Abstract
Lectins are a structurally heterogeneous group of proteins or glycoproteins with at least one noncatalytic domain binding reversibly to a specific mono- or oligosaccharide. Monocot mannose-binding lectins are an extended superfamily of structurally and evolutionarily related proteins. In this study, we evaluated anti-inflammatory and antinociceptive effects of monocot lectin from the Canna limbata seeds (CLL). To accomplish this, CLL was purified and subjected to pharmacological assays: abdominal writhing induced by acetic acid, formalin, hot plate and Zymosan A-induced peritonitis tests. The CLL was purified by chromatographic chitin column, and the relative mass of 21 kDa observed in electrophoresis was confirmed by electrospray mass spectrometry, which also revealed that purified CLL consists of a dimer having a weight of 49,676 Da. The CLL showed nociceptive activity in the acetic acid test as well as peripheral antinociceptive response. The CLL also showed anti-inflammatory effect with the reduction of inflammation in the formalin test and neutrophil migration into the peritoneal cavity. This is the first report of anti-inflammatory activity for a monocot lectin, and it suggests a new pharmacological tool to understand inflammatory and antinociceptive processes mediated through lectins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are a structurally heterogeneous group of proteins or glycoproteins with at least one noncatalytic domain binding reversibly to a specific mono- or oligosaccharide [1]. They are distributed ubiquitously in nature, ranging from microorganisms to plants and animals [2]. They have been proved excellent and versatile macromolecular tools for the study of normal or transformed cell surfaces, the isolation of glycoconjugates, and uses in other areas of biomedical science [3].
Monocot mannose-binding lectins (MMBLs), or agglutinins, are an extended superfamily of structurally and evolutionarily related proteins [4]. The hallmark of these MMBLs is the presence of a domain with three potential carbohydrate binding pockets, each generated by a QXDXNXVXY motif. Most are built from two-to-four identical or homologous subunits, although some are monomeric [5]. The monocot lectins were given special attention by the specificity and biological activities presented by this group of proteins such as proinflammatory and lymphocyte activation activities [6]; antiproliferative activity toward some cancer cells such as prostatic carcinoma, lung cancer and mastocarcinoma [7, 8]; inhibitory effects against respiratory syncytial virus, influenza A (H1N1, H3N2 and H5N1) and B viruses [9]; and insecticidal activities [10]. Most of the monocot lectins have molecular weight of about 10 to 18 kDa with specificity for mannose, GlcNAc and oligomers of GlcNAc and GalNAc [11].
In recent years, several studies have shown the anti-inflammatory and antinociceptive potential of plant lectins [12–17]. The important role of sugar residues was also demonstrated in these studies, since the observed activities were reversed when the lectins were associated with their specific binding sugars. It has been proposed that the anti-inflammatory effects elicited by exogenous lectins result from competitive blocking of glycosylated selectin binding sites on the membranes of leukocytes and/or endothelial cells [18]. However, the only monocot lectins described thus far in the literature are Arisaema erubescenscom and Arum maculatum [18, 19] in the context of their proinflammatory activity. The present work represents the first description of anti-inflammatory activity of a monocot lectin. Using a mouse model, this study aimed to isolate the protein and investigate the antinociceptive and anti-inflammatory effects of Canna limbata lectin (CLL).
Methods
Plant Material
Seeds of C. limbata were collected from plants grown in South of Brazil (Pelotas, Rio Grande do Sul). The botanical identification was carried out at the Department of Biology, Universidade Federal do Ceará (UFC).
Lectin Purification
Mature seeds of C. limbata were ground into a fine powder using a coffee mill. The protein was extracted with 20 mM phosphate buffer containing 50 mM NaCl, (PBS) at pH 7.2 (1:3, w/v) at 25 °C for 2 h. The protein extract obtained was centrifuged (10,000 × g, 5 min, 4 °C). The supernatant (crude extract) was precipitated with ammonium sulfate (0–50 % and 50–90 % saturation) and centrifuged, and the pellet was resuspended and dialyzed in 20 mM PBS, pH 7.2. The active fraction (50–90 % saturation) was applied to a chitin column previously equilibrated with extraction buffer. After removing unbound material (peak I), the lectin was eluted with 50 mM acetic acid (peak II). Peak II was submitted to an anionic exchange chromatography in a DEAE-Sephacel column equilibrated with 20 mM phosphate buffer at pH 7.2. The retained proteins were eluted by the same buffer in a linear NaCl gradient 0–1 M. The purification process was monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in the absence and presence of reducing conditions (β-mercaptoethanol) as described elsewhere by Laemmli [20], and the purified lectin was used for mass spectrometry (MS) analysis and pharmacological assays.
Hemagglutination Activity
Hemagglutination assays were carried out as described in Moreira and Perrone [21], using serial dilutions with rabbit erythrocytes, either native or treated with proteolytic enzymes (trypsin or papain). Results were expressed in hemagglutinating units (HU), with one HU being defined as the smallest amount (mg) of protein per milliliter capable of inducing visible agglutination.
MW Determination by MS
The molecular mass of CLL was determined by electrospray ionization MS (ESI-MS) using a hybrid mass spectrometer (Synapt HDMS system, Waters, Milford, USA) operating in positive ion mode at 10,000 resolution. Protein solution (10 ρmol/ml) was infused into the system using the built-in syringe drive at a flow rate of 10 ml/min. The capillary voltage and the cone voltage were set at 3 kV and 40 V, respectively. The source temperature was maintained at 100 °C, and nitrogen was used as a drying gas (flow rate: 150 l/h). Data acquisition was done with the Mass Lynx 4.0 software, and the multiply charged spectra were deconvoluted using maximum entropy techniques.
Protein Content
Protein content was determined as described by Bradford [22] using bovine serum albumin (BSA) as the standard protein. Absorbance at 280 nm was also used to estimate protein concentration in the chromatographic fractions.
Drugs and Reagents
The drugs and reagents used in the experiments included acetic acid (Merck), dipyrone (Sigma Chemical), morphine sulfate (Dimorf-Cristália), indomethacin (Merck), gum arabic (Sigma Chemical) and Tween 80 (Sigma). A 2.5 % formaldehyde (Merck) solution was prepared using saline (0.9 % NaCl). The plant material was suspended in Tween 80 and gum arabic media in all experiments and administered orally (p.o.) at 100 mg/kg. Dipyrone and indomethacin (p.o.) and morphine subcutaneously (s.c.) were used as reference drugs. The s.c. administration route was used for morphine because of the improved effect of this drug when administered s.c. The animals in the control group received gum arabic (p.o.).
Animals
A random selection of Swiss mice (n = 6) of both genders weighing 25–30 g was obtained from the experimental animal facility of the Federal University of Alagoas (UFAL). The animals were fasted for 8 h prior to the experiments to prevent food from interfering with the absorption of the substances administered in the study. The study protocols were previously approved by the UFAL Research Ethics Committee under entry #015102/2010-51. All experiments were conducted following current recommendations for the handling of laboratory animals and ethical guidelines for studies on experimental pain in conscious animals [23].
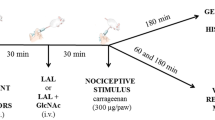
Abdominal Writhing Induced by Acetic Acid
In the abdominal writhing test, peripherally mediated pain was induced by 0.6 % acetic acid, as described by Koster et al. [24]. Mice were treated with C. limbata lectin (CLL) at 0.1, 1, 5 and 10 mg/kg, oral (p.o.), or reference drug dipyrone at 33.3 mg/kg, intraperitoneally (i.p.), 30 min before i.p. administration of 0.6 % acetic acid (10 ml/kg body weight). Ten minutes after administration of the acid, the number of constrictions was counted for 20 min. The writhing response consists of a contraction of the abdominal muscle together with a stretching of the hind limbs. Results were expressed as mean ± SEM, the number of writhings per 20 min.
Formalin Test
The technique used to induce nociception was adapted from Hunskaar and Hole [25]. Mice were first treated with CLL (0.1, 1, 5 and 10 mg/kg, p.o.), and 30 min thereafter, they received 20 μl of formalin 1.5 % s.c. (v/v in distilled water) in right hind paw. Soon after administration of formalin, the time (s) that animals spent licking the injected paws was counted for 5 min (phase 1, neurogenic), and after 15 min, a second observation was made for an additional 5 min (phase 2, inflammatory). The control groups received, respectively, indomethacin, 35.7 mg/kg, p.o., as reference drug, and saline intravenously (i.v.) 30 min before formalin injection. Results were expressed as mean ± SEM, the licking time in seconds.
Hot Plate Test
Following the methodology described by Kuraishi [26], mice were placed on a plate heated to 55 °C (±1 °C), and a measurement was made of the time they remained on the plate until proof of stereotyped behavior in reaction to pain (licking or jumping). The basal time (0 time) was measured before treatment, and the animals that did not respond by the end of 20 s were eliminated from the test. Soon after, treatment was undertaken with CLL (0.1, 1, 5 and 10 mg/kg, p.o.), morphine (4.3 mg/kg, s.c.) or saline (i.v.), and the reaction times were recorded in time intervals of 30, 60, 90, 120 and 150 min after treatment, with a cutoff time of 45 s to avoid animal paw lesion. Results were expressed as mean ± SEM, the reaction time in seconds.
Zymosan A-induced Peritonitis Test
Either CLL (0.1, 1, 5 and 10 mg/kg) or reference drug indomethacin (35.7 mg/kg), diluted in sterile saline (0.9 %, NaCl), was administered i.v. (retro orbital plexus) 30 min before i.p. injection of the inflammatory stimulus (0.5 ml Zymozan A 2 mg/ml). The control groups received sterile saline (i.v). Mice were sacrificed 4 h after Zymozan A injection, and the peritoneal cavity was washed with 2 ml PBS solution. The peritoneal fluid was collected, and both total and differential leukocyte counts were carried out, according to Leite et al. [27]. Results were expressed as mean ± SEM, the number of cells × 106/ml peritoneal fluid.
Statistical Analysis
The statistical significance of the differences between the experimental groups and the controls was determined with ANOVA using the Prisma® software, followed by Dunnett’s test. The level of statistical significance was set at p < 0.05 (*) and p < 0.01 (**). The results were expressed as mean values ± standard error.
Results
Lectin Purification and Mass Characterization
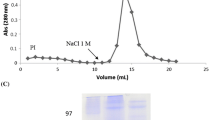
CLL was purified by affinity chromatography on a chitin column. The fraction (50–90 %) from the crude extract after affinity chromatography in chitin column showed an unretained fraction (peak I, fractions 1–21), which was washed with 20 mM phosphate buffer containing 50 mM NaCl at pH 7.2. Peak II (fractions 25–31), was eluted with 50 mM acetic acid monitored at 280 nm (Fig. 1a) and showed hemagglutinating activity (see Table 1). To optimize purification, peak II was applied into a DEAE column connected to high-performance liquid chromatography, which resulted in two fractions (PI and PII) where fraction PII contained all the hemagglutinating activity, thereby yielding the purified lectin, named CLL. PII was monitored by SDS-PAGE, and CLL revealed a band with relative mass of 50 kDa in the absence of reducing agents, but after treatment with β-mercaptoethanol, the mass found was 21 kDa, indicating the presence of at least a disulfide bond connecting two monomers (Fig. 1b). Accordingly, analysis by ESI-MS indicated a molecular weight of 49,676 Da (data not shown) which would match the mass of the dimer.
Abdominal Writhing Induced by Acetic Acid
In the abdominal writhing test (Fig. 2), all concentrations of CLL significantly reduced the number of writhes. When compared to controls receiving saline and acetic acid alone (35.4 ± 2.93 writhes), the administration of dipyrone inhibited writhing by 71.75 ± 3.68 % (p < 0.01). However, administration of CLL (0.1, 1, 5 and 10 mg/kg, p.o.) significantly inhibited writhing by 60.3, 52.1, 70.8 and 76.1 %, respectively, relative to controls.
Formalin Test
To better evaluate the antinociceptive profile of CLL, an assay was performed to measure the nociception induced by formalin. During the neurogenic stage (0–5 min) of the formalin test, the CLL did not significantly reduce paw licking time when compared to controls receiving formalin alone (Fig. 3). However, during the inflammatory phase (15–30 min), CLL administration at 0.1, 1, 5 and 10 mg/kg significantly reduced paw licking time by 48.08 ± 15.2 % (p < 0.01), 66.11 ± 20.5 % (p < 0.01), 69.63 ± 25.7 % (p < 0.01) and 79.5 ± 17.8 % (p < 0.01), respectively, whereas administration of indomethacin reduced paw licking time by 70.1 ± 15.8 % (p < 0.01; Fig. 4).
Hot Plate Test
In order to evaluate the central antinociceptive activity of CLL, a hot plate model (54 ± 1 °C) was used to reveal possible opioid analgesic activity [28]. However, in the hot plate test, CLL did not significantly reduce the reaction latency of the thermal stimulus. In contrast, morphine increased reaction time at 30, 60, 90, 120 and 150 min (2 ± 0.4 s; 6.9 ± 1.4 s; 6.8 ± 1.1 s; 3.6 ± 0.4 s, respectively; Fig. 5).
Zymosan A-induced Peritonitis Test
In animals receiving Zymosan A alone, leukocyte levels increased by 8.65 ± 3.1 × 106 cells/ml. Six hours after administration of Zymosan A, cell migration was significantly reduced in animals treated with indomethacin (64.36 %) and CLL in 0.1 mg/kg (29.68 %; **p < 0.01), 1 mg/kg (41.74 %; **p < 0.01), 5 mg/kg (48.90 %; **p < 0.01) and 10 mg/kg (43.74 %; **p < 0.01) concentrations (Fig. 6).
Effect of CLL (0.1, 1, 5 and 10 mg/kg, p.o) on the Zymosan A-induced peritonitis test (reference drug indomethacin). Zymosan A was administered at a dose of 2 mg/ml, i.p., and the positive control (indomethacin) was administered at a dose of 35.7 mg/kg, p.o. The results are expressed as mean values ± SEM (n = 6; *p < 0.05, **p < 0.01 on one-way ANOVA, followed by Dunnett’s test)
Discussion
Monocot lectins are proteins that generally have molecular mass of around 13 kDa. Regarding their carbohydrate recognition domain (CRD), many of these lectins bind preferentially to mannose and are called MMBLs [4]. However, other monocot lectins which have specificity for different carbohydrates unrelated to mannose have been described, e.g., lectins from Arisaema consanguineum, Arisaema curvature, Sauromatum guttatum and Gonatanthus pumilus. The activity of these lectins is inhibited by asialofetuin only, while simple sugars/derivatives, including chitin, porcine mucin and fetuin, showed no interaction [11]. CLL is a chitin-binding lectin extracted from the seeds of C. limbata, which is not classified as an MMBL, and does not show binding for mannose or related sugars. It also differs from the other monocot lectins by having relative mass of 21 kDa in the presence of a reducing agent, which could explain the mass found by electrospray ionization of 49,676 Da, indicating a presence of disulfide bonds connecting two monomers. Lectins that usually bind to 1,6-branched GlcNAc containing N-glycans can represent a more potent defense protein against insects, fungi, nematodes, and bacteria than the similar ones [29].
Several studies have shown anti-inflammatory potential of plant lectins [12, 13, 17]. However, few studies have demonstrated the potential for antinociceptive activity of plant lectins, and this kind of study is better characterized for the algae lectins [30–35].
Intraperitoneal injection of acetic acid produces pain via activation of chemosensitive nociceptors, or irritation of visceral surfaces, which leads to the release of histamine, bradykinin, prostaglandins, and serotonin [36]. These mediators activate chemosensitive nociceptors that contribute to the development of inflammatory pain. CLL reduced writhing in 60.3 %, 52.1 %, 70.8 % and 76.1 % of mice (Fig. 2) at concentrations of 0.1, 1, 5 and 10 mg/kg, respectively, suggesting that its antinociceptive effect could be related to the inhibition of mediators released in response to acetic acid.
These results are corroborated by those obtained by Figueiredo et al. [37] who evaluated the effect of lectin of Canavalia boliviana on the writhing induced by acetic acid, where C. boliviana lectin significantly inhibited writhing in a dose-dependent manner (60 %, 63 % and 64 % at concentrations of 1, 5 and 10 mg/kg, respectively). Other studies with Dioclea virgata seed lectins showed a reduction in pain by 34 % when administered in a concentration of 10 mg/kg [38]. Nunes [39] also demonstrated that the lectin from Canavalia grandiflora seeds showed antinociceptive activity in mice by inhibition of nociceptive mediators. These results corroborate those in the present study, showing that the lectin from C. limbata has efficient antinociceptive activity and is, therefore, a candidate for analgesic therapy.
To better assess its antinociceptive activity profile, CLL was tested by formalin-induced nociception. As used in this model, formalin produces a distinct biphasic response such that analgesic drugs can act differently during the first and second stages of the assay. This test is a reliable and valid model of nociceptive sensitivity to various classes of analgesic drugs [40]. The first phase (neurogenic) may result from direct stimulation of chemical nociceptors, while the second phase of peripheral inflammation is dependent on changes in central nociception [41]. In the first phase of this test, the time that the animal spent licking the paw in response to formalin in the control group was 60.4 ± 3.0 s. This time was not reduced after treatment with CLL (Fig. 3). This neurogenic phase is highly sensitive to opioid agents [25], suggesting that CLL does not have any antinociceptive effect on opioid receptors in cells of the central nervous system.
Plant lectins, both locally and i.v. injected, have been classically associated with proinflammatory and anti-inflammatory actions, respectively [19, 42–44]. In the second phase of the formalin test (inflammatory phase), CLL significantly reduced inflammation compared to controls, suggesting that this protein has an anti-inflammatory action. It has been proposed that the anti-inflammatory effects elicited by exogenous lectins are the result of competitive blocking of glycosylated selectin binding sites on the membranes of leukocytes and/or endothelial cells [18]. Several endogenous lectins are recognized among the adhesion molecules that actively participate in inflammatory responses such as these lectins (L-, P- and E-selectin) [45].
Some studies show that the lectins have peripheral antinociceptive effect and therefore act on inflammatory pain [32, 46]. In studies of Silva et al. [34], Nunes et al. [39] and Vieira et al. [31], lectins presented significant inhibitory effect in both phases of the formalin test, but this inhibition was predominant in the second, i.e., inflammatory, stage. In the hot plate test, CLL did not increase the latency time of the animals on the plate, suggesting the absence of central antinociceptive opioid receptors. Consequently, the antinociceptive activity of this lectin is related to its peripheral anti-inflammatory effect, an activity profile similar to that of nonsteroidal anti-inflammatory drugs. These results reinforce the findings in the assay of formalin-induced nociception, which showed no antinociceptive profile during the neurogenic phase. Bitencourt et al. [32] demonstrated that the Hypnea cervicornis lectin was unable to increase residence time of the animals on the plate to a statistically significant degree after administration at different concentrations, thus confirming our results. However, other studies with lectins of the species Brythamnion seaforthii, Brythamnion triquetrym, Amansia multifida and Caulerpa cupressoides demonstrate that these lectins significantly increased residence time of animals on the plate, implicating possible involvement of the opioid system [30, 31, 35]. In conclusion, certain lectins have antinociceptive effects only on the peripheral system, while other lectins have an effect on the central nervous system. This difference in nociceptor response could be explained by the specificity for different sugars that could enhance the lectin–carbohydrate interaction and hide cell receptors due to steric hindrance. Lectins have thus become important tools for studying inflammatory cellular events, and a relationship between lectin structures and biological effects has been proposed [47]. Studies have demonstrated the ability of plant lectins to activate cells of the immune system by different mechanisms [45, 48]. In this context, we investigated the in vivo inhibitory effect of CLL on leukocyte migration, an important inflammatory cellular event. CLL decreased the inflammatory response evoked by peritonitis via inhibition of neutrophil migration at all concentrations tested. Since a concentration of 5 mg/kg has inhibitory activity of more than 48.90 %, it seems clear that its efficacy is not dependent on dosage (Fig. 6). Previous studies have shown the ability of some lectins to reduce the rolling and adhesion of neutrophils on the endothelium, possibly by blocking interactions of adhesion molecules present on neutrophils (L-selectins) and endothelial cells [44]. Only proinflammatory activities are ascribed to monocot lectins, e.g., lectin mannose binding from Arum maculatum, which induces neutrophil migration into the peritoneal cavity, indicating that the inhibitory activity of lectin is associated with a specific sugar and, in turn, showing that this activity is directly related to the CRD [19]. Another example is the monocot lectin from the tubers of Arisaema erubescenscom that showed proinflammatory activity by paw edema and neutrophil migration models in rats, which significantly increased the concentration of nitric oxide (NO), prostaglandin E2 (PGE2) and tumor necrosis factor alpha (TNF-α) in peritoneal fluid [48]. As described by Cunha et al. [49], the migration of neutrophils to the inflamed site is a key step for the release of the proinflammatory cytokines TNF-α and IL-1β, as well as mediators that act directly on nociceptors, such as PGE2 and sympathetic amines.
Conclusion
C. limbata lectin (CLL) was purified by affinity using a chitin column with relative mass of 21 kDa. Electrospray MS confirmed that purified CLL consists of a dimer weighing 49,676 Da. CLL showed nociceptive activity in the acetic acid test, and peripheral antinociceptive response was observed. Anti-inflammatory effect of CLL was also observed with the reduction of inflammation in the formalin test (inflammatory phase) and neutrophil migration into the peritoneal cavity compared to controls. This is the first report of anti-inflammatory activity for a monocot lectin, and it suggests a new pharmacological tool to understand both inflammatory and antinociceptive processes mediated through lectins.
References
Peumans, W. J., & Van Damme, E. J. M. (1995). Plant Physiology, 109, 347–352.
Van Damme, E. J. M., Peumans, W. J., Barre, A., & Rougé, P. (1998). Critical Reviews in Plant Sciences, 17, 575–692.
Sharon, N., & LIS, H. (2004). Glycobiology, 14, 11.
Afolabi-Balogun, N. B., Inuwa, H. M., Ishiyaku, M., Bakare-Odunoola, M. T., & Nok, A. J. (2012). Infection, Genetics and Evolution, 12, 1508–1512.
Ghequire, M. G. K., Loris, R., & Mot, R. (2012). Biochemical Society Transactions, 40, 1553–1559.
Dhuna, V., Dhuna, K., Singh, J., Saxena, A. K., Agrawal, S. K., & Kamboj, S. S. (2010). Advances in Bioscience and Biotechnology, 1, 79–90.
Luo, Y., Xu, X., Liu, J., Li, J., Sun, Y., Liu, Z., Van Damme, E., Balzarini, J., & Bao, J. (2007). Journal of Biochemistry and Molecular Biology, 40, 358–367.
Yao, J., Zhao, X., Zhang, H., Liao, Z., Chen, F., Song, J., Sun, X., & Tang, K. (2003). Cell Research, 13, 301–308.
Ooi, L., Ho, W. S., Ngai, K. L., Tian, L., Chan, P. K., Sun, S. S., & Ooi, V. E. (2010). Journal of Biosciences, 35, 95–103.
Kaur, M., Singh, K., Rup, P. J., Saxena, A. K., Khan, R. H., Ashraf, M. T., Kamboj, S. S., & Singh, J. (2006). Archives of Biochemistry and Biophysics, 445, 156–165.
Shangary, S., Singh, J., Kamboj, S. S., Kamboj, K. K., & Sandhu, R. S. (1995). Phytochemistry, 40, 449–455.
Alencar, N. M. N., Teixeira, E. H., Assreuy, A. M. S., Cavada, B. S., Flores, C. A., & Ribeiro, R. A. (1999). Mediators of Inflammation, 8, 107–113.
Alencar, N. M., Cavalcante, C. F., Vasconcelos, M. P., Leite, K. B., Aragão, K. S., Assreuy, A. M., Nogueira, N. A., Cavada, B. S., & Vale, M. R. (2005). Journal of Pharmacy and Pharmacology, 57, 912–922.
Alencar, N. M. N., Oliveira, R. S. B., Figueiredo, J. G., Cavalcante, I. J. M., Matos, M. P. V., Cunha, F. Q., Nunes, J. V. S., Bomfim, I. R., & Ramos, M. V. (2010). Inflammation Research, 56, 245–254.
Assereuy, A. M. S., Shibuya, M. D., Martins, G. J., De Souza, M. L. P., Cavada, B. S., Moreira, R. A., Oliveira, J. T. A., Ribeiro, R. A., & Flores, C. A. (1997). Mediators of Inflammation, 6, 201–210.
Mota, M. R. L., Criddle, D. S. N., Alencar, N. M. N., Gomes, R. C., Meireles, A. V. P., Santi-Gadelha, T., Gadelha, C. A. A., Oliveira, C. C., Benevides, R. G., CAVADA, B. S., & Assereuy, A. M. S. (2006). Naunyn-Schmiedeberg's Archives of Pharmacology, 374, 1–10.
Santi-Gadelha, T., Gadelha, C. A., Aragão, K. S., Oliveira, C. C., Mota, M. R. L., Gomes, R. C., De Freitas, P. A., Toyama, M. H., De Oliveira, T. D., Alencar, N. M. N., Criddle, D. N., Assreuy, A. M. S., & Cavada, B. S. (2006). Biochemical and Biophysical Research Communications, 350, 1050–1055.
Assereuy, A. M. S., Martins, G. J., Moreira, M. E. F., Brito, G. A. C., Cavada, B. S., Ribeiro, R. A., & Flores, C. A. (1999). Journal of Urology, 161, 1988–1993.
Alencar, N. M. N., Assreuy, A. M. S., Alencar, V. B. M., Melo, S. C., Ramos, M. V., Cavada, B. S., Cunha, F. Q., & Ribeiro, R. A. (2003). Int. J. Biochem. Cell. B., 35, 1674–1681.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Moreira, R. A., & Perrone, J. C. (1977). Plant Physiology, 59, 783–787.
Bradford, M. M. A. (1976). Analytical Biochemistry, 72, 248–254.
Zimmermann, M. (1983). Pain, 16, 109–110.
Koster, R., Anderson, M., & De Beer, E. J. (1959). Federation Proceedings, 18, 412–416.
Hunskaar, S., & Hole, K. (1987). Pain, 30, 103–114.
Kuraishi, Y. (1983). Brain Research, 273, 245–252.
Leite, D. F. P., Echevarria-lima, J., Ferreira, S. C., Calixto, J. B., & Rumjanek, V. M. (2007). Journal of Leukocyte Biology, 82, 630–637.
Le Bars, D., Gozarium, M., & Cadden, S. W. (2001). Pharmacological Reviews, 53, 597–652.
Santi-Gadelha, T., Rocha, B. A. M., Oliveira, C. C., Aragão, K. S., Marinho, E. S., Gadelha, C. A. A., Toyama, M. H., Pinto, V. P. T., Nagano, C. S., Delatorre, P., Martins, J. L., Galvani, F. R., Sampaio, A. H., Debray, H., & Cavada, B. S. (2008). Applied Biochemistry and Biotechnology, 150, 97–111.
Viana, G. S. B., Freitas, A. L. P., Lima, M. M. L., Vieira, L. A. P., Andrade, M. C. H., & Benevides, N. M. B. (2002). Brazilian Journal of Medical and Biological Research, 35, 713–722.
Vieira, L. A. P., Freitas, A. L. P., Feitosa, J. P. A., Silva, D. C., & Viana, G. S. B. (2004). Brazilian Journal of Medical and Biological Research, 37, 1071–1079.
Bitencourt, F. S., Figueiredo, J. G., Mota, M. R. L., Bezerra, C. C. R., Silvestre, P. P., Vale, M. R., Nascimento, K. S., Sampaio, A. H., Nagano, C. S., Saker-Sampaio, S., Farias, W. R. L., Cavasda, B. S., Assreuy, A. M. S., & Ealencar, N. M. N. (2008). Naunyn-Schmiedeberg’s Archives of‘Pharmacology, 377, 139–148.
Neves, S. A., Freitas, A. L., Sousa, B. W., Rocha, M. L., Correia, M. V., Sampaio, D. A., & Viana, G. S. (2007). Brazilian Journal of Medical and Biological Research, 40, 127–134.
Silva, L. M. C. M., Lima, V., Holansdo, M. L., Pinhewiro, P. G., Rosdriguesj, A. G., Lima, M. E. P., & Benevides, N. M. B. (2010). Biological & Pharmaceutical Bulletin, 33, 830–835.
Vanderlei, E. S., Patoilo, K. K., Lima, N. A., Lima, A. P., Rodrigues, J. A., Silva, L. M., Lima, M. E., Lima, V., & Benevides, N. M. (2010). International Immunopharmacology, 10, 1113–1118.
Garcia, M. D., Fernandez, M. A., Alvarez, A., & Saenz, M. T. (2004). Journal of Ethnopharmacology, 91, 29–73.
Figueiredo, J. G., Bitencourt, F. S., Beserra, I. G., Texeira, C. S., Luz, P. B., Bezerra, E. H. S., Mota, M. R. L., Assereuy, A. M. S., Cunha, F. Q., Cavada, B. S., & Alencar, N. M. N. (2009). Naunyn-Schmiedeberg's Archives of Pharmacology, 380, 407–414.
Delatorre, P., Rocha, B. A. M., Simoes, R. C., Pereira Junior, F. N., Silva, H. C., Bezerra, E. H. S., Bezerra, M. J. B., Marinho, E. S., Gadelha, C. A. A., Santi-Gadelha, T., Farias, D. L., Assreuy, A. M. S., Marques Domingos, G. F. O., Nagano, C. S., & Cavada, B. S. (2011). Applied Biochemistry and Biotechnology, 164, 741–754.
Nunes, B. S., Resonnet, N. S., Dal-Secco, D., Vieira, S. M., Cavada, B. S., Teixeira, E. H., Moura, T. R., Clemente-Napimoga, J. T., Cunha, F. Q., & Napimoga, M. H. (2009). Naunyn-Schmiedeberg's Archives of Pharmacology, 379, 609–616.
Morteza-Semnani, K., Saledi, M., Hamidian, M., Vafamehr, H., & Dehpgur, A. R. (2002). Journal of Ethnopharmacology, 80, 181–186.
Tjølsen, A., Berge, O. G., Hunskaar, S., Rosland, J. H., & Hole, K. (1992). Pain, 51, 5–17.
Alencar, N. M. N., Assreuy, A. M. S., Criddle, D. N., Souza, E. P., Soares, P. M., Havt, A., Aragão, K. S., Bezerra, D. P., Ribeiro, R. A., & Cavada, B. S. (2004). Protein & Peptide Letters, 11, 195–200.
Gomes, J. C., Ferreira, R. R., Cavada, B. S., Moreira, R. A., & Oliveira, J. T. (1994). Agents and Actions, 41, 132–135.
Rocha, B. A. M., Delatorre, P., Oliveira, T. M., Benevides, R. G., Pires, A. F., Souza, A. A. S., Souza, L. A. G., Assereuy, A. M. S., Debray, M. H., Azevedo, W. F., Sampaio, A. H., & Cavada, B. S. (2011). Biochimie, 93, 806–816.
Bevilacqua, M. P., & Nelson, R. M. (1993). Selectins. Journal of Clinical Investigation, 91, 379–387.
Napimoga, M. H., Cavada, B. S., Alencar, N. M., Mota, M. L., Bitencourt, F. S., Alves-Filho, J. C., Grespan, R. B., Gonçalves, R. B., Clemente-Napimoga, J. T., De Freitas, A., Parada, C. A., Ferreira, S. H., & Cunha, F. Q. (2007). International Immunopharmacology, 7, 824–835.
Rangel, T. B. A., Assreuy, A. M. S., Pires, A. F., Carvalho, A. U., Benevides, R. G., Simoes, R. C., Silva, H. C., Bezerra, M. J. B., Nascimento, A. S. F., Nascimento, K. S., Nagano, C. S., Sampaio, A. H., Delatorre, P., Rocha, B. A. M., Fernandes, P. M. B., & Cavada, B. S. (2011). Molecules, 16, 5087–5103.
Liu, X. Q., Wu, H., Yu, H. L., Zhao, T. F., Pan, Y. Z., & Shi, R. J. (2011). Molecules, 16, 9480–9494.
Cunha, T. M., Verri, W. A. J. R., Schivo, I. R., Napimoga, M. H., Parada, C. A., Poole, S., et al. (2008). Journal of Leukocyte Biology, 83, 824–832.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araújo, T.S., Teixeira, C.S., Falcão, M.A.P. et al. Anti-inflammatory and Antinociceptive Activity of Chitin-binding Lectin from Canna Limbata Seeds. Appl Biochem Biotechnol 171, 1944–1955 (2013). https://doi.org/10.1007/s12010-013-0470-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0470-1