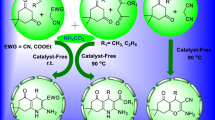
Abstract
A simple, clean, and economical methodology for the synthesis of acridine-1,8-dione and hexahydroquinoline derivatives via Hantzsch-type condensation has been described. This study highlights the development of a new green pathway for the preparation of substituted 1,4-dihydropyridines derivatives. The mild, cheap, and nontoxic potassium dihydrogen phosphate (KH2PO4) is proved to be an efficient catalyst for the above multi-component reaction to get excellent yields. Widely available and mostly benign catalyst, eco-friendly solvent, and easy purification are among the several attractive features.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acridine-1,8-diones (1,8-dioxodecahydroacridines) are an important class of nitrogen heterocyclic compounds containing a 1,4-dihydropyridine parent nucleus [1]. They are the active pharmaceutical ingredients (APIs) and versatile reactive intermediates in synthetic and medical chemistry [2]. There are many reports about them possessing a wide range of pharmaceutical activities, such as antimicrobial [3–5], antimalarial [6–8], antitumor [9], anticancer [10], antibacterial [11], fungicidal [12, 13], and DNA binding properties [14], and they are prescribed as calcium channel blockers [15]. Their derivatives have been used in chemotherapy for the treatment of cancer [6–8] and cardiovascular diseases [14]. In addition, acridinediones exhibit intense fluorescence efficiency allowing them to be used as laser dyes [16–20], photo sensitizers, and initiators [21–23]. Moreover, they have attracted much interest due to their unique photochemical and electrochemical behavior [24, 25]. There are some reports about the application of acridinediones in organic light-emitting diodes (OLED) [26]. Usual synthetic route for the synthesis of acridinediones is Hantzsch type reaction between aldehydes, β-diketones, and ammonium acetate or appropriate primary amines [27, 28] in the presence of various catalysts, like Zn(OAc)2·2H2O [18], CeCl3·7H2O [25], 4-dodecylbenzenesulfonic acid (DBSA) [29], polyphosphoric acid [30], triethylbenzylammonium chloride [31], proline [32], FeCl3-SiO2 [33], methanesulfonic acid [34], zeolite [35], N-propylsulfamic acid [36], ceric ammonium nitrate (CAN) [7], silica-bonded S-sulfonic acid [37], amberlyst-15 [38, 39], carbon-based solid acid [40], p-toluenesulfonic acid [41], InCl3 [42], MCM-41-SO3H [43], silica-supported preyssler nanoparticles [44], silica-supported polyphosphoric acid [45], In(OTf)3 [46], cetyltrimethylammonium bromide (CTAB) [47], FSG-Hf(NPf2)4 [48], tetrabutylammonium hexatungstate [49], nano-Fe3O4 [50], nano-ZnO [51], amberlite IR-120H [52], oxalic acid [53], sulfonic acid-functionalized silica [54, 55], [MIMPS]3PW12O40 [56], ionic liquids [57–63], and microwave irradiations [64–69]. Although impressive successes have been achieved, there is still a great desire for more green, general, efficient, feasible, high-yielding, and cost-effective methods using new and cheap catalysts for the synthesis of this class of compounds [70–74]. Considering the above facts, substantial investigations have been done to introduce novel catalysts and chemical reagents in the last 2 years [1, 70–83]. The Hantzsch condensation is also one of the most prominent methods to prepare polyhydroquinolines which also contain the 1,4-dihydropyridine moiety [84, 85]. Thus, the development of a green, simple, and efficient method for the synthesis of polyhydroquinoline derivatives is also an active area of research [86–90].
The green processes generally involve the use of efficient, cost-effective, and biodegradable catalysts in combination with non-toxic and non-inflammable media [79]. Recently, organic reactions conducted in the mixture of ethanol and water have attracted a great deal of attentions. Because aqueous ethanol possesses the charming properties of water such as non-toxic, inexpensive, safe, abundantly available, and environmentally benign [91, 92] and could overcome the poor ability of using pure water as a solvent to solubilize organic reactants [32]. Many organic transformations can be carried out smoothly in a water–ethanol mixture [93–96], including the preparation of acridinediones [1, 32, 48, 63, 79]. Potassium dihydrogen phosphate (KH2PO4), a mild, cheap, non-toxic, and eco-friendly inorganic salt, has been proved to be an effective catalyst in organic reactions [97–100]. Being soluble in water, this catalyst gives the reaction an easy workup and the commercial availability of this catalyst provides clean conversion [98]. As a part of our program aimed at developing useful and environment-friendly synthetic methods in the presence of KH2PO4 as catalyst [101, 102], here, we wish to extend the synthetic applicability of this catalyst in the synthesis of acridine-1,8-diones and hexahydroquinolines via a one-pot multi-component strategy of various substituted aldehydes with 1,3-dicarbonyl compounds and ammonium acetate in aqueous ethanol.
Experimental
General
Unless otherwise stated, all reagents were obtained from commercial sources and were used without further purification. Melting points were determined on a Beijing Tech X-5 melting point detector and were uncorrected. The IR spectra were measured with a Bruker Shimadzu IR-460 spectrometer. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 MHz.
Typical experimental procedure for synthesis of 4
Aromatic aldehyde (0.5 mmol), dimedone (1 mmol), ammonium acetate (0.85 mmol), KH2PO4 (0.025 mmol), and 1 mL aqueous ethanol (EtOH:H2O 3:1, v/v) were added to a 10-mL pressure tube and this solution was stirred at 120 °C for 5 h. After completion of the reaction, 0.5 mL water was added and the mixture was vigorously stirred for a moment at 120 °C. Then, the reaction mixture was cooled and filtered on a Büchner funnel. The precipitate was washed with 30% cold aqueous ethanol without further purification to afford the desired acridine-1,8-dione, except for 4n and 4p which were recrystallized from EtOH to give the pure product.
Typical experimental procedure for synthesis of 5
A similar procedure was followed for the preparation of hexahydroquinolines. A mixture of aldehyde (0.5 mmol), ethyl acetoacetate (0.5 mmol), dimedone (0.5 mmol), and ammonium acetate (1.0 mmol) in 1 mL aqueous ethanol (EtOH:H2O 3:1 v/v) was stirred at 100 °C for 5 h. On completion of the reaction, the mixture was added 0.7 mL water and vigorously stirred for a moment at 120 °C. Then, the reaction mixture was cooled and filtered on a Büchner funnel. The precipitate was washed with 30% cold aqueous ethanol without further purification to afford the desired product.
Results and discussion
As a starting point for the development of our methodology, we commenced our work to discover the best experimental conditions by choosing the three-component condensation of benzaldehyde (0.5 mmol), dimedone (1 mmol), and ammonium acetate (0.6 mmol) as a model reaction. The effects of various reaction parameters, such as solvents, catalysts, and temperature, were evaluated to optimize the reaction conditions (Table 1). The solvent screening studies revealed that aqueous ethanol is truly the most suitable solvent. Although good yield was obtained when the reaction took place in ethanol, water or acetonitrile, a higher yield was obtained using EtOH and H2O (1:1, v/v) as the solvent (Table 1, entry 5). In order to improve the yield, various volumes and ratios of EtOH and H2O were screened (Table 1, entries 6–12). This reaction with 1 mL aqueous ethanol (EtOH:H2O 3:1, v/v) gave the best yield of the desired product (Table 1, entry 10). On the basis of the above results, we examined the effects of the type and amount of the catalyst (Table 1, entries 13–20). As shown in Table 1, the reaction carried out under catalyst-free condition or with other potassium salt such as KHSO4, K2HPO4, K3PO4, and K2CO3 gave inferior results. Here, the yield could not be improved by increasing the amount of KH2PO4 (Table 1, entry 19), even while decreasing the amount of catalyst to 5 mol could lead to a slightly higher yield of 83% (Table 1, entry 18). However, the product yield dropped to 76% by further decreasing the catalyst to 2 mol% (Table 1, entry 20).
We also investigated the fate of varying the molar ratio of ammonium acetate, which is summarized in Table 2. It is clear that when increasing the reagent’s loading to 0.85 mmol (1.7 equiv. of benzaldehyde), a better outcome to afford the desired product in 94% yield was observed (Table 2, entry 3). Finally, the reaction temperature and time were tested (Table 2, entries 5–8). It was found that neither decreasing nor increasing the reaction time/temperature could improve the yield. Therefore, the reaction was optimized using a cheap, safe, and environmentally benign reaction medium and catalyst.
To probe the generality as well as the effectiveness of our newly developed protocol, a broad range of aromatic aldehydes were reacted with dimedone and ammonium acetate under identical reaction conditions to furnish the corresponding acridinediones (4a–4p) in moderate to excellent yields. As we can see in Table 3, the presence of electron-withdrawing or electron-donating substituents in the same position on the ring of various aromatic aldehydes had different influences on the procedure to furnish the desired products. The electron-rich aromatic aldehydes were transformed into the corresponding products in a slightly lower yield than that of benzaldehyde (Table 3, entries 2–7). On the other hand, the introduction of weakly electron-withdrawing substituent on the aromatic ring of aldehydes accelerated the reaction rate and a similar or slightly higher yield was observed (Table 3, entries 8–13). Unfortunately, introducing the strongly electron-withdrawing group on the phenyl ring adversely affected the outcome of the reaction. Poor yields were obtained when the nitryl was present in the ortho- or para-positions of phenyl ring (Table 3, entries 14, 16). However, employing 3-nitrobenzaldehyde as substrate gave a satisfactory result.
After successfully synthesizing a series of acridinediones derivatives in good yields, we turned our attention to the synthesis of hexahydroquinoline derivatives via Hantzsch-type reaction. Then, we found a paper reported online about the synthesis of hexahydroquinoline employing CaHPO4 as a basic catalyst which could promote the formation of nucleophilic anion [103]. As we know, KH2PO4 is a mild acid salt and will catalyze this reaction in a different mechanism from CaHPO4. Sure enough, this one-pot conversion can be efficiently catalyzed by KH2PO4 to give the corresponding hexahydroquinolines 5a–5o in good yields (Scheme 1; Table 4).
The plausible mechanism for the formation of acridine-1,8-dione is outlined in Fig. 1. According to documents reported in the literature [41, 73, 75] and our experience [102], we suggest that adding water can accelerate the dissociation of dimedone to generate the nucleophilic species, due to water having a high dielectric constant [104]. On the other hand, the mild acid KH2PO4 can not only activate the carbonyl group of aldehyde but also help to form the dehydration products 1, 3, and 4 [105].
Conclusions
In summary, we have demonstrated that KH2PO4 is an efficient catalyst and that aqueous ethanol is the valid solvent for the synthesis of acridine-1,8-dione and hexahydroquinoline derivatives via Hantzsch reaction of various aromatic aldehydes with ammonium acetate and 1,3-dicarbonyl compounds. This process does not require the use of hazardous organic solvent, complicated and expensive catalysts, or tedious experimental and isolation procedures.
References
M. Nasr-Esfahani, Z. Rafiee, H. Kashi, J. Iran. Chem. Soc. 13, 1449 (2016)
S. Tu, C. Miao, Y. Gao, F. Fang, Q. Zhuang, Y. Feng, D. Shi, Synlett 2, 255 (2004)
L. Ngadi, A.M. Galy, J.P. Galy, J. Barbe, A. Cremieux, J. Chevalier, D. Sharples, Eur. J. Med. Chem. 25, 67 (1990)
Y.M. Shchekotikhin, T.G. Nikolaeva, G.M. Shub, A.P. Kriven’ko, Pharm. Chem. J. 35, 206 (2001)
A.N. Pyrko, Russ. J. Org. Chem. 44, 1215 (2008)
K. Palani, P. Ambalavanan, M.N. Ponnuswamy, P. Murugan, V.T. Ramakrishnan, Cryst. Res. 40, 277 (2005)
M. Kidwai, D. Bhatnagar, Tetrahedron Lett. 51, 2700 (2010)
M. Kawase, A. Shah, H. Gaveriya, N. Motohashi, H. Sakagami, A. Varga, J. Molnar, Bioorg. Med. Chem. 10, 1051 (2002)
S. Tu, X. Zhang, F. Shi, T. Li, Q. Wang, X. Zhu, J. Zhang, J. Xu, J. Heterocycl. Chem. 42, 1155 (2005)
S.A. Gamage, J.A. Spicer, G.J. Atwell, G.J. Finlay, B.C. Baguley, W.A. Denny, J. Med. Chem. 42, 2383 (1999)
K. Palani, D. Thirumalai, P. Ambalavanan, M.N. Ponnuswamy, V.T. Ramakrishnan, J. Chem. Crystallogr. 35, 751 (2005)
M.J. Wainwright, Antimicrob. Chemother. 47, 1 (2001)
A. Srivastava, C. Nizamuddin, Indian J. Heterocycl. Chem. 13, 261 (2004)
R.A. Janis, D.J. Triggle, J. Med. Chem. 26, 775 (1983)
O. Berkan, B. Sarac, R. Simsek, S. Yildirim, Y. Sariogli, C. Safak, Eur. J. Med. Chem. 37, 519 (2002)
P. Shanmugasundaram, K.J. Prabahar, V.T. Ramakrishnan, J. Heterocycl. Chem. 30, 1003 (1993)
N. Srividya, P. Ramamurthy, P. Shanmugasundaram, V.T. Ramakrishnan, J. Org. Chem. 61, 5083 (1996)
S. Balalaie, F. Chadegani, F. Darviche, H.R. Bijanzadeh, Chin. J. Chem. 27, 1953 (2009)
L.B. Li, S.J. Ji, Y. Liu, Chin. J. Chem. 26, 979 (2008)
M. Kaya, Y. Yildirir, L. Turker, J. Heterocycl. Chem. 46, 294 (2009)
V. Thiagarajan, P. Ramamurthy, D. Thirumalai, V.T. Ramakrishnan, Org. Lett. 7, 657 (2005)
P. Shanmugasundaram, P. Murugan, V.T. Ramakrishnan, N. Srividya, P. Ramamurthy, Heteroat. Chem. 7, 17 (1996)
H.J. Timpe, S. Ulrich, C. Decker, J.P. Fouassier, Macromolecules 26, 4560 (1993)
H. Mohan, N. Srividya, P. Ramamurthy, J.P. Mittal, J. Chem. Soc. Faraday Trans. 92, 2353 (1996)
X. Fan, Y. Li, X. Zhang, G. Qu, J. Wang, Heteroat. Chem. 18, 786 (2007)
L. Türker, A. Tapan, S. Gumus, Polycyclic Aromat. Compd. 29, 139 (2009)
S.J. Tu, Z. Lu, D. Shi, C. Yao, Y. Gao, C. Guo, Synth. Commun. 32, 2181 (2002)
G.W. Wang, C.B. Miao, Green Chem. 8, 1080 (2006)
T.S. Jin, J.S. Zhang, T.T. Guo, A.Q. Wang, T.S. Li, Synthesis 12, 2001 (2004)
H.G. Bonacorso, R.L. Drekener, I.R. Rodrigues, R.P. Vezzosi, M.B. Costa, J. Fluorine Chem. 126, 1384 (2005)
X.S. Wang, D.Q. Shi, Y.F. Zhang, S.H. Wang, S.J. Tu, Chin. J. Org. Chem. 24, 430 (2004)
K. Venkatesan, S.S. Pujari, K.V. Srinivasan, Synth. Commun. 39, 228 (2009)
H.R. Shaterian, A. Hosseinian, M. Ghashang, Phosphorus, Sulfur Silicon Relat. Elem. 183, 3136 (2008)
Y.B. Shen, G.W. Wang, Arkivoc xvi, 1 (2008)
M. Nikpassand, M. Mamaghani, K. Tabatabaein, Molecules 14, 1468 (2009)
F. Rashedian, D. Saberi, K.J. Niknam, Chin. Chem. Soc. 57, 1006 (2010)
K. Niknam, F. Panahi, D. Saberi, M. Mohagheghnejad, J. Heterocycl. Chem. 47, 292 (2010)
B. Das, P. Thirupathi, I. Mahender, V.S. Reddy, Y.K. Rao, J. Mol. Catal. A: Chem. 247, 233 (2006)
M. Kaya, Y. Yildirir, G.Y. Celik, Med. Chem. Res. 20, 293 (2011)
A. Davoodnia, A. Khojastehnezhad, N. Tavakoli-Hoseini, Bull. Korean Chem. Soc. 32, 2243 (2011)
A. Jamalin, R. Miri, O. Firuzi, M. Amini, A.A. Moosavi-Movahedi, A. Shafiee, J. Iran. Chem. Soc. 8, 983 (2011)
S.M. Vahdat, S. Baghery, Heterocycl. Lett. 2, 43 (2012)
S. Rostamizadeh, A. Amirahmadi, N. Shadjou, A.M. Amani, J. Heterocycl. Chem. 49, 111 (2012)
A. Javid, A. Khojastehnezhad, M. Heravi, F.F. Bamoharram, Synth. React. Inorg. Met. Org. 42, 14 (2012)
F. Moeinpour, A. Khojastehnezhad, Eur. J. Chem. 9, 504 (2012)
Q.H. To, Y.R. Lee, S.H. Kim, Bull. Korean Chem. Soc. 33, 1170 (2012)
J.J. Xia, K.H. Zhang, Molecules 17, 5339 (2012)
M. Hong, G. Xiao, J. Fluorine Chem. 144, 7 (2012)
A. Davoodnia, A. Zare-Bidaki, H. Behmadi, Chin. J. Catal. 33, 1797 (2012)
M.A. Ghasemzadeh, J. Safaei-Ghomi, H. Molaei, C. R. Chimie 15, 969 (2012)
J. Safaei-Ghomi, M.A. Ghasemzadeh, S. Zahedi, J. Mex. Chem. Soc. 57, 1 (2013)
A. Nakhi, P.T.V.A. Srinivas, M.S. Rahman, R. Kishore, G.P.K. Seerapu, K.L. Kumar, D. Haldar, M.V.B. Rao, M. Pal, Bioorg. Med. Chem. Lett. 23, 1828 (2013)
J.N. Sangshetti, P.P. Dharmadhikari, R.S. Chouthe, B. Fatema, Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2012.06.005
G.M. Ziarani, S. Mousavi, Iran. J. Chem. Chem. Eng. 32, 9 (2013)
G.M. Ziarani, A. Badiei, M. Hassanzadeh, S. Mousavi, Arab. J. Chem. 7, 335 (2014)
S.M. Vahdat, S. Khaksar, M. Akbari, S. Baghery, Arab. J. Chem. (2014). doi:10.1016/j.arabjc.2014.10.026
Y.L. Li, M.M. Zhang, X.S. Wang, D.Q. Shi, S.J. Tu, X.Y. Wei, Z.M. Zong, J. Chem. Res. 2005, 600 (2005)
D.Q. Shi, S.N. Ni, F. Yang, J.W. Shi, G.L. Dou, X.Y. Li, X.S. Wang, J. Heterocycl. Chem. 45, 653 (2008)
M. Dabiri, M. Baghbanzadeh, E. Arzroomchilar, Catal. Commun. 9, 939 (2008)
W. Shen, L.M. Wang, H. Tian, J. Tang, J.J. Yu, J. Fluorine Chem. 6, 522 (2009)
H. Alinezhad, M. Tajbakhsh, M. Norouzi, A. Baghery, J. Rakhtshah, J. Chem. Sci. 125, 1517 (2013)
A.K. Dutta, P. Gogoi, R. Borah, RSC Adv. 4, 4128 (2014)
D. Patil, D. Chandam, A. Mulik, P. Patil, S. Jagadale, R. Kant, V. Gupta, M. Deshmukh, Catal. Lett. 144, 949 (2014)
S.J. Tu, C.B. Miao, Y. Gao, Y.J. Feng, J.C. Feng, Chin. J. Org. Chem. 20, 703 (2002)
S.J. Tu, Y. Gao, C.B. Miao, T.J. Li, X.J. Zhang, S.L. Zhu, F. Fang, D.Q. Shi, Synth. Commun. 34, 1289 (2004)
Z.Q. Tang, Y. Chen, C.N. Liu, K.Y. Cai, S.J. Tu, J. Heterocycl. Chem. 47, 363 (2010)
S.K. Singh, K.N. Singh, J. Heterocycl. Chem. 48, 69 (2011)
M.G. Gündüz, F. İşli, A. El-Khouly, Ş. Yıldırım, G.S.Ö. Fincan, R. Şimşek, C. Şafak, Y. Sarıoğlu, S.Ö. Yıldırım, R.J. Butcher, Eur. J. Med. Chem. 75, 258 (2014)
A.A. Abdelhamid, S.K. Mohamed, A.M. Maharramov, A.N. Khalilov, M.A. Allahverdiev, J. Saudi Chem. Soc. 18, 474 (2014)
Z. Nasresfahani, M.Z. Kassaee, Catal. Commun. 60, 100 (2015)
A.V. Borhade, B.K. Uphade, A.G. Gadhave, Res. Chem. Intermed. 41, 1447 (2015)
P. Mahesh, K. Guruswamy, B.S. Diwakar, B.R. Devi, Y.L.N. Murthy, P. Kollu, S.V.N. Pammi, Chem. Lett. 44, 1386 (2015)
H. Alinezhad, M. Tajbakhsh, N. Ghobadi, Res. Chem. Intermed. 41, 9979 (2015)
A. Khojastehnezhad, F. Moeinpour, M. Vafaei, J. Mex. Chem. Soc. 59, 29 (2015)
A. Davoodnia, H. Norouzi, N. Tavakoli-Hoseini, A. Zare-Bidaki, Synth. React. Inorg. Met. Org. 44, 70 (2014)
M. Mokhtary, S.A.M. Langroudi, Monatsh. Chem. 145, 1489 (2014)
B. Banerjee, G. Brahmachari, J. Chem. Res. 38, 745 (2014)
I.A. Khodja, W. Ghalem, Z.I. Dehimat, R. Boulcina, B. Carboni, A. Debache, Synth. Commun. 44, 959 (2014)
H.R. Safaei, M. Safaei, M. Shekouhy, RSC Adv. 5, 6797 (2015)
S.M. Baghbanian, G. Khanzad, S.M. Vahdat, H. Tashakkorian, Res. Chem. Intermed. 41, 9951 (2015)
A. Nakhaei, A. Davoodnia, A. Morsali, Res. Chem. Intermed. 41, 7815 (2015)
S. Pradhan, B.G. Mishra, RSC Adv. 5, 86179 (2015)
E. Eidi, M.Z. Kassaee, Z. Nasresfahani, Appl. Organomet. Chem. 29, 793 (2015)
S. Otokesh, N. Koukabi, E. Kolvari, A. Amoozadeh, M. Malmir, S. Azhari, S. Afr. J. Chem. 68, 15 (2015)
M. Saha, A.K. Pal, Tetrahedron Lett. 52, 4872 (2011)
B. Maleki, R. Tayebee, M. Kermanian, S.S. Ashrafi, J. Mex. Chem. Soc. 57, 290 (2013)
N.G. Khaligh, Chin. J. Catal. 35, 1497 (2014)
S. Igder, A.R. Kiasat, M.R. Shushizadeh, Res. Chem. Intermed. 41, 7227 (2015)
B.L. Li, A.G. Zhong, A.G. Ying, J. Heterocycl. Chem. 52, 445 (2015)
A. Amoozadeh, S. Rahmani, M. Bitaraf, F.B. Abadi, E. Tabrizian, R. Soc. Chem. 40, 770 (2016)
S. Ghosh, J. Das, S. Chattopadhyay, Tetrahedron Lett. 52, 2869 (2011)
F.N. Sadeh, M.T. Maghsoodlou, N. Hazeri, M. Kangani, Res. Chem. Intermed. 41, 5907 (2015)
G. Brahmachari, B. Banerjee, ACS Sustain. Chem. Eng. 2, 411 (2014)
D.R. Chandam, A.G. Mulik, P.P. Patil, S.D. Jagdale, D.R. Patil, M.B. Deshmukh, Res. Chem. Intermed. 41, 761 (2015)
N. Shabalala, S. Maddila, S.B. Jonnalagadda, New J. Chem. 40, 5107 (2016)
M. Adib, Z. Yasaei, P. Mirzaei, Synlett 27, 383 (2016)
A. Saikia, M.G. Barthakur, M. Borthakur, C.J. Saikia, U. Bora, R.C. Boruah, Tetrahedron Lett. 47, 43 (2006)
R.S. Joshi, P.G. Mandhane, M.U. Shaikh, R.P. Kale, C.H. Gill, Chin. Chem. Lett. 21, 430 (2010)
P.G. Mandhane, R.S. Joshi, D.R. Nagargoje, C.H. Gill, Tetrahedron Lett. 51, 1490 (2010)
S. Damavandi, R. Sandaroos, Res. Chem. Intermed. 39, 1251 (2013)
C. Lyu, Y. Liu, J. Wang, X. Zhou, Chin. J. Appl. Chem. 32, 1371 (2015)
S. Gao, D. Xiao, Y. Yang, X. Wei, S. Sun, J. Lang, C. Lv, Heterocycles 92, 1698 (2016)
M.A. Bodaghifard, M. Solimannejad, S. Asadbegi, S. Dolatabadifarahani, Res. Chem. Intermed. 42, 1165 (2016)
F. Bigi, S. Carloni, L. Ferrari, R. Maggi, A. Mazzacani, G. Sartori, Tetrahedron Lett. 42, 5203 (2001)
S. Tu, F. Fang, S. Zhu, T. Li, X. Zhang, Q. Zhuang, Synlett 2004, 537 (2004)
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21403100) and Liaoning Province, the Doctoral Scientific Research Foundation (20141100).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yü, SJ., Wu, S., Zhao, XM. et al. Green and efficient synthesis of acridine-1,8-diones and hexahydroquinolines via a KH2PO4 catalyzed Hantzsch-type reaction in aqueous ethanol. Res Chem Intermed 43, 3121–3130 (2017). https://doi.org/10.1007/s11164-016-2814-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2814-2