Abstract
Biogas production from agricultural residues represents an effective and sustainable option for handling vast quantities of lignocellulosic waste for meeting the global energy demand. However, the recalcitrance of lignocellulosic biomass due to the presence of cellulose and hemicellulose in a structurally complex lignocellulosic matrix, and the crystallinity of cellulose create a hindrance in biogas production by restricting the availability of fermentable sugars to microbial action. This paper assesses the different pretreatment strategies adopted for making the lignocellulosic biomass amenable to anaerobic digestion by altering the structure of lignocellulose and eliminating lignin, thus increasing the accessibility of microbes to the easily degradable components. The review highlights the advantages and limitations of each technology—physical for size reduction (chipping, milling, extrusion, cavitation), thermal for breaking down of hydrogen bonds (conventional heating, steam explosion, microwave irradiation, hydrothermal), chemical for decreasing the crystallinity and polymerization degree of cellulose (alkalis, acids, gases, oxidizing agents, various solvents), biological for enhancing the digestibility (microbial, enzymatic), and their effect on improving the degradation efficiency and biogas yield. Further, the review discusses the role of some emerging technologies and other less explored options which may require optimization at higher scale so that the confidence in the translation of this knowledge can be increased and this abundantly available raw material can be effectively utilized. With the goal of improving the applicability of pretreatment technologies, some recommendations are put forth so that the efficiency of anaerobic digestion can be increased and the development of pretreatment technologies can be promoted on a large scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Continuous increase in energy demand due to increasing human population and depletion of fossil fuel resources have driven the attention of researchers towards renewable sources of energy (Manyi-Loh et al. 2019). Lignocellulosic biomass consisting of both virgin (terrestrial plants) and waste (agricultural) biomass is the most abundant biomass available on the earth. It is cheap and can be easily exploited for its high sugar content, thus presenting a large potential to meet the global energy demand and future energy insurance in a sustainable way (Enshaeieh et al. 2015; Kamaraj et al. 2020). Among the renewable energy options, biogas production from anaerobic digestion represents a sustainable technology for its use as an alternative to fossil fuels. However, the complex nature of lignocellulosic biomass due to the presence of hemicellulose and lignin creates a major hurdle in the accessibility to cellulose and thus biogas production from such a resource is hampered.
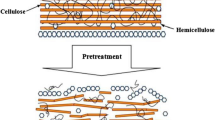
Lignocellulosic biomass conversion to biogas includes three main steps viz: pretreatment, enzymatic hydrolysis, and anaerobic digestion. Pretreatment, which is regarded as the most energy-intensive process in lignocellulose biomass conversion, alters its structure by decomposing the lignocellulosic matrix and eliminating lignin (Fig. 1). It is well known that the pretreatment of lignocellulosic biomass prior to anaerobic digestion has the potential to overcome the kinetic disadvantages of anaerobic digestion which can lead to biogas enhancement (Yu et al. 2019). Therefore, researchers across the world have devoted their attention to discovering new pretreatment technologies or modifying the existing ones for overcoming the disadvantages and shortcomings of currently available methods.
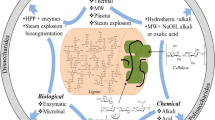
Various pretreatment technologies are available for making the lignocellulosic biomass amenable to anaerobic digestion, including physical, thermal, chemical, biological, and combined pretreatment methods in addition to some emerging technologies (Fig. 2). Several reports have discussed the different aspects of available pretreatment methods for lignocellulosic biomass, such as non-conventional pretreatment, green pretreatment for biorefinery, biological pretreatment, emerging technologies, application of nanotechnology, physical and chemical pretreatment, enzymatic pretreatment for enhanced biomethane production (Capolupo and Faraco 2016; Hassan et al. 2018; Ingle et al. 2019; Jędrzejczyk et al. 2019; Koupaie et al. 2019; Sharma et al. 2019).
Studies to date have discussed the ability of pretreatment technologies in enhancing the bioprocess efficiencies; however, certain shortcomings in biomass pretreatment technologies have restricted their large-scale commercialization. Hence, for replacing fossil fuel with cheaply exploited, abundant and renewable lignocellulosic biomass, pretreatment technologies need to be further optimized, and made fool proof in terms of the associated inherent deficiencies. This paper reviews the different physical, chemical, thermal, biological, and some new emerging as well as combined technologies for the pretreatment of lignocellulosic biomass for achieving enhancement in saccharification efficiency, which in turn can have a significant impact on the efficiency of anaerobic bioprocesses involved in the generation of biogas. The purpose of the review is to carry out a comprehensive comparison of the different pretreatment methods with reference to a critical analysis of their advantages, disadvantages, and application in biogas production. The review also puts forth some recommendations regarding the need for exploring and optimizing some new emerging technologies along with other less explored options to increase the confidence level in such technologies. This can thereby pave the path for their large-scale application in the effective utilization of the abundantly available lignocellulosic raw material.
Physical pretreatment
Physical pretreatment targets the change or modification in the structure or appearance of lignocellulosic biomass through mechanical techniques such as chipping, milling/grinding, extrusion, and cavitation. The main aim of mechanical pretreatment is to break down the encrusting material of the cell wall made of lignin and to disarray the waxy layer on the surface of the plant cell wall. An increase in the accessible contact surface area between the anaerobic microbes and the substrate is achieved, which helps in enhanced digestion of cellulose and hemicellulose fractions (Akhtar et al. 2016), and ultimately increases the biogas production from lignocellulosic material by anaerobic digestion.
Chipping
This pretreatment method is applied for reducing the size of the raw lignocellulosic biomass of agricultural and forest origin, before any other pretreatment. Employing a simple crushing method prior to any pretreatment could be useful for facilitating efficient anaerobic fermentation. Chipping is required to disarray the massive layers of lignocellulose biomass into small pieces and facilitate the various milling/grinding methods to convert lignocellulosic biomass into a fine powder. The size of the substrate is reduced to 10–30 mm after chipping which is further subjected to milling to reduce the particle size up to 0.2–2 mm (Maurya et al. 2015). Size reduction by chipping has a great effect on limiting the heat as well as mass transfer associated with large size particles (Agbor et al. 2011).
Milling/grinding
Milling pretreatment uses communication practice of ball milling, roll milling, rod milling, hammer milling, colloid milling, wet disk milling, and vibratory milling for achieving reduction in the debris size, polymerization degree, and cellulose crystallinity (Baruah et al. 2018). Simultaneously, it increases the pore size and accessible contact surface area between the anaerobes and the lignocellulose fiber. Milling is always carried out before other pretreatments to enhance the effect of pretreatment. The above characteristics of a substrate processed by milling can shorten the start-up time for fermentation (Zheng et al. 2014). It was found that when the particle size of the substrate was reduced, it could effectively resist the acidification of the system as well as increase the buffering capacity. Since milling is not feasible from an economic point of view owing to the high consumption of energy, high cost of equipment along with machinery setup, and its inability to remove lignin, it requires the incorporation of further pretreatment steps (Rizal et al. 2018).
Extrusion
The extrusion pretreatment for anaerobic digestion is a well-known method to enhance the methane production from lignocellulosic biomass. This method of pretreatment has the specific advantage of reducing the particle size and volume along with altering the physical properties compared to milling pretreatment. The benefits of extrusion have been demonstrated in the case of rice straw where this pretreatment led to a significant increase in methane production by 32.5% and 72.2% in comparison with rice straw pretreated by milling and untreated rice straw, respectively (Tsapekos et al. 2015). Recent studies by Pérez-Rodríguez et al. (2018) demonstrated methane enhancement in the range of 15.7–21.4% from extruded vine trimming shoots over untreated vine trimming shoots. The specific biogas and methane production using extrusion pretreated maize straw also showed an increase of 7.50% and 8.51% over the untreated maize straw (Kozłowski et al. 2019). Extrusion requires mild conditions such as moderate pH and temperature with a short process duration and does not produce any toxic substance. Despite these advantages, high energy consumption and high machinery cost are the bottlenecks of this method (Duque et al. 2017).
Cavitation
Pretreatment by hydrodynamic cavitation (HC) employs a highly destructive force on lignocellulosic biomass. The damage caused by cavitation to the internal structure of lignocellulose benefits the anaerobic digestion process by improving the stalk fermentation, gas production rate, and shortening the duration of maximum gas production. HC conditions of 3-bar pressure, 0.3 M NaOH, and 70 °C temperature worked best for obtaining 93.05% cellulose and 94.45% hemicellulose hydrolysis yield from sugarcane bagasse (Hilares et al. 2020). HC was also shown to increase the enzymatic digestibility of lime-treated sugarcane bagasse by 46%, whereas acoustic cavitation showed no such gain (Madison et al. 2017). Though a significant increase in methane production from HC-pretreated wheat straw was reported over untreated wheat straw control, the efficiency was noted to be lower during co-digestion of HC pretreated wheat straw mixed with cattle manure (35.6–39.4%) in comparison with the substrate pretreated by ultrasonic cavitation (59.6–64.2%) (Patil et al. 2016). However, both the pretreatments resulted in a similar solubilization of biomass (ca. 30% as CODsol) (Zieliński et al. 2019). HC assisted pretreatment method was demonstrated as a promising alternative to other mechanical pretreatment techniques due to its high efficiency of carbohydrate fraction digestibility, high energy efficiency, simple construction and configuration of the system, and the possibility of using it at large scale for pretreatment. Also, it required milder conditions and short running time, but still was a less explored method for pretreatment (Hilares et al. 2018).
Thermal pretreatment
A thermal pretreatment is a crucial approach in which the heat energy decreases the thermal stability of the substrate and the secondary explosion breaks down the hydrogen bond of complex lignocellulosic structure, exposing the surface area of cellulose for anaerobic microbes to work more efficiently (Čater et al. 2014). In addition to the lignocellulose degradation, heat energy also eliminates pathogens from agricultural waste. Two factors viz: the extent of residence time and the reaction temperature, are of paramount importance for the disintegration of complex lignocellulosic structure. Chen et al. (2011) found that a lower residence period could save energy compared to a higher residence period. Thermal pretreatment exploits the temperature range of 50–240 °C with pressure drop. Depending upon the temperature range, thermal pretreatment encompasses conventional heating, steam explosion, microwave irradiation, and hydrothermal pretreatment. The main drawback of thermal pretreatment is that the generation of soluble phenolic compounds along with toxic derivatives such as 5-hydroxymethyl 2-furfural and furfural was higher when the biomass was exposed to a temperature above 160 °C (Čater et al. 2014). Since most of the inhibitors are present in soluble form and are not separated from the liquid fraction, such inhibitors can not only hinder the enzymatic hydrolysis but also the fermentation process if the liquid fraction is to be used (Alvira et al. 2016). Insoluble lignin and exposed lignin predominantly in the solid fraction are modified to be more rigid and recalcitrant. It tends to non-productively absorb the cellulolytic enzymes and reduce the availability of active enzymes for cellulose hydrolysis. Lignin-derived phenolics also deactivate or inhibit β-glucosidase, and cellulase via precipitation and irreversible binding. Furan derivatives reduce the biological and enzymatic activities and cause oxidative damage to the cells. Meanwhile, weak acids negatively affect the cell growth due to the influx of undissociated acids into the plasma membrane and cytosol (Ko et al. 2015). Under such circumstances, there is a need for including a neutralization/detoxification step after pretreatment for the removal of inhibitors (Wang et al. 2013). Table 1 shows different thermal methods adopted for the pretreatment of lignocellulosic biomass for methane improvement by anaerobic digestion.
Conventional heating
Conventional heating in a hot air oven and autoclave is directed towards the heating of biomass and disintegration of the lignin–polysaccharide matrix. Autoclave heating leads to the formation of protuberant on the substrate that can favor subsequent enzymatic hydrolysis of the lignocellulosic biomass (Gabhane et al. 2011). High-pressure steam in an autoclave with an acidic medium showed a higher release of total sugar (glucose and xylose) from the liquid hydrolysate along with an increase in the cellulose fraction and decrease in the lignin content of residual solid chunk (Debiagi et al. 2020). Autoclaving with mild alkaline treatment has resulted in a fivefold improvement in glucose recovery from C. barbata (Obeng et al. 2019). Compositional and instrumental analysis (FESEM, XRD, and FTIR spectra) revealed that the hot air oven pretreatment led to a significant increase in the hydrolysis of lignocellulosic biomass for the anaerobic digestion (Veluchamy and Kalamdhad 2017b). Hot air oven pretreatment showed better results in terms of volatile fatty acid (VFA) production, sCOD generation, reduction in cellulose crystallinity, and increased solubilization rate in comparison with heat treatment in an autoclave, microwave oven, and hot water bath (Veluchamy and Kalamdhad 2017a). Conventional heating transfers energy from the outer region to the inner core resulting in a hot outer surface while still maintaining the inner region at a cooler temperature (Hassan et al. 2018).
Steam explosion pretreatment
Steam explosion pretreatment is an approach conducted under the high-temperature range from 200 to 220 °C coupled with a pressure drop that may disintegrate the complex matrix of lignin–polysaccharide. Steam explosion performs the hemicellulose transmutation along with crumbling of cell-wall crosslink (Siddhu et al. 2016); therefore, it is regarded as a cost-effective and environmentally friendly technology. Pretreatment at a temperature of 200 °C for 10 min showed high enzymatic hydrolysis and glucose yield corresponding to that at 190 °C, but the generation of toxic compounds at high temperature was still a major issue (Alvira et al. 2016). Wang et al. (2013) reported that the generation of toxic compounds was more in the case of corn-cob pretreated at 200 °C, which decreased subsequently with a decrease in temperature. The authors highlighted the need for the inclusion of a detoxification step before anaerobic digestion in order to neutralize the negative impact of the high level of toxic compounds. Usually, pretreatment at a high temperature promotes higher partial lignin and sugar degradation than the lower temperature, which produces soluble toxic compounds inhibitory for the fermenting microbial community and their metabolic enzymes in the subsequent steps (Alvira et al. 2016). Castro et al. (2014) reported a doubling of the inhibitor concentration after an increase in pre-treatment temperature from 175 to 195 °C which had a harsh effect on the total sugar yield and ultimately on biogas production. The steam explosion was observed to be much less useful for the pretreatment of softwood (Pielhop et al. 2016) and when applied in combination with mild acidic conditions, it hampered the thermal degradation of cellulose in addition to hindering the generation of inhibitory compounds (Zhang et al. 2019).
Microwave irradiation
Microwave irradiation relied on the non-ionizing electromagnetic radiations between 300 and 300,000 MHz with a wavelength range from 1 mm to 1 m to transfer energy to the substrate (Huang et al. 2016). Microwave irradiation converts electromagnetic radiation directly into heat energy and transfers it uniformly throughout the substrate at the molecular level (Hassan et al. 2018). Removal of a greater number of acetyl groups in the hemicellulose region, production of less acid, and higher glucopyranose generation were suggested to be the spinoff of microwave irradiation (Dai et al. 2017). One major obstacle of microwave irradiation in the pretreatment was that the low lossy dielectric properties of the biomass prevented it from absorbing electromagnetic radiation effectively until the char was produced (Salema et al. 2017). This drawback necessitated the requirement of materials that achieved a rapid heating such as activated carbon, graphite, pyrites, and charcoal. Still, it has been devoted great attention due to its uniformity and selectivity in volumetric heating accomplishment, energy efficiency, easy operation, fast heat energy transfer, brief reaction time, and low degradation and generation of secondary product. The microwave irradiation pretreatment for lignocellulosic biomass is classified into two groups viz: (a) microwave-assisted solvolysis, and (b) microwave-assisted pyrolysis.
Microwave-assisted solvolysis
Microwave-assisted solvolysis (MAS) is a chemo-thermal technique for the disintegration of lignocellulosic biomass in the presence or absence of a catalyst under mild temperature conditions (less than 200 °C). It is a promising option in which the chemical reaction is facilitated by adding a non-thermal effect to the substrate and the second exposure decreases the activation energy of the Arrhenius equation by increasing the pre-exponential factor (Lidström et al. 2001). Yunpu et al. (2016) found that in the presence of a proper catalyst, the microwave could selectively increase the biogas yield besides accelerating the degradation rate of renewable yet difficult to degrade lignin. This pretreatment method for lignin–polysaccharide pretreatment offers the following advantages including high heat efficiency and selectivity, simple operation, accessible use and control, and lower environmental pollution. However, its applicability is limited by the high capital cost, and lower application maturity (Hassan et al. 2018).
Microwave-assisted pyrolysis
Microwave-assisted pyrolysis (MAP) converts the biomass into biogas, bio-oil, and char/carbonaceous residue at high temperatures (> 400 °C) in the absence of oxygen. Corresponding to conventional heating, Huang et al. (2016) found that MAP displayed a 42% higher heating rate indicating superior conduct in terms of the requirement of less processing time to achieve the target temperature. Also, it speeds up the chemical reaction and increases the quality of biogas from different types of biomass. Lo et al. (2017) reported that MAP of feedstock biomass (corn stover, rice husk, rice straw, sugarcane peel, sugarcane bagasse, bamboo leaves, and coffee ground waste) should be more feasible and efficient economically and energetically since energy return on investment (EROI) was approximately 3.56. This study may support the practicality of MAP, considering that the minimum sustainable EROI was 3.0 (Hall et al. 2014). However, this method is still a major barrier at an industrial level for large-scale commercialization due to the pyrolysis technique. Also, the high inorganic content may contribute to decreased yield of biofuel, whereas high organic content increases the yield of biofuel but requires microwave absorbers to improve the heating. On the other side, a high aqueous fraction degrades the quality of biofuel (Tirapanampai et al. 2019).
Hydrothermal pretreatment
Hydrothermal pretreatment of lignocellulosic biomass at high temperature and pressure deletes the requirement of chemicals and corrosion-resistant material for the hydrolysis phase (Eskicioglu et al. 2017). Hydrothermal pretreatment has also been called by different names such as aqueous extraction, liquid hot water (LHW), aqueous pretreatment, aquasolv, aqueous prehydrolysis, aqueous liquefaction, autohydrolysis, and pressure cooking in water. Based on the temperature–pressure relationship, hydrothermal pretreatment is divided into subcritical and supercritical water. Subcritical water employs temperature in the range of 100–374 °C under pressure to maintain water in the liquid state (Yang et al. 2019a, b). Supercritical water exploits a temperature above 374 °C with pressure above 22.1 MPa to break down the lignin–polysaccharide matrix in the presence of inert gas (Kumar et al. 2020). Water, propane, and carbon dioxide are the most studied subcritical and supercritical fluids for this pretreatment. At high temperatures, the water dissociates into hydronium ions (H3O−, acidic) and hydroxide ions (OH−, basic), and catalyzes the hydrolysis of lignocellulosic biomass (Yang et al. 2018). During hydrothermal pretreatment, hemicellulose fraction gets converted into acetic acid, which behaves as a catalyst in the bioconversion process and leads to an enhancement in sugar recovery (He et al. 2015). Also, lignin modification, removal, or relocation all along the hydrothermal pretreatment is observed to be very effective for enzymatic hydrolysis (Simanungkalit et al. 2017). Furan derivatives such as furfural, 5-hydroxymethylfurfural, furan aldehydes, weak acids, lignin, and lignin-derived phenolics are the major inhibitory by products of hydrothermal pretreatment that hamper the enzymatic hydrolysis and fermentation in anaerobic digestion (Steinbach et al. 2017). Hydrothermal pretreatment of maple wood at 200 °C for 20 min with a solid loading rate of 230 g/L gave the maximum sugar yield. Meanwhile, the thermal pretreatment also resulted in the production of 4.1 g/L and 1.3 g/L of furfural and phenolics, respectively, which on further incubation decreased the sugar yield by 50% (Kim et al. 2011). Similar observations were made by Lin et al. (2019) who showed that sugar yield from microalgae increased from 23 to 32.2 mg/g VS with an increase in temperature from 100 to 140 °C. The study further demonstrated that an increase in temperature up to 180 °C favored higher sugar degradation into inhibitory by-products thereby leading to a decrease in sugar yield. To remove the volatile inhibitors, Ko et al. (2015) stated that over-liming (pH adjustment using alkali), sulfite addition, adsorbent treatment, and vacuum evaporation may be helpful. Though detoxification by using genetically engineered microorganisms and chemicals such as polymeric resins and activated charcoal (Ahmed et al. 2019) has attracted the attention of researchers, it requires a high manufacturing cost along with the wastage of fermentable sugars and generation of waste. Also, this method of pretreatment can lead to a change in the characteristics (molecular weight, polydispersity, hydrophobicity, and functional group) of lignin, thus increasing the adsorption behavior of cellulase towards lignin along with high energy and water consumption (Lu et al. 2016).
Chemical pretreatment
The chemical pretreatment method represents a promising alternative for decreasing the crystallinity and polymerization degree of cellulose and improving the cellulose biodegradability by eliminating hemicellulose/lignin (Behera et al. 2014). For decades, chemicals have been exploited to delignify cellulose and to enhance biomass digestibility. Chemicals ranging from alkali, acid, gases, oxidizing agents, and various solvents are used to degrade the internal lignin and hemicellulose bond from lignocellulosic biomass. Chemicals are easy for handling and operation and parade a better effect on fermentation, but the only major limitation in using chemicals for pretreatment is the generation of inhibitory compounds and secondary pollutants that requires further recovery and may hinder the fermentation process. Table 2 shows different chemical pretreatment methods adopted for pretreatment of lignocellulosic biomass for methane improvement by anaerobic digestion.
Alkali pretreatment
Alkali pretreatment results in the saponification and solvation of biomass that leads to the disintegration of crosslink between lignin, polysaccharides, and silica, thereby increasing the porosity of biomass and cellulose accessibility for microbes in biogas production. Besides, alkali also removes inhibitors of cellulose like acetyl group, uronic acid, and lignin. NaOH, KOH, Ca(OH)2, Mg(OH)2, Na2CO3, urea, ammonia, and ammonium sulfate are some alkalies commonly used in this pretreatment. Selective delignification without the loss of carbohydrates, liquid stream detoxification, and high fermentation performance due to little or no generation of inhibitors and toxins make alkali a great choice for biomass pretreatment. However, the requirement of biomass neutralization after pretreatment and the long pretreatment period have prevented the expansion of this technology (Kumari and Singh 2018).
Sodium hydroxide (NaOH), a strong base catalyst, can adequately attack the lignin- carbohydrate complex and reduce the cellulose crystallinity. During pretreatment, the OH− can effectively separate and resolve the hydrogen bond between lignin and hemicellulose and cleave the ether–ester bond of lignopolysaccharide (Jung et al. 2019). Dai et al. (2018) found that the NaOH concentration of 6% worked best for biogas production. Also, Sun et al. (2019) reported that low NaOH concentration and high-density solution strongly contributed to a higher biogas yield; however, high NaOH concentration and low dose solution could not have any significant effect on biogas yield. A decrease in the methanogenic activity and methane production as a consequence of high NaOH loading besides the high chemical recovery cost has been reported to be the cause for its restricted application in industrial practice.
To address this issue, potassium hydroxide (KOH) which also possessed strong basicity could be used under mild conditions for biomass pretreatment. As it contains potassium, it could put nutrients back into the soil after pretreatment for sustainable clean biogas production (Chi et al. 2019). Paixão et al. (2016) found that the KOH pretreatment resulted in a higher reduction in the lignin content of sugarcane bagasse up to 5% and an increase in the cellulose content up to 80% compared to NaOH that achieved a 7% lignin reduction and a 72% increase in cellulose content. 6% KOH worked effectively for wheat straw biodegradation with 26% total solids, 89% VFA, and 22% lignin, cellulose, and hemicellulose decomposition, respectively. After pretreatment, wheat straw digestate with fertilizer values of 138% potassium, 22% calcium, and 16% magnesium could be applied for soil reclamation (Jaffar et al. 2016).
Pretreatment with other milder alkalis such as calcium hydroxide [Ca(OH)2] and magnesium hydroxide [Mg(OH)2] has been also demonstrated to significantly increase the biogas yield of lignocellulose anaerobic digestion by eliminating more acetyl groups and lignin (about 30%) from the feedstock, thus promoting enhanced biomass digestibility and enzymatic saccharification (Gigac et al. 2017; Deshavath et al. 2019). Although lime pretreatment is not as strong in contrast to sodium hydroxide or ammonia, it is a low-cost and simple process, which makes this pretreatment an attractive option (Kim 2013).
Sodium carbonate (Na2CO3) has strong alkalinity in an aqueous solution and can effectively remove lignin and uronic acid in the hemicellulose which is the major obstacle in cellulose accessibility (Nosratpour et al. 2018). It can overcome the problems associated with conventional alkaline pretreatments like extensive neutralization, corrosion, and environmental hazards. Hashemi et al. (2016) revealed that 0.5 mol/L sodium carbonate worked best for safflower straw digestion with an increase in the methane yield up to 139.6 N mL/g VS added.
Urea is another commonly used reagent for biomass chemical pretreatment. There is strong evidence on pH regulation and acidity by urea during lignocellulosic digestion for methane production. Considering the advantages of urea in pH regulation, it is regarded as a potentially valuable method for further application. Pretreatment of wheat straw with 1% urea achieved the maximum methane production (305.5 L/kg VS) with 49.4% total solids, 54.5% volatile solids, 50.4% cellulose, and 47.3% hemicellulose reduction (Yao et al. 2018).
Besides NaOH, KOH, Ca(OH)2, Na2CO3, and urea, ammonia (NH3) and ammonium sulfate (NH4)2SO4 solutions are the most commonly used reagents in industries for pretreating biomass before subjecting to anaerobic digestion. The main mechanism of ammonia solution is the saponification reaction followed by cleavage of lignin–polysaccharide linkage (Fang et al. 2015). Taken together ammonia-based pretreatments include soaking in aqueous ammonia (SAA), ammonia recycled percolation (ARP), ammonia fiber expansion (AFEX), and extractive ammonia (EA). Aqueous ammonia pretreatment (SAA and ARP) has been reported to enhance the enzymatic saccharification by xylan/lignin removal with no significant change in the cellulose allomorphic structure. On the other side, anhydrous ammonia (AFEX and EA) pretreatment also enhanced the enzymatic saccharification by subtle modification in ultrastructural and physiochemical properties of the cell wall with modification in the allomorphic structure of cellulose or reduction in its crystallinity (Zhao et al. 2020). Also, ammonia-based alkaline pretreatment has been integrated most extensively as it is non-corrosive, easily recoverable and reused, non-toxic, inexpensive, offers versatile processing options, and selectively removes lignin (Li et al. 2015).
Acid pretreatment
Acid pretreatment uses sulphuric acid (H2SO4), hydrochloric acid (HCl), phosphoric acid (H3PO4), nitric acid (HNO3), nitrous acid (HNO2), maleic acid, and organic acids. Extensive studies have been conducted on the pretreatment of lignocellulosic biomass by dilute acids. This pretreatment is performed by spraying or soaking the acid solution on the surface of the raw material and then heating to a temperature between 140 and 200 °C for a certain period. Acid cleaves the glucosidic bonds which facilitates the solubilization of the hemicellulose fraction into oligomers and monomers by enzymatic hydrolysis, thus increasing their accessibility to microbes and resulting in improved biogas production. Although the use of concentrated acid was highly adapted for cellulose hydrolysis, the formation of various inhibitory compounds (furfural, 5-hydroxymethyl furfural, acetic acid, aldehyde, ketone, and phenolic acid), loss of dry matter, and corrosiveness hinder the process of methanogenesis besides adding to the capital costs due to the requirement of high priced nonmetallic containers for reactor configuration (Taherzadeh and Karimi 2008). Under severe acidic conditions, pentoses and uronic acid are dehydrated to form furfural and HMF, which are unstable and are further degraded to formic-, formid-, and levulinic acids. Besides, the acid-labile lignin bonds are rapidly split in presence of concentrated acid, thus generating a higher level of phenolics in the medium. However, the generation of inhibitors was not always higher at higher acid concentrations. For instance, Wang et al. (2020) carried out the pretreatment of cornstalk at 5% and 7% H2SO4 and found that the formation of maximum inhibitors occurred in an anaerobic digestor fed with biomass pretreated with 5% H2SO4. Therefore, the appropriate and optimum concentration of acid, reaction temperature, and retention time for biomass pretreatment are the critical parameters for reducing the inhibitor production. Nevertheless, despite the above drawbacks associated with acid pretreatment, acids also transform part of cellulose and lignin, releasing carbohydrates and oligomers (de Carvalho et al. 2015). Hence from an environmental and economic perspective, dilute acid pretreatment is more suitable and advantageous compared to the concentrated acid method due to the lower acidic waste production, less corrosive effect, and low cost of reagents (Jung and Kim 2015).
H2SO4 is the most studied acid catalyst for lignocellulosic pretreatment than the other acids due to its effectiveness. Pretreatment by 6% H2SO4 could improve the biogas yield of rice straw by 99.8% (Qin et al. 2011). Martínez-Patiño et al. (2017) revealed that olive biomass yielded maximum biofuel when pretreated in the presence of H2SO4 catalyst (4.9 g/100 g biomass) at 160 °C for 10 min. Chandrasekaran et al. (2017) found that the crystallinity index of cassava stem decreased from 63 to 52% after combined organic (oxalic acid)–inorganic (H2SO4) pretreatment. Furthermore, the major disadvantage of using H2SO4 in pretreatment was the formation of hydrogen sulfide (H2S) in due course of anaerobic fermentation which affected the biogas quality and reduced its applicability owing to the corrosive nature of H2S (Domański et al. 2020).
Maleic acid treatment of biomass has been rarely studied so far. Mosier et al. (2001) stated that maleic acid at short HRTs showed a strong effect on the substrate with low lignin percentage while it showed a similar effect on the substrate with high lignin percentage at short as well as long HRTs. Pretreatment using maleic acid requires high cost inputs; however, the fact that hardly any chemical residue was left following the pretreatment made it an attractive option for chemical pretreatment of biomass.
HCl at concentrations of 1%, 2%, and 4% (w/w) has been employed for pretreatment strategy. HCl mainly causes hemicellulose solubilization and cellulose reduction by breaking the hydrogen bonds, Van der Waal forces, and covalent bonds that hold and give a rigid structure to the biomass. Song et al. (2014) carried out the pretreatment of corn stover with seven chemicals for improving the methane yield and found that 3% H2O2 and 8% Ca(OH)2 gave maximum yield among the different chemicals in the following order 8% Ca(OH)2 > 10% NH3.H2O > 8% NaOH for alkaline pretreatment and 3% H2O2 > 4% CH3COOH > 2% H2SO4 > 2% HCl for acidic pretreatment.
H3PO4 pretreatment is useful in improving the buffering capacity of the anaerobic fermentation system (Song et al. 2014). H3PO4 improved the biodegradability of feedstock by 6.3 times which was higher than the fungal digestibility (only 4 times) (Ishola et al. 2012). Wassie and Srivastava (2016) found that H3PO4 modified teff straw showed a better performance in terms of hemicellulose removal with increased porosity and less solid structure failure. Nair et al. (2017) mentioned that the application of 1.75% H3PO4 for pretreatment resulted in the best outcome of biogas production which was about 50% higher than the untreated group.
Additionally, organic acid pretreatment of rice straw, corn stover, wheat straw, napier grass, and other crop straw has been reported for enhanced methane production. However, in contrast to previously discussed acidic pretreatments, this method is less studied due to its low effect on digestibility and no obvious benefit to anaerobic fermentation in terms of enhancement in the biogas and methane production (El-Shemy et al. 2015).
HNO3 is also a promising acid catalyst for biomass pretreatment due to its high efficiency for hemicellulose removal. Nitric acid produced from flue gases via NOx capture displayed a shorter reaction time with high saccharification efficiency (Kim et al. 2014). Furthermore, nitrate formed upon neutralization and its conversion to N2 could serve as a source of nitrogen in the fermentation process (Zhang et al. 2011). In contrast to H2SO4, HNO3 pretreatment was advantageous since it caused lower corrosion, and was observed to be much faster and effective for feedstock pretreatment (Dziekońska-Kubczak et al. 2018).
Gaseous pretreatment
Gaseous pretreatment has the following major advantage in that, it promotes uniform penetration far and wide into the substrate, thus offering uniform coverage. It has been observed that the gases preferentially attack lignin than carbohydrates causing a 50% decline in the lignin percentage which was optimal. However, when compared to the liquid medium, the gaseous medium was difficult to work with and its reuse posed some problems over the previous one (Fan et al. 1982).
Sulfur dioxide (SO2) is a gas that forms sulfurous acid when dissolved in water. Sulfurous acid binds to lignin to form lignosulphonate and the second exposure substantially depolymerizes the lignin. SO2 was reportedly suitable for effective treatment of both hardwood and softwood. SO2 impregnation prior to steam exposure led to a reduction in the carbohydrate degradation and enhanced the enzymatic digestibility of substrate resulting in more impressive pretreatment (Bura et al. 2002). Dechman et al. (2020) reported that the SO2 gas impregnation was more effective than H2SO4 in terms of uniformity, rapidness, and better recyclability. However, a high level of SO2 requirement with a long reaction period for pretreatment presents a problem in industrial expansion. Furthermore, difficulties in the processing and the disposal of sulfonated species may impede the commercialization of SO2 pretreatment (Foody et al. 2019).
Chlorine dioxide (ClO2) is an active bleaching agent that often utilizes sodium chlorite (NaClO2) and acetic acid. Acetic acid is usually added to reduce the pH of the medium and sodium chlorite in water is acidified to form ClO2 which preferentially oxidizes the lignin in the presence of polysaccharides, therefore, fulfilling the pretreatment requirement. ClO2 exhibited 2.63 times higher oxidation capacity than elemental chlorine gas, thus acting as an effective bleach. One of the major obstacles in ClO2 pretreatment was the production of highly toxic adsorbable organic halogen (AOX) from hypochlorous acid during ClO2 bleaching. However, AOX formation can be reduced by purifying the ClO2 solutions and removing chlorine gas, thus reducing the hypochlorous acid content (Yao et al. 2019).
Nitrogen oxide (NO) reacts with O2 to form NO2 followed by a reaction with water to generate HNO3 which subsequently could catalyze the hemicellulose removal with high efficiency (Fan et al. 1982). This method is relatively unexplored as evident from the very few publications.
Carbon dioxide (CO2) once dissolved in water will form HCO3− (carbonic acid), a weak acid that was capable of hydrolyzing hemicellulose and cellulose. Upon CO2 explosion with pressure, the disintegration of cellulose structure increased the surface area for enzymatic hydrolysis by anaerobes (Park and Lee 2020). Zhao et al. (2019) found that the pretreatment of agriculture residues (corn cob, corn stover, and sorghum stalk) at optimal conditions of 50–80 °C, 17.5–25 MPa for 30 min, gave maximum sugar yield, and cellulose and hemicellulose conversion with a very low level of furfural (0.25%), hydroxymethylfurfural (0.07%) and acetic acid production. CO2 pretreatment increased the glucose and C5 sugar recovery from lignocellulosic biomass. However, the efficiency of this pretreatment was eminently affected by the intrinsic features and composition of the biomass.
Oxidizing agents
Oxidative pretreatment disrupted the lignin–polysaccharides matrix by several oxidation reactions such as electrophilic substitution, oxidative cleavage of aromatic nuclei, alkyl aryl ether linkage cleavage, or displacement of the side chain (Kumari and Singh 2018). The only drawback of oxidative agents was their cost-intensive and explosive nature when used in concentrated form.
Ozone is the strongest oxidizing agent, water-soluble, and readily accessible for application after its generation from oxygen in an endothermic reaction. Ozone being electron-deficient in its terminal oxygen was able to attack lignin (an electron-rich polymer) more than carbohydrate (Coca et al. 2016). Rosen et al. (2019) revealed that a short ozonation period of 90 min delivered a better lignocellulosic conversion than a long ozonation period (6 h and beyond) that resulted in reduced conversion. Ozone is highly reactive for lignin, but its low selectivity degrades carbohydrates and generates some by-products that may be inhibitory in the downstream process. The by-products mainly included carboxylic acid which could be removed by simple water washing (Travaini et al. 2015). Simultaneously, ozone converted the nitrogen-containing polymers and phenol during anaerobic digestion, minimizing the interaction with inhibitors and significantly improving the methane yield (Si et al. 2019). Other potent inhibitors such as HMF and furfural are not found during ozonolysis pretreatment. The low inhibitory compound formation, mild condition requirement, on-site easy ozone generation and utilization, the ability of microbes to degrade ozonolysis bioproducts, chemical-free process, reduction in environmental pollution, absence of liquid phase, and avoidance of problems associated with product dilution are some advantages of ozonolysis. Nevertheless, high reactivity, flammability, corrosiveness, and toxicity of ozone, the requirement of cooling systems, high cost, and high energy demand are the major obstacles in the development of this process (Travaini et al. 2015).
Hydrogen peroxide (H2O2) pretreatment is an oxidative process which selectively attacks carbonyl and ethylene group and promotes delignification (Ho et al. 2019). Studies by Song et al. (2012) showed 4% H2O2 to be optimal for maximum biogas yield of 327.5 mL/g VS. However, the authors suggested that 3% H2O2 would both be optimal for improving the methane yield and be sustainable in the long run if the economic aspects were considered. Also, H2O2 concentration of more than 4% has been shown to cause accumulation of hydroxyl ions whose toxicity to the methanogens subsequently inhibited the anaerobic fermentation. With an increase in H2O2 concentration, biomass with initial high sugar percentages such as microalgae led to a faster degradation of sugars into by-products during the initial steps which inhibited the process of methanogenesis (Dutra et al. 2018). The main advantages of H2O2 pretreatment include low energy consumption, no generation of furfural and 5- HMF, easy acquisition and availability, no need of a special reactor for pretreatment, its compatibility with different solid loadings, and elimination of the need for antibiotics. The requirement of a large volume of water or HCl to maintain pH, generation of other inhibitors like ferulic acid, sodium acetate, p-coumaric acids due to lignin decomposition are the main drawbacks of this pretreatment method besides the high price of H2O2 (Dutra et al. 2018). Genetic strategies can be adopted for making the microbial community resistant to the inhibitors present in H2O2 pretreated biomass to obtain high biogas yield.
In wet oxidation, the transfer of hemicellulose from solid to liquid phase is promoted using air or oxygen as a catalyst with the introduction of temperature above 120 °C and pressure between 0.5 and 2 MPa for less than 30 min. Temperature plays a crucial role in enzymolysis of biomass by wet oxidation extraction as observed by Fang et al. (2017), wherein the yield of pretreated material fell from 54.9 to 42.7% by increasing the temperature from 165 to 185 °C and up to 205 °C. While, in the case of sugarcane bagasse, maximum hemicellulose (93–94%) and lignin conversion (40–50%) was obtained at 195 °C compared to the conversion rate at 185 °C (only 30% hemicellulose and 20% lignin conversion). The formation of inhibitors was maximum at 195 °C (Martín et al. 2007). Lee et al. (2020) carried out the pretreatment of oil palm empty fruit bunch at 3%, 6%, and 9% oxygen loading rate under mesophilic and thermophilic conditions. The highest methane yield (362 mL CH4/g VS) was found at 6% oxygen loading at mesophilic temperature (37 °C) over untreated one, while the methane yield decreased under thermophilic conditions (55 °C). At 3% oxygen loading, only a slight increase in the methane yield (285 mL CH4/g VS) over control (276 mL CH4/g VS) was observed due to insufficient oxygen. Pretreatment at 9% oxygen loading resulted in the lowest methane yield (284 mL CH4/g VS) due to the formation of inhibitory and toxic compounds under excessive oxidation conditions. This result supported the finding by Klinke et al. (2002) that most of the furan derivatives and phenolics were produced under the conditions of high oxygen and temperature. Its industrial application is generally revoked due to its non-selectivity in delignification with hemicellulose loss, high cost of catalyst oxygen and pressure equipment, and inhibitor generation (Kumari and Singh 2018).
Solvent extraction
Solvent extraction uses organo-solvents like ethanol, methanol, benzene, butanol, ethylene glycol, acetone, and tetrahydrofurfuryl alcohol in the presence or absence of catalyst for degradation of the lignin–carbohydrate complex. Use of alkali catalyst is preferred over the acidic catalyst due to the problems associated with acidic catalyst in hemicellulose recovery. Organic solvents demonstrate high-purity cellulose separation with higher efficiency for fractionation of hemicellulose in comparison with conventional methods. Guragain et al. (2016) carried out the fractionation of corn stover, poplar, and Douglas fir with 8 different organic solvents. He found that the combination of ethanol and isopropanol for corn stover and a mixture of glycerol and 2,3-butanediol for poplar were the most efficient organic solvents for comparatively higher sugar release than control. However, these solvents were not effective for softwood (Douglas fir) pretreatment. The benefit of using organic solvents lies in its effortless recovery by distillation and scope for recycling back into the pretreatment. However, a high boiling point for solvent recovery demands an additional energy input which is a disadvantage of this method. No commercial-scale application of organic solvent pretreatment biomass is reported.
Biological pretreatment
Biological pretreatment provides an attractive option for biogas production from lignocellulosic biomass over the physical and chemical pretreatment methods which require high energy consumption, high capital cost, and produce contaminants that may be toxic for subsequent enzymatic hydrolysis in anaerobic fermentation (Liu et al. 2014). Biological pretreatment can decrease the anaerobic digestion period, enhance the digestibility of dry matter, and gas production estimate (Mishra et al. 2018). The transformation of non-fermentable ingredients of feedstock into value-added chemical products complements one of the missing links which is needed for the accomplishment of the bio-refinery perception (Axelsson et al. 2012). Biological pretreatment employs either microorganisms (monoculture or co-culture) or enzymes (pure or in mixture form). Consequently, microbes at different stages of their growth cycle degrade complex organic molecules as their carbon and energy source (Aydin 2016). Also, enzymes formed during the pretreatment can be entrapped and used in various other processes, thus serving as co-products similar to the organic substances and lignin-derivatives, adding worth to the technique (Chen et al. 2010). The effectiveness of biological pretreatment for delignification is determined by calculating the ratio of lignin to cellulose loss that gives selective value to the lignin degradation (Kamcharoen et al. 2014). Fungal strains displaying a selectivity values less than 1.0 are not considered for lignin removal in biological pretreatment (Zhang et al. 2007). The main advantages of biological pretreatment include a requirement of mild conditions, simple equipment, and low energy consumption, low cost for downstream processing, no production of any toxic secondary compounds, and no need for chemical recycling after pretreatment. The requirement of lengthy pretreatment cycle and large area, sugar consumption, and need for efficient microbial agent are the major bottlenecks of this pretreatment method (Zabed et al. 2019).
Monoculture pretreatment
Under the circumstances of unfavorable environmental conditions such as nutrient depletion, fungi and bacteria produce extracellular enzymes and catalyze lignin breakdown by miscellaneous biochemical reactions. The fungal pretreatment includes white-rot fungi (WRF), soft-rot fungi (SRF), and brown-rot fungi (BRF). WRF and BRF are the members of the basidiomycetes and SRF belong to the ascomycetes and deuteromycetes class.
SRF usually attack lignocellulosic biomass with low lignin and high moisture content by creating unique pockets for cellulose and lignin depolymerization. Goodell et al. (2008) found that the biomass with high moisture content and high unsaturation was more susceptible to attack by SRF. The potential of some SRF like Penicillium chrysogenum, Aspergillus niger (Hamed 2013), and Trichoderma reesei (Mustafa et al. 2016) have been explored for biological pretreatment of lignocellulosic biomass. However, the prominent ones which have demonstrated exceptional capabilities for higher degradation of lignin over carbohydrates include Cadophora sp. (Chandel et al. 2015), Paecilomyces sp. (Singh et al. 2016), Botryosphaeria sp. and Fusarium oxysporum sp. (Sista Kameshwar and Qin 2018) from SRF family.
BRF cause the biomass to lose strength rapidly and primarily degrade the holocellulose component instead of lignin. These fungi are known to produce β-glucosidases to cleave cellobiose and other oligosaccharides; β-xylosidases & endoxylanases to hydrolyze hemicellulose; and endoglucanases to break down the β-1, 4-glucosidic linkages. BRF also catalyze non-enzymatic breakdown by secreting low molecular weight compounds such as hydroxybenzene derivatives, quinones, and catechols which may be involved in the partial breakdown of the lignin. These compounds catalyze the formation of hydroxyl radicals through Fenton’s reaction thereby speeding up the hydrolysis of pretreated substrates during downstream processing (Arantes et al. 2012). Coniophora puteana (Ferdeş et al. 2018), Postia placenta (Kameshwar and Qin 2018), and Gloeophyllum trabeum (Sanhueza et al. 2018) are the most reported BRF for pretreatment of lignocellulosic substrate.
WRF are also well known for their efficient lignin-degradation capability by oxidizing lignin along with increasing the enzymatic hydrolysis of polysaccharides. The enzymes associated with lignin degradation activity by WRF include laccase, lignin peroxidase (LiP), and manganese peroxidase (MnP). Some strains of WRF such as Strobiluru sohshimae, Phanerochaete chrysosporium, Trametes versicolor, and Pleurotus ostreatus can only produce ligninolytic enzymes and catalyze the selective lignin degradation without hydrolyzing cellulose or hemicellulose (Sánchez 2009). WRF include a good number of lignin oxidizing species yet the lignin degradation efficiency depends on the type of biomass, lignin percentage in biomass, fungal species, pretreatment time, and environmental factors such as temperature conditions (Liu et al. 2017). Fungal pretreatment is advantageous over other methods which is attributed to the low pretreatment expense; low reagent and energy requirement, and low downstream recovery cost, and lower generation of waste. However, a long pretreatment period and loss of saccharified sugar owing to utilization by fungi for growth are the major drawbacks associated with fungal pretreatment of lignocellulosic biomass (Zheng et al. 2014).
Although lignin degradation has been thoroughly studied in fungi, recent research has focused its inclusive interest on bacterial ligninolytic enzymes such as laccases (Singh et al. 2017), peroxidases (Falade et al. 2017), and β-etherases (Voß et al. 2020), and their delignification efficiency. De La Torre et al. (2017) compared the efficiency of bacterial (Streptomyces ipomoeae (SilA)) and fungal (Trametes villosa) laccase for detoxification and delignification of steam-exploded lignocellulosic biomass and found that the laccase from Streptomyces ipomoeae slightly decreased the lignin percentage and increased the xylose and glucose yield of hydrolysate, an outcome that was not observed in laccase produced by T. villosa. Exploring the potential of Pandoraea sp. B-6 on corn stover, Zhuo et al. (2018) found that the pre-erosion treatment of corn stover could expose more phenoxy radicals that acted as mediators for activation of laccase and manganese peroxidase and thus opened the network for efficient bacterial pretreatment. Zeng et al. (2013) studied the mechanism of lignin degradation by S. viridosporous T7A and found that its modification featured an increased S/G (syringyl:guaiacyl) ratio, reduction of carbonyl group, deduction of guaiacyl unit, and enhancement of methoxyl group. Buntić et al. (2019) carried out a study to determine the cellulolytic potential of S. meliloti strain 224 using tobacco waste as a substrate under submerged and solid-state cultivation and reported that the strain could produce both avicelase (0.131 U/mL) and carboxymethyl cellulase (1.615 U/g) for efficient conversion of biomass to biogas. Bacterial pretreatment was cost-effective, highly adaptable, required a shorter pretreatment period, and bacteria could be subjected to genetic manipulation more easily than fungi. Also, the metabolic activity and growth rates of bacteria were faster than fungi, though fungi offered potent mechanisms and enzymes for lignin removal from lignocellulosic biomass than bacteria (Rashid et al. 2017). Yet, bacteria can easily degrade cellulose and hemicellulose fractions of biomass. Table 3 shows the different fungi and bacteria reported for delignification of lignocellulosic biomass.
Co-culture pretreatment
The microbial community in the environment has the natural ability to degrade complex organic material by their synergetic metabolism which is otherwise hardly degraded by an individual microbe. Co-culture pretreatment of lignocellulosic biomass is usually done by employing microbial consortia which include bacteria-bacteria co-culture, fungi-fungi co-culture, or bacteria-fungi co-culture (Sharma et al. 2019). The requirement of maintaining aseptic conditions for pure culture, inability to execute pretreatment in an open environment, and long pretreatment times associated with monoculture pretreatment provide a new insight into using and developing the microbial consortia for biomass pretreatment to overwhelm these issues (Zabed et al. 2019). Recently, it was reported that a microbial consortia OEM2 increased the hemicellulose and lignin degradation rate along with chlorophenol detoxification (75%) within 12 days of rice straw pretreatment (Liang et al. 2018).
Microbial consortia pretreatment offers advantages of increased adaptability, increased substrate exploitation, enhanced hydrolysis productivity and efficiency, decreased pretreatment time, and improved process control in terms of better pH management (Kalyani et al. 2013). Despite having so many advantages, industrial applications of microbial consortia still face several problems related to the microbial consortia stability and their effectiveness in synergetic metabolism.
Enzymatic pretreatment
Enzymatic pretreatment includes the use of crude, purified, or semi-purified enzymes (oxidative and hydrolytic) produced by bacteria or fungi. Recently enzymatic pretreatment of biomass is gaining more interest over the other biological techniques as enzymes are not affected by the presence of other microbial metabolism and inhibitors (Wei 2016). Furthermore, the enzymes do not lose activity over a long time, can be easily recovered after pretreatment, do not require expensive chemicals and equipment for biomass processing (Ometto et al. 2014), and require shorter pretreatment time (Plácido and Capareda 2015). But the enzyme purification cost and their poor stability are the major hindrances in industrial processes (Zheng et al. 2014).
An enzyme is very specific and catalyzes a particular type of reaction, hence the efficiency of enzymatic pretreatment depends on the composition and type of biomass being treated, temperature, pH, incubation time, reactor configuration as well the enzyme used (Parawira 2012; Michalska et al. 2015). The application of specific enzymes in anaerobic digestion has shown to increase the degradability of biomass and subsequently the methane production (Weide et al. 2020). The enzymatic degradation (using laccase and versatile peroxidase) of corn flax, corn stover, hemp, and wheat straw resulted in a high biomethane production (241–288 NL/kg VS). Nevertheless, the same protocol of enzyme hydrolysis of willow and miscanthus initially showed higher release of phenolic compounds and lower biomethane potential (68.8–141.7 NL/kg VS) due to the presence of lignin. Antonopoulou et al. (2019) found that the enzymatic saccharification of acid pretreated biomass was higher than the alkali-treated one. Till now, enzymatic pretreatment of wheat grains, paper and pulp sludge, hay fibers, microalgae, sugar beet pulp silage, switchgrass, rye grain silage, sida, grass silage, and feed residues has been extensively studied for methane improvement using commercial enzymes. Crude enzyme mixtures are more promising for enzymatic pretreatment since they are easily secreted in the extracellular medium by several fungi and bacteria and are more efficient in handling technical and economic issues.
Ensiling
Ensilage was the pretreatment method introduced owing to the need for storing and preserving lignocellulosic biomass with high moisture content to make it available as a whole year’s supply for anaerobic fermentation. Ensiling pretreatment is based on the hypothesis that the microorganisms performing in ensilage conditions ferment a high percentage of water-soluble sugars in the biomass in presence of the high moisture content producing a variety of volatile organic acids that lead to a decrease in the pH of the medium (Yang 2020). This acidic pH breaks the lignin–polysaccharide bond of the biomass, reduces the loss of polysaccharides, and prevents the growth of undesired microbes (Nagle et al. 2020). Organic dry matter (ODM) losses during full-scale ensiling are one of the major issues in preserving the lignocellulosic biomass. However, mechanical pretreatment before ensiling can greatly reduce the ODM losses and lead to a higher biomethane potential (Feng et al. 2018). Chen et al. (2007) carried out the ensilage of five agricultural residues (cotton stalk, triticale hay and straw, barley straw, and wheat straw) and reported that ensilage with enzymatic pretreatment gave a combined advantage of high saccharification, holocellulose hydrolysis, and lignin removal compared to untreated biomass and ensilage pretreated biomass. Pretreatment of sorghum with hemicellulase and L. plantarum silage (HCL silage) enhanced the lactic acid concentration and residual sugar percentages by enhancing the cellulose and hemicellulose hydrolysis (Zhao et al. 2018). Ensilage is more effective for biomass containing high content of indigenous sugars like sugar beet pulp, sugar beet leaves, sweet sorghum stalk, and sweet corn stover and may not be suitable as a standalone technique for the pretreatment of feedstocks that contain no or poor water-soluble sugars such as cereal straws, hardwood, and softwood. Hence the ensiling of sugar-containing feedstocks with normal biomass may extend the applicability of this technique which was demonstrated for its efficacy in high biochemical methane potential (BMP) within a short time in comparison with the normal ensilage process (Sieborg et al. 2020). This observation was a positive attribute towards solving the issues associated with single biomass pretreatment.
Ensiling offered multiple advantages in the form of lower energy input, storage and preservation of high moisture containing biomass, diminished energy requirement for drying, the requirement of milder operating conditions, preventing carbohydrate loss by lowering pH, and providing opportunities for combined pretreatment of biomass which otherwise face difficulties if pretreated alone (Wu et al. 2018). However, this method suffers from the longer time required for pretreatment in comparison with other biological techniques with low lignin removal efficiency (Chen et al. 2007). Table 4 shows different microbial consortia, enzymes, and ensiling pretreatment applied to lignocellulosic biomass for improving the biogas generation.
Emerging technologies for pretreatment
Nowadays, with rapid advances in applied chemistry, new industrial technologies have emerged for lignocellulose pretreatment which are based on extreme and non-classical conditions such as ultrasound, gamma rays, electron beam irradiation, pulsed electric field, high hydrostatic pressure, and high-pressure homogenization. These approaches have yielded promising results for decreasing the cellulose crystallinity and have contributed to biogas enhancement from lignocellulosic biomass.
Ultrasound pretreatment of lignocellulosic feedstock results in disruption of the α-O-4 and β-O-4 linkage in lignin by the production of oxidizing chemicals which ultimately forms small cavitation bubbles on the lignocellulosic structure. The cavitation bubbles grow to a critical size, become unstable, collapse violently, and burst to break the linkages of lignin (Gonzalez-Balderas et al. 2020). It was found that in comparison with alkali pretreatment, the ultrasound-assisted delignification of coconut coir, pistachio shell, and groundnut shell resulted in an 80–100% increase in delignification efficiency with 0.5% biomass loading rate at 100 W for 70 min (Subhedar et al. 2018). The efficiency of ultrasonic pretreatment was attributed to the increase in the crystallinity and rupture of methylene/methyl groups of cellulose by decreasing the alkali metal in the material and by breaking down pits, and generation of microchannels (He et al. 2017).
Gamma rays obtained from radioisotopes (cesium-137 or cobalt-60) function by weakening the Van der Waal forces, generation of free phenoxy radical intermediates, and hydrolysis of the glycosidic bond (Loow et al. 2016). The irradiation of biomass with gamma rays prior to physical pretreatment contributes to reduced particle size and low shearing rate of the material, subsequently allowing the application of high biomass loading for the hydrolysis process (Wu et al. 2020). The appropriate dose of gamma radiation can eliminate the toxic effect of radiation compounds for biogas generation but the requirement of small and specialized chambers for safety concern limits the chunk of feedstock which can be treated per batch (Kumar et al. 2020).
The electron beam (EB) irradiation method uses irradiated accelerated electron beams to disrupt the polymer by the formation of free radicals. EB mainly depolymerizes cellulose, hence it is applied in combination with other pretreatment technologies for hemicellulose and lignin depolymerization (Hassan et al. 2018). Fei et al. (2020) stated that a high dose of irradiation decreased the cellulose crystallinity index and depolymerization with a reduction in the production of inhibitory compounds (furfural and 5-HMF). A similar study was carried out by Kumar and Tumu (2019) who found that a high dose of EB could lead to the production of excess free radicals which sheared the polymer chain in both amorphous and crystalline cellulose.
In pulsed electric field (PEF) pretreatment, membrane permeabilization in the biomass is achieved through the formation of aqueous pores on the cell membrane (electroporation) of biomass subjected to high sudden voltage bursts (5.0–20.0 kV/cm) for a short period. Wang et al. (2017) observed that the yield of reducing sugars and enzymatic hydrolysis of cellulose improved under moderate conditions of the electric field strength of 12v/m, enzyme loading of 26.68 mg/g substrate, water content 5 mL/g substrate, pH 4.5, 6 h of electrodes with overtime. PEF can increase the cellular division rate, speed up the fermentation efficiency, methanization, anaerobic digestion and biogas production, and reduce the energy requirement with selective targeting of the cell membrane (Golberg et al. 2016). PEF technologies have been used for the pretreatment of a variety of biomass but their use on seaweeds is very limited due to the high salt content of this biomass. The high salt content resulted in a high requirement of electric conductivity that led to an increase in the pulse current with unacceptable heating of the electroporated biomass beyond the energy dissipation capacity of the device (Levkov et al. 2020).
High hydrostatic pressure (HHP) pretreatment involves the exertion of high pressure for breaking the non-covalent bonds, thus changing the macromolecular conformation. It was reported that for coconut husk, HHP performance of fungal cellulase under pressurized conditions was increased by a factor of 2 (Albuquerque et al. 2016). Öztürk (2019) observed that different pressure (0.1–500 MPa) and time (5–15 min) combinations affected the celluclast activity and found that the efficiency of enzymatic hydrolysis for peanut hull was maximum at 100 MPa for 15 min. HHP positively affected the reaction rate and chemical equilibrium as seen from the enhanced enzymatic hydrolysis at pressures below that causing the denaturation in protein structure (Levkov et al. 2020).
High-pressure homogenization (HPH) is a well-known cell disruption method to homogenize the particles using a pressure pump at operating pressure of 150–200 MPa. Jin et al. (2015) found that in contrast to alkaline heat pretreatment of grass clippings, HPH could destroy the biomass microstructure to an empty-inside structure and provide the accessible surface area for enzyme attack without hemicellulose loss. The authors noted that, under high working pressure of 206.84 MPa, the particle size of lignocellulosic biomass decreased while the biomass surface area increased which led to an enhancement of the enzymatic hydrolysis efficiency as reflected from high sugar yield and bioethanol production (Choi and Lee 2016). Saelee et al. (2016) found that the HPH can also be useful for isolating nano-fibrillated cellulose from the lignocellulosic biomass.
Combined pretreatment technologies
In recent years, combining two or more pretreatment technologies has been carried out to consequently improve the pretreatment effect on biogas production. Pretreatment methods like alkali, acid, liquid hot water, and steam explosion can remove hemicellulose very effectively, while alkali and biological pretreatment parade better effect in lignin removal from lignocellulosic biomass. The physical pretreatment method reduces the size of the particle and crystallinity and reduces the acidification of the system which increases buffering capacity. Thermal and chemical pretreatment methods use temperature and chemicals to decrease the stability of lignocellulosic bonds and enhance the biogas estimates. On the other hand, biological agents utilize lignin as a source of their energy and play a vital role in biogas production.
Although, single pretreatment method can contribute significantly to biogas yield, at the same time there are series of problems associated with each of the pretreatment technologies. The degradation of renewable yet difficult to degrade lignocellulosic biomass can be increased by applying a combination of pretreatment technologies (Akhtar et al. 2016). Since each single pretreatment technology has its disadvantages, combined pretreatment technologies may not only overcome the disadvantage of pretreatment but also enhance the enzyme accessibility to cellulose and facilitate the lignin and hemicellulose recovery for the production of high-value products. Combining two pretreatment technologies can achieve the goal of mutually making up for the defect in each of the technologies with consequently improving the pretreatment effect (Yu et al. 2019). An increased sugar yield, short processing time, and less inhibitor formation are observed with combined pretreatment (Kumari and Singh 2018). Moreover, a number of combined pretreatment technologies, such as physio-chemical pretreatment (Divyalakshmi et al. 2017; Mahajan et al. 2019), thermo-chemical pretreatment (Mlaik et al. 2018), ultrasonication assisted acid pretreatment (Rehman et al. 2014), electron beam irradiation combined with ionic liquid (Jusri et al. 2019), bio-derived cholinium ionic liquids and ultrasound irradiation (Ninomiya et al. 2013), fungal pretreatment in combination with alkaline treatment (Alexandropoulou et al. 2017), extrusion combined with alkali pretreatment (Zhang et al. 2015a, b), have been researched for improving the efficiency of anaerobic digestion of lignocellulosic residues.
Critical assessment of pretreatment methods commonly adopted for major biomass
Despite the availability of a number of pretreatment options, only a few are commonly and repeatedly applied for lignocellulosic biomass before anaerobic digestion. The preference for pretreatment technology depends specifically on the type and various constituents present in biomass. An ideal pretreatment technology should possess several characteristics such as moderately low energy input, low environmental impact, minimum chemical and water use, low capital cost, maximum sugar recovery and minimum inhibitor generation from sugar degradation during pretreatment, low demand for down-stream processing like washing, detoxification and neutralization, higher pretreatment rate in short duration, and production of high biogas/biofuel and other value-added products. No single pretreatment option can provide all the advantages for enhancing biogas production since each pretreatment method is plagued by some or the other inherent limitation. Application of pretreatment method depends entirely on the proximate (percent of ash, fixed carbon, volatile fraction in dry matter, and heating value), compositional (cellulose, hemicellulose, and lignin content), and ultimate (carbon, nitrogen, hydrogen, sulfur, and oxygen percentage) properties of the biomass. For example, dilute acid pretreatment is more effective for enhancing the gas production estimate of corn and poplar tree bark as compared to sweet gum bark.
ScienceDirect displays 38,189 cumulative papers including 3,836 review articles published in the last 3 years (2018–2020) on research topic related to “pretreatment” and “biomass”. Of all the pretreatment methods adopted conventionally, chemical pretreatment is the most widely reported method for major agriculture residues (maize stuver, maize straw, rice straw, rice husk, wheat straw, corn cobs, cottonseed hairs, sugarcane leaves, sugarcane bagasse, and softwood stem) with dilute NaOH or H2SO4 apparently being most effective for methanogenesis during the fermentation process. Following the chemical pretreatment, biological pretreatment is gaining greater attention which unlike other pretreatment options has been reported to be the best for the effective valorization of biomass. Amongst the biological pretreatment methods, enzymatic pretreatment is gaining more interest owing to little or no generation of inhibitory compounds due to which it can be applied for pretreatment of a wide range of biomass. Hydrothermal pretreatment, on the other hand, is another pretreatment option that is gaining interest for the disintegration of the lignocellulose network. This technique has come to the fore as it only uses water at high temperature under pressure, and the required pH is maintained by oxidation of phosphorous and sulfur present in the biomass. The current trend is increasingly shifting towards adopting a combination of different pretreatment technologies as seen from the limited number of research articles published in the early period of the twenty-first century (only 63 publications in 2001) which have increased manifold in a very short time duration to 2492 publications in the year 2020. Figure 3 shows the comparative assessment of the number of different research articles published from the year 2018 to the year 2020 concerning the pretreatment technologies available for the processing of lignocellulosic biomass.
Effect of pretreatment on microbial community structure during biomethanation of lignocellulosic biomass
Since biogas/methane production efficiency of lignocellulosic biomass depends on the microbial metabolism, microbial communities present in the anaerobic digestion chamber are of paramount importance. The rigid network and low solubilization of lignocellulosic waste limit the hydrolysis step, and therefore the microbial activity and overall degradation efficiency. Pretreatment of lignocellulosic waste shows a pronounced effect on the increased proportion of valuable microbial community for anaerobic digestion. Figure 4 shows the effect of pretreatment on the enrichment and increase in abundance of microbial community and biogas production in anaerobic digestion of lignocellulosic biomass. This has been evidenced from numerous studies that have demonstrated the positive effect of pretreatment on microbial community structure and, hence, the performance of reactor in terms of the biogas production (Jung et al. 2016; Zhao et al. 2017; Li et al. 2017, 2019; Wang et al. 2018a, b; Westerholm et al. 2019). By exploring the advantages of biomass pretreatment effect on microbial communities and their metabolic pathways, more knowledge can be obtained to help us choose an effective and efficient pretreatment technology. Table 5 shows the different bacterial and archeal communities which became dominant by the effect of different pretreatment methods in the biogas production system. It can be seen that the Methanosarcina was able to survive most pretreatment conditions like acid, alkali, heat shock, ultrasonication, and so on. Methanoculleus, Methanobacterium, and Methanosaeta were also dominant in biogas production followed by Methanothermobacter and Methanomassiliicoccus. The methanogenic community shift during anaerobic digestion towards potent methane producing Methanosarcina and Methanosaeta suggests a key role of pretreatment in the main reaction of biogas production. Also, it is worth mentioning that pretreatment also changed the bacterial community composition mainly by the enrichment of phyla Firmicutes and Bacteroidetes. Several studies have indicated the importance of Firmicutes:Bacteroidetes ratio in anaerobic fermentation system which was positively correlated with increased biogas yield.
Besides its impact on the microbial community associated with biogas production, pretreatment of lignocellulosic biomass has also been reported for remarkable shifts in microbial community composition and abundance during biohydrogen and bioethanol production. The dominant genera during biohydrogen production from raw, untreated biomass consisted of Petrimonas, Proteiniphilum, Anaerolineaceae, and norank D8A-2 (Yang et al. 2019a, b) while those during bioethanol production included Escherichia coli, Klebsiella pneumoniae, Leuconostocaceae, Lactococcus, Weissella, and Fructobacillus; along with the dominant fungal genera—Mucor circinelloides, Candida, Hannaella, Issatchenkia and Papiliotrema (Gallagher et al. 2018). When similar biofuel production was carried out using pretreated biomass, assessment of the microbial community indicated the elimination/reduction in the abundance of all the aforementioned genera with enrichment of those which contributed to high biofuel yield. Bacteroides and Proteiniclasticum were the most abundant bacterial genera observed in biohydrogen production from pretreated waste with the relative abundance of 2.7% and 3.3%, respectively (Yang and Wang 2020). In addition to these two hydrolytic genera, Clostridium and Macellibacteroides also showed relatively high abundance in the bioreactor fed with pretreated biomass compared to the untreated control. Among the enriched bacteria, Clostridium and Proteiniclasticum were suggested for their role in contributing to higher biohydrogen yield (Zhang et al. 2015a, b; Dessì et al. 2018). A similar overall reduction in the unwanted bacterial community and increased abundance of Lactobacillus and Saccharomyces cerevisiae (potent genera for bioethanol production) was also observed in reactor fed with pretreated biomass (Ifeanyi et al. 2020). This showed that the pretreatment method before anaerobic fermentation could positively influence the evolution and formation of potential microbial communities in methane synthesis.
Scope/recommendations for future research
Biomass pretreatment is restricted by the high capital investment, reagent cost, and high energy consumption. Overcoming the drawbacks of pretreatment for the development and commercialization of large-scale industrial pretreatment plants is still a major issue. To realize the prospects of pretreatment technology, the review has opened new avenues for focussed research in the future on increasing the accessibility of lignocellulosic biomass to anaerobic digestion;
-
Implementation of combined pretreatment technologies for the efficient operation of a biogas plant with low energy consumption and secondary pollutant production
-
Development of efficient recycling system to address the issues associated with high chemical and enzyme cost and pollutant formation in biogas production.
-
A more detailed study of each pretreatment technology to overcome the bottlenecks and evaluating the techno-economic feasibility of each pretreatment method.
-
More industrial facilities and applicable equipment for pretreatment at a large scale and high efficiency need to be further researched
-
Implications of the right and effective pretreatment method to be chosen for crop straw which can increase the accessibility of microbial enzymes to cellulose. This will require the analysis of potential and existing biomass in terms of their composition and intrinsic properties before the start-up of large-scale application
-
Concentration and detoxification of hydrolysis products before the fermentation process to increase the biogas yield and reduction in the downstream processing cost needs to be studied further.
Conclusion
Knowledge about crop straw pretreatment technologies is now accumulating at an increasingly rapid pace. Given the abundant pretreatment technologies available globally and their application in various areas for treating biomass, based on the associated advantages and disadvantages, the following main conclusions can be drawn:
-
Physical pretreatment is the basic pretreatment step essential before any other pretreatment, which is aimed at breaking down the encrusting material of the cell wall and reducing the particle size of biomass. It promotes an improvement in bioprocess efficiency but the effect is intimately related to the costs.
-
Thermal pretreatment decreases the thermal stability of lignin biomass and breaks down the hydrogen bond, thus exposing the accessible surface area of cellulose for anaerobes. The only limitation of this method is that it leads to the formation of toxic derivatives at temperatures greater than 160 °C.
-
The beneficial effect of chemical pretreatment was better than physical and thermal pretreatments, but it produces secondary compounds that may be inhibitory and hinder the fermentation process.
-
Biological pretreatment plays a vital role in enhancing bioprocess like anaerobic digestion, but it needs an efficient microbial agent and suffers from the longer pretreatment period required in comparison with other methods.
-
Emerging technologies for lignocellulosic pretreatment give promising results; however high capital cost and the unavailability of comparative efficiency data of these methods on different substrates present major obstacles in commercialization.
-
Recently, combined technologies for the pretreatment of biomass have shown better performance over a single method. The synergistic application of combined pretreatment technologies can realize the future outlook for large-scale industrial applications.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Ahmed B, Aboudi K, Tyagi VK, Álvarez-Gallego CJ, Fernández-Güelfo LA, Romero-García LI, Kazmi AA (2019) Improvement of anaerobic digestion of lignocellulosic biomass by hydrothermal pretreatment. Appl Sci 9(18):3853. https://doi.org/10.3390/app9183853
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35(2):489–511. https://doi.org/10.1002/ep.12257
Albuquerque ED, Torres FAG, Fernandes AAR, Fernandes PM (2016) Combined effects of high hydrostatic pressure and specific fungal cellulase improve coconut husk hydrolysis. Process Biochem 51(11):1767–1775. https://doi.org/10.1016/j.procbio.2016.07.010
Alexandropoulou M, Antonopoulou G, Fragkou E, Ntaikou I, Lyberatos G (2017) Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J Environ Manag 203:704–713. https://doi.org/10.1016/j.jenvman.2016.04.006
Ali SS, Al-Tohamy R, Manni A, Luz FC, Elsamahy T, Sun J (2019) Enhanced digestion of bio-pretreated sawdust using a novel bacterial consortium: microbial community structure and methane-producing pathways. Fuel 254:115604. https://doi.org/10.1016/j.fuel.2019.06.012
Ali SS, Kornaros M, Manni A, Sun J, El-Shanshoury AERR, Kenawy ER, Khalil MA (2020) Enhanced anaerobic digestion performance by two artificially constructed microbial consortia capable of woody biomass degradation and chlorophenols detoxification. J Hazard Mater 389:122076. https://doi.org/10.1016/j.jhazmat.2020.122076
Alvira P, Negro MJ, Ballesteros I, González A, Ballesteros M (2016) Steam explosion for wheat straw pretreatment for sugars production. Bioethanol. https://doi.org/10.1515/bioeth-2016-0003
Antonopoulou G, Kampranis A, Ntaikou I, Lyberatos G (2019) Enhancement of liquid and gaseous biofuels production from agro-industrial residues after thermochemical and enzymatic pretreatment. Front Sustain Food Syst 3:92. https://doi.org/10.3389/fsufs.2019.00092
Arantes V, Jellison J, Goodell B (2012) Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl Microbiol Biotechnol 94(2):323–338. https://doi.org/10.1007/s00253-012-3954-y
Aski AL, Borghei A, Zenouzi A, Ashrafi N, Taherzadeh MJ (2019) Effect of steam explosion on the structural modification of rice straw for enhanced biodegradation and biogas production. BioResources 14(1):464–485
Axelsson L, Franzén M, Ostwald M, Berndes G, Lakshmi G, Ravindranath NH (2012) Jatropha cultivation in southern India: assessing farmers’ experiences. Biofuels Bioprod Biorefin 6(3):246–256. https://doi.org/10.1002/bbb.1324
Aydin S (2016) Enhancement of microbial diversity and methane yield by bacterial bioaugmentation through the anaerobic digestion of Haematococcus pluvialis. Appl Microbiol Biotechnol 100(12):5631–5637. https://doi.org/10.1007/s00253-016-7501-0
Bala R, Mondal MK (2018) Exhaustive characterization on chemical and thermal treatment of sawdust for improved biogas production. Biomass Convers Biorefin 8(4):991–1003
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Behera S, Arora R, Nandhagopal N, Kumar S (2014) Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew Sustain Energy Rev 36:91–106. https://doi.org/10.1016/j.rser.2014.04.047
Bondesson PM, Dupuy A, Galbe M, Zacchi G (2015) Optimizing ethanol and methane production from steam-pretreated, phosphoric acid-impregnated corn stover. Appl Biochem Biotechnol 175(3):1371–1388. https://doi.org/10.1007/s12010-014-1358-4
Buntić AV, Milić MD, Stajković-Srbinović OS, Rasulić NV, Delić DI, Mihajlovski KR (2019) Cellulase production by Sinorhizobium meliloti strain 224 using waste tobacco as substrate. Int J Environ Sci Technol 16(10):5881–5890. https://doi.org/10.1007/s13762-019-02230-9
Bura R, Mansfield SD, Saddler JN, Bothast RJ (2002) SO2-catalyzed steam explosion of corn fiber for ethanol production. In: Biotechnology for fuels and chemicals. Humana Press, Totowa, pp 59–72. https://doi.org/10.1007/978-1-4612-0119-9_5
Capolupo L, Faraco V (2016) Green methods of lignocellulose pretreatment for biorefinery development. Appl Microbiol Biotechnol 100(22):9451–9467. https://doi.org/10.1007/s00253-016-7884-y
Cardeña R, Moreno G, Bakonyi P, Buitrón G (2017) Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem Eng J 307:948–954. https://doi.org/10.1016/j.cej.2016.09.016
Castro E, Nieves IU, Mullinnix MT, Sagues WJ, Hoffman RW, Fernández-Sandoval MT, Tian Z, Rockwood DL, Tamang B, Ingram LO (2014) Optimization of dilute-phosphoric-acid steam pretreatment of Eucalyptus benthamii for biofuel production. Appl Energy 125:76–83. https://doi.org/10.1016/j.apenergy.2014.03.047
Čater M, Zorec M, Logar RM (2014) Methods for improving anaerobic lignocellulosic substrates degradation for enhanced biogas production. Springer Sci Rev 2(1–2):51–61. https://doi.org/10.1007/s40362-014-0019-x
Chandel AK, Gonçalves BC, Strap JL, da Silva SS (2015) Biodelignification of lignocellulose substrates: an intrinsic and sustainable pretreatment strategy for clean energy production. Crit Rev Biotechnol 35(3):281–293. https://doi.org/10.3109/07388551.2013.841638
Chandrasekaran AP, Sivamani S, Ranjithkumar V (2017) Characterization of combined organic–inorganic acid-pretreated cassava stem. Int J Environ Sci Technol 14(6):1291–1296. https://doi.org/10.1007/s13762-016-1232-8
Chen Y, Sharma-Shivappa RR, Chen C (2007) Ensiling agricultural residues for bioethanol production. Appl Biochem Biotechnol 143(1):80–92. https://doi.org/10.1007/s12010-007-0030-7
Chen S, Zhang X, Singh D, Yu H, Yang X (2010) Biological pretreatment of lignocellulosics: potential, progress and challenges. Biofuels 1(1):177–199. https://doi.org/10.4155/bfs.09.13
Chen WH, Tu YJ, Sheen HK (2011) Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl Energy 88(8):2726–2734. https://doi.org/10.1016/j.apenergy.2011.02.027
Cheng XY, Liu CZ (2010) Enhanced biogas production from herbal-extraction process residues by microwave-assisted alkaline pretreatment. J Chem Technol Biotechnol 85(1):127–131
Chi X, Liu C, Bi YH, Yu G, Zhang Y, Wang Z, Li B, Cui Q (2019) A clean and effective potassium hydroxide pretreatment of corncob residue for the enhancement of enzymatic hydrolysis at high solids loading. RSC Adv 9(20):11558–11566. https://doi.org/10.1039/C9RA01555H
Choi WY, Lee HY (2016) Effective production of bioenergy from marine Chlorella sp. by high-pressure homogenization. Biotechnol Biotechnol Equip 30(1):81–89. https://doi.org/10.1080/13102818.2015.1081407
Coca M, González-Benito G, García-Cubero MT (2016)Chemical oxidation with ozone as an efficient pretreatment of lignocellulosic materials. In: Biomass fractionation technologies for a Lignocellulosic feedstock based biorefinery. Elsevier, pp 409–429. https://doi.org/10.1016/B978-0-12-802323-5.00018-9
Dai L, He C, Wang Y, Liu Y, Yu Z, Zhou Y, Fan L, Duan D, Ruan R (2017) Comparative study on microwave and conventional hydrothermal pretreatment of bamboo sawdust: hydrochar properties and its pyrolysis behaviors. Energy Convers Manag 146:1–7. https://doi.org/10.1016/j.enconman.2017.05.007
Dai BL, Guo XJ, Yuan DH, Xu JM (2018) Comparison of different pretreatments of rice straw substrate to improve biogas production. Waste Biomass Valoriz 9(9):1503–1512. https://doi.org/10.1007/s12649-017-9950-9
de Carvalho DM, Sevastyanova O, Penna LS, da Silva BP, Lindström ME, Colodette JL (2015) Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind Crops Prod 73:118–126. https://doi.org/10.1016/j.indcrop.2015.04.021
De La Torre M, Martín-Sampedro R, Fillat Ú, Eugenio ME, Blánquez A, Hernández M, Arias ME, Ibarra D (2017) Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J Ind Microbiol Biotechnol 44(11):1561–1573. https://doi.org/10.1007/s10295-017-1977-1
Debiagi F, Madeira TB, Nixdorf SL, Mali S (2020) Pretreatment efficiency using autoclave high-pressure steam and ultrasonication in sugar production from liquid hydrolysates and access to the residual solid fractions of wheat bran and oat hulls. Appl Biochem Biotechnol 190(1):166–181. https://doi.org/10.1007/s12010-019-03092-0
Dechman J, Foody B, Iogen Corp (2020) Pretreatment of lignocellulosic biomass with sulfur dioxide and/or sulfurous acid. U.S. Patent 10,655,149
Deshavath NN, Deo BS, Boddu J, Vykuntam K, Goud VV, Veeranki VD (2019) Dilute acid pretreatment efficiency on various solid loadings and effect of different neutralizing agents on xylulosic ethanol production. In: Advances in plant & microbial biotechnology. Springer, Singapore, pp 1–7. https://doi.org/10.1007/978-981-13-6321-4_1
Dessì P, Porca E, Frunzo L, Lakaniemi AM, Collins G, Esposito G, Lens PN (2018) Inoculum pretreatment differentially affects the active microbial community performing mesophilic and thermophilic dark fermentation of xylose. Int J Hydrog Energy 43(19):9233–9245. https://doi.org/10.1016/j.ijhydene.2018.03.117
Divyalakshmi P, Murugan D, Sivarajan M, Sivasamy A, Saravanan P, Rai CL (2017) Optimization and biokinetic studies on pretreatment of sludge for enhancing biogas production. Int J Environ Sci Technol 14(4):813–822. https://doi.org/10.1007/s13762-016-1191-0
Domański J, Marchut-Mikołajczyk O, Cieciura-Włoch W, Patelski P, Dziekońska-Kubczak U, Januszewicz B, Zhang B, Dziugan P (2020) Production of methane, hydrogen and ethanol from Secale cereale L. straw pretreated with sulfuric acid. Molecules 25(4):1013. https://doi.org/10.3390/molecules25041013
Duque A, Manzanares P, Ballesteros M (2017) Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew Energy 114:1427–1441. https://doi.org/10.1016/j.renene.2017.06.050
Dutra ED, Santos FA, Alencar BRA, Reis ALS, de Souza RDFR, da Silva Aquino KA, Morais MA Jr, Menezes RSC (2018) Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: status and perspectives. Biomass Convers Biorefin 8(1):225–234. https://doi.org/10.1007/s13399-017-0277-3
Dziekońska-Kubczak U, Berłowska J, Dziugan P, Patelski P, Pielech-Przybylska K, Balcerek M (2018) Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 11(8):2153. https://doi.org/10.3390/en11082153
El-Shemy MGY, Yasin N, Gadallah MGE, Hanafi EK (2015) Effect of organic acids pretreatments on physicochemical properties and shelf life of refrigerated bolti fish: Tilapia nilotica. J Agric Vet Sci 267(3120):1–12
Enshaeieh M, Abdoli A, Madani M, Bayat M (2015) Recycling of lignocellulosic waste materials to produce high-value products: single cell oil and xylitol. Int J Environ Sci Technol 12(3):837–846. https://doi.org/10.1007/s13762-014-0687-8
Eskicioglu C, Monlau F, Barakat A, Ferrer I, Kaparaju P, Trably E, Carrère H (2017) Assessment of hydrothermal pretreatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res 120:32–42. https://doi.org/10.1016/j.watres.2017.04.068
Falade AO, Nwodo UU, Iweriebor BC, Green E, Mabinya LV, Okoh AI (2017) Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen 6(1):e00394. https://doi.org/10.1002/mbo3.394
Fan LT, Lee YH, Gharpuray MM (1982) The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. In: Microbial reactions. Springer, Berlin, pp 157–187. https://doi.org/10.1007/3540116982_4
Fang W, Weisheng N, Andong Z, Weiming Y (2015) Enhanced anaerobic digestion of corn stover by thermo-chemical pretreatment. Int J Agric Biol Eng 8(1):84–90. https://doi.org/10.3965/j.ijabe.20150801.011
Fang G, Liu S, Shen K, Lin Y (2017) Wet oxidation pretreatment of poplar waste for enhancing enzymatic hydrolysis efficiency. Pap Biomater 2:3
Fei X, Jia W, Wang J, Chen T, Ling Y (2020) Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation. Int J Biol Macromol 145:733–739. https://doi.org/10.1016/j.ijbiomac.2019.12.232
Feng L, Kristensen EF, Moset V, Ward AJ, Møller HB (2018) Ensiling of tall fescue for biogas production: effect of storage time, additives and mechanical pretreatment. Energy Sustain Dev 47:143–148. https://doi.org/10.1016/j.esd.2018.10.001
Ferdeş M, Dincă M, Zăbavă B, Paraschiv G, Munteanu M, Ionescu M (2018) Laccase enzyme production and biomass growth in liquid cultures of wood-degrading fungal strains. In: 46th international conference “Actual Tasks on Agricultural Engineering”, pp 341–388
Foody B, Tolan JS Iogen Corp (2019) Lignocellulosic conversion process comprising sulfur dioxide and/or sulfurous acid pretreatment. U.S.Patent 10,513,714.
Gabhane J, William SP, Vaidya AN, Mahapatra K, Chakrabarti T (2011) Influence of heating source on the efficacy of lignocellulosic pretreatment—a cellulosic ethanol perspective. Biomass Bioenergy 35(1):96–102. https://doi.org/10.1016/j.biombioe.2010.08.026
Gallagher D, Parker D, Allen DJ, Tsesmetzis N (2018) Dynamic bacterial and fungal microbiomes during sweet sorghum ensiling impact bioethanol production. Bioresour Technol 264:163–173. https://doi.org/10.1016/j.biortech.2018.05.053
Gigac J, Fišerová M, Stankovská M, Pažitný A (2017) Enzymatic hydrolysis of extruded wheat straw with addition of sodium hydroxide and calcium hydroxide. Wood Res 62(6):919–930
Golberg A, Sack M, Teissie J, Pataro G, Pliquett U, Saulis G, Stefan T, Miklavcic D, Vorobiev E, Frey W (2016) Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol Biofuels 9(1):94. https://doi.org/10.1186/s13068-016-0508-z
Gonzalez-Balderas RM, Velasquez-Orta SB, Valdez-Vazquez I, Ledesma MO (2020) Intensified recovery of lipids, proteins, and carbohydrates from wastewater-grown microalgae Desmodesmus sp. by using ultrasound or ozone. Ultrason Sonochem 62:104852. https://doi.org/10.1016/j.ultsonch.2019.104852
Goodell B, Qian Y, Jellison J (2008) Fungal decay of wood: soft rot—brown rot—white rot. https://doi.org/10.1021/bk-2008-0982.ch002
Guo H, Zhao Y, Chen X, Shao Q, Qin W (2019) Pretreatment of Miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb Biotechnol 12(4):787–798. https://doi.org/10.1111/1751-7915.13430
Guragain YN, Bastola KP, Madl RL, Vadlani PV (2016) Novel biomass pretreatment using alkaline organic solvents: a green approach for biomass fractionation and 2, 3-butanediol production. BioEnergy Res 9(2):643–655. https://doi.org/10.1007/s12155-015-9706-y
Hall CA, Lambert JG, Balogh SB (2014) EROI of different fuels and the implications for society. Energy Policy 64:141–152. https://doi.org/10.1016/j.enpol.2013.05.049
Hamed SAM (2013) In-vitro studies on wood degradation in soil by soft-rot fungi: Aspergillus niger and Penicillium chrysogenum. Int Biodeterior Biodegrad 78:98–102. https://doi.org/10.1016/j.ibiod.2012.12.013
Hashemi SS, Karimi K, Nosratpour MJ, Sárvári Horváth I (2016) Efficient biogas and ethanol production from safflower straw using sodium carbonate pretreatment. Energy Fuels 30(12):10592–10601
Hashemi SS, Karimi K, Mirmohamadsadeghi S (2019) Hydrothermal pretreatment of safflower straw to enhance biogas production. Energy 172:545–554. https://doi.org/10.1016/j.energy.2019.01.149
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318. https://doi.org/10.1016/j.biortech.2018.04.099
He L, Huang H, Zhang Z, Lei Z (2015) A review of hydrothermal pretreatment of lignocellulosic biomass for enhanced biogas production. Curr Organ Chem 19(5):437–446
He Z, Wang Z, Zhao Z, Yi S, Mu J, Wang X (2017) Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason Sonochem 34:136–141. https://doi.org/10.1016/j.ultsonch.2016.05.035
Hermosilla E, Rubilar O, Schalchli H, da Silva ASA, Ferreira-Leitao V, Diez MC (2018) Sequential white-rot and brown-rot fungal pretreatment of wheat straw as a promising alternative for complementary mild treatments. Waste Manag 79:240–250. https://doi.org/10.1016/j.wasman.2018.07.044
Hesami SM, Zilouei H, Karimi K, Asadinezhad A (2015) Enhanced biogas production from sunflower stalks using hydrothermal and organosolv pretreatment. Ind Crops Prod 76:449–455. https://doi.org/10.1016/j.indcrop.2015.07.018
Hilares RT, Kamoei DV, Ahmed MA, da Silva SS, Han JI, dos Santos JC (2018) A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason Sonochem 43:219–226. https://doi.org/10.1016/j.ultsonch.2018.01.016
Hilares RT, Dionízio RM, Muñoz SS, Prado CA, de Sousa Júnior R, da Silva SS, Santos JC (2020) Hydrodynamic cavitation-assisted continuous pre-treatment of sugarcane bagasse for ethanol production: effects of geometric parameters of the cavitation device. Ultrason Sonochem 63:104931. https://doi.org/10.1016/j.ultsonch.2019.104931
Ho MC, Ong VZ, Wu TY (2019) Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization—a review. Renew Sustain Energy Rev 112:75–86. https://doi.org/10.1016/j.rser.2019.04.082
Huang YF, Chiueh PT, Kuan WH, Lo SL (2016) Microwave pyrolysis of lignocellulosic biomass: heating performance and reaction kinetics. Energy 100:137–144. https://doi.org/10.1016/j.energy.2016.01.088
Ifeanyi VO, Obasi NP, Ayogu CV (2020) Study of microbial succession during bioethanol production from waste corn cob using indigenous organisms. Niger J Microbiol 34(1):5034–5043
Ingle AP, Chandel AK, Antunes FA, Rai M, da Silva SS (2019) New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioprod Biorefin 13(3):776–788. https://doi.org/10.1002/bbb.1965
Ishola MM, Millati R, Syamsiah S, Cahyanto MN, Niklasson C, Taherzadeh MJ (2012) Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules 17(12):14995–15012. https://doi.org/10.3390/molecules171214995
Jaffar M, Pang Y, Yuan H, Zou D, Liu Y, Zhu B, Korai RM, Li X (2016) Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Chin J Chem Eng 24(3):404–409. https://doi.org/10.1016/j.cjche.2015.11.005
Jędrzejczyk M, Soszka E, Czapnik M, Ruppert AM, Grams J (2019) Physical and chemical pretreatment of lignocellulosic biomass. In: Second and third generation of feedstocks. Elsevier, pp 143–196. https://doi.org/10.1016/B978-0-12-815162-4.00006-9
Jin S, Zhang G, Zhang P, Jin L, Fan S, Li F (2015) Comparative study of high-pressure homogenization and alkaline-heat pretreatments for enhancing enzymatic hydrolysis and biogas production of grass clipping. Int Biodeterior Biodegrad 104:477–481. https://doi.org/10.1016/j.ibiod.2015.08.005
Jung YH, Kim KH (2015) Acidic pretreatment. In: Pretreatment of biomass. Elsevier, pp 27–50. https://doi.org/10.1016/B978-0-12-800080-9.00003-7
Jung H, Baek G, Kim J, Shin SG, Lee C (2016) Mild-temperature thermochemical pretreatment of green macroalgal biomass: effects on solubilization, methanation, and microbial community structure. Bioresour Technol 199:326–335. https://doi.org/10.1016/j.biortech.2015.08.014
Jung W, Savithri D, Sharma-Shivappa R, Kolar P (2019) Effect of sodium hydroxide pretreatment on lignin monomeric components of Miscanthus × giganteus and enzymatic hydrolysis. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-019-00859-8
Jusri NAA, Azizan A, Zain ZSZ, Rahman AMF (2019) Effect of electron beam irradiation and ionic liquid combined pretreatment method on various lignocellulosic biomass. In: Key engineering materials, vol 797. Trans Tech Publications Ltd., pp 351–358. https://doi.org/10.4028/www.scientific.net/KEM.797.351
Kainthola J, Shariq M, Kalamdhad AS, Goud VV (2019) Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J Environ Manag 232:188–196. https://doi.org/10.1016/j.jenvman.2018.11.052
Kalyani D, Lee KM, Kim TS, Li J, Dhiman SS, Kang YC, Lee JK (2013) Microbial consortia for saccharification of woody biomass and ethanol fermentation. Fuel 107:815–822. https://doi.org/10.1016/j.fuel.2013.01.037
Kamaraj M, Ramachandran KK, Aravind J (2020) Biohydrogen production from waste materials: benefits and challenges. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02577-z
Kamcharoen A, Champreda V, Eurwilaichitr L, Boonsawang P (2014) Screening and optimization of parameters affecting fungal pretreatment of oil palm empty fruit bunch (EFB) by experimental design. Int J Energy Environ Eng 5(4):303–312. https://doi.org/10.1007/s40095-014-0136-y
Kameshwar AKS, Qin W (2018) Molecular networks of postia placenta involved in degradation of lignocellulosic biomass revealed from metadata analysis of open access gene expression data. Int J Biol Sci 14(3):237. https://doi.org/10.7150/ijbs.22868
Kim TH (2013) Pretreatment of lignocellulosic biomass. In: Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. https://doi.org/10.1002/9781118642047.ch6
Kim Y, Ximenes E, Mosier NS, Ladisch MR (2011) Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb Technol 48(4–5):408–415. https://doi.org/10.1016/j.enzmictec.2011.01.007
Kim I, Lee B, Park JY, Choi SA, Han JI (2014) Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohydr Polym 99:563–567. https://doi.org/10.1016/j.carbpol.2013.08.092
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82(1):15–26. https://doi.org/10.1016/S0960-8524(01)00152-3
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99(10):4201–4212. https://doi.org/10.1007/s00253-015-6595-0
Koupaie EH, Dahadha S, Lakeh AB, Azizi A, Elbeshbishy E (2019) Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—a review. J Environ Manag 233:774–784. https://doi.org/10.1016/j.jenvman.2018.09.106
Kozłowski K, Lewicki A, Czekała W, Wójtowicz A, Kupryaniuk K, Dróżdż D (2019) Extrusion pretreatment of maize straw—case study for a Polish biogas plants. Int Agrophys 33(4):527–535. https://doi.org/10.31545/intagr/113548
Kumar A, Tumu VR (2019) Physicochemical properties of the electron beam irradiated bamboo powder and its bio-composites with PLA. Compos B Eng 175:107098. https://doi.org/10.1016/j.compositesb.2019.107098
Kumar B, Bhardwaj N, Agrawal K, Chaturvedi V, Verma P (2020) Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process Technol 199:106244. https://doi.org/10.1016/j.fuproc.2019.106244
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
Lee JT, Khan MU, Tian H, Ee AW, Lim EY, Dai Y, Tong YW, Ahring BK (2020) Improving methane yield of oil palm empty fruit bunches by wet oxidation pretreatment: mesophilic and thermophilic anaerobic digestion conditions and the associated global warming potential effects. Energy Convers Manag 225:113438. https://doi.org/10.1016/j.enconman.2020.113438
Levkov K, Linzon Y, Mercadal B, Ivorra A, González CA, Golberg A (2020) High-voltage pulsed electric field laboratory device with asymmetric voltage multiplier for marine macroalgae electroporation. Innov Food Sci Emerg Technol 60:102288. https://doi.org/10.1016/j.ifset.2020.102288
Li Y, Merrettig-Bruns U, Strauch S, Kabasci S, Chen H (2015) Optimization of ammonia pretreatment of wheat straw for biogas production. J Chemi Technol Biotechnol 90(1):130–138. https://doi.org/10.1002/jctb.4297
Li W, Guo J, Cheng H, Wang W, Dong R (2017) Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: viability, performance and microbial community evaluation. Appl Energy 189:613–622. https://doi.org/10.1016/j.apenergy.2016.12.101
Li W, Fang A, Liu B, Xie G, Lou Y, Xing D (2019) Effect of different co-treatments of waste activated sludge on biogas production and shaping microbial community in subsequent anaerobic digestion. Chem Eng J 378:122098. https://doi.org/10.1016/j.cej.2019.122098
Liang J, Fang X, Lin Y, Wang D (2018) A new screened microbial consortium OEM2 for lignocellulosic biomass deconstruction and chlorophenols detoxification. J Hazard Mater 347:341–348. https://doi.org/10.1016/j.jhazmat.2018.01.023
Lidström P, Tierney J, Watheyb B, Westmana J (2001) Microwave assisted organic synthesis—a review. Tetrahedron 57:9225–9283
Lin R, Deng C, Ding L, Bose A, Murphy JD (2019) Improving gaseous biofuel production from seaweed Saccharina latissima: the effect of hydrothermal pretreatment on energy efficiency. Energy Convers Manag 196:1385–1394. https://doi.org/10.1016/j.enconman.2019.06.044
Liu S, Wu S, Pang C, Li W, Dong R (2014) Microbial pretreatment of corn stovers by solid-state cultivation of Phanerochaete chrysosporium for biogas production. Appl Biochem Biotechnol 172(3):1365–1376. https://doi.org/10.1007/s12010-013-0604-5
Liu X, Hiligsmann S, Gourdon R, Bayard R (2017) Anaerobic digestion of lignocellulosic biomasses pretreated with Ceriporiopsissubvermispora. J Environ Manag 193:154–162. https://doi.org/10.1016/j.jenvman.2017.01.075
Lo SL, Huang YF, Chiueh PT, Kuan WH (2017) Microwave pyrolysis of lignocellulosic biomass. Energy Proc 105:41–46. https://doi.org/10.1016/j.egypro.2017.03.277
Loow YL, Wu TY, Yang GH, Jahim JM, Teoh WH, Mohammad AW (2016) Role of energy irradiation in aiding pretreatment of lignocellulosic biomass for improving reducing sugar recovery. Cellulose 23(5):2761–2789. https://doi.org/10.1007/s10570-016-1023-x
Lu X, Zheng X, Li X, Zhao J (2016) Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stoverpretreated with liquid hot water. Biotechnol Biofuels 9(1):118. https://doi.org/10.1186/s13068-016-0531-0
Madison MJ, Coward-Kelly G, Liang C, Karim MN, Falls M, Holtzapple MT (2017) Mechanical pretreatment of biomass—Part I: acoustic and hydrodynamic cavitation. Biomass Bioenergy 98:135–141. https://doi.org/10.1016/j.biombioe.2017.01.007
Mahajan R, Nikitina A, Litti Y, Kallistova A, Nozhevnikova A, Goel G (2019) Evaluating anaerobic and aerobic digestion strategies for degradation of pretreatedpine needle litter. Int J Environ Sci Technol 16(1):191–200. https://doi.org/10.1007/s13762-017-1601-y
Manyi-Loh CE, Mamphweli SN, Meyer EL, Okoh AI (2019) Microbial anaerobic digestion: process dynamics and implications from the renewable energy, environmental and agronomy perspectives. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02380-w
Martín C, Klinke HB, Thomsen AB (2007) Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb Technol 40(3):426–432. https://doi.org/10.1016/j.enzmictec.2006.07.015
Martínez-Patiño JC, Romero I, Ruiz E, Cara C, Romero-García JM, Castro E (2017) Design and optimization of sulfuric acid pretreatment of extracted olive tree biomass using response surface methodology. BioResources 12(1):1779–1797
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. Biotechnology 5(5):597–609. https://doi.org/10.1007/s13205-015-0279-4
Michalska K, Bizukojć M, Ledakowicz S (2015) Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenergy 80:213–221. https://doi.org/10.1016/j.biombioe.2015.05.022
Mishra S, Singh PK, Dash S, Pattnaik R (2018) Microbial pretreatment of lignocellulosic biomass for enhanced biomethanation and waste management. Biotechnology 8(11):458. https://doi.org/10.1007/s13205-018-1480-z
Mlaik N, Khcharem M, Kouas M, Sayadi S, Khoufi S (2018) Improvement of anaerobic biodegradability of organic fraction of municipal solid waste by mechanical and thermochemical pretreatments. Int J Environ Sci Technol 15(9):1913–1920. https://doi.org/10.1007/s13762-017-1563-0
Mosier NS, Sarikaya A, Ladisch CM, Ladisch MR (2001) Characterization of dicarboxylic acids for cellulose hydrolysis. Biotechnol Prog 17(3):474–480. https://doi.org/10.1021/bp010028u
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy 180:661–671. https://doi.org/10.1016/j.apenergy.2016.07.135
Nagle NJ, Donohoe BS, Wolfrum EJ, Kuhn EM, Haas TJ, Ray AE, Wendt LM, Delwiche ME, Weiss ND, Radtke C (2020) Chemical and structural changes in corn stover after ensiling: influence on bioconversion. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.00739
Nair RB, Lundin M, Lennartsson PR, Taherzadeh MJ (2017) Optimizing dilute phosphoric acid pretreatment of wheat straw in the laboratory and in a demonstration plant for ethanol and edible fungal biomass production using Neurospora intermedia. J Chem Technol Biotechnol 92(6):1256–1265. https://doi.org/10.1002/jctb.5119
Nazarpour F, Abdullah DK, Abdullah N, Zamiri R (2013) Evaluation of biological pretreatment of rubberwood with white rot fungi for enzymatic hydrolysis. Materials 6(5):2059–2073. https://doi.org/10.3390/ma6052059
Negi R, Suthar S (2018) Degradation of paper mill wastewater sludge and cow dung by brown-rot fungi Oligoporus placenta and earthworm (Eisenia fetida) during vermicomposting. J Clean Prod 201:842–852. https://doi.org/10.1016/j.jclepro.2018.08.068
Ninomiya K, Ohta A, Omote S, Ogino C, Takahashi K, Shimizu N (2013) Combined use of completely bio-derived cholinium ionic liquids and ultrasound irradiation for the pretreatment of lignocellulosic material to enhance enzymatic saccharification. Chem Eng J 215:811–818. https://doi.org/10.1016/j.cej.2012.11.020
Nosratpour MJ, Karimi K, Sadeghi M (2018) Improvement of ethanol and biogas production from sugarcane bagasse using sodium alkaline pretreatments. J Environ Manag 226:329–339. https://doi.org/10.1016/j.jenvman.2018.08.058
Obeng AK, Premjet D, Premjet S (2019) Combining autoclaving with mild alkaline solution as a pretreatment technique to enhance glucose recovery from the invasive weed Chloris barbata. Biomolecules 9(4):120. https://doi.org/10.3390/biom9040120
Ometto F, Quiroga G, Pšenička P, Whitton R, Jefferson B, Villa R (2014) Impacts of microalgae pre-treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res 65:350–361. https://doi.org/10.1016/j.watres.2014.07.040
Öztürk E (2019) Effects of high hydrostatic pressure (HHP) on cellulose hydrolysis and cellulase activity, and its use for peanut hulls hydrolysis (Master's thesis, MIDDLE EAST TECHNICAL UNIVERSITY)
Paixão SM, Ladeira SA, Silva TP, Arez BF, Roseiro JC, Martins MLL, Alves L (2016) Sugarcane bagasse delignification with potassium hydroxide for enhanced enzymatic hydrolysis. RSC Adv 6(2):1042–1052. https://doi.org/10.1039/C5RA14908H
Parawira W (2012) Enzyme research and applications in biotechnological intensification of biogas production. Crit Rev Biotechnol 32(2):172–186. https://doi.org/10.3109/07388551.2011.595384
Park C, Lee J (2020) Recent achievements in CO 2-assisted and CO2-catalyzed biomass conversion reactions. Green Chem 22(9):2628–2642. https://doi.org/10.1039/D0GC00095G
Patil PN, Gogate PR, Csoka L, Dregelyi-Kiss A, Horvath M (2016) Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason Sonochem 30:79–86. https://doi.org/10.1016/j.ultsonch.2015.11.009
Pérez-Rodríguez N, García-Bernet D, Domínguez JM (2018) Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ Chem Lett 16(1):295–299. https://doi.org/10.1007/s10311-017-0668-5
Pielhop T, Amgarten J, von Rohr PR, Studer MH (2016) Steam explosion pretreatment of softwood: the effect of the explosive decompression on enzymatic digestibility. Biotechnol Biofuels 9(1):152. https://doi.org/10.1186/s13068-016-0567-1
Plácido J, Capareda S (2015) Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour Bioprocess 2(1):23. https://doi.org/10.1186/s40643-015-0049-5
Poszytek K, Ciezkowska M, Sklodowska A, Drewniak L (2016) Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front Microbiol 7:324. https://doi.org/10.3389/fmicb.2016.00324
Prapinagsorn W, Sittijunda S, Reungsang A (2017) Co-digestion of napier grass and its silage with cow dung for methane production. Energies 10(10):1654. https://doi.org/10.3390/en10101654
Qin G, Liu R, Sun C (2011) Effects of acid pretreatment on biogas fermentation of rice straw. J Shanghai Jiaotong Univ-Agric Sci 29(1):58–61
Rashid GMM, Durán-Peña MJ, Rahmanpour R, Sapsford D, Bugg TDH (2017) Delignification and enhanced gas release from soil containing lignocellulose by treatment with bacterial lignin degraders. J Appl Microbiol 123(1):159–171. https://doi.org/10.1111/jam.13470
Rehman MSU, Kim I, Kim KH, Han JI (2014) Optimization of sono-assisted dilute sulfuric acid process for simultaneous pretreatment and saccharification of rice straw. Int J Environ Sci Technol 11(2):543–550. https://doi.org/10.1007/s13762-013-0294-0
Rizal NFAA, Ibrahim MF, Zakaria MR, Abd-Aziz S, Yee PL, Hassan MA (2018) Pre-treatment of oil palm biomass for fermentable sugars production. Molecules 23(6):1381. https://doi.org/10.3390/molecules23061381
Rosen Y, Mamane H, Gerchman Y (2019) Short ozonation of lignocellulosic waste as energetically favorablepretreatment. BioEnergy Res 12(2):292–301. https://doi.org/10.1007/s12155-019-9962-3
Saelee K, Yingkamhaeng N, Nimchua T, Sukyai P (2016) An environmentally friendly xylanase-assisted pretreatment for cellulose nanofibrils isolation from sugarcane bagasse by high-pressure homogenization. Ind Crops Prod 82:149–160. https://doi.org/10.1016/j.indcrop.2015.11.064
Salema AA, Ani FN, Mouris J, Hutcheon R (2017) Microwave dielectric properties of Malaysian palm oil and agricultural industrial biomass and biochar during pyrolysis process. Fuel Process Technol 166:164–173. https://doi.org/10.1016/j.fuproc.2017.06.006
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27(2):185–194. https://doi.org/10.1016/j.biotechadv.2008.11.001
Sanhueza C, Carvajal G, Soto-Aguilar J, Lienqueo ME, Salazar O (2018) The effect of a lytic polysaccharide monooxygenase and a xylanase from Gloeophyllum trabeum on the enzymatic hydrolysis of lignocellulosic residues using a commercial cellulase. Enzyme Microbial Technol 113:75–82. https://doi.org/10.1016/j.enzmictec.2017.11.007
Shah TA, Ullah R (2019) Pretreatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int J Environ Sci Technol 16(11):7497–7508. https://doi.org/10.1007/s13762-019-02277-8
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz 10(2):235–251. https://doi.org/10.1007/s12649-017-0059-y
Shi Y, Yan X, Li Q, Wang X, Xie S, Chai L, Yuan J (2017) Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidusbasilensis B-8 without any pretreatment. Process Biochem 52:238–242. https://doi.org/10.1016/j.procbio.2016.10.004
Si B, Yang L, Zhou X, Watson J, Tommaso G, Chen WT, Liao Q, Duan N, Liu Z, Zhang Y (2019) Anaerobic conversion of the hydrothermal liquefaction aqueous phase: fate of organics and intensification with granule activated carbon/ozone pretreatment. Green Chem 21(6):1305–1318. https://doi.org/10.1039/C8GC02907E
Siddhu MAH, Li J, Zhang J, Huang Y, Wang W, Chen C, Liu G (2016) Improve the anaerobic biodegradability by copretreatment of thermal alkali and steam explosion of lignocellulosic waste. BioMed Res Int. https://doi.org/10.1155/2016/2786598
Sieborg MU, Jønson BD, Larsen SU, Vazifehkhoran AH, Triolo JM (2020) Co-ensiling of wheat straw as an alternative pre-treatment to chemical, hydrothermal and mechanical methods for methane production. Energies 13(16):4047. https://doi.org/10.3390/en13164047
Simanungkalit SP, Mansur D, Nurhakim B, Agustin A, Rinaldi N, Muryanto and Fitriady MA (2017) Hydrothermal pretreatment of palm oil empty fruit bunch. In: AIP conference proceedings, vol 1803(1). AIP Publishing LLC, p 020011. https://doi.org/10.1063/1.4973138
Singh P, Jain P, Verma R, Jagadish RS (2016) Characterization of lignin peroxidase from Paecilomyces species for decolorisation of pulp and paper mill effluent. http://nopr.niscair.res.in/handle/123456789/35155
Singh R, Hu J, Regner MR, Round JW, Ralph J, Saddler JN, Eltis LD (2017) Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci Rep 7:42121. https://doi.org/10.1038/srep42121
Sista Kameshwar AK, Qin W (2018) Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology 9(2):93–105. https://doi.org/10.1080/21501203.2017.1419296
Song Z, Yang G, Guo Y, Zhang T (2012) Comparison of two chemical pretreatments of rice straw for biogas production by anaerobic digestion. BioResources 7(3):3223–3236
Song Z, Liu X, Yan Z, Yuan Y, Liao Y (2014) Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 9(4):e93801. https://doi.org/10.1371/journal.pone.0093801
Steinbach D, Kruse A, Sauer J (2017) Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production-a review. Biomass Convers Biorefin 7(2):247–274. https://doi.org/10.1007/s13399-017-0243-0
Subhedar PB, Ray P, Gogate PR (2018) Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason Sonochem 40:140–150. https://doi.org/10.1016/j.ultsonch.2017.01.030
Sun C, Liu R, Cao W, Li K, Wu L (2019) Optimization of sodium hydroxide pretreatment conditions to improve biogas production from asparagus stover. Waste Biomass Valoriz 10(1):121–129. https://doi.org/10.1007/s12649-017-0020-0
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9(9):1621–1651. https://doi.org/10.3390/ijms9091621
Tirapanampai C, Phetwarotai W, Phusunti N (2019) Effect of temperature and the content of Na2CO3 as a catalyst on the characteristics of bio-oil obtained from the pyrolysis of microalgae. J Anal Appl Pyrol 142:104644. https://doi.org/10.1016/j.jaap.2019.104644
Travaini R, Marangon-Jardim C, Colodette JL, Morales-Otero M, Bolado-Rodríguez S (2015) Ozonolysis. In: Pretreatment of biomass. Elsevier, pp 105–135. https://doi.org/10.1016/B978-0-12-800080-9.00007-4
Tsapekos P, Kougias PG, Angelidaki I (2015) Anaerobic mono-and co-digestion of mechanically pretreated meadow grass for biogas production. Energy Fuels 29(7):4005–4010. https://doi.org/10.1021/ef5027949
Tsegaye B, Balomajumder C, Roy P (2018a) Biodegradation of wheat straw by Ochrobactrumoryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ. Sci. Pollut. Res. 25(30):30585–30596. https://doi.org/10.1007/s11356-018-3056-1
Tsegaye B, Balomajumder C, Roy P (2018b) Biodelignification and hydrolysis of rice straw by novel bacteria isolated from wood feeding termite. Biotechnology 8(10):447. https://doi.org/10.1007/s13205-018-1471-0
Tsegaye B, Balomajumder C, Roy P (2019) Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Centre 43(1):51. https://doi.org/10.1186/s42269-019-0094-x
Veluchamy C, Kalamdhad AS (2017a) Enhancement of hydrolysis of lignocellulose waste pulp and paper mill sludge through different heating processes on thermal pretreatment. J Clean Prod 168:219–226. https://doi.org/10.1016/j.jclepro.2017.09.040
Veluchamy C, Kalamdhad AS (2017b) Prerequisite—a hot air oven pretreatment for anaerobic digestion of lignocellulose waste material. CSBE/SCGAB 2017 Annual Conference Canad Inns Polo Park, Winnipeg, MB 6-10 August 2017, Paper No. CSBE17043
Voß H, Heck CA, Schallmey M, Schallmey A (2020) Database mining for novel bacterial β-etherases, glutathione-dependent lignin-degrading enzymes. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02026-19
Wang L, Fan X, Tang P, Yuan Q (2013) Xylitol fermentation using hemicellulose hydrolysate prepared by acid pre-impregnated steam explosion of corncob. J Chem Technol Biotechnol 88(11):2067–2074. https://doi.org/10.1002/jctb.4070
Wang YZ, Zhang L, Xu T, Ding K (2017) Improving lignocellulose enzymatic saccharification in a bioreactor with an applied electric field. Ind Crops Prod 109:404–409. https://doi.org/10.1016/j.indcrop.2017.08.061
Wang TT, Sun ZY, Huang YL, Tan L, Tang YQ, Kida K (2018a) Biogas production from distilled grain waste by thermophilic dry anaerobic digestion: pretreatment of feedstock and dynamics of microbial community. Appl Biochem Biotechnol 184(2):685–702. https://doi.org/10.1007/s12010-017-2557-6
Wang W, Li X, Ye D, Cai L, Shi SQ (2018b) Catalytic pyrolysis of larch sawdust for phenol-rich bio-oil using different catalysts. Renew Energy 121:146–152. https://doi.org/10.1016/j.renene.2018.01.018
Wang LQ, Cai LY, Ma YL (2020) Study on inhibitors from acid pretreatment of corn stalk on ethanol fermentation by alcohol yeast. RSC Adv 10(63):38409–38415. https://doi.org/10.1039/D0RA04965D
Wassie AB, Srivastava VC (2016) Chemical treatment of teff straw by sodium hydroxide, phosphoric acid and zinc chloride: adsorptive removal of chromium. Int J Environ Sci Technol 13(10):2415–2426. https://doi.org/10.1007/s13762-016-1080-6
Wei S (2016) The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl Microbiol Biotechnol 100(23):9821–9836. https://doi.org/10.1007/s00253-016-7926-5
Weide T, Baquero CD, Schomaker M, Brügging E, Wetter C (2020) Effects of enzyme addition on biogas and methane yields in the batch anaerobic digestion of agricultural waste (silage, straw, and animal manure). Biomass Bioenergy 132:105442. https://doi.org/10.1016/j.biombioe.2019.105442
Westerholm M, Castillo MDP, Andersson AC, Nilsen PJ, Schnürer A (2019) Effects of thermal hydrolytic pre-treatment on biogas process efficiency and microbial community structure in industrial-and laboratory-scale digesters. Waste Manag 95:150–160. https://doi.org/10.1016/j.wasman.2019.06.004
Wu ZZ, Li DY, Cheng YS (2018) Application of ensilage as a green approach for simultaneous preservation and pretreatment of macroalgae Ulva lactuca for fermentable sugar production. Clean Technol Environ Policy 20(9):2057–2065. https://doi.org/10.1007/s10098-018-1574-7
Wu X, Chen L, He W, Qi H, Zhang Y, Zhou Y, Su X, Deng M, Wang K (2020) Characterize the physicochemical structure and enzymatic efficiency of agricultural residues exposed to γ-irradiation pretreatment. Ind Crops Prod 150:112228. https://doi.org/10.1016/j.indcrop.2020.112228
Yang X (2020) Solid-state anaerobic microbial ensilage pretreatment. In: Biogas. IntechOpen. https://doi.org/10.5772/intechopen.92571
Yang G, Wang J (2020) Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: energy conversion and microbial community. Energy Convers Manag 225:113436. https://doi.org/10.1016/j.enconman.2020.113436
Yang B, Tao L, Wyman CE (2018) Strengths, challenges, and opportunities for hydrothermal pretreatment in lignocellulosic biorefineries. Biofuels Bioprod Biorefin 12(1):125–138. https://doi.org/10.1002/bbb.1825
Yang G, Yin Y, Wang J (2019a) Microbial community diversity during fermentative hydrogen production inoculating various pretreated cultures. Int J Hydrog Energy 44(26):13147–13156. https://doi.org/10.1016/j.ijhydene.2019.03.216
Yang T, Liu X, Li R, Li B, Kai X (2019b) Hydrothermal liquefaction of sewage sludge to produce bio-oil: effect of co-pretreatment with subcritical water and mixed surfactants. J Supercrit Fluids 144:28–38. https://doi.org/10.1016/j.supflu.2018.10.005
Yao Y, Bergeron AD, Davaritouchaee M (2018) Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew Energy 115:139–148. https://doi.org/10.1016/j.renene.2017.08.038
Yao S, Liu B, Nie S, Wang S, Qin C, Wang S (2019) Pretreatment of chlorine dioxide solution for pulp bleaching. J Biobased Mater Bioenergy 13(4):523–531. https://doi.org/10.1166/jbmb.2019.1886523
Yu Q, Liu R, Li K, Ma R (2019) A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew Sustain Energy Rev 107:51–58. https://doi.org/10.1016/j.rser.2019.02.020
Yuan XF, Li P, Wang H, Wang X, Cheng X, Cui Z (2011) Enhancing the anaerobic digestion of corn stalks using composite microbial pretreatment. J Microbiol Biotechnol 21(7):746–752. https://doi.org/10.4014/jmb.1011.11026
Yunpu WANG, Leilei DAI, Liangliang FAN, Shaoqi SHAN, Yuhuan LIU, Roger RUAN (2016) Review of microwave-assisted lignin conversion for renewable fuels and chemicals. J Anal Appl Pyrolysis 119:104–113. https://doi.org/10.1016/j.jaap.2016.03.011
Zabed HM, Akter S, Yun J, Zhang G, Awad FN, Qi X, Sahu JN (2019) Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew Sustain Energy Rev 105:105–128. https://doi.org/10.1016/j.rser.2019.01.048
Zeng J, Singh D, Laskar DD, Chen S (2013) Degradation of native wheat straw lignin by Streptomyces viridosporus T7A. Int J Environ Sci Technol 10(1):165–174. https://doi.org/10.1007/s13762-012-0085-z
Zhang X, Yu H, Huang H, Liu Y (2007) Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int Biodeterior Biodegrad 60(3):159–164. https://doi.org/10.1016/j.ibiod.2007.02.003
Zhang R, Lu X, Sun Y, Wang X, Zhang S (2011) Modeling and optimization of dilute nitric acid hydrolysis on corn stover. J Chem Technol Biotechnol 86(2):306–314. https://doi.org/10.1002/jctb.2529
Zhang L, Zhang L, Li D (2015a) Enhanced dark fermentative hydrogen production by zero-valent iron activated carbon micro-electrolysis. Int J Hydrog Energy 40(36):12201–12208. https://doi.org/10.1016/j.ijhydene.2015.07.106
Zhang Y, Chen X, Gu Y, Zhou X (2015b) A physicochemical method for increasing methane production from rice straw: extrusion combined with alkali pretreatment. Appl Energy 160:39–48. https://doi.org/10.1016/j.apenergy.2015.09.011
Zhang X, Zhu J, Sun L, Yuan Q, Cheng G, Argyropoulos DS (2019) Extraction and characterization of lignin from corncob residue after acid-catalyzed steam explosion pretreatment. Ind Crops Prod 133:241–249. https://doi.org/10.1016/j.indcrop.2019.03.027
Zhao X, Liu J, Liu J, Yang F, Zhu W, Yuan X, Hu Y, Cui Z, Wang X (2017) Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour Technol 241:349–359. https://doi.org/10.1016/j.biortech.2017.03.183
Zhao J, Dong Z, Li J, Chen L, Bai Y, Jia Y, Shao T (2018) Ensiling as pretreatment of rice straw: the effect of hemicellulase and Lactobacillus plantarum on hemicellulose degradation and cellulose conversion. Bioresour Technol 266:158–165. https://doi.org/10.1016/j.biortech.2018.06.058
Zhao MJ, Xu QQ, Li GM, Zhang QZ, Zhou D, Yin JZ, Zhan HS (2019) Pretreatment of agricultural residues by supercritical CO2 at 50–80° C to enhance enzymatic hydrolysis. J Energy Chem 31:39–45. https://doi.org/10.1016/j.jechem.2018.05.003
Zhao C, Shao Q, Chundawat SP (2020) Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour Technol 298:122446. https://doi.org/10.1016/j.biortech.2019.122446
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Zhong C, Wang C, Wang F, Jia H, Wei P, Zhao Y (2016) Enhanced biogas production from wheat straw with the application of synergistic microbial consortium pretreatment. RSC Adv 6(65):60187–60195
Zhou J, Yan BH, Wang Y, Yong XY, Yang ZH, Jia HH, Jiang M, Wei P (2016) Effect of steam explosion pretreatment on the anaerobic digestion of rice straw. RSC Adv 6(91):88417–88425
Zhuo S, Yan X, Liu D, Si M, Zhang K, Liu M, Peng B, Shi Y (2018) Use of bacteria for improving the lignocellulose biorefinery process: importance of pre-erosion. Biotechnol Biofuels 11(1):1–13. https://doi.org/10.1186/s13068-018-1146-4
Zieliński M, Dębowski M, Kisielewska M, Nowicka A, Rokicka M, Szwarc K (2019) Comparison of ultrasonic and hydrothermal cavitation pretreatments of cattle manure mixed with straw wheat on fermentative biogas production. Waste Biomass Valoriz 10(4):747–754. https://doi.org/10.1007/s12649-017-9977-y
Acknowledgements
Authors would like to acknowledge Director, CSIR-NEERI and AcSIR-NEERI for providing essential resources for the research work [KRC No. CSIR-NEERI/KRC/2020/ JULY/EBGD/9]. SPN and RKG are thankful to UGC, while AKS and ARC are thankful to CSIR for Senior Research Fellowship for carrying out this research. Funds from the Science and Engineering Research Board (SERB), New Delhi, for the project-EMR/2016/006589 are gratefully acknowledged.
Funding
This work was supported by the Science and Engineering Research Board (SERB), New Delhi via Grant No. EMR/2016/006589.
Author information
Authors and Affiliations
Contributions
B.J.P. performed the bulk of the literature search and data analysis and wrote the manuscript. A.A.K. developed the idea for the review article, checked and critically revised the different draft versions, did data analysis, and communicated the manuscript as a corresponding author. H.J.P. provided critical inputs on various topics covered in the review in addition to future directions in this area of research. S.P.N., R.K.G., A.R.C., A.K.S., did literature survey and provided inputs on various topics of their research in the area of municipal waste management, solid waste biomass to biofuel, pretreatment.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors have consented for publication.
Additional information
Editorial responsibility: S. K. Ponnusamy.
Rights and permissions
About this article
Cite this article
Poddar, B.J., Nakhate, S.P., Gupta, R.K. et al. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 19, 3429–3456 (2022). https://doi.org/10.1007/s13762-021-03248-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03248-8