherited form of primary JO, characterized by increased bone fragility, low bone mineral density (BMD) and extra-skeletal involvement with blue sclerae, hearing loss, and dental abnormalities [10, 11]. The clinical severity varies widely from being nearly asymptomatic with a mild predisposition to fractures, normal stature and normal lifespan, to disabling and even lethal presentations. Currently, 21 genetic variants have been described [12]. COL1A1 and COL1A2 genes, encoding the α1 and α2 chains of collagen 1, are the most commonly mutated, driving up to 90% of OI prevalence [10]. Different clinical phenotypes are caused by genetic defects compromising collagen structure or post-translational modifications affecting bone mineralization. Recently, new genetic forms of childhood-onset primary osteoporosis such as WNT1 and PLS3 mutations have been defined [13]. These new findings led to a novel molecular and pathogenetic classification, revised by the Nosology Committee of the International Skeletal Dysplasia Society in 2019 [14]. Accordingly, the diagnosis of genetic forms of JO are based on clinical presentation confirmed by genetic tests. By contrast, juvenile idiopathic osteoporosis (JIO) is a rare condition affecting prepuberal patients without a clear genetic predisposing etiology, characterized by acute onset and wide clinical spectrum, ranging from radiological evidence of osteoporosis to multiple vertebral and metaphyseal fractures, with complete recovery within 3–4 years [15, 16].
Several systemic diseases and some medications can lead to secondary JO. For example, rheumatic disorders are tightly associated with bone mass loss secondary to systemic inflammation and corticosteroid therapy. In fact, inflammatory cytokines, such as IL-1, IL-6, and TNF-α, lead to the upregulation of receptor activator of nuclear factor kappa-B ligand (RANK-L), promoting osteoclastogenesis and bone resorption [17]. A relevant association between the reduction of BMD and juvenile idiopathic arthritis (JIA), juvenile systemic lupus erythematosus, or juvenile dermatomyositis was described [18,19,20,21,22]. In addition, rheumatic patients often develop glucocorticoid-induced osteoporosis (GIO), dramatically contributing to secondary osteoporosis [23]. Indeed, glucocorticoids directly enhance bone resorption via RANK-L signal stimulation and inhibit osteoblastogenesis by blocking Wnt/β-catenin [24]. Moreover, glucocorticoids indirectly interfere with vitamin D and calcium metabolism [25]. In a cohort of 136 rheumatic patients treated with a 3-year course of glucocorticoid therapy, a higher daily average dose and a longer duration of glucocorticoids were associated with increased risk of incident vertebral fractures [26]. In addition, malabsorption due to inflammatory bowel diseases and celiac disease was also found to be related to delayed bone maturation and osteoporosis in childhood [27,28,29]. Besides, in accordance with the mechanostat theory, a low BMD secondary to the lack of mechanical stimuli was described in children affected by neuromuscular disorders [30, 31].
3 Diagnosis
The current diagnostic criteria of JO were established in 2013 according to the ISCD. The presence of a non-traumatic vertebral compression fracture (> 20% loss of vertebral height ratio) regardless of BMD or the coexistence of a history of clinically significant fractures (≥ 2 long bone fractures by the age of 10 years, or ≥ 3 long bone fractures by 19 years) with a BMD Z-score of ≤ − 2.0 are mandatory for the diagnosis of JO [4]. According to these recommendations, densitometry criteria alone are not adequate in the diagnostic work-up of JO. Indeed, the occurrence of fragility fractures with concomitant BMD Z-scores > − 2.0 was found in children with osteopenia-inducing diseases, such as leukemia, neuromuscular disorders, or rheumatic disorders [26, 32,33,34]. A further limitation of BMD assessment is the disparity in Z-scores generated by different pediatric reference databases. In 2015, the Canadian STOPP Consortium observed a significant disparity among different BMD Z-score databases used in a cohort of 186 children with leukemia and vertebral fractures, upholding the lack of validity of the BMD Z-score threshold alone in the definition of JO [32]. Moreover, areal BMD (g/cm2) can underestimate volumetric BMD (g/cm3) in children with short stature and overestimate BMD in taller ones. The use of bone mineral apparent density (BMAD) and height-for-age Z-score (HAZ) BMD represent valid tools to minimize stature impact on BMD [35]. Peripheral quantitative computed tomography (pQCT) may provide further advantage compared with DXA, as the measures obtained by this three-dimensional technique are not influenced by bone size. Furthermore, pQCT is capable of evaluating trabecular and cortical bone distinctly. However, the use of pQCT remains confined to the research field due to lack of reference data and scanning acquisition consensus [36]. Total body less head and lumbar spine are the preferred regions of interest for DXA assessment in pediatric patients as confirmed by ISCD in 2013 [36]. On the other hand, DXA assessment at other skeletal sites such as distal forearm, proximal femur, and lateral distal femur were suggested in patients with severe scoliosis or other skeletal anatomical disorders, according to updated 2019 guidelines [37]. On these bases, a careful diagnostic approach is primarily based on an accurate clinical evaluation including fracture location (with particular attention to vertebral fracture surveillance), magnitude of trauma, family history, and the presence of other risk factors. Recently, Ward et al. proposed an algorithm for the differential diagnosis of osteoporosis in children, which aims to explore genetic and metabolic defects, as well as underlying acute or chronic illnesses [2].
4 Treatment
4.1 General Measures and Prevention
Prevention strategies and removal of modifiable risk factors are the first measures to reduce bone mass loss. During intrauterine life, many factors (e.g., vitamin D status, endocrine problems, placental defects, smoking, alcohol consumption, caffeine intake) can lead to impaired skeletal mineralization and consequentially influence future peak bone mass and fracture risk. Interactions between the genome and early maternal environment may play a key role in bone physiology. It has been previously demonstrated that low birth weight, fetal growth restriction and poor childhood growth are important determinants of bone mineral content (BMC) [38, 39]. There is evidence on maternal vitamin D status during pregnancy and its association with bone outcomes in children. A double-blinded, randomized clinical trial (RCT) involving 623 pregnant women demonstrated that high-dose vitamin D supplementation (2800 IU/d) in pregnancy improved offspring bone mineralization (BMC and BMD) up to 6 years of age compared with the standard dose (400 IU/d) [40]. These results suggest that an optimization of maternal nutrition and a recommended vitamin D gestational intake should be included within preventive strategies. It is clear that, also in pediatric populations, vitamin D and calcium intake are two fundamental elements for bone health and are strongly related to BMD [41]. Vitamin D insufficiency is common in pediatric patients with primary and secondary osteopenia or osteoporosis and secondary hyperparathyroidism may contribute to bone mass loss [7]. Supplementation of vitamin D should be a priority in the management of pediatric patients with risk factors for osteoporosis or in vitamin D deficient children because it has been well demonstrated that a proper supplementation increases BMC and guarantees osteo-protection [42,43,44]. In contrast, vitamin D supplementation for healthy children with low BMD is not recommended [45]. Physical activity is another preventive strategy that can have a positive outcome on bone remodeling [46,47,48] and improve bone geometry [49]. An improvement in BMD parameters was observed in patients with JIA [50] and dermatomyositis after a 12-week supervised exercise program [51]. Finally, in patients with chronic inflammatory diseases, good control of inflammation and low disease activity result in an improvement of bone status, as reported with anti-TNF drugs in children with JIA [52, 53]. More studies are available for adult populations [54,55,56]. Regarding methotrexate, there are conflicting reports concerning bone toxicity [57,58,59,60,61]. A study of 32 children affected by JIA on therapy with methotrexate showed that low-dose treatment does not induce osteopenia, but can improve BMD, probably controlling disease activity and blocking inflammation pathways [62].
4.2 Drug Treatment
4.2.1 Bisphosphonates
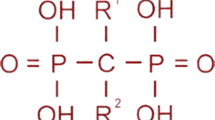
Bisphosphonates (BPs) are stable derivatives of inorganic pyrophosphate in which two phosphate groups are covalently linked to carbon group. They bind hydroxyapatite crystals and inhibit osteoclast activity. The affinity for bone matrix is conferred by the hydroxyl groups attached to the central carbon (R1 position) and by the adjacent phosphate groups, while potency for bone resorption is determined by the final structural fraction (in the R2 position). Based on the presence or not of nitrogen or amino groups in the R2 position, BPs can be classified as non–nitrogen-containing (first-generation BPs) or nitrogen-containing (second- and third-generation BPs). In first-generation BPs, a cytotoxic analog of adenosine triphosphate accumulates in osteoclasts and leads to cell death, whereas nitrogen-containing BPs promote osteoclast apoptosis inhibiting the activity of farnesyl pyrophosphate synthase. Skeletal retention of BPs depends on availability of hydroxyapatite binding sites [63]. BPs are hydrophilic medications with low gastrointestinal absorption, high distribution volume and renal excretion. After a rapid clearance from the circulation there is a long elimination phase due to a slow release from bone tissue [64]. This feature differentiates BPs from other antiresorptive drugs such as denosumab and makes side effects such as rebound hypercalcemia less likely. BPs have been used in children, following the evidence of efficacy in adults. Pamidronate, neridronate, and zoledronic acid are available for intravenous (IV) use, while alendronate and risedronate are available for oral administration. Most of the safety and efficacy reports concerning BPs in pediatric populations have been derived from studies on patients with OI.
Several studies in children with OI demonstrated increased BMD and reduced fracture risk using oral BPs [65, 66] and a randomized open-label trial showed that oral alendronate and IV pamidronate therapies are equally effective (lumbar spine BMD increase) in children with OI [67]. Oral BPs have also been used in other conditions, such as chronic inflammatory disorders, and they were effective in increasing lumbar spine BMD [68,69,70,71,72]. A recent prospective study in patients with Duchenne muscular dystrophy showed similar effect of zoledronic acid and alendronate in increasing bone mineral density and reducing bone loss [73]. Nevertheless, oral BPs have not shown the efficacy in inducing vertebral body reshaping after spine fractures [74,75,76] that can be seen with IV pamidronate [77, 78]. According to current evidence, oral BPs should be used only in patients with mild forms of OI without vertebral fractures. A recent double-blind RCT of pediatric patients with rheumatological disease with glucocorticoid-induced osteopenia showed a better improvement in lumbar spine BMD in patients receiving risedronate compared with patients treated with vitamin D supplementation [79]. However, prophylactic BP therapy (i.e., treating a low bone density in the absence of fracture) in secondary osteoporosis is not recommended currently [80]. Studies concerning oral BPs in children are summarized in Table 1 with a dosage scheme in Table 2.
In terms of IV BPs, pamidronate is the most commonly used in children. Dosage and timing of administration in the pediatric population are derived from the experience with OI. The standard dosage in children aged > 3 years consists of three infusions on consecutive days repeated every 4 months. In children under 3 years of age, the bone turnover is higher so the cycles should be closer. Pamidronate has been shown to be safe in this subgroup of patients as well [81, 82]. In children younger than 24 months, the standard dose of 0.5 mg/kg daily is repeated every 2–3 months [83]. In children aged < 1 years, a scheme of 0.5 mg/kg every 2 months was also used with good outcome [84]. Other protocols using a lower dose of pamidronate were proposed. Gandrud et al. suggest a single-day infusion of pamidronate (1 mg/kg) every 3 months. This uncontrolled observational trial of 11 children with osteoporosis (glucocorticoid-induced osteoporosis, OI, and idiopathic juvenile osteoporosis) showed an increase of spinal BMD and a reduction of fractures using low-dose BPs [85]. Another group reported efficacy of a single pamidronate infusion (30 mg if < 50 kg, 45 mg if > 50 kg) every 3 months [86]. A single retrospective study conducted in non-OI patients receiving IV pamidronate 1 mg/kg for 1 day every 3 months (4 mg/kg/year) or 1 mg/kg/day for 3 days every 4 months (9 mg/kg/year) showed a comparable increase in BMD and reduction in fragility fractures after 1 year of treatment [87]. The optimal dose of pamidronate to treat pediatric patients has not been established yet, especially for those patients with secondary osteoporosis. Large trials are needed to delineate the minimal effective dose in these patients.
The standard infusion scheme for neridronate is 1–2 mg/kg/day in a single infusion every 3–4 months [88,89,90]. Neridronate has proved effective in increasing BMD in OI patients. Idolazzi et al. found no statistically significant effect on fracture risk between OI patients treated with neridronate versus non-treated patients, although a significant reduction was observed in the mean number of fractures occurring during treatment compared with pre-treatment values [89].
For zoledronic acid, which has the highest potency among BPs, data on pediatric populations show good outcomes in terms of BMD gain and fracture rates [91]. It has also been shown to be effective in promoting vertebral reshaping [92]. The initial dose of 0.0125 mg/kg is followed by a second dose 6 weeks later of 0.0375 mg/kg [92]. A recent study comparing the efficacy of pamidronate and zoledronic acid in 40 patients with OI showed no differences between the two groups in terms of spine BMD gain and fracture rate, following 1 and 2 years of treatment [93].
Currently, neridronate is approved by the regulatory agencies (US Food and Drug Administration [FDA] and European Medicines Agency [EMA]) for use in children with OI. The IV BPs dosage schedule is summarized in Table 3. Numerous studies reassure about the safety of BPs in pediatric populations, though the majority of data are short-term. A recent retrospective study conducted in 228 pediatric patients treated with zoledronic acid showed good efficacy and safety profile [94]. The most common side effect of IV BPs (85% of patients) is the acute phase reaction, which occurs typically within 72 h from the first or second IV administration and is characterized by flu-like symptoms that respond to paracetamol or NSAIDs [95]. The acute phase reaction usually does not recur at subsequent infusions and is not an indication to stop treatment. It appears that this side effect is not dose related [96]. Hypocalcemia is another common adverse event that occurs within a few days after infusion (74% patients) [95]. Munns et al. suggested that reducing the initial zoledronic acid dose (0.0125 mg/kg instead of 0.02–0.025 mg/kg) could be effective in reducing incidence and intensity of hypocalcemia [96]. In patients with low vitamin D levels, BPs may be responsible for symptomatic hypocalcemia. To minimize the risk, it is important to ensure adequate vitamin D/calcium supplementation, especially in the days following infusion [97]. In a growing skeleton, BPs determine a typical radiological finding called ‘zebra lines’. These are transversal linear bands of increased density as a result of alternative phases of denser bone deposition and normal bone mineralization [98]. These alterations are harmless and do not induce morphological changes or have consequences for bone growth [99, 100]. BP-induced transverse lines disappear with time, supporting the view that these lines represent horizontal trabeculae that undergo remodeling [101]. Major adverse events affecting the adult population (e.g., atrial fibrillation, kidney injury, and esophageal ulceration) are not reported in children [88, 102, 103]. Osteonecrosis of the jaw after BPs in the pediatric population is a rare complication with only one report in the literature of a 15-year-old girl consequent to alendronate therapy [104]. In past years there was much concern regarding fracture healing during BP therapy. However, available evidence indicates normal fracture healing with slightly delayed osteotomy healing [105, 106]. Atypical femoral fracture has been described only in an 18-year-old male treated with IV pamidronate for 7 years, then risedronate for 2 years for X-linked osteoporosis [107], and in a 21-year-old male with OI diagnosis treated with BPs during adolescence [108]. Acquired osteopetrosis following BP therapy has also been described [109, 110]. Long-term effects of BP treatment are still unknown and data on the optimum duration of treatment with BPs are lacking. The general suggestion is to discontinue treatment after reaching a good clinical response, especially in patients with transient or modifiable risk factors [97]. In patients with persistent risk for fractures, it is recommended to continue treatment until definitive height is attained [111]. Another problem is the potential teratogenic effects of BPs; indeed, these agents can cross the placenta, resulting in fetal exposure. Up to now, only minor adverse effects have been described in newborns of pregnant woman treated with BPs and a recent study suggested that BPs have no major teratogenic effects. In this study, the rates of neonatal complications and spontaneous abortions were increased in women with bone/systemic diseases treated with BPs but this is more likely to be linked to the severity of the underlying diseases and concomitant medications rather than to antiresorptive therapy [112]. Due to the lack of definitive data, contraceptive treatment should be prescribed to adolescent girls before starting BP therapy.
4.2.2 Denosumab
Denosumab is a fully human monoclonal antibody directed against RANKL, preventing RANKL/RANK interaction on the osteoblast, which leads to the inhibition of osteoclast formation, function, and survival. It can be very useful in inflammatory diseases since cytokines and glucocorticoids can up-regulate RANK expression on osteoclast precursors and promote bone resorption [113, 114]. In children, denosumab was originally used in patients with OI or in other bone diseases (see Table 4). The pharmacokinetic and pharmacodynamic profile of denosumab in children have not been assessed yet. In dose ranging studies in adults, denosumab exhibited non-linear, dose-dependent pharmacokinetics, with lower clearance at higher doses or concentrations, and its metabolism and elimination are expected to follow the immunoglobulin clearance pathways [115]. Furthermore, the clearance seems to depend on the amount of available RANKL [116]. In the pediatric population, the increased skeletal turnover and the amount of RANKL produced by children at different ages may have an impact on pharmacokinetics [117]. Therefore, if RANKL is expressed at high concentration, the antibody will be eliminated rapidly at the standard dose of 1 mg/kg [118]. However, pediatric dosage and dosing intervals of monoclonal antibodies in children are generally readjusted taking into account body weight and surface area [119]. In patients with OI unresponsive to BP treatment, denosumab resulted in higher BMD and in a decreased fracture incidence in some studies [120,121,122,123]. The dosage used in OI patients varies from 1 mg/kg every 12 weeks to 1 mg/kg every 6 months. Hoyer-Kuhn et al. made a retrospective evaluation of an individualized biomarker-associated treatment regimen with denosumab in 10 children with classical OI who were followed for 1 year after their participation in a pilot trial (ClinicalTrials.gov identifier: NCT01799798). After a treatment period of 1 year with a fixed dose interval of 12 weeks, the following doses were given based on changes of urinary bone resorption markers. Denosumab was administered when bone resorption markers increased. In this study, increasing the intervals between drug administrations did not change vertebral shape despite a reduction of lumbar areal BMD [123]. In another study in four patients with OI, a decreased BMD was found in three out of four children after denosumab therapy but when the interval between denosumab injections was reduced, the lumbar spine-aBMD Z-score increased [118]. The suppression of bone resorption is reversible and a shorter interval seems more appropriate to ensure a constant suppression of bone resorption by osteoclasts [124]. Studies concerning OI patients are summarized in Table 4. Dosing regimens, efficacy, and side effects of denosumab for other pediatric conditions are summarized in Table 5.
Regarding safety, minor side effects such as hypocalcemia [120,121,122] have been reported. Rebound hypercalcemia is a potentially serious complication resulting from a rapid increase in bone resorption secondary to decrease in antiresorptive effect after withdrawal of denosumab and is well described in the literature. In previous reports, denosumab-associated hypercalcemia in children developed between 5.5 and 28 weeks after denosumab injection [118, 125,126,127,128,129,130,131]. This complication developed not only after treatment discontinuation but also in the interval between two denosumab injections. In accordance with this view, Trejo et al. noticed a rapid decrease in bone density when the interval between denosumab injections was extended to 6 months in two OI patients. Shortening of treatment intervals may be helpful to prevent hypercalcemia [118] but further studies are needed. No long-term data about the risk of nephrocalcinosis or calcification of coronary arteries later in life in OI patients with hypercalcemia or hypercalciuria are available. Other severe side effects like osteonecrosis of the jaw [129] have also been reported in two adolescents (aged 14 and 15 years) and a young adult (aged 40 years) received fixed-dose denosumab for giant cell tumor of bone. Radiographic appearance of zebra lines, similar to those observed with BP therapy, has also been described after denosumab administration [131,132,133]. Based on current data, growth seems to be unaffected [120,121,122, 134].
Clinical trials to evaluate the long-term effect and safety of denosumab therapy in pediatric patients are ongoing (see Table 6). At the moment, this drug is approved only in adults.
4.2.3 Specific Conditions
4.2.3.1 Osteogenesis Imperfecta
BPs are first-line therapy in children and adolescents affected by OI. They have been widely used over the years to treat OI and we currently have solid data on safety and efficacy derived through case series and RCTs. The efficacy of BPs in increasing BMD has been established by a Cochrane systematic review [135]. Data concerning fracture incidence during treatment are divergent. Three studies with oral BPs showed a reduction in relative risk or a tendency to decrease the frequency of bone fractures [65, 66, 74]. In contrast, six studies (three with oral and three with IV BPs) showed no statistically significant differences on fracture incidence between placebo and treated groups [75, 76, 90, 136,137,138]. The different treatment schemes, the small number of patients enrolled in these trials, and the differences between the various forms of OI may play an important role in explaining these discrepancies. On the other hand, no studies have reported an increased incidence of fractures with the use of BPs. Seikaly et al. reported a significant decrease in bone pain, evaluated with pain scores and the frequency of analgesic use at 12 months [66]. In other trials, no differences in bone pain between BPs and placebo were observed or pain scores were not assessed. Other treatments for OI have been proposed. A double-blind RCT involving 79 adult patients with OI demonstrated an increased areal and volumetric BMD in spine and hip in the group treated with the anabolic agent teriparatide compared with the placebo group [139]. Other evidence suggests that in patients affected by type I OI, teriparatide treatment is associated with a remarkable response in markers of bone formation [140]. Another study aimed at testing the safety and efficacy of teriparatide in patients over 18 years of age is in progress (NCT03735537). Denosumab has recently been used for the treatment of OI and seems to be very effective in increasing BMD (see Table 4).
4.2.3.2 Glucocorticoid-Induced Osteoporosis
Supplementation with vitamin D and calcium does not appear to be effective in preventing fragility fracture in patients with GIO [141, 142]. On the other hand, risedronate given preventively appears to increase BMD compared with no treatment or supplementation, but does not seem to prevent vertebral fracture progression [79]. Not all children with GIO are candidates for treatment; indeed, children who are younger and with transient glucocorticoid exposure are more likely to recover and, if they have a sufficient residual growth potential, the treatment is not necessary. In contrast, vertebral fractures are an absolute indication for BPs [3]. Since the principal manifestation of GIO is vertebral fracture, IV BPs are preferred rather than oral formulations. In fact, oral BPs have not shown efficacy in inducing vertebral body reshaping after spine fractures [74,75,76], as seen with IV pamidronate [77, 78]. In pediatric GIO, two non-randomized case-control trials (a total of 20 patients) have been performed [143, 144]. In these studies, the treated patients showed an increase of BMD Z-scores and no severe side effects were reported. Two uncontrolled studies of zoledronic acid in children with osteoporosis (including GIO) showed improvement in BMD and absence of vertebral fracture [92, 145]. In a retrospective observational study on seven boys (a total of 27 vertebral fractures) affected by Duchenne muscular dystrophy treated with glucocorticoids, IV pamidronate or zoledronic acid therapy were associated with improvement in back pain and in vertebral height ratios of previously fractured vertebral bodies. At the same time, such therapy did not appear to prevent the development of new vertebral fractures [146]. There are no consensus guidelines on when to start pharmacological therapy in children treated with high doses or for long periods with glucocorticoids. It has been suggested to start IV BPs prior to the first-ever fracture in patients at high risk [3]. A phase III, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of denosumab in pediatric subjects with GIO is ongoing (NCT03164928; active, not recruiting). In patients with rheumatic diseases, the inflammatory state can lead to bone mass loss, regardless of glucocorticoid therapy. In these patients, the principal strategy to prevent bone damage aims to control disease activity. In fact, reports in adults [54, 147, 148] and in children [52] showed that anti-TNF therapy may exert beneficial effects on bone metabolism and on bone mass acquisition.
4.2.3.3 Idiopathic Juvenile Osteoporosis
Although spontaneous remission occurs in patients with idiopathic juvenile osteoporosis, permanent bone deformities may occur. Several case reports showed the efficacy of BPs in terms of symptom resolution and improvement in bone parameters [149,150,151]. In an RCT (pamidronate vs placebo) [152] areal and volume BMD Z-scores were lower in untreated patients. During study follow-up, the incidence of new fractures was almost double in untreated compared with treated children. This study suggested that the spontaneous recovery of bone mineral status is unsatisfactory in patients with idiopathic juvenile osteoporosis and BPs can stimulate onset of the recovery phase, reducing fracture rate. Recently, a patient with vertebral spinal deformities was treated with alendronate, leading to clinical and radiological improvement [153]. No adverse events were described.
4.2.3.4 Osteoporosis-Pseudoglioma Syndrome
Osteoporosis-pseudoglioma syndrome (OPPG) is a rare autosomal recessive syndrome characterized by juvenile-onset osteoporosis and ocular abnormalities due to a low-density lipoprotein receptor-related protein 5 (LRP5) gene mutation. Based on a few published case reports, treatment with BPs improves BMD and bone pain in patients with OPPG syndrome [154,155,156,157,158]. However, Streeten et al. have demonstrated an intrinsic bone fragility despite an improvement in BMD during BP therapy. The normalization of DXA after long-term BP treatment did not correlate with the severe degree of bone fragility seen with quantitative computed tomography. In fact, they described four fractures (three femoral shafts) in three OPPG patients while on BPs, after achieving significant improvement in areal BMD [159]. These data are supported by a case series described by Papadopoulos et al. in which three of four patients with OPPG reported a fracture during BP therapy, despite an increase of BMD [160].
4.2.4 Future Treatments
Romosozumab, a humanized monoclonal antibody, promotes bone formation and inhibits bone resorption by inhibiting sclerostin, a protein involved in the regulation of bone formation. In the phase III FRAME and ARCH studies, romosozumab (210 mg once monthly) significantly reduced vertebral and clinical fracture risk versus placebo and oral alendronate in postmenopausal women with osteoporosis [161, 162]. In 2019, the FDA and EMA approved romosozumab for postmenopausal women with high risk of fracture. At the moment, a phase I, open-label, ascending multiple-dose study in children and adolescents (5–17 years old) with OI is recruiting. The aim of the study is to evaluate the pharmacokinetics, safety, tolerability, pharmacokinetics and pharmacodynamics of romosozumab in pediatric populations (NCT04545554).
Another interesting molecule is fresolimumab, an antibody that can block transforming growth factor beta (TGF-β). In studies in mice with OI, it has been shown that silencing TGF-β can lead to higher bone mass, quality, and strength [163, 164]. Fresolimumab is currently in a clinical trial in children with OI (NCT03064074).
Odanacatib, a cathepsin K inhibitor, was able to reduce the risk of fracture in the LOFT trial, but was associated with an increased risk of cardiovascular events, specifically stroke, in postmenopausal women with osteoporosis (NCT00529373) [165]. A study in a pediatric population had been planned but was cancelled following safety reports.
5 Conclusions
Although uncommon, osteoporosis may also involve young subjects. High-risk conditions should be identified and prevention strategies promptly undertaken. Management often requires a multidisciplinary team with experience in pediatric bone diseases. BPs remain the main therapeutic agent for children with significant skeletal fragility and are also useful in a large number of other conditions. Use of these agents should be managed in centers with expertise, since their long-term effects are not yet fully known.
References
Chandra A, Rajawat J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int J Mol Sci. 2021;22:3553.
Ward LM, Weber DR, Munns CF, Högler W, Zemel BS. A contemporary view of the definition and diagnosis of osteoporosis in children and adolescents. J Clin Endocrinol Metab. 2020;105:dgz294.
Ward LM. Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol. 2020;11:576.
Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom Off J Int Soc Clin Densitom. 2014;17:275–80.
Sakka SD, Cheung MS. Management of primary and secondary osteoporosis in children. Ther Adv Musculoskelet Dis. 2020;12:1759720X20969262.
Karlsland Åkeson P, Åkesson KE, Lind T, Hernell O, Silfverdal S-A, Öhlund I. Vitamin D intervention and bone: a randomized clinical trial in fair- and dark-skinned children at northern latitudes. J Pediatr Gastroenterol Nutr. 2018;67:388–94.
Bowden SA, Robinson RF, Carr R, Mahan JD. Prevalence of vitamin D deficiency and insufficiency in children with osteopenia or osteoporosis referred to a pediatric metabolic bone clinic. Pediatrics. 2008;121:e1585-1590.
Vierucci F, Saggese G, Cimaz R. Osteoporosis in childhood. Curr Opin Rheumatol. 2017;29:535–46.
Richards JB, Zheng H-F, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13:576–88.
Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe AD, et al. Osteogenesis imperfecta. Nat Rev Dis Primer. 2017;3:17052.
Forlino A, Marini JC. Osteogenesis imperfecta. Lancet Lond Engl. 2016;387:1657–71.
OMIM - Online Mendelian Inheritance in Man [Internet]. https://omim.org/ [cited 2021 Jun 28].
Kämpe AJ, Mäkitie RE, Mäkitie O. New genetic forms of childhood-onset primary osteoporosis. Horm Res Paediatr. 2015;84:361–9.
Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A. 2019;179:2393–419.
Lorenc RS. Idiopathic juvenile osteoporosis. Calcif Tissue Int. 2002;70:395–7.
Krassas GE. Idiopathic juvenile osteoporosis. Ann N Y Acad Sci. 2000;900:409–12.
Amarasekara DS, Yu J, Rho J. Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J Immunol Res. 2015;2015: 832127.
Huber AM, Ward LM. The impact of underlying disease on fracture risk and bone mineral density in children with rheumatic disorders: a review of current literature. Semin Arthritis Rheum. 2016;46:49–63.
Stagi S, Cavalli L, Signorini C, Bertini F, Cerinic MM, Brandi ML, et al. Bone mass and quality in patients with juvenile idiopathic arthritis: longitudinal evaluation of bone-mass determinants by using dual-energy X-ray absorptiometry, peripheral quantitative computed tomography, and quantitative ultrasonography. Arthritis Res Ther. 2014;16:R83.
Lim SHL, Benseler SM, Tyrrell PN, Charron M, Harvey E, Hebert D, et al. Low bone mineral density is present in newly diagnosed paediatric systemic lupus erythematosus patients. Ann Rheum Dis. 2011;70:1991–4.
Santiago RA, Silva CA, Caparbo VF, Sallum AME, Pereira RMR. Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use. Scand J Rheumatol. 2008;37:40–7.
Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, et al. RANKL:osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheumatol. 2007;56:977–83.
Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. 2014;44:47–54.
Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–83.
Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2019;30:1145–56.
LeBlanc CMA, Ma J, Taljaard M, Roth J, Scuccimarri R, Miettunen P, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30:1667–75.
Björck S, Brundin C, Karlsson M, Agardh D. Reduced bone mineral density in children with screening-detected celiac disease. J Pediatr Gastroenterol Nutr. 2017;65:526–32.
Bernstein CN, Leslie WD. Therapy insight: osteoporosis in inflammatory bowel disease—advances and retreats. Nat Clin Pract Gastroenterol Hepatol. 2005;2:232–9.
Bourges O, Dorgeret S, Alberti C, Hugot JP, Sebag G, Cézard JP. Low bone mineral density in children with Crohn’s disease. Arch Pediatr Organe Off Soc Francaise Pediatr. 2004;11:800–6.
Söderpalm A-C, Magnusson P, Ahlander A-C, Karlsson J, Kroksmark A-K, Tulinius M, et al. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord NMD. 2007;17:919–28.
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR. Bone health and osteoporosis management of the patient with duchenne muscular dystrophy. Pediatrics. 2018;142:S34–42.
Ma J, Siminoski K, Alos N, Halton J, Ho J, Lentle B, et al. The choice of normative pediatric reference database changes spine bone mineral density Z-scores but not the relationship between bone mineral density and prevalent vertebral fractures. J Clin Endocrinol Metab. 2015;100:1018–27.
Fiscaletti M, Coorey CP, Biggin A, Briody J, Little DG, Schindeler A, et al. Diagnosis of recurrent fracture in a pediatric cohort. Calcif Tissue Int. 2018;103:529–39.
Henderson RC, Berglund LM, May R, Zemel BS, Grossberg RI, Johnson J, et al. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25:520–6.
Crabtree NJ, Shaw NJ, Bishop NJ, Adams JE, Mughal MZ, Arundel P, et al. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults—the ALPHABET study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2017;32:172–80.
Adams JE, Engelke K, Zemel BS, Ward KA, International Society of Clinical Densitometry. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom Off J Int Soc Clin Densitom. 2014;17:258–74.
Weber DR, Boyce A, Gordon C, Högler W, Kecskemethy HH, Misra M, et al. The utility of DXA assessment at the forearm, proximal femur, and lateral distal femur, and vertebral fracture assessment in the pediatric population: 2019 ISCD official position. J Clin Densitom Off J Int Soc Clin Densitom. 2019;22:567–89.
Beltrand J, Alison M, Nicolescu R, Verkauskiene R, Deghmoun S, Sibony O, et al. Bone mineral content at birth is determined both by birth weight and fetal growth pattern. Pediatr Res. 2008;64:86–90.
Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2006;17:337–47.
Brustad N, Garland J, Thorsen J, Sevelsted A, Krakauer M, Vinding RK, et al. Effect of high-dose vs standard-dose vitamin D supplementation in pregnancy on bone mineralization in offspring until age 6 years. JAMA Pediatr. 2020;174:1–9.
Pan K, Tu R, Yao X, Zhu Z. Associations between serum calcium, 25(OH)D level and bone mineral density in adolescents. Adv Rheumatol Lond Engl. 2021;61:16.
Winzenberg TM, Powell S, Shaw KA, Jones G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;10: CD006944.
Thiagarajan NR, Kumar CGD, Sahoo J, Krishnamurthy S. Effect of vitamin D and calcium supplementation on bone mineral content in children with thalassemia. Indian Pediatr. 2019;56:307–10.
Solmaz I, Ozdemir MA, Unal E, Abdurrezzak U, Muhtaroglu S, Karakukcu M. Effect of vitamin K2 and vitamin D3 on bone mineral density in children with acute lymphoblastic leukemia: a prospective cohort study. J Pediatr Endocrinol Metab JPEM. 2021;34:441–7.
Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2014;29:467–78.
McVey MK, Geraghty AA, O’Brien EC, McKenna MJ, Kilbane MT, Crowley RK, et al. The impact of diet, body composition, and physical activity on child bone mineral density at five years of age-findings from the ROLO Kids Study. Eur J Pediatr. 2020;179:121–31.
Kopiczko A, Łopuszańska-Dawid M, Gryko K. Bone mineral density in young adults: the influence of vitamin D status, biochemical indicators, physical activity and body composition. Arch Osteoporos. 2020;15:45.
Krahenbühl T, Guimarães R de F, Barros Filho A de A, Gonçalves EM. Bone geometry and physical activity in children and adolescents: systematic review. Rev Paul Pediatr Orgao Soc Pediatr Sao Paulo. 2018;36:230–7.
Gannotti ME, Nahorniak M, Gorton GE, Sciascia K, Sueltenfuss M, Synder M, et al. Can exercise influence low bone mineral density in children with juvenile rheumatoid arthritis? Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Ther Assoc. 2007;19:128–39.
Omori CH, Silva CAA, Sallum AME, Rodrigues Pereira RM, Lúciade Sá Pinto A, Roschel H, et al. Exercise training in juvenile dermatomyositis. Arthritis Care Res. 2012;64:1186–94.
Simonini G, Giani T, Stagi S, de Martino M, Falcini F. Bone status over 1 yr of etanercept treatment in juvenile idiopathic arthritis. Rheumatol Oxf Engl. 2005;44:777–80.
Billiau AD, Loop M, Le P-Q, Berthet F, Philippet P, Kasran A, et al. Etanercept improves linear growth and bone mass acquisition in MTX-resistant polyarticular-course juvenile idiopathic arthritis. Rheumatol Oxf Engl. 2010;49:1550–8.
Lange U, Teichmann J, Müller-Ladner U, Strunk J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatol Oxf Engl. 2005;44:1546–8.
Veerappan SG, O’Morain CA, Daly JS, Ryan BM. Review article: the effects of antitumour necrosis factor-α on bone metabolism in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:1261–72.
Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Uña CR, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2017;28:429–46.
Georgiou KR, Scherer MA, Fan C-M, Cool JC, King TJ, Foster BK, et al. Methotrexate chemotherapy reduces osteogenesis but increases adipogenic potential in the bone marrow. J Cell Physiol. 2012;227:909–18.
Fan C, Georgiou KR, King TJ, Xian CJ. Methotrexate toxicity in growing long bones of young rats: a model for studying cancer chemotherapy-induced bone growth defects in children. J Biomed Biotechnol [Internet]; 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3085506/ [cited 2021 May 15].
May KP, Mercill D, McDermott MT, West SG. The effect of methotrexate on mouse bone cells in culture. Arthritis Rheumatol. 1996;39:489–94.
di Munno O, Mazzantini M, Sinigaglia L, Bianchi G, Minisola G, Muratore M, et al. Effect of low dose methotrexate on bone density in women with rheumatoid arthritis: results from a multicenter cross-sectional study. J Rheumatol. 2004;31:1305–9.
Cranney AB, McKendry RJ, Wells GA, Ooi DS, Kanigsberg ND, Kraag GR, et al. The effect of low dose methotrexate on bone density. J Rheumatol. 2001;28:2395–9.
Bianchi ML, Cimaz R, Galbiati E, Corona F, Cherubini R, Bardare M. Bone mass change during methotrexate treatment in patients with juvenile rheumatoid arthritis. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 1999;10:20–5.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc Mayo Clin. 2008;83:1032–45.
Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Porras AG. Clinical pharmacology of alendronate sodium. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 1993;3(Suppl 3):S13-16.
Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2013;382:1424–32.
Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J Pediatr Orthop. 2005;25:786–91.
DiMeglio LA, Peacock M. Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res Off J Am Soc Bone Miner Res. 2006;21:132–40.
Bianchi ML, Cimaz R, Bardare M, Zulian F, Lepore L, Boncompagni A, et al. Efficacy and safety of alendronate for the treatment of osteoporosis in diffuse connective tissue diseases in children: a prospective multicenter study. Arthritis Rheumatol. 2000;43:1960–6.
Lepore L, Pennesi M, Barbi E, Pozzi R. Treatment and prevention of osteoporosis in juvenile chronic arthritis with disodium clodronate. Clin Exp Rheumatol. 1991;9(Suppl 6):33–5.
El-Husseini AA, El-Agroudy AE, El-Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatr Transplant. 2004;8:357–61.
Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatol Oxf Engl. 2005;44:813–8.
Iwasaki T, Takei K, Nakamura S, Hosoda N, Yokota Y, Ishii M. Secondary osteoporosis in long-term bedridden patients with cerebral palsy. Pediatr Int Off J Jpn Pediatr Soc. 2008;50:269–75.
Zheng W-B, Dai Y, Hu J, Zhao D-C, Wang O, Jiang Y, et al. Effects of bisphosphonates on osteoporosis induced by Duchenne muscular dystrophy: a prospective study. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2020;26:1477–85.
Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet Lond Engl. 2004;363:1427–31.
Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24:1282–9.
Ward LM, Rauch F, Whyte MP, D’Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–64.
Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone. 2006;39:901–6.
Astrom E, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child. 2002;86:356–64.
Rooney M, Bishop N, Davidson J, Beresford MW, Pilkington C, Donagh JM, et al. The prevention and treatment of glucocorticoid-induced osteopaenia in juvenile rheumatic disease: a randomised double-blind controlled trial. EClinicalMedicine. 2019;12:79–87.
Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018;54:223–33.
Munns CFJ, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20:1235–43.
DiMeglio LA, Ford L, McClintock C, Peacock M. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone. 2004;35:1038–45.
Kusumi K, Ayoob R, Bowden SA, Ingraham S, Mahan JD. Beneficial effects of intravenous pamidronate treatment in children with osteogenesis imperfecta under 24 months of age. J Bone Miner Metab. 2015;33:560–8.
Lin C-H, Chien Y-H, Peng S-F, Tsai W-Y, Tung Y-C, Lee C-T, et al. Cyclic pamidronate infusion for neonatal-onset osteogenesis imperfecta. Pediatr Neonatol. 2014;55:306–11.
Gandrud LM, Cheung JC, Daniels MW, Bachrach LK. Low-dose intravenous pamidronate reduces fractures in childhood osteoporosis. J Pediatr Endocrinol Metab JPEM. 2003;16:887–92.
Steelman J, Zeitler P. Treatment of symptomatic pediatric osteoporosis with cyclic single-day intravenous pamidronate infusions. J Pediatr. 2003;142:417–23.
Martinez-Soto T, Pacaud D, Stephure D, Trussell R, Huang C. Treatment of symptomatic osteoporosis in children: a comparison of two pamidronate dosage regimens. J Pediatr Endocrinol Metab JPEM. 2011;24:271–4.
Maines E, Monti E, Doro F, Morandi G, Cavarzere P, Antoniazzi F. Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Miner Metab. 2012;30:434–8.
Idolazzi L, Fassio A, Viapiana O, Rossini M, Adami G, Bertoldo F, et al. Treatment with neridronate in children and adolescents with osteogenesis imperfecta: data from open-label, not controlled, three-year Italian study. Bone. 2017;103:144–9.
Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tatò L, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20:758–63.
Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab JPEM. 2012;25:485–91.
Ooi HL, Briody J, Biggin A, Cowell CT, Munns CF. Intravenous zoledronic acid given every 6 months in childhood osteoporosis. Horm Res Paediatr. 2013;80:179–84.
Saraff V, Sahota J, Crabtree N, Sakka S, Shaw NJ, Högler W. Efficacy and treatment costs of zoledronate versus pamidronate in paediatric osteoporosis. Arch Dis Child. 2018;103:92–4.
Lim A, Simm PJ, James S, Lee SL-K, Zacharin M. Outcomes of zoledronic acid use in paediatric conditions. Horm Res Paediatr. 2020;93:442–52.
Högler W, Yap F, Little D, Ambler G, McQuade M, Cowell CT. Short-term safety assessment in the use of intravenous zoledronic acid in children. J Pediatr. 2004;145:701–4.
Munns CF, Rajab MH, Hong J, Briody J, Högler W, McQuade M, et al. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone. 2007;41:366–70.
Marrani E, Giani T, Simonini G, Cimaz R. Pediatric osteoporosis: diagnosis and treatment considerations. Drugs. 2017;77:679–95.
Sarraf KM. Images in clinical medicine. Radiographic zebra lines from cyclical pamidronate therapy. N Engl J Med. 2011;365:e5.
Rauch F, Travers R, Munns C, Glorieux FH. Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19:1191–3.
Silva ECC, Terreri MTRA, de Castro TCM, Barbosa CPL, Fernandes ARC, Hilário MOE. Sclerotic metaphyseal lines in children and adolescents treated with alendronate. Rev Bras Reumatol. 2010;50:283–90.
Land C, Rauch F, Glorieux FH. Cyclical intravenous pamidronate treatment affects metaphyseal modeling in growing patients with osteogenesis imperfecta. J Bone Miner Res Off J Am Soc Bone Miner Res. 2006;21:374–9.
Hennedige AA, Jayasinghe J, Khajeh J, Macfarlane TV. Systematic review on the incidence of bisphosphonate related osteonecrosis of the jaw in children diagnosed with osteogenesis imperfecta. J Oral Maxillofac Res. 2013;4:e1.
Brown JJ, Ramalingam L, Zacharin MR. Bisphosphonate-associated osteonecrosis of the jaw: does it occur in children? Clin Endocrinol (Oxf). 2008;68:863–7.
Campos L, Miziara LNB, Gallottini M, Ortega K, Martins F. Medication-related osteonecrosis of the jaw in a Duchenne muscular dystrophy patient. Photodiagnosis Photodyn Ther. 2020;31: 101826.
Anam EA, Rauch F, Glorieux FH, Fassier F, Hamdy R. Osteotomy healing in children with osteogenesis imperfecta receiving bisphosphonate treatment. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30:1362–8.
Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19:1779–86.
van de Laarschot DM, Zillikens MC. Atypical femur fracture in an adolescent boy treated with bisphosphonates for X-linked osteoporosis based on PLS3 mutation. Bone. 2016;91:148–51.
Etxebarria-Foronda I, Carpintero P. An atypical fracture in male patient with osteogenesis imperfecta. Clin Cases Miner Bone Metab Off J Ital Soc Osteoporos Miner Metab Skelet Dis. 2015;12:278–81.
Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–63.
Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23:1698–707.
Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone. 2007;40:821–7.
Sokal A, Elefant E, Leturcq T, Beghin D, Mariette X, Seror R. Pregnancy and newborn outcomes after exposure to bisphosphonates: a case-control study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2019;30:221–9.
Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep. 2005;3:98–102.
Adamopoulos IE. Inflammation in bone physiology and pathology. Curr Opin Rheumatol. 2018;30:59–64.
Anonymous. Prolia [Internet]. Eur. Med. Agency. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/prolia [cited 2021 May 16].
Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50:793–807.
Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. 2017;15:283–92.
Trejo P, Rauch F, Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2018;18:76–80.
Zheng S, Gaitonde P, Andrew MA, Gibbs MA, Lesko LJ, Schmidt S. Model-based assessment of dosing strategies in children for monoclonal antibodies exhibiting target-mediated drug disposition. CPT Pharmacomet Syst Pharmacol. 2014;3: e138.
Hoyer-Kuhn H, Netzer C, Koerber F, Schoenau E, Semler O. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis. 2014;9:145.
Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfect–a first prospective trial. J Musculoskelet Neuronal Interact. 2016;16:24–32.
Kobayashi T, Nakamura Y, Suzuki T, Yamaguchi T, Takeda R, Takagi M, et al. Efficacy and safety of denosumab therapy for osteogenesis imperfecta patients with osteoporosis-case series. J Clin Med. 2018;7:479.
Hoyer-Kuhn H, Rehberg M, Netzer C, Schoenau E, Semler O. Individualized treatment with denosumab in children with osteogenesis imperfecta—follow up of a trial cohort. Orphanet J Rare Dis. 2019;14:219.
Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012;12:183–8.
Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res Off J Am Soc Bone Miner Res. 2012;27:1462–70.
Grasemann C, Schündeln MM, Hövel M, Schweiger B, Bergmann C, Herrmann R, et al. Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget’s disease. J Clin Endocrinol Metab. 2013;98:3121–6.
Gossai N, Hilgers MV, Polgreen LE, Greengard EG. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer. 2015;62:1078–80.
Setsu N, Kobayashi E, Asano N, Yasui N, Kawamoto H, Kawai A, et al. Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab. 2016;34:118–22.
Uday S, Gaston CL, Rogers L, Parry M, Joffe J, Pearson J, et al. Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J Clin Endocrinol Metab. 2018;103:596–603.
Choe M, Smith V, Okcu MF, Wulff J, Gruner S, Huisman TAGM, et al. Treatment of central giant cell granuloma in children with denosumab. Pediatr Blood Cancer. 2021;68: e28778.
Kobayashi E, Setsu N. Osteosclerosis induced by denosumab. Lancet Lond Engl. 2015;385:539.
Dunnion S, Paterson A, Johnston R. Dense sclerotic metaphyseal bands caused by denosumab therapy. Pediatr Radiol. 2020;50:877–8.
Wang HD, Boyce AM, Tsai JY, Gafni RI, Farley FA, Kasa-Vubu JZ, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. 2014;99:891–7.
Rehberg M, Winzenrieth R, Hoyer-Kuhn H, Duran I, Schoenau E, Semler O. TBS as a tool to differentiate the impact of antiresorptives on cortical and trabecular bone in children with osteogenesis imperfecta. J Clin Densitom Off J Int Soc Clin Densitom. 2019;22:229–35.
Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev [Internet]. 2016. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6611487/ [cited 2021 Jun 18].
Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, et al. Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:126–30.
Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res Off J Am Soc Bone Miner Res. 2005;20:977–86.
Chevrel G, Schott A-M, Fontanges E, Charrin JE, Lina-Granade G, Duboeuf F, et al. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res. 2006;21:300–6.
Orwoll ES, Shapiro J, Veith S, Wang Y, Lapidus J, Vanek C, et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Investig. 2014;124:491–8.
Gatti D, Rossini M, Viapiana O, Povino MR, Liuzza S, Fracassi E, et al. Teriparatide treatment in adult patients with osteogenesis imperfecta type I. Calcif Tissue Int. 2013;93:448–52.
Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol Berl Ger [Internet]. Pediatr Nephrol; 2006. https://pubmed.ncbi.nlm.nih.gov/16382319/ [cited 2021 Jun 18].
Rianthavorn P, Pisutikul K, Deekajorndech T, Tepmongkol S, Suphapeetiporn K. Prevention of bone loss in children receiving long-term glucocorticoids with calcium and alfacalcidol or menatetrenone. J Pediatr Endocrinol Metab JPEM. 2012;25:307–12.
Acott PD, Wong JA, Lang BA, Crocker JFS. Pamidronate treatment of pediatric fracture patients on chronic steroid therapy. Pediatr Nephrol Berl Ger. 2005;20:368–73.
Inoue Y, Shimojo N, Suzuki S, Arima T, Tomiita M, Minagawa M, et al. Efficacy of intravenous alendronate for the treatment of glucocorticoid-induced osteoporosis in children with autoimmune diseases. Clin Rheumatol. 2008;27:909–12.
Simm PJ, Johannesen J, Briody J, McQuade M, Hsu B, Bridge C, et al. Zoledronic acid improves bone mineral density, reduces bone turnover and improves skeletal architecture over 2 years of treatment in children with secondary osteoporosis. Bone. 2011;49:939–43.
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2012;23:2703–11.
Pazianas M, Rhim AD, Weinberg AM, Su C, Lichtenstein GR. The effect of anti-TNF-alpha therapy on spinal bone mineral density in patients with Crohn’s disease. Ann N Y Acad Sci. 2006;1068:543–56.
Seriolo B, Paolino S, Sulli A, Ferretti V, Cutolo M. Bone metabolism changes during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:420–7.
Sumník Z, Land C, Rieger-Wettengl G, Körber F, Stabrey A, Schoenau E. Effect of pamidronate treatment on vertebral deformity in children with primary osteoporosis. A pilot study using radiographic morphometry. Horm Res. 2004;61:137–42.
Melchior R, Zabel B, Spranger J, Schumacher R. Effective parenteral clodronate treatment of a child with severe juvenile idiopathic osteoporosis. Eur J Pediatr. 2005;164:22–7.
Kauffman RP, Overton TH, Shiflett M, Jennings JC. Osteoporosis in children and adolescent girls: case report of idiopathic juvenile osteoporosis and review of the literature. Obstet Gynecol Surv. 2001;56:492–504.
Baroncelli GI, Vierucci F, Bertelloni S, Erba P, Zampollo E, Giuca MR. Pamidronate treatment stimulates the onset of recovery phase reducing fracture rate and skeletal deformities in patients with idiopathic juvenile osteoporosis: comparison with untreated patients. J Bone Miner Metab. 2013;31:533–43.
Sanghai SR, Shah I. Juvenile osteoporosis in a 5-year-old girl. J Nat Sci Biol Med. 2013;4:476–7.
Celli M, D’Eufemia P, Persiani P, Turchetti A, Febbo A, D’Alfonso Y, et al. Clinical and biochemical response to neridronate treatment in a patient with osteoporosis-pseudoglioma syndrome (OPPG). Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2017;28:3277–80.
Tüysüz B, Bursalı A, Alp Z, Suyugül N, Laine CM, Mäkitie O. Osteoporosis-pseudoglioma syndrome: three novel mutations in the LRP5 gene and response to bisphosphonate treatment. Horm Res Paediatr. 2012;77:115–20.
Zacharin M, Cundy T. Osteoporosis pseudoglioma syndrome: treatment of spinal osteoporosis with intravenous bisphosphonates. J Pediatr. 2000;137:410–5.
Levasseur R. Treatment and management of osteoporosis-pseudoglioma syndrome. Expert Rev Endocrinol Metab. 2008;3:337–48.
Streeten EA, McBride D, Puffenberger E, Hoffman ME, Pollin TI, Donnelly P, et al. Osteoporosis-pseudoglioma syndrome: description of 9 new cases and beneficial response to bisphosphonates. Bone. 2008;43:584–90.
Streeten EA, Ramirez S, Eliades M, Jaimungal S, Chandrasekaran S, Kathleen R, et al. Fractures on bisphosphonates in osteoporosis pseudoglioma syndrome (OPPG): pQCT shows poor bone density and structure. Bone. 2015;77:17–23.
Papadopoulos I, Bountouvi E, Attilakos A, Gole E, Dinopoulos A, Peppa M, et al. Osteoporosis-pseudoglioma syndrome: clinical, genetic, and treatment-response study of 10 new cases in Greece. Eur J Pediatr. 2019;178:323–9.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27.
Tauer JT, Abdullah S, Rauch F. Effect of anti-TGF-β treatment in a mouse model of severe osteogenesis imperfecta. J Bone Miner Res Off J Am Soc Bone Miner Res. 2019;34:207–14.
Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20:670–5.
McClung MR, O’Donoghue ML, Papapoulos SE, Bone H, Langdahl B, Saag KG, et al. Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019;7:899–911.
Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res Off J Am Soc Bone Miner Res. 2010;25:32–40.
Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics. 2003;111:1030–6.
Marginean O, Tamasanu RC, Mang N, Mozos I, Brad GF. Therapy with pamidronate in children with osteogenesis imperfecta. Drug Des Dev Ther. 2017;11:2507–15.
George S, Weber DR, Kaplan P, Hummel K, Monk HM, Levine MA. Short-term safety of zoledronic acid in young patients with bone disorders: an extensive institutional experience. J Clin Endocrinol Metab. 2015;100:4163–71.
Al-Agha AE, Hayatalhazmi RS. Osteoporosis treatment with zoledronic acid in pediatric population at a university hospital in Western Saudi Arabia. A 13-year experience. Saudi Med J. 2015;36:1312–8.
Ward L, Bardai G, Moffatt P, Al-Jallad H, Trejo P, Glorieux FH, et al. Osteogenesis imperfecta type VI in individuals from Northern Canada. Calcif Tissue Int. 2016;98:566–72.
Upfill-Brown A, Bukata S, Bernthal NM, Felsenfeld AL, Nelson SD, Singh A, et al. Use of denosumab in children with osteoclast bone dysplasias: report of three cases. JBMR Plus. 2019;3: e10210.
Bar Droma E, Beck-Rosen G, Ilgiyaev A, Fruchtman Y, Abramovitch-Dahan C, Levaot N, et al. Positive outcomes of denosumab treatment in 2 patients with cherubism. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2020;78:2226–34.
Kawamura H, Watanabe S, Asahina I, Moriuchi H, Dateki S, Takashi I. Efficacy and safety of denosumab treatment in a prepubertal patient with cherubism. J Pediatr Endocrinol Metab JPEM. 2020;33:963–6.
Ferriero K, Shah B, Yan Y, Khatri S, Caccamese J, Napoli JA, et al. Case report: safety and efficacy of denosumab in four children with Noonan syndrome with multiple giant cell lesions of the jaw. Front Pediatr. 2020;8:515.
Lange T, Stehling C, Fröhlich B, Klingenhöfer M, Kunkel P, Schneppenheim R, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22:1417–22.
Pelle DW, Ringler JW, Peacock JD, Kampfschulte K, Scholten DJ, Davis MM, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res J Lab Clin Med. 2014;164:139–48.
Raux S, Bouhamama A, Gaspar N, Brugières L, Entz-Werlé N, Mallet C, et al. Denosumab for treating aneurysmal bone cysts in children. Orthop Traumatol Surg Res OTSR. 2019;105:1181–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants performed by any of the authors.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Costi, S., Giani, T., Orsini, F. et al. Drug Treatment of Low Bone Mass and Other Bone Conditions in Pediatric Patients. Pediatr Drugs 24, 103–119 (2022). https://doi.org/10.1007/s40272-021-00487-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00487-7