Abstract
Alkaline pretreatment was employed to enhance biogas production from asparagus stover with anaerobic digester in laboratory scale batch fermentation. Different pretreatment times (10, 18, 25 days), NaOH concentrations (2.5, 5, 7.5%), and water dose (20, 60, 100 mL) were tested to select the best pretreatment conditions. With response surface method (RSM) applied, the optimum pretreatment conditions were pretreatment time of 19d, NaOH concentration of 4.2%, water dose of 74 g.The biogas yield was predicted as 275.65 mL/g VS, while it was observed as 277.86 mL/g VS in the verification test, with the relative error of 0.80%. Further more, the verification tests show that contents of hemi-cellulose, cellulose and lignin after pretreatment were decreased by 65.20, 29.06 and 13.51%, respectively. The above results suggest that the effects of NaOH on degradations of hemi-cellulose and cellulose are higher than that on lignin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a source of clean energy and a competitive way of dealing with organic waste, biogas fermentation has long been considered bearing immense development potential in China, especially in rural areas, where agricultural waste is abundant and even superfluous. For an example, the annual yield of straw in China is about 6.81 × 109 ton [1]. But merely a small proportion of this sort of waste is handled and disposed properly such as converting into biomass energy, composting, and paper making. Most of the straws and stalks are incinerated or air-dried in the open air just for saving time and labor [1, 2]. Similarly, the amount of asparagus stover generated in the planting base, Chongming Island, Shanghai, China is estimated to around 1 × 103 ton per year. But without a proper disposal method, this agriculture waste is simply piled up on the side of country road, giving out bad smell after rotting naturally.
Agricultural waste can be converted to energy through biological and thermo-chemical conversion technologies [3, 4]. Among several ways of waste recycling, anaerobic digestion (AD) can not only yield biogas, the comparatively clean fuel with methane as the major gas, but also produce solid and liquid fertilizers. It is an ideal waste management method which combines waste reducing, recycling and reusing into one process [5]. The mixture of microorganisms in AD can theoretically realize entire degradation of organic waste, even including some inhibiting compounds such as furfural and soluble lignin, if only they are not in too high concentration [6]. Due to some technological and historical problems, the commercial production has not been completely realized so far. Nowadays, household biogas, the most feasible and prevalent biogas production pattern in rural China, accounts for only about 19% of the biogas potential of the country [2].
The physico-chemical structure of lignocellulosic agricultural wastes slows down the hydrolysis rate during AD. One method to overcome the technological obstacle is applying pretreatment, so as to obtain more hydrolytic products for subsequent biogas production. Pretreatment can help to break up the stubborn physical structure, dissolve the linear and nonlinear macromolecules and therefore improve the biodegradability of lignocellulosic materials. At present, the pretreatment methods include physical, chemical, biological and mixed ones [6]. Unlike physical and biological methods, chemical pretreatment is comparatively effective with relative low cost. Among several kinds of chemical pretreatment such as acidic, alkaline and oxidized ones, alkaline pretreatment represented by sodium hydroxide pretreatment gains more and more attention because of its operability [7].
During alkaline pretreatment, the first reactions are solvation and saponification. In this process, the raw material is swollen, thus making it more accessible to microorganisms. Then, if with a relatively high concentration of alkali, the reaction of “peeling” end-groups, alkaline hydrolysis, and polysaccharides decomposition will carry on. These reactions will greatly contribute to the later conversion [6]. Pavlostathis and Gossett (1985) reported a 100% increase in methane production from wheat straw brought by alkaline pretreatment [8]. He and Pang demonstrated that the biogas yield of rice straw (in the solid state) with 6% NaOH pretreatment was increased by 27.3–64.5% [9]. Also, a degradation of 16.4% cellulose, 36.8% hemicellulose and 28.4% lignin as well as an increase of 122.5% in water-soluble substances were observed. Also, Zhu and Wan mentioned a 37.0% higher biogas yield of corn stover with 5% NaOH-pretreatment than that of the control [10].
Currently, there are two categories of criterions for assessing the alkaline pretreatment effects. One is detecting the degradation and decomposition level of lignocelluloses, as well as the increasing level of soluble substance. In conducting this sort of valuation, the content and physicochemical characterization changes of lignin, hemicellulose, cellulose, and monosaccharide in raw material should be investigated. The other is linear comparison of fermentation indicators such as methane or biogas yield during subsequent AD between the treated and untreated. By combining the assessing criterions with scientific tests design methods, the optimal condition of alkaline pretreatment for lignocellulosic waste can be revealed.
Response surface method (RSM) is collection of mathematical and statistical techniques, which can be used in designing the tests, building models, evaluating significance of independent variables, and optimizing conditions for desirable responses [11]. It has been applied in optimizing AD conditions of methane/hydrogen production from waste water and sludge [12, 13], pretreatment conditions of certain kinds of wastes [14, 15], the culture medium conditions of culturing anaerobic microorganism [12] and so on. Often, RSM is conducted after the ‘change-one-factor-at-a-time’ method, in which the ranges of independent variables can be roughly given out when the peak response value turns up. Later, these ranges of variables will be selected to design multi-factor tests, take the RSM for example, to show the best conditions of the variables whether they interact with each other or not.
Our previous study of ‘change-one-factor-at-a-time’ tests showed that asparagus stover, the hard-to-digest lignocellulosic material, can be used for biogas production after alkaline pretreatment [16]. The objectives of our current work were to investigate the interactions among the factors and to optimize conditions of sodium hydroxide pretreatment when asparagus stover sample was used as raw material in order to increase biogas yield. The biogas yield was monitored in batch anaerobic digestion tests on lab level. The effects of different treatment conditions on biogas yield and the optimal condition for sodium hydroxide pretreatment were statistically evaluated by RSM.
Materials and Methods
Raw Material and Inoculum Preparation
The asparagus stover used in the experiments was rejected materials collected from the roadsides of asparagus planting base, located in the town of Gangyan, Chongming Island, Shanghai, P.R.China. The stover was almost asparagus rhizome, with a small part of stems and leaves. Both of which were naturally air-dried. The stover was firstly grinded by kneading miller; and then, the longer segments were cut into small pieces shorter than 2.5 cm. Before pretreatment experiments, the samples were dried in drying oven at 105 °C for 6 h, making sure its moisture content was <0.1%.
The inoculum was enriched from anaerobic sludge, which originally came from a pilot scale CSTR reactor treating pig manure in the town of Shuxin, Chongming Island, Shanghai, P.R.China. The inoculum has been acclimated to substrates of asparagus stover in four anaerobic fermentation batch tests previously. The chemical characteristics of asparagus stover (in naturally dried form) and inoculum are shown in Table 1.
Sodium Hydroxide Pretreatment
20 runs were performed in the pretreatment process. According to the tests design, each treatment involves a corresponding amount(20, 60, or 100 mL) of distilled water, an according amount(2.654, 5.263, and 8.108 g) of sodium hydroxide solid, and 100 g total solid (TS). The amount of distilled water (water dose) stands for the moisture content in the pretreatment experiment. The NaOH concentration means the amount of NaOH quantity per gram TS of the treatment in this study. The pretreating time of 10, 18 and 25 days were selected according to the results of ‘change-one-factor-at-a-time’ tests previously done in our lab [16]. The pretreating chamber of 2.5 L plastic bucket was sealed by vaseline and preservative film to avoid moisture change and rot fungi infection, and put in incubator of 25 ± 1 °C, which is close to the average value of room temperature. Each treatment was repeated twice.
Biogas Production
Biogas fermentation is conducted in 1 L flask reactor with the working volume of 0.8 L at 35 °C. A 6% solid content of the fermentation broth was used in the study. The inoculum content is 30% of the fermentation feed liquid and the ratio of inoculum to substrate was 0.02. The pH value of the feed liquid was adjusted by acetic acid to 7.2–7.5 prior to fermentation. The formed acetate was expected to give a fermentable initiation to the microorganisms, which would digest the very recalcitrant substrate full of lignocellulose. The biogas yield from the acetate was then subtracted from the biogas yield of each run of the batch AD tests. The reactor was fixed on a constant temp oscillator stirred at 100 rpm to ensure a total mixing and facilitate the diffusion of biogas. When the daily biogas yield is less than 0.1% of the accumulative biogas yield, it is deemed as the termination of fermentation tests. A blank test without asparagus stover is conducted to subtract the biogas generated from dead bacteria of inoculum. The biogas production was calculated into standard volume at STP condition (273.15 K, 101.325 KPa) [17].
Tests Design
In this study, three independent variations—pretreatment time, NaOH concentration and water dose, were selected. Biogas yield during the anaerobic fermentation was chosen as the dependent variation. In order to determine the sodium hydroxide pretreatment conditions for the maximum production of biogas, the Face-centered Central Composite Design (CCD) was employed. It allows estimating the second-order polynomial of the independent variables regarding to the response, and gives information about the interaction between independent variables in relation to the response. For statistical calculation, the variables were coded according to Eq. 1:
where Xi is the coded value of the independent variable; xi is the actual value of the independent value; x0 is the value of xi at the centre point of the investigated area; and Δxi is the step size of the independent variable. Pretreatment time (x1), NaOH concentration (x2), and water dose (x3) were chosen as three independent variables in the experimental design. The range and central point values of the independent variables, which were selected as close as possible to the optimum response values based on previous study, are shown in Table 2. The 20 runs CCD with six replicates of the centre point for biogas yield are shown in Table 3.
Statistical Model
The biogas yield was taken as the dependent variables, namely responses of the test. In order to predict the optimal point and the peak value, the second-order polynomial formulation (Eq. 2), was employed to fit the independent variables and the responses.
The statistical software package Design Expert 7.1.6 (stat-ease, Inc, USA) was employed for data regression analysis. Analysis of variance (ANOVA) was conducted to test the significances of the fitting model, the linear terms, interactive terms and the quadratic terms in the fitting model.
Analytical Methods
The biogas produced daily was recorded using water displacement method. The methane content of biogas was analyzed by gas chromatograph (GC-14B, shimadzu, Japan). Hemi-cellulose, cellulose and lignin were measured according to Goering and Van Soest [18]. The Total solid and volatile solid were detected according to the Standard Methods (APHA, 1995). The total organic carbon was analyzed by organic carbon analyzer (multi C/N 3000, Jena, Germany). The total nitrogen was determined by Kjeldahl method. The pH value was detected by pH meter (PHS-3C, Leici, Shanghai).
Results and Discussion
Optimization Analysis and Verification Test
For NaOH pretreatment-AD systems, biogas yield is the main target to be maximized. By regression analysis of the 20 runs of RSM experiment, the optimum values of selected variables were determined as pretreatment time 19 days, NaOH concentration 4.2%, and water dose 74 g. The NaOH concentration in this study was agreeable with previous studies. Many literatures reported that 2–7.5% NaOH concentration is beneficial for lignocellulosic material to decompose [9, 10, 19], though most raw materials used in previous studies belong to the grass family, such as rice, wheat and corn, while asparagus is a typical plant included in Asparagaceae.
In order to testify the validity of the authenticity of the set of optimized parameters, verification tests were performed in triplicate according to the acquired optimization results and the desirability functions. The test was carried out under the optimal conditions given by RSM.
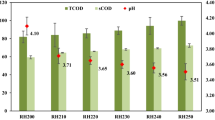
The lignocelluloses mass percentage content after pretreatment and biogas fermentation (w/w) were shown in Table 4. Compared with naturally dried raw materials, in which lignocelluloses accounted for 62.84% total weight of the asparagus stover, the lignocelluloses content after pretreatment were merely 39.76%, which means lignocelluloses have been resolved into some soluble saccharide due to NaOH pretreatment [20]. After AD, this content turned out to be 76.19%, which could be attributed to that the resolvable part of the substrate was converted into carbonic gases and volatile fatty acid [21]. Consequently, the refractory part of the substrate left took a large weight percentage of the fermented asparagus stover. Furthermore, lignin content after AD took nearly 45% percentage of the total lignocelluloses weight, while this percentage was only 17.66% in naturally dried asparagus stover and nearly 23% after NaOH pretreatment. This phenomenon suggested that NaOH exert limited effect on lignin degradation and AD could hardly utilize the under-degraded lignin, which could be supported by other studies [22]. Furthermore, it is reported that the finite soluble part of lignin also would exhibit inhibitory effect on the consequential biogas production [6].
Figure 1 shows the accumulated biogas yield of AD from asparagus stover pretreated by NaOH. It was visualized that the verification test gained 277.86 mL biogas/g VS. The maximum biogas yield predicted by RSM was 275.65 mL/g VS with desirability of 0.874.
In this study, during fermentation, the second period of rapid biogas production proceeded about 15 days after the first period of rapid biogas production. Both of which were characterized by a comparatively greater slope of the accumulated biogas yield curve. In the time slot between the two periods, besides the daily biogas yield was at a standstill, the pH value dropping was simultaneously observed, which was caused by acidogenesis and the result was presented in Fig. 2. The pH value dropped abruptly to 6.47 during the first 9 fermentation days and it did not return to the agreeable value of above 7 for biogas fermentation until the 21st day. Consequently, the lag of pH value reverting to the normal value during biogas fermentation postponed the start-up of AD and thereafter would cause the fermentation period in industrious production prolonged.
The Variation of methane content of biogas during AD from asparagus stover is shown in Fig. 3. Results of Fig. 3 showed that verification test obtained about 60–70% (v/v) methane content during its energetic fermentation period, which is indicative of the normal stage of biogas fermentation. Methane content was from 22.10 to 42.99% in the first 17 days of AD. It was not until the 21 day of fermentation that it acquired methane content above 60%, which was corresponding to the variation trends of biogas yield and pH value shown in Figs. 1 and 2. The average methane content was 53.06%.
The Statistical Analysis of RSM Experiment
Model Fitting
The experimental results of the 20 runs summarized in Table 3 were subjected to regression analysis. The Eq. 3 was obtained by using Eq. 2 to fit the experimental data. The observed biogas yield of 277.86 mL biogas/g VS in the verification test was close to the predicted value of 275.65 mL/g VS and the relative error was 0.80%, which verified the validity of the fitting model Eq C. The ANOVA for the response surface quadratic model for biogas yield were presented in Table 5.
The effects of pretreatment time, NaOH concentration and water dose on biogas yield were examined by ANOVA. The model significance (F-value and p-value) signifies the level of confidence that the selected model doesn’t derive from experimental error [23]. ANOVA of Eq. (C) indicated that the fitting model was highly significant, as the F-value of 14.05 and the value of ‘probability > F’ are <0.01. The coefficient of determination (R2) is the proportion of variation in the response due to the fitting model rather than to random error, and it is favorable that the R2 value is above 80% [24]. The R2 of Eq. 3 was 0.9267, indicating that more than 92.67% variability of the response can be explained by the model. The coefficient of variation (CV) is a ratio of the standard error (SD) to the mean value of the observed responses. If CV is <10%, the fitting model is considered reasonably reproducible. The CV of Eq. 3 is 13.52%, which means the reproduction possibility of the Eq. 3 is 87.48%, a little lower than the criterion. This could be attributed to the experimental errors and implies that the AD system of lignocellulosic material is to some extent lack of stability. However, the F-value and P-value of ‘lack of fit’ is insignificant relative to pure error. There is a 24.13% chance of a 1.95 ‘lack of fit F-value’ occurring due to noise, which means Eq. 3 is fairly fit.
ANOVA also showed the linear effect of x2 and x3, quadratic effect of x1,x2 and x3 and the interactive effect between x2 and x3 on biogas yield are significant (P < 0.05), implying these are key terms to biogas yield and the effects of x1, x2 and x3 on biogas yield are more than simple linear relations. However, the linear effect x1 and interactive effect between x1, x2 and between x1, x3 on biogas yield were not significant (P > 0.05), indicating little impact of these terms on biogas yield. Subsequently, the valid terms of Eq. 4 are involving x2, x3, x2x3 and x12, x22, x32. For improving the fitting model, Eq. 4 can be reduced to Eq. 4 as below:
The F-value and P-value of the items and R2 indicated that Eq. 4 could describe the effect of pretreatment time, NaOH concentration and water dose on the biogas yield of this study quite well. The pred-R2 was in reasonable agreement with the adj-R2. The adeq precision was 9.5565, which measures the signal to noise ratio and it is desirable when >4. The model could be used to navigate the design space in every aspect.
Interactive Effect of NaOH Concentration and Water Dose on the Biogas Yield
The response surface plots and corresponding contour plots of biogas yield are shown in Figs. 4, 5, and 6. These plots are drawn by keeping one variable at its central point level, and varying the others within the experimental range. These results showed that the high biogas yield occur at NaOH concentration around 3.0–5.8% and water dose >50 mL after pretreated for 14–23 days.
As shown in Fig. 4, the interaction between NaOH concentration and water dose suggests that in order to obtain the maximum digestibility of the raw material, the NaOH concentration needed in pretreatment system is different under different conditions of water dose, or vice versa. According to Fig. 4, at the low level of water dose, the biogas yield was considerably low and it increased first and then decreased with the increased NaOH concentration from 2.5 to 7.5% slightly. But at the high level of water dose, a considerable high biogas yield could be achieved under a relatively low level of NaOH concentration. Besides, with such water dose, high dosage of NaOH reduced the biogas production potential during AD. This may be due to the reasons that high NaOH dosage would inhibit AD because over-high Na+ level would do harm to microorganisms by disturbing their osmotic pressure balances [25]. Similarly, an increase in water dose at the low NaOH concentration led to a distinct increase in biogas yield, whereas the increase of biogas yield at the high NaOH concentration was inferior to the former. Therefore, it could be seen that besides alkaline action on lignocelluloses degradation, H2O in the pretreatment system also had positive effect on improving digestibility of lignocelluloses and thereby improving biogas yield. It is unfavorable for pretreating procedure if moisture content is deficient. Sufficient moisture content in pretreatment system would save the alkaline dosage and achieve the similar results for pretreatment and biogas production. In short, at low NaOH concentration, high water dose resulted in more biogas yield; at high water dose, low NaOH concentration promoted biogas yield; high NaOH concentration and low water dose would not benefit biogas yield.
Results in this study showed that RSM is effective in optimizing NaOH pretreatment conditions for AD from asparagus stover. However, the validity of the quadratic regression model by RSM was merely amenable to the designed range of raw data. It could not be used universally to reckon biogas yield from asparagus stover after NaOH pretreatment. So the scale-up tests determining pretreatment parameters should be conducted in the further studies.
Conclusions
This study focused on applying the RSM to optimize conditions of NaOH pretreatment on asparagus stover, so as to improve its biogas yield during AD. Based on the central faced CCD, the optimized NaOH pretreatment conditions were determined as pretreatment time of 19 days, NaOH concentration of 4.2%, water dose of 74 g. At the optimized conditions, the maximum biogas yield of 277.86 mL/g VS was acquired in verification test with the relative error of 0.80% compared with the predicted value of 275.65 mL/g VS. This indicated the fact that the quadratic model could be applied to predict the biogas yield from asparagus stem after NaOH pretreatment. The high correlation between the predicted and tested values indicates the validity of the fitting model. The results suggest that RSM offers an efficient and feasible approach for optimizing NaOH pretreatment parameters and as a result improving biogas yield during AD from some refractory agricultural waste.
References
Chen, Y., Yang, G., Sweeney, S., Feng, Y.: Household biogas use in rural China: a study of opportunities and constraints. Renew. Sust. Energ. Rev. 14, 545–549 (2010)
Ministry of Agriculture.: China’s rural biogas project planning (2006–2010), Science and Technology Education Department of Ministry of Agriculture [in Chinese], Beijing (2007)
Liu, R.: Biomass energy engineering. Chemical Engineering Press, Beijing (2009)
Liu, S., Xie, Q., Zhang, B., Cheng, Y., Liu, Y., Chen, P., Ruan, R.: Fast microwave-assisted catalytic co-pyrolysis of corn stover and scum for bio-oil production with CaO and HZSM-5 as the catalyst. Bioresour. Technol. 204, 164–170 (2016)
Katuwal, H., Bohara, A.K.: Biogas: a promising renewable technology and its impact on rural households in Nepal. Renew. Sust. Energ. Rev. 13, 2668–2674 (2009)
Hendriks, A.T.W.M., Zeeman, G.: Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 100, 10–18 (2009)
Lin, Y., Wang, D., Wu, S., Wang, C.: Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard Mater. 170, 366–373 (2009)
Pavlostathis, S.G., Gossett, J.M.: Alkaline treatment of wheat straw for increasing anaerobic biodegradability. Biotechnol. Bioeng. 27, 334–344 (1985)
He, Y., Pang, Y.: Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energ. Fuel. 22(4), 2775–2781 (2008)
Zhu, J., Wan, C., Li, Y.: Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 101(19), 7523–7528 (2010)
Draper, N., John, J.A.: Response-surface designs for quantitative and qualitative variables. Technometrics. 30(4), 423–428 (1988)
Zinatizadeh, A.A.L., Mohamed, A.R., Abdullah, A.Z., Mashitah, M.D., Isa, M.H., Najafpour, G.D.: Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM). Water Res. 17(40), 3193–3208 (2006)
Wang, J., Wan, W.: Optimization of fermentative hydrogen production process by response surface methodology. Int. J. Hydrogen Energy. 33, 6976–6984 (2008)
Jeya, M., Zhang, Y., Kim, I., Lee, J.: Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour. Technol. 100, 5155–5161 (2009)
Bhaskar, N., Mahendrakar, N.S.: Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresour. Technol. 10(99), 4105–4111 (2008)
Sun, C., Liu, R., Qin, G.: Experiments on pretreatment and anaerobic digestion of asparagus stalk for biogas production. Trans. CSAM. 8(41), 1000–1298 (2010) (In Chinese)
Sun, C., Liu, R., Cao, W., Yin, R., Mei, Y., Zhang, L.: Impacts of alkaline hydrogen peroxide pretreatment on chemical composition and biochemical methane potential of agricultural crop stalks. Energ. Fuel. 29(8), 4966–4975 (2015)
Goering, H.K., van Soest, P.J.: Forage Fiber Analysis (Apparatus, Reagents, Procedures, and Some Applications). Agricultural Handbook, No. 379. United States Department of Agriculture, Washington, DC (1970)
Zheng, M., Li, X., Li, L., Yang, X., He, Y.: Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol. 100, 5140–5145 (2009)
Xiao, B., Suna, X.F., Sun, R.: Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stabil. 74, 307–319 (2001)
Chanakya, H. N., Venkatsubramaniyam, R., Modak, J.: Fermentation and methanogenic characteristics of leafy biomass feedstocks in a solid phase biogas fermentor. Bioresour. Technol. 62, 71–78 (2001)
Klimiuk, E., Pokój, T., Budzyn ski, W., Dubis, B.: Theoretical and observed biogas production from plant biomass of different fibre contents. Bioresour. Technol. 101, 9527–9535 (2010)
Khuri, A.I., Cornell, J.A.: Response Surface Design and Analyses. Marcel Dekker Inc, New York (1987)
Oglekar, A.M., May, A.T.: Product excellence through design of experiments. Cereal Food World. 32, 857–868 (1987)
Chen, Y., Cheng, J.J., Creamer, K.S.: Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 99, 4044–4064 (2008)
Acknowledgements
Financial support from National Natural Science Foundation of China through contract (Grant No. 51376121) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C., Liu, R., Cao, W. et al. Optimization of Sodium Hydroxide Pretreatment Conditions to Improve Biogas Production from Asparagus Stover. Waste Biomass Valor 10, 121–129 (2019). https://doi.org/10.1007/s12649-017-0020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0020-0