Abstract
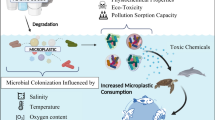
In freshwater environments, microbial assemblages attached to submerged substrates play an essential role in ecosystem processes such as primary production, supported by periphyton, or organic matter decomposition, supported by microbial communities attached to leaf litter or sediments. These microbial assemblages, also called biofilms, are not only involved in nutrients fluxes but also in contaminants dynamics. Biofilms can accumulate metals and organic contaminants transported by the water flow and/or adsorbed onto substrates. Furthermore, due to their high metabolic activity and their role in aquatic food webs, microbial biofilms are also likely to influence contaminant fate in aquatic ecosystems. In this review, we provide (1) a critical overview of the analytical methods currently in use for detecting and quantifying metals and organic micropollutants in microbial biofilms attached to benthic substrata (rocks, sediments, leaf litter); (2) a review of the distribution of those contaminants within aquatic biofilms and the role of these benthic microbial communities in contaminant fate; (3) a set of future challenges concerning the role of biofilms in contaminant accumulation and trophic transfers in the aquatic food web. This literature review highlighted that most knowledge on the interaction between biofilm and contaminants is focused on contaminants dynamics in periphyton while technical limitations are still preventing a thorough estimation of contaminants accumulation in biofilms attached to leaf litter or sediments. In addition, microbial biofilms represent an important food resource in freshwater ecosystems, yet their role in dietary contaminant exposure has been neglected for a long time, and the importance of biofilms in trophic transfer of contaminants is still understudied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
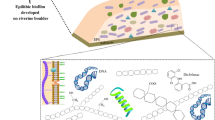
Freshwater environments host microbial biomass that can aggregate and attach to submerged inorganic (like rock, gravel, sediment) or organic (like leaf litter, macrophytes) substrates. These microbial assemblages, called “biofilms” (Watnick and Kolter 2000), are composed of eukaryotic (e.g., microalgae, fungi, protozoa) and prokaryotic (e.g., bacteria, cyanobacteria) microorganisms (Battin et al. 2016) whose form, distribution, and metabolism (i.e., autotrophy, heterotrophy, mixotrophy) are highly dependent, among other factors, on nature of the substrate (Fig. 1), light intensity, and availability of dissolved organic and inorganic nutrients (Sabater et al. 2006; Ylla et al. 2009). Autotrophic biofilms (or periphyton), which grow on inert surfaces like cobbles exposed to light, are generally dominated by diatoms, cyanobacteria, and green algae, whereas heterotrophic biofilms can be found attached to sediments or organic substrates like leaf litter and are dominated by bacteria and fungi. Biofilms are embedded within a self-produced matrix of extracellular polymeric substances (EPS) that is made up of (exo)polysaccharides and a variety of proteins, glycoproteins, and glycolipids together with high amounts of extracellular DNA (Flemming et al. 2007) and even suspended particulate matter and detritus from the surrounding environment (Flemming 1995). Biofilms are thus characterized by high structural complexity allowing multiple interactions with contaminants (Battin et al. 2003). In addition, due to their high metabolic activity and their role in aquatic food webs, microbial biofilms are likely to influence contaminant fate in aquatic ecosystems.
Schematic representation of the theoretical distribution of aquatic microbial biofilm communities according to the kind of immersed substrates under light conditions (adapted from Pesce et al. 2017)
Here we review the distribution of contaminants within aquatic biofilms and the role of these benthic microbial communities in contaminant fate. The contaminants we cover are metals (e.g., copper, mercury, cadmium, etc.) and organic micropollutants (e.g., pesticides, pharmaceuticals, and other man-made substances).
Diverse and ubiquitous contamination of lakes and rivers (e.g., Fent et al. 2006; Pal et al. 2010; Murray et al. 2010) exposes aquatic microbial biofilms to a potential accumulation of substances transported by the water flow (in a dissolved form or bound to suspended organic and inorganic matter) and/or adsorbed onto benthic substrates (sediment, leaf litter). Therefore, the kind of substrate where biofilms develop has a huge influence on their mode of exposure to contaminants (in terms of nature, quantity, bioavailability) as well as their role in subsequent contaminant transfers through aquatic food webs. However, investigations on this topic are fragmentary (i.e., one substrate/one contaminant) and difficult to unify in a common framework since they lack real representativeness for contaminant mixtures in complex systems harboring diverse substrates and/or microbial communities.
By their complexity, microbial biofilms can have multiple interactions with contaminants (Fig. 2) and therefore influence its fate in the environment. All microbial biofilms have the ability to neutralize contaminants by sorption (i.e., passive sequestration through interaction with biological matter), accumulation (i.e., increased active internalization in cells), and sequestration for metals (i.e., formation of insoluble precipitates through interaction with microbial metabolites) (Barkay and Schaefer 2001) or microbial transformation for organic substances (Edwards and Kjellerup 2013; Carles et al. 2017). All these interactions are susceptible to occur either within the cells or extracellularly within the EPS matrix. Microbial biofilms offer a diverse range of sorption sites including cationic and anionic sites as well as lipophilic/hydrophobic regions, as contaminants can bind to EPS, cellular membranes, cell walls, and more. In addition, enzymatic machinery required for contaminant transformation can either be present intracellularly or be excreted in the extracellular matrix. Metal distribution within microbial biofilms has been investigated for the last 25 years, and work continues with ongoing analytical developments. However, investigations into the sorption and/or accumulation of organic contaminants in microbial biofilms still run into technical limits, such as the large amount of biofilm needed to ensure reliable quantification of accumulated contaminants according to analytical level of detection.
Biofilm matrix features a high degree of microheterogeneity, which enables microbial biofilms to concurrently harbor a high diversity of contaminants (Flemming 1995). For instance, the presence of uronic acids (such as d-glucuronic, d-galacturonic, and mannuronic acids) was found to facilitate the sorption of various cationic metals (e.g., Pb2+, Cu2+) (Flemming 1995). Sorption of organic contaminants is partly driven by hydrophobic interactions, and so octanol-water partition coefficient (Kow) is often used to estimate the sorption capacity of organic contaminants in microbial biofilms. However, other types of interactions also occur (e.g., ionic, electrostatic, etc.) and thus need to be considered.
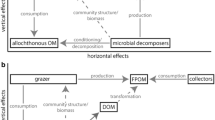
Microbial biofilms influence contaminant fate in aquatic ecosystems through their contribution to biotransformation processes and trophic transfers. Indeed, both phototrophic and heterotrophic biofilms are foundational to aquatic food webs. Phototrophic biofilms generate biomass from light energy and carbon dioxide, thus providing organic substrates and oxygen (Roeselers et al. 2008), while heterotrophic biofilms are able to decompose various organic materials and thus play a key role in nutrient fluxes in aquatic ecosystems (Romani and Sabater 2001; Battin et al. 2003). Accordingly, while periphytic biofilms are at the base of “green” food webs supported by primary production (Danger et al. 2008; Zou et al. 2016), biofilms formed on organic substrates play a functionally pivotal role in “brown” food webs based on allochthonous organic matter decomposition (Hall and Meyer 1998). The spatial proximity between autotrophic and heterotrophic microorganisms also drives carbon and nutrient cycling within periphytic biofilms where autotrophic biomass and activity can stimulate the development and activity of heterotrophic microbial communities (Romani et al. 2004). This same pattern has been observed in detritus-based food webs where primary production can stimulate leaf litter decomposition by microbial heterotrophs (Danger et al. 2013). In aquatic environments, “green” and “brown” food webs thus tend to connect through complex interactions (Zou et al. 2016). Biofilm assemblages play a key role in interconnecting between these “green” and “brown” food webs that are foundational to ecosystem functioning (Krumins et al. 2013; Zou et al. 2016) (Fig. 3). Whatever the substrate and food web involved, these biofilm assemblages are consumed by various microbial predators (e.g., amoeba, ciliate, rotifers; Neury-Ormanni et al. 2016) and meso-/macrofauna (Alvarez and Peckarsky 2005; Guasch et al. 2016) or fish (Schneck et al. 2013), which means that contaminants bioaccumulated in microbial biofilms are likely to be transferred to higher trophic levels in the food web (Singh et al. 2006).
During the last decade, research on the role of microbial biofilms in contaminant fate and transfer has mainly focused on metal accumulation in periphytic biofilms (e.g., Ancion et al. 2010; Fabure et al. 2015; Pesce et al. 2018) and on the degradation of organic contaminants in sediments (e.g., Pesce et al. 2009, 2013; Trinh et al. 2012). Nevertheless, recent studies have highlighted the potential role of periphytic and leaf litter biofilms in organic contaminant accumulation and trophic transfer (e.g., Kohušová et al. 2011; Ruhí et al. 2016). Furthermore, the daughter directive 2008/105/EU of the Water Framework Directive and Guidance Document No. 25 both recognize the importance of monitoring and preserving the sediment compartment to preserve aquatic ecosystems (European Commission 2010). However, scarce few studies have focused on contaminant accumulation in sediment microbial communities.
Scientific literature on contaminant bioaccumulation in microbial biofilms remain dispersed, insofar as most of the studies available are focusing on one type of contaminants (e.g., metals, pesticides, or pharmaceuticals) or on biofilm growing on one type of substratum (either cobbles, sediments, or leaf litter). An overview of the methods available to detect, identify, quantify, and localize contaminants accumulated in biofilms growing on different types of substrata is clearly missing. Such a methodological review is nevertheless helpful to identify conceptual and technological limitations as well as to guide method development effort. Since analytical methods are the first essential step to measure contaminant bioaccumulation in microbial biofilms, an overview of those methods and its specificities is essential to facilitate the accurate interpretation of bioaccumulation results.
In this context, the present review aims to provide a critical report of:
-
The analytical methods currently on use for detecting and quantifying contaminants in microbial biofilms developing in different benthic substrata (Sect. 2)
-
The current state of knowledge and the future challenges concerning the role of biofilms in contaminant accumulation (Sects. 3 and 5) as well as in trophic transfers in the aquatic food web (Sects. 4 and 5)
2 Analytical Methods for Quantification of Contaminants in Microbial Biofilms
A short overview of the current analytical methods used for quantification of metals and organic micropollutants in microbial biofilms is available in Table 1.
2.1 Metals
Concurrent to analytical developments, studies dealing with bioaccumulation in microbial biofilms have applied mainly to metal contaminants. Thus, in the early 1990s, methods such as atomic absorption spectrometry (AAS; Avery and Tobin 1993) or radiotracers (White and Gadd 1987; Avery et al. 1993) were developed in order to quantify metal (e.g., Zn, Fe, Cu, or Cs) concentrations in microorganisms (typically cyanobacteria, algae, or fungi). The appropriate method was selected according to different criteria, such as accuracy, sensitivity, amount of sample available, and the need or not to identify the location of the contaminant in the microorganism structure (White and Gadd 1995). The democratization of inductively coupled plasma optical emission and inductively coupled plasma mass spectrometry (ICP-OES and ICP-MS) allowing high-sensitivity analysis of several elements at the same time led to a large number of studies on Cu, Zn, Pb, and Cd bioaccumulation in microbial biofilms (e.g., Meylan et al. 2003; Farag et al. 2007; Bradac et al. 2009, 2010). The bulk of research on metal bioaccumulation in microbial biofilms was conducted on periphytic biofilms collected in situ on rock and/or gravel (e.g., Ancion et al. 2010) or on artificial substrates (low-density polyethylene membranes (Fechner et al. 2012); glass discs (Ivorra et al. 1999); glass slides (Morin et al. 2008)) beforehand immersed in the water column to allow biofilm colonization. These artificial substrates were also used in microcosm experiments to obtain enough biological material to combine metal analysis and toxicity tests under controlled exposure conditions (Fechner et al. 2011; Kim et al. 2012; Lambert et al. 2012).
To assess total metal concentration in the microbial biomass, hot (100°C) concentrated nitric acid digestion (by using a heating plate or a microwave oven) is commonly used to extract total metal content from samples that had previously been oven-dried at 50°C or freeze-dried (e.g., Morin et al. 2008; Fechner et al. 2012). To better discriminate between intracellular and extracellular metal bioaccumulation in microbial assemblage, biofilms are first flushed with a solution of ethylenediaminetetraacetic acid (EDTA) at 4 mM during 10 min (e.g., Meylan et al. 2004; Bradac et al. 2009; Arini et al. 2012; Fabure et al. 2015). This step removes the metals adsorbed to cell membranes and a fraction of the inorganic complexes in the biofilm structure (Meylan et al. 2003). The amount of intracellular metal content in the microbial assemblage is then deduced by analyzing the two fractions (raw and EDTA-washed) of a sample. To better identify metal (Al, Cu, Zn, and Pb) site within the EPS matrix and better characterize the exposure of microbial cells to metals, Aguilera et al. (2008) proposed subsequent extractions and centrifugation steps to separate first the “colloidal fraction” (extracted with distilled water), then the “capsular fraction” (extracted with NaCl at 80°C, ultrapure water at 30°C, Dowex at 4°C, or crown ether at 4°C), and finally the cellular debris from microbial biofilms. Metal content can then be quantified independently in each of the three collected fractions to determine the amount of intracellular metal (in the cellular debris) as well as the metal concentration in the EPS matrix (in the colloidal and capsular fractions). The colloidal fraction includes carbohydrates and proteins that are loosely bound to microorganisms, whereas the capsular fraction contains tightly bound compounds. However, Aguilera et al. (2008) showed that no single extraction method was able to extract all the potential EPS components with the same efficiency.
There have been several developments to assess metal accumulation and distribution in freshwater periphytic communities, but none in microbial communities from sediment or from leaf litter. Indeed, studies dealing with metal contamination and microbial communities associated to these substrates are only based on total analysis of the metal in the whole sediment (e.g., Farag et al. 2007; Kohušová et al. 2011) or leaf litter (e.g., Sridhar et al. 2008; Schaller et al. 2011), including attached biofilms. The analytical procedures commonly used include a first step of extraction with aqua region or nitric acid, for instance, on fresh or dried sediment/leaf litter. Then, metal concentrations in the extracts are measured by conventional analytical methods (ICP-OES, ICP-MS, or AAS). To date, and to the best of our knowledge, no study has been conducted to separate and specifically assess metal concentrations in microbial communities from sediment or leaf litter.
Besides traditional fractionation and extraction methods, imaging techniques have been developed to investigate interactions between metals and biofilms and to visualize metals within the biofilm structure. Analytical electron microscopy techniques such as transmission electron microscopy (TEM), often coupled with energy-dispersive X-ray spectrometry, have been used to identify metal bioaccumulation in microbial biofilms, in particular in bioremediation studies in which biofilms are used as a sink to accumulate metals from contaminated waters (Mattila et al. 1997; Miller et al. 2012). For example, this technique allowed Vilchez et al. (2011) to show that Cr(III) bioaccumulated in the EPS matrix of microbial biofilms, while Pb(II) was detected in both the EPS matrix and the microbial biofilm cells. In order to determine macromolecules and metals composition in EPS from microbial biofilms, TEM can also be coupled to electron energy loss spectrometry, which is a complex technique for measuring atomic composition and chemical binding and speciation, even for lower elements (C, O). The main drawback of TEM is that sample preparation often requires dehydration, creating artifacts such as particle shrinkage or aggregation (Dynes et al. 2006a). Furthermore, the high energy of TEM causes radiation damage in biological samples, which leads to spectral distortions, making high-resolution mapping difficult (Hitchcock et al. 2008).
Other imaging techniques such as confocal laser scanning microscopy (CLSM) and scanning transmission X-ray microscopy (STXM) are especially well suited for biofilm studies as they can be applied to fully hydrated biological materials and reduce radiation damage (see Neu et al. 2010 for a comparative review on those techniques applied to biofilms). In CLSM, metal binding to specific fluorescent probes allows detection and localization of those contaminants within biofilms (for a description of the different probes available, see the review by Hao et al. 2013). In particular, the metal-sensitive probe Newport Green has been successfully used to investigate Ni and Zn bioaccumulation in river biofilm (Wuertz et al. 2000; Lawrence et al. 2019). The combination of different probes can be particularly useful to compare localization of various metals on microbial aggregates (Hao et al. 2016). STXM, which uses near-edge X-ray absorption fine structure as the contrast mechanism, provides spatially resolved quantitative information on the distribution of elements, macromolecules, and redox states in the biofilm matrix (Lawrence et al. 2003; Behrens et al. 2012). According to Lawrence et al. (2016), STXM is “capable of mapping the biochemical composition of bacteria and biofilms at the subcellular scale […] as well as speciation of metals.” Comprehensive reviews on STXM applied to biofilms have found that it holds relevancy for investigating metal (Cu, Fe, Mn, Ni) speciation in biofilm matrices (Neu et al. 2010; Behrens et al. 2012). Dynes et al. (2006a) used STXM to highlight the close association of Ni with Mn-oxides and the role of EPS in the sequestration of metals in aquatic microbial biofilms. This technique also allowed Lawrence et al. (2012, 2016) to follow the dissolution and fate of Cu nanoparticles (Lawrence et al. 2012) and the fate and speciation of Ce, TiO2, and Cu in river biofilms (Lawrence et al. 2016). Yang et al. (2016) characterized the biotransformation of selenium oxyanions by biofilms using STXM and X-ray fluorescence imaging (at higher energies than STXM). Finally, STXM image sequences revealed that Fe localization (on the cell surface or within the EPS matrix) was speciation-dependent in a monospecific biofilm of Pseudomonas aeruginosa (Hunter et al. 2008). The main limitations of the STXM techniques are their low sensitivity and limit of resolution (25–50 nm against 4 nm for TEM techniques). Indeed, to our knowledge, very few STXM studies have been conducted at environmentally relevant concentrations: one study reports results on Mn, Fe, and Ni with water concentrations ranging from 0.01 to 0.02 mg Mn L−1, 0.02 to 0.06 mg Fe L−1, and 1 to 10 mg Ni L−1, respectively (Hitchcock et al. 2009).
The combination of different imaging techniques remains essential to determine metal distribution in biofilms and better understand the interactions between metals, cellular components, and extracellular material (van Hullebusch et al. 2003). In a recent study, Lawrence et al. (2019) combined CLSM with different fluorescent probes, scanning electron microscopy, and X-ray microprobe analyses to show that Ni was mainly associated to EPS in biofilm and was four times more concentrated around specific microcolonies than in the rest of the microbial community.
2.2 Organic Contaminants
In contrast to the substantial research on metal distributions within periphytic biofilms, there is a dearth of studies dealing with the accumulation in microbial assemblages of organic contaminants. These studies mainly focus on pesticides, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and more recently pharmaceuticals, hormones, and parabens. And the few studies published fail to detail analytical methods for the quantification of organic contaminants in the biofilm following both laboratory and in situ exposure (Schorer and Eisele 1997; Headley et al. 1998; Lawrence et al. 2001; Writer et al. 2011; Kohušová et al. 2011; Ruhí et al. 2016). Due to the high complexity of the biofilm matrix, nonselective solvent extraction methods generate analytical interferences; therefore biofilm contamination by organic contaminants is preferably estimated indirectly from contamination in water (Wang et al. 2002; Proia et al. 2013a, b). To directly measure the concentration of organic contaminants in periphytic biofilms, samples are first collected from the surfaces of stones or artificial substrates and then freeze-dried before analysis (Wang et al. 1999; Huerta et al. 2016). Organic contaminants are commonly extracted from the whole biofilm matrix without fractionation to avoid matrix destruction and cell lysis. However, Chaumet et al. (2019a) recently proposed a physical extraction method to separate the diffusible from the cell-bound EPS and microorganism fractions to further measure pesticide concentrations accumulated in each of these two fractions. Solvent extraction of organic contaminants from dried biofilm is then performed by pressurized liquid extraction (Writer et al. 2011; Huerta et al. 2016), ultrasonic extraction (Headley et al. 2001), shaking extraction (Wang et al. 1999; Du et al. 2015), or Soxhlet extraction (Schorer and Eisele 1997). Organic extracts are sometimes purified by solid-phase extraction (Coat et al. 2011; Writer et al. 2011; Du et al. 2012, 2015) or directly analyzed by chromatographic techniques (Headley et al. 2001). Liquid or gas chromatography coupled to mass spectrometry (LC-MS or GC-MS, depending on the compounds) is preferred to achieve the requisite selectivity and sensitivity (Coat et al. 2011; Dobor et al. 2012; Du et al. 2012; Huerta et al. 2016; Ruhí et al. 2016). As an example, liquid chromatography with UV detection was not sufficiently sensitive for analysis of N-methyl pyrrolidinone in biofilm extracts with a limit of quantification (LOQ) at 100 ng g−1, whereas LC-MS was able to reach a LOQ of 2 ng g−1 (Headley et al. 2001; Huerta et al. 2016). While current identification and quantification methods generally include extraction and purification steps, Headley et al. (1995) proposed in the 1990s a method based on the direct injection of a small biofilm sample using an insertion probe. Subsequent detection and identification of contaminants and metabolites were performed by tandem mass spectrometry (MS-MS) (Headley et al. 1995). Unfortunately, the absence of separation of biofilm components prior to sample introduction in the ion source induced interferences that limit the detection of a wide range of contaminants at low-level concentration.
As for metal detection (Sect. 2.1), imaging techniques, such as CLSM and STXM (Neu et al. 2010), have been successfully used to investigate organic contaminant bioaccumulation in biofilms (Lawrence et al. 2001, 2016; Dynes et al. 2006b). These nondestructive imaging techniques were applied on hydrated biofilm samples and allowed direct observation of contaminants localization in the complex structure of preserved biofilms. Fluorescence was usually used to detect contaminant with CLSM, either by investigating fluorescent contaminants (Wolfaardt et al. 1994) or by using specific probes targeting the contaminant investigated (Lawrence et al. 2001). Contaminant identification by STXM was probe-independent and based on comparison with suitable reference spectra (Neu et al. 2010). Thus, coupling CLSM with monoclonal antibodies specific to atrazine, Lawrence et al. (2001) showed that bioaccumulation of this pesticide in river biofilms resulted from atrazine sorption to specific microcolonies. Using STXM, Dynes et al. (2006b) revealed differences in bioaccumulation patterns of the antimicrobial chlorhexidine between pennate and centric diatoms within a complex microbial biofilm. To our knowledge, STXM has not been used for quantification of organic contaminant accumulation in biofilms; however the optical density obtained by STXM reflected the amount of contaminant and could therefore be used for relative comparison between samples (Dynes et al. 2006b). Up to now, these techniques have only been applied on complex microbial biofilms exposed in laboratory at relatively higher concentrations of organic contaminants than those found in the aquatic environments.
Only a few studies have reported on the evaluation of analytical method performances such as recoveries and LOQ (Coogan et al. 2007; Huerta et al. 2016) or matrix effects (Headley et al. 2001; Ruhí et al. 2016). The relatively low amounts of contaminants sorbed on biofilms (trace levels) and the limited biomass collected (often <200 mg) mean that LOQ values are generally in the same order of magnitude as the measured concentrations. As an example, Huerta et al. (2016) reported LOQs from 0.07 to 6.7 ng g−1 for pharmaceuticals (in 200 mg of phototrophic biofilms), whereas most of the values measured in biofilm samples were between 1.8 and 22 ng g−1. Matrix effects due to the specificity of the analyzed biofilm are also under-addressed. To illustrate, when Huerta et al. (2016) compared analyses of biofilm extracts against the initial solvent mixture spiked with different contaminants at the same concentration level, they observed either ion suppression or ion enhancement depending on organic contaminant, thus demonstrating that concentrations values in biofilm extracts can be biased (i.e., over- or underestimated) due to matrix effects. For quantification purposes, matrix-matched calibration together with added internal surrogates (ideally labeled compounds) is thus advocated to compensate for these matrix effects (Huerta et al. 2016; Ruhí et al. 2016).
3 Contaminant Bioaccumulation in Microbial Biofilms from Freshwater Ecosystems
Microbial biofilms can adsorb and accumulate both metals and organic contaminants. The nature of the contaminant, the surrounding environmental conditions, and the type of biofilm have all been found to influence contaminant bioaccumulation patterns. Note that bioaccumulation cannot be apprehended in the same way in periphytic biofilms, which are easily detached from their growth substrate, nor in biofilms strongly attached to leaf litter or fine detritus in sediments, which makes it difficult to specifically quantify the contaminants accumulated in the microbial biomass.
3.1 Contaminant Bioaccumulation in Periphytic Biofilms
Natural periphytic biofilms have been found to host a large variety of contaminants. Current knowledge on bioaccumulation in periphyton is illustrated and discussed here based on a meta-analysis of 24 published studies (Table S1). The data collected gather field and laboratory experiments including simultaneous quantification of contaminants in water and periphyton (Fig. 4) with biofilms sampled at various stages of maturity. Most of this data comes from chronic exposures, but some pulsed exposures are also included. To estimate uptake efficiency, bioconcentration factors (BCFs) were used as a proxy and were calculated as the ratio between the concentration measured in the biofilm and the dissolved concentration in the medium (Wang et al. 1999) (see Fig. 5 and Table S1 for details). BCF calculations rely on accurate estimation of chemical concentrations in both surface water and biofilm and are thus strongly influenced by LOQs – for instance, high LOQs in water could lead to an overestimated BCF (Arnot and Gobas 2006).
Concentration of contaminants in periphytic biofilms in μg g−1 of biofilms (dry weight) (n = 400), data from 24 published studies. Plain circles stand for observations from the field, stars for observations from laboratory experiments. PAHs polycyclic aromatic hydrocarbons, PCBs polychlorinated biphenyls, HCH hexachlorocyclohexane, TBEP tris(butoxyethyl)phosphate, DCPU N-(3,4-dichlorophenyl) urea, DCPMU N-(3,4-dichlorophenyl)-N-(methyl) urea, DDTs dichlorodiphenyltrichloroethane
Bioconcentration factor, expressed as log(BCF), for periphytic biofilms (n = 304), data from 22 published studies. Plain circles stand for observations from the field, stars for observations from laboratory experiments. PAHs polycyclic aromatic hydrocarbons, PCBs polychlorinated biphenyls, HCH hexachlorocyclohexane, TBEP tris(butoxyethyl)phosphate, DCPU N-(3,4-dichlorophenyl) urea, DCPMU N-(3,4-dichlorophenyl)-N-(methyl) urea, DDTs dichlorodiphenyltrichloroethane
In periphyton, the bioaccumulation of any type of contaminant results from a dynamic process (Chaumet et al. 2019a, b). The concentration of a contaminant within periphyton tends to an equilibrium between uptake, elimination, and biotransformation and is also influenced by growth-related dilution effects. Contaminant uptake in biofilm depends strongly on the nature of the chemical (metal vs. organic) and on the 3D architecture and composition of the biofilm. First, contaminants enter EPS via passive diffusion and can then either be accumulated/transformed in this extracellular matrix or taken up within the cells (by passive diffusion and facilitated or active transport). The complex composition of the EPS matrix of biofilms offers many adsorption sites for both polar and hydrophobic contaminants (Schorer and Eisele 1997; Flemming and Wingender 2001). Biofilms take up both metals and organic contaminants that can then be stored (extra- or intracellularly) and/or transformed by the community. Periphytic biofilms can accumulate very high concentrations of metals, particularly aluminum, iron, and zinc, which can be found at up to 24–28 mg g−1 of dry biofilm (Fig. 4, Table S1). Advanced analytical methods, including imaging techniques (see Sect. 2), have afforded a relatively precise mapping of metals in periphyton, which can be found in different microenvironments of periphytic biofilms depending on the metal form/speciation and the characteristics of the microenvironment. Precipitates can be found in the biofilm matrix at the cell surfaces (Brown et al. 1998), while positively charged metal ions can accumulate in negatively charged cell walls and EPS. Metal speciation is also reported to influence the site of metal bioaccumulation (bound to membrane vs. EPS; Hunter et al. 2008). Biofilms have also evolved enzymatic mechanisms of metals reduction (Lloyd 2003) or metal sequestration via thiol-rich polypeptides known as phytochelatins able to sequester excess intracellular metals in a stable, detoxified form (e.g., Lavoie et al. 2012). Depending on the metal and environmental conditions, they are able to store and concentrate large amounts of metals that are potentially transferable to higher trophic levels.
As stated earlier, there is less data available on the bioaccumulation of organic contaminants in periphytic biofilms. However, the pattern seems to be that these contaminants tend to accumulate at lower final concentrations (Fig. 4), but some more efficiently (i.e., with higher BCFs), than metals (Fig. 5, Table S1). These differences could be driven by differences in exposure concentrations; although concentrations of organic contaminants in surface water were not often reported, they were generally found at lower levels than for metals. Thus, in studies for which concentrations in both biofilms and surface water were reported, all median dissolved concentrations of metals were superior to 1 μg L−1 (except for mercury), while the median dissolved concentrations for all the organic contaminants studied were below 1 μg L−1. Organic contaminants tend to get adsorbed to the organic matter trapped in the biofilm, EPS, and cell membrane due to their specific chemical properties and high lipid content (Wolfaardt et al. 1998; DeLorenzo et al. 2001; Métivier et al. 2013). The bioaccumulation of organic contaminants in biofilms is driven by their hydrophobicity, which can be estimated by their partition coefficient between octanol and water, called log Kow (Fig. 6). Log Kow correlates positively with the log BCFs of organic contaminants (Pearson correlation coefficient; n = 70; r2 = 0.42, p < 0.05), and models have been developed to predict contaminant bioaccumulation in biofilms based on physical-chemical properties (Ruhí et al. 2016). Besides their hydrophobicity, organic contaminants possess other specific characteristics than can also help them bioaccumulate. For instance, the highest BCF values were observed for halogenated contaminants such as hexachlorobenzenes (BCF up to 147,000 L g−1), PCBs (BCF up to 56,000 L g−1), or per- and polyfluoroalkylated substances (BCF up to 10,000 L g−1) (Fig. 5, Table S1), suggesting that the compounds’ high electron affinity with living cells is also strongly involved in its accumulation. Nevertheless, validation of this hypothesis remains bottlenecked by technical limitations, as the quantification of intracellular organic contaminants in microbial biofilms is not yet possible (Sect. 2.2). Non-organochlorine pesticides and pharmaceuticals measured in periphytic biofilms generally show lower BCFs (BCF < 3.6 L g−1) than metals and fluorinated or organochlorine contaminants (e.g., DDT, dichlorodiphenyltrichloroethane; BCF up to 78,550 L g−1). The lower concentration of these contaminants in periphytic biofilms could also be explained by different biodegradation processes for different substances. For instance, studies have shown that periphytic microbial communities can partially transform and/or mineralize pesticides (e.g., the phenylurea herbicide diuron (Pesce et al. 2009) and glyphosate (Carles et al. 2019)) and antibiotics (e.g., the sulfonamide antibiotics sulfamethazine and sulfamethoxazole (Vila-Costa et al. 2017)). Metabolites are thus sometimes found in biofilms (e.g., atrazine metabolites (Lawrence et al. 2001) or glyphosate metabolites such as aminomethylphosphonic acid (Carles et al. 2019)), but it is not always possible to discriminate those produced through biodegradation by the biofilm itself from those that were already present in the water column before being bioaccumulated. Additionally, the lower BCFs observed for the non-organochlorine pesticides (i.e., glyphosate, diuron, and its metabolites) could also be explained by higher exposure concentrations since those values were applied in laboratory experiments (contrary to BCF values for pharmaceuticals obtained in the field); further field study investigating pesticides bioaccumulation in natural periphytic biofilms is therefore required to confirm those first observations.
Bioconcentration factor, expressed as log(BCF) of organic contaminants in periphytic biofilms vs. log Kow of the accumulated contaminants (n = 70). Linear regression: log(BCF) = 0.51 ∗ log Kow − 1.21 (r2 = 0.42, p < 0.01). Data from nine published studies. Data points circled in red are observations from laboratory experiments; all other points are observations from field studies. DCPU N-(3,4-dichlorophenyl) urea, DCPMU N-(3,4-dichlorophenyl)-N-(methyl) urea, TBEP tris(butoxyethyl)phosphate
Each step of the bioaccumulation process is influenced by a number of factors such as exposure duration and concentration, physical-chemical conditions, and biofilm composition. Hydrology and geomorphology are also likely to influence bioaccumulation especially by driving contaminants repartition between aquatic compartments (surface water, particulate matter, sediment, periphytic biofilm).
In the dataset analyzed, exposure duration was not significantly correlated with metal BCFs, which argues for fast adsorption of metals in the biofilm matrix. Among the metals considered, aluminum had the highest uptake efficiency even at low exposure concentrations (BCF up to 31,800 L g−1), irrespective of exposure duration (1–35 days). Corcoll et al. (2012) hypothesized that the amount of Al accumulated was thus “background” content for the biofilms studied. In contrast, Pb accumulation above a certain exposure concentration (exceeding more than ten times the criteria for chronic exposure concentration as defined by the US Environmental Protection Agency) dropped strongly with log BCF < −1. Exposure concentrations influence metal bioaccumulation; thus a positive correlation (Pearson correlation coefficient; n = 238; r2 = 0.28, p < 0.05) was found between metal concentration in biofilm and dissolved metal concentrations (Fig. 7). Nevertheless, our database, in line with several studies, also highlighted that BCF in biofilms is not always positively correlated with exposure concentrations. Indeed, logBCFs tend to decrease with increasing dissolved metal concentration in particular in laboratory experiments (Fig. S1) questioning the pertinence of using this parameter as a proxy of exposure. Indeed, metal speciation, including complexation, in the dissolved fraction can influence bioaccumulation (Meylan et al. 2004; Bradac et al. 2009, 2010; Dranguet et al. 2017). Thus, Meylan et al. (2004) showed that Zn accumulation in periphytic biofilm was mainly driven by dissolved Zn concentrations, while weakly complexed Cu controlled its bioaccumulation in microbial biofilms. High amounts of suspended metal-contaminated particulate matter can also get entrapped directly by the biofilm matrix, thus driving further accumulation of large additional amounts of metals (Morin et al. 2008). Therefore, metal BCF calculations based on dissolved concentrations only may underestimate the correlation between exposure and bioaccumulation values (intracellular content and BCF).
Discrepancies between exposure, measured as dissolved concentrations, and bioaccumulation, measured as BCF, were also found for some organic contaminants. A study on per- and polyfluoroalkylated substances by Munoz et al. (2016) found inverse correlations between exposure concentrations and BCFs along a gradient of contamination concentrations in the Seine River. This divergence could also be the consequence of a saturation of cellular binding sites at high exposure concentrations, with a possible influence of competition between contaminants in mixtures. Indeed, competitive sorption is likely to occur in the environment due to the co-occurrence of multiple contaminants in surface waters. For instance, the accumulation rate of the organosulfur fungicide isoprothiolane in two microalgae (Scenedesmus quadricauda, Aulacoseira granulata) and one cyanobacterium (Microcystis aeruginosa) decreased in presence of other pesticides (the herbicide p-nitrophenyl 2,4,6-trichlorophenyl ether and the insecticide O,O-dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate) in the mixture (Guanzon et al. 1996). This phenomenon is more likely to occur in laboratory experiments in which biofilms are usually exposed to higher concentrations than those found in the environment. Indeed, in our dataset BCF calculated from laboratory experiments were generally lower than those from field experiment (Figs. 5, 6 and S1).
The influence of environmental factors on metal accumulation in periphytic biofilm has been reviewed by Guasch et al. (2010). In particular, metal speciation is influenced by a range of physicochemical factors (including pH, salinity, and nutrients), affecting their bioavailability (Meylan et al. 2003) and subsequent accumulation and toxicity for microbes. Biofilm characteristics (community composition, biomass, organic matter content, EPS content) can also influence the bioavailability and therefore the accumulation and toxicity of contaminants (Berglund 2003; Berglund et al. 2005; Lambert et al. 2016; Pesce et al. 2018). In river biofilms, the sorption of certain contaminants such as triazines or metals has been attributed to specific bacterial colonies producing an EPS matrix with a unique composition (Lünsdorf et al. 1997; Lawrence et al. 2001). A change in community composition can modify lipid content and therefore influence the accumulation of organic compounds such as PCBs according to their high log Kow value (Wang et al. 1999). Finally, toluene accumulation in a bacterial biofilm has been shown to increase negatively charged carboxyl groups in EPS and might thus enhance biofilm ability to accumulate cations such as metal ions (Schmitt et al. 1995).
Through their capacity to uptake contaminants from the surface water, periphytic biofilms can also be viewed as passive samplers of contaminants in surface waters. This has prompted the idea that identifying and quantifying the contaminants accumulated within the biofilm could be a monitoring strategy for surveillance of aquatic ecosystem contamination by both organic contaminants (e.g., PAHs; Froehner et al. 2012) and metals (Leguay et al. 2016). However, following equilibrium partitioning theory, contaminants accumulated in biofilms are also likely to diffuse back in the water when their dissolved concentration has dropped. Sorption and desorption kinetics have been studied for some contaminants such as PCBs and PAHs (Bertini 2016) and for a handful of pesticides (Headley et al. 1998) and antibiotics (Wunder et al. 2011). Therefore, due to the dynamic processes involved in contaminant accumulation in biofilms and the potential influence of many abiotic and biological parameters, the use of bioaccumulation in biofilms as an indicator of contamination has been challenged, in particular for organics (Bertini 2016). Further investigations are needed to better determine the timeframe integrated by contaminant accumulation in biofilms as a function of chemical, biological, and environmental properties.
3.2 Contaminant Bioaccumulation in Sediment or Leaf Litter Microbial Communities
Up to now, data on in situ bioaccumulation of metals and organic contaminants in submerged microbial communities associated with sediments, leaves, or drift particulate matter has always included both biotic accumulation and abiotic sorption on the substratum. As stated earlier, this is partly due to the fact that microbial communities cannot be easily detached from these substrates and that the microbial biomass obtained is still very limited (and not sufficient for chemical analyses).
The distribution of organic contaminants in the different river compartments (water, sediment, and leaf litter) is influenced by the hydrology and geomorphology of the system as well as by the physical and chemical properties of the contaminant. For instance, suspended and bed sediments in the San Joaquin River and its tributaries (in one of the most productive agricultural regions of the USA) serve as a sink for hydrophobic contaminants (e.g., PAHs, DDT), whereas water-soluble herbicides (e.g., atrazine, simazine, dimethyl tetrachloroterephthalate) are mostly present in the dissolved phase of the water column (Pereira et al. 1996). A similar pattern of pesticide distribution has been observed in rivers in Europe (e.g., Fernandez et al. 1999) and Asia (e.g., Chen et al. 2006). Pesticide dissipation in water can be enhanced or reduced by the presence of sediments and according to the properties of pesticide molecules (Laabs et al. 2007). Sediments can also accumulate pharmaceuticals. An extensive study in four Spanish rivers (Ebro, Llobregat, Júcar, and Guadalquivir) highlighted the presence of endocrine disruptors accumulated in sediments at concentrations up to 7 ng g−1 (Gorga et al. 2015). Similar levels of the hormone β-estradiol were also quantified in sediments of the River Ouse (UK) (Labadie and Hill 2007), whereas lower levels were reported in three rivers in the Tianjin area (China) (Lei et al. 2009). Several antibiotics from urban sources and aquaculture activities (e.g., sulfamethazine, sulfamethoxazole, norfloxacin, among others) have been detected in the sediments of the Pearl River Estuary (South China) at concentrations ranging from 1 to 8 ng g−1 (Liang et al. 2013). While contaminant accumulation in sediments contributes to the removal of toxic substances from the surface, this apparent remediation is only temporary since those contaminants can later be remobilized following changes in redox conditions leading to the redissolution from sediment and diffusion from pore water and/or during intense hydrological events, as shown by Domagalski et al. (2010) for pyrethroid insecticides in different rivers. Flash-flood events in the Ebro river basin were also found to mobilize huge amounts of hexachlorobenzene, DDT, and PCBs largely exceeding existing regulatory reference values established for sediments (Quesada et al. 2014).
Sediments are also recognized as an important sink of metals in freshwater environments. Indeed, numerous studies have reported the accumulation of metal contaminants in sediments from various kinds of ecosystems including streams (e.g., Rodrigues and Formoso 2006), estuaries, and large rivers (e.g., Hamzeh et al. 2016), artificial reservoirs (e.g., García-Ordiales et al. 2016), and natural lakes (e.g., Gascón Díez et al. 2017) all over the world, from Europe (e.g., Thevenon et al. 2011) to the USA (e.g., Garvin et al. 2017), South America (e.g., Smolders et al. 2003), Asia (e.g., Liao et al. 2017), and Africa (e.g., Kilunga et al. 2017). While anthropogenic activities can explain part of this contamination, metals are also naturally present in sediments as geogenic particulate components (Ho et al. 2013). The ubiquity of metal contamination in freshwater sediments is illustrated in a report summarizing the results of an extensive chemical survey (567 sampling stations) designed to measure metal concentrations in sediments from various fluvial ecosystems dotted across France (INERIS 2010). The reported median, average, and maximum concentrations (in mg kg−1 dry weight sediment) were, respectively, 7.3, 12.4, and 1,005 for As; 0.7, 10.2, and 7,285 for Cd; 36.0, 52.1, and 5,300 for Cr; 21.7, 48.5, and 4,330 for Cu; 0.1, 1.2, and 200 for Hg; 19.0, 26.8, and 2,380 for Ni; 32.6, 122.0, and 50,420 for Pb; and 130.0, 446.0, and 142,500 for Zn (INERIS 2010).
Comparatively, there has been less effort to investigate organic and inorganic contaminant accumulation in submerged leaf litter. This could be explained by the ephemeral presence of the substratum in the ecosystem but also by the fact that studies addressing contamination gradients are mostly focused on downstream contaminated sections where riparian vegetation is often poor. However, leaf litter has been proven to adsorb metals (Sridhar et al. 2001) and a range of herbicide and fungicide molecules (Passeport et al. 2013; Vallée et al. 2014; Rossi et al. 2018). The sorption potential of these contaminants on leaf substrates may depend on their stage of decomposition (e.g., Dimitrov et al. 2014 for the fungicide tebuconazole). Leaf litter accumulated in rivers has comparatively similar (and/or greater) pesticide adsorption capacities to sediments (Margoum et al. 2006; Passeport et al. 2011). Vallée et al. (2014) revealed that straw has greater retention potential than sediments and soils for three herbicides and three fungicides in constructed wetlands. These results show the importance of organic carbon content and nature in the pesticides sorption process. A tracer injection experiment was conducted in the field in a “wet” forest buffer zone to test its potential for reducing loads of glyphosate, isoproturon, metazachlor, azoxystrobin, epoxiconazole, and cyproconazole (Passeport et al. 2014). Results confirmed that leaf litter layer thickness was a key parameter that influences the potential for delaying and reducing pesticide transfers and increasing their degradation.
As observed for periphytic biofilms and discussed above, bioaccumulation of organic contaminants in sediments, leaves, or drift particulate matter can be influenced by substratum characteristics and/or environmental factors. A field study in the Pearl River Estuary (South China) found that sediment total organic carbon and water pH were the most important factors influencing the dynamics of distribution of the antibiotics norfloxacin and erythromycin between water and sediments, respectively (Liang et al. 2013). Different environmental parameters, including pH and temperature, were also shown to be important drivers of metal accumulation in sediments (Lin and Chen 1998; Saeedi et al. 2011; Li et al. 2013), in which the sorption, release, and transport of metals are important processes influencing the chemical quality of water bodies (Tao et al. 2005; Fan et al. 2007).
After sorption to sediments and leaf litter material, organic contaminants can be partially or totally biodegraded by the attached microbial communities. Thus, two species of aquatic hyphomycetes (Heliscus lugdunensis, Clavariopsis aquatica) typically associated with submerged leaf litter were found to biotransform 1-naphthol (Augustin et al. 2006) or technical nonylphenol (Sole et al. 2008). Recently, leaf-associated communities from sites downstream of agricultural areas were shown to exhibit a greater potential to degrade the maize herbicide nicosulfuron compared to those from upstream communities, and this was partly explained by the pre-exposure history of these communities to the contaminant (Carles et al. 2017). In these microbial communities, Carles et al. (2018) isolated an ascomycete fungus (Plectosphaerella cucumerina AR1) capable of transforming nicosulfuron into its two major metabolites (2-amino-4,6-dimethoxypyrimidine and 2-(aminosulfonyl)-N,N-dimethyl-3-pyridinecarboxamide) in the presence of glucose. Microbial biofilms from river sediments have also been shown to degrade nonylphenol (Wang et al. 2014) and the herbicides diuron (Pesce et al. 2009) and isoproturon (Trinh et al. 2012), among others.
3.3 Contaminant Distribution in Different Kinds of Biofilms and Potential Contribution to Trophic Transfer in Aquatic Ecosystems
Periphytic, sediment, and leaf litter biofilms can all act as both sink and source of contaminants for the aquatic ecosystem. Only a few studies have set out to investigate in situ the relationship between contaminant concentrations in periphytic biofilms and in sediments in contaminated rivers, and the focus has been exclusively on metals (Farag et al. 1998, 2007; Holding et al. 2003; Kohušová et al. 2011; Ancion et al. 2013). Conceptually, any sound assessment of contaminant bioaccumulation in microbial biofilms and its consequences on trophic transfers of contaminants in aquatic ecosystems needs to account for the ecological dynamics affecting each type of aquatic biofilm. Periphytic biofilms follow a several-stage life cycle from colonization and co-adhesion to final detachment (due to biological mechanisms and/or physical constraints), and so contaminant bioaccumulation in these microbial communities can be viewed as transient. Bioaccumulation of contaminants on leaf litter is also transient (according to the leaf litter decay rates) and seasonally specific as it depends on the availability of plant litter (e.g., litter fall peaks in autumn in temperate rivers). In sediments, contaminants are accumulated in microbial biofilms attached to sediment but also complexed with inert material. The specific bioaccumulation of microbial biofilms in this accumulation cannot be, however, distinguished from the total accumulation in sediments because of methodological difficulties in discriminating the biotic from the abiotic fraction of sediments. Nevertheless, due to both physicochemical and biological characteristics, sediments can store contaminants for much longer, and can thus be viewed as a more stable compartment, even if disruptions (flood events, changes in pH or redox potential, etc.) can trigger releases.
By taking up contaminants from the surface water, microbial biofilms help to “clean” the water. But they can only completely degrade a few organic contaminants (the in situ efficiency of this kind of process being still unknown), and so the remaining bulk of accumulated contaminants (and/or their metabolites) will be either resuspended in the water column in a dissolved or complexed form (e.g., following biofilm detachment or sediment mobilization) or get transferred to higher trophic levels.
4 Contaminant Transfer from Microbial Biofilms Through Food Webs
In freshwater ecosystems, microbial biofilms are an important food resource (in both the green and brown food webs; Fig. 3), even if potentially contaminated by metals or organic contaminants. Microcosm and field studies conducted to investigate the role and importance of these biofilm communities in contaminant trophic transfer have revealed important differences in terms of contaminant fate through food webs.
4.1 Current Approaches Used to Follow Contaminants Through Food Webs
Microcosm experiments are commonly used to reproduce simple food webs under controlled conditions in order to limit any confounding factors likely to influence the bioaccumulation process (e.g., temperature, nutrients, pH, ionic composition) and explore the fate of the contaminants. These microcosm experiments typically consist in exposing animals to a food source, namely, a single (often algal) microorganism species or natural biofilms, spiked with a contaminant. For instance, simplified food chains, i.e., from primary producers to consumers such as crustaceans, insects, bivalves, and more rarely fish, have been reproduced in microcosm experiments to determine the trophic transfer of metals or metallic nanoparticles (Croteau et al. 2005; Conley et al. 2009; Komjarova and Blust 2009; Prokes et al. 2012; Golding et al. 2013; Kim et al. 2016). These microcosm studies usually contaminate the food resource in an independent spiking process before introducing it into the microcosm. For instance, algae spiked with metals have been used to demonstrate trophic transfers of a variety of metals to bivalves or crustaceans (Croteau et al. 2005; Goulet et al. 2007; Komjarova and Blust 2009). Experimental studies can more precisely gauge contaminant fates by spiking with labeled contaminants. Radioisotopes of metals have been used to label food sources and thus trace species-specific bioaccumulation dynamics by monitoring the radioactivity in consumers after pulse-chase feeding (Conley et al. 2009; Golding et al. 2013). Food-resource enrichment by stable isotopes of metals makes an interesting alternative approach to determine the relative contributions of food and water to metal contamination in grazers (Komjarova and Blust 2009). Indeed, isotopic ratio measurements can be used in microcosm experiments to characterize and model the physiological mechanisms involved in the assimilation of metals by the consumers from the biofilm they ingested (Croteau et al. 2005). To our knowledge, the trophic transfer of organic contaminants from biofilms to higher-level organisms has not yet been studied in microcosm experiments.
Microcosm experiments do provide accurate information on the dietary dynamics of contaminants in simplified food chains, but they are often specific to a prey/consumer pair, and consequently only partially reflect the environmental complexity governing trophic transfers (e.g., multiple food sources, biofilm structure, nutrient loads, and more). In addition, the choice of the microorganism(s) used as a contaminated food source and the spiking method remain challenging tasks. Indeed, metals assimilation by consumers has been reported to be closely related to microbial species and their respective ability to bioaccumulate metals, to metal distribution in the microbial cells, and obviously to community structure (Goulet et al. 2007; Conley et al. 2009; Komjarova and Blust 2009; Golding et al. 2013). On one hand, the influence of community complexity is omitted when monospecific biofilms are used as a food source. On the other hand, the acclimatization and exposure of a field-collected biofilm to laboratory conditions are likely to provoke structural and morphological changes in this complex food source (Fechner et al. 2011; Barral-Fraga et al. 2016). In most food web experiments, microbial biofilms are contaminated prior to introduction in the microcosm (e.g., Conley et al. 2009; Komjarova and Blust 2009; Xie et al. 2010; Kim et al. 2012; Li et al. 2012; Perrier et al. 2018; Hudson et al. 2019); nevertheless a few studies, usually in complex mesocosms, have also investigated the effect on trophic transfer of concomitant contamination of food resource and media, thus mimicking field conditions (e.g., Pinder et al. 2011; Cleveland et al. 2012; Kim et al. 2016; Friesen et al. 2017; Park et al. 2018).
One way to go beyond the limitations of microcosm experiments is to follow contaminant fate directly in the field. Indeed, various studies have investigated the bioaccumulation of metals or organic compounds in natural environments by collecting a variety of organisms including – but not limited to – microbial biofilms (Croteau et al. 2005; Vinot and Pihan 2005; Walters et al. 2008, 2015; Coat et al. 2011; Jardine et al. 2013; Ruhí et al. 2016). Understanding trophic enrichment with a contaminant in the field first requires an accurate description of local food web structures. Hence, stable carbon and nitrogen isotopes in biological tissues and microbial biofilms are usually analyzed (Croteau et al. 2005; Walters et al. 2008, 2015). Besides isotopic ratio measurements, lipid content assessment is recommended for studies focused on hydrophobic contaminants such as PCBs (Walters et al. 2008; Coat et al. 2011). Although using stable isotopes of C and N offers interesting perspectives to learn prey and consumer trophic positions and thus demonstrate trophic transfer through food webs, the technique does require sophisticated and expensive equipment (Burns and Ryder 2001). Moreover, the results of stable isotope measurements in microbial biofilms represent a “mean” of different signatures (bacteria, algae), which could be a limiting factor for determining accurate relationships between selective grazers and their specific food source within biofilm communities.
4.2 Role of Microbial Biofilms in Contaminant Transfers Through Aquatic Food Webs
The food web interactions of microbial biofilms concern insects, gastropods, fish, and shrimps. Some of these grazers may exhibit food preferences and thus preferentially consume specific microbial groups or taxa. For instance, the shrimp Paratya australiensis was shown to specifically reduce diatom biomass in grazed biofilms, indirectly enhancing the green algal growth (Burns 1997). Besides affecting the composition of biofilms, grazers can also impact their 3D architecture (Robson and Barmuta 1998). However, in return, grazers can be influenced by the nutritional quality of the biofilm as well as its contaminant content. It is well known that microbial biofilms are a primary food resource in aquatic ecosystems, yet few studies have investigated contaminants transfers from microbial biofilms through aquatic food webs and the resulting bioaccumulation through trophic transfer (e.g., Jardine et al. 2013; Walters et al. 2015), or feedback-loop control of other ecosystem components on contaminant concentrations in periphytic communities (Roessink et al. 2010). In addition, most of these studies focused on the trophic transfer of contaminants from periphytic biofilms to primary consumers and, more rarely, to predators, which limits the assessment of biomagnification processes (i.e., increasing contaminant concentrations with increasing trophic levels) through food webs involving contaminated biofilms. The biomagnification factor (BMF), which is calculated based on the assumption that contaminant concentration in a consumer depends on contaminant concentration in its prey (sometimes corrected for trophic-level difference between the consumer and its prey; Fisk et al. 2001), serves to convey this process. A BMF > 1 corresponds to a magnification of contaminant concentrations in consumers/predators. BMFs adjusted to trophic positions can be calculated using the following equation:
where Cconsumer(i) is concentration of contaminant in the consumer, Cdiet(i) is concentration of contaminant in the diet, δ15Nconsumer(i) is trophic level of consumer, and δ15Ndiet(i) is trophic level of diet.
Both essential (e.g., Cu, Zn, Se) and nonessential (e.g., Hg, Cd, As) metals are readily absorbed by primary producers and can be transferred from microbial biofilms to higher trophic levels (i.e., macroinvertebrates and predators) where they could exert adverse effects (Croteau et al. 2005; Magellan et al. 2014; Walters et al. 2015; Hepp et al. 2017). For instance, the consumption of spiked biofilms was reported to be a significant route of exposure to Cd for the amphipod Hyalella azteca (Conley et al. 2009) and to Se for the insect Centroptilum triangulifer (Golding et al. 2013). However, biomagnification was only observed for Hg, Zn, and sometimes Se (Farag et al. 2007; Conley et al. 2009; Jardine et al. 2012, 2013). Biomagnification of methylmercury (MeHg) from microbial biofilms to primary consumers (Jardine et al. 2013) and to fish (Walters et al. 2015) is a relatively well-studied phenomenon, and resulting MeHg BMFs ranging from 1 to 31 have been reported in different sites (Jardine et al. 2013). Various studies have also highlighted the influence of environmental parameters on Hg biomagnification throughout the food web (Jardine et al. 2012, 2013). In particular, low pH led to an increase in Hg supply for primary producers, which then also increased biomagnification at higher trophic levels (fish). Due to biomagnification processes, even low levels of water contamination can lead to high concentrations of toxic metals in wildlife. Walters et al. (2015) found that Se and Hg concentrations in top-level organisms from a large food web (including organic matter, benthic biofilm, invertebrates, and fish) regularly exceeded the exposure risk thresholds for wildlife, thus revealing the ecosystem risks due to trophic transfers of Hg and Se in aquatic food webs.
Organisms have evolved internal mechanisms for metals regulation that can complicate efforts to map these trophic transfers. As essential metals are actively regulated by freshwater organisms in order to maintain internal concentrations, gauging the enrichment of essential metals between trophic levels is far from straightforward and linked to species-specific biological needs. During exposure to labeled algae in microcosms, Cu was found to mainly accumulate by dietary route in the bivalve Corbicula fluminea, whereas it was preferentially absorbed by aqueous route in the crustacean Daphnia magna (Croteau et al. 2005; Komjarova and Blust 2009). Species-specific differences in biomagnification were also found for Se, which was biomagnified from contaminated periphytic biofilms to a primary consumer: the mayfly Centroptilum triangulifer (Conley et al. 2009). In addition, after assimilation of Se from contaminated biofilms, mayflies transferred about 46% of their Se body burdens into their eggs, resulting in a reduction of fecundity at environmentally relevant concentrations (Conley et al. 2009). Conversely, although primary producers were found to be potential sources of Se contamination for their direct consumers (i.e., mussels, shrimps, or macroinvertebrates), Se enrichment through trophic levels was not significant in various field studies in the Mirgenbach reservoir (France; Vinot and Pihan 2005), in the San Joaquin River (USA; Croteau et al. 2005), or in the Colorado River (USA; Walters et al. 2015). These differences may be at least partially explained by the high variability in Se concentrations among macroinvertebrate species, as observed in a field study by Walters et al. (2015). Zn is another essential metal with toxicity at high concentrations that was also found to transfer through the food web (Farag et al. 2007) and biomagnify in the mayfly Centroptilum triangulifer (Kim et al. 2012). While Zn concentration in mayfly larvae was 16–19-fold higher than in the labeled contaminated periphyton used as a food resource, Zn concentration in adults was only threefold to eightfold higher than in diet (Kim et al. 2012). Therefore, dietary bioaccumulation dynamics in biofilm consumers depend on the species, its growth stage, and its subsequent physiological abilities to assimilate and/or eliminate metals.
Moreover, there may be only very limited trophic transfer for metals or metallic nanoparticles. For example, titanium nanoparticles were fairly highly accumulated in sediment biofilm but barely transferred from biofilms to river snails and Chinese muddy loaches, with BMFs of just 0.01–0.02 and 0.04–0.05, respectively (Kim et al. 2016), highlighting the low degree of bioaccumulation of TiO2 in these consumers.
Organic contaminants, and in particular persistent chemicals, are likely to be transferred and/or biomagnified from microbial biofilms to higher trophic levels. Concentrations of tris(2-butoxyethyl) phosphate (TBEP), a flame retardant quantified consistently across all food web compartments, were found to increase with trophic levels (Ruhí et al. 2016). Indeed, in a Mediterranean river food web, microbial biofilms accumulated the lowest amount of TBEP, whereas intermediate concentrations of TBEP were found in the primary consumer Ancylus, and the highest concentrations were found in the omnivore filter-feeding Hydropsyche and the macroinvertebrate predator Phagocata (Ruhí et al. 2016). Similarly, concentrations of the drug carbamazepine in a stream food web including biofilms, invertebrates, and vertebrates were correlated with trophic position (Du et al. 2014). PCB concentrations were also significantly correlated with trophic level, as described in Walters et al. (2008, 2011), with an average BMF close to 1.6. The hydrophobicity of PCBs (i.e., their Kow value) strongly modulates their trophic transfer and biomagnification (Walters et al. 2008, 2011). Whereas the influence of Kow on biomagnification varies substantially across food webs, model predictions of standardized Kow-based BMFs remain consistent with field observations (Walters et al. 2011). Like metals, some organic contaminants were also found to be biomagnified slightly (e.g., low increase of chlorpyrifos concentrations from biofilm to snail; Lundqvist et al. 2012) or not (e.g., diclofenac, gemfibrozil (Ruhí et al. 2016); diphenhydramine (Du et al. 2015)) through the food web.
Exposure scenarios and environmental conditions are also likely to influence contaminant fate through food webs. For instance, TiO2 nanoparticles accumulated much more in biofilm after sequential low-dose exposures than after a single high-dose exposure (Kim et al. 2016). On one hand, environmental conditions may influence contaminant uptake and dynamics in microbial biofilms and thus modulate the amount available for further trophic transfer. On the other hand, microbial biofilm quality as a food resource (e.g., C/N) is also likely to influence trophic transfers of nutrients and contaminants. In addition, C/N ratio can also be modulated by environmental factors (e.g., flow velocity; Coat et al. 2011), while dissolved organic matter (DOM) concentrations in surrounding water can modify contaminant bioavailability for primary consumers and thus trophic transfers within the food web. Lundqvist et al. (2012) showed that increasing DOM concentrations in water led to increasing sorption of chlorpyrifos in biofilms. However, despite the resulting high concentrations in biofilms, dietary uptake of chlorpyrifos by snails remained relatively low, and the results showed that the combined presence of biofilms and of medium- and high-DOM concentrations reduced the share of chlorpyrifos bioavailable for snails. Testing two other insecticides (carbofuran and lindane) exhibiting lower hydrophobicity levels than chlorpyrifos on a low-to-high-DOM concentration gradient, Lundqvist et al. (2012) also demonstrated that the accumulation of pesticides by the snails was influenced by both DOM concentrations and pesticide hydrophobicity.
Microbial biofilms are an important food resource in freshwater ecosystems, and our literature analysis highlights their potential to accumulate contaminants and modify their bioavailability, thus influencing their transfers through food webs. However, the role of biofilms in dietary contaminant exposure has been neglected for a long time, and the importance of biofilms in trophic transfers of those potentially toxic substances is still understudied, leaving a critical lack of knowledge, especially but not exclusively concerning biofilms attached to sediments or organic substrates such as leaf litter.
5 Conclusions and Future Challenges
This literature review, which illustrates the dynamic interactions between contaminants and biofilms in aquatic ecosystems, underscores widely different levels of knowledge according to the kind of substrate where the biofilm grows and the food web pathway(s) to which it contributes. Accordingly, while studies on periphyton show that biofilms are able to accumulate a wide range of metals and organic contaminants, the role of biofilms in the distribution of contaminants among different aquatic compartments (surface water, periphyton, sediments, leaf litter) and the associated biota remains unclear and has not, to our knowledge, been quantified. This is mainly due to the fact that we still lack methods to specifically assess and measure contaminants in microbial communities of complex solid matrices such as sediment or leaf litter. Moreover, even though several studies have attempted to investigate chemical concentrations simultaneously in different aquatic compartments (sediment, surface water, and biofilm; Kohušová et al. 2011), there is still a lack of data on the role of microbial communities in contaminant accumulation (or release) processes in sediments and organic substrates. This issue remains a bottleneck, given the abovementioned methodological limitations. Field or microcosm surveys including systematic characterization of the contamination in these different aquatic compartments combined with laboratory experiments quantifying sorption/desorption rates under various controlled conditions are now needed to better estimate the role played by biofilms in contaminant fluxes within the aquatic ecosystem and their food webs.
Contaminant adsorption, storage or sequestration, transformation, and, finally, release in the environment are still mainly investigated in periphyton studies using metals and a few organics as model compounds. However, there is a gap of knowledge on the transfer kinetics of contaminants in periphytic assemblages and their resulting toxic effects (Chaumet et al. 2019a, b). To better understand the fate of organic contaminants in periphyton, the development and validation of sensitive and specific high-performance analytical methods combined with the measurement of intracellular concentrations of organic contaminants, using novel partitioning methods (Chaumet et al. 2019a), are the first technical challenges to overcome.
Microcosm experiments have brought valuable insights into contaminant transfer from biofilms to consumers, but future research should now aim to push beyond these relatively simple models and attempt to address the real-world complexity, i.e., both contaminant transfer from biofilm to upper trophic levels and ecological interactions with other ecosystem components (Roessink et al. 2010), as well as the influence of environmental factors such as temperature, organic matter, and so on.
Future studies need to consider the potential effects of global change and specifically how (1) shifts in water contamination patterns (i.e., land-use change, evolving agricultural practices, antibiotic resistances), (2) climate change (i.e., global warming, droughts, floods), and (3) the presence of invasive species (i.e. top-down versus bottom-up effects) can affect contaminant bioaccumulation by biofilms and consequences on trophic transfer – a challenge that also raises new questions requiring further interdisciplinary research bridging environmental chemistry, ecotoxicology, and ecology.
6 Summary
Freshwater environments host microbial biomass that can aggregate and attach to different kinds of submerged substrates (rock, sediment, leaf litter). These microbial assemblages, which are called biofilms, can accumulate the contaminants transported by the water flow and/or adsorbed onto the substrates where they develop. Furthermore, due to their high metabolic activity and their role in aquatic food webs, microbial biofilms are also likely to influence contaminant fate in aquatic ecosystems.
Here, by focusing on metals and organic micropollutants, we provide a critical overview of the analytical methods currently in use for detecting and quantifying these contaminants in microbial biofilms developing in different benthic substrata, together with a look at the state of current knowledge and future challenges concerning the role of biofilms in contaminant accumulation and trophic transfers in the aquatic food web.
From this literature review emerge the following issues:
-
Concurrent to analytical developments, studies dealing with bioaccumulation in microbial biofilms have been applied mainly to metal contaminants, and there is still only limited knowledge on the accumulation of organic contaminants in these microbial assemblages.
-
A large variety of metals and organic contaminants has been found in natural periphytic biofilms, which grow on inert surfaces like cobbles exposed to light. In contrast, due to technical limitations, data on the bioaccumulation of contaminants in submerged microbial communities associated with sediments, leaves, or drift particulate matter has always included both biotic accumulation and abiotic sorption on the substratum.
-
Microbial biofilms represent an important food resource in freshwater ecosystems, yet their role in dietary contaminant exposure has been neglected for a long time, and the importance of biofilms in trophic transfer of contaminants is still understudied, leaving a critical lack of knowledge especially but not exclusively concerning biofilms attached to sediments or organic substrates such as leaf litter.
These issues pose a set of challenges that need to be overcome in order to better:
-
Characterize the accumulation of contaminants in sediment and organic-substrate biofilms
-
Evaluate the role played by biofilms in contaminants fluxes within aquatic ecosystems and aquatic food webs
-
Assess how different environmental pressures such as shifts in water contamination, climate change, or the presence of invasive species (i.e., top-down versus bottom-up effects) may affect contaminant bioaccumulation in biofilms and the resulting consequences in food webs
Abbreviations
- AAS:
-
Atomic absorption spectrometry
- BCF:
-
Bioconcentration factor
- BMF:
-
Biomagnification factor
- CLSM:
-
Confocal laser scanning microscopy
- DDT:
-
Dichlorodiphenyltrichloroethane
- DNA:
-
Deoxyribonucleic acid
- DOM:
-
Dissolved organic matter
- EDTA:
-
Ethylenediaminetetraacetic acid
- EPS:
-
Extracellular polymeric substances
- GC-MS:
-
Gas chromatography-mass spectrometry
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- K ow :
-
Octanol-water partition coefficient
- LC-MS:
-
Liquid chromatography-mass spectrometry
- LOQ:
-
Limit of quantification
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PCBs:
-
Polychlorinated biphenyls
- STXM:
-
Scanning transmission X-ray microscopy
- TBEP:
-
Tris(2-butoxyethyl) phosphate
- TEM:
-
Transmission electron microscopy
References
Aguilera A, Souza-Egipsy V, Martin-Uriz PS, Amils R (2008) Extraction of extracellular polymeric substances from extreme acidic microbial biofilms. Appl Microbiol Biotechnol 78:1079–1088. https://doi.org/10.1007/s00253-008-1390-9
Alvarez M, Peckarsky BL (2005) How do grazers affect periphyton heterogeneity in streams? Oecologia 142:576–587. https://doi.org/10.1007/s00442-004-1759-0
Ancion PY, Lear G, Lewis GD (2010) Three common metal contaminants of urban runoff (Zn, Cu & Pb) accumulate in freshwater biofilm and modify embedded bacterial communities. Environ Pollut 158:2738–2745. https://doi.org/10.1016/j.envpol.2010.04.013
Ancion PY, Lear G, Dopheide A, Lewis GD (2013) Metal concentrations in stream biofilm and sediments and their potential to explain biofilm microbial community structure. Environ Pollut 173:117–124. https://doi.org/10.1016/j.envpol.2012.10.012
Arini A, Feurtet-Mazel A, Maury-Brachet R et al (2012) Field translocation of diatom biofilms impacted by Cd and Zn to assess decontamination and community restructuring capacities. Ecol Indic 18:520–531
Arnot JA, Gobas F (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257–297. https://doi.org/10.1139/a06-005
Augustin T, Schlosser D, Baumbach R et al (2006) Biotransformation of 1-naphthol by a strictly aquatic fungus. Curr Microbiol 52:216–220. https://doi.org/10.1007/s00284-005-0239-z
Avery SV, Tobin JM (1993) Mechanism of adsorption of hard and soft metal ions to Saccharomyces cerevisiae and influence of hard and soft anions. Appl Env Microbiol 59:2851–2856
Avery SV, Codd GA, Gadd GM (1993) Transport kinetics, cation inhibition and intracellular location of accumulated caesium in the green microalga Chlorella salina. Microbiology 139:827–834. https://doi.org/10.1099/00221287-139-4-827
Barkay T, Schaefer J (2001) Metal and radionuclide bioremediation: issues, considerations and potentials. Curr Opin Microbiol 4:318–323. https://doi.org/10.1016/s1369-5274(00)00210-1
Barral-Fraga L, Morin S, Rovira MDM et al (2016) Short-term arsenic exposure reduces diatom cell size in biofilm communities. Environ Sci Pollut Res 23:4257–4270. https://doi.org/10.1007/s11356-015-4894-8
Battin TJ, Kaplan LA, Newbold JD, Hansen CME (2003) Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426:439–442. https://doi.org/10.1038/nature02152
Battin TJ, Besemer K, Bengtsson MM et al (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14:251–263. https://doi.org/10.1038/nrmicro.2016.15
Behrens S, Kappler A, Obst M (2012) Linking environmental processes to the in situ functioning of microorganisms by high-resolution secondary ion mass spectrometry (NanoSIMS) and scanning transmission X-ray microscopy (STXM). Environ Microbiol 14:2851–2869. https://doi.org/10.1111/j.1462-2920.2012.02724.x
Berglund O (2003) Periphyton density influences organochlorine accumulation in rivers. Limnol Oceanogr 48:2106–2116
Berglund O, Nyström P, Larsson P (2005) Persistent organic pollutants in river food webs: influence of trophic position and degree of heterotrophy. Can J Fish Aquat Sci 62:2021–2032. https://doi.org/10.1139/f05-115
Bertini G (2016) Memory effect of aquatic biofilms in the partitioning of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in water streams. Int J Environ Sci Dev 7:921–927. https://doi.org/10.18178/ijesd.2016.7.12.905
Bradac P, Behra R, Sigg L (2009) Accumulation of cadmium in periphyton under various freshwater speciation conditions. Environ Sci Technol 43:7291–7296. https://doi.org/10.1021/es9013536
Bradac P, Wagner B, Kistler D et al (2010) Cadmium speciation and accumulation in periphyton in a small stream with dynamic concentration variations. Environ Pollut 158:641–648. https://doi.org/10.1016/j.envpol.2009.10.031
Brown DA, Beveridge TJ, Keevil CW, Sheriff BL (1998) Evaluation of microscopic techniques to observe iron precipitation in a natural microbial biofilm. FEMS Microbiol Ecol 26:297–310. https://doi.org/10.1111/j.1574-6941.1998.tb00514.x
Burns A (1997) The role of disturbance in the ecology of biofilms in the River Murray, South Australia. PhD thesis, University of Adelaide
Burns A, Ryder DS (2001) Potential for biofilms as biological indicators in Australian riverine systems. Ecol Manag Restor 2:53–64. https://doi.org/10.1046/j.1442-8903.2001.00069.x
Carles L, Rossi F, Joly M et al (2017) Biotransformation of herbicides by aquatic microbial communities associated to submerged leaves. Environ Sci Pollut Res 24:3664–3674. https://doi.org/10.1007/s11356-016-8035-9
Carles L, Rossi F, Besse-Hoggan P et al (2018) Nicosulfuron degradation by an Ascomycete fungus isolated from submerged Alnus leaf litter. Front Microbiol 9:3167. https://doi.org/10.3389/fmicb.2018.03167
Carles L, Gardon H, Joseph L et al (2019) Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ Int 124:284–293. https://doi.org/10.1016/j.envint.2018.12.064
Chaumet B, Morin S, Hourtané O et al (2019a) Flow conditions influence diuron toxicokinetics and toxicodynamics in freshwater biofilms. Sci Total Environ 652:1242–1251. https://doi.org/10.1016/j.scitotenv.2018.10.265
Chaumet B, Morin S, Boutry S, Mazzella N (2019b) Diuron sorption isotherms in freshwater biofilms. Sci Total Environ 651:1219–1225. https://doi.org/10.1016/j.scitotenv.2018.09.286
Chen SJ, Luo XJ, Mai BX et al (2006) Distribution and mass inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in sediments of the Pearl River estuary and the northern South China Sea. Environ Sci Technol 40:709–714. https://doi.org/10.1021/es052060g
Cleveland D, Long SE, Pennington PL et al (2012) Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Sci Total Environ 421–422:267–272. https://doi.org/10.1016/j.scitotenv.2012.01.025
Coat S, Monti D, Legendre P et al (2011) Organochlorine pollution in tropical rivers (Guadeloupe): role of ecological factors in food web bioaccumulation. Environ Pollut 159:1692–1701
Conley JM, Funk DH, Buchwalter DB (2009) Selenium bioaccumulation and maternal transfer in the mayfly Centroptilum triangulifer in a life-cycle, periphyton-biofilm trophic assay. Environ Sci Technol 43:7952–7957. https://doi.org/10.1021/es9016377
Coogan MA, Edziyie RE, La Point TW, Venables BJ (2007) Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67:1911–1918
Corcoll N, Bonet B, Morin S et al (2012) The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river: a translocation experiment. Ecol Indic 18:620–631. https://doi.org/10.1016/j.ecolind.2012.01.026
Croteau MN, Luoma SN, Stewart AR (2005) Trophic transfer of metals along freshwater food webs: evidence of cadmium biomagnification in nature. Limnol Oceanogr 50:1511–1519
Danger M, Lacroix G, Oumarou C et al (2008) Effects of food-web structure on periphyton stoichiometry in eutrophic lakes: a mesocosm study. Freshw Biol 53:2089–2100. https://doi.org/10.1111/j.1365-2427.2008.02031.x
Danger M, Cornut J, Chauvet E et al (2013) Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94:1604–1613. https://doi.org/10.1890/12-0606.1
DeLorenzo ME, Scott GI, Ross PE (2001) Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20:84–98. https://doi.org/10.1002/etc.5620200108
Dimitrov MR, Kosol S, Smidt H et al (2014) Assessing effects of the fungicide tebuconazole to heterotrophic microbes in aquatic microcosms. Sci Total Environ 490:1002–1011. https://doi.org/10.1016/j.scitotenv.2014.05.073
Dobor J, Varga M, Záray G (2012) Biofilm controlled sorption of selected acidic drugs on river sediments characterized by different organic carbon content. Chemosphere 87:105–110. https://doi.org/10.1016/j.chemosphere.2011.11.067
Domagalski JL, Weston DP, Zhang MH, Hladik M (2010) Pyrethroid insecticide concentrations and toxicity in streambed sediments and loads in surface waters of the San Joaquin valley, California, USA. Environ Toxicol Chem 29:813–823
Dranguet P, Le Faucheur S, Cosio C, Slaveykova VI (2017) Influence of chemical speciation and biofilm composition on mercury accumulation by freshwater biofilms. Environ Sci Process Impacts 19:38–49. https://doi.org/10.1039/c6em00493h
Du B, Perez-Hurtado P, Brooks BW, Chambliss CK (2012) Evaluation of an isotope dilution liquid chromatography tandem mass spectrometry method for pharmaceuticals in fish. J Chromatogr A 1253:177–183. https://doi.org/10.1016/j.chroma.2012.07.026
Du B, Haddad SP, Luek A et al (2014) Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos Trans R Soc B Biol Sci 369:20140058. https://doi.org/10.1098/rstb.2014.0058
Du B, Haddad SP, Scott WC et al (2015) Pharmaceutical bioaccumulation by periphyton and snails in an effluent-dependent stream during an extreme drought. Chemosphere 119:927–934. https://doi.org/10.1016/j.chemosphere.2014.08.044
Dynes JJ, Tyliszczak T, Araki T et al (2006a) Speciation and quantitative mapping of metal species in microbial biofilms using scanning transmission X-ray microscopy. Environ Sci Technol 40:1556–1565. https://doi.org/10.1021/es0513638
Dynes JJ, Lawrence JR, Korber DR et al (2006b) Quantitative mapping of chlorhexidine in natural river biofilms. Sci Total Environ 369:369–383. https://doi.org/10.1016/j.scitotenv.2006.04.033
Edwards SJ, Kjellerup BV (2013) Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl Microbiol Biotechnol 97:9909–9921. https://doi.org/10.1007/s00253-013-5216-z
European Commission (2010) Common Implementation Strategy for the Water Framework Directive (2000/60/EC): Guidance Document No. 25 on chemical monitoring of sediment and biota under the Water Framework Directive
European Commission (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J L 226
European Commission (2015) Commission Implementing Decision 2015/495 of 20 March 2015 establishing a watch list of substances for union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council (notified under document C(2015) 1756) text with EEA relevance. Off J L 78
Fabure J, Dufour M, Autret A et al (2015) Impact of an urban multi-metal contamination gradient: metal bioaccumulation and tolerance of river biofilms collected in different seasons. Aquat Toxicol 159:276–289. https://doi.org/10.1016/j.aquatox.2014.12.014
Fan Q, He J, Xue H et al (2007) Competitive adsorption, release and speciation of heavy metals in the Yellow River sediments, China. Environ Geol 53:239–251. https://doi.org/10.1007/s00254-007-0638-5
Farag AM, Woodward DF, Goldstein JN et al (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene River Basin, Idaho. Arch Environ Contam Toxicol 34:119–127
Farag AM, Nimick DA, Kimball BA et al (2007) Concentrations of metals in water, sediment, biofilm, benthic macroinvertebrates, and fish in the Boulder River watershed, Montana, and the role of colloids in metal uptake. Arch Environ Contam Toxicol 52:397–409. https://doi.org/10.1007/s00244-005-0021-z
Fechner LC, Gourlay-France C, Tusseau-Vuillemin M-H (2011) Low exposure levels of urban metals induce heterotrophic community tolerance: a microcosm validation. Ecotoxicology 20:793–802. https://doi.org/10.1007/s10646-011-0630-4
Fechner LC, Gourlay-France C, Bourgeault A, Tusseau-Vuillemin M-H (2012) Diffuse urban pollution increases metal tolerance of natural heterotrophic biofilms. Environ Pollut 162:311–318. https://doi.org/10.1016/j.envpol.2011.11.033
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159. https://doi.org/10.1016/j.aquatox.2005.09.009
Fernandez MA, Alonso C, Gonzalez MJ, Hernandez LM (1999) Occurrence of organochlorine insecticides, PCBs and PCB congeners in waters and sediments of the Ebro River (Spain). Chemosphere 38:33–43. https://doi.org/10.1016/s0045-6535(98)00167-2
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya Marine Food Web. Environ Sci Technol 35:732–738. https://doi.org/10.1021/es001459w
Flemming HC (1995) Sorption sites in biofilms. Water Sci Technol 32:27–33. https://doi.org/10.1016/0273-1223(96)00004-2
Flemming H-C, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPSs) – part I: structural and ecological aspects. Water Sci Technol 43:1–8
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells”. J Bacteriol 189:7945–7947. https://doi.org/10.1128/JB.00858-07
Friesen V, Doig LE, Markwart BE et al (2017) Genetic characterization of periphyton communities associated with selenium bioconcentration and trophic transfer in a simple food chain. Environ Sci Technol 51:7532–7541. https://doi.org/10.1021/acs.est.7b01001
Froehner S, Machado KS, Dombroski LF et al (2012) Natural biofilms in freshwater ecosystem: indicators of the presence of polycyclic aromatic hydrocarbons. Water Air Soil Pollut 223:3965–3973. https://doi.org/10.1007/s11270-012-1164-y
García-Ordiales E, Esbrí JM, Covelli S et al (2016) Heavy metal contamination in sediments of an artificial reservoir impacted by long-term mining activity in the Almadén mercury district (Spain). Environ Sci Pollut Res 23:6024–6038. https://doi.org/10.1007/s11356-015-4770-6
Garvin EM, Bridge CF, Garvin MS (2017) Screening level assessment of metal concentrations in streambed sediments and floodplain soils within the Grand Lake Watershed in Northeastern Oklahoma, USA. Arch Environ Contam Toxicol 72:349–363. https://doi.org/10.1007/s00244-017-0376-y
Gascón Díez E, Corella JP, Adatte T et al (2017) High-resolution reconstruction of the 20th century history of trace metals, major elements, and organic matter in sediments in a contaminated area of Lake Geneva, Switzerland. Appl Geochem 78:1–11. https://doi.org/10.1016/j.apgeochem.2016.12.007
Golding LA, Borgmann U, Dixon DG (2013) Cadmium bioavailability to Hyalella azteca from a periphyton diet compared to an artificial diet and application of a biokinetic model. Aquat Toxicol 126:291–298. https://doi.org/10.1016/j.aquatox.2012.09.016
Gorga M, Insa S, Petrovic M, Barcelo D (2015) Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci Total Environ 503:69–86
Goulet RR, Krack S, Doyle PJ et al (2007) Dynamic multipathway modeling of Cd bioaccumulation in Daphnia magna using waterborne and dietborne exposures. Aquat Toxicol 81:117–125. https://doi.org/10.1016/j.aquatox.2006.11.008
Guanzon NG Jr, Fukuda M, Nakahara H (1996) Accumulation of agricultural pesticides by three freshwater microalgae. Fish Sci 62:690–697
Guasch H, Atli G, Bonet B et al (2010) Discharge and the response of biofilms to metal exposure in Mediterranean rivers. Hydrobiologia 657:143–157. https://doi.org/10.1007/s10750-010-0116-z
Guasch H, Ricart M, López-Doval J et al (2016) Influence of grazing on triclosan toxicity to stream periphyton. Freshw Biol 61:2002–2012. https://doi.org/10.1111/fwb.12797
Hall RO, Meyer JL (1998) The trophic significance of bacteria in a detritus-based stream food web. Ecology 79:1995–2012. https://doi.org/10.1890/0012-9658(1998)079[1995:ttsobi]2.0.co;2
Hamzeh M, Ouddane B, Clérandeau C, Cachot J (2016) Spatial distribution and toxic potency of trace metals in surface sediments of the Seine Estuary (France). Clean (Weinh) 44:544–552. https://doi.org/10.1002/clen.201500301
Hao L, Li J, Kappler A, Obst M (2013) Mapping of heavy metal ion sorption to cell-extracellular polymeric substance-mineral aggregates by using metal-selective fluorescent probes and confocal laser scanning microscopy. Appl Environ Microbiol 79:6524–6534. https://doi.org/10.1128/AEM.02454-13
Hao L, Guo Y, Byrne JM et al (2016) Binding of heavy metal ions in aggregates of microbial cells, EPS and biogenic iron minerals measured in-situ using metal- and glycoconjugates-specific fluorophores. Geochim Cosmochim Acta 180:66–96. https://doi.org/10.1016/j.gca.2016.02.016
Headley JV, Peru KM, Lawrence JR, Wolfaardt GM (1995) MS/MS identification of transformation products in degradative biofilms. Anal Chem 67:1831–1837
Headley JV, Gandrass J, Kuballa J et al (1998) Rates of sorption and partitioning of contaminants in river biofilm. Environ Sci Technol 32:3968–3973. https://doi.org/10.1021/es980499l
Headley JV, Peru KM, Friesen DA, Neu T (2001) Liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry determination of N-methylpyrrolidinone in riverine biofilms. J Chromatogr A 917:159–165. https://doi.org/10.1016/S0021-9673(01)00687-2
Hepp LU, Pratas JAMS, Graça MAS (2017) Arsenic in stream waters is bioaccumulated but neither biomagnified through food webs nor biodispersed to land. Ecotoxicol Environ Saf 139:132–138. https://doi.org/10.1016/j.ecoenv.2017.01.035
Hitchcock AP, Dynes JJ, Johansson G et al (2008) Comparison of NEXAFS microscopy and TEM-EELS for studies of soft matter (vol 39, pg 311, 2008). Micron 39:741–748. https://doi.org/10.1016/j.micron.2007.09.010
Hitchcock AP, Dynes JJ, Lawrence JR et al (2009) Soft X-ray spectromicroscopy of nickel sorption in a natural river biofilm. Geobiology 7:432–453. https://doi.org/10.1111/j.1472-4669.2009.00211.x
Ho HH, Swennen R, Cappuyns V et al (2013) Geogene versus anthropogene origin of trace metals in sediments in Cua Luc Estuary and Ha Long Bay, Vietnam. Estuar Coasts 36:203–219. https://doi.org/10.1007/s12237-012-9562-3
Holding KL, Gill RA, Carter J (2003) The relationship between epilithic periphyton (biofilm) bound metals and metals bound to sediments in freshwater systems. Environ Geochem Health 25:87–93. https://doi.org/10.1023/a:1021205101133
Hudson ML, Costello DM, Daley JM, Burton GA (2019) Species-specific (Hyalella azteca and Lymnea stagnalis) dietary accumulation of gold nano-particles associated with periphyton. Bull Environ Contam Toxicol 103:255–260. https://doi.org/10.1007/s00128-019-02620-2
Huerta B, Rodriguez-Mozaz S, Nannou C et al (2016) Determination of a broad spectrum of pharmaceuticals and endocrine disruptors in biofilm from a waste water treatment plant-impacted river. Sci Total Environ 540:241–249. https://doi.org/10.1016/j.scitotenv.2015.05.049
Hunter RC, Hitchcock AP, Dynes JJ et al (2008) Mapping the speciation of iron in Pseudomonas aeruginosa biofilms using scanning transmission X-ray microscopy. Environ Sci Technol 42:8766–8772. https://doi.org/10.1021/es801642z
INERIS (2010) Qualité chimique des sédiments fluviaux en France. Synthèse des bases de données disponibles. Rapport d’étude. N° INERIS-DRC-10-105335-04971A, 99 pp
Ivorra N, Hettelaar J, Tubbing GMJ et al (1999) Translocation of microbenthic algal assemblages used for in situ analysis of metal pollution in rivers. Arch Environ Contam Toxicol 37:19–28. https://doi.org/10.1007/s002449900485
Jardine TD, Kidd KA, Rasmussen JB (2012) Aquatic and terrestrial organic matter in the diet of stream consumers: implications for mercury bioaccumulation. Ecol Appl 22:843–855
Jardine TD, Kidd KA, O’Driscoll N (2013) Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquat Toxicol 132:46–52. https://doi.org/10.1016/j.aquatox.2013.01.013
Kilunga PI, Sivalingam P, Laffite A et al (2017) Accumulation of toxic metals and organic micro-pollutants in sediments from tropical urban rivers, Kinshasa, Democratic Republic of the Congo. Chemosphere 179:37–48. https://doi.org/10.1016/j.chemosphere.2017.03.081
Kim KS, Funk DH, Buchwalter DB (2012) Dietary (periphyton) and aqueous Zn bioaccumulation dynamics in the mayfly Centroptilum triangulifer. Ecotoxicology 21:2288–2296. https://doi.org/10.1007/s10646-012-0985-1
Kim JI, Park HG, Chang KH et al (2016) Trophic transfer of nano-TiO2 in a paddy microcosm: a comparison of single-dose versus sequential multi-dose exposures. Environ Pollut 212:316–324. https://doi.org/10.1016/j.envpol.2016.01.076
Kohušová K, Havel L, Vlasák P, Tonika J (2011) A long-term survey of heavy metals and specific organic compounds in biofilms, sediments, and surface water in a heavily affected river in the Czech Republic. Environ Monit Assess 174:555–572. https://doi.org/10.1007/s10661-010-1478-4
Komjarova I, Blust R (2009) Application of a stable isotope technique to determine the simultaneous uptake of cadmium, copper, nickel, lead and zinc by the water flea Daphnia magna from water and the green algae Pseudokirchneriella subcapitata. Environ Toxicol Chem 28:1739–1748
Krumins JA, van Oevelen D, Bezemer TM et al (2013) Soil and freshwater and marine sediment food webs: their structure and function. Bioscience 63:35–42. https://doi.org/10.1525/bio.2013.63.1.8
Laabs V, Wehrhan A, Pinto A et al (2007) Pesticide fate in tropical wetlands of Brazil: an aquatic microcosm study under semi-field conditions. Chemosphere 67:975–989. https://doi.org/10.1016/j.chemosphere.2006.10.067
Labadie P, Hill EM (2007) Analysis of estrogens in river sediments by liquid chromatography-electrospray ionisation mass spectrometry – comparison of tandem mass spectrometry and time-of-flight mass spectrometry. J Chromatogr A 1141:174–181. https://doi.org/10.1016/j.chroma.2006.12.045
Lambert AS, Morin S, Artigas J et al (2012) Structural and functional recovery of microbial biofilms after a decrease in copper exposure: influence of the presence of pristine communities. Aquat Toxicol 109:118–126. https://doi.org/10.1016/j.aquatox.2011.12.006
Lambert AS, Dabrin A, Morin S et al (2016) Temperature modulates phototrophic periphyton response to chronic copper exposure. Environ Pollut 208:821–829. https://doi.org/10.1016/j.envpol.2015.11.004
Lavoie I, Lavoie M, Fortin C (2012) A mine of information: benthic algal communities as biomonitors of metal contamination from abandoned tailings. Sci Total Environ 425:231–241. https://doi.org/10.1016/j.scitotenv.2012.02.057
Lawrence JR, Kopf G, Headley JV, Neu TR (2001) Sorption and metabolism of selected herbicides in river biofilm communities. Can J Microbiol 47:634–641. https://doi.org/10.1139/w01-061
Lawrence JR, Swerhone GDW, Leppard GG et al (2003) Scanning transmission X-ray, laser scanning, and transmission electron microscopy mapping of the exopolymeric matrix of microbial biofilms. Appl Environ Microbiol 69:5543–5554. https://doi.org/10.1128/aem.69.9.5543-5554.2003
Lawrence JR, Dynes JJ, Korber DR et al (2012) Monitoring the fate of copper nanoparticles in river biofilms using scanning transmission X-ray microscopy (STXM). Chem Geol 329:18–25. https://doi.org/10.1016/j.chemgeo.2011.07.013
Lawrence JR, Swerhone GDW, Dynes JJ et al (2016) Soft X-ray spectromicroscopy for speciation, quantitation and nano-eco-toxicology of nanomaterials. J Microsc 261:130–147. https://doi.org/10.1111/jmi.12156
Lawrence JR, Swerhone GDW, Neu TR (2019) Visualization of the sorption of nickel within exopolymer microdomains of bacterial microcolonies using confocal and scanning electraon microscopy. Microbes Environ 34:76–82. https://doi.org/10.1264/jsme2.ME18134
Leguay S, Lavoie I, Levy JL, Fortin C (2016) Using biofilms for monitoring metal contamination in lotic ecosystems: the protective effects of hardness and ph on metal bioaccumulation. Environ Toxicol Chem 35:1489–1501. https://doi.org/10.1002/etc.3292
Lei BL, Huang SB, Zhou YQ et al (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Li M, Costello DM, Allen Burton G (2012) Interactive effects of phosphorus and copper on Hyalella azteca via periphyton in aquatic ecosystems. Ecotoxicol Environ Saf 83:41–46. https://doi.org/10.1016/j.ecoenv.2012.06.004
Li H, Shi A, Li M, Zhang X (2013) Effect of pH, temperature, dissolved oxygen, and flow rate of overlying water on heavy metals release from storm sewer sediments. J Chem. https://www.hindawi.com/journals/jchem/2013/434012/abs/. Accessed 2 May 2019
Liang XM, Chen BW, Nie XP et al (2013) The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 92:1410–1416
Liao J, Chen J, Ru X et al (2017) Heavy metals in river surface sediments affected with multiple pollution sources, South China: distribution, enrichment and source apportionment. J Geochem Explor 176:9–19. https://doi.org/10.1016/j.gexplo.2016.08.013
Lin J-G, Chen S-Y (1998) The relationship between adsorption of heavy metal and organic matter in river sediments. Environ Int 24:345–352. https://doi.org/10.1016/S0160-4120(98)00012-9
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425. https://doi.org/10.1016/s0168-6445(03)00044-5
Lundqvist A, Bertilsson S, Goedkoop W (2012) Interactions with DOM and biofilms affect the fate and bioavailability of insecticides to invertebrate grazers. Ecotoxicology 21:2398–2408. https://doi.org/10.1007/s10646-012-0995-z
Lünsdorf H, Brümmer I, Timmis KN, Wagner-Döbler I (1997) Metal selectivity of in situ microcolonies in biofilms of the Elbe river. J Bacteriol 179:31–40
Magellan K, Barral-Fraga L, Rovira M et al (2014) Behavioural and physical effects of arsenic exposure in fish are aggravated by aquatic algae. Aquat Toxicol 156:116–124. https://doi.org/10.1016/j.aquatox.2014.08.006
Margoum C, Malessard C, Gouy V (2006) Investigation of various physicochemical and environmental parameter influence on pesticide sorption to ditch bed substratum by means of experimental design. Chemosphere 63:1835–1841. https://doi.org/10.1016/j.chemosphere.2005.10.032
Mattila K, Carpen L, Hakkarainen T, Salkinoja-Salonen MS (1997) Biofilm development during ennoblement of stainless steel in Baltic Sea water: a microscopic study. Int Biodeterior Biodegrad 40:1–10
Métivier R, Bourven I, Labanowski J, Guibaud G (2013) Interaction of erythromycin ethylsuccinate and acetaminophen with protein fraction of extracellular polymeric substances (EPS) from various bacterial aggregates. Environ Sci Pollut Res 20:7275–7285. https://doi.org/10.1007/s11356-013-1738-2
Meylan S, Behra R, Sigg L (2003) Accumulation of copper and zinc in periphyton in response to dynamic variations of metal speciation in freshwater. Environ Sci Technol 37:5204–5212. https://doi.org/10.1021/es034566+
Meylan S, Behra R, Sigg L (2004) Influence of metal speciation in natural freshwater on bioaccumulation of copper and zinc in periphyton: a microcosm study. Environ Sci Technol 38:3104–3111. https://doi.org/10.1021/es034993n
Miller AZ, Dionisio A, Braga MAS et al (2012) Biogenic Mn oxide minerals coating in a subsurface granite environment. Chem Geol 322:181–191. https://doi.org/10.1016/j.chemgeo.2012.07.005
Morin S, Duong TT, Herlory O et al (2008) Cadmium toxicity and bioaccumulation in freshwater biofilms. Arch Environ Contam Toxicol 54:173–186
Munoz G, Fechner LC, Geneste E et al (2016) Spatio-temporal dynamics of per and polyfluoroalkyl substances (PFASs) and transfer to periphytic biofilm in an urban river: case-study on the River Seine. Environ Sci Pollut Res 25:23574–23582. https://doi.org/10.1007/s11356-016-8051-9
Murray KE, Thomas SM, Bodour AA (2010) Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ Pollut 158:3462–3471. https://doi.org/10.1016/j.envpol.2010.08.009
Neu TR, Manz B, Volke F et al (2010) Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol 72:1–21. https://doi.org/10.1111/j.1574-6941.2010.00837.x
Neury-Ormanni J, Vedrenne J, Morin S (2016) Who eats who in biofilms? Exploring the drivers of microalgal and micro-meiofaunal abundance. Bot Lett 163:83–92
Pal A, Gin KY-H, Lin AY-C, Reinhard M (2010) Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ 408:6062–6069. https://doi.org/10.1016/j.scitotenv.2010.09.026
Park HG, Kim JI, Chang KH et al (2018) Trophic transfer of citrate, PVP coated silver nanomaterials, and silver ions in a paddy microcosm. Environ Pollut 235:435–445. https://doi.org/10.1016/j.envpol.2017.12.104
Passeport E, Benoit P, Bergheaud V et al (2011) Selected pesticides adsorption and desorption in substrates from artificial wetland and forest buffer. Environ Toxicol Chem 30:1669–1676. https://doi.org/10.1002/etc.554
Passeport E, Tournebize J, Chaumont C et al (2013) Pesticide contamination interception strategy and removal efficiency in forest buffer and artificial wetland in a tile-drained agricultural watershed. Chemosphere 91:1289–1296
Passeport E, Richard B, Chaumont C et al (2014) Dynamics and mitigation of six pesticides in a “Wet” forest buffer zone. Environ Sci Pollut Res 21:4883–4894. https://doi.org/10.1007/s11356-013-1724-8
Pereira WE, Domagalski JL, Hostettler FD et al (1996) Occurrence and accumulation of pesticides and organic contaminants in river sediment, water and clam tissues from the San Joaquin River and tributaries, California. Environ Toxicol Chem 15:172–180. https://doi.org/10.1897/1551-5028(1996)015<0172:oaaopa>2.3.co;2
Perrier F, Baudrimont M, Mornet S et al (2018) Gold nanoparticle trophic transfer from natural biofilm to grazer fish. Gold Bull 51:163–173. https://doi.org/10.1007/s13404-018-0241-4
Pesce S, Fabrice ML, Rouard N, Montuelle B (2009) Potential for microbial diuronmineralisation in a small wine-growing watershed: from treated plots to lotic receiver hydrosystem. Pest Manag Sci 65:651–657. https://doi.org/10.1002/ps.1729
Pesce S, Margoum C, Rouard N et al (2013) Freshwater sediment pesticide biodegradation potential as an ecological indicator of microbial recovery following a decrease in chronic pesticide exposure: a case study with the herbicide diuron. Ecol Indic 29:18–25. https://doi.org/10.1016/j.ecolind.2012.12.014
Pesce S, Lyautey E, Foulquier A (2017) Réponse structurelle et fonctionnelle des communautés microbiennes hétérotrophes benthiques aux contaminants: quelle conséquence pour le fonctionnement de l’écosystème? In Bernard C, Mougin C, Péry A (eds) Ecotoxicologie, des communautés au fonctionnement des écosystèmes. ISTE Editions, pp 31–54
Pesce S, Lambert AS, Morin S et al (2018) Experimental warming differentially influences the vulnerability of phototrophic and heterotrophic periphytic communities to copper toxicity. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.01424
Pinder JE, Hinton TG, Taylor BE, Whicker FW (2011) Cesium accumulation by aquatic organisms at different trophic levels following an experimental release into a small reservoir. J Environ Radioact 102:283–293. https://doi.org/10.1016/j.jenvrad.2010.12.003
Proia L, Lupini G, Osorio V et al (2013a) Response of biofilm bacterial communities to antibiotic pollutants in a Mediterranean River. Chemosphere 92:1126–1135. https://doi.org/10.1016/j.chemosphere.2013.01.063
Proia L, Osorio V, Soley S et al (2013b) Effects of pesticides and pharmaceuticals on biofilms in a highly impacted river. Environ Pollut 178:220–228. https://doi.org/10.1016/j.envpol.2013.02.022
Prokes R, Vrana B, Klanova J (2012) Levels and distribution of dissolved hydrophobic organic contaminants in the Morava River in Zlin District, Czech Republic as derived from their accumulation in silicone rubber passive samplers. Environ Pollut 166:157–166
Quesada S, Tena A, Guillen D et al (2014) Dynamics of suspended sediment borne persistent organic pollutants in a large regulated Mediterranean River (Ebro, NE Spain). Sci Total Environ 473:381–390
Robson BJ, Barmuta LA (1998) The effect of two scales of habitat architecture on benthic grazing in a river. Freshw Biol 39:207–220. https://doi.org/10.1046/j.1365-2427.1998.00271.x
Rodrigues MLK, Formoso MLL (2006) Geochemical distribution of selected heavy metals in stream sediments affected by tannery activities. Water Air Soil Pollut 169:167–184. https://doi.org/10.1007/s11270-006-1925-6
Roeselers G, van Loosdrecht MCM, Muyzer G (2008) Phototrophic biofilms and their potential applications. J Appl Phycol 20:227–235. https://doi.org/10.1007/s10811-007-9223-2
Roessink I, Moermond CTA, Gillissen F, Koelmans AA (2010) Impacts of manipulated regime shifts in shallow lake model ecosystems on the fate of hydrophobic organic compounds. Water Res 44:6153–6163. https://doi.org/10.1016/j.watres.2010.07.013
Romani AM, Sabater S (2001) Structure and activity of rock and sand biofilms in a Mediterranean stream. Ecology 82:3232–3245
Romani AM, Guasch H, Munoz I et al (2004) Biofilm structure and function and possible implications for riverine DOC dynamics. Microb Ecol 47:316–328. https://doi.org/10.1007/s00248-003-2019-2
Rossi F, Pesce S, Mallet C et al (2018) Interactive effects of pesticides and nutrients on microbial communities responsible of litter decomposition in streams. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02437
Ruhí A, Acuña V, Barceló D et al (2016) Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci Total Environ 540:250–259. https://doi.org/10.1016/j.scitotenv.2015.06.009
Sabater S, Guasch H, Munoz I, Romani A (2006) Hydrology, light and the use of organic and inorganic materials as structuring factors of biological communities in Mediterranean streams. Limnetica 25:335–348
Saeedi M, Hosseinzadeh M, Rajabzadeh M (2011) Competitive heavy metals adsorption on natural bed sediments of Jajrood River, Iran. Environ Earth Sci 62:519–527. https://doi.org/10.1007/s12665-010-0544-0
Schaller J, Brackhage C, Mkandawire M, Dudel EG (2011) Metal/metalloid accumulation/remobilization during aquatic litter decomposition in freshwater: a review. Sci Total Environ 409:4891–4898. https://doi.org/10.1016/j.scitotenv.2011.08.006
Schmitt J, Nivens D, White DC, Flemming H-C (1995) Changes of biofilm properties in response to sorbed substances – an FTIR-ATR study. Water Sci Technol 32:149–155. https://doi.org/10.1016/0273-1223(96)00019-4
Schneck F, Schwarzbold A, Melo AS (2013) Substrate roughness, fish grazers, and mesohabitat type interact to determine algal biomass and sediment accrual in a high-altitude subtropical stream. Hydrobiologia 711:165–173. https://doi.org/10.1007/s10750-013-1477-x
Schorer M, Eisele M (1997) Accumulation of inorganic and organic pollutants by biofilms in the aquatic environment. Water Air Soil Pollut 99:651–659. https://doi.org/10.1023/a:1018384616442
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14:389–397. https://doi.org/10.1016/j.tim.2006.07.001
Smolders AJP, Lock RAC, Van der Velde G et al (2003) Effects of mining activities on heavy metal concentrations in water, sediment, and macroinvertebrates in different reaches of the Pilcomayo River, South America. Arch Environ Contam Toxicol 44:0314–0323. https://doi.org/10.1007/s00244-002-2042-1
Sole M, Kellner H, Brock S et al (2008) Extracellular laccase activity and transcript levels of putative laccase genes during removal of the xenoestrogen technical nonylphenol by the aquatic hyphomycete Clavariopsis aquatica. FEMS Microbiol Lett 288:47–54. https://doi.org/10.1111/j.1574-6968.2008.01333.x
Sridhar KR, Krauss G, Barlocher F et al (2001) Decomposition of alder leaves in two heavy metal-polluted streams in Central Germany. Aquat Microb Ecol 26:73–80. https://doi.org/10.3354/ame026073
Sridhar KR, Barlocher F, Wennrich R et al (2008) Fungal biomass and diversity in sediments and on leaf litter in heavy metal contaminated waters of Central Germany. Fundam Appl Limnol 171:63–74. https://doi.org/10.1127/1863-9135/2008/0171-0063
Tao F, Jiantong L, Bangding X et al (2005) Mobilization potential of heavy metals: a comparison between river and lake sediments. Water Air Soil Pollut 161:209–225. https://doi.org/10.1007/s11270-005-4284-9
Thevenon F, Graham ND, Chiaradia M et al (2011) Local to regional scale industrial heavy metal pollution recorded in sediments of large freshwater lakes in Central Europe (lakes Geneva and Lucerne) over the last centuries. Sci Total Environ 412–413:239–247. https://doi.org/10.1016/j.scitotenv.2011.09.025
Trinh SB, Hiscock KM, Reid BJ (2012) Mechanistic insights into the role of river sediment in the attenuation of the herbicide isoproturon. Environ Pollut 170:95–101. https://doi.org/10.1016/j.envpol.2012.05.026
Vallée R, Dousset S, Billet D, Benoit M (2014) Sorption of selected pesticides on soils, sediment and straw from a constructed agricultural drainage ditch or pond. Environ Sci Pollut Res 21:4895–4905. https://doi.org/10.1007/s11356-013-1840-5
van Hullebusch ED, Zandvoort MH, Lens PNL (2003) Metal immobilisation by biofilms: mechanisms and analytical tools. Rev Environ Sci Biotechnol 2:9–33. https://doi.org/10.1023/B:RESB.0000022995.48330.55
Vila-Costa M, Gioia R, Aceña J et al (2017) Degradation of sulfonamides as a microbial resistance mechanism. Water Res 115:309–317. https://doi.org/10.1016/j.watres.2017.03.007
Vilchez R, Gomez-Silvan C, Purswani J et al (2011) Characterization of bacterial communities exposed to Cr(III) and Pb(II) in submerged fixed-bed biofilms for groundwater treatment. Ecotoxicology 20:779–792. https://doi.org/10.1007/s10646-011-0629-x
Vinot I, Pihan JC (2005) Circulation of copper in the biotic compartments of a freshwater dammed reservoir. Environ Pollut 133:169–182. https://doi.org/10.1016/j.envpol.2004.03.002
Walters DM, Fritz KM, Johnson BR et al (2008) Influence of trophic position and spatial location on polychlorinated biphenyl (PCB) bioaccumulation in a stream food web. Env Sci Technol 42:2316–2322
Walters DM, Mills MA, Cade BS, Burkard LP (2011) Trophic magnification of PCBs and its relationship to the octanol-water partition coefficient. Environ Sci Technol 45:3917–3924. https://doi.org/10.1021/es103158s
Walters DM, Rosi-Marshall E, Kennedy TA et al (2015) Mercury and selenium accumulation in the Colorado River food web, Grand Canyon, USA. Env Toxicol Chem 34:2385–2394. https://doi.org/10.1002/etc.3077
Wang H, Kostel JA, St. Amand AL, Gray KA (1999) 2. The response of a laboratory stream system to PCB exposure: study of periphytic and sediment accumulation patterns. Water Res 33:3749–3761. https://doi.org/10.1016/S0043-1354(99)00126-8
Wang W, Wang W, Zhang X, Wang D (2002) Adsorption of p-chlorophenol by biofilm components. Water Res 36:551–560. https://doi.org/10.1016/S0043-1354(01)00267-6
Wang Z, Yang YY, Sun WM et al (2014) Nonylphenol biodegradation in river sediment and associated shifts in community structures of bacteria and ammonia-oxidizing microorganisms. Ecotoxicol Environ Saf 106:1–5
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182:2675–2679. https://doi.org/10.1128/jb.182.10.2675-2679.2000
White C, Gadd GM (1987) The uptake and cellular distribution of zinc in Saccharomyces cerevisiae. Microbiology 133:727–737. https://doi.org/10.1099/00221287-133-3-727
White C, Gadd GM (1995) Determination of metals and metal fluxes in algae and fungi. Sci Total Environ 176:107–115. https://doi.org/10.1016/0048-9697(95)04836-7
Wolfaardt GM, Lawrence JR, Headley JV et al (1994) Microbial exopolymers provide a mechanism for bioaccumulation of contaminants. Microb Ecol 27:279–291. https://doi.org/10.1007/BF00182411
Wolfaardt GM, Lawrence JR, Robarts RD, Caldwell DE (1998) In situ characterization of biofilm exopolymers involved in the accumulation of chlorinated organics. Microb Ecol 35:213–223. https://doi.org/10.1007/s002489900077
Writer JH, Ryan JN, Barber LB (2011) Role of biofilms in sorptive removal of steroidal hormones and 4-nonylphenol compounds from streams. Environ Sci Technol 45:7275–7283. https://doi.org/10.1021/es2008038
Wuertz S, Müller E, Spaeth R et al (2000) Detection of heavy metals in bacterial biofilms and microbial flocs with the fluorescent complexing agent Newport Green. J Ind Microbiol Biotechnol 24:116–123. https://doi.org/10.1038/sj.jim.2900784
Wunder DB, Bosscher VA, Cok RC, Hozalski RM (2011) Sorption of antibiotics to biofilm. Water Res 45:2270–2280. https://doi.org/10.1016/j.watres.2010.11.013
Xie L, Funk DH, Buchwalter DB (2010) Trophic transfer of Cd from natural periphyton to the grazing mayfly Centroptilum triangulifer in a life cycle test. Environ Pollut 158:272–277. https://doi.org/10.1016/j.envpol.2009.07.010
Yang SI, George GN, Lawrence JR et al (2016) Multispecies biofilms transform selenium oxyanions into elemental selenium particles: studies using combined synchrotron X-ray fluorescence imaging and scanning transmission X-ray microscopy. Environ Sci Technol 50:10343–10350. https://doi.org/10.1021/acs.est.5b04529
Ylla I, Borrego C, Romani AM, Sabater S (2009) Availability of glucose and light modulates the structure and function of a microbial biofilm. FEMS Microbiol Ecol 69:27–42. https://doi.org/10.1111/j.1574-6941.2009.00689.x
Zou K, Thebault E, Lacroix G, Barot S (2016) Interactions between the green and brown food web determine ecosystem functioning. Funct Ecol. https://doi.org/10.1111/1365-2435.12626
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Table S1
Experimental data (from 34 published articles) including chemical concentration in surface water, sediment, and biofilm as well as calculated BCF (L g−1). NA not applicable, n.d. not determined (XLSX 462 kb)
Fig. S1
Bioconcentration factor, expressed as log(BCF) of metals in periphytic biofilms vs. dissolved concentrations of metals in surface water (μg L−1). Data points circled in red are observations from laboratory experiments; all other points are observations from field studies (n = 218; data from 14 published studies) (ZIP 6 kb)
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bonnineau, C. et al. (2020). Role of Biofilms in Contaminant Bioaccumulation and Trophic Transfer in Aquatic Ecosystems: Current State of Knowledge and Future Challenges. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 253. Reviews of Environmental Contamination and Toxicology, vol 253. Springer, Cham. https://doi.org/10.1007/398_2019_39
Download citation
DOI: https://doi.org/10.1007/398_2019_39
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52540-8
Online ISBN: 978-3-030-52541-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)