Abstract
The present work reports the synthesis of novel 5-Oxo-5,6,7,8-tetrahydroquinoline and 2,5-dioxo-5,6,7,8-tetrahydroquinoline derivatives containing enaminone system and bearing a sulfonamide moiety. The newly synthesized compounds were designed in compliance with the general pharmacophoric requirements for carbonic anhydrase inhibiting anticancer drugs, as this may play a role in their anticancer activity. Twelve of the newly synthesized compounds were evaluated for their in vitro anticancer activity against human breast cancer cell line (MCF7). Compounds 5c, 7, 10, and 12c showed IC50 values (0.048, 0.040, 0.041, 0.044 μM, respectively) comparable to that of the reference drug doxorubicin (IC50 = 0.04 μM). On the other hand, compounds 12a, 12d, and 16b exhibited better activity than doxorubicin with an IC50 values (0.025, 0.036, 0.015 μM, respectively).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, researchers have been showing great interest in the enaminone family of compounds. These compounds possess a great potential as multipurpose synthetic intermediate in organic synthesis, in heterocyclic synthesis (Michael et al. 1999) and as they showed a wide variety of medicinal effects such as cardiovascular effects (García et al. 2012), anti-inflammatory (El-Hashim et al. 2010), antiviral (El-Sabbagh and Rady 2009), anti-tussive (El-Hashim et al. 2001), antimicrobial (Abbas and Farghaly 2010), and anticonvulsant effects (Edafiogho et al. 2009; Eddington et al. 2000, 2002; Jackson et al. 2012).
On the other hand, quinoline and its derivatives are very much used in pharmaceuticals such as an anticancer agents (Creaven et al. 2010; Lu et al. 2010; Gao et al. 2010; Perin et al. 2011; Wang et al. 2012), antioxidant (Korrichi et al. 2009), antifungal (Creaven et al. 2010; Kouznetsov et al. 2012), anti-inflammatory (Ghodsi et al. 2010; Chen et al. 2011; Kumar et al. 2012), antibacterial (Garudachari et al. 2012), antimalarial (Pretorius et al. 2013), antiviral (Carta et al. 2011; Guo et al. 2011), and for depression of schizophrenia (Daniel 2007). Also, it was found that quinoline compounds containing enaminone moiety show more potent biological activities, especially as antiviral (Ahmed et al. 2010; Vandurm et al. 2009), antibacterial (Jayagobi et al. 2011), antitumor (Ghorab et al. 2009, 2010; Alqasoumi et al. 2009, 2010a, b; Al-Said et al. 2011), antimicrobial (Makawana et al. 2012), and hypotensive activity (El-Sabbagh et al. 2010).
Additionally, sulfonamides have attracted a great attention as antitumor agents since many of aryl/heteroaryl sulfonamides were reported to act as antitumor agents through a variety of mechanisms, as well as the most prominent mechanism was the inhibition of carbonic anhydrase isozyme (CA) (Ghorab et al. 2009, 2010; Al-Said et al. 2011).
In this respect, we reported here the preparation of some quinoline derivatives containing enaminone system and bearing sulfonamide moiety, then we had tested their in vitro growth inhibitory activities against human cultured breast carcinoma cell lines (MCF7) in comparison to doxorubicin (DOX) which is one of the most effective antitumor agents, hoping to obtain more active and less toxic anticancer agents.
Results/Discussion
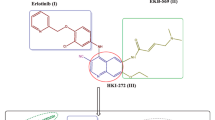
A general pharmacophore (Fig. 1) for the compounds acting as CA inhibitors has been reported by Thiry et al. (2006) from the analysis of the CA active site and from the structure of inhibitors described in the literature by Supuran et al. (2003). This pharmacophore includes the structural elements that are required to be present in the compounds in order to act as CA inhibitors. This includes the presence of a sulfonamide moiety which coordinates with the zinc ion of the active site of the CA and the sulfonamide is attached to a scaffold, which is usually a benzene ring. The side chain might possess a hydrophilic link which is able to interact with the hydrophilic part of the active site and a hydrophobic moiety which can interact with the hydrophobic part of the CA active site (Ghorab et al. 2010). We have synthesized new compounds (Schemes 1–4), and two examples that showing their compliance with the above-mentioned pharmacophore model is represented in Fig. 2.
Chemistry
Synthesis of 5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl)benzenesulfonamide derivatives
Enaminone 3 was obtained from the condensation of 5,5-dimethyl-1,3-cyclohexandione 1 with sulfanilamide 2 in absolute ethanol under reflux for 3 h, (Ghorab et al. 2009), it was also obtained in 90% yield by using ultrasonic irradiation at 80 °C for 20 min. Treatment of enaminone 3 with different arylidene malononitrile derivatives 4a–c in ethanol containing a catalytic amount of triethylamine, as a base catalyst, yielded the corresponding hexahydro quinoline derivative 5a–c (Scheme 1). Compounds 5a–c were also obtained by ultrasonic irradiation at 80 °C under the same condition in better yield. Table 1 shows a slight increase in the reaction yield in a relatively short reaction time in the presence of ultrasonic irradiation. These results confirm that ultrasonic irradiation played a crucial role in the enhancement of the rapid synthesis of hydro quinoline derivatives 5a–c. The structures of these compounds were established based on their elemental analysis and their spectral data. The fourier transform infrared spectroscopy (FTIR) spectra for compound 5a revealed four bands at 3469, 3408, 3347, and 3334 cm−1 for two NH2 groups, in addition to the absorption band at 2177 for C≡N, besides the characteristic bands for SO2 group at 1357–1190 cm−1. The 1H nuclear magnetic resonance (NMR) spectrum of compound 5a in (dimethyl sulfoxide (DMSO)-d6) showed the two methyl groups as two singlet signals at δ H 0.75 and 0.90 ppm, the two methylene proton appeared as a pair of singlet signal at δ H 1.07 and 2.09 ppm, and C4-H proton appeared as singlet signal at δ H 4.45 ppm. Moreover, two singlet signals at δ H 5.80 and 8.54 ppm for NH2 and SO2NH2 groups, respectively, are exchangeable with D2O, and the aromatic protons appear as a complex pattern from δ H 6.89–8.06 ppm.
Moreover, refluxing of compound 5a with chloroacetyl chloride in dimethylformamide (DMF) for 20 h yielded the quinoline derivative 6 (Scheme 2). The structure of the resulted compound was confirmed by elemental and spectral analysis. It’s FTIR spectra showed absorption band at 2227 cm−1 C≡N, a band at 1653 cm−1 and a broadband appeared at 1699 cm−1 for 3C=O groups. 1H NMR spectrum in (DMSO-d6) for compound 6 showed the disappearance of NH2 signals at δ H 5.89 ppm and a new two singlet bands at δ H 2.71 and 2.87 ppm for 2COCH2Cl.
When compound 5a was refluxed in acetic anhydride for 1 h the fused pyrimido[4,5-b]quinoline system 7 was obtained in a good yield. The spectral and elemental data of compound 7 confirmed the assigned structures. The FTIR spectra of this particular sample show the disappearance of the cyano group band, while two bands at 1697 and 1669 cm−1 for 2C=O groups were recorded. On the other hand, it’s 1H NMR spectrum in (DMSO-d6) showed singlet band at δ H 1.89 ppm for 5H for COCH3 and C9-H2, singlet band at δ H 2.07 ppm for 5H for CH3 at C2 and C7-H2. Tow singlet bands at δ H 10.37 and 11.95 ppm, which is corresponding to NH and OH, respectively were disappeared on adding D2O (Scheme 2).
In addition, the fusion of compound 5a with ethyl cyanoacetate yielded the corresponding acetamide derivative 8 (Scheme 2). The structure of compound 8 was confirmed by elemental and spectral analysis. The FTIR showed bands at 3312, 3229, and 3123 cm−1 for NH and NH2 groups. The new cyano groups at 2260 cm−1 were observed and two bands at 1743 and 1680 cm−1 for two C=O groups were recorded. 1H NMR spectrum in (DMSO-d6) showed the CH2CN protons as singlet together with the C4-H at δ H 3.94 ppm, and NH proton appeared as singlet signal at δ H 10.17 ppm which is disappeared on adding D2O. In addition to that, 4-(2-amino-5-(2-aminoethylamino)-3-cyano-4-(4-fluorophenyl)-7,7-dimethyl-7,8-dihydroquinolin-1(4H)-yl) benzenesulfonamide 9 was obtained by the treatment of compound 5a with ethylenediamine in the presence of carbon disulfide (Scheme 2). The structure of compound 9 was proved on the basis of its spectral and elemental data. FTIR spectra showed a broadband from 3337–3193 cm−1 for NH and NH2 groups, while C≡N appeared at 2201 cm−1. 1H NMR spectrum in (DMSO-d6) showed singlet signal for C8-H2 at δ H 1.88 ppm, two new singlet signals at δ H 2.38 and 2.51 ppm for NH and NH2 beside the original C2-NH2 at δ H 5.77 ppm, and SO2NH2 at δ H 8.30 ppm. On the other hand, the two methylene protons appeared as a pair of triplet bands at δ H 3.63 and 3.81 ppm, while the C6-H appeared with Ar–H in the range from δ H 6.65 to 8.13 ppm.
Additionally, the octahydrobenzo[b][1,8] naphthyridine derivative 10 was obtained by the reaction of compound 5a with ethyl acetoacetate (Scheme 2). The structure of the compound obtained was confirmed with their spectral and elemental analysis. The FTIR spectra of compound 10 showed NH and NH2 absorption band at 3304, 3256, and 3225 cm−1, and three absorption bands at 1713, 1660, and 1632 cm−1 for three different CO groups. 1H NMR spectrum in (DMSO-d6) of this compound revealed two singlet signals at δ H 1.16 and 1.22 ppm due to 2CH3 group at C8, two singlet signals at δ H 2.24 and 2.55 ppm for 2CH2 of C9 and C7, respectively. The new singlet signal at δ H 2.35 ppm due to COCH3, more over two singlet bands at δ H 3.57 and 4.09 ppm for C3-H and C5-H, respectively. A singlet signal at δ H 4.24 ppm due to NH proton at C4, and a signal at δ H 15.72 ppm for OH of the iminol structure 10b (Fig. 3), which were disappeared on adding D2O. In addition, a complex pattern appeared at δ H 6.04–8.50 ppm due to aromatic protons together with SO2NH2 and endocyclic NH. The observed iminol structure may be attributed to the gain of energy enhanced by intramolecular hydrogen bonding and the rate at which this tautomer interconvert is slow compared with the inherent time scale of NMR spectroscopy.
Synthesis of 2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide derivatives
In one pot reaction, a mixture of diamidone 1, sulfanilamide 2, ethyl cyanoacetate, and benzaldehyde derivatives 11a–f, were heated under reflux in the presence of ethanol to give the corresponding 2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide derivatives 12a–f (Scheme 3). The same reaction was repeated by using ultrasonic irradiation instead of the conventional method, the same products 12a–f as examined by thin layer chromatography (TLC) were obtained in shorter time and better yield (Table 2). The structures of the resulted compounds were proved by FTIR, 1H NMR, 13C NMR and elemental analysis. Compound 12a as an example, it’s FTIR spectra showed bands at 3372 and 3236 cm−1 for NH2 group and a band at 2226 cm−1 for CN group, and two bands at 1715 and 1690 cm−1 for 2C=O groups. In addition 1H NMR spectrum of compound 12a in (DMSO-d6) showed two singlet signals at δ H 0.87 and 1.02 ppm for 2CH3 groups, pair of doublets bands at δ H 2.03 and 2.23 ppm for C6 protons and pair of doublet bands at δ H 2.46 and 2.51 ppm of C8 protons, a singlet band at δ H 8.30 ppm for SO2NH2 group (exchangeable with D2O), and the aromatic protons appear as complex pattern from δ H 6.99–7.55 ppm.
Fusion of compound 12a with dimethylformamide/dimethylacetal (DMF/DMA) in a sand bath for 6 h gave the corresponding enaminone 13 in 94% yield (Scheme 4). The structure of compound 13 is assigned based on the elemental analysis and spectral data. 1H NMR spectrum in (CDCl3) showed the absence of C6 protons, a new two singlet signals at δ H 3.04 and 3.15 ppm for –N(CH3)2, and a new singlet signal at δ H 7.71 ppm for olefinic CH. Treatment of enaminone 13 with guanidine, (liberated in situ from guanidine nitrate in the presence of sodium carbonate) under fusion in a sand bath gave 4-(2-amino-9-cyano-10-(4-fluorophenyl)-5,5-dimethyl-8-oxo-5,6-dihydropyrido[2,3-h]quin-azolin-7(8H)-yl)benzenesulfonamide 14 in 97% yield. The structure of compound 14 is assigned based on its spectral and elemental analysis data. FTIR spectra for compound 14 showed two broadband peaks at 3351 and 3201 cm−1 for 2NH2 groups, and at 1666 cm−1 for CO group. 1H NMR spectrum in (DMSO-d6) showed the absence of –N(CH3)2 signals, and a new, D2O exchangeable –NH2 protons at δ H 5.80 ppm. The C4-H proton appears in a complex pattern with aromatic protons and –SO2NH2 protons in a range from δ H 6.53–8.30 ppm.
Also, enaminone 13 undergo cyclo-condensation on treatment with hydrazine derivatives under reflux in a sand bath to afford compounds 15a,b (Scheme 4). The structure of compounds 15a,b were assigned depending on their spectral data and elemental analysis. FTIR spectra of compound 15a showed a band at 1620 cm−1 for C=O. The 1H NMR spectrum in (CDCl3) for compound 15b showed the disappearance of the –N(CH3)2 protons at δ H 3.04 and 3.15 ppm, the ph-H and the CH of pyrazole ring appeared with the complex pattern from δ H 6.64–8.09 ppm.
The behavior of 4-(3-cyano-6-((dimethylamino)methylene)-4-(4-fluorophenyl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl) benzenesulfonamide 13 toward some amino pyrazole derivatives was also investigated. Thus, when enaminone 13 was treated with amino pyrazole derivatives under fusion in the sand bath in the presence of catalytic amount of Triethylamine (TEA), it afforded the corresponding derivatives 16a,b (Scheme 4). The structure of compounds 16a,b was confirmed on the basis of their spectral data and elemental analysis. The 1H NMR spectrum in (CDCl3) of compound 16a showed a new singlet signal at δ H 2.67 ppm for C2-CH3, the phenyl protons appeared in the complex pattern with aromatic protons and SO2NH2 protons ranged from δ H 7.17–8.00 ppm. The C5 proton for the fused pyrazolo pyrido derivative appeared at δ H 8.97 ppm.
In vitro cytotoxic screening
In the present work, 12 of the newly synthesized compounds (5a,c), (7), (8), (10), (12a–d), (14), (15b), (16b) were selected to evaluate their in vitro growth inhibitory activities against human cultured breast carcinoma cell lines (MCF7) in comparison to DOX, which is one of the most efficient antitumor agents. According to the resultant data presented in Table 3, which shows the in vitro cytotoxic activity of the selected synthesized compounds, some compounds exhibit significant activity compared to the reference drug. From the results in Table 3, it was found that the quinoline derivatives 12a,d and 16b (IC50 = 0.036, 0.025, 0.036, 0.015 μM, respectively) were the most potent compounds in this screening, and exhibited a higher cytotoxic activity when compared with the reference drug DOX (IC50 = 0.04 μM). Compounds 5c, 7, 10, and 12c (IC50 = 0.048, 0.040, 0.041, 0.044 μM, respectively) were nearly as active as DOX, compounds 5a, 8, 12b, 14, and 15b showed lower IC50 values than that of the reference drug, ranging from 0.055–0.088 μM.
Conclusion
In this work, we have synthesized novel quinoline derivatives containing enaminone system and bearing a sulfonamide moiety using both classical and sonicated methods. Selected examples of these newly synthesized compounds were investigated against their in vitro anticancer activity against human breast cancer cell line (MCF7). Some of these new compounds exhibited significant anticancer activity, when compared to DOX as a reference drug. Since it was reported that compounds bearing a free sulfonamide group may show potent CA inhibition activity, which is considered to be an interesting target for the design of anticancer agents, the results obtained from the anticancer screening may provide a suggestion that the synthesized compounds may act as CA inhibitors that could contribute to their anticancer activity.
Experimental section
General
All melting points (m.p.) were measured on a Mel-Temp apparatus and were uncorrected. TLC was performed on aluminum silica gel 60 F254 (E-Merk). The spots were detected by iodine and UV light absorption. Infrared spectra were recorded for the compounds in an FTIR, Perkin Elmr SP 100 Spectrometer. 1H NMR and 13C spectra were recorded on Burker WM 400 and 600 MHz spectrometer using TMS (0.00 ppm) or the signal of the deuterated solvent was used as internal standard. Reactions that carried out by ultrasonic irradiation was done using Daihan (Wiseclean, D-40 MHz) ultrasonic bath. Microanalysis was performed by Perkin Elmer elemental analyzer. Biological activity tests were performed at the National Cancer Institute, Cairo, Egypt.
Typical procedure for the reactions
Synthesis of 4-(5,5-dimethyl-3-oxocyclohex-1-enylamino)benzenesulfonamide (3)
Method A: silent reaction
Prepared according to reported procedure (Ghorab et al. 2010).
Method B: sonicated reaction
A mixture of diamidone 1 (0.14 g, 1 mmol) and sulfanilamide 2 (0.172 g, 1 mmol) in ethanol (7 ml) was sonicated at a frequency of 40 KHz for 20 min at 80 °C. Then the reaction mixture was cooled in ice bath and the collected mass was filtered off, dried, and crystallized from ethanol to give enaminone 3 as white crystals (0.265 g, 90% yield); m.p. 235–237 °C. FTIR, cm−1: 3400, 3310, 3290 (NH, NH2); 1630 (C=O); 1357–1150 (SO2).
Synthesis of 4-(2-amino-3-cyano-4-(aryl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl) benzenesulfonamide (5a–c)
Method A: silent reaction
A mixture of compound 3 (2.94 g, 10 mmol) and different arylidene malononitrile 4a–c (10 mmol) in EtOH (20 ml) containing three drops of TEA was refluxed for 6–14 h (until disappearance of starting material as examined by TLC). The reaction mixture was filtered while hot and the solid obtained was filtered off and dried.
Method B: sonicated reaction
A mixture of compound 3 (0.294 g, 1 mmol) and different arylidene malononitrile 4a–c (1 mmol) in EtOH (7 ml) containing one drop of TEA was sonicated at a frequency of 40 KHz for 30–60 min at 80 °C. Then the reaction mixture was filtered and the collected mass was filtered off and dried.
4-(2-Amino-3-cyano-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl)benzenesulfonamide (5a): It was crystallized from ethanol as red crystals (0.29 g, 62% yield); m.p. 119–120 °C. FTIR, cm−1: 3469, 3408, 3347, 3334 (2NH2); 2177 (C≡N); 1630 (C=O); 1357–1190 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.75, 0.90 (6H, 2s, 2CH3); 1.07 (2H, s, C8-H2); 2.09 (2H, s, C6-H2); 4.45 (1H, s, C4-H); 5.80 (2H, s, NH2); 6.89–8.06 (8H, complex pattern, Ar–H); 8.54 (2H, s, SO2NH2). 13C NMR (600 MHz, DMSO-d6) δ C: 15.00 (2CH3); 18.00 (C7); 57.00 (C4); 79.00 (C8); 81.22 (C6); 81.24 (C3); 112.47 (C4′); 113.21, 128.06, 130.05, 160.2 (of p-flouro phenyl ring); 114.20, 127.45, 133.55, 133.62 (of p-SO2NH2 phenyl ring); 117.04 (CN); 160.20 (C8′); 164.36 (C2); 194.00 (C5). Anal. calcd. for C24H23FN4O3S (466.53): C, 62.09; H, 4.97; N, 12.01%. Found: C, 61.99; H, 4.85; N, 11.95%.
4-(2-Amino-3-cyano-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl)benzenesulfonamide (5b): It was crystallized from isopropanol to give dark red crystals (0.45 g, 92% yield); m.p. 85–87 °C. FTIR, cm−1: 3460, 3371, 3337, 3243 (2NH2); 2190 (C≡N); 1679 (C=O); 1344–1190 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.70, 0.90 (6H, 2s, 2CH3); 1.35 (2H, s, C8-H2); 2.07 (2H, s, C6-H2); 4.37 (1H, s, C4-H); 5.79 (2H, s, NH2); 6.56–8.29 (8H, complex pattern, Ar–H); 8.34 (2H, s, SO2NH2). 13C NMR (600 MHz, DMSO-d6) δ C: 30.67 (2CH3); 45.69 (C7); 52.92 (C4); 58.00 (C8); 61.66 (C6); 79.00 (C3); 112.39 (C4′); 115.58, 129.98, 130.16, 134.13 (of p-SO2NH2 phenyl ring); 118.18 (CN); 123.63, 127.39, 142.93, 148.33 (of p-NO2 phenyl ring) 151.89 (C8′); 165.05 (C2); 194.00 (C5). Anal. calcd. for C24H23N5O5S (493.14): C, 58.41; H, 4.70; N, 14.19%. Found: C, 58.49; H, 4.50; N, 14.13%.
4-(2-Amino-3-cyano-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinolin-1(4H)-yl)benzenesulfonamide (5c): It was crystallized from ethanol to give pale yellow crystals (0.45 g, 93% yield); m.p. 148–149 °C. FTIR, cm−1: 3475, 3380, 3311, 3235 (2NH2); 2218 (C≡N); 1623 (C=O); 1314–1149 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.93, 1.01 (6H, 2s, 2CH3); 2.06 (2H, s, C8-H2); 2.21 (2H, s, C6-H2); 3.69 (H, s, C4-H); 3.87 (3H, s, O–CH3); 5.79 (2H, s, NH2); 6.56–7.97 (8H, complex pattern, Ar–H); 8.37 (2H, s, SO2NH2). Anal. calcd. for C25H26N4O4S (478.17): C, 62.74; H, 5.48; N, 11.71%. Found: C, 62.94; H, 5.39; N, 11.64%.
Synthesis of 2-chloro-N-(2-chloroacetyl)-N-(3-cyano-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-1-(4-sulfamoylphenyl)-1,4,5,6,7,8-hexahydroquinolin-2-yl)acetamide (6)
A mixture of compound 5a (0.466 g, 1 mmol) and chloroacetyl chloride (0.08 ml, 1 mmol) in dimethyl formamide (20 ml) was refluxed for 1 h. The reaction mixture was poured onto cold water and the solid obtained was filtered off and dried. It was crystallized from ethanol to give brown crystals (0.28 g, 40% yield); m.p. 126–128 °C. FTIR, cm−1: 3356, 3336 (NH2); 2227 (C≡N); br. 1699, 1653 (3C=O); 1303–1150 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 1.07 (6H, s, 2CH3); 2.53 (2H, s, C8-H2); 3.22 (2H, s, C6-H2); 2.71, 2.87 (4H, 2s, 2COCH2Cl); 3.99 (1H, s, C4-H); 6.56–7.93 (8H, complex pattern, Ar–H), 8.30 (2H, s, SO2NH2). Anal. calcd. for C28H25Cl2FN4O5S (618.09): C, 54.29; H, 4.07; N, 9.04%. Found: C, 54.34; H, 4.00; N, 8.71%.
Synthesis of N-(4-(5-(4-fluorophenyl)-4-hydroxy-2,8,8-trimethyl-6-oxo-6,7,8,9-tetrahydropyrimido[4,5-b]quinolin-10(5H)-yl) phenylsulfonyl) acetamide (7)
A solution of compound 5a (0.466 g, 1 mmol) in acetic anhydride (20 ml) was refluxed for 1 h, the reaction mixture was then concentrated, the solid obtained was filtered off and dried. It was crystallized from methanol to give pale yellow crystals (0.48 g, 87% yield); m.p. 245–246 °C. FTIR, cm−1: 3332, 3274 (NH, OH); 1697, 1669 (2C=O); 1371, 1156 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.75, 0.90 (6H, 2s, 2CH3); 1.89 (5H, s, C9-H2 and COCH3), 2.07 (5H, s, C7-H2 and C2-CH3); 3.15 (1H, s, C5-H); 7.16–8.54 (8H, m, Ar–H); 10.37 (1H, s, NH); 11.95 (1H, s, OH). Anal. calcd. for C28H27FN4O5S (550.17): C, 61.08; H, 4.94; N, 10.18%. Found: C, 61.22; H, 5.01; N, 9.97%.
Synthesis of 2-cyano-N-(3-cyano-4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-1-(4-sulfamoylphenyl)-1,4,5,6,7,8-hexahydroquinolin-2-yl)acetamide (8)
A mixture of compound 5a (0.466 g, 1 mmol) and ethyl cyanoacetate (10 ml) was refluxed together for 5 h. The formed solid mass was filtered off and dried. It was crystallized from methanol to give brown crystals (0.49 g, 92% yield); m.p. 227–228.5 °C. FTIR, cm−1: 3312, 3229, 3123 (NH2, NH); 2260 (C≡N); 1743, 1680 (2C=O); 1360–1150 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.75, 0.90 (6H, 2s, 2CH3); 1.20 (2H, s, C8-H2); 2.06 (2H, s, C6-H2); 3.98 (3H, s, CH2CN and C4-H); 7.27–7.78 (8H, complex pattern, Ar–H); 8.29 (2H, s, SO2NH2); 10.50 (1H, s, NH). 13C NMR (600 MHz, DMSO-d6:CDCL3) δ C: 23.00 (CH 2 CN); 26.93 (2CH3); 59.49 (C7); 60.74 (C4); 63.3 (C8); 66.91 (C6); 68.08 (C3); 112.00 (C4′); 114.80, 138.84, 130.00, 161.04 (p-F phenyl ring); 119.21, 130.20, 130.60, 141.24 (P-SO2NH2 phenyl ring); 126.97 (CH-CN); 152.00 (C8′); 156.00 (C2); 172.43 (NHCO); 195 (C5). Anal. calcd. for C27H24FN5O4S (533.15): C, 60.78; H, 4.53; N, 13.13%. Found: C, 60.90; H, 4.32; N, 13.02%.
Synthesis of 4-(2-amino-5-(2-aminoethylamino)-3-cyano-4-(4-fluorophenyl)-7,7-dimethyl-7,8-dihydroquinolin-1(4H)-yl)benzenesulfonamide (9)
A mixture of compound 5a (0.466 g, 1 mmol) and ethylenediamine (7 ml) was refluxed in carbon disulfide (7 ml) for 3 h. The reaction mixture was cooled and then poured onto cold water. The solid obtained was filtered off and dried. It was washed with ethyl acetate, crystallized from ethanol to give brown crystals (0.234 g, 46% yield); m.p. 159–161 °C. FTIR, cm−1: 3337–3193 (NH, NH2); 2201 (C≡N); 1324–1190 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 1.04 (6H, s, 2CH3); 1.88 (2H, s, C8-H2); 2.38, 2.51 (3H, 2s, NH and NH2); 3.63, 3.81 (4H, 2t, 2CH2); 4.30 (1H, s, C4-H), 5.77 (2H, s, C2-NH2); 6.56–8.13 (9H, complex pattern, Ar–H and C6-H); 8.3 (2H, s, SO2NH2). Anal. calcd. for C26H29FN6O2S (508.21): C, 61.40; H, 5.75; N, 16.52%. Found: C, 61.54; H, 5.40; N, 16.28%.
Synthesis of 4-(3-acetyl-5-(4-fluorophenyl)-4-imino-8,8-dimethyl-2,6-dioxo-1,2,3,4,6,7,8,9-octahydrobenzo[b][1,8]naphthyridin-10(5H)-yl)benzenesulfonamide (10)
A mixture of compound 5a (0.466 g, 1 mmol) and ethyl acetoacetate (10 ml) was refluxed together for 5 h. The formed solid mass was filtered off and dried. It was washed by ethylacetate, crystallized from toluene to give black crystals (0.38 g, 70% yield); m.p. 130–132 °C. FTIR, cm−1: 3304, 3256, 3225 (2NH, NH2); 1713, 1660, 1632 (3C=O); 1349–1198 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 1.16, 1.22 (6H, 2s, 2CH3); 2.24 (2H, s, C9-H2); 2.55 (2H, s, C7-H2); 2.35 (3H, s, COCH3); 3.57 (1H, s, C3-H); 4.09 (1H, s, C5-H); 4.24 (1H, s, C4-NH); 6.04–8.50 (9H, complex pattern, Ar–H and endocyclic NH and SO2NH2); 15.72 (1H, s, OH of the iminol structure). Anal. calcd. for C28H27FN4O5S (550.17): C, 61.08; H, 4.94; N, 10.18%. Found: C, 61.27; H, 5.03; N, 10.21%.
Synthesis of 4-(3-cyano-4-(aryl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin 1(2H)-yl) benzenesulfonamide (12a–f)
Method A: silent reaction
A mixture of diamidone 1 (0.14 g, 1 mmol) with sulfanilamide 2 (0.172 g, 1 mmol), different aromatic aldehydes 11a–f (1 mmol) and ethyl cyanoacetate (0.12 ml, 1 mmol) in ethanol (10 ml) was refluxed for 3–30 h, the obtained solid filtered off and dried.
Method B: sonicated reaction
A mixture of diamidone 1 (0.14 g, 1 mmol) with sulfanilamide 2 (0.172 g, 1 mmol), different aromatic aldehydes 11a–f (1 mmol) and with ethyl cyanoacetate (0.12 ml, 1 mmol) in ethanol (10 ml) was sonicated at a frequency of 40 KHz for 10–50 min at 80 °C. Then the collected mass was filtered off and dried.
4-(3-Cyano-4-(4-fluorophenyl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12a): It was crystallized from ethanol to give colorless crystals (0.395 g, 85% yield); m.p. 149–150 °C. FTIR, cm−1: 3372, 3236 (NH2); 2226 (C≡N); 1715, 1690 (2C=O); 1305–1145 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.87, 1.02 (6H, 2s, 2CH3); 2.03, 2.23 (2H, 2d, C6-H2); 2.46, 2.51 (2H, 2d, C8-H2); 6.99–7.55 (8H, complex pattern, Ar–H); 8.30 (2H, s, SO2NH2). 13C (600MHz, DMSO-d6) δ C: 26.45, 28.57 (2CH3); 31.85 (C7); 32.69 (C8); 49.9 (C6); 112 (C4′); 114.26, 129.35, 162.12 (of p-flouro phenyl ring); 114.4 (C3); 115.3 (CN); 126.00, 129.41, 142.51, 142.53 (of p-SO2NH2 phenyl ring); 159.59 (C8′); 161.19 (C2); 167.87 (C4); 195.83 (C5). Anal. calcd. for C24H20FN3O4S (465.12): C, 61.92; H, 4.33; N, 9.03%. Found: C, 62.03; H, 4.18; N, 8.95%.
4-(3-Cyano-7,7-dimethyl-4-(4-nitrophenyl)-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12b): It was crystallized from ethanol to give pale yellow crystals (0.472 g, 96% yield); m.p. 165.5–167 °C. FTIR, cm−1: 3459, 3342 (NH2); 2224 (C≡N); 1716, 1615 (2C=O); 1505 (NO2 arom.); 1344–1190 (SO2). 1H NMR (600 MHz, CDCL3) δ H: 1.20, 1.34 (6H, s, 2CH3); 1.42 (2H, s, C6-H2); 1.52 (2H, s, C8-H2); 6.80–8.36 (10H, complex pattern, Ar–H and SO2NH2). 13C (600 MHz, CDCl3) δ C: 14.11 (2CH3); 20.00 (C7); 63.37 (C6 and C8); 107.38 (C4′); 114.00 (C3); 114.54 (CN); 123.80, 129.00, 131.52, 149.00 (of p-SO2NH2 phenyl ring); 124.00, 131.90, 136.90, 149.72 (of p-NO2 phenyl ring); 151.50 (C8′); 151.75 (C2); 161.40 (C4); 194.00 (C5). Anal. calcd. for C24H20N4O6S (492.11): C, 58.53; H, 4.09; N, 11.38%. Found: C, 58.59; H, 4.02; N, 11.23%.
4-(3-Cyano-4-(4-methoxyphenyl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12c): It was crystallized from ethanol to give pale yellow crystals (0.453 g, 95% yield); m.p. 136–138 °C. FTIR, cm−1: 3461, 3315 (NH2); 2264 (C≡N); 1687, 1624 (2C=O); 1299–1144 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.88, 1.01 (6H, 2s, 2CH3); 2.02, 2.22 (2H, 2d, C6-H2); 2.42, 2.53 (2H, 2d, C8-H2) 3.66 (3H, s, OCH3); 5.78–7.47 (10H, complex pattern, Ar–H and SO2NH2). 13C NMR (600 MHz, DMSO-d6) δ C: 26.46 (2CH3); 28.63 (C7); 31.85 (C8); 32.31 (C6); 54.86 (OCH3); 112.41 (C4′); 113.06, 127.38, 129.98, 161.91 (of p-OCH3 phenyl ring); 115.70 (C3 and CN); 127.00, 128.56, 138.46, 151.88 (of p-SO2NH2 phenyl ring); 157.29 (C8′); 159.04 (C2); 168.04 (C4); 195.91 (C5). Anal. calcd. for C25H23N3O5S (477.14): C, 62.88; H, 4.85; N, 8.80%. Found: C, 63.01; H, 4.72; N, 8.69%.
4-(4-(4-Chlorophenyl)-3-cyano-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12d): It was crystallized from ethanol to give white crystals (0.381 g, 82% yield); m.p. 145–147 °C. FTIR, cm−1: 3476, 3371 (NH2); 2265 (C≡N); 1745, 1687 (2C=O); 1369–1143 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.87, 1.01 (6H, 2s, 2CH3); 2.02, 2.23 (2H, 2d, C6-H2); 2.42, 2.51 (2H, 2d, C8-H2); 5.79–7.56 (10H, complex pattern, Ar–H and SO2NH2). 13C NMR (600 MHz, DMSO-d6) δ C: 26.53 (2CH3); 28.65 (C7); 31.39 (C8); 33.03 (C6); 112.49 (C4′); 115.10 (C3 and CN); 127.46, 129.63, 145.43, 162.39 (of p-SO2NH2 phenyl ring); 127.72, 130.05, 130.34 (of p-chlorophenyl ring); 151.96 (C8′); 159.16 (C2); 167.91 (C4); 195.99 (C5). Anal. calcd. for C24H20ClN3O4S (481.09): C, 59.81; H, 4.18; N, 8.72%. Found: C, 59.92; H, 3.99; N, 8.65%.
4-(4-(4-Bromophenyl)-3-cyano-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12e): It was crystallized from ethanol to give yellow crystals (0.367 g, 70% yield); m.p. 213–215 °C. FTIR, cm−1: 3333, 3233 (NH2); 2263 (C≡N); 1743, 1682 (2C=O); 1369–1149 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 1.04 (6H, s, 2CH3); 1.10, 1.2 (2H, 2d, C6-H2); 2.00, 2.1 (2H, 2d, C8-H2); 6.70–7.78 (10H, complex pattern, Ar–H and SO2NH2). Anal. calcd. for C24H20BrN3O4S (525.04): C, 54.76; H, 3.83; N, 7.98%. Found: C, 54.95; H, 3.80; N, 8.01%.
4-(3-Cyano-4-(4-(dimethylamino)phenyl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl)benzenesulfonamide (12f): It was washed by petroleum ether to give yellow crystals (0.39 g, 80% yield); m.p. 122–123 °C. FTIR, cm−1: 3400, 3373 (NH2); 2208 (C≡N); 1699, 1593 (2C=O); 1274, 1227 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.90, 1.08 (6H, 2s, 2CH3); 1.19, 1.33 (2H, 2d, C6-H2); 2.15, 2.20 (2H, 2d, C8-H2); 3.45 (6H, s, N(CH3)2); 6.59–8.1 (10H, complex pattern, Ar–H and SO2NH2).13C NMR (600 MHz, DMSO-d6) δ C: 27.57, 29.09 (2CH3); 30.94 (C7); 32.72 (C8); 40.67 (N(CH3)2); 50.76 (C6); 81.45, 117.22, 128.76, 158.20 (of p-N(CH3)2 phenyl ring); 112.30 (C4′); 113.01 (C3 and CN); 114.01, 128.65, 134.27, 148.79 (of p-SO2NH2 phenyl ring); 161.01 (C8′); 162.87 (C2); 169.34 (C4); 196.58 (C5). Anal.calcd. for C26H26N4O4S (490.17): C, 63.66; H, 5.34; N, 11.42%. Found: C, 63.80; H, 5.16; N, 11.24%.
Synthesis of 4-(3-cyano-6-((dimethylamino) methylene)-4-(4-fluorophenyl)-7,7-dimethyl-2,5-dioxo-5,6,7,8-tetrahydroquinolin-1(2H)-yl) benzenesulfonamide (13)
A mixture of 12a (4.65 g, 10 mmol) and DMF/DMA (2 ml, 16.7 mmol) was fused together for 6 h at 100 °C. The obtained solid by cooling was crystallized from ethanol to give yellow crystals (0.48 g, 94% yield); m.p. 238–239 °C. FTIR, cm−1: 3387, 3347 (NH2); 2206 (C≡N); 1666, 1625 (2C=O); 1337–1153 (SO2). 1H NMR (600 MHz, CDCL3) δ H: 1.09, 1.63 (6H, 2s, 2CH3); 2.48 (2H, s, C8-H2); 3.04, 3.15 (6H, 2s, N(CH3)2); 7.71 (1H, s, olifinic H); 6.80–8.60 (10H, complex pattern, Ar–H and SO2NH2). 13C (600 MHz, CDCL3) δ C: 28.55 (C7); 30.94 (2CH3); 35.63 (C8); 41.59 (N(CH3)2); 108.00 (C3 and C4′); 109.65 (CN); 113.93, 119.70, 196.40 (of p-flouro phenyl ring); 117.75, 127.90, 139.89, 141.28 (of p-SO2NH2 phenyl ring); 128.67 (C6); 149.61 (CHN(CH3)2); 158.86 (C2); 159.10 (C8′); 200.56 (C4); 207.03 (C5). Anal. calcd. for C27H25FN4O4S (520.16): C, 62.29; H, 4.84; N, 10.76%. Found: C, 62.41; H, 4.81; N, 10.55%.
Synthesis of 4-(2-amino-9-cyano-10-(4-fluorophenyl)-5,5-dimethyl-8-oxo-5,6-dihydropyrido[2,3-h]quinazolin-7(8H)-yl)benzenesulfonamide (14)
A mixture of enaminone 13 (0.52 g, 1 mmol) and guanidine nitrate (0.122 g, 1 mmol) and sodium carbonate (0.12 g, 1 mmol) were fused at 180 °C for 2 h. The obtained solid on cooling was crystallized from chloroform to give brown crystals (0.5 g, 97% yield); m.p. 112–114 °C. FTIR, cm−1: 3351–3201 (2NH2); 2226 (C≡N); 1666 (C=O); 1336–1219 (SO2). 1H NMR (600 MHz, DMSO-d6) δ H: 0.98, 1.04 (6H, 2s, 2CH3); 2.92 (2H, s. C6-H2); 5.80 (2H, s, NH2); 6.53–8.30 (11H, complex pattern, Ar–H and C4-H and SO2NH2). Anal. calcd. for C26H21FN6O3S (516.14): C, 60.45; H, 4.10; N, 16.27%. Found: C, 60.49; H, 4.01; N, 16.17%.
Synthesis of 4-(8-cyano-9-(4-fluorophenyl)-4,4-trimethyl-7-oxo-4,5-dihydro-1H-pyrazolo[3,4-f]quinolin-6(7H)-yl)benzenesulfonamide derivatives (15a,b)
General method
A mixture of enaminone 13 (1 g, 2 mmol) and hydrazine derivatives (2 mmol) were fused together for 2–4 h, the residue obtained was crystallized.
4-(8-Cyano-9-(4-fluorophenyl)-1,4,4-trimethyl-7-oxo-4,5-dihydro-1H-pyrazolo[3,4-f]quinolin-6(7H)-yl)benzenesulfonamide (15a): It was crystallized from ethanol to give brown crystals (0.417 g, 83% yield); m.p. 101–103 °C. FTIR, cm−1: 3323, 3270 (NH2); 2353 (C≡N); 1620 (C=O); 1220–1155 (SO2). 1H NMR (600 MHz, CDCl3) δ H: 1.10, 1.20 (6H, 2s, 2CH3); 2.17 (2H, s, C5-H2); 3.80 (3H, s, NCH3); 6.7–8.01 (11H, complex pattern, Ar–H and SO2NH2). Anal. calcd. for C26H22FN5O3S (503.14): C, 62.02; H, 4.40; N, 13.91%. Found: C, 62.22; H, 4.35; N, 13.74%.
4-(8-Cyano-9-(4-fluorophenyl)-4,4-dimethyl-7-oxo-1-phenyl-4,5-dihydro-1H-pyrazolo[3,4-f]quinolin-6(7H)-yl)benzenesulfonamide (15b): It was crystallized from dioxane to give brown crystals (0.45 g, 80% yield); m.p. 141–142 °C. FTIR, cm−1: 3450, 3348 (NH2); 2202 (C≡N); 1620 (C=O); 1340–1180 (SO2). 1H NMR (600 MHz, CDCL3) δ H: 1.24 (6H, s, 2CH3); 2.17 (2H, s, C5-H2); 6.64–8.09 (16H, complex pattern, Ar–H and SO2NH2). Anal. calcd. for C31H24FN5O3S (565.16): C, 65.83; H, 4.28; N, 12.38%. Found: C, 66.03; H, 4.08; N, 12.22%.
Synthesis of 4-(10-Cyano-11-(4-fluorophenyl)-6,6-dimethyl-9-oxo-6,7-dihydropyrazolo[1,5-a]pyrido[2,3-h]quinazolin-8(9H)-yl) benzenesulfonamide derivatives (16a,b)
General method
A mixture of enaminone 13 (0.52 g, 1 mmol) and amino pyrazole derivatives (1 mmol), and few drops from TEA was fused together at 130 °C for 5 h. The obtained mass was crystallized.
4-(10-Cyano-11-(4-fluorophenyl)-2,6,6-trimethyl-9-oxo-3-phenyl-6,7-dihydropyrazolo[1,5-a]pyrido[2,3-h]quinazolin-8(9H)-yl) benzenesulfonamide (16a): It was crystallized from toluene to give brown crystals (0.49 g, 79% yield); m.p. 226–227 °C. FTIR, cm−1: 3480, 3397 (NH2); 2197 (C≡N); 1663 (C=O); 1221, 1159 (SO2). 1H NMR (600 MHz, CDCL3) δ H: 1.24 (6H, s, 2CH3); 2.59 (2H, s, C7-H2); 2.67 (3H, s, C2-CH3); 7.17–8.00 (15H, complex pattern, Ar–H and SO2NH2); 8.97 (1H, s, C5-H). 13C NMR (600 MHz, CDCL3) δ C: 14.00 (CH3 at C2); 29.27 (2CH3 at C6); 40.84 (C6); 50.70 (C7); 112.18 (C11′); 113.2 80 (C10); 114.78; 128.63, 128.72, 162.56 (of p-flouro phenyl ring); 115.39, 129.03, 131.24, 147.04 (of p-SO2NH2 phenyl ring); 115.75 (CN); 116.37 (C3); 127.04, 128.87, 129.10, 131.10 (of phenyl at C3); 129.80 (C3′); 129.9 (C5′); 146.41 (C2); 151.00 (C7′); 151.72 (C5); 156.62 (C9); 159.00 (C11″); 194.4 (C11). Anal. calcd. for C35H27FN6O3S (630.18): C, 66.65; H, 4.32; N, 13.33%. Found: C, 66.76; H, 4.21; N, 13.17%.
4-(2-(4-Chlorophenyl)-10-cyano-11-(4-fluorophenyl)-6,6-dimethyl-9-oxo-6,7-dihydropyrazolo[1,5-a]pyrido[2,3-h]quinazolin-8(9H)-yl)benzenesulfonamide (16b): The obtained mass was crystallized from ethanol to give brown crystals (0.47 g, 72% yield); m.p. 117–118 °C. FTIR, cm−1: 3336, 3254 (NH2); 2263 (C≡N); 1652 (C=O); 1221, 1159 (SO2). 1H NMR (600 MHz, CDCL3) δ H: 1.16, 1.17 (6H, 2s, 2CH3); 2.60 (2H, s, C7-H2); 6.64 (C3-H); 6.84–8.10 (14H, complex pattern, Ar–H and SO2NH2); 8.98 (C5-H). 13C NMR (600 MHz, CDCL3) δ C: 27.62 (2CH3); 37.31 (C6); 50.52 (C7); 95.97 (C3); 113.49 (C11′); 114.01 (C10); 114.79 (CN); 115.64, 127.73, 128.02, 147.32 (of p-flouro phenyl ring); 116.37, 128.38, 128.93, 129.17 (of p-SO2NH2 phenyl ring); 128.17, 128.22, 128.50, 128.63 (of p-chloro phenyl ring); 128.63 (C5′); 129.52 (C7′); 129.90 (C3′); 130.19 (C2); 130.92 (C5); 158.72 (C9); 169.00 (C11″); 170.00 (C11). Anal. calcd. for C34H24ClFN6O3S (650.13): C, 62.72; H, 3.72; N, 12.91%. Found: C, 62.93; H, 3.56; N, 12.83%.
In vitro cytotoxic screening
Twelve analogs (5a,c), (7), (8), (10), (12a–d), (14), (15b), (16b) were selected as representative examples to evaluate their in vitro inhibitory effects against cellular proliferation in human cultured breast carcinoma cell line using DOX as a reference drug. Breast cancer cell lines (MCF7) were obtained from Cell Bank in National Cancer Institute, Cairo, Egypt. The potential cytotoxicity of the selected newly synthesized derivatives was done by SRB using the method of (Skehan et al. 1990) as follows: Cells were plated in a 96-multiwell plate (104 cells/well) for 24 h before treatment with compounds to allow attachment of the cell to the wall of the plate. Different concentrations of the compound under test (5, 12.5, 25, and 50 μg/ml) were added to the cell monolayer triplicate wells which were prepared for each individual dose. Monolayer cells were incubated with the compounds for 48 h at 37 °C and in an atmosphere of 5% CO2. After 48 h, cells were fixed, washed, and stained with Sulpho-Rhodamine-B stain. Excess stain was washed with acetic acid and attached stain was recovered with Tris-EDTA buffer. Color intensity was measured in an ELISA reader. Measurements were done six times and averaged. The relation between surviving fraction and drug concentration is plotted to get the survival curve of each tumor cell line after the specified compound.
References
Abbas EMH, Farghaly TA (2010) Synthesis, reactions, and biological activity of 1,4-benzothiazine derivatives. Monatsh Chem 141:661–667
Ahmed N, Brahmbhatt KG, Sabde S, Mitra D, Singh IP, Bhutani KK (2010) Synthesis and anti-HIV activity of alkylated quinoline 2,4-diols. Bioorg Med Chem 18:2872–2879
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Ghorab MM, Noaman E (2010b) Discovering some novel tetrahydroquinoline derivatives bearing the biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 45:1849–1853
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Hamed MM, Noaman E, Ghorab MM (2009) Synthesis and biological evaluation of 2-Amino-7,7-dimethyl4-substituted-5-oxo-1-(3,4,5-trimethoxy)-1,4,5,6,7,8-hexahydro-quinoline-3-carbonitrile derivatives as potential cytotoxic agents. Bioorg Med Chem Lett 19:6939–6942
Alqasoumi SI, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM (2010a) Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active sulfonamide moiety as a new class of antitumor agents. Eur J Med Chem 45:738–744
Al-Said MS, Ghorab MM, Al-Dosari MS, Hamed MM (2011) Synthesis and In-vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem 46:201–207
Carta A, Briguglio I, Piras S, Corona P, Boatto G, Nieddu M, Giunchedi P, Marongiu ME, Giliberti G, Iuliano F, Blois S, Ibba C, Busonera B, Colla PL (2011) Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors. Bioorg Med Chem 19:7070–7084
Chen Z, W Y, Liu Y, Yu L, Zhou L, Yang S, Lai L (2011) Quinoline-4-methyl esters as human nonpancreatic secretory phospholipase A2 inhibitors. Bioorg Med Chem 19:3361–3366
Creaven BS, Duff B, Egan DA, Kavanagh K, Rosair G, Thangella VR, Walsh M (2010) Anticancer and antifungal activity of Copper(II) complexes of quinolin-2(1H)-one-derived schiff bases. Inorg Chim Acta 363:4048–4058
Daniel L (2007) The organic chemistry of drug synthesis. Wiley, Hoboken, pp 167–171
Edafiogho IO, Phillips OA, Udo EE, Samuel S, Rethish B (2009) Synthesis, antibacterial and anticonvulsant evaluations of some cyclic enaminones. Eur J Med Chem 44:967–975
Eddington ND, Cox DS, Roberts RR, Butcher RJ, Edafiogho IO, Stables JP, Cooke N, Goodwin AM, Smith CA, Scott KR (2002) Synthesis and anticonvulsant activity of enaminones. 4. Investigations on isoxazole derivatives. Eur J Med Chem 37:635–648
Eddington ND, Cox DS, Roberts RR, Stables JP, Powell CB, Scott KR (2000) Enaminones-versatile therapeutic pharmacophores. Further advances. Curr Med Chem 7:417–436
El-Hashim A, Yousefi S, Edafiogho I, Raghupathy R, Yousif M, Simon HU (2010) Anti-inflammatory and immunosuppressive effects of the Enaminone E121. Eur J Pharmacol 632:73–78
El-Hashim AZ, Edafiogho IO, Jaffal SM (2001) Anti-tussive and Bronchodilator mechanisms of action for the Enaminone E121. Life Sci 89:378–387
El-Sabbagh OI, Rady HM (2009) Synthesis of new acridines and hydrazones derived from cyclic β-diketone for cytotoxic and antiviral evaluation. Eur J Med Chem 44:3680–3686
El-Sabbagh OI, Shabaan MA, Kadry HH, Saad Al-Din E (2010) New octahydroquinazoline derivatives: synthesis and hypotensive activity. Eur J Med Chem 45:5390–5396
Gao M, Wang M, Miller KD, Hutchins GD, Zheng QH (2010) Synthesis and in-vitro biological evaluation of carbon-11-labeled quinoline derivatives as new candidate PET radioligands for cannabinoid CB2 receptor imaging. Bioorg Med Chem 18:2099–2106
García GC, Bejarano BP, Ruiz IL, Nieto MA´G (2012) Comparison of representational spaces based on structural information in the development of QSAR models for benzylamino enaminone derivatives. SAR QSAR Environ Res 23:751–774
Garudachari B, Satyanarayana MN, Thippeswamy B, Shivakumar CK, Shivananda KN, Hegde G, Isloor AM (2012) Synthesis, characterization and antimicrobial studies of some new quinoline incorporated benzimidazole derivatives. Eur J Med Chem 54:900–906
Ghodsi R, Zarghi A, Daraei B, Hedayati M (2010) Design, synthesis and biological evaluation of new 2,3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem 18:1029–1033
Ghorab MM, Ragab FA, Heiba HI, Arafa RK, El-Hossary EM (2010) In-vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur J Med Chem 45:3677–3684
Ghorab MM, Ragab FA, Noaman E, Heiba HI, El-Hossary EM (2009) Synthesis of some novel quinolines and pyrimido [4,5-b] quinolines bearing a sulphonamide moiety as potential anticancer and radioactive agents. Drug Res 57:795–803
Guo RH, Zhang Q, Maa YB, Huang XY, Luo J, Wang LJ, Geng CA, Zhang XM, Zhou J, Jiang ZY, Chen JJ (2011) Synthesis and biological assay of 4-aryl-6-chloro-quinoline derivatives as novel non-nucleoside anti-HBV agents. Bioorg Med Chem 19:1400–1408
Jackson PL, Hanson CD, Farrell AK, Butcher RJ, Stables JP, Eddington ND, Scott KR (2012) Enaminones 12. An explanation of anticonvulsant activity and toxicity per linus pauling’s clathrate hypothesis. Eur J Med Chem 51:42–51
Jayagobi M, Raghunathan R, Sainath S, Raghunathan M (2011) Synthesis and antibacterial property of pyrrolopyrano quinolinones and pyrroloquinolines. Eur J Med Chem 46:2075–2082
Korrichi L, Dalila B, Dalila S (2009) Quinolines antioxydant activity structure activity relationship. Eur J Bio Sci 1:32–36
Kouznetsov VV, Gómez CMM, Derita MG, Svetaz L, Olmo Ed, Zacchino SA (2012) Synthesis and antifungal activity of diverse C-2 pyridinyl and pyridinylvinyl substituted quinolines. Bioorg Med Chem 20:6506–6512
Kumar KS, Kumar SK, Sreenivas BY, Gorja DR, Kapavarapu R, Rambabu D, Krishna GR, Reddy CM, Rao MVB, Parsa KVL, Pal M (2012) C–C bond formation at C-2 of a quinoline ring: synthesis of 2-(1H-indol-3-yl) quinoline-3-carbonitrile derivatives as a new class of PDE4 inhibitors. Bioorg Med Chem 20:2199–2207
Lu CM, Chen YL, Chen HL, Chen CA, Lu PJ, Yang CN, Tzeng CC (2010) Synthesis and antiproliferative evaluation of certain Indolo[3,2-c]quinoline derivatives. Bioorg Med Chem 18:1948–1957
Makawana JA, Patel MP, Patel RG (2012) Synthesis and in-vitro antimicrobial activity of N-arylquinoline derivatives bearing 2-morpholinoquinoline moiety. Chin Chem Lett 23:427–430
Michael J, de Koning C, Gravestock D, Hosken G, Howard A, Jungmann C, Krause R, Parsons A, Pelly S, Stanbury T (1999) Enaminones: versatile intermediates for natural product synthesis. Pure Appl Chem 71:979–988
Perin N, Uzelac L, Piantanida I, Zamola GK, Kralj M, Hranjec M (2011) Novel biologically active nitro and amino substituted benzimidazo [1,2-a]quinolines. Bioorg Med Chem 19:6329–6339
Pretorius SI, Breytenbach WJ, Kock Cd, Smith PJ, N’Da DD (2013) Synthesis, characterization and antimalarial activity of quinoline–pyrimidine hybrids. Bioorg Med Chem 21:269–277
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer 82:1107–1112
Supuran CT, Scozzafava A, Casini A (2003) Carbonic anhydrase inhibitors. Med Res Rev 23:146–189
Thiry A, Ledecq M, Cecchi A, Dogne JM, Wouters J, Supuran CT, Masereel B (2006) Indanesulfonamides as carbonic anhydrase inhibitors. Toward structure-based design of selective inhibitors of the tumor-associated isozyme CA IX. J Med Chem 49:2743–2749
Vandurm P, Cauvin C, Guiguen A, Georges B, Van KL, Martinelli V, Cardona C, Mbemba G, Mouscadet JF, Hevesi L, Lint CV, Wouters J (2009) Structural and theoretical studies of [6-bromo-1-(4-fluorophenylmethyl)-4(1H)-quinolinon-3-yl)]-4-hydroxy-2-oxo-3-butenoïc acid as HIV-1 integrase inhibitor. Bioorg Med Chem Lett 19:4806–4809
Wang L, witalska M, Mei ZW, Lu WJ, Takahara Y, Feng XW, El-Sayed I, Wietrzyk J, Inokuchi T (2012) Synthesis and in-vitro Antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-Indolo[2,3-b]quinolines; structure activity relationships of neocryptolepines and 6-methyl congeners. Bioorg Med Chem 20:4820–4829
Acknowledgements
The authors acknowledge with thanks the NMR unit at King Abdulaziz University for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ahmed, N.S., Badahdah, K.O. & Qassar, H.M. Novel quinoline bearing sulfonamide derivatives and their cytotoxic activity against MCF7 cell line. Med Chem Res 26, 1201–1212 (2017). https://doi.org/10.1007/s00044-017-1850-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1850-9