Abstract
The lectin from seeds of Dioclea virgata (DvirL) was purified in a single step affinity chromatography, sequenced by tandem mass spectrometry and submitted to crystallization and biological experiments. DvirL has a molecular mass of 25,412 ± 2 Da and the chains β and γ has 12,817 Da ± 2 and 12,612 Da ± 2, respectively. Primary sequence determination was assigned by tandem mass spectrometry and revealed a protein with 237 amino acids and 87% of identify with ConA. The protein crystals were obtained native and complexed with X-Man using vapor-diffusion method at a constant temperature of 293 K. A complete X-ray dataset was collected at 1.8 Å resolution. DvirL crystals were found to be orthorhombic, belonging to the space group I222, with a unit cell parameters a = 647.5 Å, b = 86.6 Å, c = 90.2 Å. Molecular replacement search found a solution with a correlation coefficient of 77.1% and an R factor of 44.6%. The present study also demonstrated that D. virgata lectin presents edematogenic and antinociceptive activities in rodents electing this protein as a candidate to structure/function analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are carbohydrate-binding proteins of non-immune origin that specifically recognize diverse sugar structures and mediate a variety of biological process [1]. Lectins from the Diocleinae sub-tribe demonstrate a high degree of similarity and belong to well-studied lectin leguminous family. Despite being highly homologous, they present significant differences in many biological activities [2].
DvirL was obtained from Dioclea virgata seeds, commonly known as pixuma beans. DvirL is not a glycoprotein as all many other lectins of the same sub-tribe investigated, e.g., Dioclea lehmanni seed lectin [3], and was partially biochemically characterized [4]. A molecular characterization and crystallization was previously performed [5], which results in the determination of 50 residues N-terminal sequence and obtaining of tetragonal crystals that diffracted to maximum resolution of 2.9 Å but the data were not consistent to solve crystal structure.
Although, there are many similar structural features between D. virgata lectin and the other Diocleinae lectins, differences have been found in some of their biological activities such as lymphocyte stimulation and γ-interferon production [6], edematogenic effects [7], histamine release experiments [8] and anti-inflammatory effect in the peritonitis and paw edema models induced by carrageenan in rats [9]. Some of these lectins are much more potent than Canavalia ensiformis lectin (ConA) and Phasheolus vulgaris agglutinin (PHA), traditionally used as excellent standard for immunological studies. The reasons for such marked differences cited above are unknown yet. Despite some minor differences in their primary and three-dimensional structures, it remains unclear why this group of proteins diverges considerably in many biological properties [2, 10, 11].
Leguminous lectins from the Diocleinae sub-tribe also present anti- or pro-inflammatory effect depending on the administration route used. They are inhibitory when applied systemically [12, 13], but they elicit stimulatory responses if locally injected [14, 15]. The anti-inflammatory effect of DvirL, injected intravenously, was already demonstrated in two acute experimental models of inflammation [9, 12], but there are no data describing its effect by local injection in model of inflammation, either its action in model of nociception.
The present work reports the mass spectrometry analysis and complete amino acid sequence, crystallization, and preliminary X-ray diffraction analysis of DvirL, describing its antinococeptive and edematogenic effects.
Material and Methods
Protein Purification
D. virgata seeds were collected at municipality of João Pessoa in Paraíba state in Northeast of Brazil. The seeds were ground to a fine powder in a coffee mill and the soluble proteins were extracted at 298 K by continuous stirring with 0.15 M NaCl [1:10(w:v)] for 4 h, followed by centrifugation at 10,000 ×g at 277 K for 20 min. The supernatant was applied onto a Sephadex G-50 column (10 × 50 cm) previously equilibrated with 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2. The unbound material was eluted with 0.15 M NaCl at a flow rate of 45 mL.h−1 until the absorbance at 280 nm of the effluent stabilized at 0.05. The retained fraction was eluted with 0.1 M glycine buffer pH 2.6 and dialyzed exhaustively against Milli-Q™ water [4]. This fraction was freeze-dried and used for further experiments.
MW Determination by Mass Spectrometry
The molecular mass of DvirL was determined by electrospray ionization using hybrid quadrupole/ion mobility separator/orthogonal acceleration–time of flight instrument (the Synapt HDMS system—Waters Corp., Milford, USA). The capillary voltage and the cone voltage were set at 3 kV and 40 V, respectively. Protein suspension (10 pmol/μL) was infused into the system at a flow rate of 10 μL/min. The source temperature was maintained at 373 K and nitrogen was used as a drying gas (flow rate about 150 L/h). The acquisition of data was performed by Mass Lynx 4.0 software and the multiply charge spectra were deconvoluted using maximum entropy techniques [16].
Protein Digestion and Tandem Mass Spectrometry Analysis
The protein was submitted to SDS-PAGE and the bands were excised from the gel and then bleached in a solution of 50 mM ammonium bicarbonate in 50% acetonitrile. The bands were then dehydrated in 100% acetonitrile and dried in a speedvac (LabConco). The protein bands were digested with a solution of 50 mM ammonium bicarbonate containing trypsin (Promega) or chymotrypsin (Sigma; 1:50 w/w; enzyme:substrate ratio) at 310 K overnight. The peptides were then extracted in a solution of 50% acetonitrile with 5% formic acid and then concentrated in speedvac. The peptides were separated by C18 chromatography column (75 μm × 100 μm) using a nanoAcquity™ system and eluted with acetonitrile gradient (10–85%), containing 0.1% formic acid. The liquid chromatography was connected to a nanoelectrospray mass spectrometer source (SYNAPT HDMS system—Waters Corp., Milford, USA). The mass spectrometer was operated in positive mode, using a source temperature of 355 K and capillary voltage at 3.5 kV. The instrument was calibrated with double protonated ion of glucofibrinopeptide B (M + 2H+) 2+ = 785.84. The LC-MS/MS experiment was used according to DDA (direct data analysis) function selecting for the experiments of MS/MS double or triple charged precursor ions, which were fragmented by collision-induced decomposition (CID) using a ramp collision energy which varied according to the charge state of precursor ion. The data were processed and analyzed using the Proteinlynx™ (Waters) and the search parameters were peptide mass fingerprint and fragmentation pattern of peptides. Some peptides sequences were obtained by de novo sequencing.
Primary Structure Analysis
Primary sequence alignments with secondary structure prediction were performed at ESPript 2.2 [17]. Phylogenetic tree and identity score were performed at ClustalW [18]. Theoretical pI was determinate by ProtParam [18].
Crystallization and Data Collection
The native lectin was resuspended in 1 mM Tris–HCl buffer pH 7.0 containing 5 mM CaCl2 and 5 mM MnCl2 in a final concentration of 10 mg mL−1. In order to obtain the lectin complexed with X-Man, freeze-dried native lectin was resuspended in Milli-Q™ water in a final concentration of 12 mg mL−1 and 3 mM of X-Man (5-bromo-4-chloro-3-indolyl-α-d-mannose) was added to protein suspension. X-Man complexed lectin was incubated at 310 K for 1 h before the crystallization experiments. The crystals grew in Linbro™ plates of 24 well at room temperature (293 K) by the vapor-diffusion method [19] in hanging drops using Crystal Screen I and II (Hampton Research, Riverside, CA, USA). Each well containing 300 μL of reservoir solution and the drops were composed of equal volumes (1 μl) of protein solution and reservoir solution.
X-ray data were collected from a single crystal cooled to a temperature of 100 K. Crystals were soaked in a cryoprotectant solution made of water (70%) and PEG 400 (30%) to avoid ice formation and were submitted to X-ray diffraction at a wavelength of 1.42 Å using a synchrotron-radiation source [MX1 station, Laboratório Nacional de Luz Síncrotron (LNLS), Campinas, Brazil]. A complete data set was obtained using a CCD (MAR Research) in 180 frames with an oscillation range of 1°. The data set was indexed and integrated using MOSFLM [20]. The intensities reduced through SCALA [21]. The phase problem was solved by the molecular replacement method using the program MolRep [22].
Biological Assays
Animals, Drugs, and Reagents
Male Swiss mice (20–25 g) were grown and housed (n = 10 per cage) in rooms with a controlled 12/12 h light/dark cycle, at 298 K with free access of food and water. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Universidade Estadual do Ceará (UECE-No 0559924-4), Fortaleza-CE, Brazil, in accordance with the Guide for the Care and Use of Laboratory Animals of the US Department of Health and Human Services (NIH publication no 85–23, revised 1985). Animals were deprived of food but with free access to drinking water for 12 h prior to the nociception test, which was performed at 295–299 K in adequate acoustic environmental conditions.
α-d-Glucose and formalin were purchased from Sigma (St. Louis, MO). Drugs were solubilized directly in sterile saline.
Paw Edema Model
Paw volume was measured immediately before subcutaneous (s.c.) injection of sterile saline (0.1 mL/100 g body mass), as negative control or DvirL (0.1, 1, and 10 mg/kg), as the inflammatory stimuli (zero time) into the animals hind paw and at selected time intervals (0.5, 1, 2–7 h) thereafter by hydroplethysmometry. Results were expressed as the variation in paw volume (mL) or area under curve—AUC (arbitrary units) in relation to the basal volume measured at zero time [23].
DvirL, at the most active dose (10 mg/kg), was incubated associated with its binding sugar glucose (0.1 M) for 30 min at 310 K prior administration. Glucose was individually incubated at the same conditions as controls.
Formalin Test
Formalin (20 μL; 2.5% v/v) was injected in the animals right hind paws and the time (s) that animal spent licking its paws was recorded at initial (0–5 min) and late (15–30 min) phases [24]. Animals were treated with saline, or DvirL (0.1, 1 and 10 mg/kg) by intravenous (i.v.) route 60 min before formalin. The antinociceptive activity was expressed as reduction of licking time.
Statistical Analysis
Results were presented as mean ± S.E.M. (n = 8) animals per group. Differences (P < 0.05) were analyzed using one-way analysis of variance ANOVA followed by the Bonferroni test.
Results and Discussion
Primary Sequence Analysis
DvirL has apparent molecular mass about 25 kDa by SDS-PAGE (data not show) as lectins from Dioclea guianensis (Dgui), Dioclea rostrata (DRL), Dioclea grandiflora (DGL), and Dioclea violacea (DVL) [5].
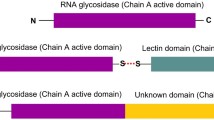
DvirL is composed by three chains (α, β, and γ) like concanavalin A (ConA). This protein is expressed as a pre-pro-protein (Ntermsignal peptide + γ chain + linker peptide + β chain + Ctermsignal peptide) that is cleaved in γ and β products. The active protein is a final fused product (α chain) in an inverse order of these two smaller chains (β + γ chains) without the signal and linker peptides [25, 26]. The α-chain has a molecular mass of 25,412 ± 2 Da by mass spectrometry and the chains fragments have 12,817 Da ± 2 for β-chain and 12,612 Da ± 2 for γ-chain (Fig. 1).
The complete sequence was determined by tandem mass spectrometry and consists of 237 amino acids residues, which β-chain comprises residues 1–118 and γ-chain 119–237 (Fig. 2). The protein sequence data reported in this paper will appear in the UniProt Knowledgebase under the accession number P58907. The theoretical pI calculated to this protein is 5.26. Table 1 displays all sequenced peptides and their respective molecular mass and a representative sequenced peptide shows the carbohydrate-binding loop sequence (Fig. 3).
DvirL has 84% of identity with ConA (SwissProt accession code: P02866), 94% with DVL (SwissProt accession code: P58909) and DGL (SwissProt accession code: A9J251), 95% with DRL (SwissProt accession code: P58908) and 97% with Dgui (SwissProt accession code: P81637), the last one differs only in five residues: 32(V/I), 155(Q/E), 158(R/K), 162(D/S), 164(S/D; Fig. 4a).
The phylogenetic analysis based on primary sequences shows a monophyletic group of proteins, and basically, three paraphyletic groups in it (Fig. 4b). The first (DvirL and Dgui) sharing characteristics such dimer–tetramer equilibrium and affinity by biantenary carbohydrates, the second group (DGL and DVL) have no affinity to biantenary carbohydrates and are homotetramers at pH above 4.5 and a third and more primitive group, only composed of DRL shows dimer–tetramer equilibrium and has no affinity to biantenary carbohydrates as synapomorphy of the group.
Diocleinae lectins could be divided into two groups corresponding to their affinities for biantennary complex carbohydrates where DGL, DVL and DRL would be one group (low affinity to biantenary carbohydrates with terminal GlcNAc) and Dvir and Dgui in another group (affinity to monodeoxy and tetradeoxy trimannoside analogs) [27].
It has been also reported that the pH-dependent dimer/tetramer equilibrium has a huge influence in the oligosaccharides recognition and lectin biological activities [5]. As previously described, DvirL behaves as a tetrameric assembly at pH of 6.5 or higher. DGL and DVL only organizes their monomers as homotetramers at pH higher than 4.5 both DvirL and Dgui exhibit dimer/tetramer equilibrium between pH 4–6 [5] and was proposed that Asn131 residue is a key amino acid in pH-dependent oligomerization [28]. Moreover, Nagano and co-workers [29] suggested that 123(A/E) 131(N/H) 133(Q/K) are important modifications for dimer/tetramer equilibrium property.
Crystallization, Data Collection, and Preliminary X-ray Structure Analysis
DvirL native crystals were obtained after a week using conditions No. 06, 49, and 50 from Hampton Crystal Screen I. Conditions 49 and 50 were chosen to be optimized due to similarities between them, both composed by the same salt (lithium sulfate) and precipitant (PEG 8000). The concentration of lithium sulfate was modified from 0.125 to 0.875 M and the PEG 8000 varied from 2% to 12%. After a week, suitable crystals for diffraction experiments were obtained on lithium sulfate concentrations from 0.1 to 0.5 M and PEG 8000 from 10% to 12% (Fig. 5a).
DvirL X-Man complexed crystals were obtained after 2 or 3 weeks using condition No. 15, 17, and 32 from Hampton Crystal Screen II. Suitable crystals (Fig. 5b) for diffraction experiments were obtained direct from the condition No. 15 of the Hampton Crystal Screen II (0.5 M ammonium sulfate, 0.1 M sodium citrate tribasic dihydrate pH 5.6 and 1.0 M lithium sulfate monohydrate). The X-Man complexed with DvirL enhanced the crystallization and permitted more accurate X-ray data diffraction analysis. Crystallization process must be optimized in presence of X-Man because the improvement of carbohydrate-binding site domain stabilization, which can classify it as a regular associated compound to glucose/mannose lectin crystallization.
The crystals obtained provided a data set extending to 2.45 (native crystal) and 1.8 Å (X-Man complexed crystal) resolution which was scaled in the resolution range 33.35–2.45 and 21.33–1.80 Å, respectively. DvirL crystal belongs to the centered orthorhombic space group I222. The Matthews coefficient values (2.37 Å3 Da−1 Native, 2.48 Å3 Da−1 X-Man complexed) [30] was calculated based on the molecular weight of 25.4 kDa, indicated a solvent content of 48.15% (native) and 50.36% (X-Man complexed), which corresponds to the presence of a monomer in the asymmetric unit. Data collection statistics are summarized in Table 2.
The preliminary crystal structure of DvirL was determined by standard molecular replacement methods using the program MOLREP [22]. Various monomers were tested for the replacement and the best result was obtained with the structural model of DRL (PDB code 2ZBJ) [31]. The best solution had a final correlation coefficient of 0.771 and an R factor of 0.446.
Comparing crystals asymmetric unities, DvirL, Dgui, and DRL are presented as monomers, only DGL is presented as dimer in the asymmetric unit. These results suggest that DvirL has a crystal packing similar to both Dgui and DRL, regarding the carbohydrate-binding site and the interdimeric contacts. Structural analyses are necessary to confirm this hypothesis and to enlighten the understanding of the pH-dependent dimer–tetramer equilibrium and the carbohydrate recognition in Diocleinae lectins. Intending to continue this study, the crystal structure resolution is in progress.
DvirL Induces Edematogenic Effect Via Lectin Domain
The s.c. injection of DvirL elicited a dose dependent paw edema at the highest doses tested (1 and 10 mg/kg; Fig. 6a, b). DvirL edematogenic effect was initiated at 30 min and maintained significant until 5 h compared to control (Fig. 6a). Among all doses tested, the maximal edematogenic effect of DvirL was attained at 10 mg/kg, increasing the paw volumes of 4× (12.628 ± 1.647) compared to control (Saline: 3.101 ± 835 arbitrary units; Fig. 6b). The edematogenic effect of DvirL at 10 mg/kg was inhibited by its association with glucose (6.210 ± 1.982 arbitrary units). Furthermore, glucose did not elicit edema per se (Fig. 6c).
DvirL induces edematogenic effect via lectin domain. Animals received DvirgL (0.1; 1; 10 mg/kg; s.c.), glucose (0.1 M), DvirL (10 mg/kg) + glucose or saline (0.01 μL/paw). Edema was measured before (time zero) and at 1/2, 1, 2–7 h. Mean ± S.E.M. (n = 8) *p < 0.05 versus saline; # p < 0.05 versus DvirgL
The edematogenic effect of DvirL corroborates other findings in which inflammatory activities of plant lectins were shown [32, 33]. The time course of edema induced by DvirL was compared to those of classical inflammatory substances dextran and carrageenan. Dextran elicits a short-duration osmotic edema with maximal effect at 30 min [34], whilst carrageenan induces a longer-lasting edema with intense leukocyte infiltrate [35].
Based on these, our investigation suggests that the edema induced by DvirL involves cell infiltration. In addition, the importance of carbohydrate sites was also demonstrated on various effects elicited by Diocleinae lectins in inflammation [9, 12, 13]. Our results demonstrated that the edematogenic activity of DvirL was reversed by its specific sugar, showing close relationship between its pro-inflammatory action and specific sugar binding properties and confirming the pattern of response among Diocleinae lectins. Structure/function analysis and comparisons with other legume lectins would permit classify this group of protein based on their activity through inflammatory process.
DvirL Presents Antinociceptive Effect
DvirL inhibited the paw licking induced by formalin at 10 mg/kg in the first phase (29.88 ± 3.79 s) by 34% and at 1 mg/kg in the second phase (125.00 ± 11.45 s) by 36% (Fig. 7a, b).
DvirL inhibits the nociception induced by formalin. Dose–response curve (a, b). Animals received DvirL (0.1; 1; 10 mg/kg) or saline by intravenous (i.v.) route 30 min before formalin 37%. The time (s) that animals spent licking its paws was recorded at initial (0–5 min) and late (15–30 min) phases after formalin. Mean ± S.E.M. (n = 8). *p < 0.05 compared to formalin
In general, it is accepted that centrally acting drugs, such as narcotics, inhibit both phases of the formalin-induced nociception, and that peripherally acting anti-inflammatory drugs inhibit mainly the second phase [36, 37]. The effect of DvirL, in both phases of the formalin test, pointed to a mixed antinociceptive mechanism, either peripheral or central. Accordingly, such mechanism has been demonstrated for the lectin from Canavalia grandiflora [15]. This hypothesis elects DvirL as a protein candidate to understand anti-inflammatory central action of lectins throughout crystallographic studies.
Conclusion
DvirL is a 25,412-Da protein which associates as a tetramer and is synthesized as a single product (α-chain) originated of a circular permutation post-translational process, which cleaves the pre-pro-protein in two small chains (β- and γ- chains). The α-chain polypeptide has 237 amino acids and is classified as a ConA like lectin based on their primary sequence identity and phylogenetic analysis. DvirL was crystallized to permit obtaining the crystal structure and indicate some features about carbohydrate recognition and dimer/tetramer association. The X-Man complexed with DvirL enhanced the crystallization and permitted more accurate X-ray data diffraction analysis. The present study also demonstrated that D. virgata lectin presents edematogenic and antinociceptive activities in rodents. The edematogenic effect was shown to occur via interaction with the lectin domain. These findings elect this protein as future issue of structure/function relationship analysis.
References
Sharon, N., & Lis, H. (2004). Glycobiology 14, 11, 53R–62R.
Cavada, B. S., Barbosa, T., Arruda, S., Grangeiro, T. B., & Barral-Netto, M. (2001). Current Protein & Peptide Science, 2, 123–135.
Perez, G., Perez, C., Sousa-Cavada, B., Moreira, R. A., & Richardson, M. (1991). Phytochemistry, 30, 2619–2621.
Cavada, B. S., Ramos, M. V., Cordeiro, E. F., Grangeiro, T. B., Carvalho, A. F. F. U., & Moreira, R. A. (1996). R Bras Fisiol Veg, 8, 37–42.
Calvete, J. J., Thole, H. H., Raida, M., Urbanke, C., Romero, A., Grangeiro, T. B., et al. (1999). Biochimica et Biophysica Acta, 1430, 367–375.
Barral-Netto, M., Santos, S. B., Barral, A., Moreira, L. I. M., Santos, C. F., Moreira, R. A., et al. (1992). Immunological Investigations, 21, 297–303.
Bento, C. A. M., Cavada, B. S., Oliveira, J. T. A., Moreira, R. A., & Barja-Fidalgo, C. (1993). Agents and Actions, 38, 48–54.
Gomes, J. C., Rossi, R. R., Cavada, B. S., Moreira, R. A., & Oliveira, J. T. A. (1994). Agents and Actions, 41, 132–135.
Alencar, N. M. N., Teixeira, E. H., Assreuy, A. M., Cavada, B. S., Flores, C. A., & Ribeiro, R. A. (1999). Mediators Inflamm, 8, 107–113.
Moreno, F. B. M. B., Delatorre, P., Freitas, B. T., Rocha, B. A. M., Souza, E. P., FacoÂ, F., et al. (2004). Acta Crystallographica, D60, 1493–1495.
Gadelha, C. A. A., Moreno, F. B. M. B., Santi-Gadelha, T., Cajazeiras, J. B., Da Rocha, B. A. M., Assreuy, A. M. S., et al. (2005). Journal of Structural Biology, 152, 185–194.
Assreuy, A. M. S., Shibuya, M. D., Martins, G. J., Souza, M. L. P., Cavada, B. S., Moreira, R. A., et al. (1997). Mediators of Inflammation, 6, 201–210.
Assreuy, A. M., Martins, G. J., Moreira, M. E. F., Brito, G. A. C., Cavada, B. S., & Ribeiro, R. A. (1999). T J Urol, 161, 1988–1993.
Assreuy, A. M. S., Fontenele, S. R., Freitas, P. A., Fernandes, D. C., Rodrigues, N. V., Bezerra, E. H., et al. (2009). Naunyn-Schmiedebergs Archives of Pharmacology, 380, 509–521.
Nunes, B. S., Rensonnet, N. S., Dal-Secco, D., Vieira, S. M., Cavada, B. S., Teixeira, E. H., et al. (2009). Naunyn-Schmiedebergs Archives of Pharmacology, 379(6), 609–616.
Ferrige, A. G., Seddon, M. J., Green, B. N., Jarvis, S. A., & Skilling, J. (1992). Disentangling electrospray spectra with maximum entropy. Rapid Communications in Mass Spectrometry, 6, 707–711.
Gouet, P., Robert, X., & Courcelle, E. (2003). Nucleic Acids Research, 31, 3320–3323.
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wikins, M. R., Appel, R. D., et al. (2005). Protein Identification and Analysis Tools on the ExPASy Server. In J. M. Walker (Ed.), The Proteomics Protocols Handbook (pp. 571–607). Humana.
Jancarik, J., & Kim, S. H. (1991). Journal of Applied Crystallography, 24, 409–411.
Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBMNewsl. Protein Crystallogr.
Evans, P. R. (1997). Joint CCP4 and ESF-EACBM Newsletter, 33, 22–24.
Vargin, A., & Teplyakov, A. (1997). Journal of Applied Crystallography, 30, 1022–1025.
Landucci, E. C. T., Antunes, E., Donato, J. L., Faro, R., Hyslop, S., & Marangoni, S. (1995). British Journal of Pharmacology, 114, 578–583.
Hunskaar, S., & Hole, K. (1987). Pain, 30, 103–14.
Cunningham, B. A., Hemperly, J. J., Hopp, T. P., & Edelman, M. (1979). Favin versus concanavalin A: circularly permuted amino acid sequences. Proceedings of the National Academy of Science, 76(7), 3218–3222.
Carrington, D. M., Aufiet, A., & Hanke, D. E. (1985). Nature, 313, 64–67.
Dam, T. K., Cavada, B. S., Grangeiro, T. B., Santos, C. F., Sousa, F. A. M., Oscarson, S., et al. (1998). The Journal of Biological Chemistry, 273, 12082–12088.
Wah, D. A., Romero, A., Gallego del Sol, F., Cavada, B. S., Ramos, M. V., Grangeiro, T. B., et al. (2001). Journal of Molecular Biology, 310, 885–894.
Nagano, C. S., Calvete, J. J., Barettino, D., Pérez, A., Cavada, B. S., & Sanz, L. (2008). The Biochemical Journal, 409, 417–28.
Matthews, B. W. (1968). Journal of Molecular Biology, 33, 491–487.
Oliveira, T. M., Delatorre, P., Rocha, B. A. M., Souza, E. P., Nascimento, K. S., Bezerra, G. A., et al. (2008). Journal of Structural Biology, 164(2), 177–182.
Bento, C. A. M., Cavada, B. S., Oliveira, J. T. A., Moreira, R. A., & Barja-Fidalgo, C. (1993). Agents and Actions, 38, 48–54.
Alencar, N. M. N., Assreuy, A. M. S., Criddle, D. N., SouzaA, E. P., Soares, P. M. G., Havt, A., et al. (2004). Protein and Peptide Letters, 11, 195–200.
Lo, T. N., Almeida, A. P., & Beaven, M. A. (1982). The Journal of Pharmacology and Experimental Therapeutics, 221, 261–267.
Di Rosa, M., Giround, J. P., & Willoughby, D. (1971). The Journal of Pathology, 104, 15–29.
Santos-de-Oliveira, R., Dias-Baruffi, M., Thomaz, S. M., Beltramini, L. M., & Roque-Barreira, M. C. (1994). Journal of Immunology, 153, 1798–1807.
Tjølsen, A., & Hole, K. (1997). Animal models of analgesia. In A. Dickenson & J. Besson (Eds.), The pharmacology of pain (Vol. 130/I, pp. 1–20). Berlin: Springer.
Acknowledgments
This work was supported by Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico—FUNCAP, Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, National Synchrotron Light Laboratory—LNLS, Brazil. BSC and PD are senior investigators of CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delatorre, P., Rocha, B.A.M., Simões, R.C. et al. Mass Spectrometry and X-ray Diffraction Analysis of Two Crystal Types of Dioclea virgata Lectin: An Antinociceptive Protein Candidate to Structure/Function Analysis. Appl Biochem Biotechnol 164, 741–754 (2011). https://doi.org/10.1007/s12010-011-9170-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9170-x