Abstract
Objective and design
Sodium channels are highly expressed in nociceptive sensory neurons during hypernociceptive conditions. Based on the presence of a glycosidic portion in the sodium channel β subunit associated to the antinociceptive effect of leguminous lectins via lectin domain, this study investigated the antinociceptive activity of the lectin isolated from Lonchocarpus araripensis seeds (LAL) in mice behavioral models and in NaV current in the nociceptor of rat dorsal root ganglion (DRG).
Material/methods
LAL antinociceptive activity and the participation of opioid system, lectin domain and sodium channels were evaluated in Swiss mice models of nociception (formalin, capsaicin, hot plate, tail flick, von Frey) and in primary cultures of Wistar rats neurons of DRG (patch clamp).
Results
LAL presented inhibitory effects in the nociception induced by chemical and mechanical, but not by thermal stimuli and reduced total Na+ current. LAL activity was inhibited by the lectin association with its binding sugar N-acethyl-glucosamine.
Conclusion
LAL inhibits peripheral hypernociception by mechanisms that involve the lectin domain, inflammatory mediators and Na+ channels. The innovative inhibitory action of leguminous lectins on NaV current brings new insights for the investigation of sodium channels role in nociception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nociceptor sensitization by inflammatory mediators leads to an increased response to noxious stimulus, characteristic of hypernociception, that occurs through several mechanisms that include the modulation of the activity of Na+, K+ and Ca2+ channels, decreasing the nociceptor threshold excitability [1–3].
Tetrodotoxin-resistant sodium channels (TTX-R) are considered important analgesic therapeutic targets, since they are specifically found in nociceptive sensory neurons and highly expressed in hypernociceptive conditions [4, 5]. The structure of these channels includes two subunits (α and β), being the subunit α the pore-forming, and the subunit β modulates the channel activity [6]. Besides, the subunit β regulates the Na+ channel expression in the plasma membrane, acting as a cell adhesion molecule [7]. Importantly, the β subunit has a glycosidic portion that could be a possible lectin target, due to the lectin property of specifically and reversibly binding to carbohydrates [8]. However, no data in the literature are referred to the modulator role of lectins in nociception via Na+ channels.
Leguminous lectins presenting binding specificity for manose-glucose have shown antinociceptive activity in mice involving carbohydrate recognition sites [9–11]. In respect to lectins with binding specificity for N-acethyl-glucosamine, the lectin isolated from Lonchocarpus sericeus had been studied in a model of inflammatory hypernociception, demonstrating antinociceptive effect via inhibition of cytokine and chemokine production [12]. Besides, the lectin studied here, isolated from Lonchocarpus araripensis seeds (LAL) showed anti-inflammatory effect [13]. It is known that the increased activity on Na+ channel is involved in the mechanism of inflammation [1]. Thus, the present investigation was undertaken to assess the antinociceptive activity of LAL in mice behavioral models and its mechanism of action, including a possible contribution of voltage-dependent Na+ (NaV) current inhibition on rat dorsal root ganglion.
Materials and methods
Animals
Male Swiss mice (20–25 g) and Male Wistar rats (150–250 g) were maintained with free access to food and water. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of Ceara (CEUA/UECE No. 10130208-8/40), Fortaleza, CE, Brazil, in accordance with the Guide for the Care and Use of Laboratory Animals of the US Department of Health and Human Services (NIH publication No. 85-23, revised 2011).
Lectin
The seed lectin of Lonchocarpus araripensis BENTH (LAL—family Leguminosae, subfamily Papilionoideae, tribe Dalbergieae, subtribe Lonchocarpinae) was isolated by affinity chromatography in a chitin column followed by ion exchange chromatography on DEAE Sephacel. The lectin activity was determined by hemagglutinating assay against rabbit erythrocytes.
A voucher specimen of Lonchocarpus araripensis had been deposited in the Herbarium Prisco Bezerra, Federal University of Ceará (EAC18636).
Drugs
Formalin, λ-carragenan, tumor necrosis factor-alpha (TNF-α), prostaglandin E2 (PGE2), serotonin (5-HT), capsaicin, bradykinin (BK), epinephrine and N-acetyl-glucosamine (GlcNAc) were purchased from Sigma (St. Louis, USA). Morphine was obtained from Cristália (São Paulo, Brazil) and naloxone from Narcan—Rhodia Pharma (São Paulo, Brazil). Drugs and LAL were solubilized directly in sterile saline (NaCl 0.15 M), except for capsaicin, which was dissolved in 5 % absolute ethanol.
Behavioral models of nociception
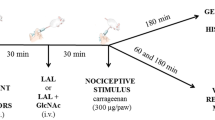
LAL (0.1–10 mg/kg) was administered intravenously (i.v.) in mice 30 min before chemical, thermal or mechanical nociceptive agents. Control animals received saline in the substitution of LAL.
Chemical nociception (Capsaicin and Formalin tests)
Capsaicin (20 μl; 1.6 μg/paw) was injected in the right hind paws, and the time (s) in which animals spent licking its paws was recorded at 0–5 min of observation [14].
Formalin (20 μl; 2.5 % v/v) was injected in the right hind paws, and the time (s) in which the animals spent licking its paws was recorded at phase 1 (0–5 min) and phase 2 (15–30 min) [15]. The opioid system participation was assessed by the treatment with naloxone (13.7 μmol/kg, intraperitoneal; i.p.) 15 min prior administration of LAL (10 mg/kg; i.v.) or morphine (5 mg/kg, subcutaneous; s.c.). Thirty minutes later the test was performed [16]. To investigate the involvement of lectin domain, LAL (10 mg/kg) was previously incubated with its binding sugar GlcNAc (0.1 M) for 1 h at 37 °C, to allow lectin–sugar interactions. Lectin or sugar was individually incubated at the same conditions as controls.
Thermal nociception (Hot plate and Tail flick tests)
Mice were placed over a hot plate at 55 ± 0.5 °C up to 25 s. The reaction latency of thermal stimuli (time displayed before appearance of licking and shaking hind paws or jumping) was recorded. Animals showing a reaction time greater than 10 s were discarded [17].
Mice tails were immersed in a water bath (50 °C), and the reaction latency of thermal stimuli (time displayed before appearance of tail flick reaction) was recorded [18]. Two basal latency measures were performed up to 10 s before test.
In both tests, the latency time was recorded immediately before and 30, 60, 90, 120, 150 and 180 min after treatment.
Mechanical nociception (von Frey test)
Mice were placed individually in boxes of elevated wire mesh platforms to allow access to the ventral surface of the hind paws [19]. The frequency of paw withdrawal in response to 6 applications (100 %) of von Frey filament (0.8 g) was determined before (basal value) and 30, 60 or 180 min after intraplantar s.c. Injection of nociceptive stimulus (50 μl): carrageenan (300 μg/paw), BK (100 nmol/paw), 5-HT (100 μg/paw), PGE2 (30 nmol/paw), TNF-α (5 ng/paw) and epinephrine (5 μg/paw).
Locomotor tests (Rotarod and Open field)
Locomotor coordination was assessed in mice selected 24 h before the experiment, being excluded those that did not remain in the Rotarod at 22 rpm for two consecutive periods of 60 s [20]. The number of falls and the time of permanence on the apparatus were recorded.
Spontaneous locomotion was verified by the introduction of mice in a square open field (30 cm side, 9 equal squares each of 10 cm). The number of each animal field crossing was observed for 4 min [21].
Electrophysiological assay
Rat dorsal root ganglions (DRG) were placed in Ca2+/Mg2+-free Hank’s balanced salt solution (HBSS, mM) [137.93 NaCl, 4 NaHCO3, 0.3 Na2HPO4, 5.33 KCl, 0.44 KH2PO4 and 5.6 glucose, pH adjusted to 7.4 with NaOH], incubated at 37 °C for 75 min with 1.0 mg/ml collagenase type I, and for 15 min with 2.5 mg/ml trypsin. DRG neurons were freed from tissue by gentle trituration in Dulbecco’s Modified Eagle’s Medium supplemented with 10 % fetal bovine serum, 100 U/ml streptomycin and 0.1 mg/ml penicillin. Cells were plated on coverslips coated with poly-d-lysine 0.01 % and incubated at 37 °C for 48 h in air atmosphere containing 5 % CO2 [22].
The whole-cell patch-clamp configuration in voltage-clamp mode was used to register DRG Na+ current. A perfusion pipette was positioned in the vicinity of the cell to be challenged. Thick-walled flint glass tubing (outside diameter 1.5 mm, inside diameter 1.1 mm, Perfecta, SP, Brazil) was pulled with a Flaming/Brown type puller (P-97 micropipette puller model, Sutter instruments, Novato, CA, USA). The electrode resistance attained 1–3 MΩ after the filling solution: 10 mM NaCl, 100 mM CsCl, 10 mM HEPES, 11 mM EGTA, 10 mM TEA-Cl, and 5 mM MgCl2 (pH 7.2 adjusted with CsOH). Axopatch 200B amplifier driven by Clampex 10.2 software (Molecular Devices, Sunnyvale, CA, USA) was used for recording [23]. Experimental time points: control; test solution perfusion (30 mM NaCl, 90 mM choline-Cl, 10 mM CsCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM TEA-Cl, 0.2 mM CdCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4 adjusted with TEA-OH); test solution containing LAL (10–500 μg/ml). Capacitance and leakage subtraction were performed using a P/6 subtraction protocol. Na+ current was sampled at 40 kHz, low-pass filtered at 5 kHz. Data acquisition was performed using computer hardware (Digidata 1440A model, Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Data were expressed as Mean ± SEM (n = 6–8). Behavior assays were analyzed by one-way ANOVA followed by Bonferroni test and electrophysiological assays by ANOVA followed by Holm-Sidak test. The values of p < 0.05 were considered significant.
Results
LAL inhibits chemical nociception induced by formalin and capsaicin
In response to formalin, paw licking time was increased in both phases (phase 1: 55.3 ± 4.98 s; phase 2: 241.66 ± 32.95 s) compared to animals that received 50 µl of saline intraplantar (P1: 1.6 ± 2.8 s; P2: 1.6 ± 2.0 s). In phase 1, LAL decreased the licking time by 52 % (26.09 ± 2.52 s) at 10 mg/kg. In phase 2, LAL decreased the licking time at all doses tested: 0.1 mg/kg (129.417 ± 26.10 s) by 46 %; 1 mg/kg (72.38 ± 17.54 s) by 70 %; 10 mg/kg (49.81 ± 18.91 s) by 79 % (Fig. 1a). Similarly, LAL (10 mg/kg) reduced by 50 % (34.5 ± 5.70 s), and the paw licking time induced by capsaicin (69.12 ± 10.02 s) (Fig. 1c). For the subsequent tests, LAL was used at 10 mg/kg.
LAL inhibits chemical nociception. a, b Formalin test: LAL (0.1, 1 or 10 mg/kg) or LAL (10 mg/kg) + GlcNAc (0.1 M) was administered 30 min before 2.5 % formalin (s.c.); Naloxone (13.7 μmol/kg) was injected i.p. 15 min before LAL (10 mg/kg) or morphine (13.3 μmol/kg, s.c.). Morphine was administered 15 min before formalin. c Capsaicin test: LAL (10 mg/kg; i.v.) was administered 30 min before capsaicin (1.6 μg/paw; s.c.). Mean ± SEM (n = 6–8). One-way ANOVA/Bonferroni. *p < 0.05 vs. formalin or capsain; # p < 0.05 vs. LAL
The antinociceptive effect of LAL at 10 mg/kg (phase 1: 26.23 ± 2.57 s; phase 2: 49.81 ± 18.91) was inhibited by GlcNAc (phase 1: 52 ± 7.52 s; phase 2: 195.04 ± 28.02 s) (Fig. 1a), but was not altered by the opioid antagonist naloxone (phase 1: 17.11 ± 1.79; phase 2: 27.33 ± 9.70 s) (Fig. 1b). In contrast, the antinociceptive effect of morphine (phase 1: 2.36 ± 0.43 s; phase 2: 1.7 ± 0.57 s) was inhibited by naloxone (phase 1: 37 ± 1.62; phase 2: 240.8 ± 11.95 s).
LAL inhibits hypernociception
Carrageenan and TNF-α increased animals paw withdrawal in response to mechanical stimulation with von Frey filaments. At 30 min, LAL (10 mg/kg) reduced in 82 % the response elicited by carrageenan (74.0 ± 3.4 % vs. LAL 39.55 ± 7.68 %) and abolished that of TNF-α (66.66 ± 8.13 % vs. LAL 22.91 ± 3.05 %). At 180 min, LAL (10 mg/kg) reduced in 63 % the response of carrageenan (72.88 ± 7.00 % vs. LAL 49.97 ± 7.27 %) and in 76 % that of TNF-α (63.89 ± 9.036 % vs. LAL 35.41 ± 6.99 %). There were no differences in the interval of 60–180 min (Fig. 2a).
LAL inhibits hypernociception. LAL (10 mg/kg; i.v.) was administered 30 min before s.c. stimuli injection: a carrageenan (300 μg) or TNF-α (5 ng); b BK (10 nmol), PGE2 (30 nmol), 5-HT (100 μg) or epinephrine (5 μg). Mean ± SEM (n = 6–8). One-way ANOVA/Bonferroni. *p < 0.05 vs. zero time; #
p < 0.05 vs. stimulus
The hypernociceptive effect of BK (80.95 ± 7.65 %) and 5-HT (90.47 ± 3.36 %) at 30 min was also reduced by LAL (10 mg/kg) in 100 % (29.16 ± 6.09 %) and in 87 % (40.47 ± 3.36 %), respectively (Fig. 2b). In addition, LAL (10 mg/kg) reduced in 80 % (43.75 ± 7.67 %) the hypernociception induced by PGE2 (79.16 ± 4.16 %) and in 82 % (37.47 ± 4.17 %) that of epinephrine (62.47 ± 7.55 %) (Fig. 2b).
LAL per se did not alter the response in naïve/normal mice, showing that the lectin did not present nociceptive effect.
LAL does not inhibit nociception induced by thermal stimulus
LAL (10 mg/kg), unlike morphine, did not alter the latency of animals response to thermal stimulation in the hot plate (50 °C) or the tail flick latency after immersion in a water bath (50 °C), at any time evaluated (Table 1).
LAL does not interfere with motor coordination or spontaneous locomotion
LAL (10 mg/kg) did not alter the time of animals permanence in the Rotarod [LAL 57.5 ± 3.65 s (n = 7) vs. Saline 59.67 ± 7.46 s (n = 6)] or the number of crossing in the open field [LAL 94.53 ± 9.55 (n = 8) vs. Saline 103.16 ± 11.13 (n = 6)].
LAL attenuated the NaV current
LAL (10 and 500 μg/ml) reversibly and time-dependently attenuated in 20 % the NaV current in DRG neurossomas of small diameters (18–30 μm) held at −80 mV with 200 ms conditioning pulse at −120 mV delivered prior to 100 ms depolarizing test pulse at 10 mV in 5 s intervals (Fig. 3a, b). In addition, the evaluation of the sodium channel kinetic of inactivation showed that LAL (10 and 500 μg/ml) significantly accelerated the slow and fast component of the NaV current decay time course, described by a sum of two exponential functions representing the contribution of each component [24]. In the presence of LAL, the slow component was 65.27 ± 15.96 % at 10 μg/ml and 72.63 ± 6.83 % at 500 μg/ml, while the fast component was 72.63 ± 5.57 % at 10 μg/ml and 75.03 ± 4.95 % at 500 μg/ml compared to control (100 %) (Fig. 3c).
LAL reduces total Na+ current of small-sized DRG neurons. a Representative current traces of INatotal under control, after 60 s exposure to LAL (500 μg/ml) or washout; b Normalized Na+ current in the presence of LAL; c normalized time constants bi-exponential sommatory of the slow and fast channel components. Mean ± SEM (n = 5–6). ANOVA/Holm-Sidak. *p < 0.05 vs. Control
Discussion
This study demonstrated that the leguminous lectin of L. araripensis presented inhibitory effects in behavioral models of nociception, induced by chemical (formalin, capsaisin) or mechanical (von Frey) stimuli, but not by thermal stimuli (hot plate, tail flick). It suggests that several mechanisms contribute to LAL antinociceptive effect, as discussed below. In addition, LAL reduced total Na+ current, and its antinociceptive activity was associated with its sugar recognition site, but not with the opioid pathway.
The inhibitory effect of LAL demonstrated in both the phases of formalin test suggests the lectin interference in either neurogenic or inflammatory nociceptive pathways. These data are in accordance with the literature that describes the same pattern of behavior of other lectins isolated from the Diocleinae subtribe [9–11, 25, 26]. It is important to emphasize that, similar to LAL, most of these studies showed the participation of the lectin domain. In addition, the LAL neurogenic effect, demonstrated in the first phase of formalin (phase 1), was corroborated with that obtained in the capsaicin test that evaluates neurogenic nociception, pointing that LAL plays a role in the pain of such origin. However, LAL predominantly inhibited the inflammatory phase of formalin (phase 2), suggesting the involvement of pain peripheral components and inflammatory mediators in the lectin antinociceptive effect. In fact, Pires [13] had shown the anti-inflammatory activity of LAL via attenuation of neutrophil–endothelium interactions implying prostaglandin E2, nitric oxide and tumor necrosis factor-alpha.
In this line, of reasoning the present study demonstrated that LAL inhibited the hypernociception induced by inflammatory mediators, such as TNF-α and BK, which initiate the release of other inflammatory mediators and contribute to pain maintenance [27]. LAL also inhibited the hypernociception induced by PGE2 and epinephrine, considered final hypernociceptive mediators [27]. Accordingly, the literature has shown that a lectin isolated from another specie of Lonchocarpus (L. sericeus) inhibited selectively the second phase of formalin test and the hypernociception stimulated by carrageenan via reduction of neutrophil migration and inflammatory cytokines [12, 28].
Furthermore, the demonstration that LAL does not interfere in the latency of response to the thermal stimulus (hot plate and tail flick) and the lack of reversal of its antinociceptive effect by the opioid antagonist morphine (formalin test) lead to the possible exclusion of central mechanisms in the LAL activity. It is generally believed that centrally active drugs, such as morphine, inhibit both phases of formalin response, and that anti-inflammatory drugs inhibit mainly the second phase [29]. Although the well-described central antinocicetive effect (via opioid system) of the lectins from Canavalia boliviana [25] and Canavalia brasiliensis [11] in models using thermal stimuli, LAL nor L. sericeus lectin showed effect in these models [12].
The depressing activity of various analgesic drugs on central nervous and muscular systems can reduce animal motor coordination and the expression of nociceptive behaviors [3]. Similar to other plant lectins [10, 11, 25], the demonstration that LAL unaltered motor coordination of animals in the Rotarod test nor spontaneous locomotion in the open field test, suggested the selectivity of its antinociceptive response. In addition, the mice treatment with LAL by intravenous route during nine consecutive days was well tolerated by mice, since there was no mortality or systemic alterations, such as in hematological and biochemical parameters [13], a profile also demonstrated for another lectin isolated from a leguminous plant belonging to the same genera as L. sericeus lectin [12].
Due to the inhibitory action of LAL in the nociception induced by stimuli that lead to the depolarization of primary nociceptors, i.e., activation of TTX-R and the consequent alteration in sodium current, such as inflammatory mediators (TNF-α, BK, and PGE2) and neurogenic substances (capsaicin and formalin), allow us to hypothesize a direct action in TTX-R sub-population. In fact, the literature has shown nociceptor sensitization by inflammatory mediators via decrease of the nociceptor threshold excitability, facilitating hypernociception [1], in part via modulation of Na+, K+ and Ca2 + channels activity [2, 3]. Besides, TTX-R has been considered important analgesic therapeutic targets [30], and the cells used in this study are within the group of neurossomas of small diameter (18–30 μm) (DRG), associated with nociception [31, 32]. Nevertheless, we cannot exclude the hypothesis that LAL acts on other neuronal population. Further studies using pharmacological modulators are being conducted to deeply investigate this issue. Besides, TTX-R has been considered important analgesic therapeutic targets [30], and the cells used in this study are within the group of neurossomas of small diameter (18–30 μm) (DRG), predominantly associated with nociception and expressing TTX-R and TTX-S sodium channels [31, 32]. Nevertheless, we cannot exclude the hypothesis that the LAL acts on other neuronal population, although the LAL absence of alteration in motricity suggests that its effect is largely restricted to a sub-population of Na+ channels, such as TTX-R.
It is to mention that the LAL inhibited only 20 % of total Na+ current amplitude. This prompts the question: is not the effect in magnitude too small to exert blockade of cell excitability, and therefore, the component of nociception due to cell excitability alteration? We do not have a precise answer to that. It is known that the protocol used here to evaluate the inhibition (from −120 mV conditioning pulse to 10 mV) measures predominantly channel pore blockade. However, this protocol did not evaluate inhibitory effect through the alteration of kinetic mechanism (displacement of steady-state inactivation curve), which is likely to have occurred, since time constants of current inactivation were decreased. For example, 1,8-cineole blocks only approximately 20 % of total Na current with this protocol, but at −60 mV resting potential, this inhibition is largely increased [33]. In any case, even if maximal LAL Na+ current inhibition was restricted to 20 % only, it probably contributed to other mechanisms to the antinociceptive effect.
It is important to highlight that the β subunit of NaV, responsible for the kinetics and voltage dependence of the channel activation and inactivation, presents a glycosidic site [7], being a possible target for LAL, since the demonstrated involvement of the lectin domain in the antinociceptive activity. Thus, the inhibitory action of a plant lectin on NaV current is innovative and brings new insights for its use as biotechnological tool in the investigation of the role of sodium channels in nociception. It is important to highlight that the β subunit of NaV, responsible for the kinetics and voltage dependence of the channel activation and inactivation, presents a glycosidic site [7], being a possible target for LAL, since the demonstrated involvement of the lectin domain in the antinociceptive activity. This is coherent with the suggestion that LAL acts on the Na+ channel inactivation kinetics. Thus, the inhibitory action of LAL on NaV current is innovative and brings new insights for its use as a biotechnological tool in the investigation of plant lectin on nociception.
In conclusion, the leguminous lectin isolated from L. araripensis inhibits peripheral hypernociception by mechanisms that are likely to involve the lectin domain, anti-inflammatory mediators and Na+ channels.
References
Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164.
Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;15:4080–5.
Souza AL, Moreira FA, Almeida KR, Bertollo CM, Costa KA, Coelho MM. In vivo evidence for a role of protein kinase C in peripheral nociceptive processing. Br J Pharmacol. 2002;135:239–47.
England JD, Gamboni F, Ferguson MA, Levinson SR. Sodium channels accumulate at the tips of injured axons. Muscle Nerv. 1994;17:593–8.
Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–59.
Patton DE, Isom LL, Catterall WA, Goldin AL. The adult rat brain beta 1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J Biol Chem. 1994;269:17649–55.
Isom LL. Beta subunits: players in neuronal hyperexcitability? Novartis Found Symp. 2002;241:124–38.
Sharon N, Lis H. Lectins–proteins with a sweet tooth: functions in cell recognition. Essays Biochem. 1995;30:59–75.
Holanda FR, Coelho-de-Souza AN, Assreuy AM, Leal-Cardoso JH, Pires AF, Nascimento KS, Cavada BS, Santos CF. Antinociceptive activity of lectins from Diocleinae seeds on acetic acid-induced writhing test in mice. Protein Pept Lett. 2009;16:1088–92.
Pinto NV, Santos CF, Cavada BS, do Nascimento KS, Pereira Junior FN, Pires AF, Assreuy AM. Homologous Canavalia lectins elicit different patterns of antinociceptive responses. Nat Prod Commun. 2013;8:1621–4.
de Freitas Pires A, Assreuy AM, Lopes EA, Celedônio NR, Soares CE, Rodrigues NV, Sousa PL, Benevides RG, Nagano CS, Cavada BS, Leal-Cardoso JH, Coelho-De-Souza AN, Santos CF. Opioid-like antinociceptive effects of oral administration of a lectin purified from the seeds of Canavalia brasiliensis. Fund Clin Pharmacol. 2013;27:201–9.
Napimoga MH, Cavada BS, Alencar NM, Mota ML, Bittencourt FS, Alves-Filho JC, Grespan R, Gonçalves RB, Clemente Napimoga JT, Parada CA, Ferreira SH, Cunha FQ. Lonchocarpus sericeus lectin decreases leukocyte migration and mechanical hypernociception by inhibiting cytokine and chemokines production. Int Immunopharmacol. 2007;7:824–35.
Pires AF, Rodrigues NV, Soares PM, Ribeiro RA, Aragao KS, Marinho MM, Silva MT, Cavada BS, Assreuy AM. A novel N-acetyl-glucosamine lectin of Lonchocarpus araripensis attenuates acute cellular inflammation in mice. Inflamm Res. 2016;65:43–52.
Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31:1279–85.
Hunskaar S, Role K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–14.
Vaz ZR, Cechinel Filho V, Yunes RA, Calixto JB. Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4,6-dimethoxy benzofuran, a novel xanthoxyline derivative on chemical and thermal models of nociception in mice. J Pharmacol Exp Ther. 1996;278:304–12.
Eddy NB, Leimbach D. Synthetic analgesics. II. dithienylbutenyl and dithienylbutylamines. J Pharmacol Exper Ther. 1953;107:385–93.
D’amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–9.
Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–7.
Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficits in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;19(46):208–9.
Capaz FR, Vasconcellos LE, De Moraes S, Neto JP. The open field: a simple method to show ethanol withdrawal symptoms. Arch Int Pharmacodyn Ther. 1981;251:228–36.
Joca HC, Cruz-Mendes Y, Oliveira-Abreu K, Maia-Joca RP, Barbosa R, Lemos TL, Lacerda Beirão PS, Leal-Cardoso JH. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J Nat Prod. 2012;75:1511–7.
Leal-Cardoso JH, da Silva-Alves KS, Ferreira-da-Silva FW, dos Santos-Nascimento T, Joca HC, de Macedo FH, de Albuquerque-Neto PM, Magalhães PJ, Lahlou S, Cruz JS, Barbosa R. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur J Pharmacol. 2010;645:86–93.
Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993;463:39–56.
Figueiredo JG, da Silveira Bitencourt F, Beserra IG, Teixeira CS, Luz PB, Bezerra EH, Mota MR, Assreuy AM, De Queiroz Cunha F, Cavada BS, De Alencar NM. Antinociceptive activity and toxicology of the lectin from Canavalia boliviana seeds in mice. N-S Arch Pharmacol. 2009;380:407–14.
Delatorre P, Rocha BA, Simões RC, Pereira-Júnior FN, Silva HC, Bezerra EH, Bezerra MJ, Marinho ES, Gadelha CA, Santi-Gadelha T, Farias DL, Assreuy AM, Marques-Domingos GF, Nagano CS, Cavada BS. Mass spectrometry and X-ray diffraction analysis of two crystal types of Dioclea virgata lectin: an antinociceptive protein candidate to structure/function analysis. Appl Biochem Biotech. 2011;164:741–54.
Cunha TM, Verri WA Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci. 2005;102:1755–60.
Alencar NMN, Teixeira EH, Assreuy AMS, Cavada BS, Flores CA, Ribeiro RA. Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediat Inflamm. 1999;8:107–13.
Tj∅lsen A, Hole K. Animal models of analgesia. In: Besson MJ, Deckeson A, editors. The pharmacology of pain. Heidelberg: Springer; 1997. p. 21–41.
Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14.
Scroggs RS, Fox AP. Multiple Ca+2 currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci. 1992;12:1789–801.
dos Santos-Nascimento T, Veras KM, Cruz JS, Leal-Cardoso JH. Inhibitory effect of Terpinen-4-ol on voltage-dependent potassium currents in rat small sensory neurons. J Nat Prod. 2015;78(2):173–80.
Ferreira-da-Silva FW, da Silva-Alves KS, Alves-Fernandes TA, Coelho-de-Souza AN, Leal-Cardoso JH. Effects of 1,8-cineole on Na(+) currents of dissociated superior cervical ganglia neurons. Neurosci Lett. 2015;595:45–9.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, Fundação Cearense de Amparo a Pesquisa-FUNCAP and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES. Leal-Cardoso JH, Cavada BS and Assreuy AM are senior investigators of CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ji Zhang.
Rights and permissions
About this article
Cite this article
Amorim, R.M.F., Pires, A.F., dos Santos-Nascimento, T. et al. The leguminous lectin of Lonchocarpus araripensis promotes antinociception via mechanisms that include neuronal inhibition of Na+ currents. Inflamm. Res. 65, 701–708 (2016). https://doi.org/10.1007/s00011-016-0951-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0951-0