Abstract
Osteoporosis-pseudoglioma syndrome (OPPG) is a rare autosomal-recessive disorder, characterized by severe osteoporosis and early-onset blindness. Loss of function mutations in the gene encoding low-density lipoprotein receptor-related protein 5 (LRP5) have been established as the genetic defect of the disease. We report the clinical and genetic evaluation of ten OPPG cases in eight related nuclear families and their close relatives. Bone mineral density (BMD) in OPPG patients was assessed by dual-energy X-ray absorptiometry (DXA). Genotyping of LRP5 gene and targeted detection of index mutation were performed by DNA direct sequencing. Four patients were introduced to bisphosphonates. Mutational screening of LRP5 gene revealed the c.2409_2503+79del deletion in homozygous state, expected to result in a truncated protein. Among 44 members of the pedigree, 10 (22%) were identified homozygous and 34 (59%) heterozygous for this mutation. All patients had congenital blindness and 7 of them had also impaired bone mineral density. Four of them received bisphosphonates and responded with decreased bone pain and improvement in BMD; however, 3 patients presented with one fracture during treatment.
Conclusion: The current study presents the molecular and clinical profiles of 10 new OPPG cases, being part of an extended pedigree. Patients who received bisphosphonate treatment responded well with increase in their BMD, though fractures occurred during therapy.
What is known: • OPPG syndrome is a rare genetic disorder characterized by congenital blindness and juvenile osteoporosis. • Loss of function mutations in the gene encoding low-density lipoprotein receptor-related protein 5 (LRP5) is the genetic defect of the disease. | |
What is new: • Genetic and clinical phenotype of 10 new OPPG patients. • The ten new OPPG patients presented with phenotypical variability in osseous manifestations. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis-pseudoglioma syndrome (OPPG; ΟΜΙΜ 259770) is a rare autosomal-recessive disorder characterized by impaired bone accrual and defective regression of the fetal ocular fibrovascular system (pseudoglioma) [1]. Although phenotypic variability—even among siblings—has been described, the clinical hallmark of the disease is the combination of severe early-onset osteoporosis and vitreoretinal pathology, leading to skeletal fragility in early childhood and congenital or neonatal blindness [7, 10, 24].
Loss of function mutations in the gene encoding low-density lipoprotein receptor-related protein 5 (LRP5), either in homozygous or compound heterozygous state, was established as the genetic defect of the disease in 2001 [10]. LRP5 variants are associated with normal bone mineral density (BMD) variation [8, 11]. Furthermore, inactivating mutations of LRP5 in different areas than for OPPG are also responsible for the recessive form of familial exudative vitreoretinopathy (FEVR), prompting the concept that both disorders lie in the same phenotypic spectrum [16].
Currently, over seventy OPPG cases have been reported, with a disease incidence estimated of 1/2,000,000 in USA [1, 21]. A significant proportion of the cases emerges in populations with high rate of consanguinity [14]. Herein, we report 10 new OPPG cases from 8 nuclear families, all members of an extended pedigree. Mutational analysis revealed that the affected individuals carry the c.2409_2503+79del deletion in the LRP5 gene in homozygous state. The clinical features of these patients and the response to treatment with bisphosphonates in four of them are presented.
Materials and methods
Subjects and laboratory measurements
In this study, we included patients referred to our department with clinical signs of OPPG and their close relatives. In total, 44 individuals, 10 patients and 34 members of their families, participated. The protocol was approved by the Institutional Review Board of the “Attikon” University Hospital. Written informed consent was obtained from the participants or the parents of the affected children. Clinical examination of the patients included the evaluation of somatometric features (i.e., height, weight) and musculoskeletal development. Ophthalmological examination was performed to all OPPG patients. Biochemical analysis, including calcium, phosphorus, alkaline phosphatase, parathyroid hormone (PTH), 25-hydroxyvitamin D, and lipid profile, was conducted during the initial evaluation of each patient.
Bone mineral density (BMD) was measured at the lumbar spine (L1-L4) by dual-energy X-ray absorptiometry (DXA), using a pediatric Hologic software (Hologic 2005, Bedford, MA). The precision in vitro was 0.36%, using an appropriate spine phantom of the manufacturer. BMD is expressed in grams per square centimeter and sex- and age-adjusted values (Z scores). The World Health Organization’s DXA-based definitions of osteopenia and osteoporosis are in terms of T scores in adults. However, in children, premenopausal women, and men under the age of 50, the Z score, which represents the standard deviation from age- and gender-matched controls, was used to assess normalcy of BMD. In our study, Z score better than − 2.0 was defined as “normal,” <− 2.0 with no fracture was considered “low BMD for age,” and <− 2.0 with fracture was defined as “osteoporosis.” All DXA scans were analyzed by the same person, in a semi-automatic fashion, including manual modifications of the regions of interest.
Genotyping
DNA was isolated from whole peripheral blood using standard procedures. Primers were designed for the genotyping of the entire LRP5 gene (23 exons) using a web-based primer design program. Sequencing analysis was performed on all PCR products of our first patient sample (patient B). Once the genetic alteration was detected in one of the exons of the gene, we performed targeted analysis on the other members of the pedigree. RNA was extracted from peripheral blood of two patients (patients B and C). Total RNA was isolated using NucleoSpin RNA Blood (Macherey-Nagel). RT PCR was performed using the primers 5′-GACGTCAGCCTGAAGACCAT-3′ and 5′-TGAACAGCAAGAAGGTGGTGGG-3′ and the Transcriptor First-Strand cDNA synthesis kit (Roche). Amplicons (expected length of 775 and 550 bp for normal and mutated, respectively) were sequenced.
Results
Overall, 44 individuals (L1-L34, A-H) participated in the study, 21 males and 23 females. The extended 6-generation pedigree of our proband (patient B) is illustrated in Fig. 1. All participants belonged to Roma ethnic minority, residing in Athens (originated from Larissa, a city in central Greece). Most of them were related, and at least three consanguineous marriages were observed, between second- or third-degree relatives. Three of the confirmed patients (E, G1, and G2) were children of consanguineous marriages. The relation of a single nuclear family with the rest of the kindred was not able to be determined. However, a common ancestor is strongly suggested by the family members and the segregation of the mutation.
Mutational analysis of the LRP5 gene in the first patient (Β) led to the identification of the c.2409_2503+79del (G804_G835delfsX49) genetic change. The exact frames of this deletion were determined by analysis of the respective cDNA sequence. The mutation would be expected to lead to a truncated protein of 853 amino acids (aa) compared to the normal protein of 1615 aa. PCR and sequencing analysis of the members of the pedigree revealed 10 patients (A-H) homozygous for this mutation (22.0%) while 26 were identified to harbor one mutant allele (59.0%). During the study, we conducted prenatal screening in two families and we identified one subject as being homozygous and the other heterozygous for the mutation. Τhe parents of the homozygous fetus chose to have the gestation aborted.
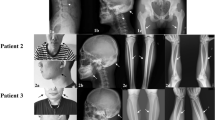
Clinical features and BMD evaluation in the OPPG patients are presented in Table 1. The age of each patient at first evaluation varied from 6 months to 9 years. The mean duration of patients’ follow-up was 3.2 years. Overall, the physical examination revealed short stature in 4 patients. Five patients presented with microcephaly and patient B only with poor weight gain.
All ten patients presented with persistent hyperplasia of the primary vitreous (pseudoglioma), 9 patients with congenital blindness, 6 with retinal detachment, 4 with microphthalmia, 1 with bulbis atrophy, and 1 with ocular cataracts. Patient D had undergone surgical correction of retinal detachment in the right eye at the age of six months and is the only one preserving minimal light perception. Ophthalmologic evaluation in all patients revealed no changes over time. LRP5 mutation heterozygotes had no history of eye anomalies.
Regarding osseous manifestations (or skeletal disease), a broader spectrum of phenotypes was recorded, with variability of BMD measurements (Table 1). Seven of the patients presented with severe osteoporosis. Patients F2 and G2 did not undergo DXA evaluation, as patient F2 was encased in a social institution and follow-up was interrupted, while G2 patient’s parents did not consent. However, both patients suffered from skeletal pain. Delayed gait and muscle hypotonia were noticed in 6 patients. Interestingly, patient G1 was diagnosed with ligamentous laxity but had no findings of osteoporosis at 9 years of age, with a BMD Z score of − 1.2. Also, patient G1 showed premature puberty and preauricular tags. Patients B, C, D, and H sustained bone fractures from the age of 2, 3, and 2 years, and 11 months of age, respectively. LRP5 mutation heterozygotes had no history of fractures.
Cognitive dysfunction was noticed in 8 out of 10 patients, showing social and expressive language delay after the age of 2 years. However, participating children did not undergo an evaluation by a pediatric psychiatrist or by a developmental pediatrician.
Biochemical data in the first visit are shown in Table 2. Biochemical analysis for calcium homeostasis and lipid profile was within normal range. Levels of 25-hydroxy-vitamin D were low in 9 patients. All were treated with 800 IU vitamin D3 daily and oral calcium. Pertaining to calcium dose, those less than 3 years of age received 500 mg/day, those over the age of 3 years old 750 mg/day, and patients who sustained bone fractures 1000 mg/day.
Four families (of patients B, C, D, and H) agreed to bisphosphonate therapy. Patients B, C, D, and H were treated with bisphosphonates (intravenous pamidronate and/or oral alendronate) from the age of 2, 3, 7, and 1 year old, for a total time duration of 4, 2, 3, and 2 years, respectively. Intravenous pamidronate was administered at a dose of 1 mg/kg/day every 3 months for 3 consecutive days and oral alendronate at a dose of 35 mg weekly in patients weighing less than 30 kg and 70 mg weekly in patients more than 30 kg. Patients B and D were initially treated with intravenous pamidronate for 18 months, which subsequently was altered to oral alendronate regimen. The therapeutic approach was modified on the basis of oral versus intravenous administration benefits, which minimizes hospitalization. Besides, alendronate is a more potent anti-resorptive agent and fever or epigastralgia are not common side effects. The patients responded to therapy with decreased bone pain and improvement in BMD Z scores, as shown in Table 1. However, patients B, C, and D did sustain a low-impact peripheral fracture 2 years after the initiation of bisphosphonate therapy (Table 1), while patient B was not able to walk at the age of 6 years old. The follow-up laboratory assessment in all patients showed maintenance of normal calcium homeostasis (data not shown).
Discussion
Molecular analysis revealed a high prevalence of LRP5 mutation (G804_G835delfsX49) within 8 related families (59.0%) of an extended pedigree. Patients’ phenotype was typical of OPPG syndrome, with blindness in infancy and severe early-onset osteoporosis. Also, most of the patients presented with muscle hypotonia and 4 of them with short stature. Bisphosphonate treatment, administrated to 4 patients, led to pain recession and BMD improvement, though 3 of them did sustain one fracture under therapy. Previously, a patient compound heterozygous for the G804_G835delfsX49 and R1708X mutations has been reported. Although not thoroughly presented, he also exhibited a severe phenotype with congenital blindness, bone fragility from 2 years of age, and intellectual disability [1]. We are unaware of any previous reports presenting patients homozygous for the above-mentioned deleterious mutation.
Pertaining to inactivating mutations reported so far, roughly 50% are missense and the rest nonsense and frame-shift variations [15]. Splicing mutations and intragenic deletions are presented with a rate of approximately 5% each [6, 15]. The mutation reported herein is located in the third β-propeller domain of LRP5 protein. The deletion of 79 nucleic bases at exon 11 generated a truncated protein which is expected to be nonfunctional, lacking pivotal protein domains, both cytoplasmatic and transmembrane. Experimental data on truncated LRP5 proteins depleted of the above-mentioned domains suggest an impaired post-translational process, leading to an aberrant intracellular protein trafficking [6, 15] or to a protein more vulnerable to degradation [18, 23].
Even though a broad allelic heterogeneity in OPPG patients has been recorded, the phenotype of patients does not seem to present significant variation regarding the final ocular deficit. Indeed, all our OPPG patients presented with persistent hyperplasia of the primary vitreous (pseudoglioma) whereas 9 with congenital blindness. The role of LRP5 in eye development is intricate and not fully elucidated. It involves a variety of mechanisms including Norrin signal [1, 10] and stabilization of b-catenin [27], possibly leading to impaired macrophage-mediated endothelial cell apoptosis [13].
LRP5 gene has emerged as a key regulator of bone metabolism via the Wnt signal pathway. A plethora of mutations in this gene have been associated with a spectrum of altered bone density phenotypes [7]. Moderate heterogeneity has been described in either compound OPPG patients or even within the same family [5, 24]. Α significant variability in bone mass was also noted among our homozygous OPPG patients. The mean initial BMD Z score in 8 patients was − 4.05 (range from − 7.8 to − 1.2), in accordance with previously published OPPG studies [1, 24]. However, patient B exhibited severe osteoporosis with early, low-impact fractures and a lumbar spine Z score at − 7.8 SD at the age of 2 years old, while patient H exhibited fracture early, from infancy. On the contrary, patient G1 had no history of fracture at the age of 9 years old and a BMD Z score at − 1.2 SD. The above-mentioned findings indicate variable expressivity and marked intra-familial variability of osseous phenotype in the reported family. Many factors might have contributed to this divergence, such as genetic modifiers [21] and genetic-environmental interference [5, 6].
Cognitive dysfunction was impaired in 8 of 10 patients. Unfortunately, participating children did not undergo an evaluation by a pediatric psychiatrist or by a developmental pediatrician. In the largest cohort reported to date, cognitive dysfunction has been described in 8 out of 30 OPPG patients [1]. So far, no correlation of a specific protein domain with mental impairment has been identified [1, 15]. We assume that genetic factors in combination with environmental influences may have contributed to the increased occurrence of cognitive dysfunction in our cohort.
Vitamin D deficiency [25(OH)D < 20 ng/ml] was observed in all patients, except for patient H. Given the pivotal role of vitamin D on calcium metabolism [20], as well as the indications of its involvement in enhancing the expression of the Wnt signaling [9], vitamin D status should be examined and maintained in normal range in OPPG patients. OPPG has also been linked to other metabolic disorders, such as hypercholesterolemia [22]. Our patients had no laboratory findings of hypercholesterolemia, unlike few other OPPG patients previously reported [15]. Three patients had slightly elevated triglycerides; however, lipids were not measured in unaffected family members, in order to further clarify this finding.
Although no curative therapy currently exists, bisphosphonates have demonstrated remarkable results on osseous manifestations and on areal BMD. Bisphosphonates primarily inhibit osteoclastic bone resorption and have been proved safe in patients with OPPG [3, 4, 17, 24, 26, 28]. Consistent with previous studies, our four treated patients reported reduced bone pain, increased their BMD, and did not experience any adverse effects. However, this increase in BMD did not prove to be clinically significant, since 3 out of the 4 treated patients suffered from fractures 2 years after the initiation of bisphosphonate treatment.
In the literature, other patients have also been reported to demonstrate substantial bone fragility under bisphosphonate therapy, even after increase in BMD to normal range [2, 26]. This indicates that bisphosphonates are not an ideal treatment of OPPG. Furthermore, it is under question if areal DXA is an adequate method to thoroughly evaluate bone metabolism of OPPG patients [25]. Streeten et al. found that though there was an increase in areal BMD, in OPPG patients treated with bisphosphonate, the trabecular volumetric BMD remained low, and therefore, in the long-term, no significant improvement occurs [25]. This fact emphasizes the progressive nature of osteoporosis in OPPG patients. Taken all together, additional anabolic regimens are essential to improve bone quality in OPPG patients. Teriparatide—a drug not FDA approved for children—has been administered in an adult and an adolescent patient [2], while lithium ([19], NCT01108068) and anti-sclerostin therapy [12] are being investigated as possible regimens in OPPG syndrome.
There are limitations in this study, which are as follows: (a) cognitive dysfunction in our cohort was not evaluated by a specialist or a specific tool; (b) follow-up DXA data was not available for all patients not treated with bisphosphonates; (c) data on bisphosphonate treatment is insufficient to make any conclusions on the effect on fractures; (d) DXA data was not available for LRP5 mutation heterozygotes; and (e) lipids were not measured in unaffected family members.
The current study presents the clinical and genetic evaluation of 10 new OPPG cases. Patients who received bisphosphonate treatment responded well with increase in their BMD, though fractures occurred during therapy. The osseous phenotypic variability among patient-carriers of the same LRP5 mutation and their response to treatment underline the complex relation between Wnt pathway and bone metabolism.
Abbreviations
- aa:
-
amino acids
- BMD:
-
bone mineral density
- DXA:
-
dual-energy X-ray absorptiometry
- FEVR:
-
familial exudative vitreoretinopathy
- LRP5:
-
low-density lipoprotein receptor-related protein 5
- OPPG:
-
osteoporosis-pseudoglioma syndrome
References
Ai M, Heeger S, Bartels CF, Schelling DK (2005) Clinical and molecular findings in osteoporosis-pseudoglioma syndrome. Am J Hum Genet 77:741–753
Arantes HP, Barros ER, Kunii I, Bilezikian JP, Lazaretti-Castro M (2011) Teriparatide increases bone mineral density in a man with osteoporosis pseudoglioma. J Bone Miner Res 26:2823–2826
Barros ER, Dias da Silva MR, Kunii IS, Lazaretti-Castro M (2008) Three years follow-up of pamidronate therapy in two brothers with osteoporosis-pseudoglioma syndrome (OPPG) carrying an LRP5 mutation. J Pediatr Endocrinol Metab 21:811–818
Bayram F, Tanriverdi F, Kurtoglu S et al (2006) Effects of 3 years of intravenous pamidronate treatment on bone markers and bone mineral density in a patient with osteoporosis-pseudoglioma syndrome (OPPG). J Pediatr Endocrinol Metab 19:275–279
Cheung WM, Jin LY, Smith DK et al (2006) A family with osteoporosis pseudoglioma syndrome due to compound heterozygosity of two novel mutations in the LRP5 gene. Bone 39:470–476
Chung BD, Kayserili H, Ai M, Freudenberg J, Üzümcü A, Uyguner O, Bartels CF, Höning S, Ramirez A, Hanisch FG, Nürnberg G, Nürnberg P, Warman ML, Wollnik B, Kubisch C, Netzer C (2009) A mutation in the signal sequence of LRP5 in a family with an osteoporosis-pseudoglioma syndrome (OPPG)-like phenotype indicates a novel disease mechanism for trinucleotide repeats. Hum Mutat 30:641–648
Cui Y, Niziolek PJ, MacDonald BT et al (2011) Lrp5 functions in bone to regulate bone mass. Nat Med 17:684–691
Ferrari SL, Deutsch S, Antonarakis SE (2005) Pathogenic mutations and polymorphisms in the lipoprotein receptor-related protein 5 reveal a new biological pathway for the control of bone mass. Curr Opin Lipidol 16:207–214
Fretz JA, Zella LA, Kim S, Shevde NK, Pike JW (2007) 1,25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene's transcriptional start site. J Steroid Biochem Mol Biol 103:440–445
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GCM, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, de Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523
Hartikka H, Makitie O, Mannikko M et al (2005) Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res 20:783–789
Jacobsen CM (2017) Application of anti-sclerostin therapy in non-osteoporosis disease models. Bone 96:18–23
Kato M, Patel MS, Levasseur R, Lobov I, Chang BHJ, Glass DA II, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314
Koay MA, Brown MA (2005) Genetic disorders of the LRP5-Wnt signalling pathway affecting the skeleton. Trends Mol Med 11:129–137
Laine CM, Chung BD, Susic M, Prescott T, Semler O, Fiskerstrand T, D'Eufemia P, Castori M, Pekkinen M, Sochett E, Cole WG, Netzer C, Mäkitie O (2011) Novel mutations affecting LRP5 splicing in patients with osteoporosis-pseudoglioma syndrome (OPPG). Eur J Hum Genet 19:875–881
Lara-Castillo N, Johnson ML (2015) LRP receptor family member associated bone disease. Rev Endocr Metab Disord 16:141–148
Levasseur R (2008) Treatment and management of osteoporosis-pseudoglioma syndrome. Expert Rev Endocrinol Metab 3:337–348
Levasseur R, Lacombe D, de Vernejoul MC (2005) LRP5 mutations in osteoporosis-pseudoglioma syndrome and high-bone-mass disorders. Joint Bone Spine 72:207–214
Mosekilde L, Torring O, Rejnmark L (2011) Emerging anabolic treatments in osteoporosis. Curr Drug Saf 2011(6):62–74
Papadopoulou A, Gole E, Nicolaidou P (2013) Hereditary rickets. How genetic alterations explain the biochemical and clinical phenotypes. Endocr Metab Immune Disord Drug Targets 13:324–334
Qin M, Hayashi H, Oshima K, Tahira T, Hayashi K, Kondo H (2005) Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 26:104–112
Saarinen A, Saukkonen T, Kivela T et al (2010) Low density lipoprotein receptor-related protein 5 (LRP5) mutations and osteoporosis, impaired glucose metabolism and hypercholesterolaemia. Clin Endocrinol 72:481–488
Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY (2000) Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol Cell 5:877–882
Streeten EA, McBride D, Puffenberger E, Hoffman ME, Pollin TI, Donnelly P, Sack P, Morton H (2008) Osteoporosis-pseudoglioma syndrome: description of 9 new cases and beneficial response to bisphosphonates. Bone 43:584–590
Streeten EA, Ramirez S, Eliades M, Jaimungal S, Chandrasekaran S, Kathleen R, Holmes Morton D, Puffenberger EG, Herskovitz R, Leonard MB (2015) Fractures on bisphosphonates in osteoporosis pseudoglioma syndrome (OPPG): pQCT shows poor bone density and structure. Bone 77:17–23
Tuysuz B, Bursali A, Alp Z, Suyugul N, Laine CM, Makitie O (2012) Osteoporosis-pseudoglioma syndrome: three novel mutations in the LRP5 gene and response to bisphosphonate treatment. Horm Res Paediatr 77:115–120
Xia CH, Liu H, Cheung D, Wang M, Cheng C, du X, Chang B, Beutler B, Gong X (2008) A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet 17:1605–1612
Zacharin M, Cundy T (2000) Osteoporosis pseudoglioma syndrome: treatment of spinal osteoporosis with intravenous bisphosphonates. J Pediatr 137:410–415
Author information
Authors and Affiliations
Contributions
Iordanis Papadopoulos contributed to the acquisition of data and molecular analysis. Evangelia Bountouvi contributed to the analysis and interpretation of data and drafting of the manuscript. Dr. Anna Papadopoulou performed the molecular studies and contributed to the study design, interpretation of the results, and editing of the manuscript. Dr. Attilakos, Dr. Dinopoulos, and Dr. Nikolaidou contributed to the conception of the study and clinical evaluation of the patients and reviewed the manuscript. Dr. Gole contributed to the molecular analysis. Dr. Peppa performed the bone mineral density measurements.
Corresponding author
Ethics declarations
The protocol was approved by the Institutional Review Board of the University Hospital “Attikon.”
Financial disclosure
The authors have no financial relationships relevant to this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from the participants and the parents of the participating children.
Conflicts of interest
All the authors declare that they have no conflicts of interest.
Additional information
Communicated by Peter de Winter
Rights and permissions
About this article
Cite this article
Papadopoulos, I., Bountouvi, E., Attilakos, A. et al. Osteoporosis-pseudoglioma syndrome: clinical, genetic, and treatment-response study of 10 new cases in Greece. Eur J Pediatr 178, 323–329 (2019). https://doi.org/10.1007/s00431-018-3299-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-018-3299-3