Abstract
The Generation R Study is a population-based prospective cohort study from fetal life until adulthood. The study is designed to identify early environmental and genetic causes and causal pathways leading to normal and abnormal growth, development and health during fetal life, childhood and adulthood. The study focuses on six areas of research: (1) maternal health; (2) growth and physical development; (3) behavioural and cognitive development; (4) respiratory health and allergies; (5) diseases in childhood; and (6) health and healthcare for children and their parents. Main exposures of interest include environmental, endocrine, genetic and epigenetic, lifestyle related, nutritional and socio-demographic determinants. In total, n = 9,778 mothers with a delivery date from April 2002 until January 2006 were enrolled in the study. Response at baseline was 61 %, and general follow-up rates until the age of 6 years exceed 80 %. Data collection in mothers, fathers and children include questionnaires, detailed physical and ultrasound examinations, behavioural observations, and biological samples. A genome and epigenome wide association screen is available in the participating children. From the age of 5 years, regular detailed hands-on assessments are performed in a dedicated research center including advanced imaging facilities such as Magnetic Resonance Imaging. Eventually, results forthcoming from the Generation R Study contribute to the development of strategies for optimizing health and healthcare for pregnant women and children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Generation R Study is a population-based prospective cohort study from fetal life until young adulthood. The study is designed to identify early environmental and genetic causes, and causal pathways leading to normal and abnormal growth, development and health during fetal life, childhood and adulthood. The background has been described in detail previously [1–3]. Specific interest is in the effects of fetal and early childhood conditions on health and disease in later life [4–7]. The study focuses on six areas of research: (1) maternal health; (2) growth and physical development; (3) behavioural and cognitive development; (4) respiratory health and allergies; (5) diseases in childhood; and (6) health and healthcare for children and their parents. Main exposures of interest include environmental, endocrine, genetic and epigenetic, lifestyle related, nutritional and socio-demographic determinants.
The main outcomes and exposures are presented in Tables 1 and 2. Main outcomes studied in the Generation R Study are new or well known risk factors in childhood or adulthood for cardiovascular disease, type 2 diabetes, obesity, asthma, neurological diseases and psychopathology. Many studies focused on these risk factors have been recently published in the European Journal of Epidemiology [8–98]. Results forthcoming from the Generation R Study should contribute to the development of strategies for optimizing health and healthcare for pregnant women and children.
Study area
The Generation R Study is conducted in Rotterdam, the second largest city in the Netherlands. Rotterdam is situated in the Western part of the Netherlands on almost 80 km south from Amsterdam, the capital of the Netherlands. The total population consists of about 600,000 inhabitants of almost 150 different ethnicities. The study area is well defined by postal codes and covers more than half of the cities inhabitants (almost 350,000 inhabitants) [99]. The largest ethnic groups in this population are the Dutch (56 %), Surinamese (9 %), Turkish (7 %), Moroccan (6 %), Dutch Antillean (3 %) and Cape Verdian (3 %) groups [100]. The percentages of the non-Dutch groups are higher in younger age groups.
Study design
Overview
The study is a population-based prospective cohort study from fetal life onwards. Mothers with an expected delivery date between April 2002 and January 2006 were eligible. Extensive assessments are performed in mothers, fathers and children. Measurements during pregnancy were conducted in two well-equipped research centers in the study area, with a close collaboration with midwives and hospitals, and planned in early pregnancy (gestational age <18 weeks), mid-pregnancy (gestational age 18–25 weeks) and late pregnancy (gestational age >25 weeks). These measurements are generally considered as first, second and third trimester measurements. The fathers were assessed once during pregnancy of their partner. The specific timing of these measurements depended on the gestational age at enrolment [2]. The children form a prenatally recruited birth-cohort that will be followed until young adulthood. In the preschool period, which in the Netherlands refers to the period from birth to the age of 4 years, data collection was performed by a home-visit at the age of 3 months, and by repeated questionnaires and routine child health centres visits. Additional detailed measurements of fetal and postnatal growth and development have been conducted in a randomly selected subgroup of Dutch children and their parents at a gestational age of 32 weeks and postnatally at the ages of 1.5, 6, 14, 24, 36 and 48 months in a dedicated research center. From the age of 5 years onwards, regular detailed hands-on assessments are performed on all children in a dedicated research center that includes advanced imaging facilities.
Study cohort
Eligibility, enrolment and response
Eligible mothers were those who were resident in the study area at their delivery date and had a delivery date from April 2002 until January 2006. We aimed to enrol mothers in early pregnancy (gestational age <18 weeks) but enrolment was allowed until birth of their child. Midwives and obstetricians informed eligible mothers about the study at their first prenatal visit in routine care, handed out the information package and asked these mothers to make an appointment for their first ultrasound examination. The study staff contacted these mothers by phone for additional information about the study and in person at the ultrasound examination to obtain informed consent. Mothers who could not be approached in pregnancy were approached in the first months after birth of their child when newborns visited the routine child health centers [2]. The fathers were not approached directly by the study staff but the mothers were informed about the importance of involvement of the fathers in the study.
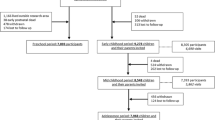
In total, 9,778 mothers were enrolled in the study (Fig. 1). Of these mothers, 91 % (n = 8,879) was enrolled in pregnancy. Partners from mothers enrolled in pregnancy were invited to participate. In total, 71 % (n = 6,347) of all fathers was enrolled. Of all participating mothers, data are available from early pregnancy in 72 % (n = 7,069), mid-pregnancy in 16 % (n = 1,594), late pregnancy in 2 % (n = 216) and from birth of their child in 9 % (n = 899). Of all pregnant women who were enrolled, 94 (n = 8,356), 6 (n = 515) and 0.1 % (n = 8) were first, second and third pregnancies in the study, respectively. A total of 1,232 pregnant women and their children were enrolled in the subgroup of Dutch children for additional detailed studies until the age of 4 years. Estimation of the precise number of eligible pregnant women in the study area is difficult since there is no satisfactory registry of pregnancies. Therefore, it was not attempted to identify overall response rates based on pregnant women, but the response rate was based on the number of children at is birth 61 %. Ethnicity and education of participating mothers and partners was defined according the classification of Statistics Netherlands [100–104]. The largest ethnic groups were the Dutch, Surinamese, Turkish and Moroccan groups. Both household income and highest followed educational level in mothers and fathers in the study cohort suggest a selection towards a higher socioeconomic status than in the whole study area [104]. This pattern is similar as in other large scale cohort studies [105].
Follow-up studies
As described above, 9,778 mothers were enrolled in the study and gave birth to 9,749 known live born children. During the preschool period (0–4 years), the logistics of the postnatal follow-up studies were embedded in the municipal routine child care system and restricted to only part of the study area due to logistical constraints. In total 1,166 children lived outside this definite study area at birth and were therefore not approached for the postnatal follow-up studies during the preschool period. Of the remaining 8,583 children, 690 (8 %) parents did not give consent for the preschool period studies, leaving 7,893 children for the preschool period follow-up studies.
From the age of 5 years onwards (school age period), we invited all 9,276 children from the original cohort of 9,749 children to participate in follow-up studies. This invitation was independent of their home address and participation in the preschool period. Of the 473 children who were not invited, 52 children died during follow-up, 106 children had withdrawn during follow-up and 315 children were lost to follow-up during the preschool period. In total, 8,305 children (90 % of those who were invited (n = 9,276) and 85 % of the original cohort (n = 9,749)) still participate in the study from the age of 5 years, of whom n = 6,690 visited the research center at the median age of 6.0 years.
Measurements
Data collection during pregnancy
Physical examinations were planned at each visit in early pregnancy, mid-pregnancy and late pregnancy and included height, weight and blood pressure measurements of both parents (Fig. 1). Since there was a wide range of gestational age at each visit, these measurements are used in the analyses as gestational age-adjusted measurements.
Mothers received four postal questionnaires and father received one postal questionnaire during pregnancy (Table 3). Topics in these questionnaires were:
-
Mother 1: medical and family history, previous pregnancies, quality of life, life style habits, housing conditions, ethnicity, and educational level;
-
Mother 2: diet, including macronutrients and micronutrients;
-
Mother 3: current pregnancy, quality of life, life style habits, and psychopathology;
-
Mother 4: current pregnancy, quality of life, life style habits, working conditions, household income, and self-esteem;
-
Father: medical history, family history, life style habits, educational level, and psychopathology.
Blood samples were collected in early (mother, father) and mid-pregnancy (mother) and at birth (child). Procedures for collection, processing and storage of biological samples have been described previously in detail [104]. Maternal and cord blood samples have been used for measuring dietary biomarkers (folate, homocystein, total vitamin B12, free vitamin B12) levels; angiogenesis biomarkers (soluble fms-like tyrosine kinase-1 (sFlft-1), Placental growth factor (PlGF)); thyroid hormone levels (thyroid-stimulating hormone (TSH), free thyroxine (FT4)) and thyroid antibody levels, and inflammation markers (high-sensitivity C-reactive protein (hs CRP) [106–111]. Urine samples of mothers have been collected from February 2004 until November 2005 and are stored for future measurements. Urine samples have been used for measurement of Chlamydia trachomatis, cannabis, pesticides, bisphenol A, and phthalates levels [112–116].
Fetal ultrasound examinations were performed at each prenatal visit. These ultrasound examinations were used for both establishing gestational age and assessing fetal growth patterns. These methods have previously been described in detail [117, 118]. Longitudinal curves of all fetal growth measurements (head circumference, biparietal diameter, abdominal circumference and femur length) were created resulting in standard deviation scores for all of these specific growth measurements. Placental haemodynamics including resistance indices of the uterine and umbilical arteries have been measured in second and third trimester [119]. Detailed measurements of fetal brain, lung, heart and kidney development have been assessed in the subcohort [120–122].
The obstetric records of mothers have been looked up in the hospitals and mid-wife practices to collect information about pregnancy outcomes. Specialists in the relevant field code items in these records [123] (Table 4).
Data collection during the preschool period
At the age of 3 months, home visits were performed to assess neuromotor development using an adapted version of Touwen’s Neurodevelopmental examination and to perform a home environment assessment [124, 125]. Information about growth (length (height), weight, head circumference) was collected at each visit to the routine child health centres in the study area using standardized procedures [126] (Table 4).
During the preschool period, parents received 8 questionnaires. One questionnaire was specifically for fathers. Items included in these questionnaires and their references are demonstrated in Table 5 [127–179]. Response rates based on the number of send questionnaires are shown in Fig. 1. Not all children received each questionnaire due to logistical constraints and implementation of questionnaires after the first group of children reached a certain age. Thus, although response rates may be similar, the absolute number of completed questionnaires differs between different ages. Response rates presented in Fig. 1 are based on the number of send questionnaires.
During the preschool period, children participating in the subgroup have been invited six times to a dedicated research center. Measurements at these visits included physical examinations (height, weight, head circumference, skinfold thickness and waist—hip ratio, Touwen’s Neurodevelopmental Examination) and ultrasound examinations (brain, cardiac and kidney structures) [180–184]. Dual X Energy Absorptiometry (DXA) scanning and Fractional exhaled Nitric Oxide (FeNO) measurements have been performed in a smaller subgroup [185, 186]. Blood pressure was measured at the age of 24 months [187]. Observations of parent–child interaction and behaviour, such as executive function, heart rate variability, infant-parent attachment, moral development, and compliance with mother and child have been repeatedly performed and with father and child once [188–192]. Biological materials have been collected if parents gave consent [193–196].
Data collection during the school period
From the age of 5 years onwards, we invite all participating children to a well-equipped and dedicated research center in the Erasmus Medical Center—Sophia Children’s Hospital every 3 years (age 6 years visit completed, age 9 years visit ongoing, and age 12 and 15 years visits planned). Currently, the total visit takes about 3 hours and all measurements are grouped in thematic 20–30 min blocks. Clinically relevant results are discussed with the parents and, if needed, children or mothers are referred to their general practitioner, paediatrician or other relevant health care provider.
At each age, we collect data with questionnaires dealing with the growth, health and physical and mental development of the children. Also we collect information on childhood diet and behaviour (Table 5). These questionnaires are being sent to the primary caregiver. From the age of 9 years, children also receive their own questionnaires.
The measurements at the research center are focused on several health outcomes including asthma, bacterial carriage, behaviour and cognition, body composition, bone health, eye and tooth development, immune status, heart and vascular development, hearing and language development, kidney growth and function, obesity, and physical appearance. Cardiovascular, metabolic and bone measurements are also conducted in mothers (Table 6).
We use various advanced imaging techniques including ultrasound and Doppler (GE LOGIQ E9, Milwaukee, WI, USA) for measuring thoracic and abdominal structures, Dual X Absorptiometry for measuring body composition and bone mineral density (iDXA scanner, GE Healthcare, Madison, WI), and 3.0 Tesla Magnetic Resonance Imaging (MRI) (Discovery MR750, GE Healthcare, Milwaukee, WI, USA) for brain imaging in subgroups of the study. Children in our study are scanned using standard imaging and positioning protocols. They are wearing light clothing without metal objects while undergoing the body scanning. Thus far, Magnetic Resonance Imaging (MRI) has been used for brain imaging in subgroups of the study using a hospital-based 3.0 Tesla MRI scanner (GE Healthcare, Milwaukee, WI, USA) [197]. We use a mock scanner, in which the children can practice to lie within the MRI scanner in a friendly way and get used to the scanner procedures. The scanner is operated by trained research technicians and all imaging data are collected according to standardized imaging protocols. Changes or updates in hardware have been avoided. Changes or updates in software configuration are minimized and regular checks with phantoms are performed to secure validity of cross-subject and cross-scan comparisons. Imaging is performed without administration of contrast agents. All imaging data are stored on a securely backed-up research picture archiving system, using programmed scripts to check for completeness of the data received. The current scanning protocol includes a 3D T1-weighted sequence, 2D PD-weighted sequence, diffusion tensor imaging (DTI), and resting state functional MRI. Total scanning time amounts to approximately 35 min. We expect to have a dedicated 3.0 Tesla MRI scanner (MR 750w, GE Healthcare, Milwaukee, WI, USA) in the Generation R research center and start with brain, lung, cardiovascular, and body fat scanning in the full cohort at age 9 years early 2013.
DNA and genome and epigenome biobank
DNA from both parents and children (cord blood) has been extracted and is used for several genotype studies. Genetic data have been generated by taqman analyses and a genome wide association scan (GWAS) using the Illumina 670 K platform in the children [3]. For genotyping, we used the infrastructure of the Genetic Laboratory of the Department of Internal Medicine (www.glimdna.org) that was also used for creation of the GWAS datasets of the Rotterdam Study, a prospective cohort study among more than 10,000 adults [198, 199]. The GWAS dataset underwent a stringent QC process, which has been described in detail previously [3]. Most GWAS analyses are strongly embedded in the Early Growth Genetics (EGG) Consortium and Early Genetics and Longitudinal Epidemiology (EAGLE) Consortium, in which several birth cohort studies combine their GWAS efforts focused on multiple outcomes in fetal life, childhood and adolescence [199–204]. These efforts have already led to successful identification of various common genetic variants related to birth weight, infant head circumference, childhood obesity and atopic dermatitis. DNA from parents is used for genotyping for candidate gene or replication studies. Recently, we started with measuring DNA methylation on a genome wide level in cord blood samples using the Illumina 450 K Infinium BeadChip, which contains 485,553 methylation sites at a single nucleotide resolution. The Illumina 450 K BeadChip provides a very good genomic coverage and requires low amounts of DNA making it ideal for use in large cohorts. We plan to measure DNA methylation at different ages to identify specific critical windows and to relate DNA methylation with expression markers and clinical outcomes.
Ethical issues
The general design, all research aims and the specific measurements in the Generation R Study have been approved by the Medical Ethical Committee of the Erasmus Medical Center, Rotterdam. New measurements will only be embedded in the study after approval of the Medical Ethical Committee. Participants are asked for their written informed consent for the four consecutive phases of the study (prenatally, birth to 4 years, 4–12 years, and from 12 years onwards). At the start of each phase, mothers and their partners receive written and oral information about the study. Even with consent of the parents, when the child is not willing to participate actively, no measurements are performed.
Follow-up and retention strategies
Thus far, loss to follow-up seems to be limited and is lower than 10 %. Major efforts are made to keep the children and parents involved in the study and to minimize loss to follow-up. Several strategies have been implemented and are currently part of the study design:
-
Addresses: new addresses of participants, which are known by the municipal health service are forwarded to the study staff;
-
Newsletters: participants receive two to four newsletters per year, in which several results of the study are presented and explained, questions of participants are answered and new research initiatives are presented;
-
Presents and discounts: all children who visit our research center receive small presents. Also, discount offers are regularly presented in the newsletter;
-
Transport costs: all costs for transport and parking related to visits to the research center are paid by Generation R;
-
Reminders for questionnaires: when the questionnaire has not been returned within 3 weeks, a kind reminder letter is send to the parents. After 6 weeks, when the questionnaire has still not been returned, the parents receive a phone call. If necessary, help for completing the questionnaire is offered and the importance of filling in the questionnaire is explained once more during this phone call;
-
Individual feedback: if clinically relevant, all results of hands-on measurements are discussed with the parents at the visit. If necessary, follow-up appointments with the general practitioner or pediatrician are planned;
-
Support for ethnic minorities: all study materials such as questionnaires, newsletters, website, and information folders are available in three languages (Dutch, English, and Turkish). Furthermore, staff from different ethnic minorities is available and able to verbally translate these materials into Arabic, French and Portuguese. With this, the study staff is able to communicate to all participants.
-
Care-cases: children and parents who showed low response rates for different measurements, showed difficulties in completing questionnaires or require additional explanation or support are considered as care-cases. Care–cases have a more individual based approach and are pro-actively contacted by one dedicated member of the study staff.
-
Home visits: We bring a visit to children and parents who cannot be contacted by phone or letter. Most visits are planned in the evenings to have higher chances that both parents and children are at home;
New methods for contacting participants, including use of internet and e-mail, are currently explored in subgroups [80].
Data management and privacy protection
Data collected by measurements in the research centers are directly entered onto written forms and into the electronic database. Data collected by questionnaires are scanned and manually entered into an electronic database by a commercial bureau. Random samples of all questionnaires are double checked by study staff members to monitor the quality of this manual data entry process. The percentage of mistakes is kept as low as possible and does not exceed 3 % per questionnaire. Open text fields are entered into the electronic database exactly as they are filled in on the questionnaires. In a secondary stage, these open text fields are cleaned and coded by a specialist in the relevant field. All measurements are centrally checked by examination of the data including their ranges, distributions, means, standard deviations, outliers and logical errors. Data outliers and missing values are checked on the original forms. The data of one specific measurement are only distributed for analyses after data collection and preparation is completed for that measurement for the whole cohort. Datasets needed for answering specific research questions are centrally built from different databases. All information in these datasets that enables identification of a particular participant (including identification number used for the logistics of the study, names and dates) is excluded before distribution to the researchers. The datasets for researchers include subject unique identification numbers that enable feedback about one subject to the data manager but do not enable identification of that particular subject.
Statistical power
Due to expected missing values and loss to follow-up, most analyses in the study are not based on data in all subjects. Therefore, power calculations demonstrated in Tables 7 and 8 are based on 7,000 subjects in the whole cohort and 700 subjects in the subgroup. The presented power calculations are rather conservative since most studies will assess the effects of continuously instead of dichotomous measured exposures and studies may be focused on outcomes collected in more than only 1 year. Furthermore, the Generation R Study has a large number of measurements repeated over time, which may increase the accuracy of measuring the true underlying value and may thereby increase the statistical power for these measurements.
Collaboration
The Generation R Study is conducted by several research groups from the Erasmus Medical Center in close collaboration with the Erasmus University Rotterdam and the Municipal Health Service Rotterdam area. Since the data collection is still ongoing and growing, the number of collaborating research groups in and outside the Netherlands is expected to increase. Various research project are performed as part of ongoing European or world wide collaboration projects. The study has an open policy in regard to collaboration with other research groups. Request for collaboration should primarily be pointed to Vincent Jaddoe (v.jaddoe@erasmusmc.nl). These requests are discussed in the Generation R Study Management Team regarding their study aims, overlap with ongoing studies, logistic consequences and financial contributions. After approval of the project by the Generation R Study Management Team and the Medical Ethical Committee of the Erasmus Medical Center, the collaborative research project is embedded in one of the research areas supervised by the corresponding principal investigator.
References
Hofman A, Jaddoe VW, Mackenbach JP, Moll HA, Snijders RF, Steegers EA, Verhulst FC, Witteman JC, Büller HA. Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol. 2004;18:61–72.
Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study: design and cohort update until the age of 4 years. Eur J Epidemiol. 2008;23(12):801–11.
Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study: design and cohort update 2010. Eur J Epidemiol. 2010;25(12):823–40.
Jaddoe VW. Fetal nutritional origins of adult diseases: challenges for epidemiological research. Eur J Epidemiol. 2008;23(12):767–71.
Geelhoed JJ, Jaddoe VW. Early influences on cardiovascular and renal development. Eur J Epidemiol. 2010;25(10):677–92.
Bakker H, Jaddoe VW. Cardiovascular and metabolic influences of fetal smoke exposure. Eur J Epidemiol. 2011;26(10):763–70.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73.
Adamsson Eryd S, Smith JG, Melander O, Hedblad B, Engstrom G. Inflammation-sensitive proteins and risk of atrial fibrillation: a population-based cohort study. Eur J Epidemiol. 2011;26(6):449–55.
Alberts VP, Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Stricker BH, et al. Heart failure and the risk of stroke: the Rotterdam Study. Eur J Epidemiol. 2010;25(11):807–12.
Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol. 2011;26(11):877–86.
Borne Y, Engstrom G, Essen B, Sundquist J, Hedblad B. Country of birth and risk of hospitalization due to heart failure: a Swedish population-based cohort study. Eur J Epidemiol. 2011;26(4):275–83.
Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: a meta-analysis. Eur J Epidemiol. 2011;26(11):833–50.
Daniel M, Paquet C, Auger N, Zang G, Kestens Y. Association of fast-food restaurant and fruit and vegetable store densities with cardiovascular mortality in a metropolitan population. Eur J Epidemiol. 2010;25(10):711–9.
Faramawi MF, Caffrey JL, Amanzadeh J, Sharpa LD, Qualls-Hampton R. Cystatin C estimated renal dysfunction predicts T wave axis deviation in US adults: results from NHANES III. Eur J Epidemiol. 2011;26(2):101–7.
Honjo K, Iso H, Inoue M, Tsugane S. Adult height and the risk of cardiovascular disease among middle aged men and women in Japan. Eur J Epidemiol. 2011;26(1):13–21.
Jacobsen BK, Knutsen SF, Oda K, Fraser GE. Parity and total, ischemic heart disease and stroke mortality. the Adventist Health Study, 1976–1988. Eur J Epidemiol. 2011;26(9):711–8.
Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman J. Distribution of echocardiographic parameters and their associations with cardiovascular risk factors in the Rotterdam Study. Eur J Epidemiol. 2010;25(7):481–90.
Kataja-Tuomola M, Sundell J, Mannisto S, Virtanen MJ, Kontto J, Albanes D, et al. Short-term weight change and fluctuation as risk factors for type 2 diabetes in Finnish male smokers. Eur J Epidemiol. 2010;25(5):333–9.
Lehto HR, Lehto S, Havulinna AS, Ketonen M, Lehtonen A, Kesaniemi YA, et al. Sex differences in short- and long-term case-fatality of myocardial infarction. Eur J Epidemiol. 2011;26(11):851–61.
Lindman AS, Veierod MB, Tverdal A, Pedersen JI, Selmer R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study. Eur J Epidemiol. 2010;25(11):789–98.
Lindschou Hansen J, Tolstrup JS, Jensen MK, Gronbaek M, Tjonneland A, Schmidt EB, et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur J Epidemiol. 2011;26(6):439–47.
Marsh RW. Predicting cardiovascular events using three stage Discriminant Function is much more accurate than Framingham or QRISK. Eur J Epidemiol. 2011;26(12):915–8.
Morkedal B, Romundstad PR, Vatten LJ. Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: the HUNT-II study. Eur J Epidemiol. 2011;26(6):457–61.
Pan XQ, Zhang YH, Liu YY, Tong WJ. Interaction between the C(-344)T polymorphism of CYP11B2 and alcohol consumption on the risk of essential hypertension in a Chinese Mongolian population. Eur J Epidemiol. 2010;25(11):813–21.
Qin L, Knol MJ, Corpeleijn E, Stolk RP. Does physical activity modify the risk of obesity for type 2 diabetes: a review of epidemiological data. Eur J Epidemiol. 2010;25(1):5–12.
Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253–60.
Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25(2):95–102.
Sonestedt E, Wirfalt E, Wallstrom P, Gullberg B, Orho-Melander M, Hedblad B. Dairy products and its association with incidence of cardiovascular disease: the Malmo diet and cancer cohort. Eur J Epidemiol. 2011;26(8):609–18.
Strandhagen E, Berg C, Lissner L, Nunez L, Rosengren A, Toren K, et al. Selection bias in a population survey with registry linkage: potential effect on socioeconomic gradient in cardiovascular risk. Eur J Epidemiol. 2010;25(3):163–72.
Yetkin E. Increased risk of stroke after the diagnosis of heart failure: is it a paradox of initiating heart failure treatment? Eur J Epidemiol. 2011;26(5):429–30.
Zhang HL, Yang Y, Wu J. Can prevalence of apolipoprotein E epsilon 4 allele explain the geographical variation of coronary heart disease mortality rates in Western Europe? Eur J Epidemiol. 2010;25(12):897–8.
Alatupa S, Pulkki-Raback L, Hintsanen M, Ravaja N, Raitakari OT, Telama R, et al. School performance as a predictor of adulthood obesity: a 21-year follow-up study. Eur J Epidemiol. 2010;25(4):267–74.
Altmaier E, Kastenmuller G, Romisch-Margl W, Thorand B, Weinberger KM, Illig T, et al. Questionnaire-based self-reported nutrition habits associate with serum metabolism as revealed by quantitative targeted metabolomics. Eur J Epidemiol. 2011;26(2):145–56.
Beyerlein A, Ruckinger S, Toschke AM, Schaffrath RA, von Kries R. Is low birth weight in the causal pathway of the association between maternal smoking in pregnancy and higher BMI in the offspring? Eur J Epidemiol. 2011;26(5):413–20.
Bot M, Spijkerman AM, Twisk JW, Verschuren WM. Weight change over five-year periods and number of components of the metabolic syndrome in a Dutch cohort. Eur J Epidemiol. 2010;25(2):125–33.
Cerqueira C, Knudsen N, Ovesen L, Laurberg P, Perrild H, Rasmussen LB, et al. Doubling in the use of thyroid hormone replacement therapy in Denmark: association to iodization of salt? Eur J Epidemiol. 2011;26(8):629–35.
Durmus B, Ay L, Hokken-Koelega AC, Raat H, Hofman A, Steegers EA, et al. Maternal smoking during pregnancy and subcutaneous fat mass in early childhood. The Generation R Study. Eur J Epidemiol. 2011;26(4):295–304.
Engeland A, Bjorge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, et al. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol. 2011;26(2):157–63.
Ghasemi A, Zahediasl S, Azizi F. Nitric oxide and clustering of metabolic syndrome components in pediatrics. Eur J Epidemiol. 2010;25(1):45–53.
Joseph J, Svartberg J, Njolstad I, Schirmer H. Risk factors for type 2 diabetes in groups stratified according to metabolic syndrome: a 10-year follow-up of the Tromso Study. Eur J Epidemiol. 2011;26(2):117–24.
Kowall B, Rathmann W, Strassburger K, Heier M, Holle R, Thorand B, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol. 2010;25(6):393–402.
Laaksonen MA, Knekt P, Rissanen H, Harkanen T, Virtala E, Marniemi J, et al. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur J Epidemiol. 2010;25(2):115–24.
Lagiou P, Samoli E, Lipworth L, Lagiou A, Fang F, Rossi M, et al. Energy intake during pregnancy in relation to offspring gender by maternal height. Eur J Epidemiol. 2011;26(1):39–44.
Lehto SM, Ruusunen A, Niskanen L, Tolmunen T, Voutilainen S, Viinamaki H, et al. Elevated depressive symptoms and compositional changes in LDL particles in middle-aged men. Eur J Epidemiol. 2010;25(6):403–9.
Liu G, Zhu H, Dong Y, Podolsky RH, Treiber FA, Snieder H. Influence of common variants in FTO and near INSIG2 and MC4R on growth curves for adiposity in African- and European-American youth. Eur J Epidemiol. 2011;26(6):463–73.
Moebus S, Gores L, Losch C, Jockel KH. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol. 2011;26(9):719–28.
Montonen J, Drogan D, Joost HG, Boeing H, Fritsche A, Schleicher E, et al. Estimation of the contribution of biomarkers of different metabolic pathways to risk of type 2 diabetes. Eur J Epidemiol. 2011;26(1):29–38.
Morseth B, Emaus N, Wilsgaard T, Jacobsen BK, Jorgensen L. Leisure time physical activity in adulthood is positively associated with bone mineral density 22 years later. The Tromso study. Eur J Epidemiol. 2010;25(5):325–31.
Nagaya T, Yoshida H, Takahashi H, Kawai M. Heart rate-corrected QT interval in resting ECG predicts the risk for development of type-2 diabetes mellitus. Eur J Epidemiol. 2010;25(3):195–202.
Schottker B, Raum E, Rothenbacher D, Muller H, Brenner H. Prognostic value of haemoglobin A1c and fasting plasma glucose for incident diabetes and implications for screening. Eur J Epidemiol. 2011;26(10):779–87.
Schuur M, Henneman P, van Swieten JC, Zillikens MC, de Koning I, Janssens AC, et al. Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. Eur J Epidemiol. 2010;25(8):561–8.
van Noord C, Dorr M, Sturkenboom MC, Straus SM, Reffelmann T, Felix SB, et al. The association of serum testosterone levels and ventricular repolarization. Eur J Epidemiol. 2010;25(1):21–8.
Castello A, Rio I, Sandin-Vazquez M, Bolumar F. Shortening of gestational length among native-born and immigrants in Spain (1997–2008). Eur J Epidemiol. 2011;26(7):563–70.
Feart C, Peres K, Samieri C, Letenneur L, Dartigues JF, Barberger-Gateau P. Adherence to a Mediterranean diet and onset of disability in older persons. Eur J Epidemiol. 2011;26(9):747–56.
Fraga AM, Fraga GP, Stanley C, Costantini TW, Coimbra R. Children at danger: injury fatalities among children in San Diego County. Eur J Epidemiol. 2010;25(3):211–7.
Heys M, Jiang C, Schooling CM, Zhang W, Cheng KK, Lam TH, et al. Is childhood meat eating associated with better later adulthood cognition in a developing population? Eur J Epidemiol. 2010;25(7):507–16.
Huisman M, Araya R, Lawlor DA, Ormel J, Verhulst FC, Oldehinkel AJ. Cognitive ability, parental socioeconomic position and internalising and externalising problems in adolescence: findings from two European cohort studies. Eur J Epidemiol. 2010;25(8):569–80.
Liu J, Blair SN, Teng Y, Ness AR, Lawlor DA, Riddoch C. Physical activity during pregnancy in a prospective cohort of British women: results from the Avon longitudinal study of parents and children. Eur J Epidemiol. 2011;26(3):237–47.
Reuser M, Willekens FJ, Bonneux L. Higher education delays and shortens cognitive impairment: a multistate life table analysis of the US Health and Retirement Study. Eur J Epidemiol. 2011;26(5):395–403.
Strand BH, Cooper R, Hardy R, Kuh D, Guralnik J. Lifelong socioeconomic position and physical performance in midlife: results from the British 1946 birth cohort. Eur J Epidemiol. 2011;26(6):475–83.
Thomas S, Heinrich S, von Kries R, Radon K. Exposure to radio-frequency electromagnetic fields and behavioural problems in Bavarian children and adolescents. Eur J Epidemiol. 2010;25(2):135–41.
Autenrieth CS, Baumert J, Baumeister SE, Fischer B, Peters A, Doring A, et al. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol. 2011;26(2):91–9.
Behrens G, Leitzmann MF, Sandin S, Lof M, Heid IM, Adami HO, et al. The association between alcohol consumption and mortality: the Swedish women’s lifestyle and health study. Eur J Epidemiol. 2011;26(2):81–90.
Bellocco R, Jia C, Ye W, Lagerros YT. Effects of physical activity, body mass index, waist-to-hip ratio and waist circumference on total mortality risk in the Swedish National March Cohort. Eur J Epidemiol. 2010;25(11):777–88.
Berard E, Bongard V, Arveiler D, Amouyel P, Wagner A, Dallongeville J, et al. Ten-year risk of all-cause mortality: assessment of a risk prediction algorithm in a French general population. Eur J Epidemiol. 2011;26(5):359–68.
Carlsson AC, Theobald H, Wandell PE. Health factors and longevity in men and women: a 26-year follow-up study. Eur J Epidemiol. 2010;25(8):547–51.
Empana JP, Bean K, Guibout C, Thomas F, Bingham A, Pannier B, et al. Paris Prospective Study III: a study of novel heart rate parameters, baroreflex sensitivity and risk of sudden death. Eur J Epidemiol. 2011;26(11):887–92.
Faeh D, Braun J, Tarnutzer S, Bopp M. Obesity but not overweight is associated with increased mortality risk. Eur J Epidemiol. 2011;26(8):647–55.
Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol. 2011;26(7):537–46.
Kerneis S, Boelle PY, Grais RF, Pavillon G, Jougla E, Flahault A, et al. Mortality trends in systemic sclerosis in France and USA, 1980–1998: an age-period-cohort analysis. Eur J Epidemiol. 2010;25(1):55–61.
Kjelsberg E, Laake P. Is the high mortality risk in sentenced offenders independent of previous imprisonment? Eur J Epidemiol. 2010;25(4):237–43.
Kowall B, Rathmann W, Heier M, Giani G, Peters A, Thorand B, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol. 2011;26(8):637–45.
Kvamme JM, Wilsgaard T, Florholmen J, Jacobsen BK. Body mass index and disease burden in elderly men and women: the Tromso Study. Eur J Epidemiol. 2010;25(3):183–93.
Mackenbach JP, Slobbe L, Looman CW, van der Heide A, Polder J, Garssen J. Sharp upturn of life expectancy in the Netherlands: effect of more health care for the elderly? Eur J Epidemiol. 2011;26(12):903–14.
Neovius K, Rasmussen F, Sundstrom J, Neovius M. Forecast of future premature mortality as a result of trends in obesity and smoking: nationwide cohort simulation study. Eur J Epidemiol. 2010;25(10):703–9.
Niedhammer I, Bourgkard E, Chau N, Lorhandicap Study G. Occupational and behavioural factors in the explanation of social inequalities in premature and total mortality: a 12.5-year follow-up in the Lorhandicap study. Eur J Epidemiol. 2011;26(1):1–12.
Oksuzyan A, Crimmins E, Saito Y, O’Rand A, Vaupel JW, Christensen K. Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. Eur J Epidemiol. 2010;25(7):471–80.
Pedersen GS, Mortensen LH, Andersen AM. Ethnic variations in mortality in pre-school children in Denmark, 1973–2004. Eur J Epidemiol. 2011;26(7):527–36.
Puddu PE, Menotti A, Tolonen H, Nedeljkovic S, Kafatos AG. Determinants of 40-year all-cause mortality in the European cohorts of the Seven Countries Study. Eur J Epidemiol. 2011;26(8):595–608.
Regidor E, Astasio P, Ortega P, Martinez D, Calle ME, de la Fuente L. Healthy and unhealthy migrant effect on the mortality of immigrants from wealthy countries residing in Spain. Eur J Epidemiol. 2011;26(4):265–73.
Savela S, Koistinen P, Tilvis RS, Strandberg AY, Pitkala KH, Salomaa VV, et al. Leisure-time physical activity, cardiovascular risk factors and mortality during a 34-year follow-up in men. Eur J Epidemiol. 2010;25(9):619–25.
Tamakoshi K, Yatsuya H, Tamakoshi A, Group JS. Early age at menarche associated with increased all-cause mortality. Eur J Epidemiol. 2011;26(10):771–8.
Tolonen H, Laatikainen T, Helakorpi S, Talala K, Martelin T, Prattala R. Marital status, educational level and household income explain part of the excess mortality of survey non-respondents. Eur J Epidemiol. 2010;25(2):69–76.
Waller K, Kujala UM, Rantanen T, Kauppinen M, Silventoinen K, Koskenvuo M, et al. Physical activity, morbidity and mortality in twins: a 24-year prospective follow-up. Eur J Epidemiol. 2010;25(10):731–9.
Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25(6):375–84.
Ngo AD, Taylor R, Roberts CL. Paternal exposure to Agent Orange and spina bifida: a meta-analysis. Eur J Epidemiol. 2010;25(1):37–44.
Risselada R, Lingsma HF, Bauer-Mehren A, Friedrich CM, Molyneux AJ, Kerr RS, et al. Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the International Subarachnoid Aneurysm Trial (ISAT). Eur J Epidemiol. 2010;25(4):261–6.
Sellier E, Surman G, Himmelmann K, Andersen G, Colver A, Krageloh-Mann I, et al. Trends in prevalence of cerebral palsy in children born with a birthweight of 2,500 g or over in Europe from 1980 to 1998. Eur J Epidemiol. 2010;25(9):635–42.
Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–55.
Both MI, Overvest MA, Wildhagen MF, Golding J, Wildschut HI. The association of daily physical activity and birth outcome: a population-based cohort study. Eur J Epidemiol. 2010;25(6):421–9.
Diouf I, Charles MA, Thiebaugeorges O, Forhan A, Kaminski M, Heude B, et al. Maternal weight change before pregnancy in relation to birthweight and risks of adverse pregnancy outcomes. Eur J Epidemiol. 2011;26(10):789–96.
Greenwood DC, Alwan N, Boylan S, Cade JE, Charvill J, Chipps KC, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol. 2010;25(4):275–80.
Gudmundsson P, Andersson S, Gustafson D, Waern M, Ostling S, Hallstrom T, et al. Depression in Swedish women: relationship to factors at birth. Eur J Epidemiol. 2011;26(1):55–60.
Timmermans S, Bonsel GJ, Steegers-Theunissen RP, Mackenbach JP, Steyerberg EW, Raat H, et al. Individual accumulation of heterogeneous risks explains perinatal inequalities within deprived neighbourhoods. Eur J Epidemiol. 2011;26(2):165–80.
Cnattingius S, Svensson T, Granath F, Iliadou A. Maternal smoking during pregnancy and risks of suicidal acts in young offspring. Eur J Epidemiol. 2011;26(6):485–92.
Danziger PD, Silverwood R, Koupil I. Fetal growth, early life circumstances, and risk of suicide in late adulthood. Eur J Epidemiol. 2011;26(7):571–81.
Slachtova H, Gehring U, Hoek G, Tomaskova H, Luttmann-Gibson H, Moshammer H, et al. Parental education and lung function of children in the PATY study. Eur J Epidemiol. 2011;26(1):45–54.
Gimeno D, Delclos GL, Ferrie JE, De Vogli R, Elovainio M, Marmot MG, et al. Association of CRP and IL-6 with lung function in a middle-aged population initially free from self-reported respiratory problems: the Whitehall II study. Eur J Epidemiol. 2011;26(2):135–44.
Centre for Research and Statistics, Rotterdam (COS). http://www.cos.rotterdam.nl; 2012.
Statistics Netherlands. Allochtonen in Nederland 2004. Voorburg/Heerlen: Statistics Netherlands; 2004.
Troe EJ, Raat H, Jaddoe VW, Hofman A, Looman CW, Moll HA, Steegers EA, Verhulst FC, Witteman JC, Mackenbach JP, The Generation R Study. Explaining differences in birthweight between ethnic populations. BJOG. 2007;114:1557–65.
Silva LM, Jansen PW, Steegers EA, Jaddoe VW, Arends LR, Tiemeier H, Verhulst FC, Moll HA, Hofman A, Mackenbach JP, Raat H. Mother’s educational level and fetal growth: the genesis of health inequalities. Int J Epidemiol. 2010;39(5):1250–61.
Raat H, Wijtzes A, Jaddoe VW, Moll HA, Hofman A, Mackenbach JP. The health impact of social disadvantage in early childhood; the Generation R Study. Early Hum Dev. 2011;87(11):729–33.
Statistics Netherlands. Standaard Onderwijsindeling 2003. Voorburg/Heerlen: Statistics Netherlands; 2004.
Jacobsen TN, Nohr EA, Frydenberg M. Selection by socioeconomic factors into the Danish National Birth Cohort. Eur J Epidemiol. 2010;25(5):349–55.
Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22:917–23.
Bergen NE, Jaddoe VW, Timmermans S, Hofman A, Lindemans J, Russcher H, Raat H, Steegers-Theunissen RP, Steegers EA. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG. 2012;119(6):739–51.
Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr. 2012;95(6):1413–21.
Coolman M, Timmermans S, de Groot CJ, Russcher H, Lindemans J, Hofman A, Geurts-Moespot AJ, Sweep FC, Jaddoe VV, Steegers EA. Angiogenic and fibrinolytic factors in blood during the first half of pregnancy and adverse pregnancy outcomes. Obstet Gynecol. 2012;119(6):1190–200.
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, Tiemeier H. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the Generation R Study. J Clin Endocrinol Metab. 2010;95(9):4227–34.
Ernst GD, de Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA, Jaddoe VW. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. Am J Obstet Gynecol. 2011;205(2):132.e1–12.
Rours GI, Verkooyen RP, Willemse HF, van der Zwaan EA, van Belkum A, de Groot R, Verbrugh HA, Ossewaarde JM. Use of pooled urine samples and automated DNA isolation to achieve improved sensitivity and cost-effectiveness of large-scale testing for Chlamydia trachomatis in pregnant women. R J Clin Microbiol. 2005;43:4684–90.
Rours GI, de Krijger RR, Ott A, Willemse HF, de Groot R, Zimmermann LJ, et al. Chlamydia trachomatis and placental inflammation in early preterm delivery. Eur J Epidemiol. 2011;26(5):421–8.
Rours GI, Duijts L, Moll HA, Arends LR, de Groot R, Jaddoe VW, Hofman A, Steegers EA, Mackenbach JP, Ott A, Willemse HF, van der Zwaan EA, Verkooijen RP, Verbrugh HA. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol. 2011;26(6):493–502.
El Marroun H, Tiemeier H, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC. Agreement between maternal cannabis use during pregnancy according to self-report and urinalysis in a population-based cohort: the Generation R Study. Eur Addict Res. 2011;17(1):37–43.
Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP, Steegers EA, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R Study. Environ Res. 2008;108:260–7.
Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31:388–96.
Gaillard R, de Ridder MA, Verburg BO, Witteman JC, Mackenbach JP, Moll HA, et al. Individually customised fetal weight charts derived from ultrasound measurements: the Generation R Study. Eur J Epidemiol. 2011;26(12):919–26.
Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Second and third trimester placental haemodynamics and the risks of pregnancy complications. The Generation R Study. Am J Epidemiol. 2012 (In press).
Verburg BO, Jaddoe VW, Wladimiroff JW, Hofman A, Witteman JC, Steegers EA. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation. 2008;117:649–59.
Verburg BO, Geelhoed JJ, Steegers EA, Hofman A, Moll HA, Witteman JC, Jaddoe VW. Fetal kidney volume and its association with growth and blood flow in fetal life: the Generation R Study. Kidney Int. 2007;72:754–61.
Roza SJ, Steegers EA, Verburg BO, Jaddoe VW, Moll HA, Hofman A, Verhulst FC, Tiemeier H. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol. 2008;168(10):1145–52.
Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010 Feb 26.
van Batenburg-Eddes T, de Groot L, Arends L, de Vries A, Moll HA, Steegers EA, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Does gestational duration within the normal range predict infant neuromotor development? Early Hum Dev. 2008;84:659–65.
Rijlaarsdam J, Stevens GW, van der Ende J, Arends LR, Hofman A, Jaddoe VW, Mackenbach JP, Verhulst FC, Tiemeier H. A brief observational instrument for the assessment of infant home environment: development and psychometric testing. Int J Methods Psychiatr Res. 2012;21(3):195–204.
Burgmeijer RJF, Merkx JAM. Pakket...En hoe pakt het uit: Ouder en Kindzorg tussen wetenschap en praktijk. Nederlands Congres Ouder en Kindzorg. Assen, the Netherlands: van Gorcum; 1999.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF- 36). I. Conceptual framework and item selection. Med Care 1992;30:473–83.
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613.
Carver DJ, Chapman CA, Thomas VS, Stadnyk KJ, Rockwood K. Validity and reliability of the Medical Outcomes Study Short Form-20 questionnaire as a measure of quality of life in elderly people living at home. Age Ageing. 1999;28:169–74.
Israel AC, Roderick HA. A measure of the stability of family activities: an initial examination. Assessment. 2001;8(4):417–24.
De Brock AJLL, Vermulst AA, Gerris JRM, Abidin RR. Nijmeegse Ouderlijke Stress Index. Lisse 1992: Swets en Zeitlinger b.v.
Gerris JR, Boxtel DA, Vermulst AA, Janssens JM, van Zuthpen RA, Felling AJ. Parenting in Dutch families, University of Nijmegen, Institute of Family Studies, Nijmegen, The Netherlands; 1993.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10 item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-6.
Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605.
Epstein N, Baldwin L, Bishop D. The McMaster family assessment device. J Marital Fam Ther 1983;9(2):171–80.
van Rossem L, Oenema A, Steegers EA, Moll HA, Jaddoe VW, Hofman A, Mackenbach JP, Raat H. Are starting and continuing breastfeeding related to educational background? The Generation R Study. Pediatrics. 2009;123(6):e1017–27.
Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:(1):e18–25.
Wardle J, Guthrie CA, Sanderson S, Rapoport LJ. Development of the Children’s Eating Behaviour Questionnaire. Child Psychol Psychiatry. 2001;42:963–70.
Landgraf JM, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res. 1998;7(5):433–45.
van der Horst K, Oenema A, van de Looij-Jansen P, Brug J. The ENDORSE study: research into environmental determinants of obesity related behaviors in Rotterdam schoolchildren. BMC Public Health. 2008;8:142.
Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36(3):201–10.
Veldhuis L, Struijk MK, Kroeze W, Oenema A, Renders CM, Bulk-Bunschoten AM, et al. ‘Be active, eat right’, evaluation of an overweight prevention protocol among 5-year-old children: design of a cluster randomised controlled trial. BMC Public Health. 2009;9:177.
Owens J, Maxim R, McGuinn M, Nobile C, Msall M, Alario A. Television-viewing habits and sleep disturbance in school children. Pediatrics. 1999;104(3):e27.
Raat H, Botterweck AM, Landgraf JM, Hoogeveen WC, Essink-Bot ML. Reliability and validity of the short form of the child health questionnaire for parents (CHQ-PF28) in large random school based and general population samples. J Epidemiol Community Health. 2005;59:75–82.
Raat H, Mohangoo AD, Grootenhuis MA. Pediatric health-related quality of life questionnaires in clinical trials. Curr Opin Allergy Clin Immunol. 2006;6(3):180–5.
Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the infant and toddler quality of life questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16(3):445–60.
Raat H, van Rossem L, Jaddoe VW, Landgraf JM, Feeny D, Moll HA, Hofman A, Mackenbach JP. The Generation R Study: a candidate gene study and genome-wide association study (GWAS) on health-related quality of life (HRQOL) of mothers and young children. Qual Life Res. 2010;19(10):1439–46.
Duijts L, Jaddoe VW, Hofman A, Steegers EA, Mackenbach JP, de Jongste JC, Moll HA. Maternal smoking in prenatal and early postnatal life and the risk of respiratory tract infections in infancy. The Generation R Study. Eur J Epidemiol. 2008;23(8):547–55.
Jenkins MA, Clarke JR. Validation of a questionnaire and bronchial hyper-responsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–16.
Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91.
Weiland SK, Bjorksten B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP, et al. Phase II of the international study of asthma and allergies in childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24(3):406–12.
Flohr C, Weinmayr G, Weiland SK, Addo-Yobo E, Annesi-Maesano I, Bjorksten B, et al. How well do questionnaires perform compared with physical examination in detecting flexural eczema? Findings from the international study of asthma and allergies in childhood (ISAAC) phase two. Br J Dermatol. 2009;161(4):846–53.
Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J Bone Miner Res. 2008;23(2):173–9.
van Beelen ME, Beirens TM, Struijk MK, den Hertog P, Oenema A, van Beeck EF, Raat H. BeSAFE’, effect-evaluation of internet-based, tailored safety information combined with personal counselling on parents’ child safety behaviours: study design of a randomized controlled trial. BMC Public Health. 2010;10:466. doi:10.1186/1471-2458-10-466.
Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37.
Vogel I, Verschuure H, van der Ploeg CP, Brug J, Raat H. Estimating adolescent risk for hearing loss based on data from a large school-based survey. Am J Public Health. 2010;100(6):1095–100.
St James-Roberts I. Persistent crying in infancy. J Child Psychol Psychiatry. 1989;30(2):189–95.
van den Berg MP, van der Ende J, Crijnen AA, Jaddoe VW, Moll HA, Mackenbach JP, Hofman A, Hengeveld MW, Tiemeier H, Verhulst FC. Paternal depressive symptoms during pregnancy are related to excessive infant crying. Pediatrics. 2009;124(1):e96–103.
Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. 2003;26:64–86.
Putnam SP, Rothbart MK. Development of short and very short forms of the Children’s Behavior Questionnaire. J Pers Assess. 2006;87(1):102–12.
Kochanska G, DeVet K, Goldman M, Murray K, Putnam SP. Maternal reports of conscience development and temperament in young children. Child Dev. 1994;65(3):852–68.
Ireton H, Glascoe FP. Assessing children’s development using parents’ reports. The Child Development Inventory. Clin Pediatr. 1995;34:248–55.
St James-Roberts I, Wolke D. Differences between maternal and objective ratings of difficult neonatal behavioural style: implications for temperament research and clinical perspectives. J Reprod Infant Psychol. 1983;1:53–60.
St James-Roberts I, Wolke D. Convergences and discrepancies, among mothers’ and professionals’ assessments of difficult neonatal behaviour. J Child Psychol Psych All Discipl; 1989;29:21–42.
Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington: University of Vermont Department of Psychiatry; 1991.
Verhulst FC, Van der Ende J, Koot HM. Manual for the CBCL/4-18. Rotterdam, the Netherlands: Erasmus Universiteit/ Dept of Child and Adolescent Psychiatry, Sophia Children’s Hospital; 1996.
Walker LS, Garber SJ, van Slyke DA. Development and validation of the pain response inventory for children. Psychol Assess. 1997;9:392–405.
Wolff NJ, Darlington AS, Hunfeld JA, Verhulst FC, Jaddoe VW, Moll HA, Hofman A, Passchier J, Tiemeier H. The association of parent behaviors, chronic pain, and psychological problems with venipuncture distress in infants: the Generation R study. Health Psychol. 2009;28(5):605–13.
Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, et al. Pain in children and adolescents: a common experience. Pain. 2000;87(1):51–8.
Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP. MacArthur communicative development inventories; user’s guide and technical manual. San Diego: Singular Publishing Group, Inc.; 1983.
Saudino KJ, Dale PS, Oliver B, Petrill SA, Richardson V, Rutter M, Simonoff E, Stevenson J, Plomin R. The validity of parent-based assessment of cognitive abilities of 2-year-olds. Br J Develop Psychol. 1998;16:349–363.
Isquith PK, Gioia GA, Espy KA. Executive function in preschool children: examination through everyday behavior. Dev Neuropsychol. 2004;26:403–22.
Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–6.
Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–33.
St James-Roberts I, Halil T. Infant crying patterns in the first year: normal community and clinical findings. J Child Psychol Psychiatry. 1991;32:951–968.
Hoekstra RA, Vinkhuyzen AA, Wheelwright S, Bartels M, Boomsma DI, Baron-Cohen S, et al. The construction and validation of an abridged version of the autism-spectrum quotient (AQ-Short). J Autism Dev Disord. 2011;41(5):589–96.
Parker JG, Low CM, Walker AR, Gamm BK. Friendship jealousy in young adolescents: individual differences and links to sex, self-esteem, aggression, and social adjustment. Dev Psychol. 2005;41(1):235–50.
Veldhuis L, Struijk MK, Kroeze W, Oenema A, Renders CM, Bulk-Bunschoten AM, Hirasing RA, Raat H. ‘Be active, eat right’, evaluation of an overweight prevention protocol among 5-year-old children: design of a cluster randomised controlled trial. BMC Public Health. 2009;9:177.
Wijtzes AI, Jansen W, Kamphuis CB, Jaddoe VW, Moll HA, Tiemeier H, Verhulst FC, Hofman A, Mackenbach JP, Raat H. Increased risk of exceeding entertainment-media guidelines in preschool children from low socioeconomic background: the Generation R Study. Prev Med. 2012. doi:10.1016/j.ypmed.2012.07.023.
Ay L, Hokken-Koelega AC, Mook-Kanamori DO, Hofman A, Moll HA, Mackenbach JP, Witteman JC, Steegers EA, Jaddoe VW. Tracking and determinants of subcutaneous fat mass in early childhood: the Generation R Study. Int J Obes. 2008;32:1050–9.
Durmus B, Mook-Kanamori DO, Holzhauer S, Hofman A, van der Beek EM, Boehm G, Steegers EA, Jaddoe VW. Growth in foetal life and infancy is associated with abdominal adiposity at the age of 2 years: the Generation R Study. Clin Endocrinol (Oxf). 2010;72(5):633–40.
Roza SJ, Govaert PP, Vrooman HA, Lequin MH, Hofman A, Steegers EA, Moll HA, Jaddoe VW, Verhulst FC, Tiemeier H. Foetal growth determines cerebral ventricular volume in infants. The Generation R Study. Neuroimage. 2008;39(4):1491–8.
de Jonge LL, van Osch-Gevers L, Willemsen SP, Steegers EA, Hofman A, Helbing WA, Jaddoe VW. Growth, obesity, and cardiac structures in early childhood: the Generation R Study. Hypertension. 2011;57(5):934–40.
Geelhoed JJ, Verburg BO, Nauta J, Lequin M, Hofman A, Moll HA, Witteman JC, van der Heijden AJ, Steegers EA, Jaddoe VW. Tracking and determinants of kidney size from fetal life until the age of 2 years: the Generation R Study. Am J Kidney Dis. 2008. doi:10.1053/j.ajkd.2008.07.030.
Ay L, Van Houten VA, Steegers EA, Hofman A, Witteman JC, Jaddoe VW, Hokken-Koelega AC. Fetal and postnatal growth and body composition at 6 months of age. J Clin Endocrinol Metab. 2009;94(6):2023–30.
Gabriele C, Asgarali R, Jaddoe VW, Hofman A, Moll HA, de Jongste JC. Smoke exposure, airway symptoms and exhaled nitric oxide in infants: the Generation R Study. Eur Respir J. 2008;32:307–13.
van Houten VA, Steegers EA, Witteman JC, Moll HA, Hofman A, Jaddoe VW. Fetal and postnatal growth and blood pressure at the age of 2 years. The Generation R Study. J Hypertens. 2009;27(6):1152–7.
Dierckx B, Tulen JH, van den Berg MP, Tharner A, Jaddoe VW, Moll HA, Hofman A, Verhulst FC, Tiemeier H. Maternal psychopathology influences infant heart rate variability: generation R Study. Psychosom Med. 2009;71(3):313–21.
Ghassabian A, Herba CM, Roza SJ, Govaert P, Schenk JJ, Jaddoe VW, Hofman A, White T, Verhulst FC, Tiemeier H. Infant brain structures, executive function, and attention deficit/hyperactivity problems at preschool age. A prospective study. J Child Psychol Psychiatry. 2012. doi:10.1111/j.1469-7610.2012.02590.x.
Tharner A, Luijk MP, Raat H, Ijzendoorn MH, Bakermans-Kranenburg MJ, Moll HA, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. Breastfeeding and its relation to maternal sensitivity and infant attachment. J Dev Behav Pediatr. 2012;33(5):396–404.
Kok R, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Velders FP, Linting M, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. The role of maternal stress during pregnancy, maternal discipline, and child COMT Val158Met genotype in the development of compliance. Dev Psychobiol. 2012. doi:10.1002/dev.21049.
Kok R, van Ijzendoorn MH, Linting M, Bakermans-Kranenburg MJ, Tharner A, Luijk MP, Székely E, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. Attachment insecurity predicts child active resistance to parental requests in a compliance task. Child Care Health Dev. 2012. doi:10.1111/j.1365-2214.2012.01374.x.
Labout JA, Duijts L, Arends LR, Jaddoe VW, Hofman A, de Groot R, Verbrugh HA, Hermans PW, Moll HA. Factors associated with pneumococcal carriage in healthy dutch infants: the generation R study. J Pediatr. 2008;153:771–6.
Labout JA, Duijts L, Lebon A, de Groot R, Hofman A, Jaddoe VV, et al. Risk factors for otitis media in children with special emphasis on the role of colonization with bacterial airway pathogens: the Generation R Study. Eur J Epidemiol. 2011;26(1):61–6.
Duijts L, Bakker-Jonges LE, Labout JA, Jaddoe VW, Hofman A, Steegers EA, Van Dongen JJ, Hooijkaas H, Moll HA. Perinatal stress influences lymphocyte subset counts in neonates. The Generation R Study. Pediatr Res. 2008;63:292–8.
Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Kirschbaum C, Verhulst FC, Tiemeier H. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Horm Behav. 2010;57(2):247–54.
Langeslag SJ, Schmidt M, Ghassabian A, Jaddoe VW, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White TJ. Functional connectivity between parietal and frontal brain regions and intelligence in young children: The Generation R Study. Hum Brain Mapp. 2012. doi:10.1002/hbm.22143.
Hofman A, van Duijn CM, Franco OH, Ikram MA, Janssen HL, Klaver CC, Kuipers EJ, Nijsten TE, Stricker BH, Tiemeier H, Uitterlinden AG, Vernooij MW, Witteman JC. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol. 2011;26(8):657–86. doi:10.1007/s10654-011-9610-5.
Ikram MA, van der Lugt A, Niessen WJ, Krestin GP, Koudstaal PJ, Hofman A, et al. The Rotterdam Scan Study: design and update up to 2012. Eur J Epidemiol. 2011;26(10):811–24.
Freathy RM, Mook-Kanamori DO, Sovio U, Prokopenko I, Timpson NJ, Berry DJ, Warrington NM, Widen E, Hottenga JJ, Kaakinen M, Lange LA, Bradfield JP, Kerkhof M, Marsh JA, Magi R, Chen CM, Lyon HN, Kirin M, Adair LS, Aulchenko YS, Bennett AJ, Borja JB, Bouatia-Naji N, Charoen P, Coin LJ, Cousminer DL, de Geus EJ, Deloukas P, Elliott P, Evans DM, Froguel P, Genetic Investigation of ATC, Glaser B, Groves CJ, Hartikainen AL, Hassanali N, Hirschhorn JN, Hofman A, Holly JM, Hypponen E, Kanoni S, Knight BA, Laitinen J, Lindgren CM, Meta-Analyses of G, Insulin-related traits C, McArdle WL, O’Reilly PF, Pennell CE, Postma DS, Pouta A, Ramasamy A, Rayner NW, Ring SM, Rivadeneira F, Shields BM, Strachan DP, Surakka I, Taanila A, Tiesler C, Uitterlinden AG, van Duijn CM, Wellcome Trust Case Control C, Wijga AH, Willemsen G, Zhang H, Zhao J, Wilson JF, Steegers EA, Hattersley AT, Eriksson JG, Peltonen L, Mohlke KL, Grant SF, Hakonarson H, Koppelman GH, Dedoussis GV, Heinrich J, Gillman MW, Palmer LJ, Frayling TM, Boomsma DI, Davey Smith G, Power C, Jaddoe VW, Jarvelin MR, Early Growth Genetics C, McCarthy MI. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet. 2010;42(5):430–5.
Taal HR, St Pourcain B, Thiering E, Das S, Mook-Kanamori DO, Warrington NM, Kaakinen M, Kreiner-Moller E, Bradfield JP, Freathy RM, Geller F, Guxens M, Cousminer DL, Kerkhof M, Timpson NJ, Ikram MA, Beilin LJ, Bonnelykke K, Buxton JL, Charoen P, Chawes BL, Eriksson J, Evans DM, Hofman A, Kemp JP, Kim CE, Klopp N, Lahti J, Lye SJ, McMahon G, Mentch FD, Muller-Nurasyid M, O'Reilly PF, Prokopenko I, Rivadeneira F, Steegers EA, Sunyer J, Tiesler C, Yaghootkar H, Cohorts for H, Aging Research in Genetic Epidemiology C, Breteler MM, Decarli C, Debette S, Fornage M, Gudnason V, Launer LJ, van der Lugt A, Mosley TH, Jr., Seshadri S, Smith AV, Vernooij MW, Early G, Lifecourse Epidemiology C, Blakemore AI, Chiavacci RM, Feenstra B, Fernandez-Banet J, Grant SF, Hartikainen AL, van der Heijden AJ, Iniguez C, Lathrop M, McArdle WL, Molgaard A, Newnham JP, Palmer LJ, Palotie A, Pouta A, Ring SM, Sovio U, Standl M, Uitterlinden AG, Wichmann HE, Vissing NH, van Duijn CM, McCarthy MI, Koppelman GH, Estivill X, Hattersley AT, Melbye M, Bisgaard H, Pennell CE, Widen E, Hakonarson H, Smith GD, Heinrich J, Jarvelin MR, Jaddoe VW. Common variants at 12q15 and 12q24 are associated with infant head circumference. Nat Genet. 2012;44:532–8.
Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, Hypponen E, Holst C, Valcarcel B, Thiering E, Salem RM, Schumacher FR, Cousminer DL, Sleiman PM, Zhao J, Berkowitz RI, Vimaleswaran KS, Jarick I, Pennell CE, Evans DM, St Pourcain B, Berry DJ, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, van der Valk RJ, de Jongste JC, Postma DS, Boomsma DI, Gauderman WJ, Hassanein MT, Lindgren CM, Magi R, Boreham CA, Neville CE, Moreno LA, Elliott P, Pouta A, Hartikainen AL, Li M, Raitakari O, Lehtimaki T, Eriksson JG, Palotie A, Dallongeville J, Das S, Deloukas P, McMahon G, Ring SM, Kemp JP, Buxton JL, Blakemore AI, Bustamante M, Guxens M, Hirschhorn JN, Gillman MW, Kreiner-Moller E, Bisgaard H, Gilliland FD, Heinrich J, Wheeler E, Barroso I, O'Rahilly S, Meirhaeghe A, Sorensen TI, Power C, Palmer LJ, Hinney A, Widen E, Farooqi IS, McCarthy MI, Froguel P, Meyre D, Hebebrand J, Jarvelin MR, Jaddoe VW, Smith GD, Hakonarson H, Grant SF, Early Growth Genetics C. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44:526–31.
Paternoster L, Standl M, Chen CM, Ramasamy A, Bonnelykke K, Duijts L, Ferreira MA, Alves AC, Thyssen JP, Albrecht E, Baurecht H, Feenstra B, Sleiman PM, Hysi P, Warrington NM, Curjuric I, Myhre R, Curtin JA, Groen-Blokhuis MM, Kerkhof M, Saaf A, Franke A, Ellinghaus D, Folster-Holst R, Dermitzakis E, Montgomery SB, Prokisch H, Heim K, Hartikainen AL, Pouta A, Pekkanen J, Blakemore AI, Buxton JL, Kaakinen M, Duffy DL, Madden PA, Heath AC, Montgomery GW, Thompson PJ, Matheson MC, Le Souef P, Australian Asthma Genetics C, St Pourcain B, Smith GD, Henderson J, Kemp JP, Timpson NJ, Deloukas P, Ring SM, Wichmann HE, Muller-Nurasyid M, Novak N, Klopp N, Rodriguez E, McArdle W, Linneberg A, Menne T, Nohr EA, Hofman A, Uitterlinden AG, van Duijn CM, Rivadeneira F, de Jongste JC, van der Valk RJ, Wjst M, Jogi R, Geller F, Boyd HA, Murray JC, Kim C, Mentch F, March M, Mangino M, Spector TD, Bataille V, Pennell CE, Holt PG, Sly P, Tiesler CM, Thiering E, Illig T, Imboden M, Nystad W, Simpson A, Hottenga JJ, Postma D, Koppelman GH, Smit HA, Soderhall C, Chawes B, Kreiner-Moller E, Bisgaard H, Melen E, Boomsma DI, Custovic A, Jacobsson B, Probst-Hensch NM, Palmer LJ, Glass D, Hakonarson H, Melbye M, Jarvis DL, Jaddoe VW, Gieger C, Genetics of Overweight Young Adults C, Strachan DP, Martin NG, Jarvelin MR, Heinrich J, Evans DM, Weidinger S, Genetics EA, Lifecourse Epidemiology C. Metaanalysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. 2012;44:187–92.
Ikram MA, Fornage M, Smith AV, Seshadri S, Schmidt R, Debette S, Vrooman HA, Sigurdsson S, Ropele S, Taal HR, Mook-Kanamori DO, Coker LH, Longstreth WT, Jr., Niessen WJ, DeStefano AL, Beiser A, Zijdenbos AP, Struchalin M, Jack CR, Jr., Rivadeneira F, Uitterlinden AG, Knopman DS, Hartikainen AL, Pennell CE, Thiering E, Steegers EA, Hakonarson H, Heinrich J, Palmer LJ, Jarvelin MR, McCarthy MI, Grant SF, St Pourcain B, Timpson NJ, Smith GD, Sovio U, Early Growth Genetics C, Nalls MA, Au R, Hofman A, Gudnason H, van der Lugt A, Harris TB, Meeks WM, Vernooij MW, van Buchem MA, Catellier D, Jaddoe VW, Gudnason V, Windham BG, Wolf PA, van Duijn CM, Mosley TH, Jr., Schmidt H, Launer LJ, Breteler MM, DeCarli C, Cohorts for H, Aging Research in Genomic Epidemiology C. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet. 2012;44:539–44.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. We thank Marjolein Kooijman, Claudia Kruithof, Natalia Loekabino, Patricia Maeijer, Ronald van den Nieuwenhof, and Karien Toebes for their study coordination. The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport. Vincent Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (ZonMw 907.00303, ZonMw 916.10159).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaddoe, V.W.V., van Duijn, C.M., Franco, O.H. et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol 27, 739–756 (2012). https://doi.org/10.1007/s10654-012-9735-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-012-9735-1