Abstract
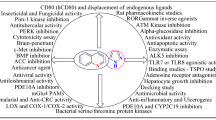
5-Oxo-hexahydroquinoline (5-oxo-HHQ) represents a biologically attractive fused heterocyclic core. Various synthetic analogs of 5-oxo-HHQ have been synthesized and assessed for different biological activities. Some derivatives have exhibited myorelaxant, analgesic, anticancer, antibacterial, antifungal, antitubercular, antimalarial, antioxidant, anti-inflammatory, multidrug resistance reversal, anti-Alzheimer, neuroprotective, antidiabetic, antidyslipidemic and antiosteoporotic activities. This review provides a comprehensive report regarding the preparation and pharmacological characterization of 5-oxo-HHQ derivatives that have been reported so far. This information will be beneficial for medicinal chemists in the field of drug discovery to design and develop new and potent therapeutical agents bearing the 5-oxo-HHQ nucleus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
5-Oxo-1,4,5,6,7,8-hexahyroquinoline (5-oxo-HHQ) (1) is a fused heterocycle which consists of a nitrogen containing doubly unsaturated six-membered nucleus, termed dihydropyridine (DHP) ring, and a cyclohexanone ring. During the last decades, compounds containing 5-oxo-HHQ core have been of great interest to the researchers due to their broad pharmacological and biological significance [1,2,3,4,5,6]. Accordingly, considerable attention has been given to the synthesis of various 5-oxo-HHQ derivatives using multicomponent reactions (MCRs) of diverse methodologies. This review provides a systematic study to assemble the chemical and pharmacological aspects of several synthesized 5-oxo-HHQ analogs reported to date.

Synthesis of 5-oxo-HHQs and the derivatives
Multicomponent condensation reactions provide the synthesis of libraries of diverse small molecules in one-pot procedure using several condensation reagents [7, 8]. 5-Oxo-HHQs are synthesized by a type of MCR called Hantzsch reaction which is used widely for the synthesis of symmetrical and unsymmetrical DHPs. The reaction includes cyclocondensation of an aldehyde, β-ketoester, 1,3-cyclohexanedione and ammonia or ammonium acetate either in acetic acid or in refluxing ethanol (Scheme 1) [9, 10]. Some modifications for this typical method have been applied in order to achieve diverse biologically active HHQs with various substitutions at all positions.
Instead of the β-ketoester compounds, β-aminocrotonate derivatives may be used for the synthesis of 5-oxo-HHQs (Scheme 2). In this case, there is no need to use ammonia as the source of nitrogen [1, 11].
N-substituted 2-amino-5-oxo-HHQs can be prepared via a one-pot three-component cyclocondensation reaction between N-substituted cyclohexane-enaminone, ethylcyanoacetate and an aldehyde in the presence of a catalytic amount of a base such as piperidine (Scheme 3) [5].
Substitution of a cyanide group at C3 of N-substituted 2-amino-5-oxo-HHQS is also possible by the reaction of N-substituted cyclohexane-enaminone, an aldehyde and malononitrile in the presence of a catalytic amount of a base such as piperidine (Scheme 4) [5, 12].
Cyclocondensation of 1,3-cyclohexanedione, different aldehydes and ammonia with various acetoacetamides leads to 5-oxo-HHQ derivatives with different carboxamide substitutions at C3 position of HHQ core (Scheme 5) [1, 3].
C3-unsubstituted 5-oxo-HHQs containing aryl moieties at C2 and C4 positions have been obtained by proceeding through reaction of 1,3-cyclohexanedione, chalcone derivatives and ammonium acetate in methanol or ethanol as solvents (Scheme 6) [13, 14]. The reaction is also possible by a solid-state green synthetic route without using solvent and catalyst at 80 °C in high yields [15].
The traditional synthetic methods suffer from numerous disadvantages such as low yields, long reaction time, use of volatile organic solvents and harsh reaction conditions. Therefore, in recent years, an increasing focus has been put in the discovery of green synthetic approaches toward the synthesis of 5-oxo-HHQs. In this vein, new synthetic strategies using more effective energy sources and less harmful solvents as well as reproducible and biodegradable catalysts to achieve the 5-oxo-HHQ scaffold have been developed. The use of ultrasound and microwave irradiations, grinding technique, solvent-free approaches, ionic liquids, reusable nano-catalysts, organocatalyst and nano-metal organic frameworks has been reported in some studies [16,17,18,19,20,21,22,23,24,25]. Some synthetic routes reported in the literatures are summarized in Table 1.
Biological activities of various functionalized 5-oxo-HHQs
Calcium channel modulatory activity
L-type calcium channel modulatory activity
L-type channels are responsible for regulating contractility in muscle cells [80]. Blockers and activators of L-type calcium channels are commonly used for treatment of cardiovascular diseases [81]. Since the discovery of the 1,4-DHPs, such as nifedipine and Bay K 8644 as potent calcium channel blockers and activators, many DHP analogs have been synthesized in order to investigate the structure–activity relationships and to find more effective compounds [82,83,84,85,86,86]. In this vein, some studies with the aim of fixing one carbonyl group in an antiperiplanar position by anellation at the DHP structure and introduction of the 1,4-DHP moiety into condensed systems have been done and revealed that 5-oxo-HHQ core, the condensed ring system of the DHP structure, could be proposed as a considerable scaffold in the field of drug discovery as potential cardiovascular agents [87,88,89,90,91,92,92].
U. Rose described the synthesis and calcium modulatory evaluation of some 5-oxo-HHQ derivatives. The racemic hexahydroquinolines 2 and 3 showed positive inotropic effects at the electrically stimulated left guinea pig atrium and suppressed BaCl2-induced contractions of the guinea pig ileum dose dependently with activity rates comparable to those of nifedipine [88, 90, 93].

In 2000, Şimşk et al. synthesized a series of 2,6,6-trimethyl-3-carbomethoxy(ethoxy)-4-aryl-hexahydroquinoline analogs and evaluated their calcium antagonistic activity in rat aortic rings precontracted with 30 mM K+. It was demonstrated that substitution of the phenyl ring at C4 position with a pyridine ring resulted in increased calcium antagonistic activity, so that compound 4 displayed the highest activity among other tested derivatives [91]. In a subsequent study, 23 compounds with 2-ethyl-3-carbmethoxy-4-aryl-5-oxo-6,6-dimethyl-hexahydroquinoline structure have been evaluated and compounds 5, 6, 7, 8, 9 and 10 showed good calcium antagonistic activity on isolated rat ileum lamb carotid artery [92]. Moreover, in 2007, Şimşk et al. reported the synthesis and evaluation of some novel 3-alkyloxycarbonyl-4-(disubstituted)aryl-5-oxo-hexahydroquinoline derivatives and found that introduction of a second electron-withdrawing substituent into the phenyl ring increased the activity. Results indicated that compound 11, containing 5-chloro-2-nitrophenyl, was the most active compound comparable to nifedipine as the positive control [1].

A number of diethylaminocarbonyl-5-oxo-hexahydroquinoline derivatives have been synthesized and evaluated by Kısmetli et al. for calcium antagonistic activity on isolated rat ileum and lamb carotid artery. The results indicated that in isolated rat ileum, compounds 12, 13 and 14 were found to be more active than nicardipine at a concentration of 10−5 mol/L and in lamb carotid artery studies, at the concentration 10−4 mol/L compounds 14 and 15 showed greater inhibition than nicardipine [94].

2-Methyl-4-(1-methyl-5-nitro-2-imidazolyl)-5-oxo-hexahydroquinolines bearing alkyl, cycloalkyl and aryl carboxylates at C3 position (16) were synthesized by Miri et al. All compounds exhibited calcium antagonist activity on guinea pig ileum longitudinal smooth muscle, and some of the compounds showed agonistic effect on guinea pig auricle [11].

The synthesis and evaluation of various 6-amino-1,4-dihydropyridines, such as ethyl 6-amino-4-aryl-5-cyano-1,4-dihydro-2-methyl-3-pyridinecarboxylic acids and 2-amino-7,7-dimethyl-5-oxo-4-aryl-hexahydroquinoline-3-carbonitriles, were described by León et al. [95]. 5-Oxo-HHQs 17 and 18 were the best blockers of the Ca2+ overload induced by depolarization with high K+ of SH-SY5Y neuroblastoma cells, with values of 63.8% and 50.4%, respectively.

Gupta and Misra [31] focused on difluoro-substituted hexahydroquinolines bearing 6,6- or 7,7-dimethyl substitutions while containing methyl/ethyl carboxylates and carboxamide moieties at C3 position. The most potent compound was 19 (86.8%), whereas nicardipine exhibited 69.6% inhibition of barium chloride-induced contraction. Derivatives containing 6,6-dimethyl were more active than the 7,7-dimethyl analogs, and carboxamide analogs exhibited less activity than compounds with carboxylate moieties.

Bülbül et al. [96] explored relaxant responses (Emax) of a series of HHQs with various carboxylates including methyl, ethyl, isobutyl, tertbutyl, allyl, benzyl and 2-methoxyethyl carboxylates on isolated strips of rabbit sigmoid colon circular smooth muscle and demonstrated that 5-oxo-HHQ derivatives containing 2-methoxyethyl carboxylate such as compound 20 were the most active compounds.

El-Khouly et al. [97] screened several 4-indolylhexahydroquinolines for their spasmolytic activities on isolated rat ileum. The obtained results indicated that introduction of the indolyl ring did not lead to significant activity; however, inserting bromine on the indole ring, as in compound 21, improved the mentioned activity. Moreover, it was observed that compounds bearing methyl substituent instead of the ethyl group in ester function are more active analogs.

Very recently, Kumar et al. [98] introduced compound 22 as a potent positive inotrope agent by performing in vivo evaluations. Furthermore, docking analysis revealed that the compound binds with the calcium channels even more toughly than Bay K 8644. The active site of the receptor contains residues LEU 26, VAL 27, LEU 29, VAL 31 and TYR 33 of chain A and VAL 56, TYR 59, LEU 60, LEU 159, LEU 162, TYR 163 and PHE 166 of chain B, which make good contacts with the ligand. A hydrogen bond between the hydroxyl group of TYR 163 and carbonyl oxygen of the ligand is also formed.

Synthesis and myorelaxant activity of several 5-oxo-HHQ derivatives bearing bulky 3-pyridylmethyl carboxylate at C3 (23) have been performed by Şafak et al. The results indicated that all compounds made concentration-dependent relaxation on isolated rabbit gastric fundus [99].

Stereoselective calcium channel modulatory activity of 5-oxo-HHQs
5-Oxo-HHQ derivatives have a critical asymmetric center at C4 position of DHP ring. As C4 position of HHQ core is chiral, this case causes activity differences depending on the isomers as it was reported for asymmetric DHP analogs; one enantiomer may show agonist activity, while the other one may serve as an antagonist. Calcium agonist and antagonists bind to the same receptor and replace each other in a competitive manner [100]. Nifedipine (antagonist) and Bay K 8644 (agonist) bind a specific DHP receptor, but Bay K 8644 affects opposite to those of nifedipine [101,102,103,103]. The R-enantiomer of 5-oxo-4-phenyl-1,4,5,7-tetrahydrofuropyridine-3-carboxylate bearing 4-oxo-2-phenyl-4H-thiochromen-8-yl moiety at C4 was found to be an agonist, while the S-enantiomer was found to be antagonist. Moreover, S-enantiomer is 50-fold more active than R-enantiomer [104]. Rose and Drage [89] reported the synthesis of enantiomerically pure HHQs of the structural type 24 and proved that the two enantiomers demonstrated calcium antagonistic activities on smooth muscles; however, the S-enantiomer was the tenfold more potent than the R-enantiomer. Furthermore, R-enantiomer exhibited positive inotropic effects on electrically stimulated atria.

T-type and N-type calcium channel inhibitory activity: analgesia activity
Cav3.2 (T-type) and Cav2.2 (N-type) calcium channels, the most important members of voltage-gated calcium channels, are responsible for the processing of peripheral nociceptive information [105]. N-type channels are highly expressed in afferent nerve terminals and control neurotransmitters’ (such as glutamate and substance P) releases. However, T-type channels are expressed both along the afferent fiber and in a subset of nerve terminals and these channels both regulate afferent fiber excitability and appear to contribute to low-threshold neurotransmitter release. T-type calcium channel activity is increased in afferent fibers in several chronic pain conditions such as diabetic neuropathy, spinal nerve injury and irritable bowel syndrome [106,107,108,109,110,110]. It is reported that also Cav3.3 sub-type (L-type) involved in peripheral pain signaling [111].
Bladen et al. optimized the reported L-type inhibitors with 5-oxo-HHQ scaffold by adding dimethyl groups, changing carboxylate moieties at C3 position and altering the substituents on the phenyl ring. It was discovered that modification of carboxylate moiety not only regulates the blocking affinity for both L-type and T-type channels but also allows for the development of HHQs with 30-fold selectivity for T-type channels over the L-type. Compounds 25 and 26 were introduced as selective T-type calcium channel blockers that reduce inflammatory and neuropathic pain in mouse models. The two compounds exhibited high affinity to Cav3.2 channels and preferential inhibition over Cav1.2 [112, 113]. 27 was a broad spectrum inhibitor of voltage-gated calcium channels that inhibited both Cav1.2 L- and Cav3.2 T-type calcium channels equipotently. Moreover, 27 effectively inhibited Cav3.3 (T-type) and Cav2.2 (N-type) [114].

Structure–activity relationships of HHQs as modulators of calcium channels
According to the data presented in above sections, a structure–activity relationship (SAR) can be deduced for 5-oxo-HHQs as modulators of calcium channels (Fig. 1):
-
1.
Nitrogen atom should be unsubstituted in the HHQ nucleus.
-
2.
The substituents at C2 position should be small groups such as methyl or ethyl or primary amine.
-
3.
The compounds having carboxylate groups at the 3-position are the most effective compounds. The methyl esters were found to be more active than ethyl esters. It is reported that compounds bearing 2-methoxyethyl carboxylates are also effective inhibitors; however, inserting tertbutyl, isobutyl, allyl, benzyl and hexyl esters would reduce the activity. Converting linear carboxylate group to annulated one caused the furoquinoline derivatives to be less active than their hexahydroquinoline analogs. Carboxylate groups can be replaced by other electron-withdrawing groups such as nitrile or carboxamides. Methyl pyridine carboxylate function allows for the development of selective inhibitors of T-type channels.
-
4.
As the C4 position of 5-oxo-HHQ core is chiral and DHP receptors are stereoselective, one enantiomer may serve as the more potent calcium channel antagonist. The aryl group on C4 position is the basic requirement for optimal activity. In addition, replacing phenyl ring with pyridyl or nitroimidazole moieties leads to active compounds. Type and position of the substitution on the benzene ring is of great importance. Ortho and meta electron-withdrawing substitutions including NO2, F, Cl, Br and CF3 are preferred.
-
5.
5-Oxo-HHQ derivatives containing 6,6-dimethyl are reported to be more active than the 7,7-dimethyl analogs.
Anticancer activities
Development of new synthetic cytotoxic agents by medicinal chemists is an ongoing approach for cancer treatment [115]. 5-Oxo-HHQ derivatives fused or hybridized with various biologically active cytotoxic structures have demonstrated potent anticancer and antitumor activity.
Taking into account that trimethoxybenzene moiety has been reported to be crucial to obtain relevant cytotoxic and antitubulin responses [116, 117], Alqasoumi et al. reported the synthesis and cytotoxicity evaluation of many new hybrid compounds comprising 5-oxo-HHQ pharmacophore, bearing cyanide substituent at C3 position with different moieties at C4 position and 3,4,5-trimethoxyphenyl at nitrogen atom of HHQ core as potential tubulin inhibitors. 2-Amino-7,7-dimethyl-5-oxo-4-(2-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-hexahydroquinoline-3-carbonitrile (28) showed the highest potency against Ehrlich ascites carcinoma (EAC) cell line with an IC50 value of 13.0 μM, which was better than that of doxorubicin as the reference drug. Unexpectedly, molecular docking analysis revealed that this compound did not exert its cytotoxic activity through the inhibition of the tubulin polymerization [2].

Some studies focused on synthesis and evaluation of the cytotoxicity and radioprotective activity of novel series of 5-oxo-HHQ derivatives bearing a sulfonamide moiety [118,119,120,121,122,122]. As several compounds containing sulfonamide group were found to possess potent carbonic anhydrase inhibitory activity [123], docking analysis was performed to confirm the potential inhibitory effect of the most potent compounds on this enzyme. Some considerable structural modifications are depicted in Figs. 2 and 3. Cytotoxicity estimation of the compounds on EAC cell line revealed that compound 29 bearing phenyl and NH2 groups at C4 and C2, respectively, did not show cytotoxicity. However, replacing NH2 with acetamide (30), phenylurea (31), phenylthiourea (32) and imino-phenyl-dihydropyrimidine-thione ring (33) would enhance the cytotoxic potential (Fig. 2) [121]. In addition, compounds bearing substituted 4-phenyl moiety with chloro (34), nitro (35) or bromo (36) groups, especially at the para position, showed remarkable in vitro cytotoxic activity (Fig. 3) [118]. In another study, the cytotoxicity of HHQs with 2,4-dichlorophenyl group at C4 was evaluated and it was revealed that phenylacetamide (38), benzenesulfonamide (39 and 40) substitutions at C2 and fusing DHP ring with 4-imino-phenyl-dihydropyrimidine-thione and 4-amino-dihydropyrimidin-one rings (41 and 42) would greatly improve the activity (Fig. 3) [119]. Later in 2012, Ghorab et al. [124] reported novel quinoline and pyrimidoquinoline derivatives, containing 4-bromophenyl substitution on nitrogen atom of central core (such as compounds 43, 44 and 45) as potential cytotoxic agents (on MCF-7 cells) with synergistic effects of γ-radiation.

10-(4-Chlorophenyl)-9-(4-methylphenyl)-3,3,6,6-tetramethyl-decahydroacridin-1,8dione (46) was screened against hepatocellular carcinoma cells (HepG2) and exhibited an IC50 value of 4.4 mg/mL [125].

Paidepala et al. [126] reported catalyst-free efficient synthesis of 5-oxo-HHQ using polyethylene glycol (PEG) as a solvent and evaluated their cytotoxicity. Compound 47 was found to display promising cytotoxicity against three human cancer cell lines including MCF-7, human cervical cancer cells (HeLa) and human neuroblastoma cells (SK-NSH).

Sangani et al. [127] synthesized pyrazole–quinoline–pyridine hybrids and showed that some of them have excellent anticancer activity against A549 (human lung adenocarcinoma) and HepG2 cancer cells. Compound 48 showed good cytotoxic potential on the two cell lines, and it was found to be the most effective inhibitor of epidermal growth factor receptor (EGFR).

The synthesis and cytotoxic screening of new spirocyclic 2-oxindole derivatives of 2-amino-hydroquinolin-5-one have been reported. It was noted that substituting the cyanide function at C3 with an ester moiety improved the cytotoxic activity. Moreover, compounds 49 and 50 were found to be the most active members that demonstrated apoptotic inhibition of the proliferation of MCF-7 cells through DNA fragmentation, induction of the tumor suppressor protein p53, induction of caspase-9, and finally the inhibition of angiogenesis by decreasing vascular endothelial growth factor expression and secretion [128].

Costa Cabrer et al. [129] described the synthesis and antiproliferative activity of novel hybrid 3-substituted polyhydroquinoline–fatty acids. The most potent compound, the stearic fatty alkyl derivative (51), which contains 3-hydroxyphenyl at C4, reduced glioma cell viability by 40% at 5 µM.

It has been shown very recently that compound 52 bearing a benzenesulfonamide moiety is a potent cytotoxic agent against MCF-7 cell line with an IC50 value of 0.041 μM, comparable to that of the reference drug doxorubicin (IC50 = 0.040 μM) [130].

Structure–activity relationships of HHQs as anticancer agents
According to the above mentioned studies, a SAR could be proposed for 5-oxo-HHQ derivatives as cytotoxic agents as follows:
-
1.
It can be stated that aromatic substitutions on nitrogen atom of HHQ core, such as 3,4,5-trimethoxyphenyl, benzenesulfonamide, 4-chlorophenyl, 4-bromophenyl and pyridine, would improve the cytotoxicity.
-
2.
The derivatives having NH2 group at C2 position of the central core are effective compounds. Replacing NH2 with acetamide, benzamide, benzenesulfonamide, phenylthiourea and dioxopyrrolidin moieties causes a noticeable increase in the cytotoxicity of 5-oxo-HHQs. Moreover, fusing DHP ring with some pyrimidine derivatives such as 4-imino-phenyl-dihydropyrimidine-thione and 4-amino-dihydropyrimidin-one rings would improve the cytotoxicity.
-
3.
Nitrile and alkyl ester moieties are preferred at C3 position (COOEt > CN). Introducing amide or carboxyl functional groups at this position would diminish the activity [120].
-
4.
Generally, it can be deduced that placing electron-withdrawing groups such as Cl, Br and NO2 on para position of 4-phenyl ring would improve cytotoxic activity.
-
5.
Introducing methyl substitutions on 6-position of HHQ nucleus led to an increase in the activity.
Antibacterial, antifungal, antitubercular and antimalarial activities
Abdel-Gawad et al. reported the synthesis and biological activity evaluation of a new series of N-naphthyl substituent hydroquinolines and pyrimidoquinolines and stated that compounds 53, 54 and 55 demonstrated remarkable antifungal activities against Saccharomyces cerevisiae compared with fungicide mycostatine. They also proved that these structures are radio resistant and sterilization by gamma irradiation may prove to be applicable [131]. In a subsequent study, the team synthesized and evaluated some novel thieno-quinoline, quinolino-thieno-pyrimidine and pyrido-thieno-quinoline analogs and found 56 and 57 to be nearly as active as mycostatine [132].

Sabbagh et al. [125] identified decahydroacridin-1,8-dione 58 bearing a 3-nitrophenyl group and 5-oxo-HHQ 59 having a 2,4-dichlorophenyl moiety as highly active compounds against Gram-positive and Gram-negative bacteria based upon using the disk diffusion method.

Thirty-two new N-(hetero) aryl-substituted 5-oxo-HHQ compounds containing 4-functionally substituted 1,3-diaryl pyrazole ring at C4 position of HHQ core (60 and 61) were synthesized by Thumar et al. Most of the synthesized compounds were found to be highly active against six bacterial pathogens, including: Bacillus subtilis, Clostridium tetani, Streptococcus pneumoniae, Salmonella typhi, Vibrio cholerae, Escherichia coli, and antifungal activity, against Candida albicans. The best antibacterial activities were obtained against Clostridium tetaniand and Bacillus subtilis [133].

Ladani et al. [134] described the synthesis and antimicrobial activity of several polyhydroquinolines bearing the tetrazolo-quinolone moiety at C4 position. The results revealed that some of the derivatives (62 and 63) possess high fungicidal activity against R. oryzae comparable to griseofulvin.

Some novel biquinoline derivatives (64) bearing a thiazole moiety were prepared by Shan et al., and the compounds were tested for their antibacterial (against E. Coli, B. subtilis and Staphylococcus aureus) and antifungal (against A. niger, F. oxysporum and R. oryzae) activities. Most of the compounds displayed moderate activity against all the mentioned strains [135].

Singh et al. [56] investigated the antibacterial and antifungal activity of some novel 1,2,3-triazole-linked 5-oxo-HHQ and reported compound 65 as the best antifungal and antibacterial derivative with an MIC of 64 mg/mL against the Gram-positive bacterial strains B. subtilis and S. aureus as compared with the standard ciprofloxacin and 55.8% inhibition of mycelial growth against the fungal strain Aspergillus flavus and 51.1% against Aspergillus nigeras compared with the standard fluconazole.

In an attempt to involve biologically active polyhydroquinoline, pyrazole and imidazole in one molecule, Kalaria et al. [5] designed and synthesized a new library of polyhydroquinoline derivatives. Compounds were evaluated for their in vitro antibacterial, antifungal, antimalarial and antitubercular activities. In this regard, 66, 67, 68 and 69 were introduced, respectively, as the strongest antibacterial, antifungal, antitubercular and antimalarial agents of the series in comparison with the standard drugs.

Kanani and Patel [136] synthesized a new category of biquinoline derivatives and proved that compounds 70 and 71 exhibited excellent antimicrobial activity. Furthermore, compound 72 with 91% inhibition at 6.25 μg/mL against M. tuberculosis H37Rv was found to be a potent antitubercular agent.

Sangani et al. studied the inhibitory effect of some pyrazole–quinoline–pyridine hybrids against β-ketoacyacyl carrier protein synthase II (FabH) of E. coli which is the essential enzyme for fatty acid biosynthesis. The most active compound was reported to be 5-oxo-hexahydroquinoline-3-carbonitrile derivative 73 which exhibited MIC of 1.56 μg/mL against E. coli (more effective than penicillin G and comparable to kanamycin B) and inhibited FabH with IC50 value of 3.1 μM [127].

Gohil et al. [137] synthesized a new series of triazole/tetrazole hybrids-based biquinoline derivatives bearing aromatic trifluoromethyl moiety at N − 1 position as antimicrobial and antitubercular agents. Compounds 74, 75, 76 and 77 were found to be the most potent antimicrobial and antituberculosis members.

Sapariya et al. prepared some 5-(phenylthio) pyrazole-hexahydroquinoline derivatives and evaluated the synthesized compounds for the in vitro antibacterial, antitubercular and antimalarial activities. Most of the derivatives displayed remarkable antibacterial activities. The results suggested that compound 85 could be a promising candidate for a new class of antimicrobial agents in future. Compounds 81, 82 and 83 were found to be superior antituberculosis agents against M. tuberculosis H37Rv with 94%, 95% and 91% inhibitory activity at 250 µg/mL concentration, respectively. The compounds 78, 79, 80, 83, 84 and 85 with IC50 in the range of 0.042–0.097 μg/mL exhibited noticeable antimalarial activity against P. falciparum as compared to quinine with IC50 of 0.268 μg/mL [138].

Bhatt et al. [139] synthesized some derivatives containing 1,3-diphenyl pyrazole moieties and showed that compounds 86, 87, 88 and 89 presented broad spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria as compared with the reference drug ciprofloxacin. Moreover, compound 88 was found to have promising antifungal activity against A. clavatus and C. albicans which is noticeably higher than that of the standard drugs nystatin and griseofulvin.

Some 4-indolyl-5-oxo-hexahydroquinoline derivatives possessing various alkyl carboxylate groups were synthesized by Baydar et al. and were tested against Mycobacterium tuberculosis H37Rv. It was concluded that introduction of ethyl or isopropyl carboxylates moieties at C3 position would enhance the activity. Molecular docking analysis of compounds with M. tuberculosis enoyl reductase (InhA) revealed that InhA might be the possible target enzyme as compounds were well accommodated in the enzyme’s active site [140].
Vanaerschot et al. [6] screened 3825 compounds from the Genomics Institute of Novartis Research Foundation malaria box and identified three lead compounds having HHQ scaffold (90, 91 and 92) as potent gametocytocidal inhibitors. It was proved that the compounds were potent in vitro transmission blockers. In vivo studies demonstrated the ability of lead HHQs to suppress plasmodium berghei blood-stage parasite proliferation.

Antioxidant activity
A series of 5-oxo-hexahydroquinoline-3-carboxylates bearing different substitutions at 4-phenyl were tested for their antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino bis (3-ethylenzothiazoline-sulfonic acid) diammonium salt (ABST+) radical scavenging assays. It was reported that insertion of methoxy groups at the phenyl ring improved the antioxidant activity. Compounds 93 and 94 were found to be as the most potent derivatives [49].

Montes-Avila et al. studied DPPH radical scavenging activity of some hydropyridines (dihydropyridines, polyhydroquinolines and polyhydroacridine derivatives) and concluded that polyhydroquinolines were the most active compounds among the studied hydropyridines. A schematic of SAR representation of these compounds is illustrated in Fig. 4. In particular, 95 and 96 having 5-oxo-HHQ scaffold were the most potent derivative and exhibited 53.5 and 55.1% DPPH scavenging activity at 100 μg/mL, while 97 possessing hexahydroacridine-dione structure showed 30.0% DPPH scavenging activity at the same concentration [141].
Structure–activity relationships of HHQs as antioxidant agents
Considering the above-mentioned studies, a brief SAR can be presented for 5-oxo-HHQ derivatives as antioxidants as follows:
-
1.
5-Oxo-HHQs bearing carboxylate moiety on C3 demonstrate antioxidant activity and modification of the nucleus to DHP or hydroacridine diminishes the activity.
-
2.
Hydroxyl and alkoxy substituents at 3, 4 and 5 positions of the C4-phenyl ring lead to derivatives with enhanced antioxidant activity.
Singh et al. [56] obtained novel 1,2,3-triazole-linked 5-oxo-HHQ via an eco-friendly one-pot five-component synthesis procedure under ultrasonic and microwave irradiation in PEG 400. The antioxidant activity was evaluated using DPPH assay, and compounds 98–101 showed good antioxidant activity at 0.8 μmol/mL concentration as compared with the standard BHT. It was observed that the antioxidant activity enhanced due to the presence of R2=OCH3 at the phenyl ring.

COX-2 inhibitory and anti-inflammatory activities
Cyclooxygenase (COX), also known as prostaglandin synthase (PGH), is a potent mediator of inflammation which catalyzes the first step of the biosynthesis of PGG2 from arachidonic acid to generate PGH2 [142]. It is well established that there are at least two COX isozymes, COX-1 and COX-2. COX-1 is mainly associated with prostaglandin production in gastric mucosa, but COX-2 is upregulated in response to inflammatory stimuli and is involved in pathologic processes [143]. Generally, nonsteroidal anti-inflammatory drugs inhibit both COXs and, consequently, lead to undesirable side effects [144].
A new class of 5-oxo-HHQ derivatives possessing a SO2Me pharmacophore at the para position of the C2 or C4 phenyl ring was designed and assessed as selective cyclooxygenase-2 (COX-2) inhibitors by Zarghi et al. The obtained results indicated that compounds having a MeSO2 group at the para position of the C2 phenyl ring were more selective COX-2 inhibitors compared to their corresponding regioisomers bearing a MeSO2 at the para position of C4 phenyl ring. Furthermore, introduction of a methoxy function at the para position of the C2 or C4 phenyl ring enhanced the potency and COX-2 selectivity. Accordingly, compounds 102 and 103 were presented as the most potent COX-2 inhibitors with high COX-2 selectivity index. In contrast, incorporation of Cl, Br or NO2 at C2 phenyl and C4 phenyl decreased COX-2 inhibitory potency and selectivity. Molecular docking study of compound 102 in active site of COX-2 indicated that the SO2Me moiety on the C2 phenyl ring accommodated into the secondary COX-2 binding site [13]. Later in 2015, they reported 4-(4-(methylsulfonyl)phenyl)-5-oxo-hexahydroquinoline derivatives containing alkyl substituents at C2 position and alkyl carboxylates at C3 position of 5-oxo-HHQ core. Compound 104 with IC50 value of 0.30 μM had the strongest COX-2 inhibitory activity. It was proved that placing larger groups such as propyl and phenyl at C2 position led to significant loss in activities. Modification of ethylcarboxylate to the large-sized benzylcarboxylate group would diminish the activity [145]. Very recently, Akbari et al. [146] modeled COX-2 inhibitory activities of the above-mentioned 5-oxo-HHQs by quantitative structure–activity relationship using step-wise multiple linear regression method. According to QSAR models results, BEHm6 (highest eigenvalue n. 6 of Burden matrix/weighted by atomic masses), Mor03u (signal 03/unweighted) and IVDE (mean information content on the vertex degree equality) were important factors controlling the COX-2 inhibitory activity.

Teng et al. [147] reported the synthesis and endometritis anti-inflammatory activity (carrageenan-induced paw edema) of 4-aryl-5-oxo-hexahydroquinoline derivatives and find out compounds 105 and 106 with electron-donating groups at the 4-phenyl ring demonstrated potent activities.

Abd-Allah et al. [148] studied the anti-inflammatory activity of novel hydroacridines by the carrageenan-induced paw edema standard method in rats and revealed that electron-donating alkyl groups in hydroacridines could increase their anti-inflammatory activity. The highly alkylated bishydroacridine-1,8-dione 107 exhibited high anti-inflammatory potency more than standard employed indomethacin.

P-gp-mediated multidrug resistance (MDR) reversal activity
Multidrug resistance (MDR) is a major impediment to successful chemotherapy, which may occur due to the over-expression of ATP-binding cassette membrane transporter family members including P-glycoprotein (P-gp), the breast cancer resistance protein and the multidrug resistance-associated protein 1 in cancer cells [149]. The over-expression of P-gp, in cancer cells, leads to reduced accumulation of chemotherapeutic drugs and results in ineffective chemotherapy. Consequently, discovering small molecules as P-gp inhibitors seems to be a promising approach for overcoming MDR in cancer cells. P-gp substrates include amphipathic compounds, lipid soluble compounds and compounds with aromatic rings [150, 151].
In this direction, Shahraki et al. [3] synthesized several 5-oxo-HHQ derivatives containing nitrophenyl moieties at C4 and different carboxamide substituents at C3 and evaluated them for their ability to inhibit P-gp using a flow cytometry assay to measure the amount of rhodamine 123 (Rh123) accumulations in uterine sarcoma cells that over-express P-gp (MES-SA/DX5). Compounds with 2-nitrophenyl moiety, such as 108 with 4.6-fold Rh123 accumulation relative to the negative control at 25 μM, demonstrated good activity.

Our team extended the work to synthesize and screen twenty-five analogs bearing different pyridyl methyl carboxylates at C3 and different substituents at C4 as P-gp inhibitors. Derivatives having phenyl moiety with electron-withdrawing substitution (such as nitro, cyano, chloro and bromo moieties) at C4 position of HHQ core presented the highest P-gp inhibitory activity. Compounds 109 and 110 which showed 6.2- and 7.4-fold Rh123 accumulation relative to the negative control at 25 μM, respectively, were even more potent than verapamil as the positive control (5.5-fold Rh123 accumulation relative to the negative control) [152]. In a subsequent study, we studied the inhibitory activity of 5-oxo-HHQ derivatives containing 2-pyridyl ethyl carboxylate, 2-pyridyl propyl carboxylate and 3-pyridyl propyl carboxylate moieties at C3. Accordingly, compounds 111 and 112 were among the most promising modulators of P-gp transporter [153].

Structure–activity relationships of 5-oxo-HHQs as MDR reversal agents
According to the above-mentioned studies, a SAR could be proposed for 5-oxo-HHQ derivatives as MDR reversal agents as follows (Fig. 5):
-
1.
Derivatives bearing the pyridyl alkyl carboxylate moieties at position 3 are better inhibitors of P-gp than the compounds having carboxamide substituents, and more lipophilic pyridyl ethyl carboxylate and pyridyl propyl carboxylate substituents are better P-gp modulators than pyridyl methyl carboxylate substituents. 3-Pyridyl propyl carboxylate substitution led to the most active derivatives.
-
2.
Introducing alkyl and hetero aromatic moieties at the C4 position would diminish the activity, while aromatic moieties with electron-withdrawing substitutions such as NO2 (as a hydrogen bond acceptor function), Cl and Br (due to their lipophilicity) would improve the MDR reversal activity.
-
3.
Converting six-membered cyclohexenone ring to five-membered would reduce the activity.
Transforming growth factor β inhibitory activity
Transforming growth factor β (TGFβ) is a cytokine that regulates many cellular functions including cell proliferation, apoptosis, differentiation, angiogenesis and wound healing [154, 155]. The TGFβ pathway is a promising therapeutic target for a variety of diseases such as cancer, fibrosis and autoimmune diseases. TGFβ signaling occurs following initial binding of TGFβ superfamily ligands to the TGFβ receptor type II (TGFβR2) that recruits and phosphorylates TGFβ receptor type I (TGFβR1). The type I receptor then phosphorylates SMADs which bind the coSMAD SMAD4. SMAD/coSMAD complexes mount up in the nucleus, act as transcription factors and contribute to the regulation of target gene expression [156, 157].
Willems et al. [158] screened a mouse embryonic stem cell (ESC)-based differentiation assay against a small molecule library and introduced 113 as the first selective TGFβ inhibitor (IC50 = 0.4–0.8 μM), which induced proteasomal degradation of the TGFβR2 and inhibited TGFβ-induced mesoderm formation from mouse ESCs during early differentiation. In the subsequent study, they reported the synthesis and structure–activity relationship (SAR) studies of 50 selected 1,4-DHPs based on the “hit” compound 113. Applying SAR-optimized substitution pattern on TGFβ inhibition, compound 114 was discovered as the most potent derivative (IC50 = 170 nM), which is almost as potent as the reported TGFβR1 inhibitor SB-431542 (IC50 = 66 nM) [159].

Anti-Alzheimer and neuroprotective activities
Alzheimer’s disease (AD) is a neurodegenerative disease with diverse etiologies including amyloid-β (Aβ) deposits, tau protein aggregation, oxidative stress, or low levels of acetylcholine that are thought to play significant roles in the disease [160, 161]. Using acetylcholinesterase inhibitors and, consequently, increasing the acetylcholine levels in the brain is the primary therapeutic methodology in management of AD [162]. Moreover, regulating the entrance of Ca2+ through calcium channels could be a good strategy to prevent cell death, as Ca2+ overload and dysfunction, involved in the pathogenesis of AD, augments Aβ formation and cell death [163, 164].
León et al. [165] discovered a series of new tacrine–HHQ hybrids (115–119) that inhibited acetylcholinesterase, calcium entry, and showed neuroprotection profile. Compounds 115–119 were introduced as a new family of molecules for the management of AD.

The diaryl-HHQ 120 displayed moderate neuroprotective and antioxidant activity and was also a potent inhibitor of calcium entry [14].

Antidiabetic and antidyslipidemic activities
Non-insulin-dependent diabetes mellitus (Type-2 diabetes) is primarily characterized by insulin resistance and abnormal insulin secretion which causes hyperglycemia [166]. Dyslipidemia is connected to insulin insensitivity and associated with increased atherosclerosis susceptibility [167].
A series of 2,4-disubstituted polyhydroquinoline derivatives have been synthesized and evaluated for their antidiabetic and antidyslipidemic activity in various in vivo and in vitro models by A. Kumar. Some derivatives such as 121, 122 and 123 exhibited antihyperglycemic activity in sucrose-loaded model (SLM) and streptozotocin (STZ-S)-induced β-cell-damaged diabetic model of Sprague–Dawley strain male albino rats comparable to the standard drugs with remarkable lipid and triglyceride modulating activity in C57BL/KsBom-db mouse (db/db). Compound 123 was a potent protein tyrosine phosphatase 1B (PTP-1B) inhibitor, whereas compounds 121 and 122 having carboxylic group inhibited the glycogen phosphorylase more efficiently than PTP-1B [4].

Antiosteoporotic activity
Applying the molecular hybridization approach, Sashidhara et al. [168] discovered compound 124 as a potent antiosteoporotic agent which increased bone mass density and volume, expression of osteogenic genes, bone formation rate, mineral apposition rate, improved the trabecular microarchitecture and decreased bone turn over markers in an ovariectomized rodent model for postmenopausal osteoporosis. It was also proved that coumarin–HHQ were more effective than individual coumarins or nifedipine and benzofuran–HHQ hybrids. Very recently, the team designed some benzofuran–HHQ hybrids and evaluated them for bone anabolic activities. Compound 125 was introduced as the most promising derivative, and it was stated that benzofuran–HHQ hybrids were more active than their individuals. Compound 125 significantly stimulate bone morphogenic protein-2 and osteoblast differentiation, increase alkaline phosphatase activity and enhance osteoblasts by improving mineralization activity in extracellular matrix. Furthermore, the compound triggers the regeneration and healing properties in bone compared to the vehicle-treated group in a drill hole fracture (defect) model [169].

Conclusions
So far, several derivatives of 5-oxo-HHQ scaffold have been synthesized and numerous studies have been done on the biological effects of such compounds. The 5-oxo-HHQ derivatives display versatile biological and pharmacological activities, i.e., cardiovascular, myorelaxant, analgesia, anticancer, antibacterial, antifungal, antitubercular, antimalarial, antioxidant, anti-inflammatory, MDR reversal, neuroprotective, antidiabetic, antidyslipidemic and antiosteoporotic activities. Studies on the pharmacological activities of 5-oxo-HHQ derivatives along with the chemistry involved in those activities, which are compiled in this review, would be helpful in designing and developing new therapeutic agents.
According to reported various biological effects and structural diversity of 5-oxo-HHQs, some general considerations seem to come out. While the presence of hydrogen on N1 and small groups on C2 and C3 positions of 5-oxo-HHQ core for calcium channel and COX-2 inhibitory activities are requirements, most of the cytotoxic derivatives have bulky and fused substitutions on these positions. It is well established that C4-aryl or C4-heteroaryl is a necessity in all reported biological activities and by placing various substitutions on these groups the biological effect could be improved or diminished. As an example, placing alkoxy and hydroxyl moieties on 4-phenyl improves the antioxidant potential of 5-oxo-HHQs, whereas in the case of MDR reversal activity this action reduces the effect.
Cytotoxic activity as well as MDR reversal potential of 5-oxo-HHQ derivatives may bring about new horizons in cancer treatment. In vivo studies reveal some derivatives are promising antimalarial, antidiabetic, antidyslipidemic and antiosteoporotic agents. We believe that 5-oxo-HHQs deserve more investigation, above all in the field of interaction with receptors and therapeutical targets as well as in their use as the scaffold for the preparation of antimalarial, antidiabetic, antidyslipidemic, antiosteoporotic and anti MDR agents.
References
Simsek R, Öztürk GS, Vural IM, Gündüz MG, Sarıoǧlu Y et al (2008) Synthesis and calcium modulatory activity of 3-alkyloxy-carbonyl-4-(disubstituted) aryl-5-oxo-1,4,5,6,7,8-hexa-hydroquinoline derivatives. Arch Pharm 341:55–60. https://doi.org/10.1002/ardp.200700087
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Hamed MM, Noaman E et al (2009) Synthesis and biological evaluation of 2-amino-7,7-dimethyl 4-substituted-5-oxo-1-(3,4,5-trimethoxy)-1,4,5,6,7,8-hexahydro-quinoline-3-carbonitrile derivatives as potential cytotoxic agents. Bioorg Med Chem Lett 19:6939–6942. https://doi.org/10.1016/j.bmcl.2009.10.065
Shahraki O, Edraki N, Khoshneviszadeh M, Zargari F, Ranjbar S et al (2017) Novel 5-oxo-hexahydroquinoline derivatives: design, synthesis, in vitro P-glycoprotein-mediated multidrug resistance reversal profile and molecular dynamics simulation study. Drug Des Dev Therapy 11:407. https://doi.org/10.2147/DDDT.S119995
Kumar A, Sharma S, Tripathi VD, Maurya RA, Srivastava SP et al (2010) Design and synthesis of 2,4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg Med Chem 18:4138–4148. https://doi.org/10.1016/j.bmc.2009.11.061
Kalaria PN, Satasia SP, Raval DK (2014) Synthesis, characterization and pharmacological screening of some novel 5-imidazopyrazole incorporated polyhydroquinoline derivatives. Eur J Med Chem 78:207–216. https://doi.org/10.1016/j.ejmech.2014.02.015
Vanaerschot M, Lucantoni L, Li T, Combrinck JM, Ruecker A et al (2017) Hexahydroquinolines are antimalarial candidates with potent blood-stage and transmission-blocking activity. Nat Microbiol 2:1403. https://doi.org/10.1038/s41564-017-0007-4
Sunderhaus JD, Martin SF (2009) Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem Eur J 15:1300–1308. https://doi.org/10.1002/chem.200802140
Alvim HG, da Silva Júnior EN, Neto BA (2014) What do we know about multicomponent reactions? mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv 4:54282–54299. https://doi.org/10.1039/C4RA10651B
Hantzsch A (1882) Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak. Justus Liebigs Annalen der Chemie 215:1–82. https://doi.org/10.1002/jlac.18822150102
Safak C, Erdemli I, Sunal R (1993) Synthesis of some 1,4,5,6,7,8-hexahydroquinoline derivatives and their calcium antagonistic activity. Arzneimittelforschung 43:1052–1055. https://doi.org/10.1002/chin.199507151
Miri R, Javidnia K, Mirkhani H, Hemmateenejad B, Sepeher Z et al (2007) Synthesis, QSAR and calcium channel modulator activity of new hexahydroquinoline derivatives containing nitroimidazole. Chem Biol Drug Des 70:329–336. https://doi.org/10.1111/j.1747-0285.2007.00565.x
Lichitsky B, Dudinov A, Krayushkin M (2001) Reaction of 3-aminocyclohex-2-en-1-ones with arylidenemalononitriles: synthesis of N-substituted 1,4,5,6,7,8-hexahydroquinolin-5-ones. Arkivoc 9:73–79. https://doi.org/10.3998/ark.5550190.0002.911
Zarghi A, Sabakhi I, Topuzyan V, Hajimahdi Z, Daraie B (2014) Design, synthesis and biological evaluation of 5-oxo-1,4,5,6,7,8 hexahydroquinoline derivatives as selective cyclooxygenase-2 inhibitors. Iran J Pharm Res 13:61
Tenti G, Egea J, Villarroya M, León R, Fernández JC et al (2013) Identification of 4,6-diaryl-1,4-dihydropyridines as a new class of neuroprotective agents. MedChemComm 4:590–594. https://doi.org/10.1039/c3md20345j
Jin T-S, Yin Y, Liu L-B, Li T-S (2006) Solid state synthesis of 5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives without using solvent and catalyst. Arkivoc 14:28–34. https://doi.org/10.3998/ark.5550190.0007.e05
Ahluwalia VK, Goyal B, Das U (1997) One-pot syntheses of 5-oxo-1,4,5,6,7,8-hexahydroquinolines and pyrimido [4, 5-b] quinolines using microwave irradiation and ultrasound. J Chem Res Synop 7:266. https://doi.org/10.1039/a608002b
Tajbakhsh M, Alinezhad H, Norouzi M, Baghery S, Akbari M (2013) Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J Mol Liq 177:44–48. https://doi.org/10.1016/j.molliq.2012.09.017
Kumar S, Sharma P, Kapoor KK, Hundal MS (2008) An efficient, catalyst-and solvent-free, four-component, and one-pot synthesis of polyhydroquinolines on grinding. Tetrahedron 64:536–542. https://doi.org/10.1016/j.tet.2007.11.008
Moosavi-Zare AR, Goudarziafshar H, Ghaffari L (2017) Nano–Mn-[4-nitrophenyl-salicylaldimine-methyl pyranopyrazole] Cl2 as a new nanostructured Schiff base complex and catalyst for the synthesis of hexahydroquinolines. Appl Organomet Chem 31:e3845. https://doi.org/10.1002/aoc.3845
Rao GD, Nagakalyan S, Prasad G (2017) Solvent-free synthesis of polyhydroquinoline derivatives employing mesoporous vanadium ion doped titania nanoparticles as a robust heterogeneous catalyst via the Hantzsch reaction. RSC Adv 7:3611–3616. https://doi.org/10.1039/C6RA26664A
Pasinszki T, Krebsz M, Lajgut GG, Kocsis T, Kótai L et al (2018) Copper nanoparticles grafted on carbon microspheres as novel heterogeneous catalysts and their application for the reduction of nitrophenol and one-pot multicomponent synthesis of hexahydroquinolines. New J Chem 42:1092–1098. https://doi.org/10.1039/C7NJ03562D
Zhaleh S, Hazeri N, Faghihi MR, Maghsoodlou MT (2016) Chitosan: a sustainable, reusable and biodegradable organocatalyst for green synthesis of 1,4-dihydropyridine derivatives under solvent-free condition. Res Chem Intermediat 42:8069–8081. https://doi.org/10.1007/s11164-016-2579-7
Moloi S, Maddila S, Jonnalagadda SB (2017) Microwave-irradiated one-pot synthesis of quinoline derivatives catalyzed by triethylamine. Res Chem Intermediat 43:6233–6243. https://doi.org/10.1007/s11164-017-2986-4
Moosavi-Zare AR, Goudarziafshar H, Ghaffari L (2017) Nano-Mn-[4-nitrophenyl-salicylaldimine-methyl pyranopyrazole] Cl2 as a new nanostructured Schiff base complex and catalyst for the synthesis of hexahydroquinolines. Appl Organomet Chem 31:e3845. https://doi.org/10.1002/aoc.3845
Anil B, Ozokan KG, Kaban S, Sahin E, Kazaz C (2016) Synthesis and spectral investigation of some new hetaryl-substituted hydroquinolinone derivatives. Magn Reson Chem 54:184–189. https://doi.org/10.1002/mrc.4361
Dyachenko V, Nesterov V, Krivokolysko S, Litvinov V (1997) Convenient method for synthesis of functionally substituted hexahydroquinolines. Molecular and crystal structure of 4-isopropyl-7,7-dimethyl-5-oxo-3-cyano-2-cyanomethylthio-1,4,5,6,7,8-hexahydroquinoline. Chem Heterocycl Compd 33:684–690. https://doi.org/10.1007/BF02291800
Suarez M, Verdecia Y, Ochoa E, Martín N, Martínez R et al (2000) Synthesis and structural study of novel 1,4,5,6,7,8-hexahydroquinolines. J Heterocycl Chem 37:735–742. https://doi.org/10.1002/jhet.5570370411
Litvinov V, Krivokolysko S, Rusanov E (2001) Cascade heterocyclization in the synthesis of new sulfur-containing 1,4-dihydropyridines and 1,4,5,6,7,8-hexahydroquinolines. Dokl Chem 377:94–100. https://doi.org/10.1023/A:1019205013304
Khabazzadeh H, Sheikhshoaie I, Saeid-Nia S (2010) A molybdenum (VI) complex as an efficient catalyst for the synthesis of dihydropyridines. Transit Met Chem 35:125–127. https://doi.org/10.1007/s11243-009-9304-y
Gao S, Tsai CH, Tseng C, Yao C-F (2008) Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron 64:9143–9149. https://doi.org/10.1016/j.tet.2008.06.061
Gupta V, Misra U (2008) Synthesis and cardiovascular activity of difluro-substituted hexahydroquinoline. Med Chem Res 17:437–444. https://doi.org/10.1007/s00044-007-9078-8
Song S-J, Shan Z-X, Jin Y (2010) One-pot synthesis of hexahydroquinolines via Hantzsch four-component reaction catalyzed by a cheap amino alcohol. Synth Commun 40:3067–3077. https://doi.org/10.1080/00397910903370626
Okoro CO, Ogunwale MA, Siddiquee T (2012) Synthesis of some new fluorinated hexahydroquinoline and acridinedione derivatives in trifluoroethanol. Appl Sci 2:368–374. https://doi.org/10.3390/app2020368
Frolov K, Dotsenko V, Krivokolysko S (2013) Synthesis and alkylation of n-methylmorpholinium 4-aryl-3-cyano-5-oxo-1,4,5,6,7,8-hexahydroquinoline-2-selenolates. Chem Heterocycl Compd 49:1146–1150. https://doi.org/10.1007/s10593-013-1356-4
Ghasemzadeh MA, Safaei-Ghomi J (2014) An efficient, one-pot synthesis of polyfunctionalised dihydropyridines catalysed by AgI nanoparticles. J Chem Res 38:313. https://doi.org/10.3184/174751914X13976454726953
Hussein E (2015) Ammonium chloride-catalyzed four-component sonochemical synthesis of novel hexahydroquinolines bearing a sulfonamide moiety. Russ J Org Chem 51:54–64. https://doi.org/10.1134/S1070428015010091
Abdelmoniem AM, Ramadan MA, Ghozlan SAS, Abdelhamid IA (2017) New synthesis of N-(1H-pyrazol-5-yl)-hexahydroquinoline-3-carbonitrile and octahydropyrazolo [4′, 3′: 5, 6] pyrimido [1, 2-a] quinoline-6-carbonitrile derivatives from the cyclic β-enaminones. J Heterocycl Chem 54:1193–1198. https://doi.org/10.1002/jhet.2692
Hong M, Cai C, Yi W-B (2010) Hafnium(IV) bis (perfluorooctanesulfonyl) imide complex catalyzed synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction in fluorous medium. J Fluor Chem 131:111–114. https://doi.org/10.1016/j.jfluchem.2009.10.009
Khojastehnezhad A, Moeinpour F, Davoodnia A (2011) PPA-SiO2 catalyzed efficient synthesis of polyhydroquinoline derivatives through Hantzsch multicomponent condensation under solvent-free conditions. Chin Chem Lett 22:807–810. https://doi.org/10.1016/j.cclet.2010.12.051
Kang SR, Lee YR (2013) Efficient one-pot synthesis of spirooxindole derivatives bearing hexahydroquinolines using multicomponent reactions catalyzed by ethylenediamine diacetate. Synthesis 45:2593–2599. https://doi.org/10.1055/s-0033-1338506
Tu S, Zhang J, Zhu X, Zhang Y, Wang Q et al (2006) One-pot synthesis of hexahydroquinolines via a four-component cyclocondensation under microwave irradiation in solvent free conditions: a green chemistry strategy. J Heterocycl Chem 43:985–988. https://doi.org/10.1002/jhet.5570430425
Ko S, Yao C-F (2006) Ceric ammonium nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron 62:7293–7299. https://doi.org/10.1016/j.tet.2006.05.037
Song G, Wang B, Wu X, Kang Y, Yang L (2005) Montmorillonite K10 clay: an effective solid catalyst for one-pot synthesis of polyhydroquinoline derivatives. Synth Commun 35:2875–2880. https://doi.org/10.1080/00397910500297255
Zhang X-L, Sheng S-R, Liu X-L, Liu X-L (2007) Solvent-free liquid-phase synthesis of polyhydroquinoline derivatives under microwave irradiation. Arkivoc 13:79–86. https://doi.org/10.3998/ark.5550190.0008.d11
Singh SK, Singh KN (2010) Glycine-catalyzed easy and efficient one-pot synthesis of polyhydroquinolines through Hantzsch multicomponent condensation under controlled microwave. J Heterocycl Chem 47(1):194–198. https://doi.org/10.1002/jhet.308
X-y Qin, T-s Jin, Z-x Zhou, T-s Li (2010) Silica sulfuric acid: an efficient and versatile catalyst for one-pot synthesis of substituted 5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives. Asian J Chem 22(2):1179
Wang JX, Shen GL, Shi DQ (2011) An efficient three-component synthesis of hexahydroquinoline derivatives in ionic liquid. J Heterocycl Chem 48:1145–1148. https://doi.org/10.1002/jhet.703
Yang X-H, Zhang P-H, Zhou Y-H, Liu C-G, Lin X-Y et al (2011) Synthesis and antioxidant evaluation of novel 4-aryl-hexahydroquinolines from lignin. Arkivoc 10:327–337. https://doi.org/10.3998/ark.5550190.0012.a27
Aswin K, Logaiya K, Sudhan PN, Mansoor SS (2012) An efficient one-pot synthesis of 1,4-dihydropyridine derivatives through Hantzsch reaction catalysed by melamine trisulfonic acid. J Taibah Univ Sci 6:1–9. https://doi.org/10.1002/jhet.703
Surasani R, Kalita D, Rao AD, Yarbagi K, Chandrasekhar K (2012) FeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction. J Fluor Chem 135:91–96. https://doi.org/10.1016/j.jfluchem.2011.09.005
Heravi MRP, Mehranfar S, Shabani N (2014) One-pot multicomponent synthesis hexahydroquinoline derivatives in Triton X-100 aqueous micellar media. Comptes Rendus Chim 17:141–145. https://doi.org/10.1016/j.crci.2012.11.017
Zare A, Abi F, Moosavi-Zare AR, Beyzavi MH, Zolfigol MA (2013) Synthesis, characterization and application of ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient catalyst for the preparation of hexahydroquinolines. J Mol Liq 178:113–121. https://doi.org/10.1016/j.molliq.2012.10.045
Khazaei A, Zolfigol MA, Moosavi-Zare AR, Afsar J, Zare A et al (2013) Synthesis of hexahydroquinolines using the new ionic liquid sulfonic acid functionalized pyridinium chloride as a catalyst. Chin J Catal 34:1936–1944. https://doi.org/10.1016/S1872-2067(12)60678-0
Abdolmohammadi S (2013) Study of the catalytic activity of Zr (HPO4)2 in the synthesis of hexahydroquinoline derivatives under solvent-free conditions. Z Naturforsch B 68:195–200. https://doi.org/10.5560/znb.2013-2237
Rostamnia S, Nuri A, Xin H, Pourjavadi A, Hosseini SH (2013) Water dispersed magnetic nanoparticles (H2O-DMNPs) of γ-Fe2O3 for multicomponent coupling reactions: a green, single-pot technique for the synthesis of tetrahydro-4H-chromenes and hexahydroquinoline carboxylates. Tetrahedron Lett 54:3344–3347. https://doi.org/10.1016/j.tetlet.2013.04.048
Singh H, Sindhu J, Khurana JM, Sharma C, Aneja KR (2013) A facile eco-friendly one-pot five-component synthesis of novel 1,2,3-triazole-linked pentasubstituted 1,4-dihydropyridines and their biological and photophysical studies. Aust J Chem 66:1088–1096. https://doi.org/10.1071/CH13217
Zolfigol MA, Baghery S, Moosavi-Zare AR, Vahdat SM, Alinezhad H et al (2014) Synthesis of the first nano ionic liquid 1-methylimidazolium trinitromethanide [HMIM] C(NO2)3 and its catalytic use for Hanztsch four-component condensation. RSC Adv 4:57662–57670. https://doi.org/10.1039/C4RA09117E
Rostamnia S, Hassankhani A, Hossieni HG, Gholipour B, Xin H (2014) Brønsted acidic hydrogensulfate ionic liquid immobilized SBA-15:[MPIm][HSO4]@ SBA-15 as an environmentally friendly, metal-and halogen-free recyclable catalyst for Knoevenagel–Michael-cyclization processes. J Mol Catal A Chem 395:463–469. https://doi.org/10.1016/j.molcata.2014.09.017
Mansoor SS, Aswin K, Logaiya K, Sudhan PN, Malik S (2014) Silica-supported perchloric acid (HClO4–SiO2): a mild, reusable and highly efficient heterogeneous catalyst for multicomponent synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction. Res Chem Intermed 40:357–369. https://doi.org/10.1007/s11164-012-0968-0
Abaszadeh M, Seifi M, Asadipour A (2015) Ultrasound promotes one-pot synthesis of 1,4-dihydropyridine and imidazo[1, 2-a] quinoline derivatives, catalyzed by ZnO nanoparticles. Res Chem Intermed 41:5229–5238. https://doi.org/10.1007/s11164-014-1624-7
Ahmed K, Jain AK, Dubey B, Shrivastava B, Sharma P et al (2015) p-TSA catalyzed synthesis of 4-aryl-2,7,7-trimethyl-5-oxo–phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamides derivatives as CNS active agents. Der Pharma Chemica 7:52–65
Ghorbani M, Noura S, Oftadeh M, Narimani M, Behbodi K (2015) Preparation, characterization and application of novel ionic liquid as an efficient and reusable catalyst for the solvent-free synthesis of hexahydroquinolines. J Mol Liq 209:224–232. https://doi.org/10.1016/j.molliq.2015.06.011
Ghorbani M, Shaterian HR, Noura S, Khammar F, Behbodi K et al (2015) Effective preparation of hexahydroquinolines under ambient and solvent-free conditions. J Mol Liq 204:15–20. https://doi.org/10.1016/j.molliq.2015.01.013
Moosavi-Zare AR, Zolfigol MA, Zarei M, Zare A, Afsar J (2015) Design, characterization and application of silica-bonded imidazolium-sulfonic acid chloride as a novel, active and efficient nanostructured catalyst in the synthesis of hexahydroquinolines. Appl Catal A 505:224–234. https://doi.org/10.1016/j.apcata.2015.08.004
Zolfigol MA, Yarie M (2015) Synthesis and characterization of novel silica-coated magnetic nanoparticles with tags of ionic liquid. Application in the synthesis of polyhydroquinolines. RSC Adv 5:103617–103624. https://doi.org/10.1039/C5RA23670C
Karimi-Jaberi Z, Azadi M (2015) Efficient synthesis of 2,4-diaryl hexahydroquinoline-5-one derivatives in the presence of triethylamine. Res Chem Intermed 41:6741–6747. https://doi.org/10.1007/s11164-014-1773-8
Khazaei A, Sarmasti N, Seyf JY, Tavasoli M (2015) Synthesis of hexahydroquinoline (HHQ) derivatives using ZrOCl2·8H2O as a potential green catalyst and optimization of reaction conditions using design of experiment (DOE). RSC Adv 5:101268–101275. https://doi.org/10.1039/C5RA16102A
Maleki A, Kamalzare M, Aghaei M (2015) Efficient one-pot four-component synthesis of 1,4-dihydropyridines promoted by magnetite/chitosan as a magnetically recyclable heterogeneous nanocatalyst. J Nanostruct Chem 5:95–105. https://doi.org/10.1007/s40097-014-0140-z
Ghorbani M, Noura S, Oftadeh M, Zolfigol M (2015) Novel ionic liquid [2-Eim] HSO4 as a dual catalytic-solvent system for preparation of hexahydroquinolines under green conditions. RSC Adv 5:55303–55312. https://doi.org/10.1039/C5RA09666A
Sakram B, Sonyanaik B, Ashok K, Rambabu S (2016) Polyhydroquinolines: 1-sulfopyridinium chloride catalyzed an efficient one-pot multicomponent synthesis via Hantzsch condensation under solvent-free conditions. Res Chem Intermed 42:7651–7658. https://doi.org/10.1007/s11164-016-2559-y
Tabrizian E, Amoozadeh A (2016) A unique approach to magnetization of metal oxides: nano-Fe3O4@ TDI@ TiO2 as a highly efficient, magnetically separable and recyclable heterogeneous nanocatalyst. Catal Sci Technol 6:6267–6276. https://doi.org/10.1039/C6CY00316H
Goli-Jolodar O, Shirini F, Seddighi M (2016) Introduction of a novel nanosized N-sulfonated Brönsted acidic catalyst for the promotion of the synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. RSC Adv 6:26026–26037. https://doi.org/10.1039/C6RA04148E
Mirsafaei R, Delzendeh S, Abdolazimi A (2016) Synthesis and characterization of reusable nano-order SO3H-KIT-5 as a heterogeneous catalyst for eco-friendly synthesis of 1,4-dihydropyridines. Int J Environ Sci Technol 13:2219–2226. https://doi.org/10.1007/s13762-016-1037-9
Yü S-J, Wu S, Zhao X-M, Lü C-W (2017) Green and efficient synthesis of acridine-1,8-diones and hexahydroquinolines via a KH2PO4 catalyzed Hantzsch-type reaction in aqueous ethanol. Res Chem Intermed 43:3121–3130. https://doi.org/10.1007/s11164-016-2814-2
Sobhani S, Zarifi F, Skibsted J (2017) Ionic liquids grafted onto graphene oxide as a new multifunctional heterogeneous catalyst and its application in the one-pot multi-component synthesis of hexahydroquinolines. New J Chem 41:6219–6225. https://doi.org/10.1039/C7NJ00063D
Tabrizian E, Amoozadeh A (2017) Sulfamic acid-functionalized nano-titanium dioxide as a novel and highly efficient heterogeneous nanocatalyst for one-pot and solvent-free synthesis of hexahydroquinolines. J Chin Chem Soc 64:331–336. https://doi.org/10.1002/jccs.201600802
Taghavi Fardood S, Ramazani A, Golfar Z, Joo SW (2017) Green synthesis of Ni–Cu–Zn ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. Appl Organomet Chem 31:e3823. https://doi.org/10.1002/aoc.3823
Abaszadeh M, Seifi M (2017) Crown ether complex cation ionic liquids: synthesis and catalytic applications for the synthesis of tetrahydro-4 H-chromene and 1,4-dihydropyridine derivatives. J Sulfur Chem 38:440–449. https://doi.org/10.1080/17415993.2017.1293058
Azarifar D, Abbasi Y, Badalkhani O (2018) N-(3-silyl propyl) diethylene triamine N,N′,N″-tri-sulfonic acid immobilized on Fe3−xTixO4 magnetic nanoparticles: a new recyclable heterogeneous nanocatalyst for the synthesis of hexahydroquinolines. Appl Organomet Chem 32:e3939. https://doi.org/10.1002/aoc.3939
Zamponi GW (1997) Antagonist binding sites of voltage-dependent calcium channels. Drug Dev Res 42:131–143. https://doi.org/10.1002/(SICI)1098-2299(199711/12)42:3/4%3c131:AID-DDR4%3e3.0.CO;2-R
Dolphin AC (2006) A short history of voltage-gated calcium channels. Br J Pharmacol 147:S56–S62. https://doi.org/10.1038/sj.bjp.0706442
Gaudio AC, Korolkovas A, Takahata Y (1994) Quantitative structure-activity relationships for 1,4-dihydropyridine calcium channel antagonists (nifedipine analogues): a quantum chemical/classical approach. J Pharm Sci 83:1110–1115. https://doi.org/10.1002/jps.2600830809
Edraki N, Mehdipour AR, Khoshneviszadeh M, Miri R (2009) Dihydropyridines: evaluation of their current and future pharmacological applications. Drug Discov Today 14:1058–1066. https://doi.org/10.1016/j.drudis.2009.08.004
Shekari F, Sadeghpour H, Javidnia K, Saso L, Nazari F et al (2015) Cytotoxic and multidrug resistance reversal activities of novel 1,4-dihydropyridines against human cancer cells. Eur J Pharmacol 746:233–244. https://doi.org/10.1016/j.ejphar.2014.10.058
Mojarrad JS, Miri R, Knaus EE (2004) Design and synthesis of methyl 2-methyl-7,7-dihalo-5-phenyl-2-azabicyclo [4.1. 0] hept-3-ene-4-carboxylates with calcium channel antagonist activity. Bioorg Med Chem 12:3215–3220. https://doi.org/10.1016/j.bmc.2004.03.063
Saddala MS, Kandimalla R, Adi PJ, Bhashyam SS, Asupatri UR (2017) Novel 1,4-dihydropyridines for L-type calcium channel as antagonists for cadmium toxicity. Sci Rep 7:45211. https://doi.org/10.1038/srep45211
Stout DM, Meyers A (1982) Recent advances in the chemistry of dihydropyridines. Chem Rev 82:223–243. https://doi.org/10.1021/cr00048a004
Rose U (1990) Vinylogous and coupled hexahydroquinolinones and dihydropyridines with calcium-modulating effects. Pharm Acta Helv 65:178–185 PMID:2167485
Rose U, Draeger M (1992) Synthesis, configuration, and calcium modulatory properties of enantiomerically pure 5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylates. J Med Chem 35:2238–2243. https://doi.org/10.1021/jm00090a014
Rose U (1990) Hexahydrochinolinone mit calciummodulatorischem Effekt-Synthese und pharmakologische Wirkung. Arch Pharm 323:281–286. https://doi.org/10.1002/ardp.19903230506
Şimşek R, İsmailoğlu UB, Şafak C, Sahin-Erdemli İ (2000) Synthesis and calcium antagonistic activity of 2,6,6-trimethyl-3-carbomethoxy (ethoxy)-4-aryl-1,4,5,6,7,8-hexahydroquinoline derivatives. Il Farmaco 55:665–668. https://doi.org/10.1016/S0014-827X(00)00086-0
Şimşek R, Şafak C, Erol K, Sirmagül B (2001) Studies on calcium antagonist activities of 2-ethyl-3-carbmethoxy-4-aryl-5-oxo-6,6-dimethyl-1,4,5,6,7,8-hexahydroquinoline derivatives. Arzneimittelforschung 51:959–963. https://doi.org/10.1055/s-0031-1300145
Rose U (1989) Kalzium-modulatoren von Typ anellierter Dihydropyridine. Synthese und pharmakologische Wirkung. Arzneim Forsch Drug Res 39:1393–1398
Kısmetli E, Şafak C, Erol K, Sırmagül B, Linden A (2004) Studies on 3-diethyl-aminocarbonyl-1,4;5,6,7,8-hexahydroquinoline derivatives and their calcium channel antagonistic activities in vitro. Arzneimittelforschung 54:371–375. https://doi.org/10.1055/s-0031-1296986
León R, de los Ríos C, Marco-Contelles J, López MG, García AG et al (2008) Synthesis of 6-amino-1,4-dihydropyridines that prevent calcium overload and neuronal death. Eur J Med Chem 43:668–674. https://doi.org/10.1016/j.ejmech.2007.06.001
Bülbül B, Öztürk GS, Vural M, Şimşek R, Sarioğlu Y et al (2009) Condensed 1,4-dihydropyridines with various esters and their calcium channel antagonist activities. Eur J Med Chem 44:2052–2058. https://doi.org/10.1016/j.ejmech.2008.10.008
El-Khouly A, Gündüz M, Cengelli C, Şimşek R, Erol K et al (2013) Microwave-assisted synthesis and spasmolytic activity of 4-indolylhexahydroquinoline derivatives. Drug Res 63:579–585. https://doi.org/10.1055/s-0033-1348261
Kumar R, Yadav N, Lavilla R, Blasi D, Quintana J et al (2017) Synthesis, pharmacological evaluation and molecular docking of pyranopyrazole-linked 1,4-dihydropyridines as potent positive inotropes. Mol Divers 21:1–14. https://doi.org/10.1007/s11030-017-9738-7
Şafak C, Gündüz MG, İlhan SÖ, Şimşek R, İşli F et al (2012) Synthesis and myorelaxant activity of fused 1, 4-dihydropyridines on isolated rabbit gastric fundus. Drug Dev Res 73:332–342. https://doi.org/10.1002/ddr.21024
Mahmoudian M, Richards WG (1986) A conformational distinction between dihydropyridine calcium agonists and antagonists. J Chem Soc Chem Commun 10:739–741. https://doi.org/10.1039/C39860000739
Görlitzer K, Schmidt E (1991) Anellierte Lactone aus Bay-K-8644 und Dihydropyridin-Nebenprodukten der Hantzsch-Synthese. Arch Pharm 324:879–886. https://doi.org/10.1002/ardp.2503241111
Franckowiak G, Bechem M, Schramm M, Thomas G (1985) The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol 114:223–226. https://doi.org/10.1016/0014-2999(85)90631-4
Hof R, Rüegg U, Hof A, Vogel A (1985) Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol 7:689–693. https://doi.org/10.1097/00005344-198507000-00012
Bossert F, Vater W (1971) Dihydropyridine, eine neue Gruppe stark wirksamer Coronartherapeutika. Naturwissenschaften 58:578. https://doi.org/10.1007/BF00598745
Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW et al (2014) Calcium-permeable ion channels in pain signaling. Physiol Rev 94:81–140. https://doi.org/10.1152/physrev.00023.2013
Bourinet E, Francois A, Laffray S (2016) T-type calcium channels in neuropathic pain. Pain 157:S15–S22. https://doi.org/10.1097/j.pain.0000000000000469
Jacus MO, Uebele VN, Renger JJ, Todorovic SM (2012) Presynaptic Cav3. 2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J Neurosci 32:9374–9382. https://doi.org/10.1523/JNEUROSCI.0068-12.2012
Yue J, Liu L, Liu Z, Shu B, Zhang Y (2013) Upregulation of T-type Ca2+ channels in primary sensory neurons in spinal nerve injury. Spine 38:463–470. https://doi.org/10.1097/BRS.0b013e318272fbf8
Cao XH, Byun HS, Chen SR, Pan HL (2011) Diabetic neuropathy enhances voltage-activated Ca2+ channel activity and its control by M4 muscarinic receptors in primary sensory neurons. J Neurochem 119:594–603. https://doi.org/10.1111/j.1471-4159.2011.07456.x
Marger F, Gelot A, Alloui A, Matricon J, Ferrer JFS et al (2011) T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci 108:11268–11273. https://doi.org/10.1073/pnas.1100869108
Li H (2006) Intrathecal administration of Cav3. 2 and Cav3. 3 antisense oligonucleotide reverses tactile allodynia and thermal hyperalgesia in rats following chronic compression of dorsal root of ganglion. Acta Pharmacol Sin 27:1547–1552. https://doi.org/10.1111/j.1745-7254.2006.00461.x
Bladen C, Gündüz MG, Şimşek R, Şafak C, Zamponi GW (2014) Synthesis and evaluation of 1,4-dihydropyridine derivatives with calcium channel blocking activity. Pflug Arch Eur J Phys 466:1355–1363. https://doi.org/10.1007/s00424-013-1376-z
Bladen C, Gadotti VM, Gündüz MG, Berger ND, Şimşek R et al (2015) 1,4-Dihydropyridine derivatives with T-type calcium channel blocking activity attenuate inflammatory and neuropathic pain. Pflug Arch Eur J Phys 467:1237–1247. https://doi.org/10.1007/s00424-014-1566-3
Gadotti VM, Bladen C, Zhang FX, Chen L, Gündüz MG et al (2015) Analgesic effect of a broad-spectrum dihydropyridine inhibitor of voltage-gated calcium channels. Pflug Arch Eur J Phys 467:2485–2493. https://doi.org/10.1007/s00424-015-1725-1
Ranjbar S, Edraki N, Khoshneviszadeh M, Foroumadi A et al (2018) Design, synthesis, cytotoxicity evaluation and docking studies of 1,2,4-triazine derivatives bearing different arylidene-hydrazinyl moieties as potential mTOR inhibitors. Res Pharm Sci 13:1–11. https://doi.org/10.4103/1735-5362.220962
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S et al (2006) Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem 49:3033–3044. https://doi.org/10.1021/jm0512903
Cushman M, Nagarathnam D, Gopal D, He HM, Lin CM et al (1992) Synthesis and evaluation of analogs of (Z)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl) ethene as potential cytotoxic and antimitotic agents. J Med Chem 35:2293–2306. https://doi.org/10.1021/jm00090a021
Ghorab MM, Ragab FA, Noaman E, Heiba HI, El-Hossary EM (2008) Utility of 4-(5,5-dimethyl-3-oxo-cyclohex-l-enylamino) benzenesulfonamide in the synthesis of novel quinolines as possible anticancer and radioprotective agents. Arzneimittelforschung 58:35–41. https://doi.org/10.1055/s-0031-1296464
Ghorab MM, Ragab FA, Heiba HI, Arafa RK, El-Hossary EM (2010) In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido [4,5-b] quinolines bearing a sulfonamide moiety. Eur J Med Chem 45:3677–3684. https://doi.org/10.1016/j.ejmech.2010.05.014
Al-Said MS, Ghorab MM, Al-Dosari MS, Hamed MM (2011) Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem 46:201–207. https://doi.org/10.1016/j.ejmech.2010.11.002
Ghorab MM, Ragab FA, Noaman E, Heiba HI, El-Hossary EM (2007) Synthesis of some novel quinolines and pyrimido [4,5-b] quinolines bearing A sulfonamide moiety as potential anticancer and radioprotective agents. Arzneimittelforschung 57:795–803. https://doi.org/10.1055/s-0031-1296682
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM (2010) Novel quinolines and pyrimido [4,5-b] quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 45:738–744. https://doi.org/10.1016/j.ejmech.2009.11.021
Supuran CT (2017) Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 12:61–88. https://doi.org/10.1080/17460441.2017.1253677
Ghorab MM, Ragab FA, Heiba HI, Nissan YM, Ghorab WM (2012) Novel brominated quinoline and pyrimidoquinoline derivatives as potential cytotoxic agents with synergistic effects of γ-radiation. Arch Pharmacal Res 35:1335–1346. https://doi.org/10.1007/s12272-012-0803-6
Sabbagh OIE, Shabaan MA, Kadry HH, Al-Din ES (2010) Synthesis of new nonclassical acridines, quinolines, and quinazolines derived from dimedone for biological evaluation. Arch Pharm 343:519–527. https://doi.org/10.1002/ardp.200900296
Paidepala H, Nagendra S, Saddanappu V, Addlagatta A, Das B (2014) Catalyst-free efficient synthesis of polyhydroquinolines using polyethylene glycol as a solvent and evaluation of their cytotoxicity. Med Chem Res 23:1031–1036. https://doi.org/10.1007/s00044-013-0706-1
Sangani CB, Makawana JA, Zhang X, Teraiya SB, Lin L et al (2014) Design, synthesis and molecular modeling of pyrazole–quinoline–pyridine hybrids as a new class of antimicrobial and anticancer agents. Eur J Med Chem 76:549–557. https://doi.org/10.1016/j.ejmech.2014.01.018
Ghozlan SA, Mohamed MF, Ahmed AG, Shouman SA, Attia YM et al (2015) Cytotoxic and antimicrobial evaluations of novel apoptotic and anti-angiogenic spiro cyclic 2-oxindole derivatives of 2-amino-tetrahydroquinolin-5-one. Arch Pharm 348:113–124. https://doi.org/10.1002/ardp.201400304
da Costa Cabrera D, Rosa SB, de Oliveira FS, Marinho MA, D’Oca CRM et al (2016) Synthesis and antiproliferative activity of novel hybrid 3-substituted polyhydroquinoline-fatty acids. MedChemComm 7:2167–2176. https://doi.org/10.1039/C6MD00425C
Ahmed N, Badahdah K, Qassar H (2017) Novel quinoline bearing sulfonamide derivatives and their cytotoxic activity against MCF7 cell line. Med Chem Res 26:1201–1212. https://doi.org/10.1007/s00044-017-1850-9
Abdel-Gawad S, El-Gaby M, Heiba H, Aly H, Ghorab M (2005) Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido [4, 5-b] quinoline derivatives. J Chin Chem Soc 52:1227–1236. https://doi.org/10.1002/jccs.200500177
El-Gaby M, Abdel-Gawad S, Ghorab M, Heiba H, Aly H (2006) Synthesis and biological activity of some novel thieno [2, 3-b] quinoline, quinolino [3′, 2′: 4, 5] thieno [3, 2-d] pyrimidine and pyrido [2′, 3′: 4, 5] thieno [2, 3-b] quinoline derivatives. Phosphorus Sulfur Silicon Relat Elem 181:279–297. https://doi.org/10.1080/104265090970322
Thumar NJ, Patel MP (2011) Synthesis and antimicrobial activity of some new N-substituted quinoline derivatives of 1H-pyrazole. Arch Pharm 344:91–101. https://doi.org/10.1002/ardp.201000010
Ladani NK, Mungra DC, Patel MP, Patel RG (2011) Microwave assisted synthesis of novel Hantzsch 1,4-dihydropyridines, acridine-1,8-diones and polyhydroquinolines bearing the tetrazolo [1, 5-a] quinoline moiety and their antimicrobial activity assess. Chin Chem Lett 22:1407–1410. https://doi.org/10.1016/j.cclet.2011.07.009
Shah NK, Shah NM, Patel MP, Patel RG (2012) Design, synthesis and antimicrobial activity of new biquinoline derivatives. J Serb Chem Soc 77:279–286. https://doi.org/10.2298/JSC110630197S
Kanani MB, Patel MP (2015) Design and synthesis of new (bis) trifluoromethyl-promoted N-aryl biquinoline derivatives as antitubercular and antimicrobial agents. Med Chem Res 24:563–575. https://doi.org/10.1007/s00044-014-1140-8
Gohil JD, Patel HB, Patel MP (2016) Ultrasound assisted synthesis of triazole/tetrazole hybrids based new biquinoline derivatives as a new class of antimicrobial and antitubercular agents. Indian J Adv Chem Sci 4:102–113
Sapariya NH, Vaghasiya BK, Thummar RP, Kamani RD, Patel KH et al (2017) Synthesis, characterization, in silico molecular docking study and biological evaluation of a 5-(phenylthio) pyrazole based polyhydroquinoline core moiety. New J Chem 41:10686–10694. https://doi.org/10.1039/C7NJ01962A
Bhatt A, Shah V, Rawal R (2017) Synthesis, characterization and docking studies of some novel dihydropyridine derivatives. Der Pharm Lettre 9:64–72. https://doi.org/10.20959/wjpr20179-9254
Baydar E, Gündüz MG, Krishna VS, Şimşek R, Sriram D et al (2017) Synthesis, crystal structure and antimycobacterial activities of 4-indolyl-1,4-dihydropyridine derivatives possessing various ester groups. Res Chem Intermed 43:1–19. https://doi.org/10.1007/s11164-017-3087-0
Montes-Avila J, Delgado-Vargas F, Díaz-Camacho SP, Rivero IA (2012) Microwave-assisted synthesis of hydropyridines and study of the DPPH-scavenging activity. RSC Adv 2:1827–1834. https://doi.org/10.1039/C1RA01135A
Fu J-Y, Masferrer J, Seibert K, Raz A, Needleman P (1990) The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 265:16737–16740 PMID: 2120205
Garavito RM, DeWitt DL (1999) The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. BBA Mol Cell Biol Lip 1441:278–287. https://doi.org/10.1016/S1388-1981(99)00147-X
Brune K (2004) Safety of anti-inflammatory treatment—new ways of thinking. Rheumatology 43:i16–i20. https://doi.org/10.1093/rheumatology/keh104
Sabakhi I, Topuzyan V, Hajimahdi Z, Daraei B, Arefi H et al (2015) Design, synthesis and biological evaluation of new 1,4-dihydropyridine (DHP) derivatives as selective cyclooxygenase-2 inhibitors. Iran J Pharm Res 14:1087 PMID: 26664375
Akbari S, Zebardast T, Zarghi A, Hajimahdi Z (2017) QSAR modeling of COX-2 inhibitory activity of some dihydropyridine and hydroquinoline derivatives using multiple linear regression (MLR) method. Iran J Pharm Res 16:525 PMID: 28979307
Teng L-L, Zhou X-L, Ma G-Z (2016) Synthesis of 4-arylpolyhydroquinoline derivatives and evaluation of their anti-inflammatory on endometritis. Biomed Res 27:1060–1063
Abd-Allah O, Abdelhamid A, Mohamed S (2015) Synthesis and anti-inflammatory study of novel n-substituted hydro-acridine-1,8-diones and bis-hexahydroacridine-1,8-dione derivatives. Med Chem S2(2161–0444):10000. https://doi.org/10.4172/2161-0444.1000004
Thomas H, Coley HM (2003) Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 10:159–165. https://doi.org/10.1177/107327480301000207
Kathawala RJ, Gupta P, Ashby CR, Chen Z-S (2015) The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Update 18:1–17. https://doi.org/10.1016/j.drup.2014.11.002
Riganti C, Mini E, Nobili S (2015) Multidrug resistance in cancer: pharmacological strategies from basic research to clinical issues. Front Oncol 5:105. https://doi.org/10.3389/fonc.2015.00105
Ranjbar S, Firuzi O, Edraki N, Shahraki O, Saso L et al (2017) Tetrahydroquinolinone derivatives as potent P-glycoprotein inhibitors: design, synthesis, biological evaluation and molecular docking analysis. MedChemComm 8:1919–1933. https://doi.org/10.1039/C7MD00178A
Ranjbar S (2017) Design, synthesis and in vitro evaluation of cytotoxicity and MDR reversal activity of novel fused dihydropyridine-cyclohexanone or cyclopentanone derivatives containing different pyridine ester groups. Dessertation, Shiraz University of Medical Sciences
Lichtman MK, Otero-Vinas M, Falanga V (2016) Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regener 24:215–222. https://doi.org/10.1111/wrr.12398
Santibanez JF, Krstic J, Quintanilla M, Bernabeu C (2016) TGF-β signalling and its role in cancer progression and metastasis. eLS 1:1–9. https://doi.org/10.1002/9780470015902.a0025045
Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S et al (2015) Targeting the TGFβ pathway for cancer therapy. Pharmacol Therapeut 147:22–31. https://doi.org/10.1016/j.pharmthera.2014.11.001
ten Dijke P, Hill CS (2004) New insights into TGF-β—smad signalling. Trends Biochem Sci 29:265–273. https://doi.org/10.1016/j.tibs.2004.03.008
Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P et al (2012) Small molecule-mediated TGF-β type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell 11:242–252. https://doi.org/10.1016/j.stem.2012.04.025
Schade D, Lanier M, Willems E, Okolotowicz K, Bushway P et al (2012) Synthesis and SAR of b-annulated 1,4-dihydropyridines define cardiomyogenic compounds as novel inhibitors of TGFβ signaling. J Med Chem 55:9946–9957. https://doi.org/10.1021/jm301144g
Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G et al (2006) Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 65:631–641. https://doi.org/10.1097/01.jnen.0000228136.58062.bf
Ittner LM, Götz J (2011) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12:67–72. https://doi.org/10.1038/nrn2967
Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H-P, van den Bussche H (2005) Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 331:321–327. https://doi.org/10.1136/bmj.331.7512.321
Smith IF, Green KN, LaFerla FM (2005) Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium 38:427–437. https://doi.org/10.1016/j.ceca.2005.06.021
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci 3:862–872. https://doi.org/10.1038/nrn960
León R, de los Ríos C, Marco-Contelles J, Huertas O, Barril X et al (2008) New tacrine-dihydropyridine hybrids that inhibit acetylcholinesterase, calcium entry, and exhibit neuroprotection properties. Bioorg Med Chem 16:7759–7769. https://doi.org/10.1016/j.bmc.2008.07.005
Olefsky JM, Nolan JJ (1995) Insulin resistance and non-insulin-dependent diabetes mellitus: cellular and molecular mechanisms. Am J Clin Nutr 61:980S–986S. https://doi.org/10.1093/ajcn/61.4.980S
Howard BV, Howard WJ (1994) Dyslipidemia in non-insulin-dependent diabetes mellitus. Endocr Rev 15:263–274. https://doi.org/10.1210/edrv-15-3-263
Sashidhara KV, Kumar M, Khedgikar V, Kushwaha P, Modukuri RK et al (2012) Discovery of coumarin–dihydropyridine hybrids as bone anabolic agents. J Med Chem 56:109–122. https://doi.org/10.1021/jm301281e
Modukuri RK, Choudhary D, Gupta S, Rao KB, Adhikary S et al (2017) Benzofuran-dihydropyridine hybrids: a new class of potential bone anabolic agents. Bioorg Med Chem 25:6450–6466. https://doi.org/10.1016/j.bmc.2017.10.018
Acknowledgements
The support of Vice-Chancellor for Research, Shiraz University of Medical Sciences, is much appreciated. The authors wish to thank Hamid Ranjbar for his invaluable assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ranjbar, S., Edraki, N., Firuzi, O. et al. 5-Oxo-hexahydroquinoline: an attractive scaffold with diverse biological activities. Mol Divers 23, 471–508 (2019). https://doi.org/10.1007/s11030-018-9886-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9886-4