Abstract
The objective was to examine the association between age at menarche and all-cause mortality. A population-based prospective study involving 55,128 Japanese women aged 40–79 years in 1988–1990 and followed up to December 2006 was used. A total of 6,967 deaths occurred during the follow-up. Hazard ratios (HR) and 95% confidence intervals (CI) adjusted for age, smoking and drinking status, exercise, sleeping hours, parity, menopausal status, and body mass index at baseline were calculated by Cox proportional hazards model. The HRs (95% CI) of all-cause mortality were 1.16 (1.01–1.32), 1.01 (0.92–1.11), 1.00, 0.97 (0.90–1.05), 0.98 (0.91–1.05), 0.92 (0.84–1.01), and 1.05 (0.96–1.14) for women with menarche aged 9–12, 13, 14 (referent), 15, 16, 17, 18–20 years, respectively, indicating an inverse J-shaped association (P for quadratic trend <.01). Moreover, women with menarche aged ≤12 years have a significantly high risk of all-cause mortality compared with those with menarche aged ≥13 years (HR 1.17, 95% CI 1.03–1.33). Comparing between women with menarche aged ≤13 years and ≥14 years, those with earlier age at menarche had borderline significantly high risk of all-cause mortality in both comparisons (HR 1.07, 95% CI 0.99–1.15, P = .082). Japanese women with early age at menarche of ≤12 years were associated with increased risk of all-cause mortality and those with late age at menarche of ≥18 years also had a slightly higher mortality risk. These associations were independent of lifestyle, anthropometric, and reproductive factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first menstrual period, menarche, is a very important event since it may provide information related to inter-individual variations in developmental, nutritional and hormonal exposures. Early menarche could be associated with some physiological characteristics related to the metabolism of fat storage, and from there to adult obesity. Alternatively, early age at menarche could result from pre-existing risk factors in early adolescence such as high BMI. Furthermore, early and very late age at menarche could be indicative of pre-existing diseases. Age at menarche is one of the important reproductive factors related to women’s health directly or indirectly. However, little attention has been paid to the effect of this landmark on later health or morbidity except for hormone-related disease such as breast cancer [1, 2]. It is only recently that, for the purpose of prevention and management of cardiovascular disease (CVD) in women, the relationship between reproductive history and CVD risk factors has been studied [3–11].
Early menarche has been associated with many different CVD risk factors and metabolic syndrome among adolescent girls or young adult women [8–11]. There is conflicting evidence regarding relationships between age at menarche and CVD morbidity [3–7]. To our knowledge, four studies have examined the association between age at menarche and all-cause mortality among occidental people [6, 7, 12, 13]. All of these studies suggested that early age at menarche was associated with increased risk of all-cause mortality, though the confounding factors were not considered enough or sample size was not large enough to divided age at menarche into many categories. Additionally, the mean age at menarche among occidental women was comparatively low. To confirm this association, a similar study is needed in other countries or races with different genetic and socio-economic backgrounds. We report here on the impacts of recalled age at menarche on the risk of all-cause mortality using data obtained from a large sample of Japanese women participating in the Japan Collaborative Cohort Study for Evaluation of Cancer Risk; the JACC Study designed prospectively.
Methods
Study population
The Japan Collaborative Cohort Study for Evaluation of Cancer Risk, the JACC Study (sponsored by the Ministry of Education, Culture, Sports, Science, and Technology of Japan), is a nationwide multicenter collaborative study to prospectively evaluate the various risks and/or protective factors on cancer mortality and incidence. Study methods and ethical issues have been described in detail elsewhere [14, 15]. Briefly, it was initiated in 1988, and enrollment continued until the end of 1990. We enrolled 127,477 apparently healthy inhabitants from 45 areas who completed the survey questionnaire. In 22 out of 45 areas, all residents living in a given target area were regarded as study subjects. In 20 areas, those who had undertaken a basic health examination that was conducted under the Health and Medical Service Law for the Aged were invited to participate in the study. In 2 areas, the study subjects consisted of health examination examinees plus volunteers. In one area, subjects were defined based on the health checkup for atomic bomb survivors. Of the 127,477 enrolled, 110,792 (46,465 men and 64,327 women), aged 40–79 years, were followed. The study was approved by the Nagoya University Ethics Review Committee.
A self-administered questionnaire was used to assess the baseline characteristics of participants. It covered medical history and included lifestyle-related items such as physical activity, drinking and smoking, and sleeping hours per day. For women, information was obtained on menstrual factors (age at menarche and age at menopause), and reproductive variables (number of parity). In the questionnaire, weight in kilograms and height in cm were entered by participants after the words ‘Current weight and height’. Of the 64,327 women, we excluded subjects with any of the following criteria: no available data to evaluate vital status during the follow-up period (n = 2) and missing information on age at menarche (n = 6,285), height (n = 3,824), and weight (n = 2,662). In addition, we also excluded one with recalled menarche aged 6, one aged 8, and 174 women whose recorded age at menarche was greater than 20 years. In all, 9,197 subjects were excluded, and the remaining 55,128 women were included in the present study.
Follow-up
The causes and dates of death among the subjects were identified by reviewing all death certificates in each area with the permission of the Director-General of the Prime Minister’s Office (Ministry of Internal Affairs and Communications). Those who moved out of a study area were treated as censored. We followed the subjects until the end of 2006, except in 8 areas where follow-ups were discontinued at the end of 1999 or 2003.
Statistical analysis
First, we divided the subjects into 10 birth cohorts (1905–1909, 1910–1914, 1915–1919, 1920–1924, 1925–1929, 1930–1934, 1935–1939, 1940–1944, 1945–1949, and 1950–1954) to evaluate the change of age at menarche. Second, age at menarche was categorized into 9–12 (the number of subjects with menarche aged 9, 10, 11, and 12 were 9, 65, 383, and 3,127, respectively.), 13 (n = 7,924), 14 (n = 12,390), 15 (n = 12,213), 16 (n = 8,872), 17 (n = 4,789), and 18–20 (the number of subjects with menarche aged 18, 19, and 20 were 3,327, 1,364, and 465, respectively.) For each participant, the person-years of follow-up were calculated from the date of filling out the baseline questionnaire to death from any cause, moving out of the study area, or the end of the follow-up period, whichever occurred first. Cox proportional hazards modeling was used to compute the relative risks (RRs) of mortality in respective age of menarche groups, with the group aged 14 years considered as the reference category. Adjusted RRs for mortality by age at menarche were estimated in 3 models as follows: model 1, adjusted for age at baseline; model 2, as in model 1 + study area (23 areas) + smoking status (never, past, current), alcohol consumption (none, past, present), exercise: ‘How long do you exercise or engage in sports in a week?’ (≥5, 3–4, 1–2 h per week, seldom), sleeping hours per day (<7, 7–<9, ≥9), parity (0, 1, 2, 3, ≥4), and menopausal status (pre-menopause, menopause); model 3, as in model 2 + body mass index (BMI) [weight in kilograms/(height in meters)2] of 25 kg/m2 and over. These variables were assessed by the baseline questionnaire and were selected as covariates because they were known or suspected to modify the risk of mortality. In the analysis, all variables except for age at baseline were treated as categorical variables, which were entered into the model as dummy variables. Missing values for each covariate were treated as an additional category of variables and were included in the model. A linear trend of association was assessed by the regression model assigning a score (0, 1, 2, …) to the levels of each independent variable. A quadratic trend of association was assessed with the model including a quadratic term. For proportional hazards function, a log–log transformation of the survival curve was used. This transformation produced survival curves were parallel and differ because of the presence of explanatory variables.
All analyses were calculated using the SPSS 17.0 statistical package. The 95% confidence intervals (CI) were presented for all RR. All P values were based on two-sided tests, in which P < .05 was considered statistically significant.
Results
The mean (standard deviation: SD) age of the participants was 57.1 (9.9) years. The mean BMI was 22.9 (3.1). The mean and median ages at menarche were 15.0 (SD: 1.77) and 14 years, respectively. The proportion of menopausal women was 69.6%.
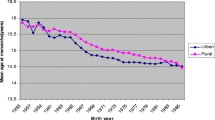
Table 1 shows the association between the mean age at menarche and birth cohort. There was a linear inverse trend (P for trend <.001). The proportion of women with menarche aged ≤12 years dramatically increased from 1.7% in women born in 1905–<1910 to 28.2% in those born in 1950–<1955. The proportion of current or past smoker (no significant trend: P = .560), current or past drinker (significantly increased trend: P < .001), taking exercise for no <1 h per week (significantly decreased trend: P < .001), sleeping 7–<9 h/day (significantly decreased trend: P < .001), and having 3 children or more (significantly decreased trend: P < .001) were significantly different across the birth cohort.
Table 2 demonstrates descriptive characteristics of study women according to age at menarche. The age at baseline increased significantly across the categories of age at menarche (P for linear trend <.001). The mean of BMI shows a significant and inverse association with age at menarche (P for linear trend <.001). There were statistically significant associations between age at menarche and smoking status (P < .001), alcohol drinking status (P < .001), exercise (P < .001), sleeping hour (P < .001), and the number of parity (P < .001) though those associations may be confounded by age at enrollment.
A total of 6,967 deaths occurred during the follow-up of 818,379 person years (mean: 14.8 years). Table 3 describes the association between age at menarche and all-cause mortality, with age at menarche categorized into 7 groups. The multivariate-adjusted HR (95% CI) of all-cause mortality for the women with menarche aged ≤12 years compared with those with menarche aged 14 years was 1.16 (1.01–1.33). The association between age at menarche and all-cause mortality was likely to show an inverse trend in comparison to the category of menarche aged 17 years but women with menarche aged ≥18 years also have a higher mortality, indicating a J-shaped association (P for quadratic trend = .003). We repeated the analysis with age at menarche as a continuous variable. The results showed the adjusted HR corresponding to the linear term for age at menarche was 0.74 (0.62–0.89) and the adjusted HR corresponding to the quadratic term was 1.01 (1.004–1.02). In addition, we combined the categories of menarche aged ≥13 years. Women with menarche aged ≤12 years have a significantly high risk of all-cause mortality compared with those with menarche aged ≥13 years [HR (95% CI): 1.17 (1.03–1.33)]. Moreover, we increased the border of age at menarche gradually. Comparing between women with menarche aged ≤13 and ≥14 years, those with earlier age at menarche had borderline significantly high risk of all-cause mortality (P = .082), whereas there was no significant difference in all-cause mortality risk in other comparisons.
In a separate analysis, we excluded women with an extreme early (aged ≤ 9 years) and extreme late (aged ≥ 18 years) age at menarche. The HRs (95% CI) of all-cause mortality were 2.45 (1.39–4.33), 1.28 (0.88–1.88), 1.10 (0.95–1.28), 1.01 (0.92–1.11), 1.00 (referent), 0.97 (0.90–1.05), 0.98 (0.91–1.06), and 0.92(0.84–1.01) for women with menarche aged 10, 11, 12, 13, 14 (referent), 15, 16, and 17 years. Thus, the linear trend was observed (P for linear trend = .004). A 1-year increase in age at menarche was associated with a 3% reduction in total mortality [HR (95% CI): 0.97 (0.95–0.99)].
Discussion
We examined the association between age at menarche and all-cause mortality among 55,128 Japanese women during the mean follow-up period of 14.8 years. We were able to compare the risk of all-cause mortality among 7 categories of age at menarche (9–12, 13, 14, 15, 16, 17, and 18–20 years) because of the large number of women in the study. Consequently, not only earlier age at menarche but also high age at menarche was associated with an increased mortality, indicating an inverse J-shaped association between age at menarche and all-cause mortality.
To our knowledge, only four studies examined the association between age at menarche and all-cause mortality previously [6, 7, 12, 13]. The summary of respective study deserves to be mentioned to clarify the similarity and difference between our study and the 4 previous studies. The study by Jacobsen et al. [12] followed up 61,319 Norwegian women aged 32–74 for 37 years, though the important confounding factors such as smoking status and body size were not collected. The main findings were that mortality rate ratios of menarche aged ≤11, 12, 13, 14, 15, 16, 17, and 18–19 years were 1.13, 1.08, 1.02, 1.00 (referent), 0.97, 0.96, 0.94, and 1.02, respectively (P for second-order term <.001). They also conducted a cohort study of 19,462 Californian Seventh-Day Adventist women aged 26–101 and followed-up from 1976 to 1988 [7]. Mortality rate ratios of menarche aged 9–10, 11, 12, 13, 14, 15, 16, and 17–18 years were 1.45, 1.20, 1.11, 1.00 (referent), 0.96, 0.96, 1.11, and 0.88, respectively, after adjustment for the confounding factors (P for linear trend <.001). Lakshman et al. [6] examined a population-based cohort study of 15,807 Caucasian women living in Norfolk, United Kingdom, aged 40–79 years at recruitment in 1993–1997 and followed up to 2007. HRs of menarche aged 8–11, 12, 13, 14, and 15–18 years were 1.00 (referent), 0.81, 0.86, 0.87, and 0.82, respectively (P for linear trend = .039). However, potentially important factors such as menopausal status were not collected. Giles et al. [13] studied this association in 1,031 women aged 65–103 years drawn from the general Australian population over 15 years of follow-up. HR of menarche aged <12 years was 1.25 compared with menarche aged ≥12 years after adjusting for lifestyle and reproductive variables. We were able to definitely confirm these previous results because our study included very large population and collected many covariates such as lifestyle factors, reproductive factors, and anthropometric indexes.
The association of earlier age at menarche with increased all-cause mortality was observed in all previous studies and our own. There are some speculations concerning the mechanism underlying this association. Many researchers have demonstrated that age at menarche was inversely linked to body mass in adulthood [8–11]. This association was also found in our study. As high BMI lead to increased mortality, they could be relevant confounders. However, adjustment for them did not influence the association between age at menarche and mortality. Some studies reported that early menarche was associated with increased cardiovascular risk factors among young and middle-aged women [8–11] though we did not have sufficient information on risk factors like blood glucose and lipids, and blood pressure. But, it is debatable whether or not it is correct to adjust for these variables, which may be part of thecausal pathway. Recent study has suggested that early menarche may not a determinant of an unfavorable cardiovascular profile in itself, but may reflect negative metabolic imprinting during pre-pubescence [11]. A relatively high weight during childhood or adolescence increases the likelihood of early menarche [16, 17]. Low birth weight combined with higher BMI during childhood has recently been demonstrated to predict early age at menarche [18]. The relationship between early menarche and unhealthy body composition in female adolescent was likely to be due to the programming effect of birth weight [19]. Unfortunately, we had no data concerning body height and weight either at birth or during childhood.
Two previous studies have reported a significantly high risk of women with menarche aged <12 years for all-cause mortality compared with those with menarche aged ≥12 years [6, 13]. Although the high risk of extremely early age at menarche is obvious, the cut-off age at menarche which shows a significantly increased risk for all-cause mortality has still not been examined. We found for the first time that women with menarche aged ≤13 years had borderline significantly high mortality risk compared with those with menarche aged ≥14 years. This finding must be examined in other countries.
We also found that a high age at menarche was associated with an increased mortality, indicating an inverse J-shaped association between age at menarche and all-cause mortality. The similar finding was observed in the large population study on Norwegian women, in which 8 categories of age at menarche (≤11, 12, 13, 14, 15, 16, 17, and ≥18 years) were included [12]. Our finding that women with menarche aged ≥18 years had a significantly high risk of all-cause mortality compared with those aged 17 years was interesting biologically. Nutrition deficiencies due to general malnutrition are known to lead to menarcheal delay [20]. The women with menarche aged ≥18 years were older than the others. Some of them may have grown up under poor nutritional conditions, since the Japanese food situation was not enough when they experienced their menarche. And the effect may cause them to have increased mortality in adulthood. In other speculation, they may have been in unhealthy medical conditions such as chronic disease which causes decreased weight. Recent study suggested that underweight should be a predictor of diabetes in itself in older adults [21]. The proportion of women with menarche aged ≥18 years in our study (9.4%) was higher than those in the previous study (2%). However, the effect of high age at menarche on mortality is not actually so important as the fact that mean age at menarche is decreasing in Japan currently and the proportion of menarche aged ≥18 years is also dramatically reducing from 13.2% in a cohort of 1910–<1915 to 0.9% in that of 1945–<1950.
It should be noted that women with menarche aged 17 had the lowest risk of mortality in Japan. The above-mentioned study in Norway also showed the lowest risk in the category of menarche aged 17. They might have no unhealthy condition which cause excessive late menarche and might be biologically younger than their chronological age. Further studies are needed to enhance the reliability of this fact and clarify the mechanism.
There are both strengths and limitations to our study. The strengths include large sample size. Moreover, since data on many kinds of exposure known or suspected to modify the mortality risk were collected in the present study, we were able to elucidate the independent effects of age at menarche by multivariate adjustment. One of the limitations in our study is the self-recall of age at menarche. Unfortunately, we were not able to verify the validity of self-reported age at menarche in our cohort. Other studies have shown high correlations between age at menarche by recall during middle age and the original data in childhood [22, 23]. The reproducibility has been also found to be acceptable in a previous study [24]. However, as the women must recall an event that happened many years ago, some random misclassification of age at menarche is inevitable. Such errors would likely attenuate the real strength of the associations that we observed. Second, the information on social criteria was not available in our study though early age at menarche is more frequent in socially disadvantaged women, which could explain the association with higher mortality. The third limitations arise from the reliance on self-reported measurements of weight and height. It is well known that self-reported current weight and height, and past weight are influenced by factors such as age, gender, and obesity. However, many studies have reported that they were accurate enough to use in an epidemiologic study [25, 26].
In summary, our study of a large Japanese population-based cohort provides evidence that early age at menarche is associated with an increased risk of all-cause mortality. The pathways through which age at menarche impacts subsequent survival remain to be resolved. Overall, our findings suggest that decreasing age at menarche could lead to shorter life spans. The decreased trend in age at menarche with generation is observed in many countries with major progress in medical treatment and technology that can increase life expectancy. Further study is warranted to examine factors which lead to decreased age at menarche, such as lifestyle, environmental, and social factors including obesity and family dysfunction during childhood. These factors will be different among countries and between the generations of the same country.
Abbreviations
- 95% CI:
-
95% confidence interval
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- HR:
-
Hazard ratio
- JACC:
-
Japan collaborative cohort study for evaluation of cancer risk
- RR:
-
Relative risk
References
Tamakoshi K, Yatsuya H, Wakai K, Suzuki S, Nishio K, Lin Y, et al. Impact of menstrual and reproductive factors on breast cancer risk in Japan: results of the JACC study. Cancer Sci. 2005;96(1):57–62.
Beaber EF, Holt VL, Malone KE, Porter PL, Daling JR, Li CI. Reproductive factors, age at maximum height, and risk of three histologic types of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3427–34.
Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology. 1999;10(3):255–9.
de Kleijn MJ, van der Schouw YT, van der Graaf Y. Reproductive history and cardiovascular disease risk in postmenopausal women: a review of the literature. Maturitas. 1999;33(1):7–36.
Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006;16(5):177–84.
Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–60.
Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. Int J Epidemiol. 2009;38(1):245–52.
Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Järvinen L, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the cardiovascular risk in Young Finns study. Am J Clin Nutr. 2008;87(6):1876–82.
Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90(5):2718–24.
Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27(11):1398–404.
Feng Y, Hong X, Wilker E, Li Z, Zhang W, Jin D, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–7.
Jacobsen BK, Heuch I, Kvåle G. Association of low age at menarche with increased all-cause mortality: a 37-year follow-up of 61, 319 Norwegian women. Am J Epidemiol. 2007;166(12):1431–7.
Giles LC, Glonek GF, Moore VM, Davies MJ, Luszcz MA. Lower age at menarche affects survival in older Australian women: results from the Australian Longitudinal Study of ageing. BMC Public Health. 2010;10:341.
Ohno Y, Tamakoshi A, JACC Study Group. Japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (JACC study). J Epidemiol. 2001;11(4):144–50.
Tamakoshi A, Yoshimura T, Inaba Y, Ito Y, Watanabe Y, Fukuda K, et al. Profile of the JACC study. J Epidemiol. 2005;15(Suppl 1):S4–8.
Dunger DB, Ahmed ML, Ong KK. Effects of obesity on growth and puberty. Best Pract Res Clin Endocrinol Metab. 2005;19(3):375–90.
Frisch RE. Body fat, menarche, fitness and fertility. Hum Reprod. 1987;2(6):521–33.
Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92(1):46–50.
Labayen I, Ortega FB, Moreno LA, Redondo-Figuero C, Bueno G, Gómez-Martínez S, et al. The effect of early menarche on later body composition and fat distribution in female adolescents: role of birth weight. Ann Nutr Metab. 2009;54(4):313–20.
Rees M. Menarche when and why? Lancet. 1993;342(8884):1375–6.
Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes Care. 2008;31(3):583–4.
Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. 1991;18(2):155–66.
Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–9.
Bosetti C, Tavani A, Negri E, Trichopoulos D, La Vecchia C. Reliability of data on medical conditions, menstrual and reproductive history provided by hospital controls. J Clin Epidemiol. 2001;54(9):902–6.
Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–5.
Wada K, Tamakoshi K, Tsunekawa T, Otsuka R, Zhang H, Murata C, et al. Validity of self-reported height and weight in a Japanese workplace population. Int J Obes (Lond). 2005;29(9):1093–9.
Acknowledgments
We wish to express our sincere appreciation to Drs. Kunio Aoki and Yoshiyuki Ohno, Professors Emeritus of the Nagoya University School of Medicine and former chairpersons of the JACC Study. We are also greatly indebted to Dr. Haruo Sugano, former Director of the Cancer Institute, Tokyo, who greatly contributed to the initiation of the JACC Study, Dr. Tomoyuki Kitagawa, Director Emeritus of the Cancer Institute of the Japanese Foundation for Cancer Research and former chairman of the Grant-in-Aid for Scientific Research on Priority Area ‘Cancer’ and to Dr. Kazao Tajima, Aichi Cancer Center and previous chairman of the Grant-in Aid for Scientific Research on Priority Area of Cancer Epidemiology, for their warm encouragement and support of this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Member list of the JACC Study Group: The present members of the JACC Study Group who co-authored this paper together with their affiliations are as follows: Dr. Akiko Tamakoshi (present chairperson of the study group), Aichi Medical University School of Medicine; Drs. Mitsuru Mori & Fumio Sakauchi, Sapporo Medical University School of Medicine; Dr. Yutaka Motohashi, Akita University School of Medicine; Dr. Ichiro Tsuji, Tohoku University Graduate School of Medicine; Dr. Yosikazu Nakamura, Jichi Medical School; Dr. Hiroyasu Iso, Osaka University School of Medicine; Dr. Haruo Mikami, Chiba Cancer Center; Dr. Michiko Kurosawa, Juntendo University School of Medicine; Dr. Yoshiharu Hoshiyama, University of Human Arts and Sciences; Dr. Naohito Tanabe, Niigata University School of Medicine; Dr. Koji Tamakoshi, Nagoya University Graduate School of Health Science; Dr. Kenji Wakai, Nagoya University Graduate School of Medicine; Dr. Shinkan Tokudome, National Institute of Health and Nutrition; Dr. Koji Suzuki, Fujita Health University School of Health Sciences; Dr. Shuji Hashimoto, Fujita Health University School of Medicine; Dr. Shogo Kikuchi, Aichi Medical University School of Medicine; Dr. Yasuhiko Wada, Faculty of Human Life and Environmental Science, Kochi Women’s University; Dr. Takashi Kawamura, Kyoto University Center for Student Health; Dr. Yoshiyuki Watanabe, Kyoto Prefectural University of Medicine Graduate School of Medical Science; Dr. Kotaro Ozasa, Radiation Effects Research Foundation; Dr. Tsuneharu Miki, Kyoto Prefectural University of Medicine Graduate School of Medical Science; Dr. Chigusa Date, Faculty of Human Environmental Sciences, Nara Women’s University; Dr. Kiyomi Sakata, Iwate Medical University; Dr. Yoichi Kurozawa, Tottori University Faculty of Medicine; Dr. Takesumi Yoshimura, Fukuoka Institute of Health and Environmental Sciences; Dr. Yoshihisa Fujino, University of Occupational and Environmental Health; Dr. Akira Shibata, Kurume University School of Medicine; Dr. Naoyuki Okamoto, Kanagawa Cancer Center; and Dr. Hideo Shio, Moriyama Municipal Hospital.
Rights and permissions
About this article
Cite this article
Tamakoshi, K., Yatsuya, H., Tamakoshi, A. et al. Early age at menarche associated with increased all-cause mortality. Eur J Epidemiol 26, 771–778 (2011). https://doi.org/10.1007/s10654-011-9623-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-011-9623-0