Abstract
Kappaphycus alvarezii commercial cultivation is increasing along Brazilian coast. After 2008, more than 30 farms were established at Rio de Janeiro state in Sepetiba and Ilha Grande bays. Each farm has about ten floating rafts corresponding to near 900 m of cultivation line. Understanding the effect of the environmental factors on the growth and carrageenan yield and quality of the target algae in commercial farms will subsidize the establishment of efficient management activities to be used in the farms. In this sense, the temporal variation of the K. alvarezii daily growth rate, productivity, carrageenan yield, and quality (viscosity and gel strength) were analyzed using the seedlings cultivated in the largest Brazilian commercial cultivation at Sepetiba Bay. The daily growth rate, productivity, and carrageenan yield and quality were within the commercial requirement desirable for commercial crops. The main factors that caused the breakage of the floating rafts and consequently diminished the productivity are the storms. Water temperature and salinity were the other factors that influenced the daily growth rate and carrageenan yield and quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macroalgae beds are insufficient to supply the global demand for macroalgae industries (Ask and Azanza 2002; Pickering et al. 2007). In the last decade, the world market for carrageenan has increased about 4% per year in 2009, and the volume of sales in metric tons of these hydrocolloids was 86,100, yielding US $527 million (Bixler and Porse 2010). Aquaculture is an alternative to improve source of raw material, to generate new income and social improvement for coastal communities in less developed countries, and to avoid the breakdown of natural beds. Kappaphycus alvarezii (Doty) Doty ex P.C. Silva is the most important species used in commercial aquaculture for the kappa carrageenan industry (Pickering et al. 2007; Bixler and Porse 2010).
In the 1970s, the commercial eucheumatoid species (K. alvarezii, Kappaphycus striatus (F.Schmitz) Doty ex P.C. Silva and Eucheuma denticulatum (N.L.Burman) F.S.Collins) began in the Philippines (Ask and Azanza 2002). In 1995, K. alvarezii was experimentally introduced in Brazilian southeastern, São Paulo state (Paula et al. 2002) and in 1998 was commercially cultivated at Rio de Janeiro state (Castelar et al. 2009). After 2008, when K. alvarezii has been allowed to be cultivated by the Brazilian government in Sepetiba Bay, Rio de Janeiro state, nearly 30 commercial farms were established in this state. Each farm has about ten floating rafts, 75-m long that correspond to almost 900 m of cultivation line. Nowadays, these farms provide the raw material for the Brazilian carrageenan industry (unpublished date).
The productivity of an established farm depends on the management efficiency (Santelices 1999). Information about the K. alvarezii growth, carrageenan yield and quality, cultivated in commercial farms and recognition of the effect of the environmental factors on those variables are fundamental for management activities (Ask and Azanza 2002; Hayashi et al. 2007a; Reis et al. 2007). In this sense, the temporal variation of the K. alvarezii growth, carrageenan yield and quality was analyzed in the largest Brazilian commercial cultivation at Rio de Janeiro state (Castelar et al. 2009; Marroig and Reis 2010).
Material and methods
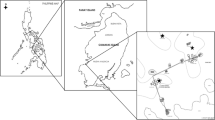
Sampling was conducted from May 2006 to February 2008, in the commercial cultivation of K. alvarezii at Marambaia Bay (23°03'50'' S and 043°52'50'' W), located south of Sepetiba bay, on the south coast of Rio de Janeiro state, Brazil (Castelar et al. 2009; Marroig and Reis 2010). This farm was surrounded by a 20-km long sandy beach without rocky shores. The substratum is predominantly silt and mud with an average depth of 2.3 m (Castelar et al. 2009). Based in Köppen’s classification, the macroclimate of Marambaia Bay is defined as tropical with rainy summers and dry winters (Aw) and Nimer’s mesoclimate is defined as tropical, hot, and super humid. The annual mean rainfall is 1,240 mm, with 37% of the rainfall occurring in summer and 15% in winter, and the annual mean of relative air humidity is 81%, decreasing in the winter with higher irradiation occurring in summer and decreasing in winter (Mattos 2005).
Cultivation structures (floating rafts)
The cultivation structures are floating rafts (Fig. 1), placed about 60 m from the sand beach, about 1.8 m in depth and perpendicular to the prevailing strong currents. There are about 100 floating rafts according to the commercial production. Each floating raft has an area of 450 m² and is composed of 30 modules (5 × 3 m each), floated by PVC tubes with the ends closed and connected to each other by polypropylene lines. In each module, 220 seedlings (100 g) are tied by tie–tie method on 11 nylon lines (long lines), corresponding to 55 m of cultivation line, 60 cm under the water’s surface. A nylon catch net (60-mm mesh) is used below the rafts to avoid seedling loss from the module. The rafts are anchored with 400 kg of cement blocks.
Abiotic data of Marambaia bay
Water temperature and salinity were measured in situ three times a week and water transparency weekly with a Secchi disk. Data from the Meteorological Station of Marambaia (located about 30 km off the Marambaia Bay) were provided by the National Institute of Meteorology. Wind velocity (m s−1) and direction (bearing) were indicative of marine hydrodynamics, and solar radiation (kJ m−2), from 6 am to 6 pm, was considered indicative of light availability (irradiance). Solar radiation was transformed to irradiance.
K. alvarezii daily growth rate and productivity
Temporal variation of the K. alvarezii daily growth rate was quantified monthly (May 2006 to February 2008), using the total wet weight of the seedlings on the floating raft. Six rafts, with production cycle between 42 and 48 days, were measured. The K. alvarezii daily growth rate of the period referred as year 1 (May 2006 to April 2007) was compared with the other one of the period called year 2 (May 2007 to February 2008). The daily growth rate was calculated by the formula: daily growth rate (% day−1) = (ln(W f/W i)/t) × 100, where W f = final wet weight; W i = initial wet weight, and t = time of cultivation (Muñoz et al. 2004; Castelar et al. 2009). The productivity was estimated by the formula: productivity (g dwt m−2 day−1) = [(dwtf − dwti)/t × (dwt/wwt)]/A, where dwtf = final mean dry weight (g); dwti = initial mean dry weight (g); t = time of cultivation; dwt = dry weight (g); wwt = wet weight (g); A = total area cultivated (m2) (Hayashi et al. 2007a). The algae were heavy wet, and the ratio was considered of approximately 35% of moisture (commercial value) for determination of the dry weight.
K. alvarezii carrageenan yield, viscosity, and gel strength
Randomly, samples of K. alvarezii were obtained monthly (August 2007 to February 2008) from six rafts (n = 6). Carrageenan yield was expressed as percentage of carrageenan from a sample of algal dry weight (Reis et al. 2008), according to the formula: carrageenan yield (%) = (W c/W s) × 100, where W c is the produced carrageenan dry mass (oven dried in 60°C to constant weight) used in the process.
Semi-refined carrageenan was obtained from the K. alvarezii by an alkaline transformation with 6% KOH solution in water bath (80°C) for 2 h and successive washing with fresh water. After that the samples were maintained in 0.06% NaClO solution for 30 min (25°C) and washed again with fresh water to remove residues and finally dried in an oven (60°C) until constant weight, a pH 8.4 was maintained during the procedure (Góes and Reis 2011; Reis et al. 2010).
The quality of the carrageenan was measured as viscosity and gel strength using a gel (n = 6) obtained by the carrageenan dilution (1:70 proportion) with distilled water, subsequently, homogenized and maintained in a water bath (80°C) for 30 min (Nova Ética, model 500/3D) and maintained at 5°C for 24 h. The viscosity was measured at 75 ± 5°C (Fungilab, model Visco Basic Plus) and the gel strength in a digital texture analyzer (Stable Micro System, standard model) (Góes and Reis 2011; Reis et al. 2010).
Statistical analysis
Normality (Shapiro Wilk’s test) and homogeneity of variance (Cochran/Levene test) tests were used to verify parametrical testing. Non-parametric data were transformed according to Zar (1996). The differences between the mean values of abiotic factors and productivity of year 1 and year 2 were verified using Student’s t test and the median values of daily growth rate using a Mann–Whitney test. The differences between the mean of carrageenan yield in the sampling period was verified using a one-way analysis of variance and the differences separated by Tukey test. The differences between the median of the K. alvarezii daily growth rate, carrageenan viscosity and gel strength, and daily abiotic data (water temperature, salinity, irradiation, and wind velocity) during the sampling period were analyzed by the non-parametric Mann–Whitney or Kruskal–Wallis test (Zar 1996).
The wind direction was grouped in quadrants related into the cardinal points for quantification. The effects of abiotic factors on K. alvarezii productivity, daily growth rate, carrageenan yield, gel strength, and viscosity were tested by the simple linear regression test, after proof of correlation (Pearson test). Statistica 6.0 StatSoft Inc. (version 6, 1999) was used. The confidence interval for the significance test was 95% (p = 0.05). Data are expressed by mean ± standard deviation.
Results
Environmental variables of Marambaia Bay, Rio de Janeiro state
The seawater temperature and salinity, wind speed, and direction (bearing) and irradiation varied during sampling period (Fig. 2; Table 1). The most frequent wind was SW and SE. There was a tendency of higher wind velocity near winter (August to September 2006 and July 2007), higher water temperature and irradiation from end of spring to summer (November to February 2006 and 2007), and drops in salinity in the spring and summer (May 2006, February to May 2006, February 2007, January and February 2008).
Irradiance, wind velocity, seawater salinity and temperature and Kappaphycus alvarezii daily growth rate and productivity of seedling sampling from May 2006 to February 2008 at Marambaia bay, Rio de Janeiro state, Brazil. Smaller squares indicate the median, rectangles indicate the quartiles (25th and 75th percentiles) and traces indicate the minimum and maximum values of the variable
When abiotic factors of year 1 were compared with the ones of year 2 (Table 1), no significant differences was observed, except irradiance that was higher in the year 1. In spite of this response, along the sampling period, in the second year the wind speed was low except in July 2007 when a wide range of values around the median occurred.
Temporal variation of K. alvarezii daily growth rate and productivity
The K. alvarezii productivity and daily growth rate varied during the sampling period and were higher in year 1 when the variations around the median were lower than year 2 (Fig. 2; Table 1). The productivity and daily growth rate tended to be higher in the end of summer to autumn (May 2006, February to April 2007) and in spring (October to November 2007). When July 2006 was compared with July 2007, K. alvarezii daily growth rate and productivity were higher in the first year, the direction of the wind was similar in both years but with low wind velocity in the first year.
Temporal variation of K. alvarezii carrageenan yield and quality
The carrageenan yield, viscosity, and gel strength were significantly different during the sampling period (Fig. 3; Table 1). Carrageenan yield was higher in the middle of the summer of 2008 (January 2008), while viscosity was higher in spring of 2007 (November 2007) and gel strength higher in spring of 2007 to summer of 2008 (November 2007 to January 2008).
K. alvarezii carrageenan yield (%), gel strength (g cm−2), and viscosity (cP) of seedlings sampling from August of 2007 to February 2008 at Marambaia bay. Smaller squares indicate the median, rectangles indicate the quartiles (25th and 75th percentiles), and traces indicate the minimum and maximum values of the variable
Effects of abiotic factors on K. alvarezii productivity, daily growth rate, carrageenan yield, gel strength, and viscosity
No correlation was observed between K. alvarezii daily growth rate and carrageenan yield. The linear regressions showed negative effect of the salinity on productivity, daily growth rate, and carrageenan yield and positive effect of the seawater temperature on productivity, daily growth rate, and salinity on viscosity (Fig. 4).
Discussion
At Marambaia Bay, the K. alvarezii daily growth rate (3.76 ± 0.79% day−1) was above 3.5% day−1 that is considered desirable for commercial cultivation (Doty 1987), and in the range obtained in other farms, 1.4% to 12% day−1 (Glenn and Doty 1990; Ohno et al. 1994; Paula et al. 1999; 2002; Hurtado et al. 2001; Muñoz et al. 2004; Bulboa and Paula 2005; Wakibia et al. 2006; Hayashi et al. 2007a, b; 2010). Probably those differences were due to the measurement method. In our study, the experimental unit was the total seedlings cultivated in one commercial floating raft (6,600 seedlings). This calculation included the loss of individuals by weather, while in other experimental studies seedlings are recorded individually and sometimes the lost seedling is not considered.
The other reason can be the differences of the environmental factors at each site, in spite of the fact that the Brazilian sites (São Paulo and Rio de Janeiro states) are in the same phytogeographical region proposed by Horta et al. (2001). This fact emphasizes the necessity of information about the crop responses to the environment in the site that the farm is being established and throughout the year to determine appropriated cultivation management practices like recommended by Ask and Azanza (2002) and Hayashi et al. (2007a). That information can also be useful to establish management activities for other farms.
Temporal variations of the growth and carrageenan quality and yield at Marambaia bay can be associated to changes in environmental factors as commented in other studies with carragenophytes (Rees 1969; Ask and Azanza (2002); Hayashi et al. 2007a, b; Reis et al. 2008, 2010; Hayashi et al. 2010). The K. alvarezii productivity (43.9 g dwt m−2 day−1) at 50 cm below the water surface, density of 12 seedlings m−2, in 44 days of cultivation observed by Hayashi et al. (2007a) was similar to our productivity results (39.32 ± 18.51 g dwt m−2 day−1). We did not find a pattern of K. alvarezii productivity and daily growth rate as was found for daily growth rate at São Paulo state, where lower values occurred in winter/spring (Paula et al. 2002; Paula and Pereira 2003) and at Santa Catarina state in winter (Hayashi et al. 2010). In this study, the fluctuations in K. alvarezii productivity and daily growth rate were associated to the positive effect of seawater temperature on growth. This evidence is in accordance with the natural occurrence of this species in sites which high seawater temperature (Hurtado et al. 2001; Paula and Pereira 2003; Ohno et al. 1996).
The negative effect of salinity on the growth (daily growth rate and productivity) was not in accordance with what was observed in other studies since the salinity (30 to 35) registered at Marambaia bay was within the range which was described to be tolerant for K. alvarezii (Doty 1987; Paula and Pereira 2003; Ask 2006; Hayashi et al. 2007a, 2010; Reis et al. 2010).
The other environmental factor that was harmful for the K. alvarezii growth at this farm was the high wind velocity associated with the southeastern wind that caused huge storms. The consequence was the breakage of the cultivation structures and seedlings lost (personal observation). This fact was confirmed in July of 2007, when the growth (productivity and daily growth rate) was compared with data from July of 2006, the productivity and daily growth rate were lower and the wind speed was high with more southeastern winds than usual while in the first year in spite of the dominance of the same wind direction the wind intensity was lower and growth was higher. Thus, the intensity of winds can influence the development of the crop in two ways: positively when it increases hydrodynamics and consequently increases the growth. This fact has been observed elsewhere and in Brazil (Doty 1987; Glenn and Doty 1990; Hayashi et al. 2010). The negative effect is the breakage of the cultivation structures (floating rafts) with consequent seedling loss.
Since there are different procedures to obtain carrageenan from the carragenophytes and also different analysis for carrageenan yield and quality, the comparison of results from different studies is not precise (Muñoz et al. 2004; Hayashi et al. 2007b). The semi-refined K. alvarezii carrageenan yield obtained at Sepetiba bay was 46.1 ± 6.1% and was within the range obtained in Brazil and other countries; 19% to 55% (Muñoz et al. 2004; Hayashi et al. 2007b, 2010; Hung et al. 2009). The gel strength from seedling sampled in Sepetiba Bay was 395.1 ± 100.6 g cm−2 and within the range of other farms from 245 to 1,960 g cm−2 (Ohno et al. 1994, 1996; Hayashi et al. 2007b; Hung et al. 2009). The viscosity of 222.6 ± 143.3 cP was in the rage obtained in other countries, from 23 to 890 cP (Ohno et al. 1994, 1996; Muñoz et al. 2004; Hung et al. 2009).
No correlation was observed between the carrageenan yield and growth (daily growth rate and productivity) of the seedlings cultivated at Sepetiba bay while the seedlings sampled at São Paulo state were negatively correlated (Hayashi et al. 2007b). The changes in carrageenan yield and quality were dependent of different abiotic factors. The effect of salinity on the carrageenan yield (negative) and carrageenan viscosity (positive) obtained from the seedlings cultivated at Marambaia bay probably were due to changes in carrageenan conformation that promotes different rheological properties to the algae preventing unfavorable environmental conditions (Rees 1969; Hayashi et al. 2007a; Reis et al. 2008). Salinity is one of the main environmental factors known to influence carrageenan yield and quality since the carrageenan is responsible for the ionic equilibrium of the cell (Percival 1979) due to the cation–anion balance to the negatively charged polysaccharides (Mariani et al. 1990). The highest carrageenan yield observed in January can be a response to huge rainfall in the summer. A similar result was observed with the native carragenophyte Hypnea musciformis in the same bay in periods of strong rainfall and was proposed the existence of a balance between carrageenan yield and quality produced by the algae for their protection against undesirable abiotic factors (Reis et al. 2008).
We conclude that there is a potential for K. alvarezii cultivation at Marambaia Bay since the growth and carrageenan yield and quality were within the commercial requirement desirable for commercial crops. The main factor that caused the breakage of the floating rafts and consequently diminished the productivity was the storms. A preventive management in this period is to strengthen the anchor, to fix better the rafts and, if possible, to harvest the seedlings. The water temperature and salinity were the other factors that influenced the daily growth rate and carrageenan yield and quality.
References
Ask EI (2006) Cultivating Cottonii and Spinosum: a “how to” guide. In: Critchley AT, Ohno M, Largo DB (eds) World seaweed resources—an authoritative reference system. ISBN 90 75000 80
Ask EI, Azanza RV (2002) Advances in cultivation technology of commercial eucheumatoid species: a review with suggestions for future research. Aquaculture 206:257–277, ETI, UK, DVD-ROM
Bixler HJ, Porse H (2010) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol. doi:10.1007/s10811-010-9529-3
Bulboa CR, Paula EJ (2005) Introduction of non-native species of Kappaphycus (Rhodophyta, Gigartinales) in subtropical waters: comparative analysis of growth rates of Kappaphycus alvarezii and Kappaphycus striatum in vitro and in the sea in south-eastern Brazil. Phycol Res 53:183–188
Castelar B, Reis RP, Moura AL, Kirk R (2009) Invasive potential of Kappaphycus alvarezii off the south coast of Rio de Janeiro state, Brazil: a contribution to environmentally secure cultivation in the tropics. Bot Mar 52:283–289
Doty MS (1987) The production and use of Eucheuma. In: Doty MS, Caddy JF, Santelices B (eds) Case studies of seven commercial seaweed resources. FAO Fisheries Technical Paper, Rome, pp 123–161, 281
Glenn EP, Doty MS (1990) Growth of the seaweeds Kappaphycus alvarezii, K. striatum and Eucheuma denticulatum as affected by environment in Hawaii. Aquaculture 84:245–255
Góes HG, Reis RP (2011) An initial comparison of tubular netting versus tie–tie methods of cultivation for Kappaphycus alvarezii (Rhodophyta, Solieriaceae) on the south coast of Rio de Janeiro State. Brazil J Appl Phycol. doi:10.1007/s10811-010-9647-y
Hayashi L, Oliveira EC, Bleicher-Lhonneur G, Boulenguer P, Pereira RTL, Seckendorff R, Shimoda VT, Leflamand A, Vallée P, Critchley AT (2007a) The effects of selected cultivation conditions on the carrageenan characteristics of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in Ubatuba Bay, São Paulo, Brazil. J Appl Phycol 19:505–511
Hayashi L, Paula EJ, Chow F (2007b) Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State, Brazil. J Appl Phycol 19:393–399
Hayashi L, Santos AA, Faria GSM, Nunes BG, Souza MS, Fonseca ALD, Barreto PLM, Oliveira EC, Bouzon ZL (2010) Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) cultivated in subtropical waters in Southern Brazil. J Appl Phycol. doi:10.1007/s10811-010-9543-5
Horta PA, Amancio E, Coimbra CS, Oliveira EC (2001) Considerações sobre a distribuição e origem da flora de macroalgas marinhas brasileiras. Hoehnea 28:243–265
Hung LD, Hori K, Nang HQ, Kha T, Hoa LT (2009) Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam. J Appl Phycol 21:265–272
Hurtado AQ, Agbayani RF, Sanares R, Castro-Mallare MTR (2001) The seasonality and economic feasibility of cultivating Kappaphycus alvarezii in Panagatan Cays, Caluya, Antique, Philippines. Aquaculture 199:295–310
Mariani P, Tolomio C, Baldan B (1990) Cell wall ultrastructure and cation localization in some benthic algae. Phycologia 29:253–262
Marroig RG, Reis RP (2010) Does biofouling influence Kappaphycus alvarezii (Doty) Doty ex Silva farming production in Brazil? J Appl Phycol. doi:10.1007/s10811-010-9602-y
Mattos CCLV (2005) Caracterização climática da Restinga da Marambaia RJ. In: Menezes LFT, Peixoto AL, Araujo DSD (eds) História Natural da Marambaia. Seropedica, Rio de Janeiro, pp 55–66
Muñoz J, Freile-Pelegrín Y, Robledo D (2004) Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán, México. Aquaculture 239:161–177
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties on cultured kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku, Japan. J Appl Phycol 8:1–5
Ohno M, Nang HQ, Hirase S (1996) Cultivation and carrageenan yield and quality of Kappaphycus alvarezii in the waters of Vietnam. J Appl Phycol 8:431–437
Paula EJ, Pereira RTL (2003) Factors affecting growth rates of Kappaphycus alvarezii (Doty) Doty ex. P. Silva (Rhodophyta, Solieriaceae) in subtropical waters of São Paulo State, Brazil. In: Chapman ARO, Anderson RJ, Vreedland VJ, Davison IR (eds) Proceedings of the 17th International Seaweed Symposium, Cape Town. Oxford University Press, Oxford, pp 381–388
Paula EJ, Pereira RTL, Ohno M (1999) Strain selection in Kappaphycus alvarezii var. alvarezii (Doty) Doty ex P. Silva (Rhodophyta, Solieriaceae) using tetraspore progeny. J Appl Phycol 11:111–121
Paula EJ, Pereira RTL, Ohno M (2002) Growth rate of the carrageenophyte Kappaphycus alvaerzii (Rhodophyta, Gigartinales) introduced in subtropical waters of São Paulo State, Brazil. Phycol Res 50:1–2
Percival E (1979) The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Brit Phycol J 4:103–117
Pickering TD, Skelton P, Sulu JR (2007) Intentional introductions of commercially harvested alien seaweeds. Bot Mar 50:338–350
Rees DA (1969) Structure, conformation and mechanims in the formation of polisaccharides gels, networks. Advan Carbohyd Chem 24:267–332
Reis RP, Bastos M, Góes HG (2007) Cultivo de Kappaphycus alvarezii no litoral do Rio de Janeiro. Panor Aqüic 17(89):42–47
Reis RP, Yoneshigue-Valentin Y, Santos CP (2008) Spatial and temporal variation of Hypnea musciformis carrageenan (Rhodophyta–Gigartinales) from natural beds in Rio de Janeiro State, Brazil. J Appl Phycol 20:1–8
Reis RP, Loureiro RR, Mesquita FS (2010) Does salinity affect growth and carrageenan yield of Kappaphycus alvarezii (Gigartinales/Rhodophyta)? Aquac Res. doi:10.1111/j.1365-2109.2010.02699.x
Santelices B (1999) Aconceptual framework formarine agronomy. Hydrobiologia 398/399:15–23
Wakibia JG, Bolton JJ, Keats DW, Raitt LM (2006) Factors influencing the growth rates of three commercial eucheumoids at coastal sites in southern Kenya. J Appl Phycol 18:565–573
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey, p 66
Acknowledgements
This research was supported by the Botanical Garden of Rio de Janeiro and Ondas Biomar Macroalgae Marine Mariculture Ltda. (Ondas Biomar Cultivo de Algas Marinhas Ltda). We express our thanks to the National Institute of Meteorology (INMET–Instituto Nacional de Meteorologia) for the meteorological data and to Denise Biato for the carrageenan analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Góes, H.G., Reis, R.P. Temporal variation of the growth, carrageenan yield and quality of Kappaphycus alvarezii (Rhodophyta, Gigartinales) cultivated at Sepetiba bay, southeastern Brazilian coast. J Appl Phycol 24, 173–180 (2012). https://doi.org/10.1007/s10811-011-9665-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9665-4