Abstract
Objective and design
The involvement of nitric oxide pathway in the antinociceptive activity of Lonchocarpus araripensis lectin (LAL) was investigated in the model of carragenan-induced hypernociception.
Methods
Swiss mice received LAL (0.01–10 mg/kg; i.v.) 30 min before s.c. injection of carragenan in the paws. For the involvement of nociceptive pathways, animals were previously treated with the blockers: NOS (L-NAME, aminoguanidine, 7-nitroindazole); soluble guanylyl cyclase (ODQ); channels of ATP-dependent K+ (glibenclamide); L-type Ca2+ (nifedipine), or Ca2+-dependent Cl− (niflumic acid). Participation of lectin domain was evaluated by injection of LAL associated with N-acetyl-glucosamine (GlcNAc). nNOS gene relative expression was evaluated in the paw tissues and nNOS immunostaining in dorsal root ganglia.
Results
LAL at all doses inhibited carrageenan-induced hypernociception (4.12 ± 0.58 g), being maximal at 10 mg/kg (3 h: 59%), and reversed by GlcNAc. At this time, LAL effect was reversed by nifedipine (39%), niflumic acid (59%), L-NAME (59%), 7-nitroindazole (44%), ODQ (45%), and glibenclamide (34%), but was unaltered by aminoguanidine. LAL increased (95%) nNOS gene expression in mice paw tissues, but not its immunoexpression in the dorsal root ganglia.
Conclusion
The antinociceptive effect of Lonchocarpus araripensis lectin involves activation of the l-arginine/NO/GMPc/K+ATP pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is an important neurotransmitter involved in the nociceptive process that contributes to the development of central sensitization in the dorsal horn of the spinal cord. However, experimental data have demonstrated the inhibitory role of NO in nociception both in peripheral and central nervous system. In vitro, the endogenous NO produced in cultured neurons of dorsal root ganglia decreases mechanosensitivity via inhibition of voltage-gated Na+ and Ca2+ channels (Chaban et al. 2001). In addition, it has been shown that NO mediates the analgesic effect of opioids and other analgesic substances (Cury et al. 2011).
Despite of the improved knowledge in the underlying pain mechanisms, the pharmacological analgesic approach is still insufficient (Su et al. 2014; Loeser and Melzack 1999). Thus, the characterization of specific pathophysiological alterations involved in inflammatory diseases and the investigation of pain modulator molecules are necessary.
Lectins isolated from seeds of leguminous plants belonging to Dalbergieae tribe (Platypodium elegans and Machaerium acutifolium) have been described to modulate the nociceptive process in animal models eliciting hypernociceptive or antinociceptive effects via interaction with carbohydrates (Nascimento et al. 2020). Although scarce, the antinociceptive mechanisms were mostly described for lectins of the genus Lonchocarpus, such as the inhibitory effect of the inflammatory nociception by L. sericeus and L. campestris lectins (Napimoga et al. 2007; Pires et al. 2019).
The lectin isolated from L. araripensis (LAL), focus of the present study, has shown pleiotropic effect in several nociceptive pathways, including a direct action on primary nociceptor fibers, inhibiting Na+ current, and also an indirect effect upon hypernociceptive mediators, such as adrenaline, bradykinin, prostaglandin E2, and TNF-α (Amorim et al. 2016). Besides, LAL presented in vivo antiinflammatory (Pires et al. 2016) and in vitro vasodilator (Pires et al. 2017) effects via NO.
This study investigated the underlying mechanism of the nitric oxide pathway in the antinociceptive activity of the lectin isolated from Lonchocarpus araripensis seeds in the mice model of hypernociception.
Materials and methods
Lectin
LAL was isolated and purified by affinity and ion-exchange chromatography (Pires et al. 2016) from seeds of Lonchocarpus araripensis BENTH (Leguminosae, Papilionaceae, Dalbergieae).
Drugs and reagents
Lambda carrageenan, N-acetyl-glucosamine (GlcNAc), L-NG-Nitroarginine Methyl Ester (L-NAME), aminoguanidine, 7-nitroindazole, 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one (ODQ), glibenclamide, nifedipine, niflumic acid, and RNAlater were purchased from Sigma (St. Louis, Missouri, USA); Anti-nNOS and anti-rabbit IgG from Biogen (São Paulo-SP, Brazil); Reverse transcriptase MMLV from GE Healthcare (São Paulo-SP, Brazil); primers Oligo (dT) and dNTP from Thermo Fisher (Waltham—Massachusetts, USA); Brazol kit from LGC Biotecnologia (Cotia—SP, Brazil); DNAse from Invitrogen® (Carlsbad—California, USA); GelRed from Biotium (Fremont—California, USA); Primers for β-actin and iNOS from Integrated DNA Technology-IDT (Coralville—Iowa, USA).
Animals
Swiss male mice (25–30 g) were maintained in adequate environmental conditions (12 h/12 h dark/white cycles, 25 °C), receiving water and food ad libitum. Experimental protocols were conducted according to the international ethic principles (National Institute of Health—NIH nº 85–23, revised in 2011) and approved by the Ethic Committee for the use of Experimental Animals of the State University of Ceará (CEUA/UECE nº 2127461/2015).
Hypernociception model: mechanical allodynia
Mice were individually placed in boxes of elevated wire mesh platforms to allow access to the ventral surface of hind paws, in which were applied 6 consecutive mechanical pressures, using a polypropylene tip (0.5 mm diameter) coupled to digital analgesimetry. The paw withdrawal response (g) was determined before (basal value), 60 and 180 min after intraplantar subcutaneous (s.c.) injection of carrageenan (300 μg/paw/50 μl) as nociceptive stimulus (Fig. 1). The reduced intensity force required to evoke paw withdrawal is indicative of hypernociception (Cunha et al. 2014).
Outline scheme of the mice treatment and experimental protocols. Mice were intravenously treated with LAL alone or associated with GlcNAc 30 min after treatment with the inhibitors (L-NAME, aminoguanidine, 7-nitroindazole, ODQ, glibenclamide, nifedipine, or niflumic acid 30 min before the administration of nociceptive stimulus (carrageenan) in mice paws. Before experiment and 60 and 180 min after carrageenan injection, the paw withdrawal response (mechanical allodynia) was measured by digital analgesimetry. Mice were euthanized 180 min after carrageenan and the paws collected to nNOS relative gene expression and immunohistochemistry
Pharmacological modulation of LAL antinociceptive effect
Animals were treated with LAL (0.01, 0.1, 10 mg/kg) by intravenous (i.v.) route 30 min before injection of carrageenan.
For evaluation of the nociceptive pathways, animals were treated s.c. or intraperitoneal (i.p.) 30 min before LAL with inhibitors of the following mediators: NOS (L-NAME; 100 mg/kg), iNOS (aminoguanidine; 50 mg/kg), nNOS (7-nitroindazole; 25 mg/kg), soluble guanylyl cyclase (ODQ; 50 μg/paw), ATP-dependent K+ channels (glibenclamide; 200 μg/paw), L-type Ca2+ channels (nifedipine; 5 mg/kg), or Ca2+-dependent Cl− channels (niflumic acid; 30 mg/kg) (Fig. 1).
The participation of the lectin domain was evaluated by previous incubation (1 h, 37 °C) of LAL (10 mg/kg) associated to its ligand sugar N-acetyl-glucosamine (0.1 M GlcNAc) before injection. GlcNAc was previously incubated in the same conditions before being injected i.v. in the animals to investigate its effect per se (Fig. 1).
Relative nNOS gene expression
Animals were euthanized 3 h after carrageenan. Total RNA was extracted from longitudinal paw tissues (100 mg), treated with DNase using 1 ml Brazol reagent (Fig. 1), resuspended in RNase-free water and the concentration determined at A260nm. RNA purity was checked at optical density ratio (OD260/OD280) between 1.8 and 2.0, being its quality and integrity analyzed in 1.2% agarose gel stained with GelRed® (Biotium).
For cDNA synthesis, total RNA (1 μg) was incubated with the following: reverse-transcriptase MMLV, primer Oligo (dT), and dNTP. Reverse transcription reaction (20 µl) was performed at 37 °C for 50 min, the enzyme denaturation at 70 °C for 15 min, followed by rapid cooling at 4 °C. cDNA concentration was determined at A260nm. RT-negative control was performed in the same conditions, but lacking reverse transcriptase.
Quantitative real-time polymerase chain reaction (qRT-PCR) was assayed using termocycler Bioer LineGene 9660 (Bioer, China) and software PCR LineGene 9660. GoTaq® qPCR Master Mix kit (Promega) was used to amplify the genes. The reaction (20 µl) included 0.2 µM of each primer, 1 μl cDNA and 10 μl of GoTaq® qPCR Master Mix. An initial cycle was performed at 95 °C for 5 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 30 s. Melting curve was performed to evaluate primers, dimers, and other artifacts. The gene expression was determined using the 2−∆∆CT method (Livak and Schmittgen 2001).
Immunohistochemistry
Animals were anesthetized i.p. with ketamine (100 mg/kg) and xylazine (10 mg/kg) 3 h after carrageenan injection into animal paws, Animals received intracardiac perfusion (paraformaldehyde 4%, 4 min) (Fig. 1), before removal and dissection of dorsal root ganglia (L3–L5). Tissues were fixed in 4% paraformaldehyde for 4 h and incubated (12 h, 4 °C) with the polyclonal anti-nNOS and with the secondary antibody IgG (30 min, r.t.) and streptavidin–biotin peroxidase (sABC). The chromogen 3,3 diaminobenzidine (DAB) peroxide was applied to tissues for 10 min, and counterstained with Mayer’s hematoxylin. For semiquantitative evaluation, areas presenting high concentration of immunostained cells (cytoplasm and nucleolus) were randomly selected in five fields/slide (400×) and scored as follows: (0) no positive cells; (1—mild) 1–33% positive cells; (2—moderate) 34–66% positive cells; (3—intense) 67–100% positive cells (HSU et al. 1981).
Statistical analysis
Statistical differences were determined by analysis of variance (one-way ANOVA) followed by Bonferroni test. Parametric data were expressed as mean ± SEM (n = 8/group or n = 3/group for gene expression). Clinical signs, histopathological, and immunohistochemical data were expressed as % frequency (f), median (maximum and minimum), and analyzed by Kruskal–Walls followed by Dunn and Chi-square tests. P < 0.05 was considered statistically significant.
Results
LAL inhibits carrageenan-induced hypernociception: reversion by N-acetyl-glucosamine
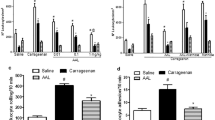
Carrageenan reduced the nociceptive threshold in response to mechanical stimulation of the animal paws at 1 h (carrageenan: 4.12 ± 0.58 vs. saline: 9. 62 ± 0 98 g) and 3 h (carrageenan: 4 ± 0.56 vs. saline: 10.62 ± 0.98 g) after its administration. The hypernociception induced with carrageenan was inhibited by LAL at 0.1 mg/kg (1 h: 4.72 ± 0.37 g; 3 h: 5.22 ± 0.37 g); 1 mg/kg (1 h: 8.62 ± 0.32 g; 3 h: 7.22 ± 0.32 g); and 10 mg/kg (1 h: 9.37 ± 0.70 g; 3 h: 9.77 ± 0.70 g) (Fig. 2a), showing maximal inhibition at 10 mg/kg (1 h: 56%; 3 h: 59%). The inhibitory effect of LAL (10 mg/kg) was reversed by the lectin association with N-acetyl-glucosamine (1 h: 5.5 ± 0.5 g; 3 h: 5.75 ± 0.75 g) (Fig. 2b). N-acetylglycosamine exhibited no effect per se.
LAL inhibits carrageenan-induced hypernociception: reversion by N-acetylglucosamine. LAL was administered i.v. 30 min before carrageenan (Cg: 300 µg/paw; s.c.). Hypernociception was evaluated 1 h and 3 h after Cg administration by digital analgesimetry. a LAL (0.01; 0.1, 1, or 10 mg/kg), b LAL (10 mg/kg), LAL + N-acetylglucosamine (GlcNAc: 0.1 M) or GlcNAc (0.1 M) was administered 30 min before Cg. Mean ± SEM (n = 6–8). An one-way ANOVA/Bonferroni: Filled circle p < 0.05 vs. saline; filled triangle p < 0.05 vs. Cg; dashed symbol p < 0.05 vs. LAL (10 mg/kg)
Nifedipine and niflumic acid reverses LAL antihypernociceptive effect
Nifedipine reversed the antihypernociceptive effect of LAL by 49% at 1 h (5.67 ± 0.56 vs. LAL: 11 ± 0.31 g) and 39% at 3 h (6.2 ± 0.37 vs. LAL: 10.2 ± 0.68 g) (Fig. 3a). Niflumic acid also inhibited the antihypernociceptive effect of LAL by 62% at 1 h (6.3 ± 0.35 vs. LAL: 16.62 ± 0.94 g) and 59% at 3 h (6.1 ± 0.37 vs. LAL: 14.75 ± 1.39 g) (Fig. 3b). The hypernociceptive profile of carrageenan (1 h: 4.12 ± 0.58 g; 3 h: 4 ± 0.56 g) was reduced by the reference drug nifedipine, both at 1 h (5.67 ± 0.56 g) and 3 h (5.73 ± 0.65 g), but was unaltered by niflumic acid.
Nifedipine and niflumic acid inhibit LAL antihypernociceptive effect. LAL (10 mg/kg) was administered i.v. 30 min before carrageenan (Cg: 300 µg/paw; s.c.). Hypernociception was evaluated 1 h and 3 h after Cg administration by digital analgesimetry. a Nifedipine (5 mg/kg; i.p.) or b Niflumic acid (30 mg/kg; i.p.) was administered 30 min before LAL. Mean ± SEM (n = 6–8). One-way ANOVA/Bonferroni: Filled circle p < 0.05 vs. Saline; filled triangle p < 0.05 vs. Cg; dashed symbol p < 0.05 vs. LAL.
L-NAME, 7-nitroindazole, ODQ, or glibenclamide, but not aminoguanidine, reverses LAL antihypernociceptive effect
LAL antihypernociceptive effect was reversed by the following blockers: L-NAME by 59% at 1 h (6.87 ± 0.58 vs. LAL: 16.62 ± 0.94 g) and 59% at 3 h (5.62 ± 0.41 g vs. LAL: 13.65 ± 1.39 g) (Fig. 4a); 7-nitroindazole by 49% at 1 h (5.01 ± 0.79 vs. LAL: 9.75 ± 0.78 g) and 44% at 3 h (6.05 ± 0.66 vs. LAL: 10.75 ± 0.59 g) (Fig. 4c); ODQ by 61% at 1 h (4.62 ± 0.37 vs. LAL: 11.87 ± 0.61 g) and 45% at 3 h (5.37 ± 0.65 vs. LAL: 9.7 ± 0.70 g) (Fig. 4d); glibenclamide by 48% at 1 h (4.5 ± 0.54 vs. LAL: 8.7 ± 0.64 g) and 34% at 3 h (5.75 ± 0.75 vs. LAL: 8.7 ± 0.88 g) (Fig. 3e). However, aminoguanidine did not alter LAL effect (Fig. 4b).
L-NAME, 7-nitroindazole, ODQ, or glibenclamide, but not aminoguanidine, reverses LAL antihypernociceptive effect. LAL (10 mg/kg; i.v.) was administered i.v. 30 min before carrageenan (Cg: 300 µg/paw). Hypernociception was evaluated 1 h and 3 h after Cg administration by digital analgesimetry. a L-NAME (10 mg/kg; i.p.), b aminoguanidine (50 mg/kg; s.c.), c 7 NI (25 mg/kg; s.c.), d ODQ (8 μg/paw; s.c.), or e glibenclamide (200 μg/paw; s.c.) was administered 30 min before LAL. Mean ± SEM (n = 6–8). One-way ANOVA/Bonferroni: Filled circle p < 0.05 vs. saline; filled triangle p < 0.05 vs. LAL
LAL increases relative nNOS gene expression in mice paw tissues stimulated with carrageenan but not its immunoexpression in the dorsal root ganglia
Total RNA quantitative profile maintained a relationship between absorbances (260/280 nm) in the range of 1.5 to 1.9. The qRT-PCR revealed that LAL increased by 95% the relative gene expression for nNOS compared to carrageenan (LAL: 76.82 vs. carrageenan: 3.49 vs. saline: 1) (Fig. 5). On the other hand, LAL did not alter nNOS immunostaining [median score: 3 (+ 3; + 3)] in the dorsal root ganglia of mice paw tissues stimulated with carrageenan [median score: 3 (+ 2; + 3) vs. saline: 2 (+ 2; + 3)] (Fig. 6).
LAL increases nNOS gene expression in mice paw tissues stimulated with carrageenan. LAL (10 mg/kg; i.v.) was administered i.v. 30 min before carrageenan (Cg: 300 µg/paw). Animals were euthanazied 3 h after Cg before collection of paw tissues. nNOS expression was evaluated by qRT-PCR. ANOVA and Bonferroni: Filled circle p < 0.05 vs. carrageenan
LAL does not alter nNOS immunoexpression in the dorsal root ganglia of mice in the model of hypernociception induced by carrageenan. LAL (10 mg/kg; i.v.) was administered i.v. 30 min before carrageenan (Cg: 300 µg/paw). Immunohistochemistry (DAB/Harris H&E, 400×), arrowheads in b, c. a Saline, b carrageenan, and c LAL. Median (maximum and minimum), Kruskal–Walls followed by Dunn and Chi-square test
Discussion
The present study demonstrates that the lectin isolated from Lonchocarpus araripensis (LAL) presents antinociceptive effect, observed in the experimental model of mechanical allodynia, that occurs via lectin domain. This is the first report that associates LAL effect and the nitric oxide pathway.
It has been previously shown that Lonchocarpus sericeus lectin inhibits carrageenan-induced hypernociception, an effect that was associated to reduced neutrophil migration and inhibition of cytokines and chemokines (Napimoga et al. 2007). Our research group also reported the antinociceptive effect of another lectin isolated from the genus Lonchocarpus (L. araripensis-LAL). LAL reduced carrageenan-induced hypernociception and that induced by several other mediators. Among these, LAL inhibited TNF-α, BK, PGE2 and adrenaline-induced hypernociception. The fact that LAL inhibits hypernociception caused by different mediators leads to a hypothesis that the mechanism of its antinociceptive action may be related to a direct action on the nociceptor or on the primary fibers, preventing the development of the hypernociceptive state. This hypothesis was justified because LAL decreased the total current produced by Na+ movement in an in vitro model of nerve transmission, corroborating the proposal of the direct-action on nociceptive fibers (Amorim et al. 2016).
In addition to the antinociceptive activity, LAL presents anti-inflammatory activity via the lectin domain, inhibiting neutrophil migration to rat peritoneal cavity and modulating inflammatory mediators, such as PGE2, TNF-α and NO (Pires et al. 2016). These inflammatory mediators produced by neutrophils also induce hypernociception. Therefore, there is a possibility that the reduction in neutrophil influx decreases the production of hypernociceptive mediators, contributes to the antinociceptive effect of LAL. However, this theory needs to be proven. It seems that LAL has a pleiotropic effect in several nociceptive pathways, being associated with the modulation of inflammatory and/or hypernociceptive mediators, including NO.
NO is considered a nociceptive neurotransmitter; however, the duality of its functions is increasingly recognized. Pharmacological experiments have shown that reduction in NO inhibits nociception in rodents (Hao and Xu 1996; Aley et al. 1998), but the injection of NO donor reduces the sensitivity to nociceptive stimuli, suggesting that endogenous NO acts on other targets than exogenous NO (Schmidtko et al. 2009). Besides, NO is a versatile molecule that contributes to the functional adaptations of nociceptive synapses in the primary sensory neuron, spinal cord, and brain (Aley et al. 1998; Ikeda et al. 2006; Miyamoto et al. 2009). This molecule has been mainly related to adaptations of nociceptive circuits due to its diffuse capacity, which allow paracrine and retrograde signaling of presynaptic neurons with neighboring cells (Ikeda et al. 2006).
The Experimental data of the present work demonstrate that the previous administration of L-NAME reversed the effect of LAL. As L-NAME is a non-selective NOS inhibitor, the effects of selective inhibitors for different NOS isoforms on LAL activity were evaluated. These results demonstrated that the antinociceptive effect of LAL may have the participation of nNOS, since the previous treatment with 7-nitroindazole reversed the lectin antinociceptive effect. Some studies highlight the involvement of nNOS in peripheral nociceptive effects, being NO production demonstrated in primary nociceptive neurons and that its peripheral antinociceptive effects are abolished in nNOS knockout rats (Cunha et al. 2010). In addition, it has been documented that the antinociceptive effect of acetylcholine, dipyrone, and diclofenac involves the participation of NO, which was not blocked by iNOS inhibitor. Besides, nNOS is involved in peripheral antinociception of many analgesic drugs (Maihöfner et al. 2000).
Studies on neuronal transmission show that nociceptive primary afferent neurons release glutamate after nociceptive stimulation. This neurotransmitter acts on NMDA receptors of postsynaptic neurons and interneurons, causing Ca2+ influx, stimulating calmodulin kinase, and producing reflex activation of nNOS (Schmidtko et al. 2009). Our results suggest that increase in intracellular calcium is an important event for nNOS activation by LAL because prior administration of the calcium channel blocker nifedipine prevented the lectin antinociceptive effect.
The intracellular influx of Ca2+ with subsequent stimulation of calmodulin kinase is a key event in the activation of nNOS (Schmidtko et al. 2009). Our experiments showed that the previous administration of calcium channel blocker prevented the antinociceptive effect of lectin. This finding reinforces the theory of the participation of nNOS in the effect of LAL.
nNOS activation induces NO production accompanied by increase in cGMP, which induces stimulation of PKG (Lewin and Walters 1999). Increased cGMP and PKG concentrations in peripheral sensory neurons promote ATP-dependent K+ channel opening, leading to repolarization and inhibition of the action potential generation (Ferreira et al. 1991; Sachs et al. 2004; Cury et al. 2011). Thus, the effect of ODQ and glibenclamide, guanylate cyclase and ATP-dependent K+ channel blockers, respectively, were investigated. The prior administration of both blockers prevented the antinociceptive effect of LAL. Therefore, the hyperpolarization of the membrane caused by the opening of potassium channels, could be an important event for the antinociceptive effect of this lectin. However, there is a need for electrophysiological experiments to reinforce this hypothesis.
The present study also demonstrated increased nNOS gene expression in the paw tissues stimulated with carrageenan. Accordingly, nNOS is expressed in dorsal and peripheral root ganglion neurons, which is amplified in inflammatory processes (Maihöfner et al. 2000). LAL significantly increased expression of the messenger RNA for nNOS relative to carrageenan, which demonstrates its direct effect on nNOS gene expression. In contrast, LAL was unable to elevate nNOS immunoexpression in the dorsal root ganglia as compared to carrageenan. An important information is that this experiment was performed three hours after i.v. injection of LAL. The synthesis of various proteins, such as nNOS is a complex process involving transcription, translation, and various regulation at different cellular levels. Thus, the gene expression does not always reflect the synthesis of the protein.
The use of non-steroidal anti-inflammatory drugs produces antinociceptive effect via inhibition of prostaglandin synthesis; however, surprisingly, the antinociceptive effect of LAL, rather than being potentiated, was prevented by prior administration of niflumic acid (non-steroidal anti-inflammatory drug used as a calcium-activated chloride channel blocker) (Collin et al. 2005). Studies show that the transmission at GABAA receptors is inhibited by niflumic acid and that calcium-activated chloride channels play an important role in pain transmission (Wang et al. 2017). Therefore, the present study demonstrated, for the first time, the participation of calcium-activated chloride channels in the antinociceptive effect of LAL; however, there is a need to conduct further studies to better understand how these channels are associated with the effect of this lectin.
In conclusion, the antinociceptive effect of the lectin isolated from Lonchocarpus araripensis seems to be associated with the activation of nNOS and involvement of ionic channels, such as calcium channel, ATP-dependent K+ channel, and calcium-activated chloride channels.
References
Aley KO, McCarter G, Levine JD (1998) Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci 18:7008–7014. https://doi.org/10.1007/s10787-020-00686-7
Amorim RMF, Pires AF, Nascimento TS, Cavada BS, Nascimento KS, Cajazeiras JB (2016) The leguminous lectin of Lonchocarpus araripensis promotes antinociception via mechanisms that include neuronal inhibition of Na+ currents. Inflamm Res 65:701–708. https://doi.org/10.1007/s00011-016-0951-0
Chaban VV, McRoberts JA, Ennes HS, Mayer EA (2001) Nitric oxide synthase inhibitors enhance mechanosensitive Ca2+ influx in cultured dorsal root ganglion neurons. Brain Res 903:74–85. https://doi.org/10.1016/s0006-8993(01)02407-6
Collin T, Chat M, Lucas MG, Moreno H, Racay P, Schwaller B et al (2005) Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci 25:96–107. https://doi.org/10.1523/JNEUROSCI.3748-04.2005
Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WAJR et al (2010) Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci 7:4442–4447. https://doi.org/10.1073/pnas.0914733107
Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S et al (2014) An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37:401–407. https://doi.org/10.1590/s0100-879x2004000300018
Cury Y, Picolo G, Gutierrez VP, Ferreira SH (2011) Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254. https://doi.org/10.1016/j.niox.2011.06.004
Ferreira SH, Duarte ID, Lorenzetti BB (1991) The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol 201:121–129. https://doi.org/10.1016/0014-2999(91)90333-l
Hao JX, Xu XJ (1996) Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain 66:313–319. https://doi.org/10.1016/0304-3959(96)03039-4
Hsu S, Raine L, Fanger H (1981) Use of avidin–biotin–peroxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580. https://doi.org/10.1177/29.4.6166661
Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T et al (2006) Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312:1659–1662. https://doi.org/10.1126/science.1127233
Lewin MR, Walters ET (1999) Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci 2:18–23. https://doi.org/10.1038/4520
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Loeser JD, Melzack R (1999) Pain: an overview. Lancet 353:1607–1609. https://doi.org/10.1016/S0140-6736(99)01311-2
Maihöfner C, Euchenhofer C, Tegeder I, Beck KF, Pfeilschifter J, Geisslinger G (2000) Regulation and immunohistochemical localization of nitric oxide synthases and soluble guanylyl cyclase in mouse spinal cord following nociceptive stimulation. Neurosci Lett 290:71–75. https://doi.org/10.1016/s0304-3940(00)01302-1
Miyamoto T, Dubin AE, Petrus MJ, Patapoutian AA (2009) TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 4:e7596. https://doi.org/10.1371/journal.pone.0007596
Napimoga MH, Cavada BS, Alencar NM, Mota ML, Bittencourt FS et al (2007) Lonchocarpus sericeus lectin decreases leukocyte migration and mechanical hypernociception by inhibiting cytokine and chemokines production. Int Immunopharmacol 7:824–835. https://doi.org/10.1016/j.intimp.2007.02.001
Nascimento KS, Silva MTL, Oliveira MV, Lossio CF, Pinto-Junior VR, Osterne VJ, Cavada BS (2020) Dalbergieae lectins: a review of lectins from species of a primitive Papilionoideae (leguminous) tribe. Int J Biol Macromol 144:509–526. https://doi.org/10.1016/j.ijbiomac.2019.12.117
Pires AF, Rodrigues NV, Soares PM, Ribeiro RA, Aragao KS et al (2016) A novel N-acetyl-glucosamine lectin of Lonchocarpus araripensis attenuates acute cellular inflammation in mice. Inflamm Res 65:43–52. https://doi.org/10.1007/s00011-015-0889-7
Pires AF, Almeida LM, Silva DHM, Marques GFO, Cajazeiras JB et al (2017) The lectin isolated from Lonchocarpus araripensis seed elicits endothelium-dependent vasorelaxation. J Health Biol Sci 5:306–310. https://doi.org/10.12662/2317-3076jhbs.v5i4.1351.p306-310.2017
Pires AF, Bezerra MM, Amorim RMF, Nascimento FLF, Marinho MM et al (2019) Lectin purified from Lonchocarpus campestris seeds inhibits inflammatory nociception. Int J Biol Macromol 125:53–60. https://doi.org/10.1016/j.ijbiomac.2018.11.233
Sachs D, Cunha FQ, Ferreira SH (2004) Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci 101:3680–3685. https://doi.org/10.1073/pnas.0308382101
Schmidtko A, Tegeder I, Geisslinger G (2009) No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci 32:339–346. https://doi.org/10.1016/j.tins.2009.01.010
Su YS, Sun WH, Chen CC (2014) Molecular mechanism of inflammatory pain. World J Anesthesiol 3:71–81. https://doi.org/10.5313/wja.v3.i1.71
Wang LJ, Wang Y, Chen MJ, Tian Z-P, Lu BH et al (2017) Effects of niflumic acid on γ-aminobutyric acid-induced currents in isolated dorsal root ganglion neurons of neuropathic pain rats. Exp Ther Med 14:1373–1380. https://doi.org/10.3892/etm.2017.4666
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, Fundação Cearense de Amparo a Pesquisa-FUNCAP and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES. Cavada BS and Assreuy AM are senior investigators of CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Assreuy, A.M.S., Amorim, R.M.F., Martins, S.L. et al. Antinociceptive effect of Lonchocarpus araripensis lectin: activation of l-arginine/NO/cGMP/K+ATP signaling pathway. Inflammopharmacol 28, 1623–1631 (2020). https://doi.org/10.1007/s10787-020-00729-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00729-z