Abstract
This work is aimed to the effective chemical pretreatment of sawdust hydrolysis for enhanced biogas production. Various chemical reagents were used for sawdust hydrolysis. NaOH was found to be the best among all in order to produce highest yield of soluble chemical oxygen demand (sCOD) and phenolic compounds. Therefore, NaOH prospective on delignification and rupture of cell wall of sawdust was determined experimentally using different approaches (NaOH addition, NaOH-microwave, and NaOH-autoclave). The NaOH-autoclave pretreatment showed pronounced effect on cellulose, hemicellulose, and lignin content of sawdust. XRD analysis revealed that 10% increase in crystallinity was observed after NaOH-autoclave treatment. SEM micrographs also depicted that cell wall surface was highly affected by NaOH-autoclave pretreatment. Optimum condition for highest lignin solubilization of 58.6% was found at 10% NaOH concentration and 90-min autoclaving time. Biogas yield was increased by 50.8% at optimum pretreatment condition in comparison to native sawdust. Rate constant and order of bioconversion into biogas was also increased after pretreatment. The maximum methane content in biogas for treated sawdust was found to be 62%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy and environment are interrelated with each other. Exploitation of fossil fuels produces greenhouse gases that contribute to global warming and climate change. An economically sustainable energy source, i.e., renewable and environment friendly is the demand of today world. Hydrogen energy is the evolution in energy system to combat the harmful effects caused by utilization of conventional fossil fuels. Biogas produced from low cost or no cost substrate can be used as an energy source.
Biogas is a mixture of primarily CO2 and CH4 produced after anaerobic digestion by microorganisms through the decomposition of organic matter. A variety of substrates from first generation to fourth generation of biofuels has been reported in literature for biogas production. Lignocellulosic biomass has been revealed out as a potential substrate for biogas production since past decade. Tectonagrandis (Teak) is a hardwood tree species and is extensively used as feedstock for manufacturing of indoor and outdoor materials due to its durability. Therefore, sawdust from wood processing mill can be used as a substrate for biogas production. Lignocelluloses basically are comprised of cellulose, hemicellulose, and lignin in variable ratios through cross linkages that resulted in complex cell wall structure from which only cellulose and hemicellulose are utilized for biofuel production, whereas lignin is rarely metabolized by microorganisms [1, 2]. Lignin forms a protective shield over cellulose and hemicellulose and hence makes them invulnerable to microorganism. It is responsible for unproductive binding to the cellulase enzyme, reduce the swelling of biomass, and hence prevent the accessibility of enzyme to substrate [3, 4]. Also, lignin can form furan compounds during degradation that can inhibit the microbial growth and hinder the production [5]. Biogas production from lignocelluloses is highly dependent on the concentration of lignin present in them [6,7,8]. Therefore, pretreatment is a crucial step to break the rigidity and increase the accessibility.
The pretreatment helps to reduce the complexity, provide large surface area to substrate, and increase porosity with the key goal of delignification [9,10,11]. Pretreatments are generally categorized as physical, mechanical, thermal, chemical, biological, and their combinations. Chemical pretreatments are effective and widely used treatment methods to overcome the recalcitrance of lignocellulosic biomass and make them appropriate for bioconversion.
In the literature, various chemical reagents have been used to break the rigidity of lignocelluloses. Acid pretreatment solubilizes the cellulose, hemicellulose, and also lignin partially [12]. Acidic reagents like HCl and H2SO4 have been extensively used along with enzymatic hydrolysis to get increased monomeric sugars from lignocelluloses for biofuel production. Wheat plant treated with dilute H2SO4 at 121 °C resulted in 15.2% lignin removal, 91.5% xylen degradation, and 15.5% higher methane yield in comparison to untreated wheat plant [13]. Dilute acid pretreatment (H2SO4) at ambient temperature caused partial solubilization of hemicelluloses, resulting in increased porosity and ethanol production (51.8%) from alfalfa stems [14]. Alkali breaks the main ether bonds, such as α-aryl ether bond, phenol-type α-alkoxy ether bond, and phenol-type β aryl ether bond present in lignin through a nucleophilic reaction mechanism that in turn causes degradation of lignin [15]. NaOH caused dissociation of lignin and carbohydrate linkages, solubilization of lignin, destruction of microfibril structures, and increased surface area of biomass that made it vulnerable to microbial enzymes [16,17,18]. In a study of optimization of NaOH pretreatment by response surface methodology for empty fruit bunches to get maximum delignification suggested the base concentration and pretreatment time were significant parameters [19]. Effectiveness of chemical pretreatment can increase with the implementation of thermal pretreatment. Wheat straw treated with N-methylmorpholine N-oxide (120 °C for 3 h), ethanol (180 °C for 1 h), and NaOH (30 °C for 24 h) resulted in 11, 15, and 15% increase in the cumulative biomethane production yield, respectively, with the alkaline pretreatment turned out to be the best to reduce lignin content in substrate [20]. Ca(OH)2 pretreatment combined with hydrothermal treatment on sugarcane bagasse resulted in 44% lignin degradation and 69% increase in methane yield in comparison to untreated sample [21]. The NaOH (10%)-microwave pretreatment caused the reduction of 56.7% in weight, 15.3% in hemicellulose, and 4.7% in lignin in the hydrolysis of paddy straws and increased the biogas yield by 54.7% [22]. The microwave assisted NaOH and Ca(OH)2 pretreatment done on catalpa sawdust resulted in high reducing sugar yield and decreased the amount of hemicellulose and lignin. Ca(OH)2 (2.25%)-microwave (400 W) treatment for 6 min followed by enzymatic hydrolysis of 96 h was found to be the ideal condition for increment in reducing sugar yield [23].
The main objective of this work was to increase biogas yield from Tectona grandis sawdust. The pretreatment effect of NaOH, HCl, H2SO4, ethanol, aqueous ammonia, and Ca(OH)2 was evaluated for sawdust hydrolysis. The effect of varied NaOH concentrations was observed with the assistance of microwave and autoclave. Moreover, optimization of pretreatment was carried out for maximum lignin solubilization. Presently, there is a lack of research regarding the use of hardwood sawdust as a substrate for biogas production and significance of such pretreatment on this substrate. Therefore, present work makes a great contribution in this regard.
2 Materials and method
2.1 Preparation and characterization of feedstock
Raw sawdust of T. grandis was procured from a local saw mill of Varanasi City, India. It was washed with distilled water to remove impurities like sand, soil, etc., and then dried in an oven at 80 °C for overnight. It was ground to reduce size and sieved, and particle size less than 36 mesh was stored for experimental work. Characterization of saw dust for total solid (TS), volatile solid (VS), moisture, fixed carbon, ash, carbon, hydrogen, nitrogen content, calorific value, cellulose, hemicellulose, and lignin was done. Inoculum used was sludge, procured from anaerobic digester of biogas plant situated at BHU, Varanasi. This plant was running on cow dung slurry. The main characteristics of sawdust and inoculum are given in Table 1.
2.2 Pretreatment of sawdust
2.2.1 Selection of suitable chemical reagent
Sawdust was treated with individual solution of HCl, H2SO4, ethanol, aqueous ammonia, NaOH, and Ca(OH)2 in order to determine effective reagent for better hydrolysis. Five grams of sawdust was added to 2% (50 ml) solutions of above-mentioned chemical reagents and incubated at 35 °C for 24 h. Experiments were performed in a 250-ml stoppered conical flask. It was filtered through Whatman filter paper no. 1, and supernatant was characterized for soluble chemical oxygen demand (sCOD) and phenolic compounds to compare the pretreatment effect of various chemical reagents.
2.2.2 NaOH pretreatment with or without thermal assistance
There were three routes of NaOH pretreatment implied in this work. In first set of experiment, it was done without thermal assistance; in second set of experiment, sawdust was treated with NaOH assisted by microwave treatment; and in third set of experiment, the sawdust was treated with NaOH with the help of autoclave treatment.
For NaOH addition without thermal treatment, 5 g of sawdust was added to the NaOH solution of variable concentration in the range of 0–12% in 250-ml glass stoppered conical flasks maintaining the TS concentration 10% and incubating at 35 °C for 24 h. For NaOH addition with thermal treatment, sawdust samples were soaked with NaOH solution having concentration in the range of 0 to 12% in 250-ml glass stoppered conical flasks with 10% TS concentration and incubated at 35 °C for 24 h. NaOH treated sawdust after 24 h was irradiated by microwave (Whirlpool MW-30 BC, USA) at 140 °C or followed by thermal treatment by using autoclave for 15 min.
NaOH-autoclave pretreatment was optimized for time in order to solubilize maximum lignin and better hydrolysis of sawdust. The autoclaving time was varied in the range of 15–120 min at 15 min interval each considering 10% NaOH as ideal concentration for NaOH-autoclave pretreatment. Evolution of COD and phenolic content were estimated after pretreatment. Quantitative estimation of compositional changes was also observed to determine the best time for autoclaving.
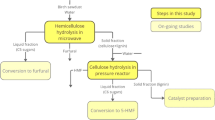
After each pretreatment, pretreated solid and liquid were separated by filtration with Whatman filter paper no 1. Figure 1 depicted the methodology of pretreatment implied for the present work. Hydrolysate of each pretreatment was stored at 4 °C until to estimate the sCOD and phenolic contents present in it. The pretreated solids were neutralized by washing with distilled water several times. It was then oven dried at 105 °C for overnight and used for compositional analysis. All the measurements were carried out in duplicates, and average value is reported.
2.3 Anaerobic digestion
Anaerobic digestion of native and treated sawdust was carried out in glass reactor of 2-L volume, containing two openings for pH and temperature measurement. The pH probe and a thermometer were inserted in reactor for pH and temperature monitoring. The reactor was connected with water displacement column through gas sampling tube. Anaerobic condition was maintained by tightly sealing the apparatus and sparging nitrogen for 15 min. The reactor was operated in batch mode (30 °C) with mesophilic conditions at pH 7.1. Substrate to inoculum ratio was maintained at 1:2 with the working volume of 1.4 L. Another identical reactor with only inoculum was also run as a control. Biogas yield was corrected by subtracting the biogas produced by control from sample yield. Experiments were performed in duplicates. The rate constant and order of bioconversion of sawdust into biogas were also determined by rate equation. The rate equation has been devised in terms of biogas yield against time as follows:
where rbio is the rate of conversion of sawdust into biogas in mL g−1VS day−1; Cbio is the biogas yield in mL g−1VS; k is the rate constant; and n is the order.
2.4 Analytical techniques
The TS, VS, ash, and moisture contents of the sample were estimated by laboratory analytical procedure (LAP) [24]. Fixed carbon was calculated by subtracting the sum of VS, ash, and moisture from 100. Carbon, hydrogen, and nitrogen contents in the sample were determined by using automatic elemental analyzer (Euro Vector EA, Italy). Calorific value of the sample was calculated with the help of bomb calorimeter (Rajdhani scientific, NSTTS Co., New Delhi, India) according to the standard protocol [25]. sCOD estimation was performed by the American Public Health Association (APHA) standard method (APHA 1998) [26]. Phenolic content of hydrolysate was found by Singleton’s Folin-Ciocalteu reagent method by taking absorbance at 765 nm using UV-VIS spectrophotometer (ELICO SL 159) [27].
The pretreated solid biomass was characterized for the presence of cellulose, hemicellulose, and lignin by estimating neutral detergent fiber (NDF) and acid detergent fiber (ADF) in accordance with Van Soest method [28]. Fourier-transform infrared spectroscopy (FTIR) absorbance spectrum was obtained by means of Nicolet 5700 FT-IR spectrometer, USA using the KBr pellet method. Spectra of native and treated sawdust were recorded in the range of 4000–400-cm−1 wavenumber. XRD diffraction was measured by X-ray diffractometer (Rigakuminiflex 600, Japan) equipped with goniometer miniflex 300/600 and detector D/tex Ultra. The diffraction pattern was obtained in the 2θ range of 5–60° at a scanning speed of 5° per min and with a step width of 0.02°. Current and voltage used were 15 mA and 40 kV, respectively. The crystallinity index (CI) was calculated according to Segal method [29], as given in equation below:
where CI is the crystallinity index; I002 is the intensity of highest peak, i.e., peak of crystalline region at 2θ ≈ 22°; and Im is the lowest intensity peak of amorphous region at 2θ ≈ 18°. The surface morphological studies were carried out by scanning electron microscope (SEM) (ZEISS EVO 18 RESEARCH, Germany) at varying magnification of ×200–5000 by using 10-kV voltage.
Measurement of biogas volume was carried out by using water displacement method, and composition of biogas was estimated by using gas chromatograph (NUCON 5765) equipped with thermal conductivity detector (TCD) and nitrogen as carrier gas with flow rate 30 ml/ min. Temperature of injector, oven, and detector was 393, 363, and 393 K, respectively. Column used for analysis was Porapak-Q, 2 m in length, and 0.25 mm in diameter.
3 Results and discussion
3.1 Physicochemical properties of native sawdust and inoculum
The important physicochemical characteristics of native sawdust and inoculum are represented in Table 1. Moisture content of sawdust was found to be 3.7%. There was 77.1% volatile matter present in sawdust. Carbon, hydrogen, and nitrogen contents were found as 50.6, 6.1, and 0.6%, respectively. Calorific value of sawdust was found to be 3206.7 kCal/kg. High moisture of 90% was found in inoculum. The pH and sCOD values of inoculum were 7.01 and 2102.5 mg/L, respectively.
3.2 Suitability of chemical reagent
The lignin solubilization is the chief requirement of pretreatment when lignocelluloses are used as a substrate for biogas production. Therefore, different reagents were used to rupture the cell wall structure and solubilize the lignin. The sCOD and phenolic compounds can be good indicators of substrate solubilization after pretreatment [30]. Disorganization of cell wall leads to the production of monomeric sugars from cellulose and hemicellulose, whereas break down of lignin forms phenolic byproducts [8, 31]. Hence, measurement of sCOD and phenolic compounds was carried out in order to evaluate the pretreatment effect. The sCOD and phenolic values for acids were found to be lower than alkali. Ethanol and NaOH treated samples contained higher values of sCOD, i.e., 9850 and 10,560 mg/L, respectively, whereas phenol concentration was found to be higher for the hydrolysate obtained from NaOH treatment (Table 2). NaOH was found to be the best pretreatment reagent among all in order to solubilize the sawdust effectively.
3.3 Effect of NaOH treatment on substrate solubilization
Potential of NaOH on substrate solubilization was evaluated as alone and in combination with microwave and autoclave treatment.
3.3.1 Effect of pretreatment on sCOD
The sCOD values were significantly increased as the concentration of NaOH was increased in all the hydrolysates, as shown in Fig. 2a. The sCOD for NaOH pretreatment was observed to increase up to 8% NaOH concentration and afterwards remained constant, whereas for NaOH-microwave and NaOH-autoclave pretreatment, it was increased up to 10% NaOH concentration and then remained almost unaltered. The autoclave assisted NaOH pretreatment caused highest yields of sCOD. It was reported previously that increase in alkali concentration and temperature as well caused solubilization of organic matter and thereby increased sCOD values [8, 32]. Accordingly, increased value of sCOD could be attributed to some alteration occurred in the structure of sawdust due to pretreatment that might be responsible for conversion of complex compounds (cellulose, hemicellulose, and lignin) into simpler sugars. Formation of volatile fatty acids (VFA) from these sugars takes place afterward.
3.3.2 Effect of pretreatment on phenolic compound
Phenolic compound in the liquid samples obtained after pretreatment followed an increasing trend as a function of concentration as illustrated in Fig. 2b. It was observed that autoclave assisted NaOH pretreatment caused a higher production of phenolic compound than other pretreatments.
3.4 Effect on composition and structure of treated sawdust
3.4.1 Effect of pretreatment on composition
Cellulose, hemicellulose, and lignin contents were estimated in native and treated sawdust by using Van Soest method, and results are represented in Table 3. The native sawdust was found to contain 49.6% cellulose, 29% lignin, and 13.1% hemicellulose. The cellulose content was slightly increased according to NaOH concentration in all solid yields after pretreatment. It was found to be in the range of 49–52, 49.9–54.5, and 49.6–60% in NaOH, NaOH-microwave, and NaOH-autoclave-treated samples, respectively. On the contrary, hemicellulose and lignin were decreased after pretreatment with increasing the NaOH concentration. The NaOH addition to the native sawdust caused degradation of esters and glycosidic side chains that resulted in lignin solubilization and swelling of biomass samples. It also demolished the acetyl and various uronic acid substitutions on hemicelluloses [33, 34]. A pretreatment is considered to be ideal that solubilizes the lignin efficiently and recovers the cellulose simultaneously [35]. From the results of this work, NaOH-autoclave pretreatment was found to be more effective in lignin solubilization as compared to other pretreatments.
3.4.2 FTIR absorption spectroscopy
The chemical composition variation in terms of functional group of sawdust before and after pretreatment was also investigated by FTIR analysis. FTIR absorption was recorded in the range of wavenumber 4000–400 cm−1 for qualitative analysis of the chemical structure of native and treated sawdust (Fig. 3). Peaks near band positions 3409, 3379, 3433, 3441, 3339, 3408, 3310, and 3424 cm−1 were observed in native and treated sawdust (Table 4). These were considered to be associated with intermolecular hydrogen bonded O▬H stretching that indicated the presence of cellulose [36,37,38]. Peaks in the range of band position 2869–2924 cm−1 were assigned to C▬H stretch that indicated the presence of cellulose [39, 40]. A new peak was generated at 2691-cm−1 band position after NaOH (10%) autoclave pretreatment that denoted CHO functional group and signified the presence of hemicellulose. The peak at band position 1739 cm−1 was associated with C〓O stretching of acetyl or carboxylic acid that represented the presence of hemicellulose and lignin [37]. A prominent peak was observed at band position 1628 cm−1. It was attributed to non-conjugated C〓C stretching and C〓O stretching vibrations of the aromatic ring that signified the presence of lignin [36, 38, 41]. The intensity of this absorption peak was found to be decreased in all treated samples; it might be due to deformation and solubilization of lignin. An absorption peak for CH2 bending was obtained at 1439 cm−1 that illustrated the presence of cellulose [38]. A new peak was appeared near 1353 cm−1 after 8% NaOH and NaOH (8%) microwave pretreatment. It was attributed to C-H deformation in cellulose and hemicellulose [42]. The peak near band position 1249 cm−1 was associated with the syringyl ring and C▬O stretch in lignin and xylan [42]. Syringyl type of lignin is the characteristic of hardwood which explains the nature of T. grandis sawdust. Its intensity was also decreased after pretreatment denoting the change in sawdust structure. The peaks near 1054–1032 cm−1 were assigned to C▬O vibrations of cellulose/hemicellulose and lignin [39, 40, 42] that described the lignocellulosic nature of sawdust.
3.4.3 Effect of pretreatment on crystallinity
In lignocellulosic biomass, cellulose presents in crystalline as well as in amorphous forms, whereas hemicellulose and lignin are considered as amorphous material [43]. XRD measurements were performed for crystallinity analysis of sawdust samples in terms of CI. XRD pattern is depicted in Fig. 4, and CI values are given in Table 5. The peak of the crystalline plane was observed at 2θ ≈ 22°, and lowest value of amorphous region was found to be at 2θ ≈ 18°. The intensity of crystalline cellulose was increased after pretreatment as shown in Fig. 4. Crystallinity pattern of this study was similar to that of the XRD spectra of NaOH-treated Spartina alterniflora [44]. The CI values were found to increase from 64 (native without NaOH addition) to 70.2% (with NaOH), 65.2 (microwave without NaOH) to 71.8% (NaOH microwave), and 66.37 (autoclave without NaOH) to 74.13% (NaOH autoclave). It might be due to peeling reaction of NaOH affected amorphous region more than crystalline region. Previous studies also reported that pretreatment of biomass resulted in the breakage of lignin-carbohydrate complex, recrystallization of cellulose, and removal of lignin and hemicellulose, which in turn caused high CI values [20, 45,46,47]. So, accordingly, increased CI values could be associated with increase in crystallinity and elimination of amorphous material from sawdust. Figure 5 signified a positive correlation of CI and cellulose content.
3.4.4 Effect on surface morphological structure of treated and native sawdust
The native and treated sawdust samples were scanned through SEM in the range of 500–5000 magnification to study the changes in surface morphology before and after pretreatment and microscopic images are given in Fig. 6. The SEM micrographs clearly illustrated the changes in the cell wall structure of native and treated sawdust. The surface structure of native sawdust was observed as rigid and compact. It seemed to be highly ordered and smooth because of lignin coating over cellulose and hemicellulose fibers [48]. Cell bundles were found to be flaky, brittle, and disaggregated in treated sawdust in comparison to native sawdust. Thermal treatment without NaOH, mainly autoclave treatment, had a minimal effect on cell surface (Fig. 6e). Whereas, combination of NaOH and thermal treatment caused significant destruction to cell wall that signified the importance of NaOH in substrate solubilization (Fig. 6d, f. As described earlier, NaOH caused distortion of cross-linkages like ester bonds between carbohydrate and lignin that solubilized a part of lignin and hemicelluloses and in turn caused the formation of cracks and holes in cell structure and exposed the cellulose. Also, its solvating nature was responsible for swelling of biomass that turned the cell wall structure disorganized and visually fragile (Fig. 6d) [49]. Therefore, disruption of cell surface could be associated with breakdown and solubilization of lignin and hemicelluloses as compositional analysis of native and treated sawdust also confirmed that lignin and hemicellulose content was decreased with increasing concentration of NaOH. The NaOH and thermal treatment broke the rigidity of sawdust by reducing lignin content, making sawdust fragile that can be vulnerable to microbial enzymes. Increased fragile nature can facilitate the accessibility of microorganisms and result in increased biogas production [50].
3.5 Optimization of time for NaOH-autoclave pretreatment
Results illustrated that NaOH autoclave pretreatment has potential to maximize the lignin solubilization and it is more effective in sawdust hydrolysis in comparison to other pretreatment methods used in the experimentation. Therefore, optimization of autoclaving time for NaOH-autoclave pretreatment was done for improving hydrolysis effect on sawdust. The 10% NaOH was selected as ideal concentration, since it turned out to be the best NaOH dose for lignin solubilization. The sCOD, phenolic content, and lignin solubilization were increased with respect to autoclaving time as illustrated in Fig. 7a. The 10% NaOH autoclave pretreatment with 90 min of autoclaving time was found to be the best pretreatment method, which solubilized 58.6% lignin as compared to native sawdust as found from Fig. 7b.
3.6 Anaerobic digestion
3.6.1 Biogas production
The effect of best pretreatment condition was examined on biodegradability and biogas production from treated sawdust. The cumulative biogas yield is represented in Fig. 8. Reactor was used to agitate daily for 1 min manually before recording the biogas volume in water displacement column. There was no production of biogas observed in first 5 days of anaerobic digestion of native sawdust. Afterwards, it was increased slowly and attained a maximum rate of 7.5 mL g−1 VS day−1 biogas production in 27 days, whereas biogas production from treated (10% NaOH and 90-min autoclaving) sawdust attained a maximum rate of 14.5 mL g−1 VS day−1 in 23 days with a lag phase of 2 days. The cumulative production of biogas from pretreated sawdust was 315 mL g−1 VS day−1, which was 50.8% more than native sawdust in 40 days of digestion period. Pretreatment solubilized a part of lignin by dissolving the carbohydrate-lignin linkages present in sawdust. Lignin solubilization made cellulose accessible to microorganisms and in turn increased the production. Previous researchers have also reported that pretreatment conditions increased the surface area of substrate and made it more vulnerable to microbial enzymes and also enhanced the biodegradability and biogas production [51]. pH observations were also noted during anaerobic digestion with the help of inserted pH probe attached with pH meter. The initial pH of digester was 7.1. The pH was decreased to 5.8 up to the 15th day of digestion. Accumulation of organic acid might have reduced the pH. It is previously reported that organic acid formation took place in earlier days of anaerobic digestion that was responsible for pH drop in digester [52]. Consumption of these organic acids by methanogens in order to produce biogas raised the pH of digester slowly afterwards. pH was found to be 7.5 after 40 days of digestion. Improvement in methane concentration improves the ignitable properties of biogas and increases its heating value [53]. Biogas production from treated sawdust attained a maximum methane concentration of 62% in 28 days of digestion.
3.6.2 Bioenergy conversion rate
The rate constants for anaerobic digestion of native and treated sawdust were calculated by using standard rate equation. The plots of rate vs. concentration of biogas are represented in Fig. 9. The value of rate constant was calculated as 1.043 mL/gVS0.592 day−1 for biogas production from native sawdust, whereas in the biogas production from treated sawdust, it was found to be 1.066 mL/gVS0.502 day−1. The value of order of conversion was also increased from 0.408 to 0.498 after pretreatment. The increased rate constant and order signified the increase in rate of sawdust conversion into biogas. Pretreatment condition might have increased the accessibility of microorganisms to substrate by breaking the recalcitrance that in turn increased the production rate and yield of biogas.
4 Conclusions
The improved biogas yield was aimed from T. grandis sawdust. Pretreatment is the rate determining step when lignocellulosic wastes are used for biogas production through anaerobic digestion. Therefore, effect of various chemical reagents was evaluated on sawdust hydrolysis. NaOH was found to be most effective chemical reagent. A significant improvement was achieved in sCOD and phenolic content in all liquids followed by pretreatment in comparison to native sawdust sample. Alterations in crystallinity, chemical composition, and morphological structure for sawdust were also seen after pretreatment. Moreover, NaOH autoclave observed to be the best pretreatment among other pretreatments in order to reduce lignin content. The 10% NaOH autoclave pretreatment with 90 min of autoclaving time resulted in maximum (58.6%) delignification. The cumulative biogas yield at optimum condition was found to be 315-mL g−1 VS that was 50.8% more than native sawdust. The rate constant and order of bioconversion were also increased after pretreatment. Pretreatment of sawdust resulted in breakdown of rigidity by lignin solubilization, and hence, biogas yield was increased and bioconversion rate was improved.
References
Alexandropoulou M, Antonopoulou G, Fragkou E, Ntaikou I, Lyberatos G (2017) Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J Environ Manag 203:704–713
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy CombusSci 42:35–53
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Biorefin 6:465–482
Siqueira G, Arantes V, Saddler JN, Ferraz A, Milagres AMF (2017) Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol Biofuels 10. https://doi.org/10.1186/s13068-017-0860-7
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Conv Bioref 4:157–191
Lo’pez MJ, Sua’rez-Estrella F, Vargas-Garcı’a MC, Lo’pez-Gonza’lez JA, Verstichel S, Debeer L, Wierinck I, Moreno J (2013) Biodelignification of agricultural and forest wastes: effect on anaerobic digestion. Biomass Bioenergy 58:343–349
Lizasoain J, Rincon M, Theuretzbacher F, Enguídanos R, Nielsen PJ, Potthast A, Zweckmair T, Gronauer A, Bauer A (2016) Biogas production from reed biomass: effect of pretreatment using different steam explosion conditions. Biomass Bioenergy 95:84–91
Pellera FM, Santori S, Pomi R, Polettini A, Gidarakos E (2016) Effect of alkaline pretreatment on anaerobic digestion of olive mill solid waste. Waste Manag 58:160–168
Saritha M, Arora A, Lata (2012) Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol 52(2):122–130
Hu Y, Hao X, Wang J, Cao Y (2016) Enhancing anaerobic digestion of lignocellulosic materials in excess sludge by bioaugmentation and pre-treatment. Waste Manag 49:55–63
Behera S, Arora R, Nandhagopal N, Kumar S (2014) Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew Sust Energ Rev 36:91–106
Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, Cherchi F (2012) Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 46:25–35
Taherdanaka M, Ziloueia H, Karimia K (2016) The influence of dilute sulfuric acid pretreatment on biogas production from wheat plant. Int J Green Energy DOI 13:1129–1134. https://doi.org/10.1080/15435075.2016.1175356
Zhou S, Weimer PJ, Hatfield RD, Runge TM, Digman M (2014) Improving ethanol production from alfalfa stems via ambient-temperature acid pretreatment and washing. Bioresour Technol 170:286–292
Xu H, Li B, Mu X (2016) Review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion. Ind Eng Chem Res 55:8691–8705
Jaffar M, Pang Y, Yuan H, Zou D, Liu Y, Zhu B, Korai RM, Li X (2016) Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Chinese J Chem Eng 24:404–409
Chandra R, Takeuchi H, Hasegawa T, Kumar R (2012) Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energ 43:273–282
Carlsson M, Lagerkvist A, Morgan-Sagastume F (2012) The effects of substrate pretreatment on anaerobic digestion: a review. Waste Manag 32:1634–1650
Zawawi AZ, Gaik LP, Sebran NH, Othman J, Shah A (2018) An optimisation study on biomass delignification process using alkaline wash. Biomass Conv Bioref 8:59–68
Mancini G, Papirio S, Lens PNL, Esposito G (2018) Increased biogas production from wheat straw by chemical pretreatments. Renew Energy 119:608–614
Mustafa AM, Li H, Radwan AA, Sheng K, Chen X (2018) Effect of hydrothermal and Ca(OH)2 pretreatments on anaerobic digestion of sugarcane bagasse for biogas production. Bioresour Technol 259:54–60
Kaur K, Phutela UG (2016) Enhancement of paddy straw digestibility and biogas production by sodium hydroxide-microwave pretreatment. Renew Energ 92:178–184
Jin S, Zhang G, Zhang P, Li F, Wang S, Fan S, Zhou S (2016) Microwave assisted alkaline pretreatment to enhance enzymatic saccharification of catalpa sawdust. Bioresour Technol 221:26–30
Ehrman T (1994) Chemical analysis and testing, laboratory analytical procedures. http://infohouse.p2ric.org/ref/40/39182.pdf. Accessed 20 Sept 2015
BIS (1975) IS: 1350-2: Methods of test for coal and coke, part II: determination of calorific value. Bureau of Indian standards, New Delhi. https://archive.org/details/gov.in.is.1350.2.1975/page/n5. Accessed 20 Sept 2015
APHA AWWA (1998) Standard methods for the examination of water and wastewater, 14th edn. APHA, Washington DC
Singleton VL, Rossi JA (1965) Colorimetry of total phenolic with phosphomolybdicphosphotungstic acid reagent. Am J End Vitic 16:144–158
Van Soest PJ, Wine RH (1968) Determination of lignin and cellulose in acid detergent fiber with permanganate. J AssocOff Anal Chem 51:750–785
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Pellera FM, Gidarakos E (2018) Chemical pretreatment of lignocellulosic agroindustrial waste for methane production. Waste Manag 71:689–703
Michalska K, Bizukoj M, Ledakowicz S (2015) Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenergy 80:213–221
Wang D, Ai P, Yu L, Tan Z, Zhang Y (2015) Comparing the hydrolysis and biogas production performance of alkali and acid pretreatments of rice straw using two stage anaerobic fermentation. BiosystEng 132:47–55
Loow YL, Wu TY, Jahim JM, Mohammad AW, Teoh WH (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics. Adv Biochem Eng Biotechnol 108:67–93
Xu F, Yu J, Tesso T, Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: a mini-review. Appl Energy 104:801–809
Taherdanak M, Zilouei H (2014) Improving biogas production from wheat plant using alkaline pretreatment. Fuel 115:714–719
Fougere D, Nanda S, Clarke K, Kozinski JA, Li K (2016) Effect of acidic pretreatment on the chemistry and distribution of lignin in aspen wood and wheat straw substrates. Biomass Bioenergy 91:56–68
Lei M, Zhang H, Zheng H, Li Y, Huang H, Xu R (2013) Characterization of lignins isolated from alkali treated prehydrolysate of corn stover. Chinese J ChemEng 21(4):427–433
Jiangtao S, Dong X, Jian L (2012) FTIR studies of the changes in wood chemistry from wood forming tissue under inclined treatment. Energy Procedia 16:758–762
Ang TN, Ngoh GC, Chua ASM, Lee MG (2012) Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol Biofuels 5(67):1–10
Sua Y, Dua R, Guo H, Cao M, Wua Q, Sua R, Qi W, He Z (2015) Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: characterization of its major components. Food Bioprod Process 94:322–330
Pandeya KK, Pitman AJ (2003) FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int Bio Deterior Biodegrad 52:150–160
Zhang Q, Huang H, Han H, Qiu Z, Achal V (2017) Stimulatory effect of in-situ detoxification on bioethanol production by rice straw. Energy 135:32–39
Chen G, Chang Z, Zheng Z (2014) Feasibility of NaOH-treatment for improving biogas production of digested Spartina alterniflora. Int Biodeter Biodegrad 93:131–137
Kristiania A, Effendib N, Aristiawana Y, Auliaa F, Sudiyania Y (2015) Effect of combining chemical and irradiation pretreatment process to characteristic of oil palm’s empty fruit bunches as raw material for second generation bioethanol. Energy Procedia 68:195–204
Lima MA, Lavorente GB, da Silva HKP et al (2013) Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: a potentially valuable source of fermentable sugars for biofuel production—part 1. Biotechnol Biofuels 6(75):1–17
Barman DN, Haque MA, Kang TH, Kim GH, Kim TH, Kim MK, Yun HD (2014) Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis trinius) straw. Environ Technol 35:232–241. https://doi.org/10.1080/09593330.2013.824009
Mohtar SS, Busu TNZTM, Noor AMM, Shaari N, Mat H (2017) An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr Polym 166:291–299
Nargotra P, Sharma V, Gupta M, Kour S, Bajaj BK (2018) Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour Technol 267:560–568
Hesamia SM, Ziloueia H, Karimia K, Asadinezhadaa A (2015) Enhanced biogas production from sunflower stalks usinghydrothermal and organosolv pretreatment. Ind Crop Prod 76:449–455
Bjornsson L, Murto M, Mattiasson B (2000) Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl Microbiol Biotechnol 54:844–849
Monte LS, Escócio VA, de Sousa ANF (2018) Study of time reaction on alkaline pretreatment applied to rice husk on biomass component extraction. Biomass Conv Bioref 8:189–197
Dabir S, Cao M, Prosser R, Tsotsis T (2017) Feasibility study of biogas reforming to improve energy efficiency and to reduce nitrogen oxide emissions. Ind Eng Chem Res 56(5):1186–1200
Acknowledgments
The authors are grateful to the Department of Chemical Engineering and Technology and Central Instrument Facility Centre, Indian Institute of Technology (Banaras Hindu University), Varanasi, India for providing facilities and added valuable contribution to this work. The authors also acknowledge the financial support extended by the Ministry of Human Resource and Development, Government of India, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bala, R., Mondal, M.K. Exhaustive characterization on chemical and thermal treatment of sawdust for improved biogas production. Biomass Conv. Bioref. 8, 991–1003 (2018). https://doi.org/10.1007/s13399-018-0342-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0342-6