Abstract
Ultrasonic irradiation is being considered not only as a green approach but also as a powerful technique for the synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives. It can be carried out by using multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes, in the presence of catalytic amounts of zinc oxide nanoparticles, in EtOH, at 80 °C. The preponderance of such a catalyst is due to its inexpensiveness, stability, and the potential of being easily obtained. Furthermore, high conversions, short reaction times, and cleaner reaction profiles are some of the advantages of this method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultrasound irradiation has been increasingly used in organic synthesis in the last three decades as an eco-friendly energy source. Compared to traditional methods, this technique is more convenient and easily controlled [1–3]. Moreover, enhanced reaction rates, formation of unadulterated products in high yields, easier manipulation, and waste minimization are some prominent features of this approach [4, 5]. More recently, ultrasonic irradiation has been used for the synthesis of azeto[2,1-d] [1, 5] benzothiazepines [6], 1,3-dipolar cycloaddition [7], and multicomponent reaction [8, 9] as a clean, practical, and usable protocol.
In this regard, multicomponent reactions (MCRs) are considered an invaluable strategy for the synthesis of many novel compounds [8–11]. The MCRs strategy depicted significant priorities over conventional linear-type synthesis, bringing about three or more simple and flexible molecules brought together to rapidly introduce structural complexity and diversity [12–15]. Because of the range of readily available starting materials and the simplicity of one-pot procedures, MCRs have been extensively used for the synthesis of heterocyclic compounds. Among these, the multicomponent synthesis of polyfunctionalized heterocyclic compounds has become more challenging in organic and medicinal chemistry [16, 17].
1,4-Dihydropyridines are an important class of compounds with a wide range of biological activities [18]. Due to being pharmacologically active and acting as an antitumor [19], calcium channel bloker [20], antitubercular [21], analgesic [22], antithrombotic [23], anti-inflammatory [24], and anticonvulsant agent [25], they are of many interests. Some of the marketed drug preparations containing 1,4-dihydropyridine moieties are Nifedipine, Amlodipine, Oxodipine, etc. (Scheme 1). In addition, imidazo[1,2-a]quinoline is a synthetically designed scaffold with a broad range of biological activities. Some of its derivatives have pharmacological properties such as antiallergic [26] and anxiolytic [27] activity (Scheme 1).
Nano-particles as heterogeneous catalysts have received considerable attention in recent years owing to their interesting structures and outstanding catalytic activities [28–31]. These nano-metal oxides have also shown high activity, strong oxidizing power, recyclability, and long-term stability [32, 33]. Nano-crystalline metal oxides such as nano-zinc oxide exhibit versatile applications as active adsorbents for gases or destruction of hazardous chemicals [34, 35], and also as catalysts in various organic transformations [8, 9, 36–39]. Hence, we decided to synthesize 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, by using ultrasound irradiation at EtOH and in the presence of catalytic amounts of zinc oxide nano-particles.
Experimental
General
Melting points were measured on a Electrothermal-9100 apparatus and are uncorrected. IR spectra were recorded on a Brucker FT-IR Tensor 27 infrared spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded on a Avance III 400 Bruker spectrometer at 400 and 100 MHz, respectively. Elemental analyses were performed using a Heracus CHN-O-Rapid analyzer. The cyclic enaminoketones 1a–d [43] and 1e, f [40] were prepared from the according to procedures described in the literature.
Preparation of ZnO nanoparticles
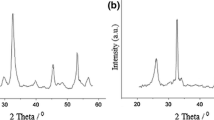
Zinc acetate dihydrate (2.19 g, 10 mmol) and CO(NH2)2 (12 g, 200 mmol) were dissolved in deionized water (200 ml) at room temperature to form a transparent solution, which was then refluxed for 12 h. It was cooled in an ice bath to stop the reaction. The precipitate was centrifuged and washed with deionized water and absolute EtOH, dried at 80 °C for 8 h, and then calcinated in a furnace in the presence of air at 400 °C for 2 h [44].
General procedure for the preparation of 1,4-dihydropyridine (4a–l) and imidazo[1,2-a]quinoline (4m–r) derivatives
A mixture of cyclic enaminoketones 1 (2 mmol), malononitrile 2 (2 mmol), aldehyde 3 (2 mmol), and ZnO nanoparticles (10 mol %) in ethanol (10 ml) was irradiated at 80 °C for the times reported in Table 3 (the progress of the reaction being monitored by TLC and was used hexane/ethyl acetate as an eluent). After completion of the reaction, the catalyst was separated from the reaction mixture by centrifugation. Then, the reaction mixture was poured into ice-cold water; the crude product was filtered, dried, and recrystallized from ethanol.
2-amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4b)
Yellow crystals; IR (KBr, ν max,cm−1): 3,392, 3,328, 3,232 (NH2, NH), 2,192 (CN), 1,654 (C=O), 1,596 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 8.82 (s, 1H, NH), 6.94 (d, 2H, 3 J HH =4 Hz, CH–Ar), 6.71 (d, 2H, 3 J HH = 4 Hz, CH–Ar), 5.65 (s, 2H, NH2), 4.16 (s, 1H, CH), 3.61 (s, 3H, OCH3), 2.32 (d, 2 J HH = 8 Hz, CH), 2.20 (d, 2 J HH =8 Hz, CH), 2.08 (d, 2 J HH =8 Hz, CH), 1.89 (d, 2 J HH =8 Hz, CH), 0.92 (s, 3H, CH3), 0.81 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ: 192.71 (C=O), 156.08, 153.27, 152.59, 137.96, 136.48, 132.71, 131.48, 113.35 (CN), 61.68 (C3), 55.63 (OCH3), 48.25 (CH2), 37.36 (CH2), 36.58 (CH), 32.69 (CMe2), 29.34 (CH3), 27.24 (CH3). Anal. calcd. for C19H21N3O2: C, 70.57; H, 6.55; N, 12.99 %. Found: C, 70.43; H, 6.48; N, 12.82 %.
2-amino-4-(4-bromophenyl)-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4c)
Yellow crystals; IR (KBr, ν max,cm−1): 3,392, 3,328, 3,232 (NH2, NH), 2,192 (CN), 1,654 (C=O), 1,596 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 8.93 (s, 1H, NH), 7.45 (d, 2H, 3 J HH =4 Hz, CH–Ar), 7.08 (d, 2H,3 J HH =4 Hz, CH–Ar), 5.82 (s, 2H, NH2), 4.30 (s, 1H, CH), 2.42 (d,2 J HH =8 Hz, CH), 2.31 (d, 2 J HH =8 Hz, CH), 2.18 (d,2 J HH =8 Hz, CH), 1.98 (d,2 J HH =8 Hz, CH), 1.02 (s, 3H, CH3), 0.90 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ: 191.89 (C=O), 155.88, 154.12, 136.70, 134.34, 132.19, 131.79, 128.86, 113.41 (CN), 60.61 (C3), 49.59 (CH2), 40.89 (CH2), 39.91 (CH), 33.53 (CMe2), 30.22 (CH3), 28.82 (CH3). Anal. calcd. for C18H18BrN3O: C, 58.08; H, 4.87; N, 11.29 %. Found: C, 57.91; H, 4.75; N, 11.05 %.
2-amino-7,7-dimethyl-5-oxo-1-phenyl-4-(p-tolyl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4e)
Yellow crystals; IR (KBr, ν max,cm−1): 3,472, 3,344 (NH2), 2,176 (CN), 1,651 (C=O), 1,590 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 7.63–7.12 (m, 9H, CH–Ar), 5.31 (s, 2H, NH2), 4.42 (s, 1H, CH), 2.21 (d, 2 J HH =4 Hz, CH), 2.17 (d, 2 J HH =4 Hz, CH), 2.00 (d,2 J HH =8 Hz, CH), 1.69 (d,2 J HH =8 Hz, CH), 1.03 (s, 3H, CH3), 0.88 (s, 3H, CH3), 0.73 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ: 193.76 (C=O), 156.23, 153.14, 135.02, 134.31, 132.25, 131.66, 130.79, 130.18, 127.77, 126.26, 120.94, 114.82 (CN), 62.70 (C3), 49.80 (CH2), 42.10 (CH2), 38.42 (CH), 34.51 (CMe2), 32.02 (CH3), 29.63 (CH3), 21.11 (CH3). Anal. calcd. for C25H25N3O: C, 78.30; H, 6.57; N, 10.96 %. Found: C, 78.08; H, 6.45; N, 10.83 %.
2-amino-4-(4-bromophenyl)-7,7-dimethyl-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4f)
White crystals; IR (KBr, ν max,cm−1): 3,456, 3,328 (NH2), 2,160 (CN), 1,648 (C=O), 1,587 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 7.63–7.23 (m, 9H, CH–Ar), 5.71 (s, 2H, NH2), 4.46 (s, 1H, CH), 2.21 (d, 2 J HH =4 Hz, CH), 2.17 (d, 2 J HH =4 Hz, CH), 2.01 (d, 2 J HH =8 Hz, CH), 1.70 (d,2 J HH =8 Hz, CH), 0.88 (s, 3H, CH3), 0.73 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6) δ: 192.25 (C=O), 157.14, 154.66, 135.29, 133.27, 132.92, 131.62, 131.43, 128.36, 126.03, 122.88, 120.95, 114.95 (CN), 61.69 (C3), 49.75 (CH2), 43.22 (CH2), 38.58 (CH), 35.90 (CMe2), 31.69 (CH3), 29.50 (CH3). Anal. calcd. for C24H22BrN3O: C, 64.29; H, 4.95; N, 9.37 %. Found: C, 64.07; H, 4.78; N, 9.19 %.
2-amino-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4g)
Yellow crystals; IR (KBr, ν max,cm−1): 3,408, 3,328, 3,232 (NH2, NH), 2,176 (CN), 1,638 (C=O), 1,596 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 8.96 (s, 1H, NH), 7.27–7.12 (m, 5H, CH–Ar), 5.75 (s, 2H, NH2), 4.34 (s, 1H, CH), 2.28–1.76 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6) δ: 192.94 (C=O), 156.59, 153.98, 137.94, 133.74, 132.82, 131.70, 130.29, 113.40 (CN), 62.12 (C3), 49.10 (CH2), 42.89 (CH2), 38.58 (CH), 25.40 (CH2). Anal. calcd. for C16H15N3O: C, 72.43; H, 5.70; N, 15.84 %. Found: C, 72.29; H, 5.52; N, 15.67 %.
2-amino-5-oxo-4-(p-tolyl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4h)
Yellow crystals; IR (KBr, ν max,cm−1): 3,424, 3,328, 3,232 (NH2, NH), 2,176 (CN), 1,651 (C=O), 1,596 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 8.93 (s, 1H, NH), 7.04 (d, 2H, 3 J HH =4 Hz, CH–Ar), 7.01 (d, 2H, 3 J HH =4 Hz, CH–Ar), 5.73 (s, 2H, NH2), 4.30 (s, 1H, CH), 2.23 (s, 3H, CH3), 2.20–1.72 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6) δ: 192.58 (C=O), 155.97, 153.22, 138.00, 133.13, 132.73, 131.85, 129.64, 113.43 (CN), 62.29 (C3), 49.28 (CH2), 40.90 (CH2), 38.36 (CH), 26.12 (CH2), 21.57 (CH3). Anal. calcd. for C17H17N3O: C, 73.10; H, 6.13; N, 15.04 %. Found: C, 72.89; H, 5.98; N, 14.88 %.
2-amino-4-(4-chlorophenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4i)
Yellow crystals; IR (KBr, ν max,cm−1): 3,392, 3,328, 3,232 (NH2, NH), 2,176 (CN), 1,657 (C=O), 1,600 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 9.00 (s, 1H, NH), 7.31 (d, 2H, 3 J HH = 4 Hz, CH–Ar), 7.14 (d, 2H, 3 J HH =4 Hz, CH–Ar), 5.81 (s, 2H, NH2), 4.35 (s, 1H, CH), 2.28–1.73 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6) δ: 193.05 (C=O), 156.11, 154.01, 135.56, 133.77, 132.79, 131.57, 127.61, 114.94 (CN), 63.14 (C3), 48.92 (CH2), 41.40 (CH2), 37.29 (CH), 25.28 (CH2). Anal. calcd. for C16H14ClN3O: C, 64.11; H, 4.71; N, 11.83 %. Found: C, 63.87; H, 4.59; N, 11.69 %.
2-amino-5-oxo-1,4-diphenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4j)
Pink crystals; IR (KBr, ν max,cm−1): 3,472, 3,296 (NH2), 2,176 (CN), 1,648 (C=O), 1,590 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 7.52–7.09 (m, 10H, CH–Ar), 5.26 (s, 2H, NH2), 4.41 (s, 1H, CH), 2.21–1.49 (m, 6H, 3CH2).). 13C NMR (100 MHz, DMSO-d6) δ: 192.75 (C=O), 155.18, 153.49, 142.28, 140.33, 135.26, 131.61, 131.23, 131.08, 130.50, 125.88, 124.68, 114.06 (CN), 62.89 (C3), 49.12 (CH2), 41.75 (CH2), 38.34 (CH), 26.60 (CH2). Anal. calcd. for C22H19N3O: C, 77.40; H, 5.61; N, 12.31 %. Found: C, 77.18; H, 5.47; N, 12.13 %.
2-amino-5-oxo-1-phenyl-4-(p-tolyl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4k)
Yellow crystals; IR (KBr, ν max,cm−1): 3,472, 3,328 (NH2), 2,192 (CN), 1,638 (C=O), 1,590 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 7.61–7.11 (m, 9H, CH–Ar), 5.32 (s, 2H, NH2), 4.46 (s, 1H, CH), 2.27 (s, 3H, CH3), 2.22–1.56 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6) δ: 192.86 (C=O), 156.82, 154.27, 136.07, 135.51, 134.52, 134.14, 133.54, 131.63, 131.32, 128.85, 125.96, 115.44 (CN), 63.23 (C3), 48.69 (CH2), 41.24 (CH2), 37.69 (CH), 25.88 (CH2), 21.46 (CH3). Anal. calcd. for C23H21N3O: C, 77.22; H, 5.96; N, 11.82 %. Found: C, 77.01; H, 5.78; N, 11.69 %.
2-amino-4-(4-chlorophenyl)-5-oxo-1-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile (4l)
Brown crystals; IR (KBr, ν max,cm−1): 3,408, 3,328 (NH2), 2,176 (CN), 1,657 (C=O), 1,600 (C=C). 1H NMR (400 MHz, DMSO-d6) δ: 7.61–7.41 (m, 5H, CH–Ar), 7.39 (d, 2H, 3 J HH =4 Hz, CH–Ar), 7.30 (d, 2H, 3 J HH =4 Hz, CH–Ar), 5.41 (s, 2H, NH2), 4.51 (s, 1H, CH), 2.29–1.56 (m, 6H, 3CH2). 13C NMR (100 MHz, DMSO-d6) δ: 193.11 (C=O), 155.12, 153.68, 140.94, 135.16, 132.03, 130.87, 130.83, 129.94, 128.51, 127.47, 126.97, 115.35 (CN), 63.69 (C3), 49.28 (CH2), 41.52 (CH2), 38.14 (CH), 25.30 (CH2). Anal. calcd. for C22H18ClN3O: C, 70.30; H, 4.83; N, 11.18 %. Found: C, 70.04; H, 4.68; N, 10.99 % .
Results and discussion
There are several methods known for the synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives; these compounds were conventionally prepared using multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes [40–42]. Although, many reported methods are effective enough, the use of expensive or poisonous catalysts, low yields, tedious work-up processes, long reaction times, and hazardous conditions make them less favorable. In addition, the catalysts are not recyclable and are destroyed during the work-up procedure. Therefore, the introduction of a mild, easy, efficient, and environmentally benign method to synthesize 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives is still needed.
In order to synthesis 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, we have investigated the multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes in EtOH, in the presence of catalytic amounts of zinc oxide nanoparticles by using ultrasound irradiation (Scheme 2).
To optimize the reaction conditions, the reaction between 3-amino-5,5-dimethylcyclohex-2-enone 1a, malononitrile 2, and benzaldehyde 3a was chosen as a model reaction. When this multicomponent reaction was carried out in the presence of ZnO nanoparticles (10 mol %), in ethanol, under ultrasound irradiation and at 80 °C, the 2-amino-7,7-dimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile 4a was obtained in 92 % yield within 30 min. This reaction was also carried out in different solvents such as toluene and acetonitrile, and the best results in terms of reaction time and yield of the desired product 4a, which was obtained when the reaction was conducted in ethanol (Table 1, entries 1–3). Decreasing the catalyst loading from 10 to 4 mol % significantly lowered the yield of the reaction (Table 1, entries 4–6). The best catalyst loading was found at 10 mol %, which gave an excellent yield of 4a after only 30 min. It is worth mentioning that the reaction temperature was optimized to 80 °C in ethanol (Table 1, entries 7–9).
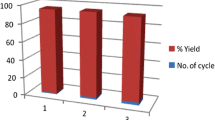
We also attempted to reuse the catalysts by a variety of methods (Table 2). Direct reuse of the catalysts (Table 2, entry 1) led to a greater than 25 % decrease in activity, while washing of the catalysts with dichloromethane, ethyl acetate, and chloroform prior to reuse also resulted in lower conversions (Table 2, entries 2–4). This phenomenon probably arose because the reactant and product were not completely desorbed from nano-ZnO and, therefore, the active sites were blocked.
According to our collected data, we decided to apply this method for synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, using a multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes under ultrasound irradiation and in the presence of Zno nanoparticles (Table 3).

Conclusions
In summary, we have reported an efficient procedure for the multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes, which leads to the synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives. This reaction was carried out in the presence of ZnO nanoparticles (10 mol %), in ethanol, by using ultrasound irradiation at 80 °C. The procedure offers several advantages including high yields, operational simplicity, and clean reaction conditions in comparison with existing methods, which makes it a useful practical process for the synthesis of these compounds.
References
T.J. Mason, J.P. Lorimer, In Sonochemistry: Theory Application and Uses of Ultrasound in Chemistry (Wiley, New York, 1988)
J.L. Luche, Synthetic Organic Sonochemistry (Plenum, New York, 1998)
H.J. Zang, M.L. Wang, B.W. Cheng, J. Song, Ultrason. Sonochem. 16, 301 (2009)
J.T. Li, M.X. Sun, Y. Yin, Ultrason. Sonochem. 17, 359 (2010)
A. Bazgir, S. Ahadi, R. Ghahremanzadeh, H.R. Khavasi, P. Mirzaei, Ultrason. Sonochem. 17, 447 (2010)
A. Dandia, R. Singh, S.L. Gupta, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1292-z
E. Chandralekha, A. Thangamani, R. Valliappan, Res. Chem. Intermed. 39, 961 (2013)
A.S. Al-bogami, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1171-7
R. Sandaroos, S. Damavandi, Res. Chem. Intermed. 39, 4167 (2013)
R.V.A. Orru, M. de Greef, Synthesis 10, 1471 (2003)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
H. Ohno, Y. Ohta, S. Oishi, N. Fujii, Angew. Chem. Int. Ed. 46, 2295 (2007)
H. Yoshida, H. Fukushima, J. Ohshita, A. Kunai, J. Am. Chem. Soc. 128, 11040 (2006)
H.A. Dondas, C.W.G. Fishwick, X. Gai, R. Grigg, C. Kilner, N. Dumrongchai, B. Kongkathip, N. Kongkathip, C. Polysuk, V. Sridharan, Angew. Chem. Int. Ed. 44, 7570 (2005)
A.R. Siamaki, B.A. Arndtsen, J. Am. Chem. Soc. 128, 6050 (2006)
E.C. Franklin, Chem. Rev. 16, 305 (1935)
F.W. Bergstrom, Chem. Rev. 35, 77 (1944)
D.M. Stout, A. Meyers, Chem. Rev. 82, 223 (1982)
R. Boer, V. Gekeler, Drugs Future 20, 499 (1995)
M. Ramesh, W.V. Matowe, J. Med. Chem. 41, 509 (1998)
S.R. Pattan, A.N. Parate, Indian J. Heterocycl. Chem. 12, 387 (2003)
Y.S. Sadanandam, M.M. Shetty, Eur. J. Med. Chem. 29, 975 (1994)
K. Cooper, M.J. Fray, J. Med. Chem. 35, 3115 (1992)
S.R. Agudoawu, E.E. Knaus, J. Heterocycl. Chem. 37, 303 (2000)
A. Shafiee, N. Rastakari, Daru 12, 81 (2004)
I.R. Ager, A.C. Barnes, G.W. Danswan, P.W. Hairsine, D.P. Kay, P.D. Kennewell, S.S. Matharu, P. Miller, P. Robson, D.A. Rowlands, W.R. Tully, R. Westwood, J. Med. Chem. 31, 1098 (1988)
S.J. Clements, G. Danswan, C.R. Gardner, S.S. Matharu, R. Murdoch, W.R. Tully, R. Westwood, J. Med. Chem. 31, 1220 (1988)
N.R. Shiju, V.V. Guliants, Appl. Catal. A Gen. 356, 1 (2009)
J. Guzman, B.C. Gates, Nano Lett. 1, 689 (2001)
B.M. Choudary, M.L. Kantam, K.V.S. Ranganath, K. Mahender, B. Sreedhar, J. Am. Chem. Soc. 126, 3396 (2004)
S. Anadan, A. Vinu, N. VenkataChalam, B. Arabindoo, V. Murugesan, J. Mol. Catal. A Chem. 256, 312 (2006)
K.J. Klabunde, R. Mulukutla, Nanoscale Materials in Chemistry (Wiley Interscience, New York, 2001)
A.J. Amali, R.K. Rana, Green Chem. 11, 1781 (2009)
R. Schlogl, S.B. Abd, Chem. Int. Ed. 43, 1628 (2003)
A.T. Bell, Science 299, 1688 (2003)
Z. Lasemi, E. Mehrasbi, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1394-7
B.M. Choudary, K. Mahendar, K.V.S. Ranganath, J. Mol. Catal. A Chem. 234, 25 (2005)
M.H. Sarvari, H. Sharghi, J. Org. Chem. 69, 2573 (2004)
F. Tamaddon, M.A. Amrollahi, L. Sharafat, Tetrahedron Lett. 46, 7841 (2005)
B.V. Lichitsky, A.A. Dudinov, M.M. Krayushkin, Arkivoc ix, 73 (2012)
S. Tu, C. Li, G. Li, L. Cao, Q. Shao, D. Zhou, B. Jiang, J. Zhou, M. Xia, J. Comb. Chem. 9, 1144 (2007)
D.S. Patel, J.R. Avalani, D.K. Raval, J. Braz. Chem. Soc. 23, 1951 (2012)
Z. Karimi-Jaberi, Z. Takmilifard, Eur. Chem. Bull. 2, 211 (2013)
M. Hosseini-Sarvari, S. Etemad, Tetrahedron 64, 5519 (2008)
Acknowledgments
The authors express their great appreciation to Kerman University of Medical Sciences, Pharmaceutics Research Center, Institute of Neuropharmacology for support of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abaszadeh, M., Seifi, M. & Asadipour, A. Ultrasound promotes one-pot synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, catalyzed by ZnO nanoparticles. Res Chem Intermed 41, 5229–5238 (2015). https://doi.org/10.1007/s11164-014-1624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1624-7