Abstract
Increasing energy demands are not only exploiting the fossil resources but, also depleting natural environment. Biofuels from lignocellulosic biomass is a renewable, ecofriendly, sustainable and could be a promising alternative to fossil fuels. However, pretreatment is an essential step to disarray the layers of lignocellulose prior to enzymatic hydrolysis. Among various pretreatments of lignocellulose, the biological pretreatment using microorganisms such as bacteria and fungi are gaining popularity due to its financial and environmental benefits. Careful selection of the suitable microbial consortium for efficient pretreatment of biomass is a critical step. The co-culture of bacteria and/or fungi in consolidated bioprocessing (CBP) is highly beneficial in the breakdown of complex biopolymers due to their high enzyme activity. Our selection of highly promising bacterial and/or fungal consortium has the ability to produce various extracellular enzymes including cellulase, hemicellulase, and lignases. It can be used in CBP for efficient biological pretreatment of lignocellulosic biomass following production of biofuels and bioproducts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an undeniable fact that human reliance on fuels to quench the thirst of liquid energy (oil, biofuels, and other liquid fuels) is increasing progressively, resulted in resource depletion and environmental pollution. British Petroleum Global (2016) has estimated that increasing human population and rising prosperity associated with emerging economies accounted for 97% of the increase in global oil consumption. The rate of oil consumption grew by 1.9 million barrels per day (b/d) that is nearly double (1.9%) than the average of 1% seen in 2014. China accounted for the largest increment in demand of 6.3% (i.e. 770,000 b/d) in oil consumption (https://www.bp.com/). Global increases of fuel demand accompanied by depletion of fossil fuels over the years and various disadvantages attached to its use has lead in search for an innovative alternative energy from renewable source like lignocellulosic biomass [1]. The lignocellulose is considered as a potential feedstock for production of biofuels and other bioproducts including various chemicals, biofibers, biopulps, enzymes, etc. [2].
The lignocellulosic biofuel is renewable, cost efficient, ecofriendly and thus creating a global priority. However, the main hurdles in utilizing lignocellulosic materials lie in the crystalline nature of cellulose sheathed by hemicellulose, degree of polymerization, biomass particle size and recalcitrance of their bonding due to protective covering of lignin which allow very less surface area for enzymatic hydrolysis [3,4,5]. Thus, to increases the digestibility of cellulose and hemicellulose, the removal or efficient breakdown of lignin from lignocellulosic biomass is usually a targeted step of pretreatment. The physical pretreatment such as milling, grinding, chipping, ultrasonic, etc. and chemical pretreatment with acids, alkali or oxidative delignification can efficiently breakdown the recalcitrant bonding in a short time thus are being extensively used in several industries. However, it requires high energy and operational cost along with chances of high risk of chemical hazards on environment. The biological pretreatment on the other hand has its very wide application and gaining its popularity because it requires low energy, has no chemicals, less pollution and higher yield. The naturally occurring bacteria and fungi secret different lignocellulolytic enzymes for efficient breakdown of biomass and help in formation of 5- and 6-carbon chain sugars. These sugars can be converted into biofuels and other various value added products [6]. This review mainly focuses on various hydrolytic enzymes and biological pretreatment methods to explore the best possible combination of microorganisms and its future perspectives in biofuel production.
Lignocellulosic Biomass
Lignocellulose is the plant biomass composed of carbohydrate polymers: cellulose (40–60%), hemicellulose (20–40%), and an aromatic polymer: lignin (10–24%) as main composition of plants cell walls [7]. The composition of lignocellulosic biomass varies from one plant species to another and their sources such as hardwoods, softwoods, and grasses (Table 1). Moreover, the composition within a single plant also differs with age, stage of growth, and conditions under which plant grows [8, 9]. The sources of lignocellulosic biomass not only include crop and forest residues, but also found in municipal solid waste, animal manures, papermill sludge, bioenergy crops and forest products. It has been estimated that about 10–50 billion ton of lignocellulosic biomass is produced annually worldwide [10]. It can be farmed for energy purposes thereby enabling higher production per unit land area and thus increasing land-use efficiency [11]. It is an abundantly available renewable resource on the earth that reduces reliance on fossil fuels by production of biofuels which is carbon neutral, alternative to petroleum and can mitigate the greenhouse gas emission. Thus, the lignocellulosic biomass has promising future and well chosen as predictable, feasible and sustainable resource for biofuels and other value added products [12].
Cellulose
Cellulose is the structural material in cell wall and composed of d-glucose subunits linked by β-1,4 glycosidic bonds [15]. The long polysaccharide chains are unbranched and arranged parallelly to form cellulose microfibrils. These cellulose molecules are the most abundant natural biopolymers found in earth. The cellulose microfibrils are tightly bound each other by inter- and intra-molecular hydrogen bonds which allow a rigid crystalline or amorphous structure. The CP-MAS study reveal the crystalline structure of cellulose has two form called Iα and Iβ [16,17,18].
Hemicellulose
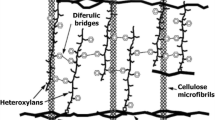
Hemicellulose is a complex carbohydrate, branched polymer consists of heterogeneous mixture of pentoses (xylose, arabinose), hexoses (mannose, glucose, galactose) and sugar acids (4-O-methyl-glucuronic, galacturonic and glucuronic acids). These sugars are linked together by β-1,4-glycosidic and sometimes by β-1,3-glycosidic bonds [19]. Its composition varies in hardwood which contain xylans and glucomannans; and softwood that contain glucomannans, xylans, arabinogalactans, xyloglucans and glucans [20, 21]. Hemicelluloses bind with cellulose microfibrils, lignin and pectin to form a cross-linked network of heterogeneous mixture of pentoses and hexoses in the cell walls [21].
Lignin
Lignin is a complex, amorphous hetero-biopolymer, insoluble in water consisting of phenylpropane units joined together by carbon–carbon and aryl–ether linkages. Lignin along with cellulose is considered the most abundant biopolymer in nature [15]. It is formed by oxidative coupling of three monolignols namely: trans-p-coumaryl alcohol, trans-coniferyl alcohol and trans-sinapyl alcohol. These monomers when form polymer, the phenylpropane units are called p-hydroxyphenyl, guaiacyl and syringyl units [22, 23]. This phenylpropanoid unit of lignin is the main bottleneck of breakdown of lignocellulosic biomass because it provides structural support, impermeability, and protection against microbial invasion [24].
Biomass and Biofuels
Biomass is an organic matter derived from living organisms. Biomass like wood, charcoal or dried animal waste has traditionally been used as unprocessed primary fuel whereas the processed secondary biofuels such as ethanol and biodiesel have been increasingly used for transportation. Biofuels are non-fossil fuels, can be divided into primary and secondary biofuels. The primary (unprocessed) biofuels such as firewood, wood chips and pellets are directly combusted in their natural form mainly for heating, cooking or electricity production. The secondary (processed) biofuels such as charcoal, bioethanol, biodiesel and biogas are produced from biomass. Depending upon the sources of feedstock used and their technological innovation, the secondary biofuels are further divided into first, second and third generation biofuels [25].
First Generation Biofuels
First generation biofuels are made from the food crops such as: sugarcane in Brazil, corn in the United States of America (USA) and beet or wheat in Europe and biodiesel made from plant oil such as: oilseed in France and Germany and from palm oil in Indonesia, Malaysia, Central America, Thailand, Africa and some other parts of the world. USA and Brazil together produced 85% (i.e. 21,793 million gallons) of ethanol and rest of the world produced only 15% (i.e. 3783 million gallons). Of which USA alone produced 14,700 million gallons (57%) and Brazil produced 7093 million gallons (28%) of ethanol (http://www.ethanolrfa.org/) [26]. However, it has some conflicting issue because of its intrinsic parts in the food chain.
Second Generation Biofuels
Second generation biofuels are manufactured from agriculture and forest residues and non-food crop feedstock including wood, organic waste, food waste and specific bioenergy crops. The study of U.S. Environmental Protection Agency (https://www.epa.gov/) showed, USA produced 2.18 million gallons of cellulosic ethanol in 2015. Similar, high potential of cellulosic ethanol can be noticed from Gao et al. [27] who estimated that 66% of agricultural residue and 34% of forest residue in China make a total of 12,693 petajoule biomass available for energy production. However, several concerns including competition and impact on arable land uses remain unchanged.
Third Generation Biofuels
Third generation biofuels are bioethanol and biodiesel manufactured from algae and sea weeds. It is of low-cost, possess high-energy, and completely renewable sources of energy. The algae-based biofuels and bioproducts have immense potentiality to replace fossil fuel and thus have promising future because of production of sustainable green energy. It has been estimated that the most efficient microalgae grown in optimized photobioreactors can produce 19,000–57,000 l of algal oil per acre per year [28]. It can grow in areas unsuitable for first and second generation crops using sewage, wastewater, and saltwater which would minimize impacts/competition on water and arable land uses. However, it has high operational cost and thus required intensive research on its further technological innovation and efficient utilization.
Pretreatment of Lignocellulosic Biomass
There are various pretreatment methods like physical, chemical, biological, and/or their combination. The purpose of any pretreatment method is to disintegrate the cellulose, hemicellulose and lignin so that the polymers are converted into smaller fragments (Fig. 1) readily accessible for enzymatic hydrolysis and other bio-refinery process to produce greater yield of various value added products (Fig. 2). However, each pretreatment method has its own advantages and disadvantages (Table 2). The physical methods (such as chipping, grinding or milling) are for mechanical breakdown of biomass that reduces the particle size and increase exposed surface area for further hydrolysis. But, it required high energy and is not cost efficient. Similarly, the application of chemicals like acids, alkalis, ozone, or peroxide in pretreatment is faster but may produce toxic substances and involves extra financial circumstances for chemicals recovery to sustain the system. Nevertheless, a combined mechanical and chemical method like steam explosion, and hot water treatments have reported a relatively cost-effective technique [29]. The biological pretreatment on the other hand is comparatively slower process and cannot easily control but in some circumstances where time is not always a major concern, it is cost effective technique, requires low energy input, no chemicals and ecofriendly [30, 31]. However, the biological method has been less investigated due to low industrial significance and limited technological progress. Nevertheless, the increasing scholarly interest on using microorganisms such as bacteria and fungi have reflected its brighter optimistic side of biological pretreatment. Selection of proper microbial consortia with diverse enzymatic composition and efficient hydrolytic activity is a challenging task. Thus, this review mainly focused on biological pretreatment of lignocellulosic biomass and their hydrolytic enzymes.
Biological Pretreatment
The naturally found wide taxonomic array of microorganisms are used in biological pretreatment. They alter or degrade lignocellulose extracellularly by secreting hydrolytic enzyme (such as hydrolases); and ligninolytic enzyme, which depolymerizes lignin [15]. Due to this the cell wall structure open up and allowing the subsequent hydrolysis of biopolymers. In biological pretreatment, the cellulose and hemicellulose are usually hydrolyzed into monomeric sugars using cellulolytic and hemicellulolytic microorganisms. The simultaneous degradation of lignocellulosic biomass followed by fermentation process are initiated at the same time which result in formation of biofuels such as ethanol, hydrogen, methane, furfural, etc. and bioproducts such as several enzymes, lactate, acetate, organic acids, etc. [39,40,41]. Some bacteria (such as Clostridium sp., Cellulomonas sp., Bacillus sp., Thermomonospora sp., Streptomyces sp. etc.) and several fungi (such as Phanerochaete chrysosporium, Trichoderma reesei, Trichoderma viride, Aspergillus niger etc.) are known to hydrolyze the natural biopolymers (Table 3).
Bacterial Pretreatment
There are many bacteria producing various biomass degrading enzymes (Table 3) used in biological pretreatment. The selection of the most efficient bacterial strains in pretreatment of lignocellulosic biomass followed by enzymatic hydrolysis and fermentation are the crucial steps during biofuel production. Unlike lignin, the cellulose and hemicellulose are comparatively easier to degrade. The cellulolytic bacteria for example Cellulomonas fimi and Thermomonospora fusca have been extensively studied for cellulase production. Similarly, cellulolytic bacteria, Paenibacillus campinasensis can survive in harsh conditions and has good potential for the pretreatment of lignocellulosic biomass [59]. There are at least 30 predominant rumen cellulolytic bacterial species (for example F. succinogenes, R. flavefaciens, and R. albus, etc.) which have a specific mechanism of adhesion to cellulose and its hydrolysis [60]. Although there are many cellulolytic anaerobic bacteria such as Clostridium thermocellum and Bacteroides cellulosolvens that produce high cellulase activity, they do not secret enough enzymatic concentration [61]. However, anaerobic bacteria like Zymomonas mobilis is a notable cellulolytic candidate and can be used in fermentation of sucrose, glucose and fructose to give high yield of ethanol [62]. The gram-positive Bacillus strains Firmicutes and the gram-negative strains Pseudomonas, Rahnella and Buttiauxella produce cellulase that shows highest activities in degrading the cellulosic materials [63]. Some bacterial strains such as Azospirillum lipoferum, and Bacillus subtilis have been reported to produce bacterial laccases thereby causing depolymerization of lignin [12]. Although, the microbial degradation of lignin has been well studied in fungi and very less studied in bacteria, the scientific communities have shown their comprehensive interest in bacterial lignin degradation [64,65,66] because of recently discovered bacterial peroxidases [67], laccases [68] and β-etherases [69] which can be used effectively in delignification.
Fungal Pretreatment
Fungi are well known microbes for their interactive effect on decaying lignocellulosic residue by their enzymes. These fungi are widely distributed in nature, most of which produces various cellulolytic [42, 70, 71], hemicellulolytic [71] and ligninolytic enzymes [72, 73]. The lignocellulolytic fungi include species from the ascomycetes (e.g. Aspergillus sp., Penicillium sp., Trichoderma reesei), basidiomycetes including white-rot fungi (e.g. Schizophyllum sp., P. chrysosporium), brown-rot fungi (e.g. Fomitopsis palustris) and few anaerobic species (e.g. Orpinomyces sp.) [74, 75]. However, the highly impermeable, resistance and recalcitrance nature of lignin; and insoluble and crystalline nature of cellulose represents a formidable challenge for enzymatic hydrolysis. The early report on T. reesei showed that it produces considerable amounts of xylanases and β-glucosidase with high cellulase activities [53]. Similarly, an extensively studied soil fungus Trichoderma longibrachiatum is one of the promising species in solubilization of crystalline cellulose because it secrets three types of cellulases: endoglucanases (e.g. carboxymethyl cellulases), exoglucanases (e.g. cellobiohydrolases), and β-glucosidases (e.g. cellobiases). These different cellulases and substrates have their complex interactions that function in a synergistic manner [6, 15, 54, 55] during hydrolysis. The lignin on the other hand has its complex intricate pathway of delignification and becoming a major hurdle to understand and selecting the efficient fungal strain. The white rot fungi (like basidiomycetes) however have its significant role in disintegration of lignin and considered as a natural lignin degrading microorganisms. They depolymerize and mineralize lignin because they secrete range of ligninolytic enzymes like laccases, lignin peroxidases and manganese peroxidases [2, 64, 76]. Otjen et al. [77] isolated 30 different wood decaying white rot fungi for lignin degradation and among these the best delignifiers reported so far were Phellinus pini-2, Pholiota mutabilis, Phlebia brevispora-1 and P. chrysosporium. However, the challenge of selecting fungal strain that effectively degrade the lignin with simultaneous cellulose recovery is persisted, and no breakthrough yet on its commercialized application.
Other Macroorganisms Pretreatment
Besides bacteria and fungi there are several other macroorganisms such as insects, worms, gastropods and ruminant animals which has strong ability to degrade lignocellulose. These macroorganisms are built up with some physiological mechanisms for breakdown of cellulosic biomass either by mechanical, enzymatic, gut flora and/or combination of these. These organisms have their own specific feeding/masticating mechanism for physical breakdown and different enzymatic components for efficient digestion of cellulose. There are diverse taxonomic groups of insects (more than 20 families representing 10 orders) such as termites (Isoptera), beetles (Coleoptera), wood wasps (Hymenoptera), crickets (Orthoptera), silverfish (Thysanura) etc., which are known to digest cellulosic biomass such as wood, leaf litters and forage [78]. The earthworms are well known for their detritus feeding behavior. Many epizoic composting earthworms, such as Eisenia fetida, Perionyxexcavates, Lumbricus rubellus, etc. can efficiently digest the organic matters [79]. The enzymatic action within the gut of earthworm accompanied by activities of microbial flora have potential in the digestion of cellulose, sugars, chitin, lignin, starch, etc. [80, 81]. Thus, the worm tea (i.e. the liquid leachate of vermicomposting) has been used as an alternative of acid pretreatment. Worm tea is considered as a microbial consortium and thus being used in biofuel production by enzymatic hydrolysis and fermentation [82]. Similarly, the microfloral consortium of gastropods and ruminant mammals also has significant role in cellulose digestion. Several studies have been carried out in microbial isolation of intestinal flora, their application in biological pretreatment of lignocellulose and bioproducts production [83,84,85].
Lignocellulose Degrading Enzymes
Cellulolytic Enzymes
Cellulase consists of endoglucanase, exoglucanase or cellobiohydrolase (CBH), and β-glucosidase, all these hydrolytic enzymes belong to glycosyl hydrolase (GH) family [86]. There are 128 GH families consisting of different cellulase enzymes and the synergistic actions of these hydrolytic enzymes catalyze the cellulose into monomeric sugar units. The endo- and exo-glucanases hydrolyze the glycosidic bonds from chain ends of cellulose to release cellobiose and some glucose. The β-glucosidases finally cleave cellobiose to glucose. Various bacteria and fungi are known to secrete endo or exo-acting cellulases that act on cellulose, resulting in release of glucose and cellobiose. So far, cellodextrin and cellobiose have their inhibitory activities during cellulose hydrolysis, the β-glucosidase is essential to break the final glycosidic bonds of cellobiose so as to produce sufficient glucose molecules [59, 87].
Hemicellulolytic Enzymes
Hemicellulases can be categories into glycoside hydrolase (GH) groups found in about 29 GH families and carbohydrate esterase (CE) groups found in about 9 CE families [88]. The GH groups hydrolyze the glycosidic bonds whereas the CE hydrolyze the ester bonds of acetate or ferulic acid groups. There are wide array of interdependent hemicellulases involve synergistically during hydrolysis of hemicellulose to form several monomeric sugars and also liberate cellulase [15, 88]. The enzymes like endo- and exo-xylanases hydrolyze the cross-linked of hemicelluloses that cleave the xylene to generate oligosaccharides [15]. The other enzymes like β-xylosidases, α-arabinofuranosidase, and esterases hydrolyze xylooligosaccharides to xylose; arabinose into furanose and pyranose forms; acetyl group into arabinose and ferulic acids respectively [21].
Ligninolytic Enzymes
The ligninolytic enzymes are a group of enzymes that degrade highly complex and recalcitrant lignin. Most of the White rot fungi possess enzymatic system to degrade the lignin [89]. They produce laccase and various peroxidases such as manganese peroxidase (MnP), lignin peroxidase (LiP) and versatile peroxidase [90, 91]. The white rot fungi are well-known producer of ligninolytic enzymes, followed by brown rot and soft rot fungi [91]. Unlike fungi, the bacteria are considered as low potential for lignin degradation. However, the three groups of bacteria namely, actinomycetes, α-proteobacteria and γ-proteobacteria are known to have ligninolytic system [92]. The bacterial ligninolytic enzymes such as laccase, lignin peroxidase (LiP), dye-decolorizing peroxidases (DyP), β-etherases, superoxide dismutases, etc. has already been discovered in different bacteria [66]. Among these above enzymes some of the most significant ligninolytic enzymes are laccase and peroxidases. Laccase is a multicopper oxidases having four copper molecules and act as oxidizing agent and cofactor. Similarly, various peroxidases have their potential to degrade different aromatic structure by involved in redox reaction [89].
Lytic Polysaccharide Monooxygenases (LPMO)
Lytic polysaccharide monooxygenase (LPMO) was initially discovered for its activity on chitin degradation [3, 93] however recently it has been known to disrupt the glycoside bonds in cellulose [3]. LPMO is copper-dependent monooxygenases [94, 95], belongs to the auxiliary activities (AA) enzyme classes. The carbohydrate-active enzyme of LPMO is classified into four AAs families AA9, AA10, AA11 and AA13 [96]. AA9 is found exclusively in fungi (Arthrobotrys oligospora, Aspergillus nidulans, Coprinopsis cinerea, etc), AA10 is predominantly found in bacteria (Bacillus cellulosilyticus, Streptomyces halstedii, Serratia marcescens, etc.) whereas AA11 and AA13 LPMOs are found in wider groups of fungi and some bacteria (http://www.cazy.org) [97]. LPMO carry out oxidative disintegration of recalcitrant polysaccharide chains in their crystalline regions so as to release oxidized oligosaccharides [93, 98]. LPMO works synergistically with hydrolytic enzymes, boost up the hydrolytic activity and increases the sugar production from lignocellulosic biomass [99].
Factors Affecting in Biological Pretreatment
There are several physical factors (such as temperature, moisture, incubation time, aeration, substrate size, accessible surface area, etc.), chemical factors (such as pH, composition of culture media, source of carbon, source of nitrogen, cellulose crystallinity, inorganic and organic compounds, roles of enzymes and hydrolysates, etc.) and biological factors (such as species of microorganism, consortia of microorganisms, their interaction and competition etc.). These factors affect the rate of biomass degradation and play a key role in changing physiochemical structure of lignocellulosic biomass. Following are some of the important factors that affect during biological pretreatment.
Temperature
The effect of temperature on microbial growth and their enzyme activities greatly varies with the different species. It is natural to produce considerable amount of heat due to some metabolic activities of microbes during fermentation. Many bacteria and fungi can grow in large spectrum of temperature gradient. Depending on their temperature preference, microorganisms are classified into three major groups: psychrophiles (–15 to 10 °C), mesophiles (20–45 °C) and thermophiles (41–122 °C). Bacteria can grow in wider range of temperature from 4 to 60 °C. The mesophilic fungi and bacteria are the most common [100] and most studied microbes of which their optimum temperature ranges from 25 to 40 °C. Many pathogenic bacteria prefer to grow in optimum temperature of 37 °C and on the other hand most thermophiles cannot grow below 45 °C. Similarly, some of the white rot ascomycetes grow in 39 °C whereas the basidiomycetes grow in 25–30 °C [101].
Moisture
The moisture content play a significant role in establishment of microbial growth, required for degradation of lignocellulose which greatly varies with types of substrate and microorganism involved in the pretreatment process [101]. Many bacteria and fungi prefer to grow in optimum moisture content ranges from 40 to 70% on solid substrates [102, 103]. It has been observed that the optimum moisture of 40 and 80% were suitable for Aspergillus niger on rice and coffee pulp respectively [102]. The fungal strain, Daedalea flavida MTCC 145 on the other hand has highest cellulose and lignin degradation due to low particle size and high moisture content (85% moisture) in solid-state fermentation [104]. Similar high optimum moisture level of 84% was recorded on white rot fungi Phlebia brevispora during pretreatment [105]. Generally, the single cell microorganism requires free water for their propagation. However, very high moisture level creates anaerobiosis and very low moisture content results in delayed microbial growth [103].
Incubation Time
The recalcitrant nature of lignocellulose is the major limiting factor in biological pretreatment which require relatively a longer incubation time for efficient delignification than other physio-chemical methods [101, 106]. It greatly varies with the biomass types and microorganisms involved in pretreatment process. The pretreatment of grass with P. chrysosporium showed significant degradation of lignin and exposing greater amount of cellulose and hemicellulose in third week of incubation time [107]. A satisfactory cellulose yield (64.3%) was obtained in 60 days’ pretreatment of corn stalk with Irpex lacteus [106]. However, the prolonged incubation period can not only degrade the lignin but also greatly reduce the amount of polysaccharide. Thus, effective enzymatic hydrolysis for higher yield of sugars and ethanol is desirable and can be achieve by optimization of incubation time.
Substrate Size and Aeration
The particle size of substrate and oxygenation play a vital role in biological pretreatment of lignocellulose. The surface area of lignocellulosic biomass comprises of external surface area, depends on particle shape and size; and internal surface area, depends on capillary structure of cellulosic fibers [35]. Mechanical reduction in particle size of lignocellulosic substrate increases the surface area thus increases the hydrolytic activity of various enzymes. The larger particle size limits fungal penetration and low diffusion of air whereas very low inter-particle space in smaller substrate decrease the aeration which hinders the growth and metabolism of microorganism [101, 104, 108]. Study on particle size of cotton stalk reviled that the D. flavida MTCC 145 have higher lignin degradation with lower cellulose loss when particle size was 5 mm [104]. Increase in aeration not only provide enough oxygen but also support in CO2 removal, heat dissipation and maintenance of humidity [2]. Thus, appropriate substrate size and high aeration are essential for enzyme production and better hydrolytic activity.
pH
The pH of culture medium has significant role in growth and metabolic activities of microorganisms. In most of the cases the pH value is generally drop after few days of microbial incubation [109], which directly influence in production of lignolytic enzymes [2, 101]. In Acinetobacter sp. the pH decreased from 7.0 to <4.0 after 10 days of incubation [109]. Most of the white rot fungi preferred slightly acidic (pH 4–5) environment for their better growth [110, 111]. It has been observed that the more ligninolytic the fungus (Vararia effuscata and Dichomitus squalens), much lower the pH with higher enzyme activity [111]. However, both decrease and increase in level of optimum pH during pretreatment result in low enzyme activity. The low pH inhibited the cellulases activity and in higher pH the enzymes will dissolve and lost their activity [112].
Structural Complexity
The lignocellulosic biomass has structural complexity due to cellulose crystallinity, cellulose sheathing by hemicellulose and complex phenylpropanoid unit of lignin. This structural complexity in plant cell wall results in recalcitrant biomass which is resistant to enzymatic and microbial deconstruction [113]. Cellulose has strong inclination to form inter and intra-molecular hydrogen bonds between the cellulosic chains [114, 115] that foster its accretion into two forms of crystalline structure called Iα and Iβ [16, 17]. Lignin on the other hand is most recalcitrant biopolymer, insoluble in water and composed of very complex network of non-fermentable phenylpropanoid units. Nonproductive binding of cellulolytic enzymes onto lignin together with protective covering of lignin and cellulose sheathing by hemicellulose act as a physical barrier for cellulase to reach the cellulose which inhibit the hydrolysis of lignocellulose [116, 117]. Thus, several studies have been concentrated on to remove the lignin and to decrease the cellulose crystallinity by different pretreatment methods for maximising the enzymatic digestibility. Significant amount of highly efficient various lignolytic enzymes are required for their synergistic effect to yield maximum monomeric sugars from cellulose and hemicellulose fractions of lignocellulosic biomass.
Loss of Polysaccharides
The major limiting factor of biological pretreatment is slow process accompanied by loss of polysaccharide [2, 118]. The considerable amount of cellulose and hemicellulose are consumed during the pretreatment process. Some of the white-rot fungi such as P. chrysosporium, C. subvermispora, Echinodontium taxodii 2538, Trametes ochracea, Irpex lacteus etc. are known to degrade the lignin but also have increased risk of loss of sugars from cellulose and hemicellulose [118]. The cellulolytic enzymes secreted by white-rot fungi are used to digest the cellulose for its own growth which result in low sugar production after enzymatic saccharification [104]. However, selection of efficient strain and optimization of culture condition can minimize the pretreatment time and sugar loss. Moreover, the technique of genetic manipulation and altering the ligninolytic or cellulolytic enzyme for efficient lignin degradation and low carbohydrate loss still need further improvement.
Microbial Co-Culture and Adaptation
A maximum enzyme activity during pretreatment is highly desirable to everyone. But, it is not always possible to produce all the lignocellulolytic enzymatic components from a single strain of any bacterium or fungus due to their limiting levels of one or the other enzymes. Despite having complexity to grow in the same culture medium several attempts have been made on co-culture of different species to understand the microbial world of communication, their secretions, adaptation and possible application on pretreatment. The ubiquitous nature of microbes and their ability to break the recalcitrant bonding of biopolymers have better functions by balancing two or more tasks in mixed populations which could otherwise become difficult for individual strains [119]. However, finding suitable microbes for co-culture is a daunting task because of their different genetic makeup, enzymatic components and ecological niche. The ecological and adaptational factors could also play a significant role in metabolic function of microorganism. It is because the microbial communities living in natural habitat can degrade the lignocellulosic components within their intricate network of food web where the whole consortia play a vital role. Three possible pretreatment combinations for bacterial and fungal cultures could be as follow.
Bacterial Co-Culture
Culture of two or more species of bacteria for efficient enzymatic hydrolysis is useful in pretreatment of lignocellulosic biomass and help in formation of biofuel and value added products. There are many bacteria belonging to Clostridium, Cellulomonas, Bacillus, Thermomonospora, Ruminococcus and Streptomyces that can produce various cellulases enzymes [14] secreted by dissimilar organisms worked together in cellulose hydrolysis [6]. Similarly, improved enzyme levels were also achieved by Chandra et al. [120] when bacterial strains Paenibacillus sp., Aneurinibacillus aneurinilyticus, and Bacillus sp. were cultured together that showed their high potential over the pure strains. High cellulose degradation was also observed by Kato et al. [121] in mixed culture of Clostridium straminisolvens and the three strains of aerobic isolates compared to that of the original microflora. Several attempts have been carried out in mixed culture of rumen bacteria [83,84,85, 122] for possible high enzymatic activities with coexistence and to find out their network relationship [123] so as to improve hydrolysis of lignocellulogic biomass. Moreover, the study on bacterial co-culture of C. thermocellum with other closely related thermophilic Clostridia has shown its significant role in hydrolysis of cellulose and hemicellulose and finally converts the sugars into biofuels, the ethanol [59].
Fungal Co-culture
Application of two or more species of fungi in biological pretreatment of lignocellulose has been in practiced from few decades. The fungal degradation in monoculture and co-culture is complex phenomenon and their metabolic interaction is not well understood [124]. Almost none of the fungi can produce significant amount of enzymes for hydrolysis at a same time [74]. However, enzymes production in co-culture sometime gets better output of enzymatic composition. For example, in separate experiment on Trichoderma reesei and Aspergillus phoenicis by Wen et al. [125] showed interesting opposite level of cellulolytic enzymes secretion: T. reesei produced high level cellulase, but low β-glucosidase whereas A. phoenicis produced low level cellulase and high β-glucosidase. On the contrary, the mixed culture of two fungi T. reesei with A. phoenicis at their optimum temperature 27 °C and pH 5.5 resulted in a high level of total cellulase and β-glucosidase production and thus showed higher enzymatic activities [125, 126] probably because of high nutrient level in the substrate [125]. There were multiple evidences of improved cellulolytic and hemicellulolytic activities in fungal co-culture [124, 127,128,129]. Furthermore, large amount of lignin degradation has also been reported so far by Chi et al. [130] in co-culture of C. subvermispora and Pleurotus ostreatus, than compared to monocultures.
Bacterial and Fungal Co-culture
This is a relatively new avenue of microbial co-culture of bacteria and fungi with the aim of producing continue enzymatic activities from a dynamic consortium. The main idea of these microbial consortia came from nature where different microorganisms live together, communicate each other and participate in interconnected network of food web within a microbial community. A study on four strains of white rot fungi (including Dichomitus squalens, Ganoderma applanatum, and two strains of Pleurotus sp.) on milled straw with addition of non-sterile soil containing soil microbes revealed that the laccase and manganese peroxidase production of Pleurotus sp. was not affected by soil microbiota and also showed high enzymatic activity in nonsterile soil [56]. It can be compared with natural biodegradation, where the non-sterile soil contains various bacteria that interact synergistically with fungal degradation of lignocellulose result in high and fast enzymatic activities [131]. Here in pretreatment of lignocellulosic biomass the fungi opened up the recalcitrant bonding of lignocellulose, hydrolyze the cellulose and hemicellulose into soluble saccharides, and the bacteria convert it into valued products. The study on bacterial and fungal co-culture has resulted in formation of different products like isobutanol using Trichoderma reesei and Escherichia coli [132] and ethanol from co-culture of Z. mobilis and Pichia stipitis [133]. Similarly, Golias et al. [134] observed high cellulase activity in co-culture of recombinant Klebsiella oxytoca P2 with Kluyveromyces marxianus, Saccharomyces pastorianus or Z. mobilis and produced more ethanol in faster rate compared to pure culture. Since, there is higher enzyme production from bacterial and fungal co-culture and thus it is likely a better alternative for efficient breakdown of lignocellulosic residue [135].
Microbial Adaptation
The selective microbial co-culture avoids competition for substrates between species [59]. However, the progress in adaptation of organisms in new environment and its evolution is determined by their population size, its survival, spread, and/or transmission of an organism within a specific ecological niche [136]. Different populations have their differences in physiological capabilities, cellular structures, and ecological niches, which can surpass its value in share adaptation [137]. The genetic makeup of organism allows them in pre-existing adaptation to either invading a new environment or advancement of adaptation characters in its existing niche. The molecular ecology of microbes based on 16S rRNA gene sequence represents a perfect molecule to study their diversity, phylogeny, evolution, and adaptation [138]. Systematic laboratory experiments on ecological aspect and molecular level are essential to understand the underlying mechanisms of adaptation of microbes in different ecological niches. It is always recommended to consider the optimal acceptable ranges of various physiochemical parameters like pH, temperature, oxygen demand and substrate of individual microbes in advance to set up their co-culture [139]. However, the survival and continue success of biologically active microbial consortia in highly competitive and hostile environment is challenging.
Commercial Potential of Biological Pretreatment
The cumulative capital investment of pretreatment facility is major financial concern among biorefinery and biofuel industries. The enzyme loading, its digestibility, production of sugars, energy consumption, quality of biofuels and bioproducts etc. are some important parameters and techno-economic bottlenecks that demands the commercial potential of treatment facility. The quality and price of bioproducts depend on types of biomass and process conditions used in the manufacturing plant [140]. The pretreatment is essential step to solubilize the biomass which offer higher cost of enzymes and other chemicals during bioconversion. Thus, the concept of consolidated bioprocessing (CBP) has been introduced as a single step process of simultaneous saccharification and fermentation for bioconversion of lignocellulosic biomass to biofuels using single microorganism or microbial consortium [141]. Many bacteria and fungi have lignolytic capability however the anaerobic, thermophilic and cellulolytic bacteria are mainly used in CBP for manufacture of second generation biofuels [142]. The thermophilic bacteria such as C. thermocellum [143,144,145] and Clostridium phytofermentans [146, 147] are well studied CBP bacteria [148]. Some other anaerobic, thermophilic bacteria such as Thermoanaerobacter sp. [149, 150] and Caldicellulosiruptor sp. [150, 151] have been studied in CBP for production of biofuels.
Concluding Remarks and Recommendations
Pretreatment plays a significant role in breakdown of lignin seal and disrupts crystalline nature of cellulose [29]. Microbial pretreatment of lignocellulosic biomass has its application in wide array of disciplines including industrial (in bioprocessing, biofuel, bio-refinery, pulp and paper industries); environmental (in wastewater treatment, biodegradation and bioremediation); and plays a vital role in carbon cycling. However, the paucity of enzymes exposure, presence of lignin and hemicellulose on cellulose surface and low accessibility on their limited surface area are the main hurdle in complete microbial degradation [4]. Therefore, the capital investment in finding suitable microbial consortia using optimization of different physiochemical parameters has been practiced. The genetic information focused on high yield of various enzymes is gaining its popularity among the scientific communities. There are some unsuccessful attempts of genetic manipulation on microorganism to secrete all possible enzymes from single genetically engineered one. However, there is no breakthrough yet, because the genetic manipulation of organisms is often a difficult part [152]. Despite this fact, the isolation of suitable strains followed by genetic engineering and co-culture of suitable microbial consortium in CBP could have possible application in large scale commercial biofuel production.
References
Hamelinck, C.N., Van Hooijdonk, G., Faaij, A.: Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28, 384–410 (2005). doi:10.1016/j.biombioe.2004.09.002
Millati, R., Syamsiah, S., Niklasson, C., Cahyanto, M.N., Ludquist, K., Taherzadeh, M.J.: Biological pretreatment of lignocelluloses with white-rot fungi and its applications: a review. BioResources 6, 5224–5259 (2011). doi:10.15376/BIORES.6.4.5224-5259
Horn, S., Vaaje-Kolstad, G., Westereng, B., Eijsink, V.G.: Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 5, 45 (2012). doi:10.1186/1754-6834-5-45
Zhang, Y.-H.P., Ding, S.-Y., Mielenz, J.R., Cui, J.-B., Elander, R.T., Laser, M., Himmel, M.E., McMillan, J.R., Lynd, L.R.: Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 97, 214–223 (2007). doi:10.1002/bit.21386
Zavrel, M., Bross, D., Funke, M., Büchs, J., Spiess, A.C.: High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour. Technol. 100, 2580–2587 (2009). doi:10.1016/j.biortech.2008.11.052
Zhou, S., Ingram, L.O.: Synergistic hydrolysis of carboxymethyl cellulose and acid-swollen cellulose by two endoglucanases (CelZ and CelY) from Erwinia chrysanthemi. J. Bacteriol. 182, 5676–5682 (2000). doi:10.1128/JB.182.20.5676-5682.2000
Putro, J.N., Soetaredjo, F.E., Lin, S.-Y., Ju, Y.-H., Ismadji, S.: Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 6, 46834–46852 (2016). doi:10.1039/C6RA09851G
Jeffries, T.W.: Biodegradation of lignin and hemicelluloses. In: Biochemistry of Microbial Degradation, pp. 233–277. Springer, Dordrecht (1994)
Chen, H.: Chemical composition and structure of natural lignocellulose. In: Biotechnology of Lignocellulose, pp. 25–71. Springer, Dordrecht (2014)
Sánchez, J., Cardona, C.A.: Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 99, 5270–5295 (2008). doi:10.1016/j.biortech.2007.11.013
Larson, E.: Biofuel Production Technologies: Status, Prospects and Implications for Trade and Development. United Nations Conference on Trade and Development, Geneva (2008)
Saritha, M., Arora, A.: Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 52, 122–130 (2012). doi:10.1007/s12088-011-0199-x
Kim, M., Day, D.F.: Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 38, 803–807 (2011). doi:10.1007/s10295-010-0812-8
Sun, Y., Cheng, J.: Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83, 1–11 (2002). doi:10.1016/S0960-8524(01)00212-7
Pérez, J., Muñoz-Dorado, J., de la Rubia, T., Martínez, J.: Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 5, 53–63 (2002). doi:10.1007/s10123-002-0062-3
Atalla, R.H., VanderHart, D.L.: Native cellulose: a composite of two distinct crystalline forms. Science. 223, 283–286 (1984)
VanderHart, D.L., Atalla, R.H.: Studies of microstructure in native celluloses using solid-state carbon-13 NMR. Macromolecules 17, 1465–1472 (1984). doi:10.1021/ma00138a009
O’Sullivan, A.C.: Cellulose: the structure slowly unravels. Cellulose 4, 173–207 (1997). doi:10.1023/A:1018431705579
Joy, J., Jose, C., Mathew, P. L., Thomas, S., Khalaf, M.N.: Biological delignification of biomass. In: Khalaf, M.N. (ed.) Green Polymers and Environmental Pollution Control, p. 271. CRC Press, Boca Raton (2016)
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30, 279–291 (2003). doi:10.1007/s10295-003-0049-x
Zhang, Z., Donaldson, A.A., Ma, X.: Advancements and future directions in enzyme technology for biomass conversion. Biotechnol. Adv. 30, 913–919 (2012). doi:10.1016/j.biotechadv.2012.01.020
Lewis, N.G., Yamamoto, E.: Lignin: occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 41, 455–496 (1990). doi:10.1146/annurev.pp.41.060190.002323
Cesarino, I., Araújo, P., Domingues Júnior, A.P., Mazzafera, P.: An overview of lignin metabolism and its effect on biomass recalcitrance. Braz. J. Bot. 35, 303–311 (2012). doi:10.1590/S0100-84042012000400003
Mussatto, S.I.: Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. Elsevier, Amsterdam (2016)
Singh Nigam, P., Singh, A.: Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 37, 52–68 (2011). doi:10.1016/j.pecs.2010.01.003
Renewable Fuels Association (RFA): Fueling a High Octane Future: 2016 Ethanol Industry Outlook. Renewable Fuels Association (RFA), Washington, D.C. (2016)
Gao, J., Zhang, A., Lam, S.K., Zhang, X., Thomson, A.M., Lin, E., Jiang, K., Clarke, L.E., Edmonds, J.A., Kyle, P.G., Yu, S., Zhou, Y., Zhou, S.: An integrated assessment of the potential of agricultural and forestry residues for energy production in China. GCB Bioenergy 8, 880–893 (2016). doi:10.1111/gcbb.12305
Demirbas, A.: Use of algae as biofuel sources. Energy Convers. Manag. 51, 2738–2749 (2010). doi:10.1016/j.enconman.2010.06.010
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96, 673–686 (2005). doi:10.1016/j.biortech.2004.06.025
Wan, C., Li, Y.: Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 30, 1447–1457 (2012). doi:10.1016/j.biotechadv.2012.03.003
Shi, J., Qing, Q., Zhang, T., Wyman, C., Lloyd, T.: Biofuels from cellulosic biomass via aqueous processing. In: Ginley, D.S., Cahen, D. (eds.) Fundamentals of Materials for Energy and Environmental Sustainability. Cambridge University Press, Cambridge (2011)
Harmsen, P.F.H., Huijgen, W.J.J., Bermúdez López, L.M., Bakker, R.: Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Energy Res. Cent. Netherlands. 10–13 (2010)
Taherzadeh, M.J., Karimi, K.: Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9, 1621–1651 (2008). doi:10.3390/ijms9091621
Conde-Mejía, C., Jiménez-Gutiérrez, A., El-Halwagi, M.: A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 90, 189–202 (2012). doi:10.1016/j.psep.2011.08.004
Maurya, D.P., Singla, A., Negi, S.: An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5, 597–609 (2015). doi:10.1007/s13205-015-0279-4
Singh, R., Shukla, A., Tiwari, S., Srivastava, M.: A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 32, 713–728 (2014). doi:10.1016/j.rser.2014.01.051
Kim, Y., Kreke, T., Mosier, N.S., Ladisch, M.R.: Severity factor coefficients for subcritical liquid hot water pretreatment of hardwood chips. Biotechnol. Bioeng. 111, 254–263 (2014). doi:10.1002/bit.25009
Bensah, E.C., Mensah, M.: Chemical pretreatment methods for the production of cellulosic ethanol: technologies and innovations. Int. J. Chem. Eng. 2013, 1–21 (2013). doi:10.1155/2013/719607
Reguera, G., Speers, A., Young, J.: Microbial electrochemical cells and methods for producing electricity and bioproducts therein. US Patent 14/705,766, 20 Aug 2015
Zhao, X.-Q., Zi, L.-H., Bai, F.-W., Lin, H.-L., Hao, X.-M., Yue, G.-J., Ho, N.W.Y.: Bioethanol from lignocellulosic biomass. In: Biotechnology in China III: Biofuels and Bioenergy, pp. 25–51. Springer, Berlin (2011)
Faik, A.: Plant cell wall structure-pretreatment the critical relationship in biomass conversion to fermentable sugars. In: Green Biomass Pretreatment for Biofuels Production. pp. 1–30. Springer, Dordrecht (2013)
Mandels, M., Reese, E.T.: Induction of cellulase in fungi by cellobiose. J. Bacteriol. 79, 816–826 (1960)
Biswas, R., Persad, A., Bisaria, V.: Production of cellulolytic enzymes. In: Bisaria, V.S., Kondo, A. (eds.) Bioprocessing of Renewable Resources to Commodity Bioproducts. pp. 105–132. Wiley, Hoboken (2014)
Narasimha, G., Sridevi, A., Viswanath, B., Chandra, S., Reddy, R.: Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. African J. Biotechnol. 5, 472 (2006)
Valaskova, V., Baldrian, P.: Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus—production of extracellular enzymes and characterization of the major cellulases. Microbiology 152, 3613–3622 (2006). doi:10.1099/mic.0.29149-0
Hatakka, A., Hammel, K.E.: Fungal biodegradation of lignocelluloses. In: Industrial Applications, pp. 319–340. Springer, Berlin (2011)
Hespell, R.: Microbial digestion of hemicelluloses in the rumen. Mol. Microbiol. 5, 362–365 (1988)
López-Mondéjar, R., Zühlke, D., Becher, D., Riedel, K., Baldrian, P.: Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 6, 25279 (2016). doi:10.1038/srep25279
Weingartner Montibeller, V., Porto de Souza Vandenberghe, L., Amore, A., Soccol, C.R., Birolo, L., Vinciguerra, R., Salmon, Xavier, Rigon, D.N., Spier, M., Faraco, V.: Characterization of hemicellulolytic enzymes produced by Aspergillus niger NRRL 328 under solid state fermentation on soybean husks. BioResources 9, 7128–7140 (2014). doi:10.15376/biores.9.4.7128-7140
Gessesse, A., Mamo, G.: High-level xylanase production by an alkaliphilic Bacillus sp. by using solid-state fermentation. Enzyme Microb. Technol. 25, 68–72 (1999)
Zorec, M., Vodovnik, M., Marinšek-Logar, R.: Potential of selected rumen bacteria for cellulose and hemicellulose degradation. Food Technol. Biotechnol. 52, 210–221 (2014)
Dehority, B.: Degradation and utilization of isolated hemicellulose by pure cultures of cellulolytic rumen bacteria. J. Bacteriol. 89, 1515–1520 (1965)
Tangnu, S.K., Blanch, H.W., Wilke, C.R.: Enhanced production of cellulase, hemicellulase, and β-glucosidase by Trichoderma reesei (Rut C-30). Biotechnol. Bioeng. 23, 1837–1849 (1981). doi:10.1002/bit.260230811
Béguin, P., Aubert, J.-P.: The biological degradation of cellulose. FEMS Microbiol. Rev. 13, 25–58 (1994). doi:10.1111/j.1574-6976.1994.tb00033.x
Nidetzky, B., Steiner, W., Claeyssens, M.: Synergistic interaction of cellulases from Trichoderma reesei during cellulose degradation. In: Enzymatic Degradation of Insoluble Carbohydrates, pp. 90–112. American Chemical Society, Washington, D.C. (1996)
Lang, E., Eller, G., Zadrazil, F.: Lignocellulose decomposition and production of ligninolytic enzymes during interaction of white rot fungi with soil microorganisms. Microb. Ecol. 34, 1–10 (1997). doi:10.1007/s002489900029
Naraian, R., Singh, D., Verma, A., Garg, S.K.: Studies on in vitro degradability of mixed crude enzyme extracts produced from Pleurotus spp. J. Environ. Biol. 31, 945–951 (2010)
Kameshwar, A.K.S., Qin, W.: Recent developments in using advanced sequencing technologies for the genomic studies of lignin and cellulose degrading microorganisms. Int. J. Biol. Sci. 12, 156–171 (2016). doi:10.7150/ijbs.13537
Maki, M., Leung, K.T., Qin, W.: The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 5, 500–516 (2009). doi:10.7150/ijbs.5.500
Miron, J., Ben-Ghedalia, D., Morrison, M.: Invited review: adhesion mechanisms of rumen cellulolytic bacteria. J. Dairy Sci. 84, 1294–1309 (2001). doi:10.3168/jds.S0022-0302(01)70159-2
Duff, S.J.B., Murray, W.D.: Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour. Technol. 55, 1–33 (1996). doi:10.1016/0960-8524(95)00122-0
Dien, B.S., Cotta, M.A., Jeffries, T.W.: Bacteria engineered for fuel ethanol production: current status. Appl. Microbiol. Biotechnol. 63, 258–266 (2003). doi:10.1007/s00253-003-1444-y
Paudel, Y.P., Qin, W.: Characterization of ovel ellulase-producing bacteria isolated from rotting wood samples. Appl. Biochem. Biotechnol. 177, 1186–1198 (2015). doi:10.1007/s12010-015-1806-9
Bandounas, L., Wierckx, N.J., de Winde, J.H., Ruijssenaars, H.J.: Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 11, 94 (2011). doi:10.1186/1472-6750-11-94
Palamuru, S., Dellas, N., Pearce, S.L., Warden, A.C., Oakeshott, J.G., Pandey, G.: Phylogenetic and kinetic characterization of a suite of dehydrogenases from a newly isolated bacterium, strain SG61-1L, that catalyze the turnover of guaiacylglycerol-β-guaiacyl ether stereoisomers. Appl. Environ. Microbiol. 81, 8164–8176 (2015). doi:10.1128/AEM.01573-15
De Gonzalo, G., Colpa, D.I., Habib, M.H.M., Fraaije, M.W.: Bacterial enzymes involved in lignin degradation. J. Biotechnol. 236, 110–119 (2016). doi:10.1016/j.jbiotec.2016.08.011
van Bloois, E., Torres Pazmiño, D.E., Winter, R.T., Fraaije, M.W.: A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl. Microbiol. Biotechnol. 86, 1419–1430 (2010). doi:10.1007/s00253-009-2369-x
Chandra, R., Chowdhary, P.: Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci. Process. Impacts 17, 326–342 (2015). doi:10.1039/C4EM00627E
Picart, P., de María, P.D., Schallmey, A.: From gene to biorefinery: microbial β-etherases as promising biocatalysts for lignin valorization. Front. Microbiol. 6, 916 (2015). doi:10.3389/fmicb.2015.00916
Sukumaran, R.K., Singhania, R.R., Pandey, A.: Microbial cellulases—production, applications and challenges. J. Sci. Ind. Res. 64, 832–844 (2005)
Ljungdahl, L.G.: The cellulase/hemicellulase system of the anaerobic fungus Orpinomyces PC-2 and aspects of its applied use. Ann. N. Y. Acad. Sci. 1125, 308–321 (2008). doi:10.1196/annals.1419.030
Arantes, V., Maria, A., Milagres, F.: The synergistic action of ligninolytic enzymes (MnP and laccase) and Fe3+ -reducing activity from white-rot fungi for degradation of Azure B. Enzyme Microb. Technol. 42, 17–22 (2007). doi:10.1016/j.enzmictec.2007.07.017
Shary, S., Kapich, A.N., Panisko, E.A., Magnuson, J.K., Cullen, D., Hammel, K.E.: Differential expression in Phanerochaete chrysosporium of membrane-associated proteins relevant to lignin degradation. Appl. Environ. Microbiol. 74, 7252–7257 (2008). doi:10.1128/AEM.01997-08
Dashtban, M., Schraft, H., Qin, W.: Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 5, 578–595 (2009). doi:10.7150/ijbs.5.578
Paudel, Y.P., Qin, W.: Two bacillus species isolated from rotting wood samples are good candidates for the production of bioethanol using agave biomass. J. Microb. Biochem. Technol. 7, 218–225 (2015). doi:10.4172/1948-5948.1000210
Guillén, F., Martínez, M.J., Gutiérrez, A., Del Rio, J.C., Camarero, S., Ferreira, P., Ruiz-Dueñas, F.J., Speranza, M., Martínez, Á.T.: Biodegradation of lignocellu- losics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 8, 195–204 (2005)
Otjen, L., Blanchette, R., Effland, M., Leatham, G.: Assessment of 30 white rot basidiomycetes for selective lignin degradation. Holzforschung 41, 343–349 (1987). doi:10.1515/hfsg.1987.41.6.343
Sun, J., Ding, S.-Y., Doran-Peterson, J.: Biomass and its biorefinery: novel approaches from nature-inspired strategies and technology. In: Biological Converstion of Biomass for Fuels and Chemicals: Exploration from Natural Utilization System, pp. 1–13. Royal Society of Chemistry, Cambridge (2014)
Pathma, J., Sakthivel, N.: Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 1, 26 (2012). doi:10.1186/2193-1801-1-26
Zhang, B., Li, G., Shen, T., Wang, J., Sun, Z.: Changes in microbial biomass C, N, and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol. Biochem. 32, 2055–2062 (2000). doi:10.1016/S0038-0717(00)00111-5
Vivas, A., Moreno, B., Garcia-Rodriguez, S., Benítez, E.: Assessing the impact of composting and vermicomposting on bacterial community size and structure, and microbial functional diversity of an olive-mill waste. Bioresour. Technol. 100, 1319–1326 (2009)
Siti Norfariha, M.N., Siti, A.I., Nur Farehah, Z.A., Renuka, R., Norli, I.: Second generation bioethanol from lignocellulosic biomass using worm tea as pretreatment. In: International Proceedings of Chemical, Biological and Environmental Engineering (IPCBEE), pp. 1–5. International Association of Computer Science and Information Technology Press (IACSIT), Singapore (2013)
Russell, J.B., Muck, R.E., Weimer, P.J.: Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 67, 183–197 (2009). doi:10.1111/j.1574-6941.2008.00633.x
Fondevila, M., Dehority, B.A.: Degradation and utilization of forage hemicellulose by rumen bacteria, singly in coculture or added sequentially. J. Appl. Bacteriol. 77, 541–548 (1994). doi:10.1111/j.1365-2672.1994.tb04399.x
Weimer, P.J., Nerdahl, M., Brandl, D.J.: Production of medium-chain volatile fatty acids by mixed ruminal microorganisms is enhanced by ethanol in co-culture with Clostridium kluyveri. Bioresour. Technol. 175, 97–101 (2015). doi:10.1016/j.biortech.2014.10.054
Henrissat, B., Davies, G.: Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7, 637–644 (1997). doi:10.1016/S0959-440X(97)80072-3
Dashtban, M., Maki, M., Leung, K.T., Mao, C., Qin, W.: Cellulase activities in biomass conversion: measurement methods and comparison. Crit. Rev. Biotechnol. 30, 302–309 (2010). doi:10.3109/07388551.2010.490938
Sweeney, M.D., Xu, F.: Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: recent developments. Catalysts 2, 244–263 (2012). doi:10.3390/catal2020244
Plácido, J., Capareda, S.: Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Bioresour. Bioprocess. 2, 23 (2015). doi:10.1186/s40643-015-0049-5
nee’Nigam, P.S., Gupta, N., Anthwal, A.: Pre-treatment of agro-industrial residues. In: Biotechnology for Agro-Industrial Residues Utilisation, pp. 13–33. Springer, Dordrecht (2009)
Niladevi, K.N.: Ligninolytic enzymes. In: Biotechnology for Agro-Industrial Residues Utilisation, pp. 397–414. Springer, Dordrecht (2009)
Bugg, T.D., Ahmad, M., Hardiman, E.M., Singh, R.: The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 22, 394–400 (2011). doi:10.1016/j.copbio.2010.10.009
Vaaje-Kolstad, G., Westereng, B., Horn, S.J., Liu, Z., Zhai, H., Sørlie, M., Eijsink, V.G.H.: An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 80, 330 (2010)
Hemsworth, G.R., Taylor, E.J., Kim, R.Q., Gregory, R.C., Lewis, S.J., Turkenburg, J.P., Parkin, A., Davies, G.J., Walton, P.H.: The copper active site of CBM33 polysaccharide oxygenases. J. Am. Chem. Soc. 135, 6069–6077 (2013). doi:10.1021/ja402106e
Aachmann, F.L., Sørlie, M., Skjåk-Bræk, G., Eijsink, V.G.H., Vaaje-Kolstad, G.: NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc. Natl. Acad. Sci. USA 109, 18779–18784 (2012). doi:10.1073/pnas.1208822109
Villares, A., Moreau, C., Bennati-Granier, C., Garajova, S., Foucat, L., Falourd, X., Saake, B., Berrin, J.-G., Cathala, B.: Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci. Rep. 7, 40262 (2017). doi:10.1038/srep40262
Levasseur, A., Drula, E., Lombard, V., Coutinho, P.M., Henrissat, B.: Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 6, 41 (2013). doi:10.1186/1754-6834-6-41
Eibinger, M., Ganner, T., Bubner, P., Rošker, S., Kracher, D., Haltrich, D., Ludwig, R., Plank, H., Nidetzky, B.: Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J. Biol. Chem. 289, 35929–35938 (2014). doi:10.1074/jbc.M114.602227
Patel, I., Kracher, D., Ma, S., Garajova, S., Haon, M., Faulds, C., Berrin, J., Ludwig, R., Record, E.: Salt-responsive lytic polysaccharide monooxygenases from the mangrove fungus Pestalotiopsis sp. NCi6. Biotechnol. Biofuels 9, 108 (2016)
Dix, N.J., Webster, J.: Fungi of extreme environments. In: Fungal Ecology, pp. 322–340. Springer, Dordrecht (1995)
Sindhu, R., Binod, P., Pandey, A.: Biological pretreatment of lignocellulosic biomass—an overview. Bioresour. Technol. 199, 76–82 (2016). doi:10.1016/j.biortech.2015.08.030
Raimbault, M.: General and microbiological aspects of solid substrate fermentation. Electron. J. Biotechnol. 1, 26–27 (1998). doi:10.4067/s0717-34581998000300007
Raghavarao, K.S.M.., Ranganathan, T., Karanth, N.: Some engineering aspects of solid-state fermentation. Biochem. Eng. J. 13, 127–135 (2003). doi:10.1016/S1369-703X(02)00125-0
Meehnian, H., Jana, A.K., Jana, M.M.: Effect of particle size, moisture content, and supplements on selective pretreatment of cotton stalks by Daedalea flavida and enzymatic saccharification. 3 Biotech 6, 235 (2016). doi:10.1007/s13205-016-0548-x
Saha, B.C., Kennedy, G.J., Qureshi, N., Cotta, M.A.: Biological pretreatment of corn stover with Phlebia brevispora NRRL-13108 for enhanced enzymatic hydrolysis and efficient ethanol production. Biotechnol. Prog. 33, 365–374 (2017). doi:10.1002/btpr.2420
Zhong, W., Yu, H., Song, L., Zhang, X.: Combined pretreatment with white-rot fungus and alkali at near room-temperature for improving saccharification of corn stalks. BioResources 6, 3440–3451 (2011). doi:10.2307/302397
Liong, Y.Y., Halis, R., Lai, O.M., Mohamed, R.: Conversion of lignocellulosic biomass from grass to bioethanol using materials pretreated with alkali and the white rot fungus Phanerochaete chrysosporium. BioResources 7, 5500–5513 (2012). doi:10.15376/biores.7.4.5500-5513
Bhargav, S., Panda, B., Ali, M., Javed, S.: Solid-state fermentation: an overview. Chem. Biochem. Eng. Q. 22, 49–70 (2008)
Marra, L.M., de Oliveira-Longatti, S.M., Soares, C.R., de Lima, J.M., Olivares, F.L., Moreira, F.: Initial pH of medium affects organic acids production but do not affect phosphate solubilization. Braz. J. Microbiol. 46, 367–375 (2015). doi:10.1590/S1517-838246246220131102
Reid, I.D.: Solid-state fermentations for biological delignification. Enzyme Microb. Technol. 11, 786–803 (1989). doi:10.1016/0141-0229(89)90052-5
Agosin, E., Odier, E.: Solid-state fermentation, lignin degradation and resulting digestibility of wheat straw fermented by selected white-rot fungi. Appl. Microbiol. Biotechnol. 21, 397–403 (1985). doi:10.1007/BF00249988
Geiger, G., Brandl, H., Furrer, G., Schulin, R.: The effect of copper on the activity of cellulase and β-glucosidase in the presence of montmorillonite or Al-montmorillonite. Soil Biol. Biochem. 30, 1537–1544 (1998)
Himmel, M.E., Ding, S.-Y., Johnson, D.K., Adney, W.S., Nimlos, M.R., Brady, J.W., Foust, T.D.: Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 (2007). doi:10.1126/science.1137016
Pu, Y., Hu, F., Huang, F., Davison, B.H., Ragauskas, A.J., Huang, C., Sherman, D., Tucker, M., Sun, L., Çetinkol, Ö., Dixon, R., Wang, Z.-Y., Templer, R., Tschaplinski, T.: Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 6, 15 (2013). doi:10.1186/1754-6834-6-15
Kondo, T., Sawatari, C., Manley, R.S.J., Gray, D.G.: Characterization of hydrogen bonding in cellulose-synthetic polymer blend systems with regioselectively substituted methylcellulose. Macromolecules 27, 210–215 (1994). doi:10.1021/ma00079a031
Mansfield, S., Mooney, C., Saddler, J.: Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 15, 804–816 (1999). doi:10.1021/bp9900864
Rahikainen, J.: Cellulase-lignin interactions in the enzymatic hydrolysis of lignocellulose. (2013)
Narayanaswamy, N., Dheeran, P., Verma, S., Kumar, S.: Biological pretreatment of lignocellulosic biomass for enzymatic saccharification. In: Fang, Z. (ed.) Pretreatment Techniques for Biofuels and Biorefineries, pp. 3–34. Springer, Berlin (2013)
Brenner, K., You, L., Arnold, F.H.: Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489 (2008). doi:10.1016/j.tibtech.2008.05.004
Chandra, R., Raj, A., Purohit, H., Kapley, A.: Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67, 839–846 (2007). doi:10.1016/j.chemosphere.2006.10.011
Kato, S., Haruta, S., Cui, Z.J., Ishii, M., Igarashi, Y.: Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol. Ecol. 51, 133–142 (2004). doi:10.1016/j.femsec.2004.07.015
Kato, S., Haruta, S., Cui, Z.J., Ishii, M., Igarashi, Y.: Stable coexistence of five bacterial strains as a cellulose-degrading community. Appl. Environ. Microbiol. 71, 7099–7106 (2005). doi:10.1128/AEM.71.11.7099-7106.2005
Kato, S., Haruta, S., Cui, Z.J., Ishii, M., Igarashi, Y.: Network relationships of bacteria in a stable mixed culture. Microb. Ecol. 56, 403–411 (2008). doi:10.1007/s00248-007-9357-4
Salimi, F., Mahadevan, R.: Characterizing metabolic interactions in a clostridial co-culture for consolidated bioprocessing. BMC Biotechnol. 13, 95 (2013). doi:10.1186/1472-6750-13-95
Wen, Z., Liao, W., Chen, S.: Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochem. 40, 3087–3094 (2005). doi:10.1016/j.procbio.2005.03.044
Madamwar, D., Patel, S.: Formation of cellulases by co-culturing of Trichoderma reesei and Aspergillus niger on cellulosic waste. World J. Microbiol. Biotechnol. 8, 183–186 (1992). doi:10.1007/BF01195843
Maheshwari, D.K., Gohade, S., Paul, J., Varma, A.: Paper mill sludge as a potential source for cellulase production by Trichoderma reesei QM 9123 and Aspergillus niger using mixed cultivation. Carbohydr. Polym. 23, 161–163 (1994). doi:10.1016/0144-8617(94)90098-1
Ahamed, A., Vermette, P.: Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochem. Eng. J. 42, 41–46 (2008). doi:10.1016/j.bej.2008.05.007
Duenas, R., Tengerdy, R.P., Gutierrez-Correa, M.: Cellulase production by mixed fungi in solid-substrate fermentation of bagasse. World J. Microbiol. Biotechnol. 11, 333–337 (1995). doi:10.1007/BF00367112
Chi, Y., Hatakka, A., Maijala, P.: Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int. Biodeterior. Biodegrad. 59, 32–39 (2007). doi:10.1016/j.ibiod.2006.06.025
Mikesková, H., Novotný, Č., Svobodová, K.: Interspecific interactions in mixed microbial cultures in a biodegradation perspective. Appl. Microbiol. Biotechnol. 95, 861–870 (2012). doi:10.1007/s00253-012-4234-6
Minty, J.J., Singer, M.E., Scholz, S.A., Bae, C.-H., Ahn, J.-H., Foster, C.E., Liao, J.C., Lin, X.N.: Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl. Acad. Sci. USA 110, 14592–14597 (2013). doi:10.1073/pnas.1218447110
Fu, N., Peiris, P., Markham, J., Bavor, J.: A novel co-culture process with Zymomonas mobilis and Pichia stipitis for efficient ethanol production on glucose/xylose mixtures. Enzyme Microb. Technol. 45, 210–217 (2009). doi:10.1016/j.enzmictec.2009.04.006
Golias, H., Dumsday, G., Stanley, G.: Evaluation of a recombinant Klebsiella oxytoca strain for ethanol production from cellulose by simultaneous saccharification and fermentation: comparison with native cellobiose-utilising yeast strains and performance in co-culture with thermotolerant yeast and Zymomonas mobilis. J. Biotechnol. 96, 155–168 (2002)
Kamsani, N., Salleh, M.M., Yahya, A., Chong, C.S.: Production of lignocellulolytic enzymes by microorganisms isolated from Bulbitermes sp. termite gut in solid-state fermentation. Waste Biomass Valoriz. 7, 357–371 (2016). doi:10.1007/s12649-015-9453-5
Preston, G.M., Haubold, B., Rainey, P.B.: Bacterial genomics and adaptation to life on plants: implications for the evolution of pathogenicity and symbiosis. Curr. Opin. Microbiol. 1, 589–597 (1998). doi:10.1016/S1369-5274(98)80094-5
Cohan, F.M., Koeppel, A.F.: The origins of ecological diversity in prokaryotes. Curr. Biol. 18, R1024–R1034 (2008). doi:10.1016/j.cub.2008.09.014
Grice, E.A., Kong, H.H., Conlan, S., Deming, C.B., Davis, J., Young, A.C., Bouffard, G.G., Blakesley, R.W., Murray, P.R., Green, E.D., Turner, M.L., Segre, J.A.: Topographical and temporal diversity of the human skin microbiome. Science 324, 80- (2009). doi:10.1126/science.1171700 1190–1192 ).
Bader, J., Mast-Gerlach, E., Popović, M.K., Bajpai, R., Stahl, U.: Relevance of microbial coculture fermentations in biotechnology. J. Appl. Microbiol. 109, 371–387 (2010). doi:10.1111/j.1365-2672.2009.04659.x
Kumar, D., Murthy, G.S.: Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 4, 27 (2011). doi:10.1186/1754-6834-4-27
van Zyl, W.H., Lynd, L.R., den Haan, R., McBride, J.E.: Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. In: Biofuels, pp. 205–235. Springer, Berlin (2007)
Singh, N., Mathur, A.S., Tuli, D.K., Gupta, R.P., Barrow, C.J., Puri, M.: Cellulosic ethanol production via consolidated bioprocessing by a novel thermophilic anaerobic bacterium isolated from a Himalayan hot spring. Biotechnol. Biofuels 10, 73 (2017). doi:10.1186/s13068-017-0756-6
Demain, A.L., Newcomb, M., Wu, J.H.D.: Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 69, 124–154 (2005). doi:10.1128/MMBR.69.1.124-154.2005
Taylor, M.P., Eley, K.L., Martin, S., Tuffin, M.I., Burton, S.G., Cowan, D.A.: Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 27, 398–405 (2009). doi:10.1016/j.tibtech.2009.03.006
Akinosho, H., Yee, K., Close, D., Ragauskas, A.: The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications. Front. Chem. 2, 66 (2014). doi:10.3389/fchem.2014.00066
Jin, M., Balan, V., Gunawan, C., Dale, B.E.: Consolidated bioprocessing (CBP) performance of Clostridium phytofermentans on AFEX-treated corn stover for ethanol production. Biotechnol. Bioeng. 108, 1290–1297 (2011). doi:10.1002/bit.23059
Jain, A., Morlok, C.K., Henson, J.M.: Comparison of solid-state and submerged-state fermentation for the bioprocessing of switchgrass to ethanol and acetate by Clostridium phytofermentans. Appl. Microbiol. Biotechnol. 97, 905–917 (2013). doi:10.1007/s00253-012-4511-4
Weimer, P.: The ruminant animal as a natural biomass-conversion platform and a source of bioconversion agents. In: Biological Conversion of Biomass for Fuels and Chemicals, pp. 248–281. Royal Society of Chemistry, Cambridge (2013)
He, Q., Hemme, C.L., Jiang, H., He, Z., Zhou, J.: Mechanisms of enhanced cellulosic bioethanol fermentation by co-cultivation of Clostridium and Thermoanaerobacter spp. Bioresour. Technol. 102, 9586–9592 (2011). doi:10.1016/j.biortech.2011.07.098
Svetlitchnyi, V.A., Kensch, O., Falkenhan, D.A., Korseska, S.G., Lippert, N., Prinz, M., Sassi, J., Schickor, A., Curvers, S.: Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol. Biofuels 6, 31 (2013). doi:10.1186/1754-6834-6-31
Chung, D., Cha, M., Guss, A.M., Westpheling, J.: Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc. Natl. Acad. Sci. USA 111, 8931–8936 (2014). doi:10.1073/pnas.1402210111
Lynd, L.R., Weimer, P.J., van Zyl, W.H., Pretorius, I.S.: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002). doi:10.1128/MMBR.66.3.506-577.2002. table of contents
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, H.K., Xu, C. & Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor 10, 235–251 (2019). https://doi.org/10.1007/s12649-017-0059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0059-y