Abstract
Neutrophil migration is responsible for tissue damage observed in inflammatory diseases and is also implicated in inflammatory nociception. The use of lectins has been demonstrated to be effective in different activities including anti-inflammatory, antimicrobial, and in cancer therapy. In this study, we addressed the potential use of a lectin from Canavalia grandiflora seeds (ConGF) to control neutrophil migration and inflammatory hypernociception. Pretreatment of the animals intravenously (15 min before) with ConGF inhibited neutrophil migration to the peritoneal cavity in a dose-dependent fashion confirmed by an inhibition of rolling and adhesion of leukocytes by intravital microscopy. Another set of experiments showed that pretreatment of the animals with ConGF inhibited the mechanical hypernociception in mice induced by the i.pl. injection of carrageenan or formalin. This anti-nociceptive effect correlated with an effective blockade of neutrophil influx, as assessed by the hind paw tissue myeloperoxidase levels. Furthermore, ConGF had important inhibitory effects on the mouse carrageenan-induced paw edema. In addition, animals treated with ConGF showed inhibition of cytokines release. In conclusion, we demonstrated that the lectin ConGF inhibits neutrophil migration and mechanical inflammatory hypernociception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutrophil migration during an inflammatory response results mainly from the release of inflammatory mediators by resident cells, which induce the rolling and adhesion of neutrophils on endothelial cells, followed by their transmigration to the extravascular space (Smith 1993). These events require interaction of reciprocal adhesion molecules present on neutrophils and endothelial cells. Rolling is mediated by E- and P-selectins (on endothelial cells) and L-selectin (on leukocytes) interacting with their respective carbohydrate ligand. Thereafter, adhesion and transmigration are mediated by CD11/CD18 complex (ß2-integrins) on leukocyte, which interacts with its ligands immunoglobulins, such as intercellular adhesion molecule-1, present mostly on endothelial cells (Mayadas and Cullere 2005).
Many new anti-inflammatory drugs aim at interfering with transendothelial migration by antagonizing surface integrins, selectins, or chemokines involved in leukocyte homing (Mackay 2008). In clinical use, since the early twentieth century, to blunt acute inflammation, colchicine prevents microtubule assembly by binding to tubulin, which inhibits chemotactic migration of neutrophils and monocytes into and within interstitial tissue (Ben-Chetrit et al. 2006). More recently, developed strategies to interfere with chemoattractant signaling show strong promise in reducing tissue damage in chronic inflammation (Mackay 2008). Likewise, competitive inhibitors of the Leukotrien B4 receptor BLT1 reduce inflammation in models of rheumatoid arthritis and atherosclerosis (Aiello et al. 2002; Mathis et al. 2007, Grespan et al. 2008)
Chemical constituents obtained from medicinal plants and other natural products have been increasingly used to treat many inflammatory diseases. In this line, lectins constitute a group of widely distributed and structurally heterogeneous carbohydrate-binding proteins that comprises distinct but evolutionary-related families (Sharon and Lis 2004). An important discovery was that due to their high and specific reactivity with carbohydrate epitopes, regardless whether these were in solution or on cellular surfaces, lectins could offer the possibility of developing useful tools and applications in bioscience and biomedicine (Pusztai et al. 2008). Leguminosae lectins constitute a protein family with related amino acid sequences that may differ from each other strongly with respect to their carbohydrate-binding specificity (Van Damme et al. 1988).
Several biological activities of plant lectins have been described (Machuka et al. 1999; Rhodes and Campbell 2002; Dube and Bertozzi, 2005; Teixeira et al. 2006, Teixeira et al. 2007). Regarding the inflammatory process, some previous works have reported the useful application of lectins as anti-inflammatory approach by inhibiting the leukocyte migration (Mota et al. 2006; Napimoga et al. 2007; Bitencourt et al. 2008), since lectins are recognized among the adhesion molecules that actively participate in those responses, such as the selectins (L-, P-, and E-selectin), the integrin CD11b/CD18 (Mac-1), CD31 (PECAM-1), and CD44 (Hébert 2000). Therefore, in the present study, we evaluated the potential use of Canavalia grandiflora seeds lectin (a d-glucose/d-mannose-specific lectin) on the inhibition of carrageenan-induced peritonitis as well the mechanisms by which this lectin inhibits neutrophil migration and inflammatory hypernociception (hyperalgesia and/or allodynia in experimental animals).
Materials and methods
Lectin isolation
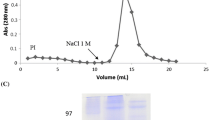
A d-glucose/d-mannose-specific lectin from seeds of C. grandiflora (ConGF) was purified by affinity chromatography on Sephadex G-50 as previously described (Ceccatto et al. 2002). By sodium dodecyl sulfate polyacrylamide gel electrophoresis, ConGF yielded three protein bands with apparent molecular masses of 29–30 kDa (alpha chain), 16–18 kDa (beta fragment), and 12–13 kDa (gamma fragment), like other related lectins from the genus Canavalia (Leguminosae), and is endotoxin free.
Animals
Male Swiss mice (30–35 g) were housed in temperature-controlled rooms (22–25°C) with access to water and food ad libitum. All experiments were conducted in accordance with the National Institutes of Health guidelines for the welfare of experimental animals and with the approval of the Ethics Committee of the University of Uberaba, Brazil. The animals were used only in a single experimental group.
Experimental procedure to evaluate neutrophil migration
For the determination of neutrophil migration to peritoneal cavity, ConGF was administered i.v. 15 min before (1, 3, or 10 mg/kg) the administration of inflammatory stimuli by intraperitoneal injection of carrageenan (Cg; Sigma, St. Louis, MO, USA) at 500 μg/cavity in naive mice.
Mice were killed 4 h after the challenge (carrageenan) administration, and the peritoneal cavity cells were harvested by washing the cavity with 3 ml of phosphate-buffered saline (PBS) containing ethylenediamine tetraacetic acid (EDTA) 1 mM. The volumes recovered were similar in all experimental groups and equated to approximately 95% of the injected volume. Total counts were performed in a Newbauer chamber, and differential cell counts (100 cells total) were carried out on cytocentrifuge (Cientec, Piracicaba, SP, Brazil) slides stained with Rosenfeld. The results are presented as the number of neutrophils per cavity.
Vascular permeability
The vascular permeability was analyzed by Evans blue test, as described previously (Thurston et al. 2000). Thirty minutes before Cg administration, Evans blue (50 mg/kg) was injected with 100 μl of saline intravenously into the ocular plexus. Mice were killed 4 h after Cg administration, and the peritoneal cavity was washed with 3 ml of PBS. Evans blue content was calculated using an Evans blue standard curve, and the absorbance of each sample was measured at 620 nm using a spectrophotometer.
Determination of myeloperoxidase activity
We evaluated the neutrophil migration to the intraplantar hind paw using the myeloperoxidase activity. Mice were pretreated 15 min before with lectin solution i.v. at 10 mg/kg in a final volume of 100 μl. The animals received an i.pl. injection of Cg (100 μg/paw; 50 µl), and neutrophil migration was measured after 3 h. The extent of neutrophil accumulation in the hind paw was measured by assaying myeloperoxidase activity as previously described (Cunha et al. 2008). Briefly, the hind paw tissue was removed and homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 1.015 M NaEDTA) followed by centrifugation at 3,000×g for 15 min. The pellet was subjected to hypotonic lyses (1.5 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide. After that, the tissue was snap-frozen in liquid nitrogen three times and was centrifuged at 10,192×g for 15 min and was re-homogenized. Myeloperoxidase activity in the resuspended pellet was assayed by measuring the change in optical density at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were calculated by comparing the optical density of hind paw tissue supernatant with a standard curve of neutrophil (>95% purity) numbers.
Carrageenan-induced mouse paw edema
Mice were pretreated 15 min before with lectin solution i.v. at 10 mg/kg in a final volume of 100 μl. The animals received a 100-μl i.pl. injection in one hindpaw (right paw) of saline 0.9% containing carrageenan (300 μg/paw). The contralateral paw (left paw) received 100 μl of saline, and it was used as a control. Edema was measured using a plethysmometer (Ugo Basile) at several time points after carrageenan injection. Edema is expressed in microliters as the difference between the right and left paws.
Real time in situ microscopic analysis for rolling and adhesion events of neutrophils in the mesenteric microcirculation
The leukocyte rolling and adhesion were examined as described previously (Fortes et al. 1991). Briefly, mice were anesthetized i.p. with tribromoethanol (250 mg/kg), and the mesenteric tissue was exposed for microscopic examination. The animals were maintained on a special board thermostatically controlled at 37°C. Images were recorded on a video recorder using a long-distance objective lens (×40) with a 0.65 numerical aperture. Vessels selected for study were third-order venules, defined according to their branch-order location within the microvascular network. These vessels corresponded to postcapillary venules, with a diameter of 10–18 μm. Rolling leukocytes were defined as the white blood cells that moved at a lower velocity than erythrocytes in the same stream and were determined at 10-min intervals 2 h after challenge with carrageenan. Adherent leukocytes were considered to be the white blood cells that remained stationary on the venular endothelium at the end of the observation period and were determined 4 h after challenge with carrageenan (Granger et al. 1989). The venular area in which the adhesion process was determined varied from 350 to 450 μm2, and the results were expressed as the number of adherent leukocyte per 100 μm2 of venule. The time points selected to determine rolling (2 h) and adhesion (4 h) processes were based on previous studies, which observed that these processes peak at these times after inflammatory stimuli injection (Dal Secco et al. 2003; Napimoga et al. 2008).
Effect of pretreatment of animals with ConGF upon mechanical hypernociception induced by Cg or formalin in mice
Separate sets of mice were tested for behavioral responses to mechanical stimuli using calibrated von Frey nylon filaments (tip diameter, 0.8 mm; Insight Equipments, Brazil). The mice were placed in an elevated plastic cage on a wire mesh floor. Von Frey hairs were applied from below to the plantar surface of right hind paw in ascending order of stimulus force (maximum, 50 g). At threshold, the mouse withdraws its paw away from the hair. Right hindpaw were tested three times, and the interval between two consecutive trials on the same paw was at least 1 min. The results are presented as the average of responses against a given stimulus force.
All mice groups tested were pretreated 15 min before with lectin solution i.v. at 10 mg/kg in a final volume of 100 μl. Vehicle was injected i.pl. and used as a negative control. In a separate set of animals, hypernociception was measured at 0 and 3 h after injection of Cg (100 μg/paw; 50 µl) into the hind paws (i.pl.). The Cg dose injected were the smallest doses that evoked maximum acute mechanical hypernociception (Napimoga et al. 2007).
Formalin-induced hypernociception behavior was assessed as described previously (Tjolsen et al. 1992). Mice were allowed to acclimate for 15 min in a plastic box and injected subcutaneously with 50 µl of 1% formaldehyde in 0.9% saline into the plantar right hindpaw of mice pretreated 15 min before with ConGF (10 mg/kg) or vehicle. The mice were observed for 60 min after the formalin injection and the paw flinches counted during the observation period time. The acute phase (phase 1) was defined as 0–10 min after injection, and the persistent (tonic) phase (phase 2) was defined as 10–40 (phase 2a) min and 40–60 (phase 2b) after injection.
Cytokine measurements assay
Mice received lectin solution i.v. at 10 mg/kg in a final volume of 100 μl, and after 2 h of Cg stimuli, peritoneal exudates were recovered to cytokine measurements. Levels of TNF-α and IL-1β were determined by enzyme-linked immunosorbent assay (ELISA) using protocols supplied by the manufacturers (R&D Systems, Minneapolis, USA). The results are expressed as picograms.
Statistical analysis
Data were expressed as mean ± SD. Statistical comparisons between groups were made using analyses of variance (ANOVA) followed by Bonferroni’s test. Significance was accepted when the P value was ≤0.05.
Results
The pretreatment with ConGF (1, 3, or 10 mg/kg; i.v.) decreased, in a dose-dependent manner (P < 0.05), the neutrophil migration in mice, induced by intraperitoneal injection of Cg (500 μg/cavity) determined 4 h later (Fig. 1a). In addition to neutrophil migration, vascular leakage is a major parameter in inflammation. Therefore, we tested the effect of ConGF in the carrageenan-induced microvascular permeability. Figure 1b shows that pretreatment (15 min, i.v.) with 10 mg/kg of ConGF significantly decreased (P < 0.05) the content of Evans blue in peritoneal fluid. To confirm the mechanisms by which the lectin decreases the neutrophil migration to the inflammatory site, we administered ConGF lectin and evaluate the leukocyte–endothelium interaction in vivo. Leukocyte–endothelium interaction (rolling and adhesion) was examined in mesenteric postcapillary venules using intravital microscopy system. The intraperitoneal injection of Cg (500 μg/cavity) caused a significant augment in rolling (Fig. 1c) and adhesion (Fig. 1d) of leukocytes on endothelium compared with the i.p. injection of saline (P < 0.05). The pre-treatment of the Cg-injected mice with ConGF, at a dose of 10 mg/kg, significantly decreased leukocyte rolling and adhesion, as shown Fig. 1c and d, respectively (P < 0.05).
Anti-inflammatory effect of ConGF on carrageenan-induced peritonitis in mice. a Mice were treated with saline (0.1 ml, i.v.) or ConGF (1, 3, or 10 mg/kg, i.v., 15 min before) and then injected i.p. with carrageenan at a dose of 500 μg/cavity. The neutrophil migration was evaluated 4 h later. The white bars represent the neutrophil migration induced by saline injected i.p. b Effects of the pretreatment with ConGF lectin on vascular permeability. Vehicle (saline) or ConGF (10 mg/kg) was injected i.v. and, 15 min later, carrageenan (500 μg/cavity). The ConGF treatment decreases leukocyte rolling and adhesion on venular endothelial cells. The mice were treated with saline (0.1 ml, i.v.) or ConGF (10 mg/kg, i.v., 15 min before) and then injected with Cg (500 μg/cavity). The leukocyte rolling (c) and adhesion (d) were evaluated by intravital microscopy in the mesentery 4 h after Cg injection. The first bar in c and d represents the rolling and adhesion, respectively, in PBS i.p.-injected animals. The values are means ± SD. *P < 0.05 compared to carrageenan group; # P < 0.05 compared to saline group (ANOVA followed by Bonferroni’s t test)
Supporting the anti-inflammatory effects of ConGF in peritonitis-induced inflammation, we observed that the concentration of the pro-inflammatory mediators interleukin (IL)-1β and tumor necrosis factor alpha (TNF-α) in the peritoneal fluid of mice challenged with Cg were decreased (Fig. 2a and b, respectively).
Effect of ConGF lectin pretreatment on cytokines IL-1β and TNF-α production on peritoneal exudates. Animals were injected with saline (control) or ConGF (10 mg/kg), and 15 min later, injection with Cg was performed. The concentrations of tested cytokines were determined by ELISA. Results are reported as means ± SD of five animals each group and are representative of two different experiments. *P < 0.05 compared to Cg-injected group; # P < 0.05 compared to saline group (ANOVA followed by Bonferroni’s t test)
We previously demonstrated that neutrophils play a critical role in carrageenan-induced mechanical hypernociception (Cunha et al. 2008). Thus, we tested if ConGF can prevent the neutrophil influx and, consequently, the Cg-induced nociception into mouse hind paw. As shown in Fig. 3a, ConGF (10 mg/kg; i.v.) given 15 min before i.pl. injection of Cg (100 μg/paw) inhibited mechanical hypernociception. This effect on inflammatory hypernociception seems to be closely associated with the capacity of ConGF to inhibit Cg-induced neutrophil migration to the plantar tissue, as evaluated by myeloperoxidase (MPO) activity assay (Fig. 3b). In addition, the treatment with ConGF significantly diminished edema formation by carrageenan (300 μg/paw) injection into mouse paw (Fig. 3c). ConGF injected i.v. at 10 mg/kg, 15 min before formalin, did not inhibited the first phase (first 10 min) of the formalin. This first phase results essentially from the direct stimulation of nociceptors. However, a significant antinociceptive effect was observed (P < 0.05) in the second phase (10–40 min) of the test (Fig. 3d), which involves a period of sensitization during which inflammatory phenomena occur (Le Bars et al. 2001).
ConGF inhibits mechanical hypernociception and neutrophil migration to the plantar tissue. Mice were treated with ConGF (10 mg/kg, i.v.) or vehicle (saline) 15 min before the i.pl. injection of carrageenan (Cg; 100 μg/paw) or saline. The hypernociceptive responses were evaluated 3 h after stimuli, followed by the collection of the plantar hind-paw tissue for MPO analysis (a and b, respectively). c Effect of treatment with ConGF (10 mg/kg, i.v.) on mouse paw edema induced by carrageenan (300 μg/paw). d To perform formalin test, ConGF was injected i.v. 15 min before formalin administration. The mice were observed for 60 min after the formalin injection, and the paw flinches were counted during the observation time period. Results are expressed as means ± SD at 10, 40, and 60 min after injection. # P < 0.05, compared with the control saline paw-injected group; * compared with the vehicle-treated group; one-way ANOVA followed by Bonferroni’s t test (n = 5)
Furthermore, mice treated i.v. with ConGF at 10 mg/kg dose, in which the lectin presented anti-inflammatory action, in a single dose scheme over seven consecutive days did not show any signal of toxicity, such as the animal’s corporal mass and unaltered urea, aspartate transaminase and alanine transaminase values (data not shown).
Discussion
New research directions in the last decade have led to major developments in the uses of plant lectins in bioscience and biomedicine. In the present study, we demonstrated that the pretreatment of mice with C. grandiflora lectin (ConGF) decreases leukocyte–endothelium interaction (rolling and adhesion), neutrophil transmigration, and also the inflammatory hypernociception in response to injection of inflammatory stimuli. The inhibition of pro-inflammatory cytokine production by treatment of the animals with ConGF correlates with the reduction of neutrophil influx to inflammatory site.
Any acute or chronic inflammation in the tissue in response to damage, infection, and in autoimmune disease prompts tissue infiltration by effector cells, including neutrophils, monocytes, T cells, and in chronic states, B cells (Friedl and Weigelin 2008). Since the lectin-mediated interactions are involved in many pathological processes, such as host–microbial interaction and inflammation, carbohydrates and exogenous lectins, by blocking these glycobiological interactions, can be potentially useful as tools or therapeutic modulators in these processes. In this study, we demonstrated that ConGF, a d-glucose/d-mannose-specific lectin can dose-dependently inhibit the neutrophil migration to peritoneal cavity by inhibiting the rolling and adherence of leukocytes to microvessel parameter keys to induce neutrophil migration (Mayadas and Cullere 2005). Besides, the inflammatory response is orchestrated by a large range of mediators able to promote vascular events, edema, and consequently, the recruitment of inflammatory cells (Mackay 2008). In line with this, we studied the mechanism by which C. grandiflora lectin could inhibit the leukocyte transmigration.
Our results demonstrated that pretreated animals with ConGF present decrease in pro-inflammatory cytokines such as TNF-α and IL-1β levels. This is important since it is well documented that both cytokines are important for neutrophil recruitment, which is a complex process that involves a sequence of molecular–mechanical events on leukocytes and endothelial cells that depends on distinct cell–cell adhesion molecules and cytokines/chemokines production (Hogg and Walker 1995). Thus, our data suggest that the in vivo anti-inflammatory effect of this lectin could be by inhibition of pro-inflammatory cytokine production at inflammatory sites, which in turn inhibit the leukocyte interaction with endothelial cells (rolling and adhesion). On the other hand, under inflammatory conditions, the resident cells such as dendritic cells, macrophages, mast cells, and lymphocytes are tissue cells that, after recognizing the inflammatory stimuli, release cytokines. These cytokines play an essential role in the development of inflammatory pain as well as other inflammatory events, such as leukocyte migration. (Cunha et al. 1992; Safieh-Garabedian et al. 1995; Cunha et al. 2005; Verri et al. 2006). The mediators involved in the genesis of inflammatory pain also play an essential role in triggering other inflammatory events, including edema and leukocyte migration. In agreement, neutrophil infiltration into the joint of arthritis patients precedes clinical signs of inflammation and is predictive of pain (Jones et al. 1991).
Considering that activated neutrophils produce and release pro-inflammatory cytokines, including TNF-α, IL-1β, and CINC-1/CXCL1, and mediators such as prostaglandins (Conti et al. 1988; Edamatsu et al. 1997; Kasama et al. 2005), which are essential to the development of inflammatory hypernociception, it is conceivable to suggest that neutrophils could be a relevant source of hypernociceptive cytokines or, alternatively, of the direct-acting hypernociceptive mediators, such as prostaglandins. Our group previously demonstrated that during the inflammatory process, the migrating neutrophils participate in the cascade of events leading to mechanical hypernociception (Cunha et al. 2008). Thus, in the present study, we tested the potential usefulness of ConGF as a pharmacological tool to inhibit neutrophil migration to mice hind paw. In fact, we observed an analgesic effect in the pretreated animals with ConGF, and this effect was correlated with lower neutrophil influx to mice hind paw confirmed by MPO activity assay. Importantly, ConGF also diminished the mice paw edema. The edema induced by carrageenan is a temporal and multimediated phenomenon involving the participation of a diversity of mediators (Ozaki 1990). In the initial phase (1/2–1 h), bradykinin, histamine, and serotonin are released by local cells. In a later phase (3–4 h), the release of prostaglandins takes place (Di Rosa et al. 1971; Morris 2003). In our study, ConGF potently inhibited the edema evoked by carrageenan, until the fourth hour after challenge, time coincident to prostaglandins release, suggesting an inhibition of this prostaglandin.
The pretreatment of mice with ConGF did also inhibit the second phase of hypernociception induced by formalin in the mice paw. Importantly, intraplantar injections of formalin produce a biphasic behavioral reaction in which the first phase results essentially from the direct stimulation of nociceptors, whereas the second involves a period of sensitization during which inflammatory phenomena occur (Le Bars et al. 2001). Opioid analgesics seem to be antinociceptive for both phases, although the second is more sensitive to these substances. In contrast, non-steroidal anti-inflammatory drugs such as indomethacin seem to suppress only the second phase, since this phase possesses inflammatory characteristics (Jourdan et al. 1997). These results reinforce the proposal that ConGF antihyperalgesic effect is mostly due to a reduction in neutrophils, which mediate the release of direct-acting hypernociceptive mediators (e.g., prostaglandins and sympathetic amines; Cunha et al. 2008).
In summary, our results demonstrate an important effect for the ConGF as a modulator of key inflammatory events occurring at the interface of leukocytes and the vascular endothelium during inflammatory responses. Furthermore, the anti-inflammatory and analgesic effects of ConGF appear to be mediated via IL-1β, TNF-α, rolling and leukocyte adhesion on endothelium reduction, and consequently, neutrophil trafficking inhibition. Together, these results identify ConGF as a potential target for novel anti-inflammatory and analgesics therapies.
References
Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ (2002) Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol 22:443–449
Ben-Chetrit E, Bergmann S, Sood R (2006) Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology 45:274–282
Bitencourt Fda S, Figueiredo JG, Mota MR, Bezerra CC, Silvestre PP, Vale MR, Nascimento KS, Sampaio AH, Nagano CS, Saker-Sampaio S, Farias WR, Cavada BS, Assreuy AM, de Alencar NM (2008) Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn-Schmiedeberg’s Arch Pharmacol 377:139–148
Ceccatto VM, Cavada BS, Nunes EP, Nogueira NA, Grangeiro MB, Moreno FB, Teixeira EH, Sampaio AH, Alves MA, Ramos MV, Calvete JJ, Grangeiro TB (2002) Purification and partial characterization of a lectin from Canavalia grandiflora benth. seeds. Protein Peptide Lett 9:67–73
Conti P, Reale M, Fiore S, Cancelli A, Angeletti PU, Dinarello CA (1988) Recombinant interleukin 1 and tumor necrosis factor acting in synergy to release thromboxane, 6-KETO-PGF1α and PGE2 by human neutrophils. Scand J Rheumatol Suppl 75:318–324
Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH (1992) The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107:660–664
Cunha TM, Verri WA Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH (2005) A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 102:1755–1760
Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ (2008) Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83:824–832
Dal Secco D, Paron JA, de Oliveira SH, Ferreira SH, Silva JS, Cunha Fde Q (2003) Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 9:153–164
Di Rosa M, Giroud JP, Willoughby DA (1971) Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–29
Dube DH, Bertozzi CR (2005) Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev 4:477–488
Edamatsu T, Xiao YQ, Tanabe J, Mue S, Ohuchi K (1997) Induction of neutrophil chemotactic factor production by staurosporine in rat peritoneal neutrophils. Br J Pharmacol 121:1651–1658
Fortes ZB, Farsk YSP, Oliveira MA, Garcia-Leme J (1991) Direct vital microscopic study of defective leukocyte–endothelial interaction in diabetes mellitus. Diabetes 40:1267–1273
Friedl P, Weigelin B (2008) Interstitial leukocyte migration and immune function. Nat Immunol 9:960–969
Granger DN, Benoit JN, Suzuki M, Grisham MB (1989) Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol 257:G683–G688
Grespan R, Fukada SY, Lemos HP, Vieira SM, Napimoga MH, Teixeira MM, Fraser AR, Liew FY, McInnes IB, Cunha FQ (2008) CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum 58:2030–2040
Hébert E (2000) Endogenous lectins as cell surface transducers. Biosci Rep 20:213–237
Hogg JC, Walker BA (1995) Polymorphonuclear leucocyte traffic in lung inflammation. Thorax 50:819–820
Jones AK, al-Janabi MA, Solanki K, Sobnack R, Greenwood A, Doyle DV, Britton KE, Huskisson EC (1991) In vivo leukocyte migration in arthritis. Arthritis Rheum 34:270–275
Jourdan D, Ardid D, Bardin L, Bardin M, Neuzeret D, Lanphouthacoul L, Eschalier A (1997) A new automated method of pain scoring in the formalin test in rats. Pain 71:265–270
Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL (2005) Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy 4:273–279
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53:597–652
Machuka J, Van Damme JEJM, Peumans WJ, Jackai LEN (1999) Effect of plant lectins on larval development of the legume pod borer; Maruca vitrata. Entomol Exp Appl 93:179–187
Mackay CR (2008) Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol 9:988–998
Mathis S, Jala VR, Haribabu B (2007) Role of leukotriene B4 receptors in rheumatoid arthritis. Autoimmun Rev 7:12–17
Mayadas TN, Cullere X (2005) Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol 26:388–395
Morris CJ (2003) Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225:115–121
Mota MR, Criddle DN, Alencar NM, Gomes RC, Meireles AV, Santi-Gadelha T, Gadelha CA, Oliveira CC, Benevides RG, Cavada BS, Assreuy AM (2006) Modulation of acute inflammation by a chitin-binding lectin from Araucaria angustifolia seeds via mast cells. Naunyn-Schmiedeberg’s Arch Pharmacol 374:1–10
Napimoga MH, Cavada BS, Alencar NM, Mota ML, Bittencourt FS, Alves-Filho JC, Grespan R, Gonçalves RB, Clemente-Napimoga JT, de Freitas A, Parada CA, Ferreira SH, Cunha FQ (2007) Lonchocarpus sericeus lectin decreases leukocyte migration and mechanical hypernociception by inhibiting cytokine and chemokines production. Int Immunopharmacol 7:824–835
Napimoga MH, Vieira SM, Dal-Secco D, Freitas A, Souto FO, Mestriner FL, Alves-Filho JC, Grespan R, Kawai T, Ferreira SH, Cunha FQ (2008) Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J Immunol 180:609–617
Ozaki Y (1990) Anti-inflammatory effects of Curcuma xanthorrhiza Roxb, and its active principle. Chem Pharm Bull 38:1045–1048
Pusztai A, Bardocz S, Ewen SW (2008) Uses of plant lectins in bioscience and biomedicine. Front Biosci 13:1130–1140
Rhodes JM, Campbell BJ (2002) Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med 8:10–16
Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ (1995) Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 115:1265–1275
Sharon N, Lis H (2004) History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14:53R–62R
Smith CW (1993) Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharmacol 71:76–87
Teixeira EH, Napimoga MH, Carneiro VA, de Oliveira TM, Cunha RM, Havt A, Martins JL, Pinto VP, Gonçalves RB, Cavada BS (2006) In vitro inhibition of Streptococci binding to enamel acquired pellicle by plant lectins. J Appl Microbiol 101:111–116
Teixeira EH, Napimoga MH, Carneiro VA, de Oliveira TM, Nascimento KS, Nagano CS, Souza JB, Havt A, Pinto VP, Gonçalves RB, Farias WR, Saker-Sampaio S, Sampaio AH, Cavada BS (2007) In vitro inhibition of oral streptococci binding to the acquired pellicle by algal lectins. J Appl Microbiol 103:1001–1006
Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD (2000) Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6:460–463
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51:5–17
Van Damme EJM, Peumans WJ, Barre A, Rouge P (1988) Plant lectins: a composite of several distinct families of structurally and evolutionarily related proteins with diverse biological roles. Crit Rev Plant Sci 17:575–692
Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH (2006) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112:116–138
Acknowledgments
This work was supported by grants from Programa de Apoio a Pesquisa–Universidade de Uberaba (PAPE-UNIUBE 2007/005) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nunes, B.S., Rensonnet, N.S., Dal-Secco, D. et al. Lectin extracted from Canavalia grandiflora seeds presents potential anti-inflammatory and analgesic effects. Naunyn-Schmied Arch Pharmacol 379, 609–616 (2009). https://doi.org/10.1007/s00210-009-0397-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-009-0397-9