Abstract
The seaweed genus Gracilaria is a potential candidate for the production of bioethanol due to its high carbohydrate content. Gracilaria is abundant throughout the world and can be found in both wild and cultivated forms. Differences in the ecological factors such as temperature, salinity, and light intensity affecting wild and cultivated specimens may influence the biochemical content of seaweeds, including the carbohydrate content. This study aimed to investigate the proximate composition and potential bioethanol production of wild and cultivated G. gigas and G. verrucosa. Bioethanol was produced using separate hydrolysis fermentation (SHF), employing a combination of enzymatic and acid hydrolysis, followed by fermentation with Saccharomyces cerevisiae ATCC 200062. The highest carbohydrate content was found in wild G. gigas. The highest galactose and glucose contents (20.21 ± 0.32 and 9.70 ± 0.49 g L−1, respectively), as well as the highest production of bioethanol (3.56 ± 0.02 g L−1), were also found in wild G. gigas. Thus, we conclude that wild G. gigas is the most promising candidate for bioethanol production. Further research is needed to optimize bioethanol production from wild G. gigas. Domestication of wild G. gigas is a promising challenge for aquaculture to avoid overexploitation of this wild seaweed resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agarophyte seaweeds belonging to the red algal genus Gracilaria are important raw materials in agar production. Since the beginning of the twentieth century, agar production has relied largely on Gelidium, as it produces a higher quality agar (less sulfated) than Gracilaria (Armisen 1995). The resulting high demand for Gelidium eventually exceeded the supply of naturally occurring Gelidium, and cultivation of the genus proved problematic. On the other hand, Gracilaria is successfully being cultivated in land-based ponds in several countries; the largest producer of which is Indonesia, followed by China and Chile (McHugh 2003; Pambudi et al. 2010; Bixler and Porse 2011, Porse and Rudolph 2017). Furthermore, Gracilaria cultivation has been steadily increasing, from 936,000 t in 2005 to 3,752,000 t in 2014 (McHugh 2003; Pambudi et al. 2010; Bixler and Porse 2011). Also, the quality of agar produced from Gracilaria can be improved by pretreating the seaweed with alkali. The successful combination of Gracilaria cultivation and alkali pretreatment has led to Gracilaria replacing Gelidium as the leading source for agar production (Armisen 1995).
The most common species of Gracilaria used for commercial agar production are G. verrucosa and G. gigas, which are obtained from both cultivation and the wild. Recently, Gracilaria species have been used as raw material for bioethanol production (Kumar et al. 2013; Meinita et al. 2013; Wu et al. 2014). The cell walls of Gracilaria consist of cellulose and complex agar polysaccharide; these, in turn, comprise a mixture of neutral polymer agarose, pyruvated agarose, and sulfated galactans, which can be hydrolyzed to sugars and fermented to ethanol. The purpose of the present study was to compare the biochemical compositions of wild and cultivated G. verrucosa and G. gigas as well as their respective potentials as raw materials for bioethanol production.
Material and methods
Preparation of seaweed samples
Cultivated samples of Gracilaria. gigas and G. verrucosa were obtained from Brebes, Central Java, Indonesia, and wild samples of the same were obtained from Garut Beach, West Java, Indonesia. The samples were washed with water and then freeze-dried.
Proximate analysis
The proximate compositions of cultivated and wild G. gigas and G. verrucosa were analyzed as follows. The total carbohydrate expressed as agar was determined by the phenol-sulfuric acid method (Kochert 1978). Total lipid was extracted using a mixture of hexane and isopropanol (3:2) and was quantified gravimetrically (Radin 1981). The amount of soluble protein in the tissue was estimated according to the method of Lowry et al. (1951) after heating a tissue suspension at 100 °C in 1 N NaOH for 2 h to obtain complete solubilization of the protein. Bovine serum albumin was used as a protein standard. The ash content was determined by weighing the residue after heating the sample for 5 h at 575 °C.
Pretreatment
H2SO4 was used to hydrolyze carbohydrates into simple sugars. Samples consisting of 10 g powdered waste agar in 100 mL of 0.2 M H2SO4 in a 250 mL flask were autoclaved at 120 °C for 15 min (Meinita et al. 2013). The acid-hydrolyzed slurries of agar wastes were then analyzed for sugar content using high-performance liquid chromatography (HPLC) and used in the next steps.
Enzymatic saccharification
The acid-hydrolyzed slurries from the previous step were adjusted to pH 5 and then saccharified using an enzyme mixture of Cellic C tec II, Viscozyme, and Cellic H tec II (Meinita et al. 2017). The saccharification was carried out at 50 °C in a water bath shaker at 130 rpm for 24 h. The resulting hydrolysates were then evaluated for sugar content using HPLC and used in the next steps.
Microorganisms and medium
Prior to fermentation, the hydrolysate samples were adjusted to pH 5 using 10 N NaOH. Saccharomyces cerevisiae ATCC 200062 was used for the fermentation. The inoculum culture was prepared in a medium containing 10 g yeast extract, 6.4 g urea, and 20 g glucose per liter (Meinita et al. 2017). The resulting yeast suspension was incubated at 30 °C in a shaking incubator at 130 rpm. Fermentation was carried out in triplicate in 100 mL Erlenmeyer flasks, each with a working volume of 50 mL. The flasks were periodically sampled for measurements of sugar and ethanol content.
Sugar and ethanol determination
Monosaccharide composition (galactose, glucose) was determined by HPLC using an Alltech IOA 1000 organic acid column (300 × 7.8 mm) equipped with an RI detector and maintained at 60 °C. Ethanol production was measured using an Agilent model 6890N Series Gas Chromatograph with a 2B-WAX column (Agilent, USA). The injection volume was 2 μL with an inlet split ratio of 30:1. The initial and maximum oven temperatures were 35 and 250 °C, respectively (Meinita et al. 2013).
Results and discussion
Proximate composition
The proximate compositions (carbohydrate, protein, lipid, and ash contents) of cultivated versus wild G. gigas and G. verrucosa are shown in Table 1. In both the cultivated and wild samples, carbohydrate and ash were the most abundant components, whereas lipid was the least abundant component. These results agree with those of previous studies on the biochemical compositions of seaweeds (Matanjun et al. 2009; Norziah and Ching 2000; Wong and Cheung 2000; Marinho-Soriano et al. 2006; Gómez-Ordóñez et al. 2010; Vergara-Rodarte et al. 2010; Syad et al. 2013).
Carbohydrate
Carbohydrate was the major component in the proximate composition of all the Gracilaria seaweeds examined. The carbohydrate content ranged from 38.38 ± 7.77% in cultivated G. verrucosa to 64.71 ± 3.74% in wild G. gigas. The highest observed carbohydrate concentration was comparatively greater than that reported by Norziah and Ching (2000) for G. changgi, but it was similar to that obtained by Marinho-Soriano et al. (2006) for G. cervicornis (63.13 ± 3.50%). For both G. verrucosa and G. gigas, the carbohydrate content was greater in the wild samples than in the cultivated samples. The range of carbohydrate values was comparable to that seen in previous studies of the carbohydrate content of red seaweeds (Norziah and Ching 2000; Wong and Cheung 2000; Marinho-Soriano et al. 2006; Matanjun et al. 2009; Gómez-Ordóñez et al. 2010; Vergara-Rodarte et al. 2010; Syad et al. 2013).
Protein
The protein content of the Gracilaria seaweeds ranged from 6.64 ± 3.21 to 12.63 ± 1.58%, comparable to the values reported by Norziah and Ching (2000) for G. changgi (6.9%) but lower than those reported by Marinho-Soriano et al. (2006) for G. cervicornis (22.96%). The protein range reported here is in agreement with that described in most Gracilaria studies. According to Briggs and Smith (1993), the protein content in most Gracilaria is between 7 and 13%.
Lipid
The lipid content of the Gracilaria seaweeds ranged from 0.58 ± 0.10 to 1.31 ± 1.25%.
The maximum lipid content was observed in wild G. gigas, followed by wild G. verrucosa, cultivated G. gigas, and cultivated G. verrucosa, in that order. Generally, seaweeds are relatively low in lipid. Previous studies of the lipid content of Gracilaria species reported values ranging from 0.028 to 3.3% (Norziah and Ching 2000; Marinho-Soriano et al. 2006; Syad et al. 2013). Compared to other biochemical components of seaweeds, lipid appears to be the least abundant. Moreover, the lipid content of tropical seaweeds is generally lower than that of subtropical seaweeds.
Ash
The ash contents of wild G. gigas, cultivated G. gigas, wild G. verrucosa, and cultivated G. verrucosa were 19.59 ± 0.15, 17.86 ± 0.98, 13.85 ± 0.99, and 12.51 ± 0.15%, respectively. These values are within the range reported for most Gracilaria seaweeds by other researchers (Norziah and Ching 2000; Marinho-Soriano et al. 2006; Syad et al. 2013). In general, ash content represents the total mineral content of the seaweeds.
In this study, the cultivated and wild G. gigas and G. verrucosa differed in protein, carbohydrate, lipid, and ash contents. These differences might be attributed to environmental parameters. Some researchers have concluded that the proximate compositions of seaweeds vary with different species and seasons (Marinho-Soriano et al. 2006; Ratana-arporn and Chirapart 2006; Matanjun et al. 2009; Denis et al. 2010).
Sugar content before and after enzymatic hydrolysis
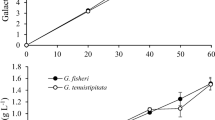
We increased the content of monosaccharides in our seaweed samples by two methods, acid hydrolysis followed by enzymatic saccharification, to convert polysaccharides into simple sugars. Previous studies (Meinita et al. 2013, 2015) showed that dilute acid hydrolysis using H2SO4 is the most effective method for hydrolyzing the polysaccharides of red seaweed. Enzymatic saccharification is an important step in bioethanol production, with many advantages over chemical saccharification. In enzymatic saccharification, mild pH and temperature are applied. These mild conditions avoid producing the toxic and/or corrosive by-products that may result from acid hydrolysis (Alvira et al. 2010). The sugar contents before and after enzymatic saccharification of wild G. verrucosa, cultivated G. verrucosa, wild G. gigas, and cultivated G. gigas are shown in Fig. 1. The galactose content of the wild G. verrucosa, cultivated G. verrucosa, wild G. gigas, and cultivated G. gigas samples after acid hydrolysis were 15.76 ± 0.18, 10.26 ± 0.16, 18.54 ± 18.54, and 14.08 ± 0.16 g L−1, respectively. After enzymatic saccharification, the galactose content of the wild G. verrucosa, cultivated G. verrucosa, wild G. gigas, and cultivated G. gigas samples were 16.84 ± 0.24, 12.49 ± 0.09, 20.21 ± 0.32, and 15.20 ± 0.16 g L−1, respectively. The glucose content of the wild G. verrucosa, cultivated G. verrucosa, wild G. gigas, and cultivated G. gigas samples after acid hydrolysis were 0.66 ± 0.02, 0.40 ± 0.03, 1.45 ± 0.03, and 1.01 ± 0.05 g L−1, respectively. After enzymatic saccharification, the glucose content of the wild G. verrucosa, cultivated G. verrucosa, wild G. gigas, and cultivated G. gigas samples were 6.47 ± 0.25, 2.67 ± 0.28, 9.70 ± 0.49, and 4.37 ± 0.16 g L−1, respectively. Based on these results, we conclude that enzymatic saccharification is the more efficient method for increasing glucose, rather than galactose, in Gracilaria seaweeds. The enzymatic hydrolysate of wild G. gigas contained the greatest concentration of glucose; suggesting wild G. gigas is the best candidate for bioethanol production; hence, it was used for the subsequent ethanol fermentation.
Fermentation and bioethanol production
Figure 2 shows the time course of ethanol fermentation in the hydrolysate of agar waste from wild G. gigas. Based on our previous study (Meinita et al. 2013), we chose S. cerevisiae ATCC20062 as the best microorganism for bioethanol production from red seaweed. This yeast utilizes primarily hexose sugars, including glucose and galactose, both of which result from the acid hydrolysis and enzymatic saccharification. The enzymatic hydrolysate of wild G. gigas contained 20.00 g L−1 galactose and 9.70 g L−1 glucose before fermentation with S. cerevisiae ATCC20062. In this ethanol fermentation, the ethanol production rate in the early phase of the culture was relatively slow but rapidly increased and reached a maximum bioethanol production (3.56 g L−1) after 24 h of fermentation, which corresponded to a 28.81% theoretical yield.
Gracilaria species may be the best agarophytes to use for bioethanol production due to their abundant carbohydrate content. Among cultivated and wild G. gigas and G. verrucosa, wild G. gigas appears to be the most promising species, based on proximate composition and sugar content after enzymatic saccharification. Carbohydrate is the most abundant component found in wild G. gigas. Acid hydrolysis, followed by enzymatic saccharification, significantly increased the sugar content of the wild G. gigas samples. Currently, most of the G. gigas supply is from the wild. Hence, domestication of G. gigas is a promising approach to aquaculture designed to facilitate pilot-scale production of bioethanol. Domestication of wild G. gigas may be needed to avoid collapse of the wild seaweed population due to overexploitation.
References
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production proscess based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Armisen R (1995) World-wide use and importance of Gracilaria. J Appl Phycol 7:231–243
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Briggs MRP, Smith SJF (1993) Macroalgae in aquaculture: an overview and their possible roles in shrimp culture. Proceedings Conference on Marine Biotechnology in the Asia Pacific, pp 137–143
Denis C, Morançais M, Li M, Deniaud E, Gaudin P, Wielgosz-Collin G, Barnathan G, Jaouen P, Fleurence J (2010) Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem 119:913–917
Gómez-Ordóñez E, Jiménez-Escrig A, Rupérez P (2010) Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res Int 43:2289–2294
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods, vol II, Physiological and biochemical methods. Cambridge University Press, Cambridge, pp 95–97
Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour Technol 135:150–156
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97:2402–2406
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
McHugh DJ (2003) A guide to the seaweed industry. FAO, Rome
Meinita MDN, Marhaeni B, Winanto T, Jeong GT, Khan MNA, Hong YK (2013) Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol 25:1957–1961
Meinita MDN, Marhaeni B, Winanto T, Setyaningsih D, Hong YK (2015) Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. J Ind Eng Chem 27:108–114
Meinita MDN, Marhaeni B, Hong YK, Jeong GT (2017) Enzymatic saccharification of agar waste from Gracilaria verrucosa and Gelidium latifolium for bioethanol production. J Appl Phycol. https://doi.org/10.1007/s10811-017-1205-4
Norziah MH, Ching CY (2000) Nutritional composition of edible seaweed Gracilaria changgi. Food Chem 68:69–76
Pambudi LT, Dyah M, Meinita N, Ariyati RW (2010) Seaweed cultivation in Indonesia : recent status. Mar Biosci Biotechnol 4:6–10
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol. https://doi.org/10.1007/s10811-017-1144-0:1-14
Radin NS (1981) Extraction of lipids with hexane-isopropanol. Method Enzymol 72:5–7
Ratana-arporn P, Chirapart A (2006) Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Nat Sci 40:75–83
Syad AN, Shunmugiah KP, Kasi PD (2013) Seaweeds as nutritional supplements: analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomed Prev Nutr 3:139–144
Vergara-Rodarte MA, Hernández-Carmona G, Rodríguez-Montesinos YE, Arvizu-Higuera DL, Riosmena-Rodríguez R, Murillo-Álvarez JI (2010) Seasonal variation of agar from Gracilaria vermiculophylla, effect of alkali treatment time, and stability of its colagar. J Appl Phycol 22:753–759
Wong KH, Cheung PCK (2000) Nutritional evaluation of some subtropical red and green seaweeds. Food Chem 71:475–482
Wu FC, Wu JY, Liao YJ, Wang MY, Shih IL (2014) Sequential acid and enzymatic hydrolysis in situ and bioethanol production from Gracilaria biomass. Bioresour Technol 156:123–131
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meinita, M.D.N., Marhaeni, B., Oktaviani, D.F. et al. Comparison of bioethanol production from cultivated versus wild Gracilaria verrucosa and Gracilaria gigas . J Appl Phycol 30, 143–147 (2018). https://doi.org/10.1007/s10811-017-1297-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1297-x