Abstract
The effects of a lectin (AaL) from seeds of Araucaria angustifolia were investigated in the model of rat paw edema. In vivo anti-and pro-inflammatory activities, role of sugar residues, inflammatory mediators and systemic toxicity were assessed. Intravenous injection of AaL (0.1–1 mg/kg) dose-dependently inhibited the dextran-induced increase in edema and vascular permeability, which were prevented by association of the lectin with its binding sugar N-acetyl-glucosamine (Glyc-Nac). AaL also significantly inhibited edema induced by serotonin (18%) and compound 48/80 (33%), but not edema induced by histamine. In contrast, when applied by the s.c. route, AaL evoked a paw edema that peaked 1 h later and was partially prevented by association with Glyc-Nac (59%) or by prior i.v. administration of the lectin itself (38.8%). This AaL edematogenic activity was significantly inhibited by pentoxifylline (44.4%) or dexamethasone (51%) and also by depletion of rat paw mast cells (45.6%), but not byl-N-nitro-arginine methyl ester or indomethacin, excluding involvement of nitric oxide and prostaglandins. Treatment of animals with a single anti-inflammatory dose of AaL (1 mg/kg, i.v.) for 7 days did not affect rat corporal mass, liver, kidney, spleen or stomach wet weight, blood leukocyte count, and urea, creatinine or serum transaminase activity. Systemic toxicity was apparent only at much higher doses (LD50=88.3 mg/kg) than those required for the anti-inflammatory effect. Summarizing, AaL exerts anti-and pro-edematogenic actions via interaction with its specific lectin domain. These actions may share a common pathway involving either activation or inhibition of inflammatory mediators from resident mast cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cells are intimately involved in the pathophysiology of allergic diseases and inflammation (Mekori and Metcalfe 2000), and their activation promotes release of chemical mediators responsible for important tissue alterations such as vasodilatation, increase in vascular permeability, broncoconstriction and neutrophil or eosinophil chemotaxis, among others (Metcalfe et al. 1997). Many of these effects are attributable to the activity of histamine, an amine formed by histidine decarboxylation, which can activate four subtypes of receptors (Marone et al. 2002). Osmotic edema induced by dextran results from alterations of the endothelium leading to fluid and protein leakage from local microvasculature (Lyons 1995) mediated by release of bradykinin and the amines histamine and 5-hydroxytryptamine (5-HT; serotonin) of mast cell origin (Moodley et al. 1982).
Lectins are glycoproteins that can recognize and reversibly bind to carbohydrates or other substances derived from sugars (Peumans and Van Damme 1995) are encountered throughout the animal and plant kingdoms. The literature to date has focused largely on the biological effects of angiosperm lectins belonging to the leguminous family, due to their comparative ease of isolation and purification (Sharon and Lis 1990). Leguminous lectins may exert both pro- (Alencar et al. 2003, 2004) and anti-inflammatory actions (Alencar et al. 1999, 2005; Assreuy et al. 1997, 1999). The important role of sugar residues was also demonstrated in these studies, since the observed activities were reversed when the lectins were associated with their specific binding sugars. A previous study has suggested that the in vitro inflammatory response induced by glucose-mannose-binding plant lectins involves activation of mast cells and release of histamine (Gomes et al. 1994). Additionally, some plant lectins with binding specificity for N-acetyl-glucosamine (Glyc-Nac) have been shown to inhibit histamine secretion from mast cells in vitro (Bach and Brashler, 1975; Matsuda et al. 1994). However, little is currently known about the effects of gymnosperm lectins (Datta et al. 1991).
We therefore decided to investigate the effect of a novel gymnosperm lectin (AaL) isolated from seeds of Araucaria angustifolia, with binding specificity for Glyc-Nac, on acute inflammation using the in vivo model of rat paw edema. AaL was administered both by i.v. and s.c. routes and the participation of inflammatory mediators and sugar residues were assessed. The potential systemic toxicity of the lectin was also evaluated.
Methods
Animals
Male Wistar rats (160–220 g) and Swiss mice (20–30 g) were maintained in rooms with a controlled 12 /12 h light/dark cycle, at a temperature of 25°C with free access of food and water. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of the State University of Ceará (UECE), Fortaleza-CE, Brazil, in accordance with international guidelines (NIH publication No. 85–23, revised 1985).
Lectin isolation
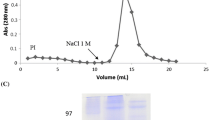
Seeds of Araucaria angustifolia, commonly known as “pinhão brasileiro”, were collected in Rio Grande do Sul (Brazil). Seeds were separated from the fruits, dried, finely ground in a coffee mill and the flour used for experimental purposes. Protein was extracted with 0.1 M glycine-HCl buffer containing 0.15 M NaCl, at pH 2.6 (1:10, w/v) at 25°C for 3 h. The protein extract obtained was centrifuged (20,000 g, 20 min, 4°C) and the supernatant applied to a chitin column previously equilibrated with extraction buffer. After removing unbound material (Peak I), the lectin was eluted at a flow rate of 30 ml h−1 with 0.1 M triethanolamine buffer, pH 11, containing 0.15 M NaCl (Peak II).
The purity of lectin preparations was monitored by SDS-PAGE (Laemmli 1970) on vertical 2 mm gel slabs of 12% polyacrylamide separation gel with 3 M Tris-HCl, pH 8.8, 1% SDS buffer and 3.5% stacking gel with 0.5 M Tris-HCl, pH 6.8, 1% SDS buffer. Samples were dissolved in 0.0625 M Tris-HCl, pH 8.3, 1% SDS buffer with 5% 2-mercaptoethanol and 0.02% 0.01 M bromophenol blue and incubated at 100°C for 10 min. Electrophoresis was conducted at a constant current of 20 mA for 4 h. Protein bands were visualized by staining with Coomassie Brilliant Blue R-250.
Drugs and reagents
Dextran sulphate, carrageenan, Glyc-Nac, α-d-mannose (Man), histamine, serotonin, compound 48/80, indomethacin, dexamethasone, pentoxifylline, l-N-nitro-arginine methyl ester (L-NAME) and Evans blue were purchased from Sigma (St. Louis, MO). The enzymatic kits used for evaluation of the lectin systemic toxicity were from LABTEST (Diagnostic Tests-Brazil).
All drugs and the lectin were solubilized in 0.15 M sterile NaCl (saline), except for indomethacin, which was dissolved in DMSO up to 10 % of the total volume and then in saline.
The investigation of the effects of AaL was guided by the editorial published for assistance of authors interested in studying pharmacological activities of compounds obtained from plant sources (Michel et al. 2005).
Rat paw edema model
Paw volume was measured immediately before s.c. injection of inflammatory stimuli (zero time) into the right hind paw of rats under light ether anesthesia (Cirino et al. 1989) and at selected time intervals (0.5, 1, 2, 3, 4, 8 and 24 h) thereafter using a hydroplethysmometer (PanLab, Barcelona-Spain). Results were expressed as the increase or reduction in paw volume (in milliliters) calculated by subtracting the basal volume measured at zero time. The area under the time-course curve (AUC; time on the x-axis and response on the y-axis) was also calculated using a trapezoidal rule and results expressed in arbitrary units (Landucci et al. 1995).
Evaluation of AaL anti-inflammatory activity in the model of paw edema induced by dextran
Edema measurement
Dextran, a classical osmotic agent (Lo et al. 1982), was injected s.c. into the animal paws at a dose of 300 μg/paw (0.1 ml/100 g body weight), 30 min after intravenous treatment of animals with AaL (0.01, 0.1 or 1 mg kg−1/100 g body weight). Positive edema controls received only dextran and negative controls received the same volume of sterile saline. Edema was measured at 0.5, 1, 2, 3 and 4 h after dextran administration.
Vascular permeability determination
The methodology employed for quantification of vascular permeability was adapted from that used by Garcia-Leme and Wilhelm (1975). Control rats were injected s.c. with 0.1 ml of saline or dextran (300 μg/paw) into the right hind paw. AaL (1 mg/kg) was injected i.v. 30 min before the animals received dextran as inflammatory stimulus. Both AaL- and saline-treated animals received Evans blue (25 mg/kg; i.v.) 1 h before sacrifice. Paws were sectioned at the ankle, weighed, placed in 2 ml formamide and incubated at 36°C for 72 h. The optical density of the extracted dye was estimated at 600 nm and the results are presented as mean±SEM (μg) of Evans blue/mg tissue using a linear regression based on a control Evans blue curve.
Pharmacological modulation of AaL anti-edematogenic effect
In this experiment, we investigated which inflammatory mediator would be inhibited by the lectin, avoiding or limiting the development of paw edema induced by dextran. Paw edema was induced by s.c. injections of histamine (100 μg), serotonin (20 μg) or compound 48/80 (10 μg/paw). These agonists participate, or elicit release of other chemical mediators involved, in the dextran-evoked paw edema (Cirino et al. 1989). AaL (1 mg/kg; i.v.) was injected into animals 30 min before edema induction.
Evaluation of AaL edematogenic activity
Paw edema was induced by s.c. injection of AaL at doses of 0.01, 0.1 and 1 mg/kg, in a final volume of 0.1 ml/100 g body weight. Control animals received the same volume of sterile saline. Edema was measured at 0.5, 1, 2, 3 and 4 h after stimulus (AaL). In the majority of experiments, negative controls were presented as a pool of 4 experiments, using 6 animals each, in order to reach n=24.
Evaluation of the involvement of sugar residues in the pro-or anti-edematogenic effect of AaL
Animals were administered s.c. (pro-inflammatory) or i.v. (anti-inflammatory) with a solution containing the most active dose of the lectin associated with 0.1 M of its specific ligand sugar (Glyc-Nac) or with a non-ligand sugar (Man) as a control, after incubation at 37°C for 30 min in order to allow binding between lectin and sugar.
Modulation of AaL edematogenic effect
Effect of AaL systemic treatment on edema formation induced by AaL local administration
AaL (1 mg/kg) was administered i.v. 30 min before animals were locally treated with the lectin itself (1 mg/kg; s.c.).
Experiments for mast cell depletion with compound 48/80
Compound 48/80 or saline was injected i.p. into animals over 4 days: on the first 3 days at 0.6 mg/kg and on day 4 at 1.2 mg/kg (Di Rosa et al. 1971; Feitosa et al. 2002). Control experiments inducing paw edema with carrageenin and dextran, agents that evoke resident mast cell degranulation (Ohta et al. 2003) were also carried out: saline or compound 48/80-i.p. treated rats received s.c. injection of dextran (300 μg/paw) or carrageenan (500 μg/paw) 24 h after the sub-chronic treatment with compound 48/80 or saline. Control of the carageenin and dextran-induced edema was made by s.c. injection of saline into animal paws.
Investigation of the inflammatory mediator involved in the AaL edematogenic effect
AaL was injected s.c. (1 mg/kg) into animal paws 24 h after sub-chronic treatment with compound 48/80 or 1 h before single dose treatment with the following pharmacological blockers: indomethacin (5 mg/kg; s.c.); dexamethasone (1 mg/kg, s.c.); pentoxifylline (90 mg/kg; s.c.) and l-NAME (300 mg/kg; i.v.) (Feitosa et al. 2002; Di Rosa et al. 1971). Positive controls were injected s.c. with AaL only and negative controls received saline (0.1 ml/100 g body weight s.c.).
LD50 determination
AaL (1, 3, 10, 30, 100, 300 mg/kg) was diluted in 0.2 ml sterile saline and injected i.v. into groups of ten mice each (five males and five females). Animals were maintained with free access to water and food and observed over a 72-h period of surveillance for abnormal patterns of behavior (piloerection, tachycardia, cyanosis, tachypnea, pruritus, convulsions, sedation and death). The percentage of death was determined and LD50 calculated using the Probits method (Miller and Tainter 1944).
Evaluation of the systemic effects of AaL in rats
Corporal mass loss, organ weight alteration, stomach ulceration, leukogram and blood chemistry parameters [concentration of total proteins, albumin, urea, creatinine, alanine amino transferase (AST) and aspartate amino transferase (ALT)] were evaluated after sub-chronic treatment with AaL. Rats were weighed before i.v. treatment with single doses of AaL (1 mg/kg) or saline for 7 consecutive days. After treatment, rats were again weighed and peripheral blood was collected for leukogram (Souza and Ferreira 1985) and biochemical dosage (determined by enzymatic and colorimetric tests—LABTEST).
After sacrifice, the liver, kidney, spleen and stomach were removed and weighed. The wet weight of each organ was expressed relative to 100 g body weight and compared to the saline-injected group (control). Possible ulcerative lesions or hemorrhage were quantified and macroscopically measured. The stomach was opened via longitudinal incision following the great curvature and exposed for evaluation of the number and grade of gastric mucosal lesions (Santucci et al. 1994).
Statistical analysis
All results were expressed as mean±SEM of 6–10 animals per group. Statistical differences between groups were analyzed using ANOVA, followed by a post hoc Duncan test for multiple comparisons. P<0.05 was taken to indicate statistical significance.
Results
Antiinflammatory effect of AaL, pharmacological modulation and involvement of sugar residues
AaL inhibits edema formation and the increase in vascular permeability induced by dextran in rats, and this effect is prevented by Glyc-Nac
Subcutaneous injection of dextran (300 μg/paw) induced intense paw edema that reached a maximal value at 30 min (0.97±0.08 ml) and decreased over the following hours after administration (Fig. 1a). The i.v. treatment of rats with AaL 30 min before local injection of dextran, significantly and dose-dependently reduced the course of dextran-induced paw edema, by 24 and 49% at doses of 0.1 and 1 mg/kg, respectively, compared to animals treated with sterile saline (Fig. 1a,b). The maximal AaL inhibitory activity (113.1±11.1 arbitrary units) was completely prevented by the i.v. administration (30 min before stimulus) of a solution containing AaL (1 mg/kg) mixed and pre-incubated at 37°C for 30 min with its specific ligand Glyc-Nac (0.1 M). However, the i.v. pre-treatment of animals with a combined solution of AaL (1 mg/kg) with Man (0.1 M), a non-ligand sugar, did not alter the lectin inhibitory effect (114.8±9.6 arbitrary units, data not shown). Moreover, 0.1 M Glyc-Nac, administered alone in rats 30 min before dextran, did not modify edema formation (198.3±13.9 arbitrary units, data not shown).
Araucaria angustifolialectin (AaL) inhibits edema and the increase in vascular permeability induced by dextran, and this effect is reversed by N-acetyl-glucosamine (Glyc-Nac). AaL (0.1 ml; 0.01; 0.1 and 1 mg/kg; i.v.)/100 g body weight or AaL (1 mg/kg) associated with 0.1 M Glyc-Nac was administered to rats 30 min before the same volume of dextran (300 μg/kg; s.c.). Animals were administered i.v. with AaL (1 mg/kg), 30 min before dextran, and with Evans blue (25 mg/kg) 1 h before sacrifice. a Dose-response curves, b area under the time-course curve (AUC), c results expressed as micrograms of Evans blue/gram paw wet weight. Positive controls received dextran only. Negative controls received sterile saline i.v. and s.c. Data are means ±SEM (n=6). * Difference (P<0.05) compared to positive control (dextran), # difference (P<0.05) compared to AaL 1 mg/kg
Additionally, dextran induced a significant increase in vascular permeability (33.3 μg Evans Blue/gram paw wet weight) compared to rats that had received sterile saline only. (Fig. 1c). The administration of AaL (1 mg/kg) 30 min before dextran (300 μg/paw; s.c.), also reduced (by approximately 30%) the increase in vascular permeability.
Effect of AaL on paw edema induced by histamine, 5-HT and compound 48/80
Histamine (100 μg/paw; s.c.) and compound 48/80 (10 μg/paw; s.c.) induced an intense edema that reached maximal values in the first 30 min after administration (0.85±0.03; 0.74±0.05 ml, respectively) and then subsequently decayed. Serotonin (5-HT; 20 μg/paw; s.c.) also evoked edema formation, which was maximal after 1 h (1.05±0.07 ml). AaL (1 mg/kg; i.v.), injected 30 min before these flogistic agents, did not reduce the time course of the edema developed by histamine (Fig. 2a) but significantly inhibited edema formation induced by compound 48/80 (Fig. 2b). The serotonin-induced edema was slightly although significantly inhibited by AaL (Fig. 2c).
AaL inhibits paw edema induced by serotonin and compound 48/80 but not by histamine. AaL [0.1 ml/100 g body weight (1 mg/kg; i.v.)] was administered in rats 30 min before the same volume of serotonin (20 μg/paw), compound 48/80 (10 μg/paw) or histamine (100 μg/paw) as stimuli. Positive controls received serotonin, compound 48/80 or histamine only. Negative controls (n=24) received sterile saline i.v. and s.c (a–c). Data are means ±SEM (n=6). * Difference (P<0.05) compared to the stimuli
Pro-inflammatory effect of AaL: pharmacological modulation and role of sugar residues
AaL induces a dose-dependent edematogenic effect that is reversed by Glyc-Nac
The s.c. injection of AaL at the same dose at which it exerts anti-inflammatory activity (0.1 and 1 mg/kg) evoked intense paw edema that peaked at the first hour after injection (0.66±0.07 ml and 0.80±0.07 ml, respectively) and decayed in the following hours. Local administration of AaL (1 mg/kg) associated with 0.1 M of Glyc-Nac, partially prevented (52.8%) the lectin edematogenic effect (Fig. 3).
Induction of paw edema by local injection of AaL is inhibited by Glyc-Nac. a AaL (0.01, 0.1 and 1 mg/kg) alone, or AaL (1 mg/kg) associated with 0.1 M Glyc-Nac was administered s.c. Negative controls (n=24) received sterile saline (0.1 ml/100 g body weight) s.c. b AUC. Data are means ±SEM (n=6). * Difference (P< 0.05) compared to negative control, # difference (P< 0.05) compared to AaL (1 mg/kg)
Intravenous injection of AaL inhibits edema formation induced by local injection of the lectin
The edema formation resulting from subcutaneous injection of AaL (1 mg/kg) was significantly (P<0.05) reduced (Fig. 4) in animals treated i.v. with the lectin, at the same dose, 30 min before its s.c. administration.
AaL inhibits paw edema formation evoked by the lectin itself. AaL (1 mg/kg) was administered i.v. 30 min before treatment of animals with AaL s.c. Positive controls received AaL only (1 mg/kg; s.c.)., negative controls (n=24) received sterile saline (0.1 ml/100 g body weight; i.v. and s.c.). Data are means ± SEM (n=6). * Difference (P< 0.05) compared to positive control
Control of rat paw mast cell depletion
Here, we analyzed the possibility of depleting resident mast cells of rat paws with compound 48/80 at the same dose used for depleting peritoneal mast cells (Ohta et al. 2003). Paw edema was induced both by carrageenan and dextran in animals sub-chronically pre-treated with compound 48/80. The edema evoked by dextran elicits release of histamine and serotonin from mast cells as the main chemical mediators, and in the edema evoked by carrageenin the role of these mediators is important only in its initial phase (Berstad 1980; Moodley et al. 1982). At 24 h after mast cell depletion, edema was induced by carrageenin (500 μg/paw) or dextran (300 μg/paw) into compound 48/80-treated and non-treated animals (injected i.p. with saline). Both dextran and carrageenin produced paw edema compared to control (injected s.c. with saline) with respective amplitudes of 118.5±9.3 (arbitrary units) and 127.7±9.6 (arbitrary units) (Fig. 5). The sub chronic treatment with compound 48/80 significantly reduced the paw edema evoked by dextran (61.7±4.8 arbitrary units) at all times analyzed (Fig. 5a,c), while the edema induced by carrageenin was significantly inhibited only in the first 2 h (Fig. 5b), but did not show statistical difference in the AUC calculation (102.8±9.8 arbitrary units; Fig. 5c).
Depletion of rat paw mast cells with compound 48/80 inhibits the paw edema induced by dextran and the initial phase of the edema induced by carrageenan. a,b Compound 48/80 was administered i.p. over 4 days (0.6 mg/kg on the first 3 days and 1.2 mg/kg on day 4). 24 h after compound 48/80 treatment, normal or 48/80 pre-treated rats were injected with carrageenan (500 μg/paw; s.c.) or dextran (300 μg/paw; s.c.). Positive controls received dextran or carrageenan only and negative controls (n=24) received saline s.c. (A and B). c AUC. Data are means ± SEM (n=6) * Difference (P< 0.05) compared to positive control
Pentoxifylline, compound 48/80 and dexamethasone inhibit the paw edema caused by AaL
Sub chronic treatment of animals with compound 48/80, and single dose treatment with pentoxifylline (1 mg/kg; s.c.) or dexamethasone (1 mg/kg; s.c.), injected 1 h before s.c. administration of AaL (1 mg/kg; s.c.), significantly inhibited by 44%, 36% and 51% respectively, the AaL-induced paw edema (123±9.75 arbitrary units). However, administration of l-NAME (300 mg/kg; i.v.) or indomethacin (5 mg/kg; s.c.), 1 h before s.c. administration of AaL did not alter its edematogenic effect (Fig. 6).
Pentoxifylline, compound 48/80 and dexamethasone inhibit the paw edema caused by AaL. Compound 48/80 was administered i.p. over 4 days (0.6 mg/kg on the first 3 days and 1.2 mg/kg on day 4). On the last day of compound 48–80 treatment, rats were administered s.c. with AaL (1 mg/kg). Pentoxifylline (pentoxi; 90 mg/kg; s.c.), dexamethasone (dexa; 1 mg/kg; s.c.), l-NAME (300 mg/kg) or indomethacin (indo; 5 mg/kg; s.c.) were injected as single doses 1 h before AaL. Negative controls (n=24) received saline (0.1 ml/100 g body weight; s.c.); positive controls received AaL s.c. only. AUC shown as means ± SEM (n=6). * Difference (P<0.05) compared to positive control
Effect of acute and sub-chronic treatment with AaL
Animals injected with AaL up to a dose of 30 mg/kg, observed for 72 h, did not exhibit any alteration in appearance or behavior (piloerection, tachycardia, cyanosis, tachypnea, pruritus, convulsions, sedation and death). Some animals showed signals of toxicity (piloerection, convulsions and death) at a dose of 100 mg/kg and the highest dose (300 mg/kg) evoked death in all animals, with LD50=88.3 mg/kg.
AaL injected in a single dose scheme (1 mg/kg; i.v., the dose at which AaL presented anti-inflammatory action) over 7 consecutive days did not affect corporal mass or wet weight of animal organs (spleen, stomach, liver, and kidney) compared to controls injected with sterile saline. All organs showed normal appearance and absence of edema over the whole course of lectin treatment. Values obtained for urea and creatinine dosage, used as parameters of renal function, did not differ from controls. Moreover, hepatic function, evaluated via the kinetics of hepatic enzymes (ALT, AST), and also the number of blood circulating leukocytes were not altered by the lectin treatment (data not shown).
Discussion
Dextran is a pro-inflammatory agent that promotes release of vasoactive amines such as histamine and serotonin, causing osmotic edema, which is characterized by an increase in vascular permeability (Berstad 1980; Moodley et al. 1982). In the present study, the s.c. injection of dextran induced intense paw edema in rats that was significantly and dose-dependently inhibited by previous i.v. treatment with Araucaria angustifolialectin (AaL). The AaL inhibitory activity was completely prevented by i.v. treatment with a combined solution of AaL and its specific ligand sugar Glyc-Nac, but not with a non ligand Man. Thus, the AaL anti-inflammatory effect appears to be completely dependent on the lectin carbohydrate recognition site. Additionally, i.v. administration of AaL at the dose at which this lectin promoted maximal anti-edematogenic activity also reduced the increase in vascular permeability resultant from the local injection of dextran.
The paw edema induced by dextran results from alterations in the endothelium leading to fluid and protein leakage from local microvasculature (Lyons 1995) mediated by release of bradykinin and the amines histamine and 5-HT (serotonin) of mast cell origin (Moodley et al. 1982). At this point, it was possible to postulate two principal mechanisms by which AaL might exert its anti-inflammatory action: (1) via inhibition of the release of biogenic amines from activated resident cells; (2) via inhibition of binding and/or activity of inflammatory mediators on their receptors.
Other plant lectins have been previously shown to recognize mast cells and to modulate histamine release in vitro (Bach and Brashler 1975). Thus, we investigated the putative participation of the principal biogenic amines of mast cell origin in the effects of AaL. Our results showed that i.v. treatment of animals with AaL significantly inhibited the time course of the edema induced both by serotonin and compound 48/80 but not that developed by histamine. These data indicate that the lectin does not antagonize histamine receptors. Since compound 48/80 is a substance that degranulates mast cells and promotes release of their contents, such as histamine and serotonin (Ohta et al. 2003), our data suggest that AaL may rather be acting as an anti-inflammatory via recognition of specific sugar/glucoconjugates on the mast cell surface, somehow stabilizing this cell membrane and preventing release of its granule contents. Serotonin is a mediator found in large amounts in enterocromafin cells of the gastrointestinal tract, mast cells, and cells of the nervous system (Dey and Hoffpauir 1984; Nagata et al. 2001). Serotonin released from inflammatory cells participates in the genesis of acute inflammatory vascular events and in various stages of the immune response (Mossner and Lesch 1998). The serotonin receptors 5-HT1 and 5-HT3 have also been associated with the modulation of mast cell degranulation (Castex et al. 1994; Coelho et al. 1998). The discrete, but significant, AaL inhibitory action on serotonin-induced paw edema might be explained in two ways: (1) AaL is a weak antagonist of serotonin receptors located in the peripheral microvasculature, (2) AaL interferes with serotonin’s autocrine effect on mast cells, preventing degranulation.
Plant lectins, locally injected, have been classically associated with inflammatory and immunostimulatory actions (Alencar et al. 2003, 2004; Gomes et al. 1994). In order to better explain the anti-inflammatory effects of AaL we examined its role as an inflammatory stimulator using a local route of administration. The s.c. injection of AaL in the same dose in which it exerts anti-inflammatory activity evoked intense paw edema that was partially prevented by the local administration of AaL associated to its ligand sugar Glyc-Nac. The AaL pro-inflammatory effect also appeared to be dependent on the recognition of specific carbohydrate sites.
Additionally, the i.v. injection of AaL inhibited the edema formation induced by local injection of the lectin itself. These results suggest that the mechanism used by AaL in evoking anti- or pro-inflammatory effects in the model of rat paw edema may follow a common pathway of activation or inhibition of inflammatory mediators from resident mast cells.
We further observed that the edema induced by AaL was inhibited in animals pre-treated sub chronically with compound 48/80, and also by treatment with single doses of dexamethasone and pentoxifylline, but not with indomethacin or l-NAME. Dexamethasone is a synthetic glucocorticoid with potent antiinflammatory and immunosuppressant properties (Jaffuel et al. 1999; Kim et al. 2003); indomethacin is a non-specific inhibitor of the cyclooxygenase enzyme activity, responsible for the arachidonic acid metabolism and synthesis of prostaglandins (Kankuri et al. 2001); l-NAME non-specifically interferes with the activity of nitric oxide synthase isoforms, inhibiting the production of nitric oxide (Moncada et al. 1991); pentoxifylline inhibits production of IL-1 and TNF-α, which participate in various events of the inflammatory response, including expression of adhesion molecules (Bernot et al. 2005; Dinarello 2000, 2002). Our results suggest that the mechanism of the AaL pro-inflammatory effect involves release of primary cytokines such as IL-1 and TNFα, and exclude the participation of prostaglandins and nitric oxide. Moreover, the inhibition of AaL edematogenic activity by compound 48/80 indicates that AaL most likely activates mast cells via histamine release (the opposite mechanism postulated for the AaL anti-inflammatory activity). At this stage, it was possible to speculate that some modifications in the native structure of AaL may occur: (1) AaL injected i.v. acts as antagonist of mast cell membrane receptors, inhibiting the release of its content granules; (2) AaL injected s.c. acts as agonist of mast cell membrane receptors, stimulating the release of its mediators.
Finally, sub chronic treatment of rats with AaL did not affect animal corporal mass or the wet weight of spleen, stomach, liver or kidney, all of which showed normal macroscopic appearance. Renal and hepatic function and the number of blood circulating leukocytes were not altered by the lectin treatment suggesting that AaL, at the dose evoking anti-inflammatory effect, did not cause secondary effects in the lymphoid tissue. Accordingly, the evaluation of acute toxicity showed undesirable effects only at doses higher than that required for the anti-inflammatory effect.
In conclusion, the lectin from seeds of Araucaria angustifolia possesses distinct effects in the in vivo experimental model of rat paw edema. The anti- or pro-inflammatory actions appear to be mediated via interaction of the lectin domain with specific carbohydrate recognition sites in mast cells, negatively or positively modulating mast cell degranulation. This novel in vivo anti-inflammatory action of plant lectins, associated with the lack of systemic toxicity, suggests the use of AaL as a biotechnological tool for construction of novel substances inhibitory to inflammatory mediator release. Such tools may have potential application in the treatment of pathological allergic conditions involving histamine release.
References
Alencar NMN, Teixeira EH, Assreuy AMS, Cavada BS, Flores CA, Ribeiro RA (1999) Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediat Inflamm 8:107–113
Alencar NMN, Assreuy AMS, Alencar VBM, Melo SC, Ramos MV, Cavada BS, Cunha FQ, Ribeiro RA (2003) The galactose-binding lectin from Vatairea macrocarpa seeds induces in vivo neutrophil migration by indirect mechanism. Int J Biochem Cell B 35:1674–1681
Alencar NMN, Assreuy AMS, Criddle DN, Souza EP, Soares PM., Havt A, Aragão KS, Bezerra DP, Ribeiro RA, Cavada BS (2004) Vatairea macrocarpa lectin induces paw edema with leukocyte infiltration. Protein Peptide Lett 11:195–200
Alencar NMN, Cavalcante CF, Vasconcelos MP, Leite KB, Assreuy AMS, Cavada BS, Nogueira NAP, Vale MR (2005) Antiinflammatory and antimicrobial effect of lectin from Lonchocarpus sericeus seeds in an experimental model of infectious peritonitis. J Pharm Pharmacol 57:912–922
Assreuy AMS, Shibuya MD, Martins GJ, Souza MLP, Cavada BS, Moreira RA, Oliveira JTA, Ribeiro RA, Flores CA (1997) Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Mediat Inflamm 6:201–210
Assreuy AMS, Martins GJ, Moreira ME, Brito GA, Cavada BS, Ribeiro RA, Flores CA (1999) Prevention of cyclophosphamide-induced hemorrhagic cystitis by glucose-mannose binding plant lectins. J Urol 161:1988–1993
Bach MK, Brashler JR (1975) Inhibiton of IgE and compound 48/80-induced histamine release by lectins. Immunology 29:371–386
Bernot D, Peiretti F, Canault M, Juhan-Vague I, Nalbone G (2005) Upregulation of TNF-alpha-induced ICAM-1 surface expression by adenylate cyclase-dependent pathway in human endothelial cells. J Cell Physiol 202:434–441
Berstad J (1980) Dextran-induced release of serotonin from isolated rat peritoneal mast cells. Acta Pharmacol Toxicol 47:213–216
Castex N, Fioramonti J, Fargeas MJ, More J, Bueno L (1994) Role of 5-HT3 receptors and afferent fibers in the effects of mast cell degranulation on colonic motility in rats. Gastroenterology 107:976–984
Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB (1989) Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci 86:3428–3432
Coelho AM, Fioramonti J, Bueno L (1998) Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Digest. Dis. Sci. 43:727–737
Datta PK, Figueroa MOC, Lajolo FM (1991) Purification and characterization of two major lectins from Araucaria brasiliensis syn. Araucaria angustifolia seeds (pinhão). Plant Physiol 97:856–862
Dey RD and Hoffpauir J (1984) Ultrastructural immunocytochemical localization of 5-hydroxytryptamine in gastric enterochromaffin cells. J Histochem Cytochem 32:661–666
Dinarello CA (2000) Interleukin-18, a proinflammatory cytokine. Eur Cytokine Network 11:483–486
Dinarello CA (2002) The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 20:1–13
Di Rosa M, Giroud JP, Willoughby DA (1971) Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–29
Feitosa RF, Melciades GB, Assreuy AMS, Rocha MF, Ribeiro RA, Lima AA (2002) The pharmacological profile of ovoalbumin-induced paw oedema in rats. Mediat Inflamm 11:155–163
Gomes JC, Ferreira RR, Cavada BS, Moreira RA, Oliveira JT (1994) Histamine release induced by glucose (mannose)-specific lectins isolated from Brazilian beans. Comparison with concanavalin A. Agents Actions 41:132–135
Jaffuel D, Demoly P, Gougat C, Mautino G, Bousquet J, Mathieu M (1999) Rifampicin is not an activator of the glucocorticoid receptor in A549 human alveolar cells. Mol Pharmacol 55:841–846
Kankuri E, Vaali K, Korpela R, Pakari I, Vapatalo H, Moilanen E (2001) Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation 25:301–310
Kim TW, Moon HB, Kim SJ (2003) Interleukin-10 is up-regulated by prolactin and serum-starvation in cultured mammary epithelial cells. Mol Cells 31:168–172
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Landucci ECT, Antunes E, Donato JL, Faro R, Hyslop S, Marangoni S, Oliveira B, Cirino G, De Nucci G (1995) Inhibition of carrageenan-induced rat paw edema by crotapotin, a polypeptide complexed with phospholipase A2. Brit J Pharmacol 114:578–583
Leme JG, Wilhelm DL (1975) The effects of adrenalectomy and corticosterone on vascular permeability responses in the skin of the rat. Brit J Exp Pathol 56:402–407
Lo TN, Almeida AP, Beaven MA (1982) Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther 221:261–267
Lyons CR (1995) The role of nitric oxide in inflammation. Adv Immunol 60:323–371
Marone G, Genovese A, Granata F, Forte V, Detoraki APA, Triggiani M (2002) Pharmacological modulation of human mast cells and basophils. Clin Exp Allergy 32:1682–1689
Matsuda K, Niitsuma A, Uchida MK, Suzuki-Nishimura T (1994) Inhibitory effects of sialic acid-or N-acetylglucosamine-specific lectins on histamine release induced by compound 48/80, bradykinin and a polyethylenimine in rat peritoneal mast cells. Jpn J Pharmacol 64:1–8
Mekori YA, Metcalfe DD (2000) Mast cells in innate immunity. Immunol Rev 173:131–140
Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77:1033–1079
Michel MC, König G, Mohr K, Simmet T (2005) Editorial guidelines for manuscripts on the pharmacology of plant extracts. Naunyn-Schmiedeberg’s Arch Pharmacol. 371:349–350
Miller LLC, Tainter TL (1944) Stimation of the DL50 and its error by means of logarithmin-probits graphpaper. Proc Soc Exp Biol Med 57:261–268
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–142
Moodley I, Mongan JL, Foreman JC (1982) Histamine release induced by dextran: the nature of the dextran receptor. Eur J Pharmacol 83:69–81
Mossner R, Lesch KP (1998) Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun 12:249–271
Nagata K, Fujimiva M, Sugiura H, Uehara M (2001) Intracellular localization of serotonin in mast cells of the colon in normal and colitis rats. Histochem J 33:559–568
Ohta Y, Kobayashi T, Inui K, Yoshino J, Nakasazawa S (2003) Protective effect of teprenone against acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. J Pharma Sci 93:337–346
Peumans WJ and Van Damme EJM (1995) Lectins as plant defense proteins. Plant Physiol 109:347–352
Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A (1994) Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut 35:909–915
Sharon N, Lis H (1990) Legume lectins-a large family of homologous proteins. FASEB J 4:3198–3208
Souza GEP, Ferreira SH (1985) Blockade by antimacrophage serum of the migration of PMN neutrophils into the inflammed peritoneal cavity. Agents Actions 17:97–103
Acknowledgements
M.R.L.M. (graduate student) and R.C. (undergraduate student) are recipients of fellowships from Fundação Cearense de Amparo à Pesquisa (FUNCAP) and CNPq, respectively. B.S.C. and A.M.S.A. are senior investigators of CNPq (Brazil).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mota, M.R.L., Criddle, D.N., Alencar, N.M.N. et al. Modulation of acute inflammation by a chitin-binding lectin from Araucaria angustifolia seeds via mast cells. Naunyn-Schmied Arch Pharmacol 374, 1–10 (2006). https://doi.org/10.1007/s00210-006-0097-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0097-7