Abstract
The seaweed industry has developed rapidly over the last decade, and carrageenan is the leading hydrocolloid in the seaweed industry. Approximately 57,500 t of carrageenan is produced annually throughout the world. As a consequence of the increase in carrageenan production, the enormous amount of waste resulting from the carrageenan industry has also increased. This study investigated the possibility of ethanol production using carrageenan solid waste from the carrageenan extraction of Kappaphycus alvarezii, the principal species used in the carrageenan industry. Optimum acid hydrolysis followed by enzymatic hydrolysis enhanced the production of both galactose and glucose. Fermentation of the enzymatic hydrolysate using Saccharomyces cerevisiae ATCC 200062 resulted in an ethanol yield of 13.8 g L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The seaweed industry which has developed rapidly over the past decade uses the commercial exploitation of seaweed as a food and as a source of three hydrocolloids: carrageenan, agar, and alginate (McHugh 2003). Of these three principle hydrocolloids, carrageenan has the highest production and sales value. The carrageenan industry supports a wide variety of products in the food, cosmetic, pharmaceutical, and dairy industries that have an estimated total annual value of US$ 518 million (Porse and Rudolph 2017).
Carrageenan is the generic name for a hydrocolloid consisting of sulfated galactan with an alternating backbone consisting of α(1-4)-anhydro-d-galactose and β(1-3)-d-galactose (Jol et al. 1999). The word “carrageenan” originates from “carrageenin,” which was used for the first time in 1829 by Stanford, a British pharmacist (Pereira et al. 2009). Carrageenans can be divided into three principal types based on their chemical structure and properties: kappa, iota, and lambda. Kappa carrageenans encompass the strongest gels of all carrageenans; hence, kappa carrageenans are important in the food, dairy, and pharmaceutical industries, where they are used as thickeners, gelling agents, and stabilizers. Most carrageenan is commercially extracted from Kappaphycus alvarezii (“cottonii”), the principal species producer of kappa carrageenans. Previous studies have shown that K. alvarezii is one of the most promising carrageenophytes for bioethanol production (Meinita et al. 2012; Khambhaty et al. 2012; Hargreaves et al. 2013). However, the utilization of K. alvarezii for bioethanol faces challenges due to biomass availability and other commercial uses as a carrageenan resource.
Large producers of carrageenan, such as CPKelco, FMC, and Cargill, are located in Europe and the US; small- to medium-size producers are located in Indonesia and the Philippines (Pambudi et al. 2010; Bixler and Porse 2011). World carrageenan production exceeds 57,500 t each year (Porse and Rudolph 2017). A significant increase in production has been observed in the carrageenan market. As a consequence of this increase in carrageenan production, the enormous amount of solid waste produced by the carrageenan industry has also been increasing each year. Solid waste resulting from carrageenan extraction may contain a large amount of polysaccharide materials. Management of solid waste resulted from industrial is a challenge, since these wastes are discarded directly to the environment which might cause several environmental problems (Guerrero et al. 2013; Marshall and Farahbakhsh 2013). However, the solid waste resulting from the carrageenan industry is not being utilized. Instead, this waste is discarded directly into the environment, which could cause several environmental problems. The utilization of carrageenan waste for bioethanol production may be a biorefinery approach that can be applied in the carrageenan industry. This study investigated the use of sequential acid hydrolysis, enzymatic hydrolysis, and fermentation of carrageenan waste from K. alvarezii for bioethanol production.
Materials and methods
Carrageenan waste material
Carrageenan waste materials are solid wastes resulting from the carrageenan extraction of the carrageenophyte, Kappaphycus alvarezii. This species is the most common raw material and is widely used in the carrageenan industry. Carrageenan waste materials collected from carrageenan extraction were freeze-dried and used for subsequent analyses.
Carrageenan extraction
The extraction of carrageenan from K. alvarezii was carried out by the alkaline method using 2 kg of dried carrageenan waste material. The wastes were boiled in 60% KOH solution and maintained at 80 °C in a water bath for 3 h. The extracts were then frozen overnight. After thawing, the extracts were washed with fresh water to remove any residue and dried in an oven (Ohno et al. 1994). Carrageenan waste was collected and used for the next step.
Carrageenan yield was determined by the following equation:
Acid hydrolysis
Acid hydrolysis was performed in a 250-mL flask by optimizing the H2SO4 concentration (0–1 M), carrageenan waste concentration (0–12%), hydrolysis time (0–45 min), and hydrolysis temperature (0–130 °C). The optimum acid hydrolysis conditions were then used for the next step.
Enzymatic hydrolysis
The carrageenan waste pretreated with acid hydrolysis was subsequently used for enzymatic hydrolysis. Optimization of the enzymatic hydrolysis of carrageenan waste was carried out for enzyme type, enzyme dosage, and incubation period. Four commercial enzymes—Celluclast, Viscozyme, Cellic C Tec II, and Cellic H Tec II—and five mixed enzymes—Celluclast + Viscozyme (1:1), Cellic C tec II + Cellic H tec II (1:1), Viscozyme + Cellic H tec II (1:1), Celluclast + Cellic H Tec II, and Cellic C Tec II + Viscozyme + Cellic H Tec II (1:1:0.1)—were purchased from Sigma (Novozymes, Denmark) and added to the carrageenan waste. Different concentrations of enzyme (0, 5, 10, 20, 30, and 40%) were applied to the acid hydrolysate of carrageenan waste. Optimization of the incubation period was performed by incubating carrageenan waste hydrolysate and measuring glucose and galactose production at 0, 3, 6, 9, 12, 24, 36, and 72 h. The pH of pretreated carrageenan waste was adjusted to 5 prior to enzymatic hydrolysis. Enzymatic hydrolysis was carried out at 50 °C in a water bath shaker at 130 rpm for 72 h. Enzymatic hydrolysates were evaluated by measuring the sugar content by high-performance liquid chromatography (HPLC) and used for the subsequent steps.
Microorganisms and medium
Prior to fermentation, hydrolysate samples were neutralized using 10 N NaOH to reach a pH = 5. Saccharomyces cerevisiae ATCC 200062 was used for fermentation. The inoculum culture was prepared in yeast medium containing 10 g yeast extract, 6.4 g urea, and 20 g glucose per liter. The yeast suspension was incubated at 30 °C with shaking at 130 rpm in an incubator. Fermentation was performed in triplicate in 100-mL Erlenmeyer flasks with a working volume of 50 mL. Samplings were conducted periodically to measure sugar and bioethanol production (Meinita et al. 2012, 2015).
Sugar, by-product, and ethanol determination
Monosaccharides (galactose and glucose) were measured by HPLC on an Alltech IOA 1000 organic acid column (300 × 7.8 mm) equipped with a refractive index detector. The column temperature was maintained at 60 °C. Ethanol production was measured using a Gas Chromatography (GC) Agilent model 6890N Series with a 2B-WAX column (Agilent Technologies, USA). The injection volume was 2 μL with an inlet split ratio of 30:1. The initial oven temperature was set at 35 °C, which can reach a maximum temperature of 250 °C. Bioethanol yield was calculated based on the following equation:
where YP/S is ethanol yield (g g−1), [EtOH]max is the highest concentration of bioethanol obtained during fermentation (g L−1), and [Sugar]ini is the total initial sugar concentration at the onset of fermentation (g L−1). The percent theoretical yield was calculated based on the following equation:
where Y% is the percent theoretical yield (%) and 0.51 is the maximum ethanol yield per unit of hexose sugar from glycolytic fermentation (g g−1).
Results and discussion
Carrageenan is the main polysaccharide found in K. alvarezii. Over 50,000 t of carrageenan is produced each year throughout the world, with a higher sales volume and sales value compared to the alginate and agar industries (Table 1). In this study, carrageenan yield was 32.95 ± 1.43% (Table 2), which is comparable to that reported elsewhere for the same alga (Ohno et al. 1994; Hayashi et al. 2007; Hayashi et al. 2011; de Góes and Reis 2012; Periyasamy et al. 2014). Carrageenan yields of 25.4–35.3% and 15–28% have been reported by Hayashi et al. (2011) and Hayashi et al. (2007), whereas Ohno et al. (1994) reported yields of 27.6–58.8% in Japan and the Philippines (Ohno et al. 1994; Hayashi et al. 2011). A carrageenan yield of 29.10–31.00% has been reported for K. alvarezii in India, while a yield of 46.1% has been recorded for K. alvarezii in Brazil (de Góes and Reis 2012; Periyasamy et al. 2014). Thus, carrageenan yield may vary according to seaweed strain, environmental parameters, geographical location, and extraction method. Some environmental parameters, such as water temperature and salinity, may also influence carrageenan yield (Hayashi et al. 2011; de Góes and Reis 2012).
In this study, the amount of carrageenan waste was 30.52 ± 0.79% (Table 2). Hence, we assumed that 30.52% or 15,260 t of seaweed raw material, which could be used as raw material for bioethanol production, remains as waste produced from the carrageenan industry each year.
Acid hydrolysis
Pretreatment is an important process for converting the structural characteristics of polysaccharides contained in carrageenan waste. This process is aimed at breaking down polysaccharides into simpler sugars and enhancing the effectiveness of enzymatic hydrolysis. The selection of a pretreatment method plays an important role, as it significantly affects the subsequent enzymatic hydrolysis and fermentability of the hydrolysate.
Dilute acid hydrolysis has been successfully applied to the pretreatment or hydrolysis of seaweed materials (Meinita et al. 2012, 2013, 2015). The benefits of using dilute sulfuric acid hydrolysis are its high reaction rates and the efficiency with which it breaks down seaweed polysaccharides. A high temperature is favorable as it produces a high yield of sugar decomposition. In this study, acid pretreatment was optimized by applying different H2SO4 concentrations (0–1 M), amounts of carrageenan waste (0–12%), hydrolysis times (0–45 min), and hydrolysis temperatures (0–130 °C).
Effect of H2SO4 concentration on glucose and galactose content
The effect of H2SO4 concentration on the hydrolysis of carrageenan waste was determined (Fig. 1a). Among the different H2SO4 concentrations tested, the highest production of galactose and glucose was obtained with 0.2 M H2SO4 under standard conditions of hydrolysis at 120 °C for 15 min (13.43 ± 1.3 g L−1 galactose and 0.30 ± 0.019 g L−1 glucose). At lower and higher concentrations, galactose and glucose concentrations decreased. This finding is in agreement with the effect of acid concentration on seaweed hydrolysis observed by Meinita et al. (2012, 2015). Thus, the higher acid concentration results in a lower amount of sugar released.
Acid hydrolysis optimization of carrageenan waste by various parameters. a Effect of H2SO4 concentrations on glucose and galactose content. b Effect of carrageenan waste concentrations on glucose and galactose content. c Effect of hydrolysis time on glucose and galactose content. d Effect of hydrolysis temperature on glucose and galactose content. Values represent the mean ± SD (n ≥ 3)
Effect of carrageenan waste concentration on glucose and galactose content
Various concentrations of carrageenan waste were treated under standard conditions of hydrolysis at 120 °C for 15 min with 0.2 M H2SO4 (Fig. 1b). The optimum amount of carrageenan waste material was determined to be 10%. When the carrageenan waste concentration was increased from 8 to 10%, the glucose obtained increased from 0.178 ± 0.02 to 0.285 ± 0.013 g L−1 and the galactose obtained increased from 7.73 ± 0.44 to 13.70 ± 2.07 g L−1. When the concentration of carrageenan waste increased to 12%, the glucose and galactose content decreased. However, with the subsequent increase in carrageenan waste concentration, the galactose and glucose content dropped dramatically.
Effect of hydrolysis time on glucose and galactose content
The optimum hydrolysis time with 0.2 M H2SO4 was 15 min under standard conditions of hydrolysis at 120 °C with 10% carrageenan waste (Fig. 1c). Increasing the hydrolysis time to longer than 15 min resulted in decreased production of galactose and glucose, suggesting that longer reaction times may degrade the sugar compounds and produce more by-product inhibitors, such as 5-hydroxy-methyl-furfural and levulinic acid (Meinita et al. 2012, 2013).
Effect of hydrolysis temperature on glucose and galactose content
The optimum galactose (13.43 ± 0.58 g L−1) and glucose (0.37 ± 0.004 g L−1) content was achieved at 120 °C for 15 min with 0.2 M H2SO4 and 10% carrageenan waste as the standard conditions. Thus, we concluded that increasing the temperature above 120 °C tends to decrease the production of both glucose and galactose.
Enzymatic hydrolysis
Enzymatic hydrolysis is an important process in bioethanol production. In this process, polysaccharides are hydrolyzed into simpler saccharides. The factors that affect enzymatic hydrolysis include the type of enzyme, the enzyme concentration, and the incubation time. In enzymatic hydrolysis, a mild pH and temperature are applied. These mild conditions will not produce toxic and corrosive by-product compounds, as seen in acid hydrolysis (Alvira et al. 2010). However, there are some important factors that may influence enzymatic hydrolysis. In this study, we considered these factors by optimizing the type of enzyme, the enzyme concentration, and the enzyme incubation time. Enzymatic hydrolysis was performed sequentially to acid hydrolysis.
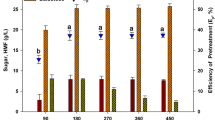
Optimization of the enzymatic hydrolysis of carrageenan waste is shown in Fig. 2. Among the different enzymes tested on carrageenan waste from the carrageenan extraction of K. alvarezii, the mixed enzyme of Cellic C tec II + Viscozyme + Cellic H tec II (1:1:0.1) was more effective than the single enzymes (Fig. 2a). The optimum galactose and glucose contents of carrageenan waste from the carrageenan extraction of K. alvarezii that saccharified using a mixed enzyme were 18.00 ± 0.50 g L−1 and 25.68 ± 0.01 g L−1, respectively. Hence, this mixed enzyme was selected for further research to determine the optimum enzyme concentration and incubation time of carrageenan waste from the carrageenan extraction of K. alvarezii.
Substrate concentration is also an important variable that has a significant effect on enzymatic hydrolysis. The optimization of enzyme concentration is shown in Fig. 2b. In general, when the substrate concentration was gradually increased from 0% (w/v) to 20% of the biomass weight, the glucose yield increased gradually. This phenomenon indicates the availability of more cellulose that can be hydrolyzed to glucose. When the substrate concentration was increased from 20% (w/v) to 40% (w/v), there was a decrease in glucose and galactose yields. The optimum galactose and glucose contents of carrageenan waste from the carrageenan extraction of K. alvarezii at a 20% enzyme concentration were 17.50 ± 0.50 and 25.33 ± 0.01 g L−1, respectively. Hence, we concluded that 20% was the optimum enzyme concentration. When the substrate concentration was increased from 20% (w/v) to 30% (w/v), there was a significant decrease in glucose yield. This optimum concentration occurs when the substrate concentration increases above its optimum value, thus becoming a limiting factor. A higher enzyme concentration may increase the viscosity of the hydrolysate, which would subsequently result in end-product inhibition and mass transfer limitations within the reaction mixture (i.e., it will produce a low glucose yield).
The effect of enzyme incubation time is shown in Fig. 2c. The time course of the enzymatic hydrolysis of carrageenan waste from the carrageenan extraction of K. alvarezii revealed an increase in glucose and galactose release at 24 h, which remained almost constant thereafter. However, the patterns of glucose and galactose release differed in the enzymatic hydrolysis of carrageenan waste. Glucose increased significantly, whereas galactose remained relatively constant. Based on this result, we conclude that enzymatic hydrolysis is more efficient at increasing glucose in carrageenan waste from the carrageenan extraction of K. alvarezii than is galactose. Sequential acid and enzymatic hydrolysis effectively convert polysaccharides into sugar and increase the sugar content in the carrageenan waste hydrolysate. Carrageenan waste from the carrageenan extraction of K. alvarezii showed a different pattern in enzymatic hydrolysis, and this may have been caused by the different compositions of the compounds; hence, the enzymes reacted differently. Thus, the combination of different types of enzyme, different enzyme concentrations, and different incubation times used in enzymatic hydrolysis greatly affects the production of sugars and the efficiency of ethanol production.
Sugars and by-products before and after enzymatic hydrolysis
The comparison of glucose and galactose production after acid hydrolysis and enzymatic hydrolysis is shown in Table 2. We found a significant increase in glucose after enzymatic hydrolysis. There was also an increase in galactose after enzymatic hydrolysis, although the increase was not significantly relative to glucose. However, one drawback of acid hydrolysis is the formation of hydroxymethylfurfural (HMF) and levulinic acid. HMF is a toxic compound that results from the degradation of hexoses, while levulinic acid is formed by the degradation of HMF. HMF and levulinic acid were formed during acid hydrolysis. After enzymatic hydrolysis, we found a decrease in both HMF and levulinic acid (Table 2). However, the amounts of HMF and levulinic detected in our study after enzymatic hydrolysis were much lower than those reported previously (Table 3). We also found that the level of glucose produced from our acid and enzymatic hydrolysis was higher than that in previous studies. Thus, we concluded that acid hydrolysis followed by enzymatic hydrolysis is a suitable method that can produce a high glucose yield and generate a small amount of by-product compounds.
Fermentation and ethanol production
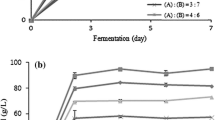
In this study, we examined the time course of main bioethanol fermentation from 50 mL enzymatic hydrolysate of carrageenan waste fermented using S. cerevisiae ATCC 200062 (Fig. 3). Our previous study found that S. cerevisiae ATCC 200062 performs well in producing bioethanol from red seaweed. The hydrolysate containing 25.89 g L−1 glucose and 17.26–25.89 g L−1 galactose was fermented using S. cerevisiae ATCC 200062 and produced 13.78 g L−1 of ethanol in 12 h.
Time course of ethanol production, galactose, and glucose during fermentation of sequential acidic-enzymatic hydrolysate of carrageenan waste. Fermentation was performed in in 100-mL Erlenmeyer flask at 30 °C. Samples were taken periodically for measurement of sugar and bioethanol production. Values represent the mean ± SD (n ≥ 3)
In the ethanol fermentation of carrageenan waste hydrolysate of K. alvarezii, the ethanol production rate increased slowly in the early phase but increased rapidly after 9 h. The ethanol production rate reached a maximum after 12 h of fermentation, and the maximum ethanol yield of 0.35 g g−1 sugars was also obtained after 12 h. The glucose was exhausted after 12 h, while galactose continued to decrease slowly and remained steady until 96 h of fermentation. The maximum ethanol concentration of 13.78 g L−1 was obtained with 69% of the theoretical yield.
The ethanol production achieved in this study was higher than that reported by previous studies on bioethanol production from seaweed (Table 3). This might be related to carrageenan content as fermentable polysaccharide in carrageenan waste, the effectiveness of hydrolysis and fermentation method. Acid and enzymatic hydrolysis play an important role in converting the structural characteristic of polysaccharide, enhancing fermentable sugar production, which will also affect ethanol production. Hence, the selection of hydrolysis is a crucial step which might determine the fermentable sugar yield and ethanol production. The major polysaccharide found in carrageenan waste hydrolysate of K. alvarezii is classified as kappa carrageenan. Chemically, kappa carrageenan consists of repeating d-galactose units, 3,6-anhydrogalactose and sulfated at C4 in the 1,3-linked galactose ring (Jol et al. 1999). Acid hydrolysis breakdown the complexity of kappa carrageenan which still remained in carrageenan waste to fermentable sugars and making remained kappa carrageenan more workable to the enzyme hydrolysis. Combination of acid and enzymatic hydrolysis resulted in higher glucose. Compared to galactose, glucose is the preferred carbon source for S. cerevisiae ATCC 200062 to produce ethanol (Meinita et al. 2017a). Glucose was thoroughly consumed by S. cerevisiae ATCC 200062 while galactose was consumed partially and remained until the end of fermentation. Sequential acid and enzymatic hydrolysis appear as the best method to optimize the glucose; hence, the ethanol production in this study is higher than the previous study which mostly only uses acid or enzymatic hydrolysis.
Biorefinery approach
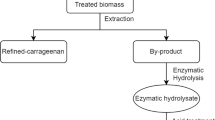
A biorefinery approach can be developed from K. alvarezii biomass and its carrageenan waste. We can extract chemicals from K. alvarezii for use in food, dairy, pharmaceuticals, cosmetics, pet food, soil fertilizers, and other bioproducts. While carrageenan waste can be utilized for bioethanol production, it contains rich sulfated galactans that can be converted into simple sugars by sequential acid and enzymatic hydrolysis. The by-products resulting from bioethanol production contain large amounts of organic matter and useful minerals that can be used as soil fertilizer or pet food (Fig. 4).
From the industrial point of view, it is very important to consider many aspects in the bioethanol process. The biggest challenges of large-scale bioethanol production are technology and the availability and sustainability of raw material. The present study showed that sequential acid and enzymatic hydrolysis followed by ethanol fermentation by S. cerevisiae ATCC 200062 can be used as an efficient technology to produce bioethanol form carrageenan waste. According to Porse and Rudolph (2017), the total carrageenan sales volume reaches approximately 57,500 t per year, with the sales value reaching US$ 518 million. Based on our study, we believe that carrageenan extraction of K. alvarezii will produce 30.52% of carrageenan waste. We estimate that the galactose and glucose resulting from enzymatic hydrolysis would be equivalent to 3481 t year−1 and 5222 t year−1, respectively. The large-scale ethanol yield from carrageenan waste hydrolysate of K. alvarezii is estimated to be approximately 3069 t year−1. The schematic of bioethanol production and estimation of ethanol production from carrageenan waste hydrolysate of K. alvarezii is shown in Fig. 4 and Table 4. The abundance of polysaccharides that remains in carrageenan waste can be used in bioethanol production. Based on technology, availability, and sustainability, carrageenan waste can be one of the best candidate raw materials for bioethanol production.
References
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Cho Y, Kim H, Kim SK (2013) Bioethanol production from brown seaweed, Undaria pinnatifida, using NaCl acclimated yeast. Bioprocess Biosyst Eng 36:713–719
de Góes HG, Reis RP (2012) Temporal variation of the growth, carrageenan yield and quality of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultivated at Sepetiba bay, southeastern Brazilian coast. J Appl Phycol 24:173–180
Guerrero LA, Maas G, Hogland W (2013) Solid waste management challenges for cities in developing countries. Waste Manag 33:220–232
Hargreaves PI, Barcelos CA, da Costa ACA, Pereira N (2013) Production of ethanol 3G from Kappaphycus alvarezii: evaluation of different process strategies. Bioresour Technol 134:257–263
Hayashi L, De Paula EJ, Chow F (2007) Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State, Brazil. J Appl Phycol 19:393–399
Hayashi L, Faria GSM, Nunes BG, Zitta CS, Scariot LA, Rover T, Felix MRL, Bouzon ZL (2011) Effects of salinity on the growth rate, carrageenan yield, and cellular structure of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultured in vitro. J Appl Phycol 23:439–447
Jol CN, Neiss TG, Penninkhof B, Rudolph B, De Ruiter GA (1999) A novel high-performance anion-exchange chromatographic method for the analysis of carrageenans and agars containing 3,6-anhydrogalactose. Anal Biochem 268:213–222
Khambhaty Y, Mody K, Gandhi MR, Thampy S, Maiti P, Brahmbhatt H, Eswaran K, Ghosh PK (2012) Kappaphycus alvarezii as a source of bioethanol. Bioresour Technol 103:180–185
Marshall RE, Farahbakhsh K (2013) Systems approaches to integrated solid waste management in developing countries. Waste Manag 33:988–1003 3
McHugh DJ (2003) A guide to the seaweed industry, FAO, Rome
Meinita MDN, Kang J-Y, Jeong G-T, Koo H-M, Park S-M, Hong Y-K (2012) Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J Appl Phycol 24:857–862
Meinita MDN, Marhaeni B, Winanto T, Jeong G-T, Khan MNA, Hong Y-K (2013) Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol 25:1957–1961
Meinita MDN, Marhaeni B, Winanto T, Setyaningsih D, Hong Y-K (2015) Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. J Ind Eng Chem 27:108–114
Meinita MDN, Marhaeni B, Hong Y-K, Jeong G-T (2017a) Enzymatic saccharification of agar waste from Gracilaria verrucosa and Gelidium latifolium for bioethanol production. J Appl Phycol 29:3201–3209
Meinita MDN, Marhaeni B, Oktaviani DF, Jeong G-T, Hong Y-K (2017b) Comparison of bioethanol production from cultivated versus wild Gracilaria verrucosa and Gracilaria gigas. J Appl Phycol 30:143–147
Nunraksa N, Rattanasaensri S, Praiboon J, Chirapart A (2018) Comparison of ethanol production from Gracilaria fisheri and Gracilaria tenuistipitata cultivated in aquaculture system in Thailand. J Appl Phycol 30:3319–3325
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties of cultured kappa- carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku, Japan. J Appl Phycol 6:1–5
Pambudi LT, Dyah M, Meinita N, Ariyati RW (2010) Seaweed cultivation in Indonesia: recent status. Mar Biosci Biotechnol 4:6–10
Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll 23:1903–1909
Periyasamy C, Anantharaman P, Balasubramanian T, Rao PS (2014) Seasonal variation in growth and carrageenan yield in cultivated Kappaphycus alvarezii (Doty) Doty on the coastal waters of Ramanathapuram district, Tamil Nadu. J Appl Phycol 26:803–810
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Sudhakar MP, Jegatheesan A, Poonam C, Perumal K, Arunkumar K (2017) Biosaccharification and ethanol production from spent seaweed biomass using marine bacteria and yeast. Renew Energy 105:133–139
Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: an optimization study. Energy 78:53–62
Acknowledgments
We thank the Biotechnology Department, College of Fisheries Sciences, Pukyong National University for their collaboration.
Funding
This work was supported by the Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meinita, M.D.N., Marhaeni, B., Jeong, GT. et al. Sequential acid and enzymatic hydrolysis of carrageenan solid waste for bioethanol production: a biorefinery approach. J Appl Phycol 31, 2507–2515 (2019). https://doi.org/10.1007/s10811-019-1755-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-1755-8