Abstract
This study has investigated the valorization of waste tobacco, as lignocellulosic biomass, for cellulase production by rhizobium belonging to genus Sinorhizobium. For the first time, Sinorhizobium meliloti strain 224 was used to produce cellulase (Avicelase and carboxymethyl cellulase) during the submerged and solid-state fermentation using tobacco waste as substrate. The effect of substrate chemical modification on enzymes production has been examined as well. The obtained optimal conditions for the maximum activity of both produced enzymes during submerged fermentation using response surface methodology were: 5 g/L of unmodified waste tobacco concentration, incubation time of 2 days and inoculum concentration of 9%. On the other hand, the use of 1 g of sodium hydroxide modified tobacco for the production of cellulase during solid-state fermentation with 10% inoculum, after 2 days of incubation at 28 °C, expressed the maximum Avicelase activity of 1.503 U/g and carboxymethyl cellulase activity of 1.615 U/g. In addition to its basic role in plant root colonization and the provision of nitrogen compounds, strain 224 can also be exploited to produce cellulases by bioconversion of plant waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most widespread water-insoluble polymer consisting of repeated units of β-d-glucopyranose interconnected by β-1,4 glycosidic bonds (Liu and Kokare 2017; Farinas 2018). Plant biomass contains a significant amount of cellulose because it is their major cell-wall polysaccharide. There are two ways to convert cellulose to glucose: chemical and enzymatic hydrolysis. The application of cellulases for cellulose hydrolysis is an environmentally friendly process, performed without by-products generation (Sukumaran et al. 2005; Juturu and Wu 2014).
Cellulases are inducible enzymes produced by a number of microorganisms including both fungi and bacteria during their growth on cellulosic materials (Quintanilla et al. 2015; Liu and Kokare 2017). Fungal cellulases include all components of the cellulase enzyme complex. They have different specificity and mode of actions and include: endoglucanases (carboxymethylcellulase-CMCase), cellobiohydrolases (exoglucanases–Avicelase) and β-glucosidas. However, in most cases, bacteria do not have a complete cellulase system. The main activity is endoglucanase that does not hydrolyze crystalline cellulose (Kuhad et al. 2011; Kumar et al. 2012).
The biotechnological potential of cellulase production is in their application in various industries, such as pulp and paper industry, textile and bioethanol industry, wine and brewery industry, food industry, animal feed industry, agricultural and detergent industry (Kuhad et al. 2011; Adrio and Demain 2014). Microbiological production of cellulases may be performed by submerged fermentation (SmF) or solid-state fermentation (SSF) technology (Farinas 2018). Various lignocellulosic residues with low-cost values can be used as sources of carbon in those processes: straw, spent grains and pulse carcasses, rice or wheat openings, bagasse, waste in the paper industry (Sukumaran et al. 2005).
Tobacco solid waste is produced during the tobacco manufacturing process in a large quantity (Wang et al. 2005; Onorevoli et al. 2018). Nowadays, the usage of electronic cigarettes (smokeless cigarette which extract nicotine without tobacco combustion and smoke) is increasing. Tobacco residues from these types of cigarettes present a new useful carbon source with lower nicotine content, and it also can be further treated with bioconversion instead of depositing. There are few studies on the use of tobacco waste for the production of enzymes, but the genus Sinorhizobium (Ensifer) was not used for this purpose up to now (Narasimha et al. 2006; Akpinar et al. 2009; Sun et al. 2010).
Rhizobia are a unique group of soil bacteria that can establish symbiotic association forming root nodules with specific host legumes. They convert atmospheric nitrogen to ammonia inside the root nodule and have a beneficial effect on plant growth (Shahzad et al. 2012). So far, enzymatic potential, especially cellulolytic, of this genus was not investigated. Only, Chen et al. (2004) noted CMCase activity for Sinorhizobium fredii CCRC 15769. On the other side, Sinorhizobium was described in Bergey’s manual as non-cellulolytic (could not utilize cellulose) (Kuykendall et al. 2015).
This work is the first study that deals with cellulolytic potential of a S. meliloti strain 224. The aim of present research was to optimize conditions for the cellulase production (Avicelase and CMCase) from the strain 224 using tobacco waste during SmF and SSF. The response surface methodology (RSM) was applied for enzymes production process optimization. A chemical modification of the lignocellulosic substrate was made, and its influence on the production of enzymes was tested. All qualitative and quantitative experiments in the present study were conducted in the period from January to May 2018 in the Department of microbiology of the Institute of Soil Science, Belgrade, Serbia.
Materials and methods
Microorganism and inoculum preparation
Sinorhizobium meliloti strain 224 is a part of the Collection of the Institute of Soil Science (ISS WDCM375-Collection of Bacteria, Institute of Soil Science, Department of Microbiology). The strain was identified to nearest species based on morphological characteristics, and sequence of the 16S rRNA encoding gene (gene accession numbers FR714444.1) was higher than 99.7% (Stajković-Srbinović et al. 2012). It was selected according to qualitative test of the growing of strain on carboxymethyl cellulose (CMC) agar plate. Semi-quantification of cellulolytic potential was done on CMC, Avicel and waste tobacco agar plates (per liter: 1 g of CMC/Avicel/tobacco waste, 3.0 g of yeast extract, 3.0 g of K2HPO4, 1.0 g of KH2PO4, 0.5 g of MgSO4 and 6.0 g of agar) (Mihajlovski et al. 2016). A loopful of bacteria, previously grown in liquid CMC medium (without agar, 24 h, 28 °C), were spot-plated on these agar plates. After incubation (4 days, 28 °C), plates were flooded with Gram’s iodine (2.0 g KI and 1.0 g iodine in 300 mL distilled water) for 3 to 5 min.
The working culture of rhizobium was prepared in Erlenmeyer flasks containing yeast mannitol broth [YMB; per liter: 10.0 of mannitol/1.0 g of CMC, 0.5 g of K2HPO4, 0.2 g of MgSO4, 0.1 g of NaCl, 0.2 g of CaCO3 and 100 mL of fresh yeast extract (30.0 g/L)] in a rotary shaker (125 rpm, 2 days, 28 °C) (Vincent 1970).
Substrate raw material and its modification
The tobacco solid waste material was dried and ground to a particle size of 0.063–0.1 mm (unmodified substrate). Its modification was done with 1% H2SO4 or NaOH in ratio 1:5 (w:v). After 2 h at room temperature (25 °C), the solid phase was separated by a vacuum pump and washed with distillate water. The resulting modified substrate was dried for overnight in an oven at 105 °C (modified substrate).
Experimental design of cellulase production
In order to evaluate the influence of selected factors on the production of cellulase as well as process optimization and statistical analysis, software Design Expert 8 (Stat-Ease, Inc) that includes Box–Behnken design (BBD) was employed. The model was described with 17 experiments and two responses, Avicelase activity (Y1, U/mL) and CMCase activity (Y2, U/mL) according to three process parameters: tobacco waste concentration (A, g/L), incubation time (B, days) and inoculum concentration (C, %). The levels of chosen parameters, independent variables, are presented in Table 1. In these experiments, rhizobium that was growing in the CMC medium was used. Multiple regressions were used to analyze BBD data and fit to a second-order polynomial regression model. The model equation of response (Y) of three independent variables listed above is given in the following equation:
where Y represents the predicted response, Xi refers to the coded levels of the independent variables, XiXj is the interaction effect, X 2 i is the square effect, β0 is a constant, βi, βii and βij are the linear, quadratic and interactive regression coefficients, respectively, and z is the number of independent variables (process parameter) (Pavlović et al. 2013; Talebi et al. 2017). Analysis of variance (ANOVA) was employed to check the adequacy of developed regression models.
Submersed fermentation
Liquid fermentation was carried out in 100-mL Erlenmeyer flasks with a predetermined amount of unmodified tobacco waste in the YMB medium. (Mannitol was replaced by waste material.) After sterilization, a predetermined amount of inoculum (growth in presence of CMC) was added and incubated on a rotary shaker (125 rpm, 28 °C). After the predetermined incubation time, the samples were centrifuged (6000×g for 15 min) and the cell-free supernatant was further tested for cellulase activity. Modified tobacco substrates were used to produce cellulases, under obtained optimal conditions, to test the effect of modification on the production of this enzyme.
Solid-state fermentation
Unmodified and modified tobacco substrates were used as a low-cost substrate for the production of cellulases by S. meliloti 224 within the SSF. SSF was performed in 100-mL Erlenmeyer flasks with 1.0 g of sterile tobacco waste [distilled water was added before sterilization in ratio 1:3 (w:v)] and 5% or 10% of rhizobium culture which was growing in YMB or CMC (1 g/L) medium. After incubation (2 days, 28 °C), 10 mL of 0.1 M tri-sodium citrate buffer (pH 4.8) was added for enzyme extraction. The samples were filtrated, and the liquid aliquot was centrifuged and analyzed for Avicelase and CMCase activity.
Enzyme assay for Avicelase and CMCase
The activity of cellulase was measured by the reduction of 3,5-dinitrosalicylic acid in the presence of glucose released by enzymatic cellulose hydrolysis (Miller 1959). Avicelase and CMCase activities were determined using 0.5 mL of enzyme sample and 0.5 mL of 1% (w/v) Avicel or CMC solution in 0.1 M tri-sodium citrate buffer (pH 4.8). The mixture was incubated in a rotary shaker (125 rpm, 50 °C). After 30 min of incubation, 1 mL of DNS reagent was added and the reaction mixture boiled, cooled and diluted by adding 5 mL of distilled water. The absorbance was read on the UV/visible spectrophotometer (UV-160A, Shimadzu Corporation, Japan) at 540 nm against a blank (non-incubated enzyme). One unit of Avicelase or CMCase activity was defined as the amount of enzyme that released 1 μmol of glucose equivalents per minute.
Results and discussion
Cellulase qualitative test
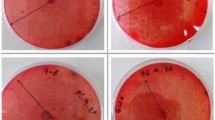
Sinorhizobium meliloti strain 224 was growing on Avicel, CMC and waste tobacco agar plate for 4 days. The presence of halo zones around bacterial colonies on these plates produced by strain 224 indicates areas of hydrolysis of used substrates (Fig. 1).
Halo zones appeared on all agar plates with the appropriate substrate. This indicates that strain 224 can use both microcrystalline (Avicel) and amorphous (CMC) cellulose, as the sole carbon source, proving it to be a true cellulolytic bacterium. The hydrolysis of both Avicel and CMC by S. meliloti has not been reported yet in the literature. Just Hu and Lin (2003) reported positive CM-cellulase activity of the sonicated cell extracts of S. fredi CCRC15769 on double-layer plate with CMC, but not in the supernatant after bacterial growth in the broth as in the present study. In addition, strain 224 can hydrolyze waste tobacco (Fig. 1c) and the hydrolysis of this waste material by S. melilot was not previously mentioned in the literature.
Fitting the process parameters
In order to achieve the maximum activity of Avicelase and CMCase, the optimal combination of three independent variables according to the experimental design matrix derived from the BBD was performed (Table 2).
By applying a multiple regression analysis for experimental data, the relationship between the responses and the three examined factors is efficiently designed as a second-order response corresponding to the surface and it is shown by the two equations [Eqs. (2), (3)]:
where the Y1 (Avicelase activity, U/mL) and Y2 (CMCase activity, U/mL) are the responses, and the A (waste tobacco concentration, g/L), B (incubation time, days) and C (inoculum concentration, %) are the independent variables.
The quadratic model was found to be the most suitable model for both responses. The analysis of variance ANOVA to determine the significance of regression model for the responses was evaluated, and the results are presented in Table 3. The model F values of 30.28 (Y1) and 91.81 (Y2) corresponding to P value < 0.0001 imply the models are significant and accurate. In addition, the term of lack of fit is nonsignificant (P > 0.05) according to model assumption which explained that quadratic models were adequate for predicting the production of cellulases. The validity of the second-order model was further investigated by the correlation coefficient (R2). It was 0.9750 for Avicelase activity and 0.9916 for CMCase activity and indicates a good correlation and fitting between the actual (experimental) and predicted values. Further, the adjusted multiple correlation coefficient (R 2adj. ) value was found to be 0.9428 and 0.9808 for Avicelase and CMCase activity, respectively. The predicted multiple correlation coefficients (R 2pred. , 0.7775 for Avicelase and 0.8971 for CMCase activity) were close to the R 2adj. values. These result values showed that the model fitted the data well. The adequate precision values of 19.19 for Avicelase activity and 34.87 for CMCase activity indicated a desirable signal-to-noise ratio.
Independent factors are designated as significant under selected conditions according to the values of model terms Prob > F < 0.0500. Significant model terms for the response Avicelase activity are A, C, BC, A2, B2 and C2, while for the response CMCase activity significant model terms are A, B, C, AC, A2 and B2 (Table 3).
Models validation
In the purpose of the model reproducibility, the validation was done by using the three points which were randomly selected from the numerical optimization results. The results were compared with the obtained (now predicted in Table 4) values from Table 2. The percentage errors between experimental and predicted values of Avicelase and CMCase activity during submersed fermentation using untreated tobacco waste were within the acceptable range (Table 4). In addition, results showed the same trends as predicted results. Thus, these results indicate that the model equation presented good agreement with the experimental result.
Effect of process variables on Avicelase activity
The maximum Avicelase activity of 0.131 U/mL was obtained under the following conditions: unmodified waste tobacco concentration of 5 g/L, 2 days of incubation time and inoculum concentration of 9% (Table 2). Avicelase activity was increased by increasing tobacco and inoculum concentrations, as well as increasing incubation time from 1 to 2 days and decreasing this parameter from 3 to 2 days (Fig. 2).
In this study, for the first time, Avicelase activity of the genus Sinorhizobium has been documented. Other studies which analyzed Avicelase activity of soil bacteria used equal or higher substrate concentrations (commercial or waste material) in order to achieve optimal Avicelase production (Abdel-Fattah et al. 2007; Martins et al. 2011; Oliveira et al. 2014). Shashidhar et al. (2018) investigated the influence of CMC concentration (2–20 g/L) on the Avicelase activity of enzymes from soil isolates Serratia marcescens WW4 and L4. Maximum activity was achieved with 5 g/L of CMC concentration. In addition to that, Acharya et al. (2008) obtained lower Avicelase activity from 0.0518 to 0.0804 U/mL produced by Aspergillus niger growing on a sawdust, for a concentration of 2.4% to 7.2% in a fermentation medium. Meanwhile, isolate CL5 (Bacillus sp.) achieved Avicelase activity of 0.108 U/mL during SmF at 37 °C with the presence of 3% of sawdust and about 10% of inoculum (Fauzi and Makky 2013). The RSM was used to obtain the maximum Avicelase activity of the natural isolate Paenibacillus chitinolyticus CKS1 using barley bran as low-cost substrate by Mihajlovski et al. (2017). Maximum Avicelase activity was 0.433 U/mL with applying 4% concentration of waste substrate during 3 days of incubation and 10% of inoculum concentration (Mihajlovski et al. 2017).
An increase in Avicelase activity with increasing inoculum concentrations, as is the case in this study, was also noted by Makky (2009). Lower inoculum concentration required longer time for the cells multiplication and utilization of the substrate to produce enzyme (Sun et al. 2010). Increasing inoculum concentration would allow rapid proliferation and biomass synthesis. Also, it can lead to a reduction in enzyme production, because after a certain limit due to exhaustion of nutrients, it could lead to a decrease in metabolic activity and cellulase production. The balance between the proliferating biomass and the available nutrient would give the optimum effect on which the synthesis of the enzymes would be maximal (Ramachandran et al. 2004; Sun et al. 2010).
The effect of different incubation times on the production of Avicelase is shown in Fig. 2. Maximum Avicelase activity over a period of 2 days, which was achieved for cellulase produced by strain 224, is the average time for reaching the maximum Avicelase activity, according to previous researches in this field. Incubation time ranged from 3 to 120 days, but it should also be noted that these cellulases are from different sources (Abdel-Fattah et al. 2007; Makky 2009; Oliveira et al. 2014; Mihajlovski et al. 2015, 2017).
Effect of process variables on CMCase activity
The maximum CMCase activity of 0.101 U/mL was obtained under the same conditions as maximum Avicelase activity: unmodified waste tobacco concentration of 5 g/L, 2 days of incubation time and inoculum concentration of 9% (Table 2). CMCase activity also increased when Avicelase activity increased, with increasing tobacco and inoculum concentrations, and decreased with incubation time of 1 to 2 and of 2 to 3 days (Fig. 3).
Among the nitrogen fixing bacteria, CMCase activities have been observed in Sinorhizobium fredii, Bacillus spharricus, Bacillus circulans, Paenibacillus sp., Gluconacetobacter (Emtiazi et al. 2007). Chen et al. (2004) demonstrated the presence of CMCase activity of S. fredii. However, the results cannot be compared with the results in this study, since the analysis of CMCase activity was performed from bacteria pellets which were resuspended in buffer (PCA; pH5.2) (Chen et al. 2004). In addition, the use of agro-industrial waste material as a substrate for the production of CMCase of the genus Sinorhizobium and their species S. meliloti was for the first time reported in the present study.
Emtiazi et al. (2007) and Pongsilp (2008) studied CMCase from Paenibacillus sp. and Rhizobium sp., which were produced with activity of 0.2 U/mL and 3 U/mL and about 2 mU/mL using 10 g/L of CMC in the growing medium, respectively. They were only tested in this CMC concentration, and the obtained CMCase activities were both higher and lower than those in the present study. Also, these activities were reached after 2 days of culture incubation, as in the present study; and after that period, the activity was decreased.
It is interesting to note that the strain 224 showed higher Avicelase than CMCase activity while growing on waste tobacco. Considering that there are no available literature data about the cellulolytic potential of the Sinorhizobium sp., it can be stated that the strain 224 is the first reported Sinorhizobium sp. with the predominant activity of Avicelase. For the efficient hydrolysis of cellulose, exoglucanases and endoglucanases should act in synergy. Therefore, the cellulases secreted by S. meliloti could be categorized as predominantly exoglucanases with complementary and lower endoglucanases activity.
On the other hand, the utilization of various agro-waste materials during SmF, such as wheat bran, cotton seed, rice bran, rice straw and pomegranate, to express the CMCase activity of Aspergillus flavus, was reported by Gomathi et al. (2012). The highest CMCase production was showed using wheat bran after 3 days of incubation, with 4% inoculum concentration and waste substrate concentration of 3%. In fact, the application of smaller amounts of inoculum provides advantages in the production of enzymes, as production costs could be reduced in this way. The maximal CMCase activity was 3.3 U/mL (Gomathi et al. 2012). In addition, Mihajlovski et al. (2017) also employed RSM to optimize the production of CMCase from P. chitinolyticus CKS1 using barley bran. The maximum CMCase activity (0.405 U/mL) was obtained with a similar inoculum concentration, lower substrate concentration of 4% and after a prolonged incubation time of 3 days (Mihajlovski et al. 2017).
Submersed fermentation using modified waste tobacco
Under optimal conditions obtained from RSM (substrate concentration 5 g/L, 10% inoculum concentration and 2 days of incubation time), the modified substrates were tested for cellulase production. The results are present in Table 5.
The modification of tobacco waste did not contribute to the improvement in the production of both enzymes during SmF, except for a slight increase in CMCase activity with applying H2SO4 pretreatment. Avicelase activities between untreated and NaOH pretreatment of substrate were similar. Pretreatment of wheat bran with H2SO4 and NaOH also did not contribute to the increase in cellulases production, as reported by Bansal et al. 2012, where the highest CMCase activity was obtained by utilizing untreated substrate.
Solid-state fermentation using tobacco waste
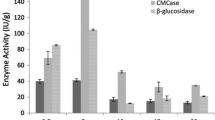
The production of enzymes Avicelase and CMCase from soil bacterial strain S. meliloti 224 by solid fermentation using unmodified and modified tobacco waste as a substrate was analyzed, and the results are presented in Fig. 4.
The use of the same amount of bacteria inoculums, which were growing in YMB (YMB culture) and CMC (CMC culture) medium before fermentation, yielded to different results. The use of YMB culture favored higher production of Avicelase, while the use of CMC culture led to higher CMCase production. Application of the acclimated culture achieved an increase in Avicelase activity by 2.9-fold and in CMCase activity by about 3.1-fold. This can be explained by the effect of a bacterial passaging on the production of cellulases, where the microorganism is forced to use cellulosic material as a source of carbon (Mihajlovski et al. 2016). In addition, the increased percentage of acclimatized inoculum increased the activity of both enzymes, Avicelase and CMCase.
The applied chemical pretreatment of the tobacco substrate improved the production of CMCase and Avicelase. The modification with NaOH was proved as a better solution for the pretreatment of substrate in the production of cellulase during SSF. Maximum Avicelase and CMCase activities reached 1.503 U/g and 1.615 U/g using the NaOH-modified tobacco waste and 10% inoculum of acclimatized bacterial culture, respectively. Application of NaOH pretreatment to tobacco waste causes swelling of the material. It further leads to an increase in the internal surface and a decrease in the degree of polymerization. The diluted NaOH treatment also causes a decrease in the crystallinity of the lignocellulosic material, which probably increases the activity of the CMCase (Karp et al. 2013). Application of the modified substrate (H2SO4 and NaOH modified) achieved an increase in Avicelase activity of 1.5- to 2.5-fold and in CMCase activity of about 1.3- and 2.1-fold, respectively.
In the literature, tobacco waste without any previous treatment was used to produce cellulase by fungi: Aspergillus terreus, A. niger, Phanerochaete chrysosporium, Trametes versicolor and Trametes hirsute (Narasimha et al. 2006; Oliveira et al. 2008; Su et al. 2016). Oliveira et al. (2008) produced CMCase utilizing black and Virginia tobacco dust waste as a substrate. During fermentation of black tobacco dust by A. niger, the obtained values for the assayed CMCase activity were very low, while fermentation with A. terreus gave the CMCase activity of 0.4163 U/mL (incubation time of 4 days, 31 °C). When Virginia tobacco dust was used as a substrate, the activities of CMCases from A. niger and A. terreus were 0.2128 U/mL and 0.3729 U/mL after 3 and 5 days of fermentation, respectively (Oliveira et al. 2008). Application of other fungi, P. chrysosporium, T. versicolor and T. hirsute in the production of cellulases during SSF of tobacco waste, gave the highest CMCase (0.51 U/mL) and Avicelase activity (0.51 U/mL) by using T. versicolor (inoculation of 10 days, 28 °C, relative humidity of 80%) (Su et al. 2016).
Conclusion
Soil bacterium Sinorhizobium meliloti strain 224 was found to be capable to produce both Avicelase and CMCase using tobacco waste as substrate with predominant Avicelase activity. Submerged fermentation favored Avicelase production and the usage of unmodified substrate. (Maximum Avicelase activity was 0.131 U/mL.) On the other hand, solid-state fermentation gave higher CMCase activities using the NaOH-modified substrate and expressed the maximum activity of 1.615 U/g. The presence of Avicelase and CMCase in strain 224 is a rare occurrence in bacteria and makes the despair significant in bioconversion of lignocelluloses waste and reduction in the amount of agro-industrial waste. The crude enzyme, produced by using the tobacco waste by strain 224, could be employed in eco-friendly processes of cellulose-based material bioconversion to useful products.
References
Abdel-Fattah YR, El-Helow ER, Ghanem KM, Lotfy WA (2007) Application of factorial designs for optimization of avicelase production by a thermophilic Geobacillus isolate. Res J Microbiol 2:13–23
Acharya PB, Acharya DK, Modi HA (2008) Optimization for cellulase production by Aspergillus niger using saw dust as substrate. Afr J Biotechnol 7:4147–4152
Adrio JL, Demain AL (2014) Microbial enzymes: tools for biotechnological processes. Biomolecules 4:117–139
Akpinar O, Erdogan K, Bostanci S (2009) Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food Bioprod Process 87:145–151
Bansal N, Tewari R, Soni R, Soni SK (2012) Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manage 32:1341–1346
Chen PJ, Wei TC, Chang YT, Lin LP (2004) Purification and characterization of carboxymethyl cellulase from Sinorhizobium fredii. Bot Bull Acad Sin 45:111–118
Emtiazi G, Pooyan M, Shamalnasab M (2007) Cellulase activities in nitrogen fixing Paenibacillus isolated from soil in N-free media. World J Agric Sci 3:602–608
Farinas CS (2018) Solid-state fermentation for the on-site production of cellulolytic enzymes and their use in the saccharification of lignocellulosic biomass. In: Pandey A, Larroche C, Soccol CR (eds) Current developments in biotechnology and bioengineering: current advances in solid-state fermentation. Elsevier, Amsterdam, pp 169–183
Fauzi NA, Makky EA (2013) Avicelase enzyme from Sawdust: isolation, production and optimization. Int J Biosci Biochem Bioinform 3:501–504
Gomathi D, Muthulakshmi C, Kumar DG, Ravikumar G, Kalaiselvi M, Uma C (2012) Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pac J Trop Biomed 2:S67–S73
Hu CY, Lin LP (2003) Characterization and purification of hydrolytic enzymes in Sinorhizobium fredii CCRC15769. World J Microb Biot 19:515–522
Juturu V, Wu JC (2014) Microbial cellulases: engineering, production and applications. Renew Sust Energ Rev 33:188–203
Karp SG, Woiciechowski AL, Soccol VT, Soccol CR (2013) Pretreatment strategies for delignification of sugarcane bagasse: a review. Braz Arch Biol Technol 56:679–689
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res. https://doi.org/10.4061/2011/280696
Kumar D, Yadav KK, Singh M, Garg N (2012) Biochemical utilization of agro-industrial lignocelluloses rich waste for cellulase production. Res J Agric Sci 44:184–191
Kuykendall LD, Hashem FM, Wang ET (2015) Sinorhizobium. In: Whitman WB (ed) Bergey’s manual of systematics of archaea and bacteria. Wiley, London, pp 1–11
Liu X, Kokare C (2017) Microbial enzymes of use in industry. In: Brahmachari G (ed) Biotechnology of microbial enzymes, 1st edn. Academic Press, New York, pp 267–298
Makky EA (2009) Avicelase production by a thermophilic Geobacillus stearothermophilus isolated from soil using sugarcane bagasse. World Acad Sci Eng Technol 57:487–491
Martins DAB, do Prado HFA, Leite RSR, Ferreira H, de Souza Moretti MM, da Silva R, Gomes E (2011) Agroindustrial wastes as substrates for microbial enzymes production and source of sugar for bioethanol production. In: Kumar S (ed) Integrated waste management-volume II. InTech, Kampala, pp 319–360
Mihajlovski KR, Carević MB, Dević ML, Šiler-Marinković S, Rajilić-Stojanović MD, Dimitrijević-Branković S (2015) Lignocellulosic waste material as substrate for Avicelase production by a new strain of Paenibacillus chitinolyticus CKS1. Int Biodeter Biodegr 104:426–434
Mihajlovski KR, Davidović SZ, Carević MB, Radovanović NR, Šiler-Marinković SS, Rajilić-Stojanović MD, Dimitrijević-Branković SI (2016) Carboxymethyl cellulase production from a Paenibacillus sp. Hem Ind 70:329–338
Mihajlovski KR, Davidović SZ, Veljović ĐN, Carević MB, Lazić VM, Dimitrijević-Branković SI (2017) Effective valorisation of barley bran for simultaneous cellulase and β-amylase production by Paenibacillus chitinolyticus CKS1: statistical optimization and enzymes application. J Serb Chem Soc 82:1223–1236
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Narasimha G, Sridevi A, Buddolla V, Subhosh CM, Rajasekhar RB (2006) Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr J Biotechnol 5:472–476
Oliveira AI, Curbelo C, Alvarez GM, Vicente AA, Teixeira JA (2008) Biological treatment of solid wastes from the tobacco industry for enzyme production. In: Valnatura: a Europe-Latin América post-graduate research network in the valorization of natural resources, pp 91–94
Oliveira LRC, Barbosa JB, Martins MLL, Martins MA (2014) Extracellular production of avicelase by the thermophilic soil bacterium Bacillus sp. SMIA-2. Acta Sci Biol Sci 36:215–222
Onorevoli B, da Silva Maciel GP, Machado ME, Corbelini V, Caramão EB, Jacques RA (2018) Characterization of feedstock and biochar from energetic tobacco seed waste pyrolysis and potential application of biochar as an adsorbent. J Environ Chem Eng 6:1279–1287
Pavlović MD, Buntić AV, Šiler-Marinković SS, Dimitrijević-Branković SI (2013) Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep Purif Technol 118:503–510
Pongsilp N (2008) The use of agricultural wastes as substrates for cell growth and carboxymethyl cellulase (cmcase) production by Bacillus subtilis, Escherichia coli and Rhizobium sp. Curr Appl Sci Technol J 8:84–92
Quintanilla D, Hagemann T, Hansen K, Gernaey KV (2015) Fungal morphology in industrial enzyme production-modelling and monitoring. In: Krull R, Bley T (eds) Filaments in bioprocesses. Springer International Publishing, Switzerland, pp 29–54
Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szakacs G, Pandey A (2004) Coconut oil cake—a potential raw material for the production of -amylase. Bioresour Technol 93:169–174
Shahzad F, Shafee M, Abbas F, Babar S, Tariq MM, Ahmad Z (2012) Isolation and biochemical characterization of Rhizobium meliloti from root nodules of Alfalfa (Medico sativa). J Anim Plant Sci 22:522–524
Shashidhar A, Arza S, Das A, Bhattacharya S, Shivakumar S (2018) Neutral Avicelase from Serratia marcescens with Denim Biofinishing Potential. J Sci Ind Res India 77:120–124
Stajković-Srbinović O, De Meyer SE, Miličić B, Delić D, Willems A (2012) Genetic diversity of rhizobia associated with alfalfa in Serbian soils. Biol Fer Soils 48:531–545
Su Y, Xian H, Shi S, Zhang C, Manik SN, Mao J, Zhang G, Liao W, Wang Q, Liu H (2016) Biodegradation of lignin and nicotine with white rot fungi for the delignification and detoxification of tobacco stalk. BMC Biotechnol 16:81
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases-production, applications and challenges. J Sci Ind Res India 64:832–844
Sun H, Ge X, Hao Z, Peng M (2010) Cellulase production by Trichoderma sp. on apple pomace under solid state fermentation. Afr J Biotechnol 9:163–166
Talebi M, Abbasizadeh S, Keshtkar AR (2017) Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf Environ 109:340–356
Vincent JM (1970) A manual for the practical study of the root nodule bacteria IBP handbook no 15. Blackwell Scientific Publications, Oxford
Wang SN, Xu P, Tang HZ, Meng J, Liu XL, Ma CQ (2005) “Green” route to 6-hydroxy-3-succinoyl-pyridine from (S)-nicotine of tobacco waste by whole cells of a Pseudomonas sp. Environ Sci Technol 39:6877–6880
Acknowledgements
The financial support for this investigation given by the Ministry of Education, Science and Technological Development of the Republic of Serbia under the Project TR 31035 and TR 37006 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interes
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: J Aravind.
Rights and permissions
About this article
Cite this article
Buntić, A.V., Milić, M.D., Stajković-Srbinović, O.S. et al. Cellulase production by Sinorhizobium meliloti strain 224 using waste tobacco as substrate. Int. J. Environ. Sci. Technol. 16, 5881–5890 (2019). https://doi.org/10.1007/s13762-019-02230-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02230-9